Abstract
In primary metabolism, fatty acid synthases (FASs) biosynthesize fatty acids via sequential Claisen-like condensations of malonyl-CoA followed by reductive processing. Likewise, polyketide synthases (PKSs) share biosynthetic logic with FAS which includes utilizing the same precursors and cofactors. However, PKS biosynthesize structurally diverse, complex secondary metabolites, many of which are pharmaceutically relevant. This digest covers examples of interconnected biosynthesis between primary and secondary metabolism in fatty acid and polyketide metabolism. Taken together, further understanding the biosynthetic linkage between polyketide biosynthesis and fatty acid biosynthesis may lead to improved discovery and production of novel drug leads from polyketide metabolites.
Keywords: Polyketide synthase, Fatty acid synthase, Microbial metabolism, Biosynthesis
Introduction
Polyketide synthases (PKS) produce secondary metabolites that have a diverse range of biological functions within the microorganisms that produce them and are particularly fruitful sources for the discovery of bioactive compounds including antibiotics (e.g. erythromycin), anticancer agents (e.g.epothilone), antiparasitics (e.g. avermectin), immunosuppressive agents (e.g.rapamycin) and others.1–3 Polyketide synthases have an evolutionary and functional relationship with fatty acid synthases from primary metabolism in that both typically catalyze the sequential decarboxylative condensations of malonyl-CoA followed by subsequent reductive processing.3,4 Over the past couple decades, our ability to bioinformatically identify biosynthetic gene clusters and make predictions regarding the chemical structure of secondary metabolites that may be produced by a microorganism has expanded greatly with the development of algorithms like antiSMASH which enables “genome mining,” or identification of biosynthetic gene clusters from motifs harbored within genome sequence data.5–7 While prediction of natural product structure has improved in recent years through the development of data mining algorithms like antiSMASH, it remains challenging to predict the chemical structure completely and accurately from sequence alone. This is largely due to the diverse repertoire of biosynthetic transformations that can occur in biology, and the degree to which nature has evolved variations on common enzymatic organizations that do not follow canonical biosynthetic “rules”.8–10 Furthermore, in genome mining it is not uncommon for pathways that are observable in the genome to be “cryptic” or “silent” (e.g., not expressed) under laboratory culture conditions, leading to further mysteries regarding mechanism of regulation.11
Throughout investigations to better understand the biosynthesis and regulation of PKS pathways, a striking feature is the degree to which these pathways are interconnected with fatty acid biosynthesis. PKSs share precursor pools (malonyl-CoA) with FAS and their domains can have similar protein folds and undergo similar chemical transformations. This digest highlights examples wherein the biosynthesis between PKS and FAS appear to be interconnected both in regulation and in some cases, potential crosstalk in biosynthetic transformations. In better understanding the biosynthetic crosstalk between these metabolic pathways, it may thus lead to better prediction of metabolite structure, production of cryptic natural products and/or expression in heterologous hosts.
Fatty acid synthase and polyketide synthase biosynthetic logic
The overall biosynthetic logic of PKS and FAS contain the same core set of enzymatic domains and enzymatic transformations. They possess a canonical set of the following domains: an acyltransferase (AT), an acyl carrier protein (ACP), a ketosynthase (KS), a ketoreductase (KR), a dehydratase (DH), an enoylreductase (ER), and a dehydratase (DH).12–14 The AT domain is responsible for selecting an extender unit (typically malonyl-CoA) for elongation of the polyketide chain.14 The AT domain then transfers the malonyl unit to an acyl carrier protein where it is tethered as a new thioester to a phosphopantetheinyl prosthetic group. The thioester undergoes a decarboxylative Claisen-like condensation through a KS domain to elongate the polyketide chain. It can then be further modified by β-carbon processing domains which include a ketoreductase which reduces the nascent beta keto group to become a β-hydroxy group, a dehydratase which dehydrates the hydroxy group to form an olefin, and an enoylreductase which reduces to the olefin to a saturated alkene (Fig. 1).
Fig. 1.

A scheme representing the enzymes involved in the biosynthesis of fatty acids and polyketides.The acyltransferase (AT) domain selects the starter unit which is then loaded onto the 4′-phosphopantetheinyl to the ACP via thioesterification. A Claisen-like condensation reaction is catalyzed by the ketosynthase (KS) domain between the ACP-bound substrate and extender unit (either acetyl or malonyl). The growing intermediate is then processed by each of the individual processing domains. The ketoreductase (KR) domain reduces the beta-keto group into a hydroxy moiety which is then followed by a dehydration reaction catalyzed by the dehydratase (DH) domain to form an alkene group. The intermediate is then further reduced by the enoylreductase (ER) transforming the alkene group into an alkane.
For FAS, these enzymatic domains and transformations can either be a set of discrete enzymes or as one “megasynthase” enzyme. Discrete enzymes wherein these domains are standalone domains are called “type II” whereas megasynthase organizations wherein a multi-domain enzyme that is held together by flexible linkers are termed “type I.”15,16 In bacteria, typically fatty acids are generated through type II FAS.16–18 Canonically, the bacterial FAS requires eight core fatty acid genes (a complex called Fab). The acyl carrier protein (ACP) is the anchor for the elongating fatty acid chain. The processing domains, which include the KS domains (FabH/FabB/FabF), the AT domain (FabD), the KR domain (FabG), the DH domains (FabZ and FabA), and the ER domain (FabI), all dock to the ACP.17 Finally, when the fatty acid chain is ready for termination, it is cleaved by thioesterase (TesA). ACP-mediated transformations further crosstalk with other pathways in primary metabolism including lipolic acid biosynthesis, biotin biosynthesis, phospholipid biosynthesis, and lipid A biosynthesis. Conversely, the second canonical arrangement of FAS is the “megasynthase” where all these domains are tethered together.16,17 There are two discrete divergently evolved pathways for generating type I FAS. Animals contain a FAS which has a homodimeric set of all the biosynthetic enzymes described above.19 The structure of the porcine FAS by Ban and coworkers revealed the overall topology of this arrangement. Fungal type I FAS, however, arose divergently in a distinct heterododecameric formation.20 Furthermore, some bacteria (such as Corynebacterium) possess a type I FAS that resembles a fungal type I FAS.21,22
PKSs, like FAS are also divided into type I and type II based on whether they are composed of discrete enzymes or megaenzymes (in addition to the type I and type II distinction, there are also type III PKS which lack ACPs and instead perform all transformations on CoA bound substrates).12,23,24 The main distinction between PKSs and FAS is the variety of structures that can be formed via these chemical transformations. Unlike FAS, PKS can have all or only a subset of reductive domains, leading to varied carbon skeletons.25 In the case of type II PKS, the length of the carbon skeleton is controlled by the ketosynthase-chain length factor (KS-CLF); a heterodimeric complex that can catalyze multiple cycles of elongation after the initial elongation of the acyl-ACP. This is also a major difference in the elongation step between type II FAS and PKS as the type II FAS contains a homodimeric KS domain.17 PKSs can also have domains that are absent in FAS such as AT domains with variant selectivity (resulting in α-branching), a methyltransferase (MT) domain which can transfer an S-adenosyl methionine unit to yield alpha methyl group substitution, and alternative reductive domains.25–27 PKSs also contain thioesterase (TE) domains that functions in other capacities than in releasing intermediates and final products. In type II PKS, the thioesterase-II (TEII) domain performs “corrective” roles to maintain the efficiency of the biosynthetic pathway.28 Furthermore, polyketide scaffolds can be further modified via post-PKS processing or “tailoring” enzymes to result in the final natural product metabolite.
While the type II FAS resembles the type II PKS in that both are comprised of standalone enzymes that dock together to form a complex enacting chemical transformations on the phosphopantetheinyl arm of the acyl carrier protein,17 type I PKSs, peculiarly more closely resemble the mammalian FAS compared to the fungal/bacterial FAS in terms of their overall homodimeric architecture.29 In type I PKS pathways, chain elongation and reductive modifications can be conducted by a multi-domain polypeptide that conducts the biosynthesis of natural products in an iterative manner or it can exist as a string of independent modules arranged according to the structure of the molecule being biosynthesized.29 There are two main classes of type I PKSs: modular PKSs and iterative PKSs.23 The modular PKSs can be further subdivided into two types: cis-AT PKSs and trans-AT PKSs.23,30 The cis-AT architecture of type I PKSs consists of having acyltransferases that are embedded within the modules of the biosynthetic pathway whereas the trans-AT PKSs have an acyltransferase domain encoded one or more separate proteins that dock into the polyketide synthase.30 Trans-AT PKSs also have a greater diversity of biosynthetic enzymatic domains harbored within them. These two type I PKS pathways appear to have evolved through divergent evolution.30 Interestingly, trans-AT PKS almost always select malonyl-CoA as a precursor, using SAM dependent methyltransferases for α-branching whereas cis-AT PKSs can integrate a wider variety of extender units31 perhaps suggesting closer evolutionary relationships to FAS chemistry.
Fatty acid synthase and polyketide synthase co-regulation
The biosynthetic ties between PKS and FAS are obvious in the use of the shared precursor malonyl-CoA as well as shared CoA pools to provide phosphopanetheinylation of ACP domains. The intricate ties between these biosynthetic pathways are revealed through efforts to improve the titer of both pathways via increasing the flux of malonyl-CoA.29,32 For example, biosensor design33 has been utilized applied towards this purpose. The connection between PKS and FAS precursor pool and regulation became all the clearer when evaluating experiments comparing inhibitors of fatty acid biosynthesis. Triclosan is a well-known inhibitor of fatty acid biosynthesis via inhibition of the fatty acid synthase enoyl reductase (FabI).34 Indeed, Heath and coworkers published several works describing the antibacterial agent Triclosan and other related 2-hydroxydiphenyl ethers that are responsible for the inhibition of fatty acid production at the FabI step.34–36 In prior studies, Heath hypothesized that enzymes involved in the catalysis of fatty acid synthesis could be the regulators based on their investigation which identified that FabI can be inhibited by long chain acyl-ACP.37 This supports the notion that FabI plays a regulatory role in the biosynthesis of fatty acids in addition to being a biosynthetic catalytic enzyme.36,37 Further supporting this hypothesis, Nodwell and coworkers sought to determine if they could use elicitor compounds to affect the expression of polyketide natural products focusing on compounds structurally related to triclosan. The compound ARC-2 also regulated the type II PKS antibiotic, actinorhodin in Streptomyces coelicolor. This investigation further demonstrated that the compound ARC-238 activated the Streptomyces antibiotic regulatory protein (SARP) gene actII-ORF4 which is a pleitropic gene which in turn triggers the biosynthesis of actinorhodin. Inhibitors of FAS resulting in induction of a type II PKS demonstrate a clear regulatory link between the two pathways that utilized shared precursors.
Another regulatory link between PKS and FAS arises from the shared post-translational modification of PKSs and FAS at their ACP domains. This post translational modification is mediated by phosphopantetheinyltransferase (pptase) enzymes (reviewed extensively by Burkart and coworkers).39 Typically, the folds of pptases are distinct between those that typically modify fatty acids versus those that typically alter secondary metabolites. AcpS was identified in the 1960s by a landmark paper demonstrating this class of enzymes40 whereas the surfactin biosynthetic gene cluster revealed an entirely distinct fold that is more associated with polyketides and other secondary metabolites. The need to have separate pptase enzyme that modify carrier proteins in an orthogonal fashion demonstrates that the intertwined precursor usage necessitates regulation for activation to appropriately partition precursor flux.
Interplay of biosynthetic enzymes
Type II
As highlighted earlier, FAS and PKS share similar enzymatic domains that are responsible for the biosynthesis and tailoring of fatty acids and polyketides, respectively. As such, there are studies that have reported the sharing of biosynthetic enzymes between both FAS and PKS. This is the case for Revill and coworkers who outlined the relationship between type II FAS and type II PKS in Streptomyces coelicolor A3.41 The type II PKS pathway found in Streptomyces coelicolor A3 is responsible for producing the blue-pigmented antibiotic actinorhodin. Revill and team were able to show that the malonyl acyltransferase, FabD, found in the type II FAS pathway was responsible for supplying the malonyl-CoA precursor to the acyl carrier protein (ACP) in the type II PKS pathway found in the same organism. With both pathways sharing common catalytic sites, there have been reports that suggest that both biosynthetic systems can be influenced by similar antibacterial compounds.34,38,42 This cross-talk between type II FAS and type II PKS and the importance of the malonylacyl transferase enzyme in bacterial metabolic pathways have also been highlighted in the biosynthesis of tetracenomycin,43 andrimid44 and FK228.45
The promiscuity of enzymatic domains does not stop at the S. coelicolor type II FAS acyltransferase (AT), FabD, however. The promiscuity of the dehydratase FabA, the keto-reductase FabG and the enoyl-reductase FabI, between the type II FAS and PKS was also studied in Streptomyces coelicolor (Fig. 2). In two separate reports, Reynolds and coworkers were able to show that FabA, FabG and FabI from the type II FAS pathway are involved in the biosynthesis of the red-pigmented natural product undecylprodiginine, produced from the type II PKS pathway in S. coelicolor.46,47 Their investigation revealed that the type II FAS enzymes did not discriminate between the acylcarrier proteins, FabC and RedQ, from the primary and secondary metabolic pathways. They also reported that the enzyme has a moderate substrate specificity and was active towards both fatty acid and polyketide biosynthetic intermediates.47
Fig. 2.

Scheme showing the chemistry conducted by the ketoreductase FabG, the dehydratase FabA and the enoyl reductase FabI in the type II FAS biosynthetic pathways. FabG performs a reduction of the beta-keto group to form an alcohol which is then dehydrated by the FabA enzyme to give an alkene group which is further reduced by the FabI enzyme to give an alkane. Each green circle represents an acyl carrier protein (ACP) enzyme.
Coupled with the crosstalk between enzymatic domains between the primary and secondary biosynthetic pathway is the structural similarity among these catalytic enzymes. In the reported alkyl quinolone signaling system that is responsible for the production of 2-heptyl-3-hydroxy-4-quinolone (PQS), a molecule required to produce secondary metabolites and extracellular enzymes, an enzyme has been identified that carries structural similarities to the E. coli ketosynthase FabH and the polyketide chalcone synthase.48,49 The enzyme, PqsD, is encoded in the pqs biosynthetic gene cluster and is responsible for the condensation and cyclization of anthranoyl-CoA and malonyl-CoA to 2,4-dihydroxyquinoline (DHQ). PqsD has also been named as a key enzyme catalyzing the condensation reaction that generates the highly active intermediate 2-aminobenzoylacetate-CoA (2-ABA-CoA) which is a precursor for secondary metabolites HHQ and PQS.48 Results from the crystal structure elucidation by Brea and coworkers suggests PqsD showed more structural relation to the β-ketoacyl-ACP synthase (FabH) and was catalytically like the polyketide chalcone synthase which catalyzes an extra chemistry beyond condensation (Fig. 3).48 Again, this shows the structural relationship between the enzymes from primary and secondary metabolic pathways.
Fig. 3.
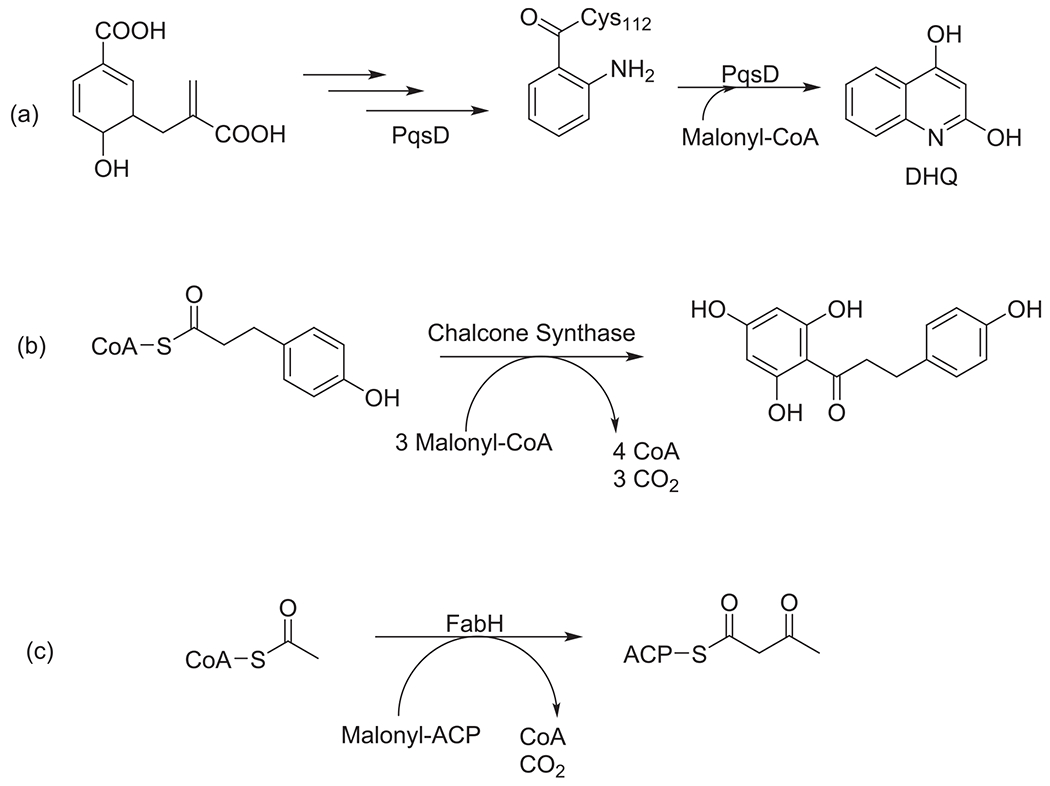
Scheme showing the condensation reaction catalyzed by PqsD in the biosynthesis of DHQ (a), chalcone synthase (b) and type II FAS enzyme FabH (c).
While the focus of this section was to highlight the interplay of enzymes between both primary and secondary pathways, there have been instances where researchers have developed different approaches to test the compatibility of enzymatic domains between the two biosynthetic systems.50 A major issue that researchers face in studying the interaction between enzymes to understand their chemistry, is the fleeting nature of these interactions.17,51,52 Because the interaction between the ACP bound intermediate with the different processing domains is so transient, researchers have employed different techniques that facilitate trapping the interactions between the proteins. Wormington and coworkers developed crosslinking 4′-phosphopantetheine analogues that were able to trap the interaction between the ACP and KS domains in E. coli type II FAS and type II PKS enterocin pathways.53 They also explored the possibility of intersystem ACP-KS interactions using cross linking analyses. While their study showed the expected preference of each KS with its cognate ACPs, they also reported an interaction between the ACP from the enterocin pathway with the KS domain FabB from the type II FAS pathway of E. coli.53 This result may suggest ACP involvement in both the type II FAS and PKS in S. maritimus.
Type I PKSs
While there is certainly a mechanistic link between the enzymes of type I and type II fatty acid synthases in general, some bacterial species use the machinery of type I PKSs to generate high molecular weight linear lipids that are used in cell wall construction and more typically associated with fatty acids. The mycolic acid-producing branch of the Actinomycetales (which includes Mycobacteria, Corynebacterial, and Norcardiae) produces anywhere from 22 to 90 carbon fatty acids that are found in the matrix of the cell wall.21,22 Hybrids between type I and type II pathways also exist in these bacteria as these types of actinomycetes lack the ability to build up longer chain fatty acids de novo. However, these pathways still serve a cellular function that is more similar to lipids typically produced by FAS in their host organisms. For example, these pathways typically comprise the bulk of lipids in the membrane,22 illustrating further splintering of biosynthetic and functional nomenclature.
What is currently unknown is the degree to which type I polyketide synthases that generate secondary metabolites or specialized metabolites (as opposed to fatty acid-like molecules in function) interface with primary metabolism.23 To date, to our knowledge, at least five pathways from diverse genera of bacteria have been discovered to have inactive acyltransferase domains. For pathways like this, the acyltransferase domain has a few peculiarities. First, it is somewhat truncated relative to a typical acyltransferase domain. But more saliently, the conserved serine of the catalytic triad is replaced with glycine (in the case of conglobatin, serine is replaced with an alanine residue), meaning that it cannot create an acyl-enzyme intermediate to transfer to the carrier protein (Fig. 4).23,54 Pathways containing such an organization are found in modules two and four of gephyronic acid (from the myxobacteria Archangium gephyra strain Ar3895 and Cystobacter violaceus strain Cb vi76),55 module 3 of bengamide (from Myxococcus virescens and various aquatic sponge symbionts),56 module 3 of conglobatin (Streptomyces conglobatus ATCC 31005,57 and module 10 of desertomycin (from Streptomycs althioticus MSM3).58 In each of these pathways, there has been no clustered trans-acyltransferase identified that could perform this chemistry instead, leading to questions regarding how the malonyl unit could be delivered by an acyltransferase enzyme. In Zhou’s investigation into the biosynthesis of Conglobatin, he hypothesized that to compensate for the inactive AT domain, the module KS2 could be conducting two successive condensations prior to passing the growing chain to the module with the inactive AT domain for reduction.57 This process is known as “stuttering” and has been reported as the mechanism used in AT-less systems such as the pathway that produces stigmatellin59 and erythromycin60 even though in the latter polyketide only side products were reported from such action. A second hypothesis is that the upstream AT domain would malonate the downstream ACP in a fashion akin to NRPS modules that lack a A domain wherein the downstream A domain aminoacylates the upstream peptidyl carrier protein (PCP) which has been observed in several NRPS pathways.61–64 A final hypothesis is that an enzyme universal to all bacteria such as one from primary metabolism like FabD could deliver the extender unit in an analogous fashion to the way it can in type II PKS pathways (Fig. 5). FabD has been shown to be a promiscuous enzyme with a plastic interface with varied ACP domains that can serve as a reaction partner.65 Indeed, in actinorhodin and other type II PKSs, the MAT domain from fatty acid biosynthesis delivers the extender unit.66 Further supporting this hypothesis, desertomycin was successfully heterologously expressed in Streptomyces lividans TK23 without supplying any acyltransferase domain, suggesting that an enzyme universal to bacteria (such as one from primary metabolism) may perform this chemistry.58 More investigation needs to be performed to determine whether this chemistry is performed via “stuttering,” upstream domain modification or through an enzyme common to all bacteria such as FabD.
Fig. 4.

Sequence alignment showing the inactive acyltransferase (AT) domains in the biosynthetic pathway of gephyronic acid, bengamide, desertomycin and conglobatin compared to an active acyltransferase (AT) domain within the same biosynthetic pathways highlighting the missing conserved serine residue. AT1_gph = acyltransferase domain in module 1 of the biosynthetic pathway of gephyronic acid with serine residue; AT2_gph = acyltransferase domain in module 2 of the biosynthetic pathway of gephyronic acid with glycine residue; AT2_ben = acyltransferase domain in module 2 of the biosynthetic pathway of bengamide with serine residue; AT3_ben = acyltransferase domain in module 3 of the biosynthetic pathway of bengamide with glycine residue; AT7_des = acyltransferase domain in module 7 of the biosynthetic pathway of desertomycin with serine residue; AT10_des = acyltransferase domain in module 10 of the biosynthetic pathway of desertomycin with glycine residue; AT1_cong = acyltransferase domain in module 1 of the biosynthetic pathway of conglobatin with serine residue; AT3_cong = acyltransferase domain in module 3 of the biosynthetic pathway of conglobatin with alanine residue.
Fig. 5.

Possible hypothesis for malonyl transfer via malonyl acyl transferase, FabD, from the type II FAS pathway supplying the extender unit to the acylcarrier protein (ACP) in module 2 of the gephyronic acid type I PKS biosynthetic pathway that contains the inactive acyltransferase (AT) domain. Each circle represents a catalytic domain in the first three modules of the biosynthetic pathway of gephyronic acid: MT = methyltransferase, GNAT = GCN5-related N-acetyltransferase, ACP = acylcarrier protein, KS = ketosynthase, AT = acyltransferase, DH = dehydratase, KR = ketoreductase, ATo = inactive acyltransferase.
As indicated earlier, type II FAS and type II PKS show a relationship where enzymatic domains are shared between the two pathways. This then raises the question of whether such a relationship is also present among the type II FAS and the type I PKS biosynthetic systems. Given the highlighted pathways mentioned above that shows a deviation from the typical “canonical” organization of the type I PKSs with no presently reported study of the mechanism behind how these systems work, developing new techniques or employing existing methods could shed light on the chemistry behind these unique pathways. Just as with the type II systems for both FAS and PKS, developing crosslinking probes coupled with the use of heterologous expression would be a great way to understand not only the mechanism of how these systems make their respective natural products but also how these systems can be manipulated to produce even more useful natural products. This will also lead to showing that the relationship between primary and secondary metabolism extends to even type I PKS biosynthetic pathways.
Conclusions
The polyketide synthase pathways play an important role, being the machinery behind the production of natural products that possess very important properties utilized in a variety of areas in our society. One major area where these useful secondary metabolites have found much success is in the field of medicine as they have been shown to be very applicable antibiotics, antifungal, immunosuppressive and anticancer agents. This digest highlights the crosstalk between the biosynthetic pathways that produce fatty acid and polyketides. Given their evolutionary relationship there have been multiple studies discussed that show the inter-relationship between FAS and PKS and their role in facilitating the production of secondary and primary metabolites, respectively. While the reports have mostly highlighted the type II FAS and PKS systems, the tools and techniques developed to investigate these systems can further extend to type I PKS systems that show unique characteristics in their organization and chemistry. Considering the diversity of secondary metabolites produced from these complex type I PKS pathways, indicates their potential for pharmaceutical discovery. Consequently, gaining a full understanding of how these systems are biosynthesized can allow for further discovery, improved heterologous expression, and engineered pathways which will enable the production of more important drug leads.
Acknowledgements
The authors thank the University of Tennessee, University of Tennessee-Oak Ridge Innovation Institute (UT-ORII) Science Alliance, and the National Institutes of Health (R15GM146192) for financial support.
Abbreviations:
- PKS
polyketide synthase
- FAS
fatty acid synthase
- AT
acyltransferase
- KS
ketosynthase
- DH
dehydratase
- KR
ketoreductase
- ER
enoylreductase
- PPTase
phosphopantetheinyltransferase
- TE
thioesterase
- MT
methyltransferase
- ACP
acyl carrier protein
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- 1.Fischbach MA, Walsh CT. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms. Chem Rev. 2006;106(8):3468–3496. 10.1021/cr0503097. [DOI] [PubMed] [Google Scholar]
- 2.El-Saber Batiha G, Alqahtani A, Ilesanmi OB, et al. Avermectin derivatives, pharmacokinetics, therapeutic and toxic dosages, mechanism of action, and their biological effects. Pharmaceuticals (Basel). 2020;13(8). 10.3390/ph13080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nivina A, Yuet KP, Hsu J, Khosla C. Evolution and Diversity of Assembly-Line Polyketide Synthases. Chem Rev. 2019;119(24):12524–12547. 10.1021/acs.chemrev.9b00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohli GS, John U, Van Dolah FM, Murray SA. Evolutionary distinctiveness of fatty acid and polyketide synthesis in eukaryotes. ISME J. 2016;10(8):1877–1890. 10.1038/ismej.2015.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blin K, Shaw S, Kloosterman AM, et al. AntiSMASH 6.0: improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021;49(W1):W29–W35. 10.1093/nar/gkab335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber T; Blin K; Duddela S; Krug D; Kim HU; Bruccoleri R; Lee SY; Fischbach MA; Müller R; Wohlleben W; Breitling R; Takano E; Medema MH AntiSMASH 3.0-a Comprehensive Resource for the Genome Mining of Biosynthetic Gene Clusters. Nucleic Acids Res. 2015, 43 (W1), W237–W243. DOI: 10.1093/nar/gkv437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medema MH, Blin K, Cimermancic P, et al. AntiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011;39. 10.1093/nar/gkr466. Web Server issue), W339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biermann F, Wenski SL, Helfrich EJN. Navigating and Expanding the Roadmap of Natural Product Genome Mining Tools. Beilstein J Org Chem. 2022;18:1656–1671. 10.3762/bjoc.18.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz-Morales P, Kopp JF, Martínez-Guerrero C, et al. Phylogenomic analysis of natural products biosynthetic gene clusters allows discovery of arseno-organic metabolites in model streptomycetes. Genome Biol Evol. 2016;8(6):1906–1916. 10.1093/gbe/evw125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sélem-Mojica N, Aguilar C, Gutiérrez-García K, Martínez-Guerrero CE, Barona-Gómez F. EvoMining reveals the origin and fate of natural product biosynthetic enzymes. Microb Genom. 2019;5(12). 10.1099/mgen.0.000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoskisson PA, Seipke RF. Cryptic or Silent? The known unknowns, unknown knowns, and unknown unknowns of secondary metabolism. MBio. 2020;11(5). 10.1128/mBio.02642-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keatinge-Clay AT. The structures of type i polyketide synthases. Nat Prod Rep. 2012;29(10):1050–1073. 10.1039/c2np20019h. [DOI] [PubMed] [Google Scholar]
- 13.Hopwood DA. Genetic contributions to understanding polyketide synthases. Chem Rev. 1997;97(7):2465–2498. 10.1021/cr960034i. [DOI] [PubMed] [Google Scholar]
- 14.Englund E, Schmidt M, Nava AA, et al. Expanding extender substrate selection for unnatural polyketide biosynthesis by acyltransferase domain exchange within a modular polyketide synthase. J Am Chem Soc. 2023. 10.1021/jacs.2c11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White SW, Zheng J, Zhang Y-M, Rock.. The structural biology of type II fatty acid biosynthesis. Annu Rev Biochem. 2005;74:791–831. 10.1146/annurev.biochem.74.082803.133524. [DOI] [PubMed] [Google Scholar]
- 16.Campbell JW, Cronan JE. Bacterial fatty acid biosynthesis: targets for antibacterial drug discovery. Annu Rev Microbiol. 2001;55:305–332. 10.1146/annurev.micro.55.1.305. [DOI] [PubMed] [Google Scholar]
- 17.Chen A, Re RN, Burkart MD. Type II fatty acid and polyketide synthases: deciphering protein-protein and protein-substrate interactions. Nat Prod Rep. 2018;35(10):1029–1045. 10.1039/c8np00040a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kassab E, Fuchs M, Haack M, Mehlmer N, Brueck TB. Engineering escherichia coli FAB system using synthetic plant genes for the production of long chain fatty acids. Microb Cell Fact. 2019;18(1):163. 10.1186/s12934-019-1217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leibundgut M, Maier T, Jenni S, Ban N. The multienzyme architecture of eukaryotic fatty acid synthases. Curr Opin Struct Biol. 2008;18(6):714–725. 10.1016/j.sbi.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Jenni S, Leibundgut M, Boehringer D, Frick C, Mikolásek B, Ban N. Structure of fungal fatty acid synthase and implications for iterative substrate shuttling. Science. 2007;316(5822):254–261. 10.1126/science.1138248. [DOI] [PubMed] [Google Scholar]
- 21.Radmacher E, Alderwick LJ, Besra GS, et al. Two functional FAS-I type fatty acid synthases in corynebacterium glutamicum. Microbiology (Reading, Engl). 2005;151(Pt 7):2421–2427. 10.1099/mic.0.28012-0. [DOI] [PubMed] [Google Scholar]
- 22.Schweizer E; Hofmann J Microbial Type I Fatty Acid Synthases (FAS): Major Players in a Network of Cellular FAS Systems. Microbiol. Mol. Biol. Rev 2004, 68 (3), 501–517, table of contents. DOI: 10.1128/MMBR.68.3.501-517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Du L. Iterative polyketide biosynthesis by modular polyketide synthases in bacteria. Appl Microbiol Biotechnol. 2016;100(2):541–557. 10.1007/s00253-015-7093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brachmann AO, Joyce SA, Jenke-Kodama H, Schwär G, Clarke DJ, Bode HB. A type II polyketide synthase is responsible for anthraquinone biosynthesis in photorhabdus luminescens. Chembiochem. 2007;8(14):1721–1728. 10.1002/cbic.200700300. [DOI] [PubMed] [Google Scholar]
- 25.Skiba MA, Sikkema AP, Fiers WD, et al. Domain organization and active site architecture of a polyketide synthase c-methyltransferase. ACS Chem Biol. 2016;11(12):3319–3327. 10.1021/acschembio.6b00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y-Q, Coughlin JM, Lim S-K, Shen B. Type I polyketide synthases that require discrete acyltransferases. Meth Enzymol. 2009;459:165–186. 10.1016/S0076-6879(09)04608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohman JR, Ma M, Osipiuk J, et al. Structural and evolutionary relationships of “AT-less” type I polyketide synthase ketosynthases. Proc Natl Acad Sci USA. 2015;112(41):12693–12698. 10.1073/pnas.1515460112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotowska M, Pawlik K. Roles of type II thioesterases and their application for secondary metabolite yield improvement. Appl Microbiol Biotechnol. 2014;98(18):7735–7746. 10.1007/s00253-014-5952-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herbst DA, Townsend CA, Maier T. The architectures of iterative type I PKS and FAS. Nat Prod Rep. 2018;35(10):1046–1069. 10.1039/c8np00039e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang B, Guo F, Huang C, Zhao H. Unraveling the iterative type I polyketide synthases hidden in Streptomyces. Proc Natl Acad Sci USA. 2020;117(15):8449–8454. 10.1073/pnas.1917664117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker PD, Weir ANM, Willis CL, Crump MP. Polyketide β-branching: diversity, mechanism and selectivity. Nat Prod Rep. 2021;38(4):723–756. 10.1039/d0np00045k. [DOI] [PubMed] [Google Scholar]
- 32.Choi JW, Da Silva NA. Improving polyketide and fatty acid synthesis by engineering of the Yeast acetyl-CoA carboxylase. J Biotechnol. 2014;187:56–59. 10.1016/j.jbiotec.2014.07.430. [DOI] [PubMed] [Google Scholar]
- 33.Malico AA, Nichols L, Williams GJ. Synthetic biology enabling access to designer polyketides. Curr Opin Chem Biol. 2020;58:45–53. 10.1016/j.cbpa.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heath RJ, Rubin JR, Holland DR, Zhang E, Snow ME, Rock CO. Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J Biol Chem. 1999;274(16):11110–11114. 10.1074/jbc.274.16.11110. [DOI] [PubMed] [Google Scholar]
- 35.Heath RJ, Yu YT, Shapiro MA, Olson E, Rock CO. Broad spectrum antimicrobial biocides target the fabi component of fatty acid synthesis. J Biol Chem. 1998;273(46):30316–30320. 10.1074/jbc.273.46.30316. [DOI] [PubMed] [Google Scholar]
- 36.Heath RJ, Rock CO. Enoyl-acyl carrier protein reductase (FabI) plays a determinant role in completing cycles of fatty acid elongation in Escherichia Coli. J Biol Chem. 1995;270(44):26538–26542. 10.1074/jbc.270.44.26538. [DOI] [PubMed] [Google Scholar]
- 37.Heath RJ, Rock CO. Regulation of fatty acid elongation and initiation by acyl-acyl carrier protein in Escherichia coli. J Biol Chem. 1996;271(4):1833–1836. 10.1074/jbc.271.4.1833. [DOI] [PubMed] [Google Scholar]
- 38.Calvelo VY, Crisante D, Elliot M, Nodwell JR. The ARC2 response in streptomcyes coelicolor requires the global regulatory genes AfsR and AfsS. Microbiology (Reading, Engl). 2021;167(5). 10.1099/mic.0.001047. [DOI] [PubMed] [Google Scholar]
- 39.Beld J, Sonnenschein EC, Vickery CR, Noel JP, Burkart MD. The phosphopantetheinyl transferases: catalysis of a post-translational modification crucial for Life. Nat Prod Rep. 2014;31(1):61–108. 10.1039/c3np70054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lambalot RH, Gehring AM, Flugel RS, et al. A new enzyme superfamily - the phosphopantetheinyl transferases. Chem Biol. 1996;3(11):923–936. 10.1016/s1074-5521(96)90181-7. [DOI] [PubMed] [Google Scholar]
- 41.Revill WP, Bibb MJ, Hopwood DA. Purification of a malonyltransferase from streptomyces coelicolor A3(2) and analysis of its genetic determinant. J Bacteriol. 1995;177(14):3946–3952. 10.1128/jb.177.14.3946-3952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Escalada MG, Harwood JL, Maillard JY, Ochs D. Triclosan inhibition of fatty acid synthesis and its effect on growth of Escherichia coli and pseudomonas aeruginosa. J Antimicrob Chemother. 2005;55(6):879–882. 10.1093/jac/dki123. [DOI] [PubMed] [Google Scholar]
- 43.Shen B, Summers RG, Gramajo H, Bibb MJ, Hutchinson CR. Purification and characterization of the acyl carrier protein of the streptomyces glaucescens tetracenomycin c polyketide synthase. J Bacteriol. 1992;174(11):3818–3821. 10.1128/jb.174.11.3818-3821.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishikawa F, Sugimoto H, Kakeya H. In vitro investigation of crosstalk between fatty acid and polyketide synthases in the andrimid biosynthetic assembly line. Chembiochem. 2016;17(22):2137–2142. 10.1002/cbic.201600410. [DOI] [PubMed] [Google Scholar]
- 45.Wesener SR, Potharla VY, Cheng Y-Q. Reconstitution of the FK228 biosynthetic pathway reveals cross talk between modular polyketide synthases and fatty acid synthase. Appl Environ Microbiol. 2011;77(4):1501–1507. 10.1128/AEM.01513-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh R, Reynolds KA. Characterization of FabG and FabI of the streptomyces coelicolor dissociated fatty acid synthase. Chembiochem. 2015;16(4):631–640. 10.1002/cbic.201402670. [DOI] [PubMed] [Google Scholar]
- 47.Singh R, Reynolds KA. Identification and characterization of FabA from the type II fatty acid synthase of streptomyces coelicolor. J Nat Prod. 2016;79(1):240–243. 10.1021/acs.jnatprod.5b00560. [DOI] [PubMed] [Google Scholar]
- 48.Bera AK, Atanasova V, Robinson H, et al. Structure of PqsD, a pseudomonas quinolone signal biosynthetic enzyme complex with anthranilate. Biochemistry. 2009;48(36):8644–8655. 10.1021/bi9009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diggle SP, Matthijs S, Wright VJ, et al. The pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem Biol. 2007;14(1):87–96. 10.1016/j.chembiol.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 50.Schnarr NA, Khosla C. Trapping transient protein-protein interactions in polyketide biosynthesis. ACS Chem Biol. 2006;1(11):679–680. 10.1021/cb600451d. [DOI] [PubMed] [Google Scholar]
- 51.Miyanaga A, Ouchi R, Ishikawa F, et al. Structural basis of protein-protein interactions between a trans-acting acyltransferase and acyl carrier protein in polyketide disorazole biosynthesis. J Am Chem Soc. 2018;140(25):7970–7978. 10.1021/jacs.8b04162. [DOI] [PubMed] [Google Scholar]
- 52.Bartholow TG, Sztain T, Patel A, et al. Elucidation of transient protein-protein interactions within carrier protein-dependent biosynthesis. Commun Biol. 2021;4(1):340. 10.1038/s42003-021-01838-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Worthington AS, Hur GH, Meier JL, Cheng Q, Moore BS, Burkart MD. Probing the compatibility of type ii ketosynthase-carrier protein partners. Chembiochem. 2008;9(13):2096–2103. 10.1002/cbic.200800198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D’Ambrosio HK, Ganley JG, Keeler AM, Derbyshire ER. A single amino acid residue controls acyltransferase activity in a polyketide synthase from toxoplasma gondii. iScience. 2022;25(6), 104443. 10.1016/j.isci.2022.104443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Young J, Stevens DC, Carmichael R, et al. Elucidation of gephyronic acid biosynthetic pathway revealed unexpected sam-dependent methylations. J Nat Prod. 2013;76(12):2269–2276. 10.1021/np400629v. [DOI] [PubMed] [Google Scholar]
- 56.Wenzel SC, Hoffmann H, Zhang J, et al. Production of the bengamide class of marine natural products in myxobacteria: biosynthesis and structure-activity relationships. Angew Chem Int Ed. 2015;54(51):15560–15564. 10.1002/anie.201508277. [DOI] [PubMed] [Google Scholar]
- 57.Zhou Y, Murphy AC, Samborskyy M, Prediger P, Dias LC, Leadlay PF. Iterative mechanism of macrodiolide formation in the anticancer compound conglobatin. Chem Biol. 2015;22(6):745–754. 10.1016/j.chembiol.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hashimoto T, Kozone I, Hashimoto J, et al. Identification, cloning and heterologous expression of biosynthetic gene cluster for desertomycin. J Antibiot. 2020;73(9):650–654. 10.1038/s41429-020-0319-0. [DOI] [PubMed] [Google Scholar]
- 59.Gaitatzis N, Silakowski B, Kunze B, et al. The biosynthesis of the aromatic myxobacterial electron transport inhibitor stigmatellin is directed by a novel type of modular polyketide synthase. J Biol Chem. 2002;277(15):13082–13090. 10.1074/jbc.M111738200. [DOI] [PubMed] [Google Scholar]
- 60.Wilkinson B, Foster G, Rudd BA, et al. Novel octaketide macrolides related to 6-deoxyerythronolide b provide evidence for iterative operation of the erythromycin polyketide synthase. Chem Biol. 2000;7(2):111–117. 10.1016/s1074-5521(00)00076-4. [DOI] [PubMed] [Google Scholar]
- 61.Magarvey NA, Haltli B, He M, Greenstein M, Hucul JA. Biosynthetic pathway for mannopeptimycins, lipoglycopeptide antibiotics active against drug-resistant gram-positive pathogens. Antimicrob Agents Chemother. 2006;50(6):2167–2177. 10.1128/AAC.01545-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Felnagle EA, Rondon MR, Berti AD, Crosby HA, Thomas MG. Identification of the biosynthetic gene cluster and an additional gene for resistance to the antituberculosis drug capreomycin. Appl Environ Microbiol. 2007;73(13):4162–4170. 10.1128/AEM.00485-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomas MG, Chan YA, Ozanick SG. Deciphering tuberactinomycin biosynthesis: isolation, sequencing, and annotation of the viomycin biosynthetic gene cluster. Antimicrob Agents Chemother. 2003;47(9):2823–2830. 10.1128/AAC.47.9.2823-2830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Du L, Sánchez C, Chen M, Edwards DJ, Shen B. The biosynthetic gene cluster for the antitumor drug bleomycin from Streptomyces Verticillus ATCC15003 supporting functional interactions between nonribosomal peptide synthetases and a polyketide synthase. Chem Biol. 2000;7(8):623–642. 10.1016/s1074-5521(00)00011-9. [DOI] [PubMed] [Google Scholar]
- 65.Misson LE, Mindrebo JT, Davis TD, et al. Interfacial Plasticity Facilitates High Reaction Rate of E Coli FAS Malonyl-CoA:ACP Transacylase, FabD. Proc Natl Acad Sci USA. 2020;117(39):24224–24233. 10.1073/pnas.2009805117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dreier J, Shah AN, Khosla C. Kinetic analysis of the actinorhodin aromatic polyketide synthase. J Biol Chem. 1999;274(35):25108–25112. 10.1074/jbc.274.35.25108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


