Abstract
Interferon (IFN)-induced antiviral responses are mediated through a variety of proteins, including the double-stranded RNA-dependent protein kinase PKR. Here we show that fibroblasts derived from PKR−/− mice are more permissive for vesicular stomatitis virus (VSV) infection than are wild-type fibroblasts and demonstrate a deficiency in alpha/beta-IFN-mediated protection. We further show that mice lacking PKR are extremely susceptible to intranasal VSV infection, succumbing within days after instillation with as few as 50 infectious viral particles. Again, alpha/beta-IFN was unable to rescue PKR−/− mice from VSV infection. Surprisingly, intranasally infected PKR−/− mice died not from pathology of the central nervous system but rather from acute infection of the respiratory tract, demonstrating high virus titers in the lungs compared to similarly infected wild-type animals. These results confirm the role of PKR as the major component of IFN-mediated resistance to VSV infection. Since previous reports have shown PKR to be nonessential for survival in animals challenged with encephalomyocarditis virus, influenza virus, and vaccinia virus (N. Abraham et al., J. Biol. Chem. 274:5953–5962, 1999; Y. Yang et al., EMBO J. 14:6095–6106, 1995), our findings serve to highlight the premise that host dependence on the various mediators of IFN-induced antiviral defenses is pathogen specific.
Interferons (IFNs) have for some time now been recognized as the cytokines responsible for conferring an antiviral state on cells of the host. These effects are mediated by the gene products activated or induced following the interaction of IFNs with their cognate receptor(s). Several pathways have been identified, the most actively studied of which include the Mx proteins, 2′-5′-adenylate synthase/RNase L system, inducible nitric oxide synthase, and double-stranded RNA (dsRNA)-activated protein kinase PKR. Each of these systems affects the viral life cycle in different fashions. The Mx proteins for example, members of the dynamin superfamily, are thought to, among other things, inhibit the transport of virus nucleocapsids into the nucleus, thereby preventing viral transcription (19). Influenza virus, vesicular stomatitis virus (VSV), and measles virus are just some of the viruses demonstrating Mx protein-dependent growth suppression (11). Interestingly, most laboratory strains of mice possess naturally occurring mutations in the two murine Mx genes cloned to date and have shown an increased susceptibility to influenza virus and VSV infections compared with that of wild-type mice (13, 14). The 2′-5′-adenylate synthase/RNase L pathway represents a multienzyme system which is activated upon the binding of dsRNA to 2′-5′-adenylate synthase, which in turn produces 2′-5′-oligoadenylates (2′-5′-A) that stimulate RNase L to degrade viral RNAs (16). RNase L has been shown previously to be protective against encephalomyocarditis virus (EMCV) (38) and vaccinia virus (8). Cytokine-induced nitric oxide synthase has been shown to be protective against several viruses including vaccinia virus (15), herpes simplex virus type 1 (25), VSV (3), and reovirus (29). Inducible nitric oxide synthase is thought to inhibit late stages of viral replication including protein synthesis (12, 18), viral protease activity (33), viral DNA synthesis (12, 26), and viral particle formation (12). PKR is an IFN-inducible serine/threonine protein kinase activated subsequent to the binding of dsRNA. Once activated, PKR inhibits protein translation by phosphorylating eukaryotic initiation factor 2α. PKR has also demonstrated a role in both virus- and stress-induced apoptosis (7, 17, 35, 37) and may thereby represent a multifunctional antiviral protein.
Two distinct homozygous disruptions for the PKR gene have been described previously by independent groups (1, 36). Yang et al. (36) challenged their PKR−/− mice with EMCV but found no difference in survival from that of wild-type animals. This group did, however, demonstrate a reduction in protective effect from pretreatment with either gamma IFN (IFN-γ) or the dsRNA analogue polyinosine-polycytosine [poly(I · C)]. These PKR−/− mice demonstrated normal IFN-α/β responses; however, mouse embryo fibroblasts (MEFs) from these animals showed impaired induction of IFN-α/β and reduced activation of NF-κB following poly(I · C) treatment. Abraham et al. (1) found no deficiency in resistance to either influenza virus or vaccinia virus in vivo, while IFN-α/β signaling, hematopoiesis, apoptosis, and eukaryotic initiation factor 2α phosphorylation were found to be indistinguishable in tissues derived from PKR−/− mice and in tissues from wild-type mice.
The present study was undertaken to examine the role of PKR in the IFN-mediated resistance to VSV infection. PKR−/− mice and MEFs derived therefrom were tested, with or without IFN pretreatment, for their ability to resist VSV infection. We show here that PKR does indeed play a crucial role in antiviral defense and may represent the dominant IFN-mediated response to VSV in vivo, particularly in the respiratory system.
MATERIALS AND METHODS
Virus.
The Indiana serotype of VSV was used throughout this study and was propagated in L929 cells.
Assaying virus production in primary MEFs.
Primary MEF cultures were established from PKR−/− (1) and BALB/c E13.5 embryos as described previously (23). For analysis of MEF susceptibility to VSV infection, MEFs were seeded to 80% confluence in 35-mm-diameter dishes containing 2 ml of α minimum essential medium (Gibco/BRL, Burlington, Ontario, Canada) supplemented with 10% fetal bovine serum (FBS) and either not treated or supplemented with various doses of IFN and then incubated for 16 h. Virus diluted in medium to an appropriate multiplicity of infection (MOI) was added to the dishes and allowed to adsorb for 30 min at 37°C. The dishes were subsequently rinsed three times with phosphate-buffered saline (PBS), overlaid with 2 ml of medium, and incubated for 8 h. For virus-laden medium from these dishes, titers were then determined as described previously (4) with minor modifications. Briefly, virus-containing media were serially diluted in α minimum essential medium supplemented with 10% FBS. Each virus inoculum was allowed to adsorb to a monolayer of L cells for 30 min at 37°C and then overlaid with 0.5% agarose in medium. Following an overnight incubation at 37°C, the agar was removed and the monolayers were fixed in 4% paraformaldehyde and stained with 0.5% methylene blue. Plaques were counted visually.
Analysis of viral protein production by 35S in vivo labeling.
MEFs were infected with VSV at an MOI of 3 PFU/cell as described above. At 2, 4, 6, and 8 h postinfection (p.i.), the dishes were rinsed with PBS and the cells were harvested and lysed directly into sodium dodecyl sulfate (SDS) sample buffer (34). One hour prior to each time point (i.e., at 1, 3, 5, and 7 h p.i.), the cells were pulsed with 50 μCi of 35S-labeled methionine-cysteine (1,000 Ci/mmol; ICN Pharmaceuticals, Costa Mesa, Calif.) in Met-Cys-deficient medium (Gibco/BRL) supplemented with 1% FBS. The lysates were boiled, and the labeled proteins were separated by SDS-polyacrylamide gel electrophoresis and visualized using a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
Experimental infection of mice.
Eight- to ten-week-old female mice were used throughout. Anesthetized mice were infected intranasally (i.n.) with VSV diluted in 50 μl of PBS into the nares of each animal. Subcutaneous infection proceeded without prior anesthetic, with a 50-μl dose of virus diluted in PBS. For intravenous (i.v.) infection, mice were catheterized into the tail vein and injected with VSV diluted in 100 μl of PBS, and then the catheter was subsequently flushed with another 100 μl of PBS to ensure a consistent dose. In all cases, mice were monitored for up to 14 days after infection for weight loss, piloerection, group huddling, dehydration, respiratory distress, and hind limb paralysis. Animals displaying hind limb paralysis or severe morbidity were scored as such and euthanatized in the interests of animal welfare.
IFN treatment of animals was by intraperitoneal injection of either mouse IFN-α/β (Cytimmune; Lee Biomolecular Research Inc., San Diego, Calif.) or mouse IFN-γ (Boehringer, Mannheim, Germany) as indicated, 24 h prior to viral infection.
Determination of tissue viral titers.
Mice were sacrificed, and organs were aseptically removed and snap frozen on dry ice. Specimens were homogenized in 2 ml of PBS on ice, and titers were determined on L-cell monolayers as described above.
RESULTS
PKR−/− MEFs are more permissive for VSV infection than are control MEFs.
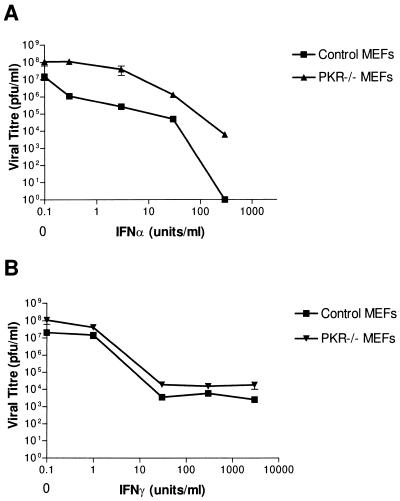
Primary MEFs derived from PKR−/− mice and control mice were assayed for their permissiveness for VSV infection (Fig. 1). IFN-treated or untreated MEFs were infected with VSV at an MOI of 0.1 PFU/cell and allowed to produce virus for 24 h. For the medium from these dishes, the titers were then determined to determine the number of infectious viral particles (PFU) produced from these cells, with or without pretreatment with IFN-α/β or IFN-γ. We observed a slight increase in the amount of virus produced from PKR−/− MEFs over that from control MEFs (Fig. 1), indicating that PKR−/− fibroblasts are moderately more permissive for VSV infection. Increasing doses of IFN-γ protected PKR−/− and control MEFs to similar degrees at each dose given, indicating no deficiency in the IFN-γ pathway (Fig. 1B). We did, however, see a consistently greater viral titer produced from infected PKR−/− MEFs than from control MEFs, regardless of IFN-γ dose. IFN-α/β-mediated responses showed a marked deficiency in PKR−/− MEFs, as doses of IFN-α/β which completely protected control MEFs (7-log reduction in titer) were able to reduce the titer in PKR−/− MEFs by only 4 logs to 104 PFU/ml (Fig. 1A).
FIG. 1.
MEFs from PKR−/− animals show a slight increase in VSV production and an IFN-α/β deficiency compared to control MEFs. Primary embryo fibroblasts from PKR−/− and BALB/c mice (control) were either untreated or treated with various doses of IFNs 18 h prior to their infection with VSV at an MOI of 0.1 PFU/cell. The titers of infectious viral particles produced from these infections were determined as described in Materials and Methods and are represented as PFU per milliliter. Untreated PKR−/− MEFs were slightly more productive for VSV infection (5- to 10-fold) than were untreated control MEFs. (A) Control MEFs showed dose-dependent reductions in titer when pretreated with IFN-α/β, with 300 IU/ml providing complete protection. PKR−/− MEFs, however, responded less well, producing between 1 and 4 logs more virus than did control MEFs, depending on the dose of IFN given. (B) IFN-γ pretreatment protected PKR−/− and control MEFs to similar degrees; however, PKR−/− MEFs consistently produced two- to fivefold-more virus than did controls regardless of IFN dose. Data represent means ± standard errors of the means of triplicate experiments.
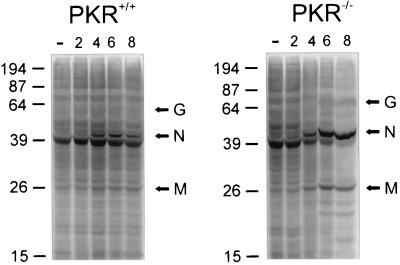
We also monitored the kinetics of infection in MEFs using [35S]methionine in vivo labeling following infection of MEFs at a dose of 3 PFU/cell. For PKR−/− MEFs (Fig. 2), we detected the appearance of viral proteins at about 4 h p.i., with bands representing VSV G, N, and M proteins increasing in intensity relative to host proteins as time progressed. Control MEFs also expressed viral proteins at 4 h p.i., but the intensity of these bands did not appear to increase appreciably during the remaining course of infection as it had for the PKR−/− MEFs. It appears from these experiments that, although the onset of infection is similar between wild-type and PKR−/− MEFs, control fibroblasts are capable of limiting the infection while, in PKR−/− cells, the kinetics of VSV replication are unabated.
FIG. 2.
SDS-polyacrylamide gel electrophoresis separation of 35S-labeled proteins synthesized in VSV-infected MEFs from PKR−/− and control animals. MEFs were either not infected (−) or infected with VSV at an MOI of 3 for 2, 4, 6, or 8 h as described in Materials and Methods. The infected cells at each time point were pulsed with [35S]methionine-cysteine for 1 h prior to being harvested and lysed. VSV proteins (G, N, and M) begin to appear in PKR−/− and control cells at 4 h p.i. While this pattern of labeled proteins remains constant in control cells up to 8 h p.i. viral proteins become the predominant translated proteins in the PKR−/− samples by 8 h p.i. Molecular masses are indicated at left of each panel in kilodaltons.
PKR−/− mice demonstrate an acute susceptibility to i.n. VSV infection.
To further investigate the role of PKR in the defense against VSV, we infected PKR−/− mice i.n. with various doses and monitored their survival. Table 1 reveals the extreme sensitivity of PKR−/− mice to i.n. VSV infection, even down to the minimum dose of 50 PFU. None of the mice lacking PKR could survive beyond 5 days p.i. This is in contrast to the PKR+/+ control mice that we tested; BALB/c, CD1, and BALB/c × 129 mouse strains, treated with the highest dose tested, all survived past 5 days. We have found the 50% lethal dose (LD50) for PKR−/− mice infected i.n. with VSV to be <15 PFU (data not shown), while the LD50 for CD1 and BALB/c × 129 mice was greater than 106 PFU (data not shown). BALB/c animals, which have been reported previously to be sensitive to VSV due to their major histocompatibility complex class I-restricted cytolytic T-cell response (9), demonstrated an LD50 of 104 PFU (our data and reference 31). Also, while the normal pathology of VSV infection in mice is manifested in the central nervous system, most notably as hind limb paralysis, all PKR−/− animals infected with VSV appeared to die of respiratory failure.
TABLE 1.
PKR−/− or PKR+/+ mice infected i.n. with various doses of VSVa
| Genetic background | Strain | i.n. dose (PFU) | No. of mice surviving at day 5/total no. of mice |
|---|---|---|---|
| PKR+/+ | BALB/c | 5 × 104 | 5/5 |
| CD1 | 5 × 104 | 5/5 | |
| BALB/c × 129 | 5 × 104 | 5/5 | |
| PKR−/− | BALB/c × 129 | 5 × 104 | 0/5 |
| 5 × 103 | 0/4 | ||
| 5 × 102 | 0/3 | ||
| 5 × 101 | 0/3 |
PKR−/− and PKR+/+ control mice were infected i.n. with various doses, and their survival was monitored over time. All PKR−/− mice succumbed to the infection between days 2 and 5 depending on the dose, while control mice remained alive beyond this point.
IFN cannot rescue PKR−/− animals from i.n. VSV infection.
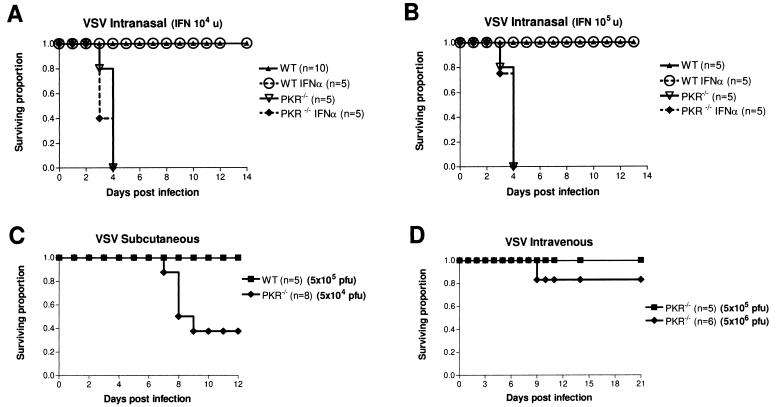
Animals nullizygous for PKR and infected with VSV by the i.n. route demonstrate a significantly decreased survival rate compared to that for control mice (Fig. 3A and B). At this dose (5 × 104 PFU i.n.), all PKR−/− mice died fairly synchronously by day 4. All these animals exhibited piloerection and rapid breathing as early as 16 h after infection, weight loss (3 to 5% of total weight per day), and squinting of the eyes (by day 2). By day 4 p.i., most animals were in respiratory distress, many with severe distress as denoted by “crackles and pops” that were heard while they were breathing. If allowed to continue, the animal died within the hour. This is in stark contrast to PKR+/+ control (BALB/c × 129) animals, which all survived past day 14 and never exhibited the respiratory distress manifested by the knockout animals. Control mice sometimes showed mild piloerection, eye squinting, and weight loss but appeared to recover from these symptoms by day 6 p.i. To investigate the contribution of PKR in the IFN resistance to VSV, both control and PKR−/− mice were pretreated with 2 × 104 or 2 × 105 U of mouse IFN-α/β 18 h prior to i.n. infection with VSV (Fig. 3A and B, respectively). BALB/c × 129 control animals again showed no mortality but also demonstrated complete alleviation of the above-mentioned symptoms at both doses of IFN following VSV infection (Fig. 3A and B). BALB/c control mice infected at the same dose of VSV (five times their LD50) were also protected with IFN pretreatment, increasing their median time to death twofold (data not shown). PKR−/− mice, however, showed no increase in survival (Fig. 3A and B), no alleviation of symptoms, and no change in median time to death with IFN pretreatment (data not shown), even at the highest dose.
FIG. 3.
PKR−/− mice are acutely sensitive to i.n. VSV infection and demonstrate a deficiency in IFN-mediated resistance. (A and B) PKR−/− and control mice (BALB/c × 129) were infected i.n. with 5 × 104 PFU of VSV and monitored for morbidity and survival over the course of 14 days, after which remaining animals were deemed to have survived the infection. PKR−/− mice showed a severe decrease in survival compared to that of control mice (wild type [WT]), succumbing by day 3 or 4, while all control mice survived the infection. IFN-α/β pretreatment (18 h prior to infection) with either 2 × 104 IU (A) or 2 × 105 IU (B) had no protective effect in PKR−/− animals. (C) PKR−/− mice infected subcutaneously (5 × 104 PFU) showed increased survival compared with i.n. infected PKR−/− animals (A and B) but were still more susceptible than subcutaneously infected control mice (BALB/c), which appeared unaffected even at a 10-fold-higher dose (5 × 105 PFU). All PKR−/− animals scored here as fatalities displayed hind limb paralysis and were euthanatized. (D) PKR−/− mice demonstrate resistance to i.v. VSV infection (5 × 105 and 5 × 106 PFU) with all animals except one surviving (one of six in the cohort infected with 5 × 106 PFU). This animal displayed signs of hind limb paralysis and was euthanatized.
Route of infection determines pathology for PKR−/− animals.
The observation that PKR−/− mice succumb to respiratory pathology rather than neuropathology was unexpected. To investigate this further, we infected both control (BALB/c) and PKR−/− animals through various routes and monitored their survival and symptoms. As expected, control animals were completely resistant to subcutaneous VSV infection at a dose of 5 × 105 PFU (Fig. 3C). These mice never showed any signs of infection or distress. In contrast, PKR−/− animals infected at a 10-fold-lower dose demonstrated a marked decrease in survival, with fewer than 40% of the animals remaining after 12 days p.i. Furthermore, these animals did not present with severe respiratory distress, as was the case with i.n. infection, but instead developed hind limb paralysis.
PKR−/− animals infected i.v. showed good resistance to VSV infection at doses as high as 5 × 106 PFU (Fig. 3D). At this dose, only one of six animals showed any signs of sickness, and it was eventually euthanatized at day 9 p.i. after developing hind limb paralysis. Tissues from this animal assayed for VSV showed virus present only in the brain at levels similar to those for i.n. infected animals (data not shown).
PKR−/− mice show high lung titers following i.n. infection.
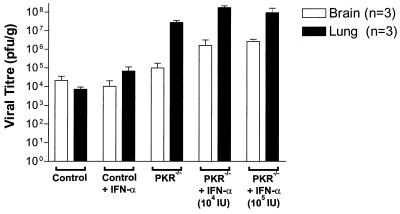
To determine the extent of viral infection, virus titers in assorted tissues were determined following infection through various routes. PKR−/− and control animals (BALB/c) were infected i.n., and on day 4 when PKR−/− animals appeared quite sick, blood, brain, lung, liver, kidney, and spleen tissues were removed aseptically and analyzed to determine the titer of virus present. Control animals showed significant titers of virus in the brain (Fig. 4) and lung while all other organs had no detectable virus (data not shown). PKR−/− mice also showed high titers of virus in the brain but, in contrast, had significantly higher titers (3 logs higher) of VSV in the lungs than did control mice (Fig. 4). As was the case with wild-type mice, all other tissues from PKR−/− animals showed no detectable infection with VSV by this method. Furthermore, pretreatment with increasing doses of IFN-α/β was not able to decrease viral titers in the lungs of PKR−/− animals infected i.n. with VSV (Fig. 4).
FIG. 4.
VSV-infected PKR−/− mice show high lung titers compared with control mice. IFN-α-treated and untreated PKR−/− and control mice were infected i.n. with 5 × 104 PFU and sacrificed for their organs on day 4 p.i. Titers in blood, spleen, kidney, liver, brain, and lung tissues were determined, and only brain and lung tissue showed any detectable virus. Although brain tissue titers from PKR−/− and control mice were similar (<105 PFU/g), lung titers in PKR−/− animals were 2 to 3 logs higher than those seen in control mice. IFN-α/β treatment of PKR−/− mice did not reduce virus titers and perhaps even resulted in a slight increase in both brain and lung tissues. Virus titers are expressed as PFU per gram of tissue. Data represent means ± standard errors of the means of triplicate experiments.
When PKR−/− animals were infected subcutaneously and assayed on day 8 p.i., we could find virus only in the brain while the lungs had very low or undetectable levels of VSV. PKR+/+ control animals, in contrast, had low titers of virus in the brain and no detectable virus in any other organs. i.v. infection with VSV again demonstrated no virus in any organs except the brain (data not shown).
DISCUSSION
Biochemical data from many laboratories have clearly defined PKR as an IFN-inducible gene product whose enzymatic activity is stimulated by dsRNA (2, 10, 20, 27). Because of these properties, PKR has been predicted to play a major role in IFN-mediated antiviral defense. Indeed, PKR demonstrated an antiviral role in cultured cells following various means of overexpression of the wild type or catalytically inactive mutants (21, 22, 28). However, previous studies from our lab and others have failed to demonstrate a definitive role for PKR on an organismal level following genetic ablation of PKR in mice (1, 36). Yang et al. (36) challenged their PKR−/− mice with EMCV (∼1,000 PFU i.v.) and found no difference in survival from that of wild-type animals, but the mice did show a diminished protective effect from pretreatment with either IFN-γ or the dsRNA analogue poly(I · C). In a recent report, Zhou et al. have also shown only a very slight difference in survival between wild-type animals and the Yang PKR−/− mice following EMCV infection (100 PFU i.v.) (39). These data indicate that, during the course of infection by i.v. injected EMVC, PKR does not play an important role in antiviral defense but can be called upon to bolster defenses if the animal is pretreated with dsRNA or IFN-γ prior to infection. Abraham et al. (1) also found no deficiency in resistance to either influenza virus or vaccinia virus in their PKR−/− mice. As mentioned above, these results were unexpected, as the biochemical evidence predicted a substantial role for PKR in antiviral defense.
We continued to investigate the role of PKR in viral resistance and have observed in this study that MEFs derived from the Abraham mouse (39) are more permissive for VSV infection than are MEFs from wild-type animals (Fig. 1). We further demonstrated that, although IFN-γ treatment was equally protective in PKR−/− MEFs and in control MEFs, IFN-α/β was much less effective in protecting MEFs devoid of PKR than in protecting PKR+/+ control cells (Fig. 1). This deficiency in IFN-α/β-mediated response in PKR−/− MEFs demonstrates the importance of PKR in the IFN-mediated resistance to VSV in fibroblasts. This is consistent with a recent report by Zhou et al. describing similar results with MEFs derived from the Yang mouse (39). They showed a decrease in IFN-α/β-mediated protection in PKR−/− MEFs compared to that in wild-type-derived MEFs (39) and further showed that the IFN-mediated resistance to VSV in cultured cells can be attributed almost exclusively to PKR, as MEFs from mice triply deficient in Mx1, RNase L, and PKR showed no increase in susceptibility to VSV over that of MEFs deficient in PKR alone (39). The differences in susceptibility between wild-type and PKR−/− cultured cells became apparent only in this study, with increased IFN pretreatment; that is to say that untreated MEFs from PKR−/− mice were only two- to fivefold more susceptible to VSV than were control MEFs. This is consistent with our in vitro data showing that, in cultured primary embryo fibroblasts at least, PKR on its own is only slightly protective against VSV infection. IFN-α/β pretreatment, however, can provide complete protection against VSV infection, but only if PKR is present.
To examine this in the whole mouse, we infected PKR−/− mice i.n. with various doses of VSV and observed a significant increase in susceptibility over that of control mice. At doses as low as 50 PFU, all of the PKR−/− mice succumbed to the infection, while all control mice survived. This was surprising considering the modest difference in susceptibility observed for cultured cells from these mice. Firstly, this demonstrates unequivocally that PKR plays a major role in host defense against VSV infection. Without PKR, a mouse cannot survive i.n. infection with even a minute inoculum of VSV (Table 1). Furthermore, since pretreatment with IFN-α/β could not rescue these animals (Fig. 3A and B) or ameliorate their symptoms, PKR may in fact be the major component for the IFN pathway mediating resistance to i.n. VSV infection. This is in good agreement with the data mentioned above for cultured cells but highlights a critical limitation of studies with tissue culture cells. Although a modest correlation was found between PKR and VSV susceptibility in MEFs, in the mouse model presented here PKR is essential for survival. This could perhaps be explained by different tissue sensitivities to the virus. In fact, i.n. infection of PKR−/− mice resulted in an acute respiratory pathology leading to the death of the animal. Tissue titers from PKR−/− mice showed high levels of virus in the lungs and brains, while control animals had equally high titers in the brain but greatly reduced titers in the lungs compared to those of PKR−/− mice (Fig. 4). This is also supported by histological analysis of tissues harvested from infected mice. The lungs of PKR−/− mice showed considerable inflammation of the alveolar ducts, while control mice showed no such pathology (data not shown). IFN-α/β treatment again showed no protective effect in PKR−/− mice, as VSV tissue titers did not decrease with increasing doses of IFN (Fig. 4). In fact, virus tissue titers appeared to increase following IFN pretreatment, although we are not convinced that this represents a true exacerbation of the infection, as increasing doses of IFN-α/β did not seem to result in consistently decreased survival (Fig. 3A and B). These data being taken together with the observed symptoms of respiratory distress displayed by PKR−/− mice during the infection, which were not evident in the control mice, it would appear that tissues in the lungs have an exquisite sensitivity to VSV infection in the absence of the pkr gene product. Interestingly, in a study by Ronni et al. (32) human lung epithelium was shown to have only a modest IFN-α/β response and a slow MxA protein accumulation following infection with influenza A virus. It may be, therefore, that lung epithelium relies heavily on PKR to dampen viral infection until humoral host defenses can be mounted.
The route of inoculation also plays a role in the course of the infection. Subcutaneous infection of PKR−/− mice with 5 × 104 PFU of VSV resulted in 40% survival compared to that for BALB/c animals, which were unaffected even at a 10-fold-higher dose (Fig. 3C). This survival rate is significantly better than that seen when PKR−/− animals were infected i.n. Interestingly, PKR−/− mice did not present with severe respiratory distress, as was the case during i.n. infection, but instead showed neuropathology manifested as hind limb paralysis. As well, PKR−/− animals demonstrated relatively high resistance to VSV infection when virus was delivered i.v. (Fig. 3D). Doses as high as 5 × 106 PFU were well tolerated by these mice, with only one animal affected and subsequently euthanatized due to signs of hind limb paralysis. When the organs of this animal were examined for the presence of VSV, it showed virus in the brain only. Therefore, route of infection determines not only the severity but also the type of pathology seen. This again suggests that PKR−/− mice may be acutely sensitive to infection of the respiratory system, as routes of infection which bypass the respiratory tract result in increased survival of these animals.
It is also formally possible that PKR−/− mice show a deficiency in resistance at the initial sites of infection, thereby determining the tropism of the virus. During the i.n. infection in wild-type animals, VSV initially infects the olfactory receptor neurons and then spreads quickly to the rest of the central nervous system (30). The respiratory epithelium appears to be relatively resistant to VSV (24). Perhaps in PKR−/− mice, however, the infection cannot be dampened by the innate immune system at the respiratory epithelium because of the lack of PKR and therefore the virus is allowed to spread to and eventually inundate the respiratory system, leading to the death of the animal. Although the exact mechanisms remain to be determined, it is clear that mice deficient in PKR do not succumb to VSV via the standard pathology of the central nervous system but die first from a massive infection and inflammation of the respiratory system.
The IFN pathway has many tools by which it protects the host from viral infections. It is becoming clear now that the host relies on each of these mechanisms to various degrees depending on the pathogen at hand. The genetic ablation of PKR in mice has allowed us to determine the contribution of this gene product to IFN-mediated defense. We have shown here that PKR appears to be a critical facet of the innate immune response protecting the host against VSV infection. Other viruses such as EMCV, influenza virus, and vaccinia virus have evolved mechanisms to defeat PKR (5, 6) and therefore have perhaps diminished the role of PKR in resisting these infections, forcing the host instead to rely on other effectors of the IFN pathway to counter these invaders.
ACKNOWLEDGMENTS
This work was funded by a grant from the National Cancer Institute of Canada. D.F.S. was supported by an Ontario Graduate Scholarship in Science and Technology (OGSST).
REFERENCES
- 1.Abraham N, Stojdl D F, Duncan P I, Methot N, Ishii T, Dube M, Vanderhyden B C, Atkins H L, Gray D A, McBurney M W, Koromilas A E, Brown E G, Sonenberg N, Bell J C. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem. 1999;274:5953–5962. doi: 10.1074/jbc.274.9.5953. [DOI] [PubMed] [Google Scholar]
- 2.Bevilacqua P C, Cech T R. Minor-groove recognition of double-stranded RNA by the double-stranded RNA-binding domain from the RNA-activated protein kinase PKR. Biochemistry. 1996;35:9983–9994. doi: 10.1021/bi9607259. [DOI] [PubMed] [Google Scholar]
- 3.Bi Z, Reiss C S. Inhibition of vesicular stomatitis virus infection by nitric oxide. J Virol. 1995;69:2208–2213. doi: 10.1128/jvi.69.4.2208-2213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cave D R, Hendrickson F M, Huang A S. Defective interfering virus particles modulate virulence. J Virol. 1985;55:366–373. doi: 10.1128/jvi.55.2.366-373.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang H, Watson J C, Jacobs B L. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc Natl Acad Sci USA. 1992;89:4825–4829. doi: 10.1073/pnas.89.11.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies M V, Elroy-Stein O, Jagus R, Moss B, Kaufman R J. The vaccinia virus K3L gene product potentiates translation by inhibiting double-stranded-RNA-activated protein kinase and phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J Virol. 1992;66:1943–1950. doi: 10.1128/jvi.66.4.1943-1950.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Der S D, Yang Y L, Weissmann C, Williams B R. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:3279–3283. doi: 10.1073/pnas.94.7.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz-Guerra M, Rivas C, Esteban M. Inducible expression of the 2-5A synthetase/RNase L system results in inhibition of vaccinia virus replication. Virology. 1997;227:220–228. doi: 10.1006/viro.1996.8294. [DOI] [PubMed] [Google Scholar]
- 9.Forger J M, III, Bronson R T, Huang A S, Reiss C S. Murine infection by vesicular stomatitis virus: initial characterization of the H-2d system. J Virol. 1991;65:4950–4958. doi: 10.1128/jvi.65.9.4950-4958.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galabru J, Hovanessian A G, Hovanessian A. Autophosphorylation of the protein kinase dependent on double stranded RNA. J Biol Chem. 1987;262:15538–15544. [PubMed] [Google Scholar]
- 11.Haller O, Frese M, Kochs G. Mx proteins: mediators of innate resistance to RNA viruses. Rev Sci Technol. 1998;17:220–230. doi: 10.20506/rst.17.1.1084. [DOI] [PubMed] [Google Scholar]
- 12.Harris N, Buller R M, Karupiah G. Gamma interferon-induced, nitric oxide-mediated inhibition of vaccinia virus replication. J Virol. 1995;69:910–915. doi: 10.1128/jvi.69.2.910-915.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin H K, Takada A, Kon Y, Haller O, Watanabe T. Identification of the murine Mx2 gene: interferon-induced expression of the Mx2 protein from the feral mouse gene confers resistance to vesicular stomatitis virus. J Virol. 1999;73:4925–4930. doi: 10.1128/jvi.73.6.4925-4930.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin H K, Yamashita T, Ochiai K, Haller O, Watanabe T. Characterization and expression of the Mx1 gene in wild mouse species. Biochem Genet. 1998;36:311–322. doi: 10.1023/a:1018741312058. [DOI] [PubMed] [Google Scholar]
- 15.Karupiah G, Xie Q W, Buller R M, Nathan C, Duarte C, MacMicking J D. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 16.Kerr I M, Brown R E. pppA2′p5′A2′p5′A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci USA. 1978;75:256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kibler K V, Shors T, Perkins K B, Zeman C C, Banaszak M P, Biesterfeldt J, Langland J O, Jacobs B L. Double-stranded RNA is a trigger for apoptosis in vaccinia virus-infected cells. J Virol. 1997;71:1992–2003. doi: 10.1128/jvi.71.3.1992-2003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y M, Son K, Hong S J, Green A, Chen J J, Tzeng E, Hierholzer C, Billiar T R. Inhibition of protein synthesis by nitric oxide correlates with cytostatic activity: nitric oxide induces phosphorylation of initiation factor eIF-2 alpha. Mol Med. 1998;4:179–190. [PMC free article] [PubMed] [Google Scholar]
- 19.Kochs G, Haller O. Interferon-induced human MxA GTPase blocks nuclear import of Thogoto virus nucleocapsids. Proc Natl Acad Sci USA. 1999;96:2082–2086. doi: 10.1073/pnas.96.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhen K L, Samuel C E. Isolation of the interferon-inducible RNA-dependent protein kinase Pkr promoter and identification of a novel DNA element within the 5′-flanking region of human and mouse Pkr genes. Virology. 1997;227:119–130. doi: 10.1006/viro.1996.8306. [DOI] [PubMed] [Google Scholar]
- 21.Lee S B, Bablanian R, Esteban M. Regulated expression of the interferon-induced protein kinase p68 (PKR) by vaccinia virus recombinants inhibits the replication of vesicular stomatitis virus but not that of poliovirus. J Interferon Cytokine Res. 1996;16:1073–1078. doi: 10.1089/jir.1996.16.1073. [DOI] [PubMed] [Google Scholar]
- 22.Lee S B, Esteban M. The interferon-induced double-stranded RNA-activated human p68 protein kinase inhibits the replication of vaccinia virus. Virology. 1993;193:1037–1041. doi: 10.1006/viro.1993.1223. [DOI] [PubMed] [Google Scholar]
- 23.Li E, Bestor T H, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 24.Lundh B, Kristensson K, Norrby E. Selective infections of olfactory and respiratory epithelium by vesicular stomatitis and Sendai viruses. Neuropathol Appl Neurobiol. 1987;13:111–122. doi: 10.1111/j.1365-2990.1987.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 25.MacLean A, Wei X Q, Huang F P, Al-Alem U A, Chan W L, Liew F Y. Mice lacking inducible nitric-oxide synthase are more susceptible to herpes simplex virus infection despite enhanced Th1 cell responses. J Gen Virol. 1998;79:825–830. doi: 10.1099/0022-1317-79-4-825. [DOI] [PubMed] [Google Scholar]
- 26.Melkova Z, Esteban M. Inhibition of vaccinia virus DNA replication by inducible expression of nitric oxide synthase. J Immunol. 1995;155:5711–5718. [PubMed] [Google Scholar]
- 27.Meurs E, Chong K, Galabru J, Thomas N S B, Kerr I M, Williams B R G, Hovanessian A G, Thomas N S, Williams B R. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. doi: 10.1016/0092-8674(90)90374-n. [DOI] [PubMed] [Google Scholar]
- 28.Meurs E F, Watanabe Y, Kadereit S, Barber G N, Katze M G, Chong K, Williams B R G, Hovanessian A G, Williams B R. Constitutive expression of human double-stranded RNA-activated p68 kinase in murine cells mediates phosphorylation of eukaryotic initiation factor 2 and partial resistance to encephalomyocarditis virus growth. J Virol. 1992;66:5805–5814. doi: 10.1128/jvi.66.10.5805-5814.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pertile T L, Karaca K, Sharma J M, Walser M M. An antiviral effect of nitric oxide: inhibition of reovirus replication. Avian Dis. 1996;40:342–348. [PubMed] [Google Scholar]
- 30.Plakhov I V, Arlund E E, Aoki C, Reiss C S. The earliest events in vesicular stomatitis virus infection of the murine olfactory neuroepithelium and entry of the central nervous system. Virology. 1995;209:257–262. doi: 10.1006/viro.1995.1252. [DOI] [PubMed] [Google Scholar]
- 31.Roberts A, Kretzschmar E, Perkins A S, Forman J, Price R, Buonocore L, Kawaoka Y, Rose J K. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J Virol. 1998;72:4704–4711. doi: 10.1128/jvi.72.6.4704-4711.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronni T, Matikainen S, Sareneva T, Melen K, Pirhonen J, Keskinen P, Julkunen I. Regulation of IFN-alpha/beta, MxA, 2′,5′-oligoadenylate synthetase, and HLA gene expression in influenza A-infected human lung epithelial cells. J Immunol. 1997;158:2363–2374. [PubMed] [Google Scholar]
- 33.Saura M, Zaragoza C, McMillan A, Quick R A, Hohenadl C, Lowenstein J M, Lowenstein C J. An antiviral mechanism of nitric oxide: inhibition of a viral protease. Immunity. 1999;10:21–28. doi: 10.1016/S1074-7613(00)80003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stojdl D F, Clarke M W. Trypanosoma brucei: analysis of cytoplasmic Ca2+ during differentiation of bloodstream stages in vitro. Exp Parasitol. 1996;83:134–146. doi: 10.1006/expr.1996.0057. [DOI] [PubMed] [Google Scholar]
- 35.Takizawa T, Ohashi K, Nakanishi Y. Possible involvement of double-stranded RNA-activated protein kinase in cell death by influenza virus infection. J Virol. 1996;70:8128–8132. doi: 10.1128/jvi.70.11.8128-8132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, Reis L F L, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams B R G, Aguet M, Weissmann C. Deficient signalling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeung M C, Liu J, Lau A S. An essential role for the interferon-inducible, double-stranded RNA-activated protein kinase PKR in the tumor necrosis factor-induced apoptosis in U937 cells. Proc Natl Acad Sci USA. 1996;93:12451–12455. doi: 10.1073/pnas.93.22.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou A, Paranjape J, Brown T L, Nie H, Naik S, Dong B, Chang A, Trapp B, Fairchild R, Colmenares C, Silverman R H. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. EMBO J. 1997;16:6355–6363. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou A, Paranjape J M, Der S D, Williams B R, Silverman R H. Interferon action in triply deficient mice reveals the existence of alternative antiviral pathways. Virology. 1999;258:435–440. doi: 10.1006/viro.1999.9738. [DOI] [PubMed] [Google Scholar]