Abstract
Epidemiological studies have shown that human immunodeficiency virus type 2 (HIV-2) is markedly less pathogenic than HIV-1 in vivo. Individuals infected with HIV-2 exhibit a remarkably slow rate of disease development, and these clinical properties have been attributed presumptively to an “attenuated” phenotype of HIV-2 itself. Here, we investigated the impact of coreceptor usage on the cytopathicity of HIV-2 and compared its pathogenic potential with that of HIV-1 in a unique human lymphoid histoculture model. We found that HIV-2 strains, as well as closely related simian immunodeficiency viruses (SIV), displayed mildly or highly aggressive cytopathic phenotypes depending on their abilities to use the coreceptor CCR5 or CXCR4, respectively. A side-by-side comparison of primary X4 HIV-1 and HIV-2 strains revealed similar, high degrees of cytopathicity induced by both HIV types. Furthermore, we found that HIV-2 coreceptor specificity for CCR5 and CXCR4 determined the target cell population for T-cell depletion in lymphoid tissue. Finally, utilization of the alternate coreceptors BOB and Bonzo did not significantly increase the cytopathic properties of HIV-2. These findings demonstrate that coreceptor preference is a key regulator of target cell specificity and the cytopathic potential of HIV-2, with indistinguishable rules compared with HIV-1. Moreover, HIV-2 strains are not characterized by an intrinsically lower cytopathicity than HIV-1 strains. Therefore, direct cytopathic potential per se does not explain the unique behavior of HIV-2 in people, highlighting that other unknown factors need to be elucidated as the basis for their lesser virulence in vivo.
Human immunodeficiency virus type 2 (HIV-2) is prevalent mainly in West Africa and is found less frequently in some Asian countries, Western Europe, and the United States. Human infection with HIV-2 is associated with eventual immunologic failure and AIDS. However, disease progression has been reported to be much slower in HIV-2-infected individuals compared with HIV-1-infected individuals, as evidenced by significantly slower rates of development of abnormal CD4+ T-cell counts and progression to AIDS (20, 25, 32, 40). This attenuated virulence of HIV-2 infection in vivo is a compelling area of investigation in which to elucidate the mechanisms of HIV-induced pathogenesis.
With the discovery of the role of viral coreceptors in cellular entry by HIV, several studies have revealed that nearly all strains of HIV-2 use CD4 together with the chemokine receptors CCR5 and/or CXCR4 (18, 26–28, 37), the molecules that have been defined as the major coreceptors for HIV-1 (reviewed in reference 2). In comparison with HIV-1, HIV-2 typically is more promiscuous in its coreceptor utilization profile (18, 26–28). Specifically, the majority of HIV-2 isolates can use the orphan receptors BOB and/or Bonzo with efficiencies comparable to their usage of CCR5 in in vitro assays, a behavior that is similar to that of the closely related simian immunodeficiency viruses (SIV) (7, 28, 39). The contribution of distinct coreceptor specificities to HIV-2 infection and pathogenesis in vivo nevertheless remains to be established. In HIV-1 infection, viral coreceptor phenotypes typically evolve during the course of infection in that CCR5-specific (R5) viruses predominate in early stages and persist throughout the course of disease, while CXCR4-using (X4) viruses emerge frequently in temporal association with rapid CD4+ T-cell decline and onset of AIDS (10, 33, 35). Several studies have indicated a similar shift of viral coreceptor specificity for CCR5 and CXCR4 during the course of HIV-2 infection (27, 28, 37).
We have shown in previous studies that coreceptor specificity substantially determines the cytopathic potential of HIV-1 and that the X4 phenotype dramatically enhances HIV-1 virulence in mature lymphoid tissue ex vivo. These findings indicated that the emergence of X4 viruses in vivo likely accelerates disease progression in HIV-1-infected individuals due to enhanced cytopathic effects in peripheral lymphoid tissue (29). We also found that additional coreceptors appear to have little impact on the T-cell depletion potential of HIV-1 strains in lymphoid tissues ex vivo (34). Given the similarities in CCR5 and CXCR4 utilization patterns and the striking difference regarding the pathogenic potential compared to HIV-1, an important question is how coreceptor preferences relate to the cytopathic potential of HIV-2.
In the present study, therefore, we used an ex vivo human lymphoid histoculture system to investigate the relationship between coreceptor preferences and cytopathicity of HIV-2. Histocultures of spleen or tonsil specimens recapitulate important aspects of HIV pathogenesis in the tissue microenvironment of major HIV production sites in vivo and have served in previous studies as a valuable model in which to study HIV-1 infection (15, 16, 29, 34). A key feature of this system for studying the impact of HIV coreceptor utilization is that lymphoid histocultures are readily susceptible to HIV infection without requiring exogenous stimulation that can alter chemokine receptor expression patterns (4). Moreover, in this system, the expression levels of CCR5 and CXCR4 on T lymphocytes remain stable throughout the culture period. In the present study, we exploited the lymphoid histoculture system to investigate the impact of coreceptor preferences on the cytopathicity of HIV-2. Our results demonstrate that coreceptor specificity markedly influences the cytopathic potential of HIV-2 and that HIV-2 can be as cytopathic as HIV-1 in mature lymphoid tissue despite its distinct clinical characteristics.
MATERIALS AND METHODS
Viruses and preparation of virus stocks.
The primary HIV-2 isolates A1958 and SLRHC (28) were provided by Beatrice Hahn, and the HIV-1 primary isolate 12/86 (10) was provided by Ruth I. Connor. The HIV-2 strain CBL20 (36) was provided by Robin Weiss, and the primary isolate HIV-2 7924A (14) was provided by Feng Gao and Beatrice Hahn via the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program. Virus stocks of HIV-1 and HIV-2 isolates were established by infection of heterologous phytohemagglutinin-activated peripheral blood mononuclear cells (PBMC) that were propagated with interleukin-2 (IL-2) as described previously (34). Viral stocks of primary SIVsmm FKl and FBo were established by cocultivation of PBMC derived from two naturally infected sooty mangabey monkeys from the Yerkes Regional Primate Research Center with activated mixed heterologous PBMC from uninfected sooty mangabeys. Donor cells for FBo cultivation were activated with concanavalin A (5 μg/ml; Sigma) for 48 h. Donor cells for FK1 cultivation were depleted of CD8 cells (Dynal) and activated with anti-monkey-CD3 antibody (1 μg/ml; Biosource) for 48 h. Both isolates were cocultured in RPMI medium containing 20% fetal calf serum and recombinant human IL-2 (100 U/ml; Chiron). The molecular clone of HIV-2 ST/SXB1 (λJSP4-27) (24) was provided by Beatrice Hahn, the molecular clone pNL4-3 was provided by Malcom Martin via the AIDS Research and Reference Reagent Program, and the molecular clone 49-5 (9) was provided by Bruce Chesebro. The molecular clones SIVmac239, SIVmac316, and SIVmac239PS were described previously (22). Infectious virus stocks were prepared as previously described (6). The p24 and p27 GAG concentrations of HIV-1, HIV-2, and SIV virus stocks were assessed by enzyme-linked immunosorbent assay (NEN Life Sciences, Beckman Coulter, Inc.).
Infection of human lymphoid tissue ex vivo.
Human tonsil and adenoid tissue removed during routine tonsillectomy (provided by San Francisco General Hospital, Kaiser-San Francisco, San Francisco, Calif., and Kaiser-San Rafael, San Rafael, Calif.) was received within 5 h of excision and was sectioned into 2- to 3-mm-thick blocks. The tissue blocks were placed onto collagen sponges in the culture medium as previously described (29), and 5 μl of clarified virus-containing media was applied on top of these tissue blocks (0.1 to 0.5 ng of p24 or p27 for HIV-1, HIV-2, and SIVsmm primary isolates and up to 5 ng of p24 or p27 per tissue block for HIV-1, HIV-2, and SIVmac recombinants) as described previously. Productive HIV infection was assessed by measuring the amount of p24 and p27 antigen that had accumulated in the culture medium during the 3 days between successive changes of medium. Infections in the depletion-kinetics experiment (see Fig. 2) were performed with 5 μl of virus-containing media per tissue block, which represented 7 to 40 50% tissue culture infectious doses as determined by terminal dilution of the virus stocks in quadruplicate on heterologous phytohemagglutinin-activated PBMC propagated with human IL-2 (5 U/ml).
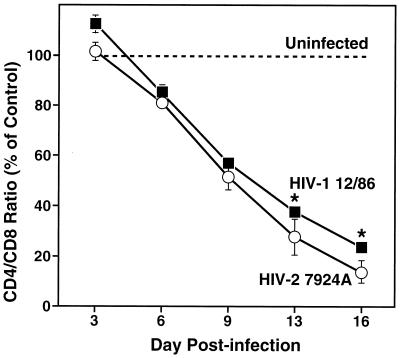
FIG. 2.
Comparable and progressive depletion of CD4+ T cells by multitropic primary isolates of HIV-1 and HIV-2 in ex vivo tonsil cultures. Ex vivo tonsil cultures were inoculated with the primary HIV-1 isolate 12/86 and the primary HIV-2 isolate 7924A, and CD4+ T-cell depletion was assessed by FACS analysis on subsequent days (3, 6, 9, 13, and 16) postinfection. Shown are mean relative CD4/CD8 ratios (n = 3) with SEM (∗, n = 2).
Assessment of CD4+ T-cell depletion by FACS analysis.
On day 12 following infection, cells were mechanically isolated from infected and uninfected control tissue and analyzed by flow cytometry (fluorescence-activated cell sorting [FACS]). Dispersed cells from infected and uninfected lymphoid histocultures were stained for cell surface markers CD3, CD4, CD8, and CCR5 as described previously (29, 34), by using the following monoclonal antibodies: anti-CD3 (clone SK7, phycoerythrin conjugated), anti-CD4 (clone SK3, fluorescein isothiocyanate conjugated), anti-CD8 (clone SK1, PerCP conjugated) (Becton Dickinson) and anti-CCR5 (clone 2D7, antigen-presenting cell conjugated) (Pharmingen). Then, 10,000 lymphocytes positive for CD3 surface marker were counted and the data were analyzed with CELLQUEST software (Becton Dickinson). To facilitate comparison among experiments, CD4+ T-cell depletion was assessed by measuring the ratio of CD4+ to CD8+ T cells. This value was normalized to the CD4/CD8 ratio of control (uninfected) samples to yield the “mean relative CD4/CD8 ratio.” Changes in the CD4/CD8 ratio represented CD4+ T-cell depletion since increases in the numbers of CD3+ CD4− CD8− cells as a result of virus-induced CD4 downregulation were insignificant in infected cultures.
Assessment of coreceptor utilization of SIVsmm isolates.
GHOST cells (NIH AIDS Research and Reference Reagent Program) expressing human CD4 and human chemokine receptors were plated overnight at 40,000 cells/well per 12-well plate. The following day, the cultures were infected with 20 to 50 ng of p27 of SIVsmm. Four days after infection, the cultures were harvested and assayed for green fluorescent protein expression by flow cytometry as an indicator of infection.
RESULTS AND DISCUSSION
Coreceptor specificity correlates with the cytopathic phenotype of HIV-2 strains in lymphoid tissue ex vivo.
In order to elucidate the impact of viral coreceptor specificity on the cytopathic potential of HIV-2, we infected lymphoid histocultures with a panel of HIV-2 isolates that were previously established to exhibit distinct coreceptor utilization patterns in vitro (7, 26, 28). As described previously (16, 29, 34), CD4+ T-lymphocyte depletion in infected cultures of human tonsillar or adenoid tissue was monitored as an indicator of virus-induced cytopathicity. The cultures were harvested on day 12 following infection, at which time the tissue was dispersed and immunostained for CD4 and CD8. FACS analysis was used to measure the ratio of CD4+ and CD8+ T cells in infected versus uninfected cultures, and CD4+ T-cell depletion was detected as a decrease of the CD4/CD8 ratio in infected cultures.
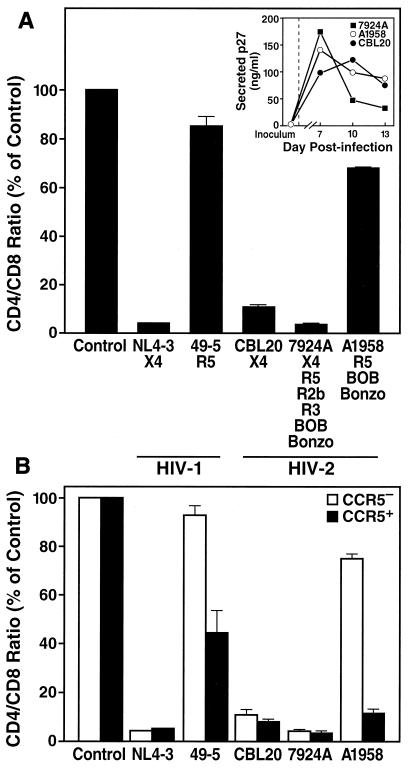
This analysis revealed striking differences regarding the cytopathic potential among different HIV-2 isolates, segregating the HIV-2 strains into two distinct phenotypes. The primary isolate A1958 depleted CD4+ T cells very mildly, as reflected in the slightly depressed CD4/CD8 ratio in infected cultures, whereas the primary isolate 7924A and the T-cell-line-adapted strain CBL20 aggressively depleted CD4+ T cells in these cultures, as reflected in severely depressed CD4/CD8 ratios (Fig. 1A). These differences in cytopathicity were observed despite similar replication kinetics of these viruses, as measured by accumulation of HIV-2 p27 antigen in the culture media between successive media changes (Fig. 1A, inset).
FIG. 1.
The cytopathic phenotype of HIV-2 is linked to coreceptor specificity in ex vivo lymphoid tissue. (A) CD4+ T-cell depletion in adenoid cultures by various HIV-2 strains and two HIV-1 recombinants was assessed by FACS analysis on day 12 postinfection. Shown are mean relative CD4/CD8 ratios (n = 3) with the SEM. Previously reported coreceptor specificities are indicated (7, 26, 28, 29). (Inset) Viral replication of the HIV-2 isolates was monitored by assessing accumulation of p27 in the culture supernatant between successive medium changes. (B) In the same infection, depletion within the CCR5+ and the CCR5− subsets of CD4+ T cells was analyzed by multiparameter FACS on day 12 postinfection. Shown are mean relative CD4/CD8 ratios (n = 3) with the SEM for CCR5+ and CCR5− T cells.
Interestingly, this marked distinction in cytopathic effect corresponded to the difference in coreceptor preferences among these HIV-2 isolates (Fig. 1A). Specifically, the mildly cytopathic strain A1958 has the R5 phenotype, whereas the highly virulent strains CBL20 and 7924A have the X4 phenotype. These results mirror the pattern typically found for HIV-1 infections in lymphoid tissue (16, 29, 34), which is demonstrated here for comparison purposes with a recombinant isogenic HIV-1 virus pair, NL4-3 and 49-5, differing solely in reciprocal specificity for CXCR4 and CCR5, respectively (9, 29, 38). Whereas the R5 strain (49-5) only mildly depressed the CD4/CD8 ratio compared to uninfected control tissue (Fig. 1A), the X4 isogenic counterpart (NL4-3) aggressively depleted CD4+ T cells in these cultures, thereby recapitulating the dramatic impact of CXCR4 specificity on cytopathicity of HIV-1.
These results indicate that viral coreceptor specificity significantly influences the cytopathic potential of HIV-2 in lymphoid tissue. Moreover, HIV-2 cytopathicity seems to be controlled in a manner very similar to that of HIV-1, with specificity for CXCR4 linked to an aggressive depletion phenotype. Furthermore, since both the CXCR4-restricted HIV-2 strain (CBL20) and the multitropic primary isolate (7924A) aggressively depleted CD4+ T cells in these cultures, specificity for CXCR4 appears to be sufficient to confer high cytopathicity to HIV-2 as it does to HIV-1.
X4 HIV-1 and HIV-2 isolates show similar kinetics of CD4+ T-cell depletion.
To define further the cytopathic potential of X4 strains of HIV-2, we performed a side-by-side comparison of the kinetics of CD4+ T-cell depletion by HIV-1 and HIV-2 in lymphoid histocultures (Fig. 2). Tonsil tissue was infected ex vivo with comparable doses of the primary isolates HIV-2 7924A and HIV-1 12/86. Both isolates were derived from patients with advanced disease and displayed expanded coreceptor usage in vitro, including specificity for both CXCR4 and CCR5 (10, 28). Infected and uninfected control tissue was harvested at various time points following infection, and CD4+ T-cell depletion was assessed at each time point. This comparison revealed that both the HIV-1 and the HIV-2 strains progressively and profoundly depleted CD4+ T cells and that they did so with comparable kinetics (Fig. 2), revealing that a lower cytopathicity is not an integral feature of HIV-2.
Coreceptor specificity determines the target cell population for CD4+ T-lymphocyte depletion by HIV-2.
To investigate further the mechanism underlying the differential cytopathicity of HIV-2 in lymphoid histocultures, we stratified the depletion analyses into coreceptor-expressing subsets of CD4+ T cells. Immunostaining of tonsil tissue and FACS analysis revealed that CXCR4 is expressed on the majority of CD4+ T cells (mean ± standard error of the mean [SEM], 88.5% ± 1.6%; n = 25), whereas CCR5 is expressed on a minor subset (mean ± SEM, 10.4% ± 0.8%; n = 25) (see also reference 17). Stratification of the T-cell depletion analysis into CCR5+ (CXCR4+) and CCR5− (CXCR4+) CD4+ T-cell subsets revealed a distinct depletion pattern for the HIV-2 isolates tested. The R5 isolate A1958 preferentially and efficiently depleted within the CCR5+CD4+ subset, whereas the X4 strain HIV-2 CBL20 and the multitropic isolate HIV-2 7924A caused profound depletion in both T-cell subsets (Fig. 1B). These results mirror those observed with the R5 (49-5) and X4 (NL4-3) strains of HIV-1 (Fig. 1B); such subset-specific effects are readily evident despite some degree of overlap between the CCR5+ and CCR5− cell populations in FACS (17). Thus, R5 strains of HIV-2 are highly cytopathic for CCR5-bearing CD4+ T cells, and X4 strains of HIV-2 are cytopathic for the larger set of CD4+ T cells that bear CXCR4, demonstrating that CD4+ T-cell depletion by HIV-2 is highly controlled by coreceptor specificity. Therefore, HIV-2 should be viewed as an intrinsically cytopathic virus, with its overall effects on the T-cell pool determined by the coreceptor properties of the particular viral strain and the cellular expression pattern of CCR5 and CXCR4 within the CD4+ T-cell population.
An attenuated HIV-2 strain displays an R5 depletion phenotype in ex vivo lymphoid tissue.
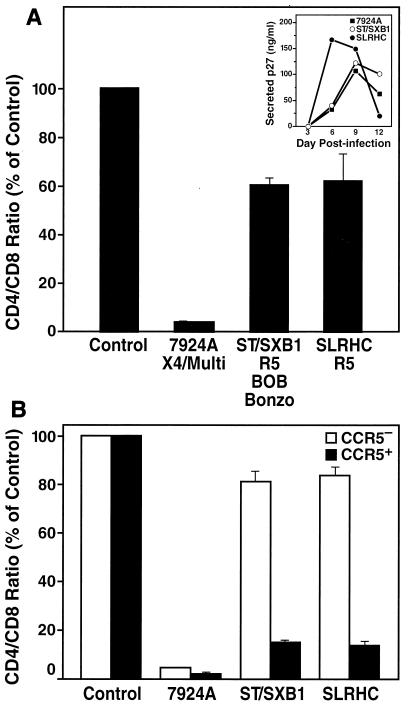
Earlier studies described an attenuated HIV-2 strain (HIV-2 ST) isolated from an asymptomatic HIV-2-infected individual in Senegal, West Africa, which exhibited a noncytopathic phenotype in vitro as indicated by the absence of virus-induced cell fusion or cell death in T-cell lines and peripheral blood lymphocytes (PBL) (23). A recent study has shown that the molecular clone of this virus, HIV-2 ST/SXB1, which displays the biological properties of the parent HIV-2 ST isolate (24), uses CCR5, BOB, and Bonzo, but not CXCR4, as coreceptor (11). Based on our finding that coreceptor preferences highly influence the cytopathic phenotype of HIV-2 in peripheral lymphoid tissue, we hypothesized that the apparent noncytopathic phenotype of HIV-2-ST/SXB1 as assessed in T-cell lines or PBL is a direct consequence of its inability to use CXCR4.
To test this hypothesis, we infected tonsil cultures with HIV-2 ST/SXB1 as well as the X4 primary HIV-2 isolate 7924A and the R5 primary HIV-2 isolate SLRHC for comparison purposes. Twelve days following infection, the tissue was harvested and CD4+ T-cell depletion in infected cultures was analyzed. As predicted, both HIV-2 ST/SXB1 and SLRHC depleted CD4+ T cells only mildly, whereas the X4 strain 7924A depleted cells quite aggressively (Fig. 3A). Furthermore, stratification of the analysis into the CCR5+ and CCR5− CD4+ T-cell subsets revealed that HIV-2 ST/SXB1 preferentially but potently depleted cells within the CCR5+ CD4+ T-cell pool, as did SLRHC (Fig. 3B), thus mirroring the typical depletion phenotype of other R5 HIV-2 and HIV-1 strains (Fig. 1) (16, 29, 34). This finding demonstrates that the reported noncytopathic and nonfusogenic properties of HIV-2 ST/SXB1 in T-cell lines or PBL are likely a consequence of its coreceptor specificity for CCR5 and its inability to use CXCR4, rather than of an intrinsically noncytopathic character. It is of interest to note that both HIV-2 ST/SXB-1 and SLRHC (Fig. 3) were derived from an asymptomatic individual, whereas HIV-2 A1958 (Fig. 1) was isolated from a patient who had progressed to AIDS. Therefore, these results also demonstrate that both early- and late-stage R5 HIV-2 isolates are similarly mildly cytopathic in secondary lymphoid tissue in contrast to X4 HIV-2 isolates, which further highlights the dominant impact of coreceptor specificity on CD4+ T-cell depletion by HIV-2. Together these findings imply that the differential expression of CXCR4 and CCR5 in the lymphoid tissues is a crucial factor for the distinct cytopathic behavior of R5 and X4 HIV-2 strains in our experiments. This principle is consistent with a recent study demonstrating that infection of rhesus monkeys with chimeric SHIV viruses carrying X4 or R5 HIV-1 envelopes led to markedly different pathogenic effects in various lymphoid tissues in vivo, which was interpreted as the result of differential expression of CCR5 and CXCR4 in the respective tissues (19).
FIG. 3.
An HIV-2 strain with attenuated cytopathicity in vitro displays the typical depletion phenotype of R5 strains. (A) Tonsil histocultures were inoculated with HIV-2 ST/SXB1, X4 HIV-2 isolate 7924A, and R5 HIV-2 isolate SLRHC. CD4+ T-cell depletion was assessed on day 12 postinfection. Previously reported coreceptor specificities are indicated (11, 28). Shown are mean relative CD4/CD8 ratios (n = 3) with SEM. (Inset) Viral replication was monitored by assessing accumulation of p27 in the culture supernatant between successive medium changes. (B) In the same infection, depletion within the CCR5+ and the CCR5− subsets of CD4+ T cells was analyzed by multiparameter FACS on day 12 postinfection. Shown are mean relative CD4/CD8 ratios (n = 3) with SEM.
SIV depletes CCR5+ CD4+ T cells in human lymphoid tissue ex vivo.
SIV is closely related to HIV-2 (8), but exhibits an interesting difference regarding its coreceptor utilization profile. SIV isolates from naturally infected, disease-resistant sooty mangabey monkeys (SIVsmm from Cercocebus torquatus atys), as well as SIV strains that cause disease in rhesus macaque monkeys (SIVmac from Macaca mulatta), universally use CCR5 as a coreceptor, and most strains exhibit additional capacities to use the orphan receptors BOB and/or Bonzo (7, 11, 12). In contrast to HIV-1 and HIV-2, however, SIV does not typically evolve to exploit CXCR4 as a coreceptor in vivo, although both macaque and sooty mangabey CXCR4 coreceptors have been demonstrated to be permissive for HIV-1 entry in vitro (7). In view of the close relationship of HIV-2 and its simian ancestor SIVsmm, the differences and similarities in coreceptor dependence among HIV-2 and SIV, and the host-dependent pathogenicity of SIV strains, we compared the CD4+ T-cell depletion phenotype of several SIV strains with that of HIV-2 in human lymphoid histocultures. It had been established earlier that human PBMC support SIV infection (13), and the cross-species infectivity of SIV was further demonstrated by the productive infection of a laboratory worker following accidental exposure to SIVmac (21). These factors provide a rationale for evaluating these viruses in the human lymphoid tissue system.
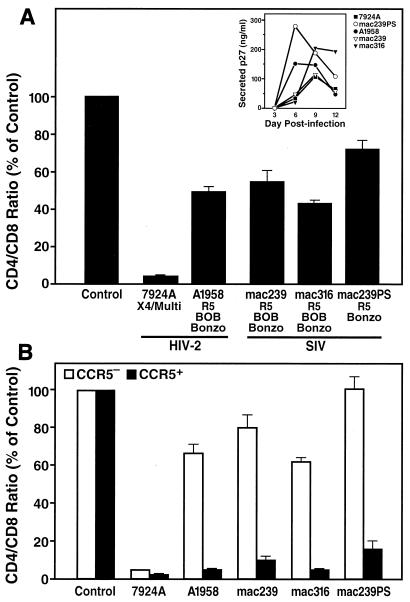
We therefore infected tonsil cultures ex vivo with two recombinant SIV strains (SIVmac239 and SIVmac316) that are highly virulent in rhesus macaques and two primary SIVsmm isolates (SIVsmm FKl and SIVsmm FBo) that were isolated from naturally infected sooty mangabeys. All four SIV strains display specificity for CCR5, BOB, and Bonzo as coreceptors (12, 30) (see Fig. 5). In addition, we performed infections with SIVmac239PS, a derivative of SIVmac239 that is impaired in BOB utilization as a result of a single amino acid substitution in the V3 region of the envelope (30). For comparison purposes we also infected cultures with the X4 primary HIV-2 7924A and the R5 HIV-2 isolate A1958. On day 12 postinfection, infected and uninfected cultures were harvested and analyzed. FACS analysis revealed that all SIVmac strains and the primary SIVsmm isolates mildly depleted CD4+ T cells in these cultures (Fig. 4A and 5A), despite robust replication (Fig. 4A and 5A, insets). Thus, SIVmac and SIVsmm isolates displayed a cytopathic phenotype comparable to that of the R5 HIV-2 A1958 strain (Fig. 4A) and R5 HIV-1 strain (Fig. 1A) (16, 29, 34), but contrasting with the aggressive depletion phenotype of the X4 HIV-2 7924A (Fig. 4A). No significant difference was observed for the cytopathic potential of SIVmac239 and SIVmac239PS, demonstrating that coreceptor specificity for BOB does not enhance viral cytopathicity in peripheral lymphoid tissue.
FIG. 5.
Mild depletion of CD4+ T cells by primary SIVsmm isolates in ex vivo lymphoid tissue. (A) Ex vivo tonsil cultures were inoculated with primary SIVsmm isolates FKl and FBo. CD4+ T-cell depletion was assessed by FACS on day 12 postinfection. Shown are mean relative CD4/CD8 ratios (n = 3) with SEM. (Inset) Viral replication was monitored by assessing accumulation of p27 in the culture supernatant between successive medium changes. (B) In the same infections, T-cell depletion analysis was stratified into CCR5+ and CCR5− CD4+ T-cell subsets by multiparameter FACS. Shown are mean relative CD4/CD8 ratios (n = 3) with SEM for CCR5+ and CCR5− T cells. (C) Coreceptor preferences of SIVsmm isolates FKl and FBo were established by infection of GHOST cells stably expressing CD4 together with various coreceptors. Shown is usage of alternative coreceptors relative to that of CCR5.
FIG. 4.
Mild depletion of CD4+ T cells by recombinant strains of SIVmac in ex vivo lymphoid tissue. (A) Ex vivo tonsil cultures were inoculated with recombinant strains SIVmac239, SIVmac316, and SIVmac239PS and primary HIV-2 isolates A1958 and 7924A. CD4+ T-cell depletion was assessed by FACS on day 12 postinfection. Shown are mean relative CD4/CD8 ratios (n = 3) with SEM. Coreceptor preferences are indicated as described previously (7, 12, 28, 30). (Inset) Viral replication was monitored by assessing accumulation of p27 in the culture supernatant between successive medium changes. (B) In the same infections, T-cell depletion analysis was stratified into CCR5+ and CCR5− CD4+ T-cell subsets by multiparameter FACS. Shown are mean relative CD4/CD8 ratios (n = 3) with SEM for CCR5+ and CCR5− T cells.
Stratification of the T-cell depletion analysis in the CCR5+ and CCR5− subsets of CD4+ T cells further revealed that the SIVmac and SIVsmm strains preferentially and rather potently depleted CCR5+ CD4+ T lymphocytes (Fig. 4B and 5B). This finding suggests that the mild overall CD4+ T-cell depletion induced by these viruses is a consequence of relative inaccessibility of the overall CD4+ T-cell population for these viruses due to restricted cellular expression of CCR5. Furthermore, the coreceptor specificity for BOB and Bonzo does not significantly increase the target cell pool for depletion and/or enhance viral cytopathicity in human lymphoid tissue compared with that of viruses with restricted specificity for CCR5. The expression patterns for BOB and Bonzo in human tissue have been not well established to date, but they clearly do not facilitate aggressive CD4+ T-cell depletion by HIV-2 or SIV in human lymphoid tissue ex vivo. This failure to augment overall CD4+ T-cell depletion is consistent with the in vivo comparison of SIVmac239 and SIVmac239PS that revealed no significant contribution of BOB utilization to viral replication or pathogenesis in the rhesus macaque model (30) and mirrors our finding that alternate coreceptors do not have a major role in T-cell depletion by HIV-1 (34).
In summary, this study demonstrates that primary and T-cell-line-adapted HIV-2 strains display distinct cytopathic phenotypes in peripheral human lymphoid tissue depending on their coreceptor specificities for CXCR4 or CCR5, respectively. As with HIV-1, specificity of HIV-2 for CCR5 alone or in combination with additional coreceptors such as BOB and Bonzo is linked to restricted overall CD4+ T-cell depletion potential, while specificity for CXCR4 is linked to a highly virulent phenotype. As assessed in ex vivo lymphoid cultures, these cytopathic properties of HIV-2 are very similar to those of HIV-1, both quantitatively and qualitatively. We also demonstrate that recombinant and primary strains of SIV, which are genetically closely related to HIV-2 but typically do not exploit CXCR4 as a coreceptor, displayed a rather mild cytopathic phenotype in human peripheral lymphoid tissue mirroring that of R5 HIV-1 and HIV-2. We further observed that an HIV-2 strain that was previously recognized as attenuated in vitro displays cytopathic properties in lymphoid tissue that are similar to these of R5 HIV-1, HIV-2, and SIV strains, indicating that viral coreceptor specificity represents a major regulator of the biological heterogeneity that has been reported for different strains of HIV-2 (1, 5). Finally, we found that coreceptor expression patterns determine the target cell population for CD4+ T-cell depletion by HIV-2 and SIV, which further emphasizes that coreceptor specificity is a key determinant of cytopathicity of HIV-2 in mature lymphoid tissue. Together, our findings strongly suggest that a lower intrinsic cytopathic potential does not underlie the remarkably slower disease progression that is described in many HIV-2-infected individuals (20, 25, 32, 40), since diverse HIV-2 strains exhibited robust CD4+ T-cell depletion potential in lymphoid tissues ex vivo that was indistinguishable from that of HIV-1; of course, studies of additional HIV-2 isolates would be useful for establishing how generalizable these findings are relative to other viral strains.
It is particularly notable that the viral load in the peripheral blood of HIV-2-infected individuals typically is much lower than that in HIV-1-infected individuals. The viral load in vivo is an indicator of viral fitness, or replicative capacity, and viral clearance in the context of the particular host environment and poorly defined viral and/or host features. It is evident that a different dynamic equilibrium of host and virus is established during infection with HIV-2 compared with HIV-1 (3, 31). Therefore, the present results strongly suggest that this equilibrium, rather than the cytopathic character of HIV-2 per se, is responsible for the attenuated effects of HIV-2 in vivo. We speculate that key host immune responses that are not apparent in short-term ex vivo lymphoid cultures operate in vivo to control HIV-2 infection relatively efficiently, as has been proposed previously (41). This conclusion should prompt further investigation to elucidate the basis of this distinct virus-host relationship as a possible foundation for strategies to modulate these processes in patients infected with HIV-1 and/or HIV-2.
ACKNOWLEDGMENTS
We thank Beatrice Hahn, Ruth I. Connor, and Robin Weiss for kindly providing viruses; Bruce Chesebro for kindly providing plasmids; and Dee J. Holthe, Cecilia Stewart, Claudette Delphis, Ursula Perotti, Sharon Hall, Jaqueline Hylton, Nancy W. Abbey, Mark D. Weinstein, and the surgical staffs at Kaiser hospitals (San Rafael, Calif., and San Francisco, Calif.) and San Francisco General Hospital for generous assistance in obtaining post-tonsillectomy samples. Recombinant IL-2 was the generous gift of Chiron Corporation. We acknowledge the technical assistance of Eric Wieder and Lisa Gibson in the conduct of these experiments and the assistance of Heather Gravois, John Carroll, and Neile Shea in the preparation of the manuscript.
B.S. is supported by the Boehringer Ingelheim Fond. M.L.P. is supported by the Biomedical Sciences Graduate Program, the California Universitywide AIDS Research Program, and the NIH Medical Scientist Training Program at UCSF. F.K. is supported by the Deutsche Forschungsgemeinschaft (SFB466), and R.M.G. and E.H.P. are supported by NIH grant P51 RR-0165. This work was supported in part by NIH grant R01-AI43695 (M.A.G.) and the J. David Gladstone Institutes (M.A.G. and R.M.G.).
REFERENCES
- 1.Albert J, Nauclér A, Böttiger B, Broliden P A, Albino P, Ouattara S A, Björkegren C, Valentin A, Biberfeld G, Fenyö E M. Replicative capacity of HIV-2, like HIV-1, correlates with severity of immunodeficiency. AIDS. 1990;4:291–295. doi: 10.1097/00002030-199004000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 3.Berry N, Ariyoshi K, Jaffar S, Sabally S, Corrah T, Tedder R, Whittle H. Low peripheral blood viral HIV-2 RNA in individuals with high CD4 percentage differentiates HIV-2 from HIV-1 infection. J Hum Virol. 1998;1:457–468. [PubMed] [Google Scholar]
- 4.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro B A, Barnett S W, Evans L A, Moreau J, Odehouri K, Levy J A. Biologic heterogeneity of human immunodeficiency virus type 2 (HIV-2) strains. Virology. 1990;178:527–534. doi: 10.1016/0042-6822(90)90350-z. [DOI] [PubMed] [Google Scholar]
- 6.Chan S Y, Speck R F, Power C, Gaffen S L, Chesebro B, Goldsmith M A. V3 recombinants indicate a central role for CCR5 as a coreceptor in tissue infection by human immunodeficiency virus type 1. J Virol. 1999;73:2350–2358. doi: 10.1128/jvi.73.3.2350-2358.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z, Gettie A, Ho D D, Marx P A. Primary SIVsm isolates use the CCR5 coreceptor from sooty mangabeys naturally infected in west Africa: a comparison of coreceptor usage of primary SIVsm, HIV-2, and SIVmac. Virology. 1998;246:113–124. doi: 10.1006/viro.1998.9174. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Telfier P, Gettie A, Reed P, Zhang L, Ho D D, Marx P A. Genetic characterization of new West African simian immunodeficiency virus SIVsm: geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J Virol. 1996;70:3617–3627. doi: 10.1128/jvi.70.6.3617-3627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesebro B, Wehrly K, Nishio J, Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992;66:6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng H, Unutmaz D, KewalRamani V, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–299. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 12.Edinger A L, Hoffman T L, Sharron M, Lee B, O'Dowd B, Doms R W. Use of GPR1, GPR15, and STRL33 as coreceptors by diverse human immunodeficiency virus type 1 and simian immunodeficiency virus envelope proteins. Virology. 1998;249:367–378. doi: 10.1006/viro.1998.9306. [DOI] [PubMed] [Google Scholar]
- 13.Fultz P N, McClure H M, Anderson D C, Swenson R B, Anand R, Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys) Proc Natl Acad Sci USA. 1986;83:5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao F, Yue L, Robertson D L, Hill S C, Hui H, Biggar R J, Neequaye A E, Whelan T M, Ho D D, Shaw G M, Sharp P M, Hahn B H. Genetic diversity of human immunodeficiency virus type 2: evidence for distinct sequence subtypes with differences in virus biology. J Virol. 1994;68:7433–7447. doi: 10.1128/jvi.68.11.7433-7447.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glushakova S, Baibakov B, Margolis L B, Zimmerberg J. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat Med. 1995;1:1320–1322. doi: 10.1038/nm1295-1320. [DOI] [PubMed] [Google Scholar]
- 16.Glushakova S, Baibakov B, Zimmerberg J, Margolis L B. Experimental HIV infection of human lymphoid tissue: correlation of CD4+ T cell depletion and virus syncytium-inducing/non-syncytium-inducing phenotype in histocultures inoculated with laboratory strains and patient isolates of HIV type 1. AIDS Res Hum Retrovir. 1997;13:461–471. doi: 10.1089/aid.1997.13.461. [DOI] [PubMed] [Google Scholar]
- 17.Grivel J C, Penn M L, Eckstein D A, Schramm B, Speck R F, Abbey N W, Herndier B, Margolis L, Goldsmith M A. Human immunodeficiency virus type 1 coreceptor preferences determine target T-cell depletion and cellular tropism in human lymphoid tissue. J Virol. 2000;74:5347–5351. doi: 10.1128/jvi.74.11.5347-5351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillon C, van der Ende M E, Boers P H, Gruters R A, Schutten M, Osterhaus A D. Coreceptor usage of human immunodeficiency virus type 2 primary isolates and biological clones is broad and does not correlate with their syncytium-inducing capacities. J Virol. 1998;72:6260–6263. doi: 10.1128/jvi.72.7.6260-6263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harouse J M, Gettie A, Tan R C, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 1999;284:816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- 20.Jaffar S, Wilkins A, Ngom P T, Sabally S, Corrah T, Bangali J E, Rolfe M, Whittle H C. Rate of decline of percentage CD4+ cells is faster in HIV-1 than in HIV-2 infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:327–332. doi: 10.1097/00042560-199712150-00003. [DOI] [PubMed] [Google Scholar]
- 21.Khabbaz R F, Heneine W, George J R, Parekh B, Rowe T, Woods T, Switzer W M, McClure H M, Murphey-Corb M, Folks T M. Brief report: infection of a laboratory worker with simian immunodeficiency virus. N Engl J Med. 1994;330:172–177. doi: 10.1056/NEJM199401203300304. [DOI] [PubMed] [Google Scholar]
- 22.Kirchhoff F, Pöhlmann S, Hamacher M, Means R E, Kraus T, Uberla K, Di Marzio P. Simian immunodeficiency virus variants with differential T-cell and macrophage tropism use CCR5 and an unidentified cofactor expressed in CEMx174 cells for efficient entry. J Virol. 1997;71:6509–6516. doi: 10.1128/jvi.71.9.6509-6516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong L I, Lee S W, Kappes J C, Parkin J S, Decker D, Hoxie J A, Hahn B H, Shaw G M. West African HIV-2-related human retrovirus with attenuated cytopathicity. Science. 1988;240:1525–1529. doi: 10.1126/science.3375832. [DOI] [PubMed] [Google Scholar]
- 24.Kumar P, Hui H X, Kappes J C, Haggarty B S, Hoxie J A, Arya S K, Shaw G M, Hahn B H. Molecular characterization of an attenuated human immunodeficiency virus type 2 isolate. J Virol. 1990;64:890–901. doi: 10.1128/jvi.64.2.890-901.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marlink R, Kanki P, Thior I, Travers K, Eisen G, Siby T, Traore I, Hsieh C C, Dia M C, Gueye E H, et al. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science. 1994;265:1587–1590. doi: 10.1126/science.7915856. [DOI] [PubMed] [Google Scholar]
- 26.McKnight A, Dittmar M, Moniz-Periera J, Ariyoshi K, Reeves J, Hibbitts S, Whitby D, Aarons E, Proudfoot A, Whittle H, Clapham P. A broad range of chemokine receptors are used by primary isolates of human immunodeficiency virus type 2 as coreceptors with CD4. J Virol. 1998;72:4065–4071. doi: 10.1128/jvi.72.5.4065-4071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mörner A, Björndal A, Albert J, Kewalramani V N, Littman D R, Inoue R, Thorstensson R, Fenyö E M, Björling E. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J Virol. 1999;73:2343–2349. doi: 10.1128/jvi.73.3.2343-2349.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owen S, Ellenberger D, Rayfield M, Wiktor S, Michel P, Grieco M, Gao F, Hahn B, Lal R. Genetically divergent strains of human immunodeficiency virus type 2 use multiple coreceptors for viral entry. J Virol. 1998;72:5425–5432. doi: 10.1128/jvi.72.7.5425-5432.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penn M L, Grivel J C, Schramm B, Goldsmith M A, Margolis L. CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue. Proc Natl Acad Sci USA. 1999;96:663–668. doi: 10.1073/pnas.96.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pöhlmann S, Stolte N, Münch J, Ten Haaft P, Heeney J L, Stahl-Hennig C, Kirchhoff F. Co-receptor usage of BOB/GPR15 in addition to CCR5 has no significant effect on replication of simian immunodeficiency virus in vivo. J Infect Dis. 1999;180:1494–1502. doi: 10.1086/315097. [DOI] [PubMed] [Google Scholar]
- 31.Popper S J, Sarr A D, Travers K U, Guèye-Ndiaye A, Mboup S, Essex M E, Kanki P J. Lower human immunodeficiency virus (HIV) type 2 viral load reflects the difference in pathogenicity of HIV-1 and HIV-2. J Infect Dis. 1999;180:1116–1121. doi: 10.1086/315010. [DOI] [PubMed] [Google Scholar]
- 32.Poulsen A G, Aaby P, Larsen O, Jensen H, Nauclér A, Lisse I M, Christiansen C B, Dias F, Melbye M. 9-year HIV-2-associated mortality in an urban community in Bissau, west Africa. Lancet. 1997;349:911–914. doi: 10.1016/S0140-6736(96)04402-9. [DOI] [PubMed] [Google Scholar]
- 33.Scarlatti G, Tresoldi E, Björndal A, Fredriksson R, Colognesi C, Deng H K, Malnati M S, Plebani A, Siccardi A G, Littman D R, Fenyö E M, Lusso P. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat Med. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 34.Schramm B, Penn M L, Speck R F, Chan S Y, De Clercq E, Schols D, Connor R I, Goldsmith M A. Viral entry through CXCR4 is a pathogenic factor and therapeutic target in human immunodeficiency virus type 1 disease. J Virol. 2000;74:184–192. doi: 10.1128/jvi.74.1.184-192.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E, van Steenwijk R P, Lange J M, Schattenkerk J K, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulz T F, Whitby D, Hoad J G, Corrah T, Whittle H, Weiss R A. Biological and molecular variability of human immunodeficiency virus type 2 isolates from The Gambia. J Virol. 1990;64:5177–5182. doi: 10.1128/jvi.64.10.5177-5182.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sol N, Ferchal F, Braun J, Pleskoff O, Tréboute C, Ansart I, Alizon M. Usage of the coreceptors CCR-5, CCR-3, and CXCR-4 by primary and cell line-adapted human immunodeficiency virus type 2. J Virol. 1997;71:8237–8244. doi: 10.1128/jvi.71.11.8237-8244.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Speck R F, Wehrly K, Platt E J, Atchison R E, Charo I F, Kabat D, Chesebro B, Goldsmith M A. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptors determined by individual amino acids within the envelope V3 loop. J Virol. 1997;71:7136–7139. doi: 10.1128/jvi.71.9.7136-7139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unutmaz D, KewalRamani V N, Littman D R. G protein-coupled receptors in HIV and SIV entry: new perspectives on lentivirus-host interactions and on the utility of animal models. Semin Immunol. 1998;10:225–236. doi: 10.1006/smim.1998.0134. [DOI] [PubMed] [Google Scholar]
- 40.Whittle H, Morris J, Todd J, Corrah T, Sabally S, Bangali J, Ngom P T, Rolfe M, Wilkins A. HIV-2-infected patients survive longer than HIV-1-infected patients. AIDS. 1994;8:1617–1620. doi: 10.1097/00002030-199411000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Whittle H C, Ariyoshi K, Rowland-Jones S. HIV-2 and T cell recognition. Curr Opin Immunol. 1998;10:382–387. doi: 10.1016/s0952-7915(98)80108-8. [DOI] [PubMed] [Google Scholar]