Abstract
A 65-year-old man with type 2 diabetes who was being treated with metformin developed lactic acidosis following excessive alcohol consumption. While an impaired renal function is a major risk factor for metformin-associated lactic acidosis (MALA), the patient's basal renal function was normal. Alcohol misuse reduces lactate clearance by utilizing nicotinamide adenine dinucleotides for ethanol oxidation, thereby promoting vulnerability to MALA. Nevertheless, as MALA in individuals with a normal renal function is extremely rare, the clinical picture of alcohol-induced MALA is unclear. We delineate the clinical picture and discuss the pathogenesis of alcohol-induced MALA based on our experience and previous case reports.
Keywords: lactic acidosis, metformin, alcohol
Introduction
Metformin is the most commonly prescribed oral antihyperglycemic agent for the treatment of diabetes mellitus worldwide and is considered the first-line therapy for newly diagnosed type 2 diabetes mellitus by the American Diabetes Association and European Association for the Study of Diabetes (1). With over 60 years of real-world global clinical experience, metformin is generally regarded as effective and safe unless there are contraindications (2,3).
Metformin-associated lactic acidosis (MALA) is a rare but serious adverse effect of metformin, with an estimated incidence of <10 events per 100,000 patient-years of exposure and a mortality rate of 30-50% (4). MALA is generally precipitated by metformin accumulation due to an impaired renal function (4). In contrast, acute alcohol intoxication is associated with impaired hepatic lactate clearance, which may increase the risk of lactic acidosis (5). However, since MALA is extremely rare in patients with a normal renal function (6), a detailed clinical picture of alcohol-induced MALA is unknown.
We herein report a case of alcohol-induced MALA in a patient with a normal renal function. Furthermore, we describe the clinical picture and discuss the pathogenesis of alcohol-induced MALA based on our experience and previous reports.
Case Report
A 65-year-old Japanese man was transported to the emergency room with the chief complaint of vomiting. His medical history included type 2 diabetes mellitus, alcoholic hepatitis, and chronic pancreatitis. There was no history of chronic kidney disease, and the outpatient serum creatinine prior to the visit was 0.9 mg/dL [estimated glomerular filtration rate (eGFR) 66 mL/min/1.73 m2]. The duration of type 2 diabetes was more than seven years, and it was complicated by microalbuminuria and distal symmetric polyneuropathy. The presence of retinopathy was uncertain.
The patient was prescribed insulin degludec (6.5 unit once daily), metformin (500 mg twice daily), sitagliptin (50 mg once daily), mitiglinide (10 mg twice daily), and voglibose (0.3 mg thrice daily). The patient had temporarily refrained from consuming alcohol six years ago during his hospitalization for pancreatitis but resumed the habit shortly thereafter. Unfortunately, he did not report persistent alcohol consumption by his physician. The patient had been on leave from work for 1 week because of low back pain syndrome, and his alcohol intake increased 2-fold compared to his normal habit (from 130 g/day to 260 g/day) during this period. The day before the visit, the patient developed anorexia but continued to consume ethanol. He continued to drink on the day of the visit and experienced multiple episodes of vomiting from midday onwards. The patient visited the emergency room on the evening of the same day.
A clinical examination revealed tachycardia (122 beats per minute) with a normal blood pressure of 117/71 mmHg. The respiratory rate was 24 breaths per minute, and transcutaneous oxygen saturation was 98% on room air. His body mass index was 17.3 kg/m2 (height, 170 cm; body weight, 50 kg). A physical examination revealed no signs of mucosal dryness. The abdomen was flat and soft with no tenderness, and the liver and spleen were not palpable.
Laboratory tests revealed acidemia (pH, 6.97) with a low serum bicarbonate level (7.9 mmol/L) and marked hyperlactatemia (serum lactate, 30.0 mmol/L). The concentration ratio of lactate to pyruvate was also markedly elevated (lactate, 217.7 mg/dL; pyruvate, 3.73 mg/dL; and lactate/pyruvate ratio, 58.4). However, the serum ketone level was mildly elevated (serum ketone, 2.1 mmol/L). Moderate renal impairment (serum creatinine, 1.40 mg/dL; eGFR, 40 mL/min/1.73 m2) and high transaminase levels with aspartate aminotransferase predominance (aspartate aminotransferase, 117 U/L; alanine aminotransferase, 34 U/L) were also observed. The prothrombin time was not prolonged (prothrombin time-international normalized ratio, 0.88), and the serum albumin level was within the normal range (serum albumin, 4.4 g/dL). Thus, there was no apparent decrease in the hepatic synthetic capacity. Hematocrit and urea nitrogen values were within normal ranges (hematocrit, 47.9%; urea nitrogen, 16 mg/dL), and intravascular dehydration was not evident (Table 1).
Table 1.
Laboratory Findings.
| Laboratory test | Results | Reference range | |
|---|---|---|---|
| pH | 6.966 | 7.35-7.45 | |
| pCO2 (mmHg) | 23.0 | 35-48 | mmHg |
| pO2 (mmHg) | 105.0 | 83-108 | mmHg |
| HCO3 (mmol/L) | 7.9 | 21-28 | mmol/L |
| AG (mmol/L) | 45.7 | 10-20 | mmol/L |
| Lactate (mmol/L) | 30.0 | 0.5-1.6 | mmol/L |
| Lactate (mg/dL) | 217.7 | 3.0-17.0 | mg/dL |
| Pyruvate (mg/dL) | 3.73 | 0.30-0.94 | mg/dL |
| Blood ketone (mmol/L) | 2.1 | <0.6 | mmol/L |
| AAc (mmol/L) | 0.925 | <0.055 | mmol/L |
| 3-HBA (mmol/L) | 2.171 | <0.085 | mmol/L |
| PT-INR | 0.88 | 0.9-1.1 | |
| WBC (×100 /µL) | 104.7 | 33-86×100 | /µL |
| Hb (g/dL) | 15.4 | 11.6-14.8 | g/dL |
| Plt (×10,000 /µL) | 16.4 | 15.8-34.8×10,000 | /µL |
| ALB (g/dL) | 4.4 | 4.1-5.1 | g/dL |
| BUN (mg/dL) | 16 | 8-20 | mg/dL |
| Cre (mg/dL) | 1.40 | 0.46-0.79 | mg/dL |
| eGFR (mL/min/1.73 m2) | 40.5 | >90 | mL/min/1.73 m2 |
| UA (mg/dL) | 7.5 | 3.7-7.8 | mg/dL |
| Na (mEq/L) | 135.9 | 138-145 | mEq/L |
| K (mEq/L) | 5.64 | 3.6-4.8 | mEq/L |
| Cl (mEq/L) | 85.5 | 101-108 | mEq/L |
| AST (U/L) | 117 | 13-30 | U/L |
| ALT (U/L) | 34 | 7-23 | U/L |
| LDH (U/L) | 348 | 124-222 | U/L |
| ALP (U/L) | 114 | 38-113 | U/L |
| γ-GTP (U/L) | 190 | 9-32 | U/L |
| Total Bil (mg/dL) | 0.54 | 0.40-1.50 | mg/dL |
| AMY (U/L) | 155 | 44-132 | U/L |
| CK (U/L) | 135 | 41-153 | U/L |
| Glu (mg/dL) | 230 | 73-109 | mg/dL |
| HbA1c (%) | 7.9 | 4.9-6.0 | % |
| CRP (mg/dL) | 0.33 | 0.00-0.14 | mg/dL |
3-HBA: 3-hydroxybutyric acid, AAc: acetoacetate, AG: anion gap, ALB: albumin, ALP: alkaline phosphatase, ALT: alanine transaminase, AMY: amylase, AST: aspartate aminotransferase, Bil: bilirubin, BUN: blood urea nitrogen, CK: creatine kinase, Cl: chloride, Cre: creatinine, CRP: C-reactive protein, eGFR: estimated glomerular filtration rate, γ-GTP: γ-glutamyl transferase, Glu: glucose, Hb: hemoglobin, HbA1c: hemoglobin A1c, HCO3: bicarbonate, K: potassium, LDH: lactate dehydrogenase, Na: sodium, pCO2: carbon dioxide partial pressure, Plt: platelet, pO2: oxygen partial pressure, PT-INR: prothrombin time and international normalized ratio, UA: uric acid, WBC: white blood cell
Based on low blood pH (<7.35) and elevated lactate levels (>5.0 mmol/L), the patient was diagnosed with lactic acidosis (7). Bedside echocardiography showed that the left ventricular contractility was preserved, and the inferior vena cava did not collapse. Based on physical, laboratory, and ultrasound findings, the patient did not have circulatory failure or tissue hypoxia (type B lactic acidosis) and was taking metformin; thus, we considered MALA (7).
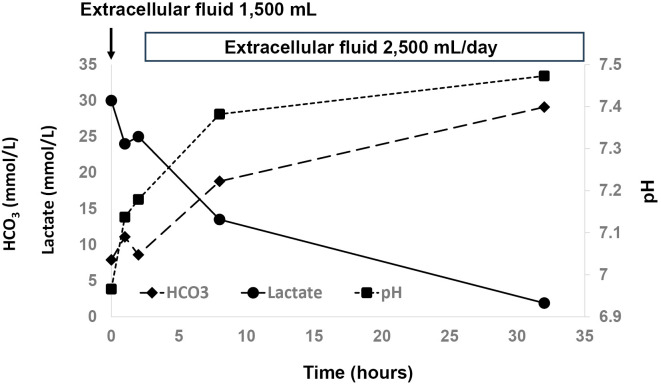
As circulation was preserved, and the urine output was maintained, the patient was infused with a crystalloid solution under careful monitoring. After the initiation of infusion, serum lactate levels continued to decrease. Metabolic acidosis resolved on day 2 of hospitalization, and serum lactate levels normalized on day 3 (Figure). Abdominal ultrasonography was performed after the patient's general condition had stabilized. No morphological changes were observed in the liver, suggestive of cirrhosis. The patient was discharged on day 17. The prescriptions at discharge were insulin glargine (10 units once daily), insulin lispro (10 units 3 times daily before each meal), and sitagliptin (50 mg once daily).
Figure.
Clinical Course.
Discussion
We herein report a case of MALA that was induced by excessive alcohol consumption. Although the patient regularly consumed large amounts of alcohol, no evidence of cirrhosis or an impaired liver function was observed. In addition, the basal renal function was normal.
Although metformin use is not contraindicated in the US or other countries, alcohol consumption may increase the risk of lactic acidosis (4,5). However, how much alcohol intake leads to MALA development is unclear. Therefore, we compiled cases of alcohol-induced MALA with no baseline renal impairment and documented amounts of alcohol intake. A literature search using the search terms ‘alcohol' and ‘metformin-associated lactic acidosis' in PubMed, Google Scholar, and Ichushi-Web (Japanese database of medical articles) identified four case reports (Table 2) (6,8-10).
Table 2.
Clinical Characteristics Of Patients With Alcohol-induced Mala.
| Age (years old) | 65 | 55 | 69 | 44 | 35 |
| Sex | Male | Male | Male | Female | Male |
| Dosage of metformin (mg/day) | 1,000 | 750 | None noted | 5,000 | 1,000 |
| Alcohol intake (g/day) | 260 | 135 | 216 | 90 | 125-150 |
| Last intake of alcohol | The day of admission | The day of admission | The day of admission | The day of admission | The day before admission |
| Serum creatinine concentration at baseline (mg/dL) | 0.77 | 0.9 | None noted | None noted | None noted |
| Serum creatinine concentration at admission (mg/dL) | 1.4 | 2.7 | 1.4 | 0.94 | None noted |
| Treatment | Infusion | Dialysis | Dialysis | Dialysis | Infusion |
| Outcome | Survived | Survived | Survived | Survived | Survived |
| Citation | Present case | [8] | [9] | [10] | [6] |
MALA: metformin-associated lactic acidosis
In patients taking metformin at a regular dose (<2,250 mg/day in Japan), alcohol consumption was >100 g/day. The amount of alcohol consumed was based on self-report. Therefore, it is possible that the patients consumed an even higher amount of ethanol than that presented in the studies. Nevertheless, it seems advisable to assume a risk of MALA when the amount of alcohol consumed exceeds 100 g/day. Therefore, the rate of alcohol consumption is important. In one study, patients with diabetes mellitus taking 3,000 mg/day of metformin ingested 30 g of ethanol in 1 min, and their blood lactate levels increased to 4 mmol/L after 1 h (11). Therefore, it is possible that even a relatively small amount of ethanol consumed in a short period may lead to the development of MALA.
None of the patients presented in Table 2 died. The prognosis of patients with MALA has been suggested to be associated with the severity of the underlying diseases (4). Thus, the mortality rate of alcohol-induced MALA in relatively young patients without chronic kidney disease or cirrhosis may be lower than the overall mortality rate of MALA.
Interestingly, the majority of the patients with alcohol-induced MALA presented in Table 2 consumed alcohol until the day of the onset. This is in contrast to alcoholic ketoacidosis (AKA), which often occurs 24-72 h after alcohol cessation and is characterized by low or undetectable blood alcohol levels (12).
The difference in onset timing may reflect differences in pathophysiology. Ethanol metabolism involves the reduction of nicotinamide adenine dinucleotide (NAD) to dihydronicotinamide dinucleotide (NADH). The increased NADH/NAD ratio converts pyruvate metabolism to lactate and inhibits gluconeogenesis (13). Indeed, in our patient, the lactate/pyruvate ratio, which is proportional to the cytoplasmic NADH/NAD ratio, was markedly elevated to 58.4 (normal range: 4-10) (14). Finally, metformin inhibits hepatic gluconeogenesis in a redox-dependent manner, which may lead to the development of lactic acidosis during acute alcohol intoxication (15).
Once ethanol ingestion ceases, the hepatic NADH/NAD ratio returns to normal, allowing gluconeogenesis to normalize. This reduces the levels of circulating lactic acid and oxaloacetate, which are gluconeogenic substrates. The depletion of oxaloacetate due to increased gluconeogenesis predisposes ketogenesis, as this substrate is essential for acetyl-CoA to enter the citric acid cycle (13,16). This mechanism is believed to be the reason for AKA development a few days after the discontinuation of alcohol intake.
One factor contributing to alcohol-induced MALA is the inhibition of gluconeogenesis and reduced lactate clearance. In contrast, in AKA, one of the contributing factors is the reversal of gluconeogenesis inhibition, which leads to oxaloacetate depletion. Considering the incompatible pathophysiologies, the likelihood of the simultaneous onset of alcohol-induced MALA and AKA appears to be low. In cases where ketoacidosis complicates alcohol-induced MALA, it may be important to consider causes of ketoacidosis other than AKA, such as diabetic ketoacidosis.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35: 1364-1379, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holman RR, Paul SK, Bethel MA, Matthews DR, Nail AW. 10-year follow up of intensive glucose control in type 2 diabetes. N Engl J Med 359: 1577-1589, 2008. [DOI] [PubMed] [Google Scholar]
- 3.ElSayed NA, Aleppo G, Aroda VR, et al. ; the American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes - 2023. Diabetes Care 46: S140-S157, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeFronzo R, Fleming GA, Chen K, Bicsak TA. Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism 65: 20-29, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Overend L, Hardy K. Metformin and alcohol. Pract Diabetes 29: 229-230, 2012. [Google Scholar]
- 6.Ryder RE. Lactic acidotic coma with multiple medication including metformin in a patient with normal renal function. Br J Clin Pract 38: 229-230, 1984. [PubMed] [Google Scholar]
- 7.Kraut JA, Madias NE. Lactic acidosis. N Engl J Med 371: 2309-2319, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Suda K, Hashimoto T, Etou T. Lactic acidosis caused by alcohol abuse in a patient with type 2 diabetes mellitus treated with metformin. Tounyoubyou (J Jpn Diabetes Soc) 49: 941-945, 2006. (in Japanese). [Google Scholar]
- 9.Fujita N, Kanao K, Shindo K, Saito Y, Takuma K. A case of circulatory failure due to metformin-associated lactic acidosis and alcoholic ketoacidosis triggered by alcohol. Nihon Rinsho Kyukyu Igakukai Zasshi (J Jpn Soc Emerg Med) 24: 743-746, 2021. (in Japanese). [Google Scholar]
- 10.Suzuki K, Okada H, Yoshida S, et al. Effect of high-flow high-volume-intermittent hemodiafiltration on metformin-associated lactic acidosis with circulatory failure: a case report. J Med Case Rep 12: 280-283, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaffalitzky de Muckadell OB, Mortensen H, Lyngsøe J. Metabolic effects of glucocorticoid and ethanol administration in phenformin- and metformin- treated obese diabetics. Acta Med Scand 206: 269-273, 1979. [DOI] [PubMed] [Google Scholar]
- 12.Duffens K, Marx JA. Alcoholic ketoacidosis - a review. J Emerg Med 5: 399-406, 1987. (in Swedish). [DOI] [PubMed] [Google Scholar]
- 13.McGuire LC, Cruickshank AM, Munro PT. Alcoholic ketoacidosis. Emerg Med J 23: 417-420, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luft FC. Lactic acidosis update for critical care clinicians. J Am Soc Nephrol 12: S15-S19, 2001. [PubMed] [Google Scholar]
- 15.Madiraju AK, Qiu Y, Perry RJ, et al. Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat Med 24: 1384-1394, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer BF, Clegg DJ. Starvation ketosis and the kidney. Am J Nephrol 52: 467-478, 2021. [DOI] [PubMed] [Google Scholar]