Abstract
Human brucellosis, one of the most common zoonoses worldwide, is rare in Japan. Brucella canis is the specific pathogen of human brucellosis carried by dogs. According to an epidemiological study of B. canis infection in Japan, B. canis is the specific pathogen of human brucellosis in dogs. We herein report a rare case of meningoencephalomyelitis caused by B. canis in a 68-year-old Japanese man. Neurobrucellosis was diagnosed based on a serum tube agglutination test and abnormal cerebrospinal fluid findings. The patient was started on targeted treatment with a combination of doxycycline and streptomycin. Although extremely rare, neurobrucellosis should be considered in patients with a fever of unknown origin and unexplained neurological symptoms.
Keywords: neurobrucellosis, Brucella canis, meningoencephalomyelitis, fever of unknown origin, doxycycline, streptomycin
Introduction
Brucellosis is a zoonotic infection that occurs worldwide and is transmissible to humans (1,2). Four species of Brucella are pathogenic to humans, each of which has specific types of animal reservoirs: Brucella melitensis (goats, sheep, and camels), B. abortus (cattle and buffalo), B. suis (pigs), and B. canis (dogs). Neurologic involvement, including involvement of the peripheral and central nervous systems (CNS), occurs in approximately 5-10% of cases (3). Human neurobrucellosis is a rare condition in Japan.
We herein report a rare case of meningoencephalomyelitis caused by B. canis in a Japanese man.
Case Report
A 68-year-old Japanese man was admitted to our hospital with complaints of appetite loss, mental alternation, a fever, chills, gait disturbance, and incontinence. Although he had traveled 10 days prior to the onset of illness in Japan and had diarrhea, he had not eaten any unpasteurized food or had contact with a sick person. He had a history of diabetes mellitus, cervical and lumbar disc herniation, drinking, smoking, and allergies to lacquer. In addition, he had previously adopted a dog up to one year ago.
Upon admission, he was febrile (body temperature, 37.9°C), his blood pressure was high at 160/120 mmHg, and his pulse rate was regular at 74 bpm. Although he was able to follow simple instructions, he had an aphasia-like language disorder (Japan Coma Scale: I-2, restlessness). Neurological findings included ataxia of the left upper and lower limbs, ataxic gait, hyperreflexia of the deep tendons of the extremities, and urinary obstruction. Myoclonus of the extremities, trunk, and head and restlessness were observed. The laboratory results were as follows: white blood cell count, 8,720 /μL (neutrophil 64%, eosinophil 1.7%, basophil 0.5%, monocyte 10.1%, and lymphocyte 23.7%); C-reactive protein level, 0.23 mg/dL; erythrocyte sedimentation rate, 21 mm/h; hemoglobin A1c level, 6.1%; and human immunodeficiency virus antibody, negative. A cerebrospinal fluid (CSF) examination revealed 100 mmH2O pressure, xanthochromia, lymphocytic pleocytosis (CSF cell count, 110 /μL), high protein concentration (236 mg/dL), glucose level of 67 mg/dL (blood glucose, 108 mg/dL), and normal IgG index (0.57; cut-off <0.7).
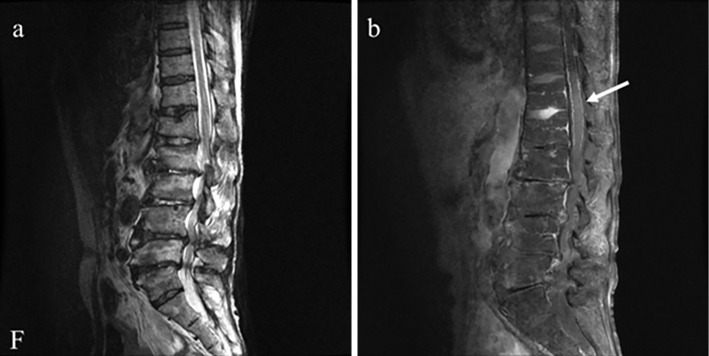
Chest radiography and brain magnetic resonance imaging (MRI) showed no abnormal findings. Electroencephalography revealed diffuse 13-Hz beta waves in the background activity, and α-blocking was not observed when the eyes were opened. In the drowsy-to-asleep state, delta waves were predominantly observed in the frontal and central lobes. During electroencephalography, myoclonus-like involuntary movements, especially in the right upper extremity, were observed from beginning to end; however, spikes and slow waves synchronized with involuntary movements were not observed. Cervical MRI showed cervical spinal canal stenosis at C4/5, and lumbar MRI revealed lumbar vertebral body deformity and spinal canal stenosis. On sagittal T1-weighted imaging with gadolinium, diffuse leptomeningeal enhancement of the lumbar spinal cord and a faint intramedullary enhancement effect were observed (Fig. 1). Enhanced whole-body computed tomography revealed an arterial wall thrombus in the right common iliac, internal iliac, and femoral arteries. Synovial capsule fluid retention was observed in the left adductor muscle near the femoral neck. No hepatomegaly, splenomegaly, or lymphadenopathy was observed. No abnormal isotope uptake was observed on bone or gallium scintigraphy.
Figure 1.
Lumbar magnetic resonance imaging findings. (a) T2-weighted imaging shows vertebral body deformity and lumbar spinal canal stenosis. (b) On sagittal T1-weighted imaging with gadolinium, diffuse leptomeningeal enhancement (arrow) of the lumbar spinal cord and a faint intramedullary enhancement effect were observed.
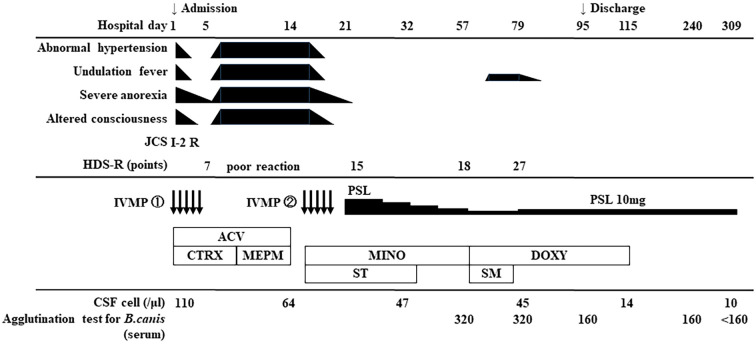
The clinical course of the patient is shown in Fig. 2. Upon admission, with an initial diagnosis of acute disseminated encephalomyelitis, intravenous methylprednisolone (m-PSL) 1,000 mg/day (IVMP) was administered for 5 days. Continuous heparin infusion was administered to treat arterial thrombosis. Microbiological treatment was empirically started with 30-mg/kg/day acyclovir (ACV) and 2-g/day ceftriaxone, followed by meropenem (MEPM) 2.0 g×3/day. Treatment with ACV or MEPM did not improve this condition. CSF polymerase chain reaction for herpes simplex virus DNA, varicella-zoster virus DNA, and Mycobacterium tuberculosis was negative. Blood and CSF cultures were negative.
Figure 2.
Clinical course of the present case. JCS: Japan Coma Scale, R: restlessness, HDS-R: Hasegawa Dementia Rating Scale-revised, IVMP: intravenous methylprednisolone, PSL: prednisolone, ACV: acyclovir, CTRX: ceftriaxone, MEPM: meropenem, MINO: minocycline, ST: sulfamethoxazole/trimethoprim, DOXY: doxycycline, SM: streptomycin, CSF: cerebrospinal fluid
Although the disease etiology was difficult to identify, corticosteroid treatment was useful in alleviating symptoms. When IVMP therapy was completed, the fever, severe anorexia, abnormal hypertension, and disturbance of consciousness recurred. On day 16 of hospitalization, a second course of IVMP therapy further improved response speed and food intake. These symptoms, including the fever, temporarily improved with steroid treatment; however, there was no sign of underlying improvement, and the antimicrobials were considered ineffective.
We therefore started administration of 800 mg/160 mg sulfamethoxazole/trimethoprim (ST) and 100-mg/day minocycline as treatment for atypical pathogens on day 16. We administered ST for Toxoplasma gondii (toxoplasmosis) and minocycline for Brucella spp. (brucellosis), Coxiella burnetii (Q fever), and Borrelia garinii (Lyme disease) as causative microorganisms. As a result of searching for a wider range of pathogens, including various parasites, T. gondii, Chlamydia spp., Borrelia spp., and Brucella spp., on day 57 of hospitalization, a serum agglutination test (SAT) for B. canis was positive (1:320), whereas that for B. abortus was negative. Brucella spp. were not isolated from blood or CSF cultures. Based on these data and abnormal neurological findings, the patient was diagnosed with meningoencephalomyelitis caused by B. canis.
Oral doxycycline at 100 mg/day for 96 days and intramuscular injection of streptomycin at 1 g/day for 16 days were administered. Tapering of oral prednisolone (PSL) (1 mg/kg/day corticosteroid) caused the recurrence of symptoms, including myoclonus; therefore, oral PSL (10 mg/day) was required as maintenance therapy. The cognitive function on the Hasegawa Dementia Scale-Revised (HDS-R) and Mini-Mental State Examination improved from 18 and 21 points, respectively, on day 57 to 27 and 28 points, respectively, on day 75. The contrast effect on spinal MRI disappeared. The patient was discharged on day 95. Additional steroids were necessary to relieve his severe medical condition, including his neurologic symptoms, and the PSL dose was tapered, along with continued administration of appropriate antimicrobials to eliminate pathogenic microorganisms. He achieved symptomatic relief and normalized CSF abnormalities and showed negative SAT results (<160). The treatment for the elimination of pathogenic antigens was completed in four months, and no neurological sequelae were observed during a long-term follow-up.
Discussion
Brucellosis is a zoonotic infection that is transmitted to humans through contact with fluids from infected animals or derived food products, such as unpasteurized milk and cheese (3,4). The epidemiology of brucellosis in Japan was systematically reported by Imaoka (5-7). In Japan, 47 cases of brucellosis were reported to health centers between April 1, 1999, and March 31, 2021 (5-7). All cases of infection aside from those involving B. canis (10 B. melitensis, 4 B. abortus, and 1 undetermined) were imported cases. Among recent cases, several foreign residents in Japan were found to be infected after a temporary stay in a brucellosis-endemic mother country. Of the 47 total cases reported, 32 involved B. canis infection (5,7), all of which were domestic infections (Japanese). In addition, epidemiological data have reported dogs carrying antibodies against B. canis, where 3-3.5% of dogs that underwent microplate agglutination testing in Japan were seropositive for B. canis (5,8). Therefore, the key point for the diagnosis of B. canis infection in the present case was to confirm the contact history with a dog.
The physical findings of B. canis infection are mostly variable and nonspecific. Acute illness usually consists of fever-related symptoms, such as night sweats, arthralgias, myalgias, lower back pain, and weight loss, as well as whole-body symptoms, such as diarrhea, weakness, fatigue, malaise, headache, dizziness, depression, and anorexia. Hepatomegaly, splenomegaly, and/or lymphadenopathy may also be observed (2-4,9-14). The fever in cases of untreated acute brucellosis can be high or slightly elevated and usually lasts from days to weeks. An irregular undulating fever has also been reported. Brucellosis can cause a fever of unknown origin. Neurologic involvement, including the peripheral nervous system and CNS, occurs in approximately 5-10% of cases (2,3,15-19). CNS involvement in brucellosis rarely occurs (approximately 3-5%) (4,9-11,18,20,21) and has a broad range of clinical manifestations. Severe complications, including acute meningitis or meningoencephalitis, Guillain-Barre syndrome-like presentation (22), the chronic peripheral form (radiculoneuropathy), and the chronic CNS form [meningoencephalitis, myelitis (23), cerebellar involvement, and cranial nerve palsies] have been reported (13,20).
The incubation period is typically one to four weeks but may occasionally extend beyond several months to years. Chronic brucellosis accounts for approximately 10-15% of all brucellosis cases (9,11,16,18). Chronic brucellosis is characterized by localized infection (generally spondylodiscitis, osteomyelitis, tissue abscesses, or uveitis) and/or relapse in patients with objective evidence of infection. Osteoarticular brucellosis is the most common location of brucellosis and frequently presents as sacroiliitis, spondylitis, and peripheral arthritis (24,25). In the present case, we confirmed that the patient was not involved in the delivery of dogs; we therefore assumed that B. canis had been transmitted through close contact with a dog or its urine (26), resulting in latent infection of the spine. The localized lumbar spine MRI lesions improved after treatment; therefore, it is likely that the lumbar spine became the source of infection. The hematogenous spread of pathogens, direct invasion of the cerebrospinal fluid space, and involvement of inflammatory mechanisms are possible.
The diagnosis usually depends on clinical features, isolation of bacteria, SAT, abnormal CSF, and serological findings. Although direct pathogen detection by blood culture is the gold standard, it is not effective for detecting positive results (4,9,10). A serum antibody test with a tube agglutination test is usually conducted to confirm the diagnosis using a blood agglutination titer of ≥1:160 (3). In the present case, positive antibody titers supported persistent B. canis infection status. Since November 2020, antibody tests are no longer available at private clinical laboratories; consultations and requests for an administrative inspection are therefore made to the National Institute of Infectious Diseases through the nearest public health center and local public health institute.
Therapeutic strategies for focal brucellosis should be individualized based on the site of involvement. Drugs with good cellular penetration are required owing to the intracellular location of the pathogen. Combination drug therapy is recommended, including an aminoglycoside to reduce the chances of relapse: doxycycline, 200 mg a day orally in 1 or 2 doses for 6 weeks plus streptomycin 1 g intramuscularly or intravenously once a day for the first 2-3 weeks (2,3,12,27-29). A combination of doxycycline and streptomycin (SM) is recommended, especially for Brucella spondylitis (3,25). Recurrence of brucellosis is also reported to occur in 5-30% of cases (3,18,27), usually at 1-6 months after the initial infection. In the present case, doxycycline (96 days) and streptomycin (16 days) were administered as targeted treatments until clinical manifestations and CSF findings had normalized. The SAT also showed negative findings.
Owing to the intracellular parasitic nature of B.canis, a positive antibody signifies a carrier. The transition detected in the antibody titer from positive to negative against B.canis supported the elimination of the antigenic pathogen; thus, the combination therapy was effective. In the present case, the period of antibiotic use was relatively long, as the patient's initial condition was severe, and the improvement of cerebrospinal fluid findings and the decrease in antibody titer after the start of doxycycline were slow. In addition, we worried about the risk of recurrence. After doxycycline was completed, we confirmed that antibody titer remained below the detection limit for a long period.
In our case, corticosteroids were evidently useful in alleviating symptoms, although they were not a targeted treatment for neurobrucellosis. In addition, corticosteroids may have contributed to the improvement of abnormal findings on spinal MRI by suppressing the pathological conditions of inflammation and edema. The administration of steroid therapy lacks consensus and is not part of routine therapy. Furthermore, no literature supports the routine use of corticosteroids. However, the use of steroids may be appropriate for neurobrucellosis (29) complicated by iritis (18), optic neuritis (19,30,31), papilledema (18,19,32), meningo-myeloradiculitis (32), myelopathy (18,20), polyneuropathy (18), cranial nerve palsies (18), demyelinating steroid-responsive neurobrucellosis (21,33), arachnoditis (20), cranial nerve involvement (20), or vasculitis involvement (34). In the present case, corticosteroids were useful in alleviating the patient's symptoms; however, the administration of appropriate antibiotics was essential for the treatment of neurobrucellosis.
The pathogenesis and immunobiology of brucellosis, including cytokines and proinflammatory reactions, have been studied (35,36). In the present case, we speculate that corticosteroids exert their effects by suppressing long-lasting inflammation of the autoimmune mechanism. Steroids were tapered after confirmation of normalization of cerebrospinal fluid findings, elimination of antigens, and absence of recurrence. Steroid tapering re-exacerbated the symptoms twice during the course, so steroid therapy for neurobrucellosis was continued even after completion of appropriate antibiotic therapy. There is no evidence regarding the timing of steroid discontinuation. Therefore, evidence regarding the role of corticosteroids in brucellosis treatment should be evaluated.
It is worth noting that this is the first detailed case report of CNS involvement; however, there are some limitations. The duration of antibiotic treatment (96 days) appears to have been too long for an infectious disease, possibly indicating excessive antibiotic treatment. According to the Japanese package insert, the daily dose of doxycycline (100 mg/day) was lower than the recommended dose (100 mg twice daily). In this case, if the recommended dose (100 mg twice daily) had been used, the treatment period might have been shortened.
In conclusion, we encountered an extremely rare case of Japanese neurobrucellosis caused by B. canis that presented as meningoencephalomyelitis. Neurobrucellosis caused by B. canis should be considered in the evaluation of patients with a fever of unknown origin and unexplained neurological symptoms. It is important to note cases with an undulant fever and ask questions regarding the patient's occupation and contact with dogs.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis 6: 91-99, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Dean AS, Crump L, Greter H, et al. Clinical manifestations of human brucellosis: a systematic review and meta-analysis. PLOS Negl Trop Dis 6: e1929, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanefi C. Gul, Hakan E. Brucellosis. Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases. 9th Edition. Bennett JE, R Dolin, MJ Blaser, Eds. ELSEVIER, 2019: 2753-2758. [Google Scholar]
- 4.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med 352: 2325-2336, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Imaoka K. Review: pulmonary brucellosis (in Japanese). In: Respiration Syndrome. 3rd Ed. IV. Nippon Rinshosha, Tokyo, 2021: 290-294. [Google Scholar]
- 6.Kawakami N, Wakai Y, Saito K, Imaoka K. Chronic brucellosis in Japan. Intern Med 58: 3179-3183, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institute of Infectious Diseases. Brucellosis [Internet]. [cited 2023 Jul 17]. Available from: https://www.niid.go.jp/niid/ja/kansennohanashi/513-brucella.html(in Japanese).
- 8.Nabeshima K, Sato S, Kabeya H, et al. Seroepidemiological survey of Brucella canis infection in dogs in Japan. Jpn J Vet Res 68: 129-132, 2020. [Google Scholar]
- 9.Buzgan T, Karahocagil MK, Irmak H, et al. Clinical manifestations and complications in 1028 cases of brucellosis: a retrospective evaluation and review of the literature. Int J Infect Dis 14: e469-e478, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Zheng R, Xie S, Lu X, et al. A systematic review and meta-analysis of epidemiology and clinical manifestations of human brucellosis in China. BioMed Res Int 2018: 5712920, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kursun E, Turunc T, Demiroglu Y, Arslan H. Evaluation of four hundred and forty seven brucellosis cases. Intern Med 52: 745-750, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis 7: 775-786, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Gul HC, Erdem H, Bek S. Overview of neurobrucellosis: a pooled analysis of 187 cases. Int J Infect Dis 13: e339-e343, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Guven T, Ugurlu K, Ergonul O, et al. Neurobrucellosis: clinical and diagnostic features. Clin Infect Dis 56: 1407-1412, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Rossi M, Tascini C, Carannante N, et al. Neurobrucellosis: diagnostic and clinical management of an atypical case. New Microbiol 41: 165-167, 2018. [PubMed] [Google Scholar]
- 16.Greenblatt D, Krupp LB, Belman AL. Parainfectious meningo-encephalo-radiculo-myelitis (cat scratch disease, Lyme borreliosis, brucellosis, botulism, legionellosis, pertussis, mycoplasma). Handb Clin Neurol 112: 1195-1207, 2013. [DOI] [PubMed] [Google Scholar]
- 17.Akdeniz H, Irmak H, Anlar O, Demiröz AP. Central nervous system brucellosis: presentation, diagnosis and treatment: presentation. J Infect 36: 297-301, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Lulu AR, Araj GF, Khateeb MI, et al. Human brucellosis in Kuwait: a prospective study of 400 cases. Q J Med 66: 39-54, 1988. [PubMed] [Google Scholar]
- 19.McLean DR, Russell N, Khan MY. Neurobrucellosis: clinical and therapeutic features. Clin Infect Dis 15: 582-590, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Shakir RA, Al-Din AS, Araj GF, et al. Clinical categories of neurobrucellosis. A report on 19 cases. Brain 110: 213-223, 1987. [DOI] [PubMed] [Google Scholar]
- 21.Bektaş O, Ozdemir H, Yılmaz A, et al. An unusual case of neurobrucellosis presenting as demyelination disorder. Turk J Pediatr 55: 210-213, 2013. [PubMed] [Google Scholar]
- 22.Alanazi A, Najjar SA, Madkhali J, et al. Acute brucellosis with a Guillain-Barre syndrome-like presentation: a case report and literature review. Infect Dis Rep 13: 1-10, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turgut M, Turgut AT, Koşar U. Spinal brucellosis: Turkish experience based on 452 cases published during the last century. Acta Neurochir (Wien) 148: 1033-1044; discussion 1044, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Turan H, Serefhanoglu K, Karadeli E, et al. Osteoarticular involvement among 202 brucellosis cases identified in Central Anatolia region of Turkey. Intern Med 50: 421-428, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Alp E, Doganay M. Current therapeutic strategy in spinal brucellosis. Int J Infect Dis 12: 573-577, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Mizutani H, Kubota N, Soumura Y, et al. Prevalence of anti-Brucella canis antibodies among dogs in Tokyo, Abstract in English. J Japan Vet Med Assoc 67: 204-207, 2014. (in Japanese). [Google Scholar]
- 27.Solera J. Update on brucellosis: therapeutic challenges. Int J Antimicrob Agents 36: S18-S20, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Skalsky K, Yahav D, Bishara J, et al. Treatment of human brucellosis: systematic review and meta-analysis of randomised controlled trials. BMJ 336: 701-704, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosilkovski M, Keramat F, Arapović J. The current therapeutical strategies in human brucellosis. Infection 49: 823-832, 2021. [DOI] [PubMed] [Google Scholar]
- 30.Elrazak MA. Brucella optic neuritis. Arch Intern Med 151: 776-778, 1991. [PubMed] [Google Scholar]
- 31.Habeeb YK, Al-Najdi AK, Sadek SA, Al-Onaizi E. Paediatric neurobrucellosis: case report and literature review. J Infect 37: 59-62, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Oueslati I, Berriche A, Ammari L, et al. Epidemiological and clinical characteristics of neurobrucellosis case patients in Tunisia. Med Mal Infect 46: 123-130, 2016. [DOI] [PubMed] [Google Scholar]
- 33.Ata F, Yousaf Z, Sharif MK, Abdallah A. Demyelinating steroid-responsive neurobrucellosis. BMJ Case Rep 13: e233798, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim EJ, Lee SJ, Ahn EY, et al. Relapsed brucellosis presenting as neurobrucellosis with cerebral vasculitis in a patient previously diagnosed with brucellar spondylitis: a case report. Infect Chemother 47: 268-271, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Figueiredo P, Ficht TA, Rice-Ficht A, et al. Pathogenesis and immunobiology of brucellosis: review of Brucella-host interactions. Am J Pathol 185: 1505-1517, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldi PC, Giambartolomei GH. Immunopathology of Brucella infection. Recent Pat Antiinfect Drug Discov 8: 18-26, 2013. [DOI] [PubMed] [Google Scholar]