Abstract
The most common cause of acute kidney injury (AKI) in multiple myeloma is light-chain cast nephropathy (LCCN), which consists of a light chain and Tamm-Horsfall protein (THP). We herein report a 46-year-old woman with hypercalcemia and AKI. A renal biopsy showed crystalline casts, which were consistent with lambda light chains but not THP. Hydration therapy and treatment to lower her serum calcium concentration were initiated immediately. She subsequently received bortezomib-based anti-myeloma therapy and recovered successfully. This was a rare case of LCCN, suggesting that hypercalcemia may play a role in the development of crystalline LCCN.
Keywords: multiple myeloma, cast nephropathy, crystalline cast, Tamm-Horsfall protein, hypercalcemia
Introduction
Multiple myeloma (MM) is a hematological disorder characterized by the presence of abundant monoclonal plasma cells in the bone marrow. Renal function impairment has been reported in 20-50% of patients with MM (1-4). Symptomatic MM is commonly complicated by acute kidney injury (AKI). The most frequent cause of AKI is light chain cast (LCC) nephropathy (LCCN), which is typically defined as the precipitation of monoclonal free light chains with Tamm-Horsfall protein (THP) (5). Because THP, also known as uromodulin, is produced by tubular epithelial cells in the distal loop of Henle, casts fill the distal tubules in the distal light chain. Crystalline LCCN is a rare morphological variant of LCCN. The pathophysiology of crystalline LCCN is not well understood, although the possibility of gene mutations in the light chain may be related to the formation of crystalline LCCN (6,7).
Hypercalcemia also causes AKI in patients with MM. AKI in hypercalcemia is thought to be mediated by renal vasoconstriction, decreased water and sodium chloride reabsorption, and calcium deposition. Therefore, AKI caused by hypercalcemia may be reversible with volume expansion and reduction in serum calcium concentration. In addition, calcium chloride may facilitate the formation of crystalline LCCN (8).
We herein report a Japanese woman with IgG-lambda-type MM-associated AKI with LCCN who showed recovery of the renal function. Hydration therapy and treatment to lower her serum calcium concentration were initiated immediately. She subsequently received bortezomib-based anti-myeloma therapy and recovered successfully. A renal biopsy revealed that the renal tubules were filled with glassy and crystalline LCCs. THP is not present in crystalline LCCs. This case indicates that an early diagnosis and intervention can help achieve recovery from renal injury, even in patients with crystalline LCCN, which is reported to be associated with a poor outcome. To our knowledge, this is the first case of crystalline LCCN with MM that also showed non-crystalline casts.
Case Report
A 46-year-old woman with hypercalcemia and severe renal dysfunction was admitted to our hospital. Six months prior to presentation, she had broken her right eighth rib during a badminton match. One month before admission, she had visited a primary care clinic with nausea and general fatigue. Despite treatment with vonoprazan, her symptoms worsened, and she was found to have an elevated serum creatinine (Cr) level of 5 mg/dL.
The patient's height and weight were 158 cm and 51 kg, respectively. Her blood pressure was 138/84 mmHg. She gained 3 kg in 2 weeks and had symmetric edema, mainly on her lower legs. Laboratory findings included the following: hemoglobin, 7.9 g/dL; white blood cell count, 8,180 /μL; platelet count, 18×104/μL; total serum protein, 7.9 g/dL; serum albumin, 3.2 g/dL; serum IgG 2,563 mg/dL: serum IgA 18 mg/dL: serum IgM 7 mg/dL: blood urea nitrogen, 44 mg/dL; serum Cr, 7.1 mg/dL [estimated glomerular filtration rate (eGFR), 5.1 mL/min/1.73 m2]; serum sodium, 139 mmol/L; serum potassium, 4.8 mmol/L; serum chloride, 99 nmol/L; serum calcium 14.2 mg/dL (serum corrected calcium, 14.8 mg/dL); serum ionized calcium, 1.92 mmol/L); serum phosphate, 6.1 mg/dL; and serum uric acid, 8.0 mg/dL. The corrected calcium formula, which we used, was the addition of 0.8 mg/dL for every 1 g decrease in serum albumin below 4 g/dL. Urinalysis confirmed 1+ proteinuria and 1+ hematuria. Red blood cells (1-4/high-power field), hyaline casts, and granular casts were found in the urine sediment. Urine protein was 2.3 g/gCr. Computed tomography revealed multiple osteolytic lesions and swelling of the kidney. Based on these findings, we suspected that she had acute kidney injury associated with hypercalcemia and a hematological disorder.
Her intact parathyroid hormone level was not elevated (19 pg/mL), and serum concentrations of parathyroid hormone-related peptide (<1.0 pg/mL) and 25-hydroxyvitamin D (<4.0 ng/mL) were suppressed. In addition, serum protein immune electrophoresis showed M protein with monoclonal IgG-lambda, and urine protein immunofixation electrophoresis showed a monoclonal free lambda spike. Her serum free light chain level was 3,460 mg/L with a kappa-to-lambda ratio of 0.13. Bone marrow aspiration showed plasma cells accounting for 10.4% of the total marrow cellularity, and a bone marrow biopsy showed hypercellular marrow with increased CD138-positive plasma cells. In addition, in situ hybridization revealed that tumor cells expressed lambda light chains. Therefore, a diagnosis of kidney failure associated with IgG-lambda-type multiple myeloma was made.
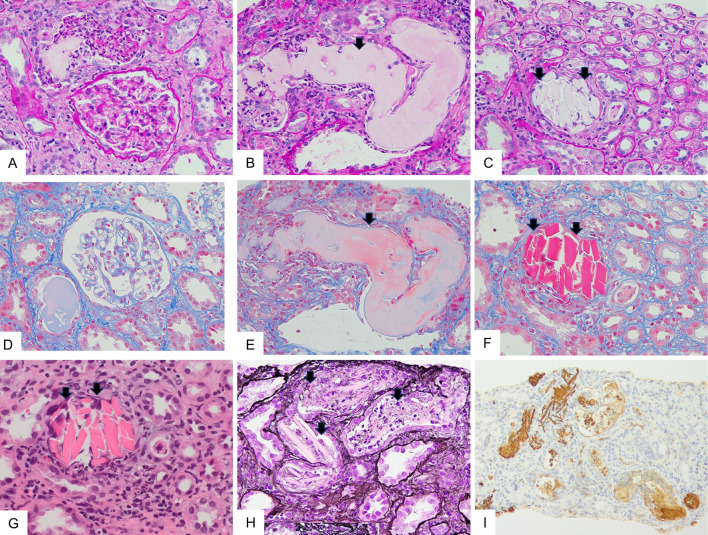
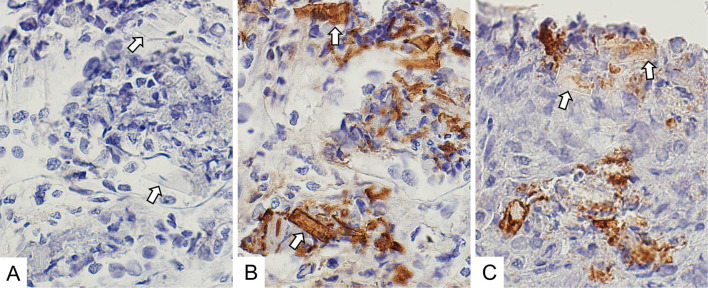
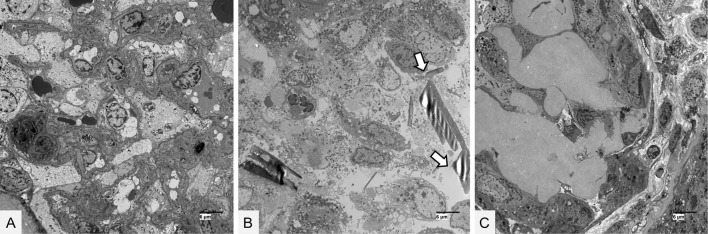
A renal biopsy showed 30 glomeruli with minor abnormalities (Fig. 1A, D). Moderate interstitial fibrosis and tubular atrophy were observed with the casts filling the tubular lumens. The casts consisted of two types: glassy casts with a fractured appearance (Fig. 1B, E) and crystalline casts with a rod-shaped or rhomboid configuration (Fig. 1C, F-H). Crystalline casts were fuschinophilic on Masson's trichrome staining (Fig. 1F) and eosinophilic on hematoxylin and eosin staining (Fig. 1G), as previously reported (6). The two types of casts were positively stained with an anti-lambda light-chain antibody (Fig. 1I). Serial biopsy sections were also processed with anti-kappa, anti-lambda, and anti-THP antibodies, as in the case of Matsumura et al. (6). The crystalline casts were positively stained with an anti-lambda light chain antibody (Fig. 2B) but not with anti-kappa light chain and anti-THP antibodies (Fig. 2A, 2C). Electron microscopy revealed no glomerular deposits (Fig. 3A). Needle-like rod-shaped intraluminal crystalline inclusions were observed in the tubules (Fig. 3B), and cell reactions were observed around the crystalline inclusions and non-crystalline casts (Fig. 3B, C).
Figure 1.
Light microscopic findings of the renal biopsy specimens. (A-C) Periodic acid-Schiff (PAS) staining (original magnification 400×). (D-F) Masson’s trichrome (MT) staining (original magnification 400×). (A and D) The glomeruli showed minor abnormalities without crystalline depositions. (B and E) Glassy casts with fractured appearance were seen in the distal tubular lumens (arrows). (C and F) Crystalline casts with rod-shaped or rhomboid configuration appearance were observed in the distal tubular lumens. Crystalline casts were fuschinophilic on MT staining but were pale on PAS staining (arrows). (G) Hematoxylin and Eosin staining (original magnification 400×). Crystalline casts were eosinophilic (arrows). (H) Periodic acid-methenamine silver staining (original magnification 400×). Crystalline casts surrounded by reactive inflammatory cells were pale (arrows). (I) Lambda light chain staining (original magnification 100×). Glassy casts and crystalline casts were positively stained.
Figure 2.
Immunohistochemical staining of the serial renal biopsy sections. (A) Kappa light chain staining (original magnification 600×). Crystalline inclusions were negatively stained (arrows). (B) Lambda light chain staining (original magnification 600×). Crystalline inclusions were positively stained (arrows). (C) Tamm-Horsfall protein staining (original magnification 600×). Crystalline inclusions were negatively stained (arrows).
Figure 3.
Electron microscopic findings of the renal biopsy specimens. (A) No electron-density deposits were found in the glomerular cells. The bar represents 6 μm. (B) Crystalline substructures were seen within the tubular lumens (arrows). The bar represents 6 μm. (C) Cell reactions were found around the noncrystalline cast. The bar represents 6 μm.
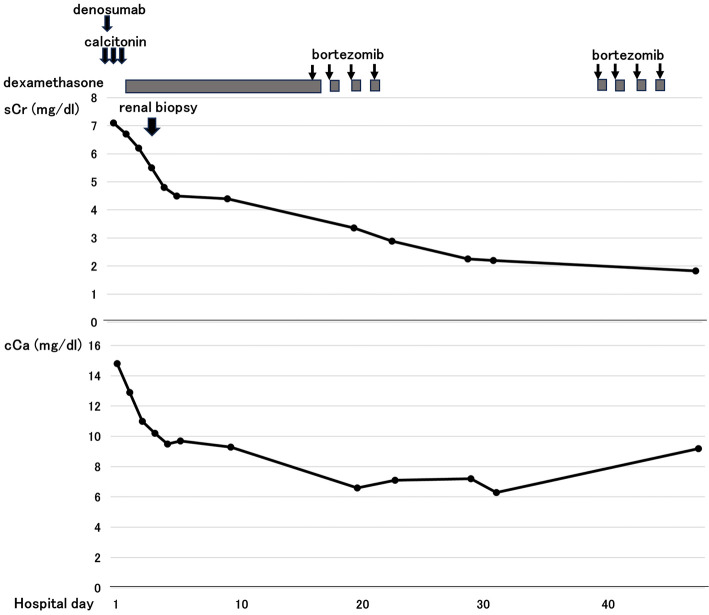
The patient was initially started on therapy with calcitonin plus hydration, followed by denosumab for hypercalcemia and dehydration. At 3 days after the initiation of therapy, her renal serum Cr had decreased from 7.1 mg/dL to 4.8 mg/dL (eGFR: from 5.6 mL/min/1.73 m2 to 8.3 mL/min/1.73 m2). The patient then received anti-myeloma treatment with bortezomib (1.3 mg/m2/therapy) and dexamethasone (20 mg/therapy). At 1.5 months after the initiation of therapy, her serum Cr level was 1.8 mg/dL (eGFR:25.1 mL/min/1.73 m2) (Fig. 4).
Figure 4.
Changes in the renal function and serum calcium concentration during the initial management. sCr: serum creatinine, cCa: serum corrected calcium
During treatment, she did not show any significant side effects, including bortezomib-induced neuropathy or lung injury. Her renal function gradually recovered, and she underwent stem cell transplantation 10 months after presentation. One year after the onset of the presentation, she was well, and her serum Cr level was 1.32 mg/dL (eGFR: 35 mL/min/1.73 m2).
Discussion
The pathophysiology of LCC is complex. In general, the circulating light chain is freely filtered through the glomerulus and forms casts upon interaction with THP (5). LCC formation is influenced by the concentration of free light chains. In patients with MM, the serum free light chain level at the diagnosis was reported to be related to the renal outcome, and LCCN occurs below a serum free light chain level of 500 mg/L (9). In the present case, the serum light chain level was 3,460 mg/L. In addition, LCC formation is influenced by factors such as the THP concentration, ionic composition of the tubule fluid, tubule fluid flow rates, and the presence of furosemide (4,5). Any concurrent conditions leading to dehydration are potential precipitating factors for LCCN. Hypercalcemia can trigger LCCN through dehydration and increase urine calcium concentration. In addition, calcium chloride is also known to facilitate the crystallization of macromolecules (8). In our case, the serum ionized calcium level increased to 1.92 mmol/L. Thus, a high concentration of free light chains and hypercalcemia may have influenced crystalline cast formation in our case.
The CDR3 hypervariable region of the free light chain binds to a nine-amino-acid sequence of THP (6,10). In the present case, the renal tubular lumens were filled with glassy noncrystalline LCCs and crystalline LCCs. Crystalline casts differ from noncrystalline casts in terms of cast formation. Non-crystalline LCCs are the result of the coprecipitation of free light chains with THP and have a fractured appearance and inflammatory cellular reactions. In an experimental model, THP was shown to bind to free light chains in variable regions (11). Crystalline LCCs are considered to form based on the structural characteristics of pathogen-free light chains through hydrophobic and hydrogen bonds (6). In fact, in our case, the crystalline casts were positive for lambda light chain staining but not THP staining. In previously reported cases of crystalline cast nephropathy, two patients were subjected to a genetic analysis, and mutations in the variable regions were found (6,7). Both cases had mutations in the CD3 region, known as the hypervariable region of the free light chain, which binds to a nine-amino acid sequence of THP. In addition, glassy non-crystalline LCCs were not found in either case. Since mutations in CD3 might reduce the affinity for THP, these findings support the hypothesis that structural changes in the variable region may affect crystalline cast formation and inhibit the binding of THP to the free light chain.
Immunohistochemical staining was performed in a previous case report of crystalline LCCN (6). As in our case, THP was not incorporated into the crystalline material. In our case, glassy noncrystalline casts were also observed. To our knowledge, this is the first case to clearly show the colocalization of two types of LCC obstructing the tubular lumens. In addition, to our knowledge, three previous reports on crystalline light chain cast nephropathy described the serum calcium levels, and none of those cases had hypercalcemia (serum calcium 8.4-9.6 mg/dL) (6,12,13). However, in contrast to the previous reports, the serum calcium level in the present case was elevated to 14.2 mg/dL (serum corrected calcium, 14.8 mg/dL). Patients with crystalline LCCN have been reported to show rapid progression and a poor prognosis (6). However, the present patient's AKI successfully resolved with hypercalcemia correction (Fig. 4). Therefore, although the exact mechanism underlying the existence of two different casts (grassy and crystalline cast) in this case was unclear, hypercalcemia and/or hypercalcemia-induced volume depletion may have played an important role in the formation of crystalline LCCN.
Recent cohort studies of patients with confirmed LCCN who required dialysis and did not recover their renal function showed a 50% survival rate at <1 year (5). In particular, patients with crystalline LCCN have been reported to show rapid progression and a poor prognosis (6). As irreversible AKI is associated with a reduced survival in patients with myeloma, an early diagnosis and immediate treatment are needed. In addition, some recent reports of crystalline LCCN, including ours, have suggested that bortezomib-based therapy might help resolve AKI (12,14). Renal failure affects the treatment options available to patients, including certain chemotherapeutic agents and hematopoietic stem cell transplantations. Our patient received appropriate treatment for LCCN and underwent hematopoietic stem cell transplantation.
In conclusion, we encountered a patient with IgG-lambda-type MM-associated AKI whose renal tubules were filled with glassy noncrystalline and crystalline casts. To our knowledge, this is the first case to clearly show the colocalization of two types of light-chain casts obstructing tubular lumens. The patient showed successful recovery of renal manifestations and eventually underwent hematopoietic stem cell transplantation. An early diagnosis, correction of hypercalcemia, hydration, and administration of bortezomib-based therapy may aid in the restoration of the renal function in AKI patients with LCCN.
Informed consent was obtained from the participant included in this article.
Author's disclosure of potential Conflicts of Interest (COI).
Takashi Uzu: Honoraria, Eli Lilly Japan, AstraZeneca and Kyowa Kirin.
Acknowledgement
The authors thank Mrs. Hiromi Kataho and Dr. Naoto Takahashi (Akita University School of Medicine) for performing the immunohistochemical analyses using anti-kappa, anti-lambda, and anti-THP antibodies on serial biopsy sections.
References
- 1.Bladé J, Fernández-Llama P, Bosch F, et al. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med 158: 1889-1893, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 78: 21-33, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Eleutherakis-Papaiakovou V, Bamias A, Gika D, et al. Renal failure in multiple myeloma: incidence, correlations, and prognostic significance. Leuk Lymphoma 48: 337-341, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Yadav P, Cook M, Cockwell P. Current trends of renal impairment in multiple myeloma. Kidney Dis (Basel) 1: 241-257, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkel KW, Cohen EP, Shirali A, Abudayyeh A; American Society of Nephrology Onco-Nephrology Forum. Paraprotein-related kidney disease: evaluation and treatment of myeloma cast nephropathy. Clin J Am Soc Nephrol 11: 2273-2279, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumura H, Furukawa Y, Nakagaki T, et al. Multiple myeloma-associated Ig light chain crystalline cast nephropathy. Kidney Int Rep 5: 1595-1602, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toly-Ndour C, Peltier J, Piedagnel R, et al. Acute renal failure with lambda light chain-derived crystals in a patient with IgD myeloma. Nephrol Dial Transplant 26: 3057-3059, 2011. [DOI] [PubMed] [Google Scholar]
- 8.McPhersona A, Gavira JA. Precipitants used in macromolecular crystallization. Acta Crystallogr F Struct Biol Commun 70: 2-20, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yadav P, Sathick IJ, Leung N, et al. Serum free light chain level at diagnosis in myeloma cast nephropathy - a multicentre study. Blood Cancer J 10: 28, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basnayake K, Stringer SJ, Hutchison CA, Cockwell P. The biology of immunoglobulin free light chains and kidney injury. Kidney Int 79: 1289-1301, 2011. [DOI] [PubMed] [Google Scholar]
- 11.Ying WZ, Allen CE, Curtis LM, Aaron KJ, Sanders PW. Mechanism and prevention of acute kidney injury from cast nephropathy in a rodent model. J Clin Invest 122: 1777-1785, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miki K, Shimamura Y, Maeda T, et al. Successful renal recovery from multiple myeloma-associated crystalline light chain cast nephropathy and accompanying acute kidney injury with early use of bortezomib-based therapy: a case report and literature review. CEN Case Rep 12: 56-62, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haider M, Salvatore SP, Kaplan J, Seshan SV. Acute kidney injury due to tubular intraluminal monoclonal light chain crystals mimicking acute pyelonephritis. Ren Fail 36: 300-305, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Piro E, Molica S. A systematic review on the use of bortezomib in multiple myeloma patients with renal impairment: what is the published evidence? Acta Haematol 126: 163-168, 2011. [DOI] [PubMed] [Google Scholar]