Abstract
A 49-year-old Japanese woman was admitted to our hospital with weight loss of 15 kg, nephrotic-range proteinuria (4.5 g/g.Cre), and hematuria over a 6-month period. She had received two doses of the coronavirus disease 2019 (COVID-19) vaccine one year before the onset of the disease, after which the estimated glomerular filtration rate increased. Laboratory tests and other tests led to a diagnosis of hyperthyroidism, and a kidney biopsy showed thrombotic microangiopathy-like glomerular microangiopathy comprising mainly glomerular endothelial cell damage. Thiamazole (30 mg) was started for the hyperthyroidism. Three months later, the thyroid function normalized, and two months later, the proteinuria and hematuria disappeared, suggesting that COVID-19 vaccination and these events were related.
Keywords: COVID-19 vaccination, glomerular microangiopathy, hyperthyroidism, TMA-like renal disease, tamoxifen
Introduction
Thyroid hormones are well known to have some function in the kidneys (1). Recently, therapeutic agents for hyperthyroidism have been reported to cause antineutrophil cytoplasmic antibody-associated vasculitis (2). Only a few publications have reported the relationship between hyperthyroidism and nephropathy (3-5), and this issue has not been discussed much. In cases where renal disease is related to hyperthyroidism, it can also be expected to improve when the thyroid function normalizes with treatment.
We encountered a case of hyperthyroidism coinciding with nephropathy onset and remission. The kidney biopsy findings in our case were different from those reported previously, and we discuss the reasons for this difference.
Case Report
A 49-year-old Japanese woman was admitted to our hospital because of weight loss of 15 kg, nephrotic-range proteinuria, and hematuria. At 41 years old, the patient had undergone surgical resection for right-sided breast cancer, after which tamoxifen had been administered until 47 years old. Six months before the current admission, no urinary abnormalities, such as proteinuria or occult blood, had been present. Two months before admission, however, the patient began to notice rapid weight loss despite eating normally.
On admission, the patient was 162 cm tall and weighed 47.3 kg. Her blood pressure was 124/80 mmHg, heart rate was 110 beats/min, and body temperature was 36.3°C. No edema was present. Rapid weight loss and tachycardia were noted, but hand tremor, increased sweating, and fullness in the thyroid region were not evident.
The findings of a complete blood count were as follows: erythrocytes, 4.4×106/μL; hemoglobin, 12.1 g/dL; leukocytes, 4,100 /μL; and thrombocytes, 21.5×104/μL. The results of blood chemistry tests were as follows: serum protein, 5.9 g/dL; serum albumin, 3.6 g/dL; serum creatinine, 0.37 mg/dL; serum cysteine C, 0.90 mg/L; estimated glomerular filtration rate (eGFR)cre (using creatinine), 140.0 mL/min/1.73 m2; eGFRcys (using cysteine C), 72.2 mL/min/1.73 m2; total cholesterol, 137 mg/dL; hemoglobin A1c, 5.8%; C-reactive protein, 0.05 mg/dL. There were no findings suggestive of hemolytic anemia, such as elevated bilirubin or lactate dehydrogenase levels or the appearance of schistocytes, or thrombocytopenia. The total complement activity (assessed as CH50) was 73 U/mL (reference range, 30-45 U/mL). Autoantibodies, including antinuclear antibody, anti-double-stranded DNA antibody, anti SS-A/B antibody, anti-neutrophil cytoplasmic antibody (MPO/PR3), were negative.
Urinary protein excretion was 4.5 g/g.Cre (calculated using creatinine excretion from spot urine), and the urinary sediment contained more than 100 erythrocytes per high-power field. Anti-thyroglobulin antibody was 40.2 IU/mL (reference value, <28 IU/mL), and anti-thyroid microsome [antithyroperoxidase (TPO)] antibody was 206.0 IU/mL (reference value, <16.0 IU/mL).
The level of thyroid-stimulating hormone (TSH) was 0.01 μlU/mL (reference range, 0.35-4.9 μlU/mL), that of free T3 (FT3) was 26.2 ng/dL (reference range, 0.7-1.48 ng/dL), that of free T4 (FT4) was 7.7 ng/dL (reference range, 0.7-1.48 ng/dL), and that of TSH receptor antibody (TRAb) was 8.8 IU/L (reference value, <2.01 IU/L) (Table).
Table.
Laboratory Test Results at Kidney Biopsy.
| At kidney biopsy | nomal range | |
|---|---|---|
| White blood cell(/μL) | 4,300 | 3,200 - 7,900 |
| Red blood cell(106/μL) | 4.4 | 3.7 - 5.0 |
| Hemoglobin(g/dL) | 12.1 | 11.3-15.0 |
| Platelet (×103/μL) | 21.5 | 155-350 |
| Total protein (g/dL) | 6.6 | 6.9-8.4 |
| Albumin (g/dL) | 3.7 | 4.1-5.1 |
| Urea nigrogen (mg/dL) | 7.2 | 8-21 |
| Creatinine (mg/dL) | 0.37 | 0.6-1.0 |
| eGFR (mL/min/1.73 m3) | 140 | ≥ 90 |
| C-reactive protein(CRP) | 0.05 | <0.3 |
| Aspartate aminotransferase(IU/L) | 17 | 11-38 |
| Alanine transaminase(IU/L) | 15 | 6-50 |
| Lactate Dehydrogenase(IU/L) | 151 | 103-190 |
| Alkaline phosphatase(U/L) | 109 | 38-113 |
| Total bilirubin (mg/dL) | 0.76 | 0.4-1.5 |
| Prothrombin time(%) | 89 | >75 |
| Activated partial thromboplastin time(sec) | 24.4 | 27-40 |
| D dimer(μg/mL) | 7.06 | <0.99 |
| Complement activities50(U/mL) | 73 | 30-46 |
| Complement3(mg/dL) | 62 | 86-160 |
| Complement4(mg/dL) | 36 | 17-45 |
| Renin activity(ng/mL/h) | 2.03 | 0.2-2.9 |
| Aldosteron concentration(ng/dL) | 3.8 | 2.9-15.9 |
| IgG(mg/dL) | 1,071 | 870-1,700 |
| IgA(mg/dL) | 139 | 110-410 |
| IgM(mg/dL) | 46 | 46-260 |
| Antinuclear antibody(ANA) | <40 | <40 |
| Anti-double-stranded DNA antibody(IU/mL) | <12 | <12 |
| Anti Sm antibody(U/mL) | 10.8 | <7.0 |
| Anti-RNP antibody(U/mL) | 1.9 | <3.5 |
| Anti-SS-A antibody(U/mL) | <1 | <1 |
| Anti-SS-B antibody(U/mL) | <1 | <1 |
| Anti-cardiolipin antibody | nagative | negative |
| Anti-neutrophil cytoplasmic antibody(MPO/PR3) | negative | negative |
| Cryoglobuline | negative | negative |
| Thyroid-stimulating hormone (TSH)(μlU/mL) | 0.01 | 0.35-4.9 |
| Free T3 (FT3)(ng/dL) | 26.2 | 0.7-1.48 |
| Free T4 (FT4)(ng/dL) | 7.7 | 0.7-1.48 |
| TSH receptor antibody (TRAb)(IU/L) | 8.8 | <2.01 |
| Thyroid microsome (or TPO) antibodie(IU/mL) | 206 | <16.0 |
| Thyroglobulin antibody | 40.2 | <28.0 |
| Hepatitis B virus(HBV) antibody | negative | negative |
| Anti-hepatitis C virus (HCV) antibody | negative | negative |
| Urinary RBC sediment(/HPF) | <1 | <1 |
| Urinary protain (g/gCr) | 2.49 | <0.15 |
| Urinary Bence Jones protein | negative | negative |
Computed tomography revealed bilateral diffuse enlargement of the thyroid glands. Ultrasonography also showed diffuse enlargement of the bilateral thyroid glands, with hypoechogenicity and abundant blood flow.
Hyperthyroidism was diagnosed, and thiamazole 30 mg/day was started immediately. One month later, a kidney biopsy was performed to evaluate nephropathy.
Kidney biopsy findings
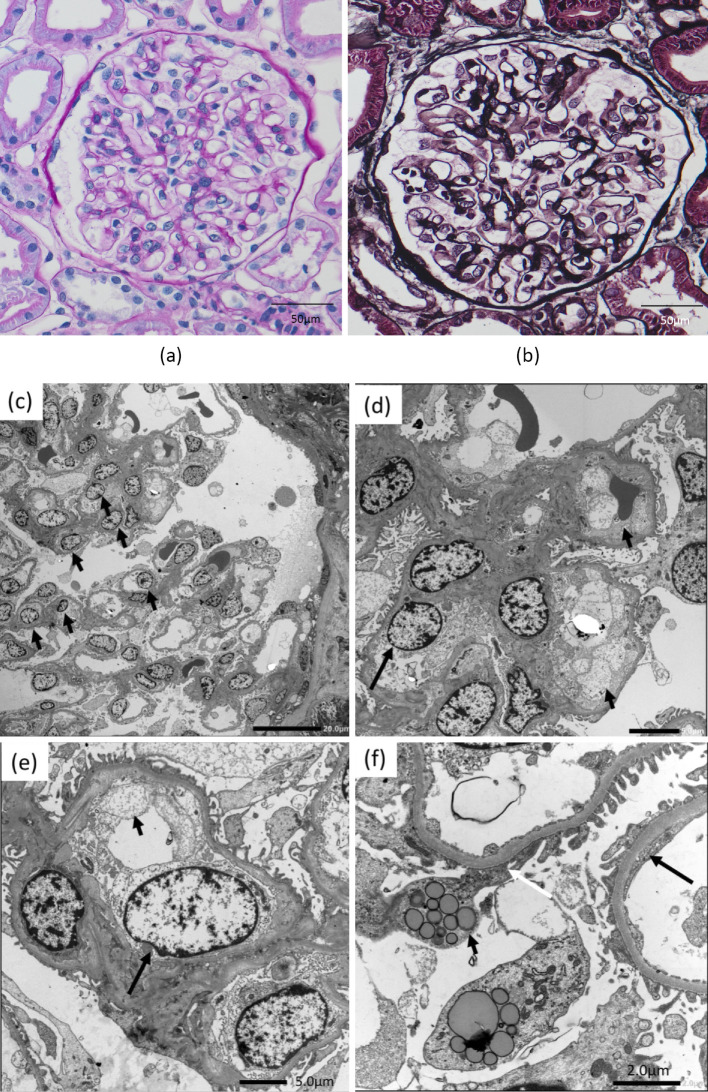
A light microscopic examination of the biopsy specimen revealed no global sclerosis in any of the glomeruli (n=27). The glomeruli and tubules appeared normal, but glomerular endothelial cell proliferation was slightly prominent (Fig. 1a, b). Immunofluorescence staining was negative for IgG, IgA, IgM, C3, and C1q. However, electron microscopy showed endothelial cell proliferation and hyperplasia at low magnification (Fig. 1c) and endothelial cell swelling with subendothelial edema and narrowing of the subendothelial space at high magnification (Fig. 1d, e). Endothelial fenestrations were not regularly arranged, and fenestration fusion was observed in many areas (Fig. 1f). The foot process was intact in some areas; however, many areas of foot process effacement were observed, and vacuolar structures were present in the podocytes (Fig. 1f). Electron-dense deposits were not observed in the mesangial or subendothelial subepithelial areas.
Figure 1.
Kidney biopsy findings. a+b: Light microscopy findings: Glomerular endothelial cell proliferation was slightly prominent. a: Periodic acid-Schiff (original magnification ×400; bar=50 μm). b: Periodic acid methenamine silver staining (original magnification, ×400; bar=50 μm). c-f: Electron microscopy findings. c: At low magnification, endothelial cell proliferation (arrow) was noted. d and e: Endothelial cell swelling (large arrow) and subendothelial edema (small arrow) were observed at high magnification. f: Endothelial fenestrations are not regularly arranged, and fenestration fusion (large arrow) is observed. Many areas of foot process effacement (white arrow) were seen. Vacuolar structures (small arrow) were present in the podocyte.
Light microscopy and immunofluorescence microscopy findings correspond to minimal change disease; however, electron microscopy findings correspond to glomerular microangiopathy or glomerular endotheliopathy, which is referred to as thrombotic microangiopathy (TMA)-like renal disease (6).
Clinical course
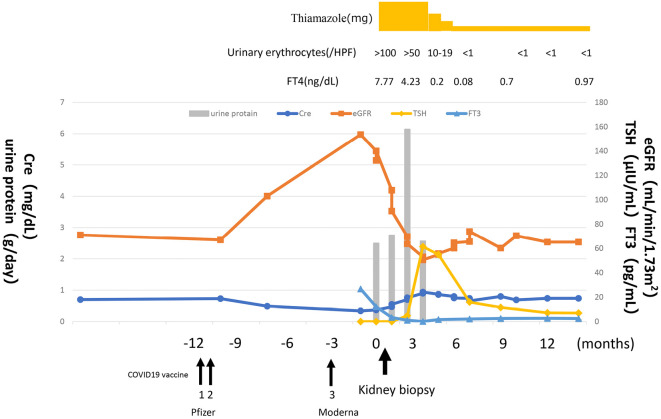
After thiamazole was started at 30 mg/day, the FT3 and FT4 levels gradually decreased and normalized after 3 months. Urinary protein and sediment erythrocytes also showed a downward trend after three months and normalized after five months. One year later, the thyroid function remained normal, with no relapse of proteinuria or hematuria. The serum albumin level increased to 4.4 g/dL. The serum creatinine level was 0.70 mg/dL, the serum cysteine C level was 0.85 mg/L, the eGFRcre was 69.3 mL/min/1.73 m2, and the eGFRcys was 85 mL/min/1.73 m2 (Fig. 2).
Figure 2.
Clinical course. After thiamazole was started at 30 mg/day, free T3 and free T4 levels gradually decreased and normalized after 3 months. Urine protein and urinary sediment erythrocytes also showed a downward trend after three months and normalized after five months. The clinical course was evaluated by assessing thyroid-stimulating hormone levels (μIU/mL), free T3 levels (pg/mL), urine protein levels (g/day), and urinary erythrocyte count (/high-power field). FT3: free T3 level, FT4: free T4 level, TSH: thyroid-stimulating hormone
We found that the patient had received two doses of the coronavirus disease 2019 (COVID-19) vaccine one year before the onset of the disease, after which the serum creatinine level started to decrease and the eGFR increased, peaking when hyperthyroidism and proteinuria appeared. Proteinuria subsequently normalized as the thyroid function normalized and the eGFR decreased and stabilized (Fig. 2).
Discussion
We encountered a patient with hyperthyroidism, proteinuria, and hematuria who was successfully treated with the antithyroid drug, thiamazole. Therefore, we searched PubMed for articles describing renal disease caused by hyperthyroidism; however, we found only a few reports, which we describe briefly below:
Weetman et al. examined 14 patients with Graves' disease who did not originally have proteinuria (3). They found proteinuria in nine patients at the onset of the disease and reported that proteinuria decreased in four of these patients after remission of Graves' disease upon treatment with isotope therapy [radioactive iodine (I-131)]. These results suggest that hyperthyroidism may be associated with proteinuria. The authors also stressed the possibility that some patients with hyperthyroidism may have proteinuria when a detailed urinalysis is performed.
Mariani et al. summarized reports on renal disorders in patients with hyperthyroidism and found that renal histopathology revealed membranous nephropathy, minimal change disease, membranoproliferative glomerulonephritis, and IgA nephropathy (1).
Neves et al. described a 46-year-old man with nephrotic syndrome and symptomatic hyperthyroidism, in whom a kidney biopsy showed membranous nephropathy (4). They reported that the thyroid function normalized after the patient started treatment with methimazole and prednisone and that proteinuria remained unchanged but then disappeared with the addition of radioiodine therapy.
Hasnain et al. treated a 63-year-old man with Graves' disease complicated by nephrotic syndrome (5). A kidney biopsy revealed minimal changes in the disease. Treatment with mercazole was not effective; therefore, total thyroidectomy was performed, which resulted in the normalization of the thyroid function and later resolution of proteinuria.
These reports suggest that hyperthyroidism may be a possible cause of proteinuria in renal disease. However, based on these reports alone, we cannot conclude that there is a strong relationship between hyperthyroidism and associated renal dysfunction, as this phenomenon is extremely rare. Further research on this topic is required.
In our patient, we suspected a relationship between hyperthyroidism and nephropathy; however, such a relationship should not be assumed and requires careful investigation of background factors. The only clue in this patient was that she had undergone surgery for breast cancer and had been receiving tamoxifen for a long time. Some reports have suggested that hypothyroidism can occur after long-term use of tamoxifen, and patients can subsequently develop hyperthyroidism as a rebound phenomenon after discontinuation of the drug (6). Our patient had also been treated with tamoxifen for six years and developed hyperthyroidism two years after its discontinuation. Thus, tamoxifen treatment may have contributed to the development of hyperthyroidism, and the course of the case indicated that hyperthyroidism and nephropathy may have been related in our patient.
The nephropathy observed in our patient was an endothelial cell disorder similar to the TMA-like lesions reported by Eremina et al. (7), who described the characteristics of renal injury caused by molecular-targeted drugs for malignant tumors. Our case was similar in that it was one of nephropathy involving drug-related factors.
Immune deposit-related membranous nephropathy, IgA nephropathy, and membranoproliferative glomerulonephritis have been reported in patients with hyperthyroidism. We encountered a case of new TMA-like glomerular microangiopathy. In previous reports, immune deposit-related diseases developed during chronic hyperthyroidism; however, in the present case, the onset of both hyperthyroidism and renal disease was rapid. If hyperthyroidism had become chronic, the patient may have developed nephropathy related to immune deposits.
If only severe proteinuria had been seen rapidly, it would correspond to minimal change disease, but the simultaneous presence of proteinuria and hematuria in this case cannot be explained by minimal change disease alone, suggesting the presence of some endocapillary proliferative glomerulonephritis, including mesangial and endothelial cell damage. In fact, the finding of foot process effacement on electron microscopy may explain the proteinuria, but the finding of endothelial cell damage may also explain the hematuria. Since hyperthyroidism has been reported to trigger a variety of nephropathies with proteinuria and hematuria, we would like to report this case as a variation of nephropathy, although the nephropathy seen in this case does not fit into the conventional nephropathy concept. The fact that proteinuria and hematuria disappeared simultaneously with normalization of the thyroid function suggests that hyperthyroidism is involved in the development of nephropathy as evidenced by a kidney biopsy.
The clinical course of this case is reviewed in terms of changes in serum creatinine and eGFR values. We additionally searched for factors contributing to its pathogenesis. We found that the patient had received two doses of the COVID-19 vaccine one year before the onset of the disease, after which the serum creatinine level started to decrease and the eGFR increased, peaking when hyperthyroidism and proteinuria appeared. Proteinuria subsequently normalized as the thyroid function normalized and the eGFR decreased and stabilized. On admission, the eGFRcre was abnormally high compared to the eGFRcys, with (eGFR)cre of 140.0 mL/min/1.73 m2 vs. eGFRcys of 72.2 mL/min/1.73 m2. This indicated increased glomerular filtration, which could be related to the development of hyperthyroidism. It is evident that nephropathy develops late in association with hyperthyroidism. COVID-19 vaccination is inferred to be related to this phenomenon. In recent years, there have been reports of COVID-19 vaccination being associated with the development of nephropathy related to various immune abnormalities (8). It is possible that COVID-19 vaccination may have contributed to hyperthyroidism and nephropathy in this case. We have also seen reports of Graves' disease developing after mRNA COVID-19 vaccination, making it plausible that a similar event happened in our case (9). Our speculation is understandable, as it has been reported that excessive thyroid hormone secretion causes an increase in the GFR, whereas hypothyroidism is associated with a decrease in the GFR (10).
In conclusion, we encountered a case of nephropathy that developed in association with rapid-onset hyperthyroidism over a period of six months. The nephropathy was accompanied by proteinuria, hematuria, and TMA-like glomerular microangiopathy, which consisted mainly of glomerular endothelial cell damage, and thus was in line with the simultaneous presence of proteinuria and hematuria. Both proteinuria and hematuria remitted two months after the hyperthyroidism remitted, suggesting that nephropathy was strongly related to hyperthyroidism. Furthermore, the involvement of COVID-19 vaccination in the development of hyperthyroidism and nephropathy was suggested.
notes
The present report conformed to the Declaration of Helsinki, and the patient provided her written informed consent for the publication of the details of her case.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
I would like to thank Toru Sanai (Fukumitsu Hospital) for his advice on preparing this paper.
References
- 1.Mariani LH, Berns JS. The renal manifestations of thyroid disease. J Am Soc Nephrol 23: 22-26, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Hasegawa J, Hoshino J, Sekine A, et al. Clinical and histological features of antineutrophil cytoplasmic antibody-associated vasculitis related to antithyroid drugs. Clin Nephrol 89: 438-444, 2018. [DOI] [PubMed] [Google Scholar]
- 3.Weetman AP, Tomlinson K, Amos N, Lazarus JH, Hall R, McGregor AM. Proteinuria in autoimmune thyroid disease. Acta Endocrinol (Copenh) 109: 341-347, 1985. [DOI] [PubMed] [Google Scholar]
- 4.Neves PDMM, Muniz MPR, Morgantetti GF, et al. Membranous nephropathy secondary to Graves' disease: a case report. Front Immunol 13: 824124, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasnain W, Stillman IE, Bayliss GP. Minimal-change renal disease and Graves' disease: a case report and literature review. NDT Plus 4: 96-98, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marina D, Rasmussen ÅK, Buch-Larsen K, Gillberg L, Andersson M, Schwarz P. Influence of the anti-oestrogens tamoxifen and letrozole on thyroid function in women with early and advanced breast cancer: a systematic review. Cancer Med 12: 967-982, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129-1136, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klomjit N, Alexander MP, Fervenza FC, et al. COVID-19 vaccination and glomerulonephritis. Kidney Int Rep 6: 2969-2978, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura F, Awaya T, Ohira M, Enomoto Y, Moroi M, Nakamura M. Graves' disease after mRNA COVID-19 vaccination, with the presence of autoimmune antibodies even one year later. Vaccines (Basel) 11: 934, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basu G, Mohapatra A. Interactions between thyroid disorders and kidney disease. Indian J Endocrinol Metab 16: 204-213, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]