Abstract
Objectives:
To identify studies promoting the use of artificial intelligence (AI) or automation with HIV pre-exposure prophylaxis (PrEP) care and explore ways for AI to be used in PrEP interventions.
Design:
Systematic review
Methods:
We searched in the US Centers for Disease Control and Prevention Research Synthesis database through November 2023 PROSPERO (CRD42023458870). We included studies published in English that reported using AI or automation in PrEP interventions. Two reviewers independently reviewed the full text and extracted data by using standard forms. Risk of bias was assessed using either the revised Cochrane risk-of-bias tool for randomized trials for randomized controlled trials or an adapted Newcastle-Ottawa Quality Assessment Scale for non-randomized studies.
Results:
Our search identified 12 intervention studies (i.e., interventions that used AI/automation to improve PrEP care). Currently available intervention studies showed AI/automation interventions were acceptable and feasible in PrEP care while improving PrEP-related outcomes (i.e., knowledge, uptake, adherence, discussion with care providers). These interventions have used AI/automation to reduce workload (e.g., directly observed therapy) and helped non-HIV specialists prescribe PrEP with AI-generated clinical decision-support. Automated tools can also be developed with limited budget and staff experience.
Conclusions:
AI and automation have high potential to improve PrEP care. Despite limitations of included studies (e.g., the small sample sizes and lack of rigorous study design), our review suggests that by using aspects of AI and automation appropriately and wisely, these technologies may accelerate PrEP use and reduce HIV infection.
Keywords: HIV, pre-exposure prophylaxis, artificial intelligence, chatbot, automation
INTRODUCTION
Despite improvements in treatment (antiretroviral therapy1) and prevention (pre-exposure prophylaxis or PrEP2), Human Immunodeficiency Virus (HIV) is still one of the principal challenges to public health. In 2021, 36,163 people in the United States (US) were newly diagnosed with HIV,3 which may be partly explained by the persistent disparities in PrEP prescription among factUS populations who can benefit from this important HIV prevention strategy.4 To meet the national goal, more innovative interventions to improve PrEP care are needed to increase PrEP coverage to 50% by 2025.5
In November 2022, OpenAI’s Chat Generative Pre-trained Transformer (ChatGPT) was released.6 Although chatbots can be as rudimentary as menu or button decision trees, ChatGPT is a complex, generative, artificial intelligence (AI) chatbot.7 AI is defined as a multidisciplinary field that focuses on developing intelligent systems (e.g., machine learning [ML], deep learning) capable of performing tasks that typically require human intelligence. Because of its real-world applications and ability to mimic a human conversation, ChatGPT has quickly become one of the most used applications in daily life, and AI and digital technology have rapidly been adopted in medical care. During the COVID-19 pandemic, scientists used AI to obtain and provide information, estimate epidemic trends, deliver care, and facilitate communication between healthcare providers and patients in virtual spaces to minimize COVID-19 exposure.8,9
The integration of AI and digital technology in healthcare has expanded to the field of HIV prevention. The United Nations Educational, Scientific and Cultural Organization created an AI chatbot named Eli to answer questions on HIV prevention in 2020,10 followed by the US government launching a new AI chatbot tool at bot.HIV.gov in 2021.11 Despite these programs, adoption of AI/automation in HIV prevention efforts has been slower than in other public health areas.12 Use of AI/automation can be unlimited, but our knowledge and use as it is related to PrEP care has been limited.12 Moreover, the White House issued a landmark Executive Order in October 2023, which raised awareness that AI can increase the risk of injuring, misleading, or otherwise harming Americans.13 For healthcare in particular, the Executive Order directs advancing the responsible use of AI to protect consumers.13 To use it appropriately and ethically, AI in PrEP care must be better understood before taking advantage of its rapid development and expansion.
This review focuses on PrEP and AI/automation. To our knowledge, this is the first systematic review to explore PrEP interventions that use AI/automation. We describe published studies that reported interventions that use AI/automation to promote PrEP care. Our review explores 1) characteristics of studies aimed to promote HIV PrEP care with the use of AI/automation, 2) how AI/automation were used in these PrEP studies, and 3) how AI/automated tools can be incorporated and implemented in PrEP interventions to improve PrEP uptake and/or persistence.
METHODS
The study protocol for the parent review was registered in PROSPERO (CRD42023458870).14 Our report followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRSIMA) Statement.15
Search
A search was conducted in the CDC’s Prevention Research Synthesis (PRS) Project database. The PRS database is a collection of HIV prevention literature focused on behavioral risk reduction, medication adherence, linkage/retention/re-engagement in HIV care, structural interventions, PrEP, and systematic reviews.16 By the end of August 2023, the PRS database had amassed ~123,000 citations. The automated search is implemented annually using the following databases: MEDLINE (OVID), EMBASE (OVID), CINAHL (EBSCOhost), Global Health (OVID), PsycINFO (OVID), and Sociological Abstracts (ProQuest) (Appendix). The search was developed in the MEDLINE (OVID) database using indexing and keyword terms cross-referenced by using Boolean logic with no language limits. The finalized search was tailored to other databases to adhere to each proprietary indexing system.17 Supplementary searches included a manual search of journals (available from the PRS website), online non-indexed databases (e.g., Scopus), gray literature (e.g., Google Scholar), and reference lists from relevant HIV literature.
For this review, the librarian searched the PRS database on August 29, 2023, for literature published 2012 to the present. There were two components used in the search. The first component looked at AI and included phrases centered on “machine learning” and “natural language.” The second component focused on PrEP. The search query looked specifically at the title, abstract, and indexing terms for any concepts related to PrEP and AI. Additionally, we searched RePORTER (https://reporter.nih.gov/) for pipeline and potential publications and ClinicalTrials.gov (https://clinicaltrials.gov/) and International Standard Randomized Controlled Trial Number registry (https://www.isrctn.com/) for studies that were registered but had not been completed or published. We also searched for any newly published literature in PubMed, Scopus, and Google Scholar by using the same search terms (searched November 2, 2023). See Appendix for full PrEP annual searches, PRS database queries, and supplementary searches conducted for this review.
Inclusion/Exclusion Criteria
Studies published in English that reported using AI/automation in PrEP interventions were included. We excluded studies that used AI/automation to improve HIV prevention in general and did not focus on PrEP. This review especially emphasized the use of AI/automation in behavioral interventions to enhance PrEP care.
Screening and Data Abstraction
A two-step approach was applied to select studies for review. First, two reviewers independently screened the citations by title and abstract. Second, two reviewers independently reviewed the full text of included citations to confirm study eligibility. Disagreements were resolved through discussion. Reviewers were trained, and all screening forms were pilot tested and revised, as necessary. Identified citations were exported to DistillerSR (a systematic review software developed by DistillerSR Inc, Ottawa, Canada) for data management and to screen studies and identify eligible studies.
For all eligible studies, two reviewers used a standard data abstraction form to extract data on population characteristics and AI/automation program-related information and outcomes. Study population characteristics were abstracted from the primary study’s participant eligibility criteria.
Risk of Bias Assessment/ Data Synthesis
For studies with outcomes to evaluate interventions, risk of bias assessment was conducted by using either the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) for randomized controlled trials (RCT)18,19 or an adapted Newcastle-Ottawa Quality Assessment Scale (NOS) for non-randomized studies.20-22 RoB 2 consisted of 28 questions with “yes,” “probably yes,” “probably no,” “no,” or “no information,” and NOS consisted of 5 (for cohort study) to 6 (for cross-sectional study) questions with “yes” or “no” responses. For RoB 2, reviewers assessed risk of bias (“low risk of bias,” “some concerns,” “high risk of bias”) based on the answers to the questions. For NOS, a total score of 5 or 6 was possible, with 3 or higher being considered as “low risk of bias” for cohort study and 4 or higher for cross-sectional study. We narratively synthesized characteristics and findings of the included studies.
RESULTS
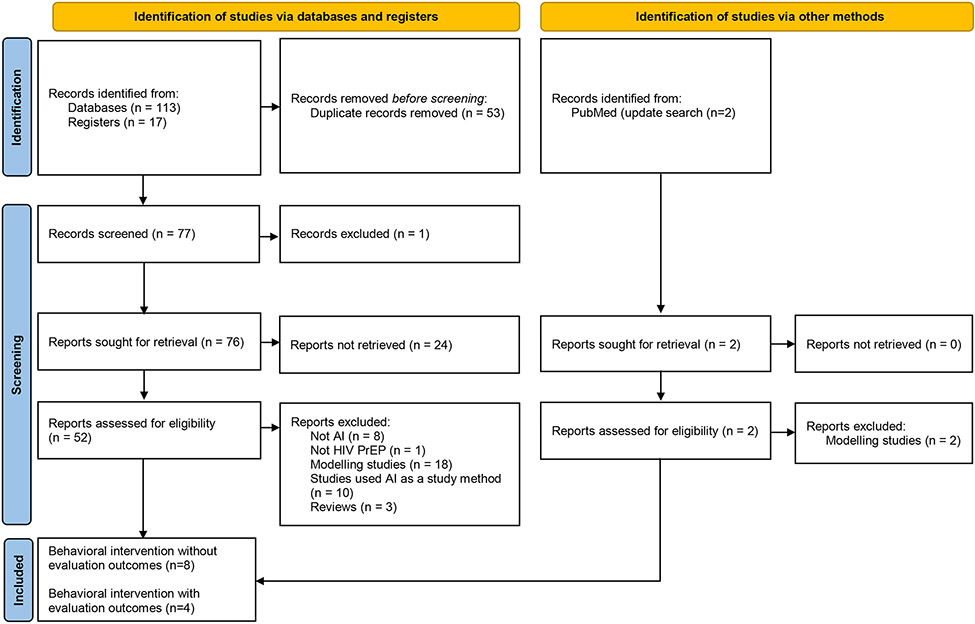
After removing duplications, 59 published citations were screened for titles and abstracts, and 10 additional citations were excluded from this review because they did not meet the inclusion criteria (Figure 1). Of these 49 studies screened for full reports, 9 additional studies were excluded because they were neither studies with AI/automation (n=8) nor HIV PrEP-relevant studies (n=1). Of the remaining 40 studies, 18 were modeling studies, 10 used AI as a study method, 9 were behavioral interventions, and 3 were reviews.
Figure 1:
Flow diagram of included studies
Additionally, 17 potential trials found in study registries were screened for titles and available information in the registries, and we identified 3 eligible interventions that were in progress but have not published results as of November 2023. Moreover, 2 newly published studies were identified with the updated search. Both were modeling studies and excluded from our review. Thus, 12 behavioral interventions (i.e., 9 published, 3 registries) were analyzed further and described in detail below.
Behavioral Interventions Overall (N=12)
Among 12 behavioral interventional, 4 types of AI/automation were identified: chatbot (AI-based or non-AI-based) (n=8),23-30 ML (n=1),31 natural language processing (NLP; building machines that can understand human language and generate text and speech) (n=1),32 and other types of AI (n=2).33,34
Behavioral Interventions Without Evaluation Outcomes (n=8)
Eight of the 12 intervention studies did not report evaluation outcomes; they were either ongoing studies (i.e., registered in trial registry but have not yet published; n=3),28-30 qualitative studies to assess perception/preference (n=2),27,31 study protocols (n=2),23,24 or an intervention description (n=1).32 Four were US-based studies,24,27,28,31 three were non-US-based,23,29,30 and one study’s location was unspecified.32 Six used chatbot,23,24,27-30 one used NLP,32 and one used ML31 (Table 1).
Table 1:
Study characteristics of behavioral interventions reporting no evaluation outcomes (N=8)
| Study Name | First Author (publication year) or Primary Investigator or ClnicalTrials.gov ID |
Study Type | Study Objectives | Intervention Characteristics and Study Design, Sample and Place |
Relevant Outcomes |
|---|---|---|---|---|---|
| Chatbot (n=6) | |||||
| HIVST-chatbot | Chen (2023) | Study protocol | Evaluate efficacy by comparing it to HIVST with online instruction and counseling, which is CDC’s PRS EBI | HIVST service with web-based real-time instruction and counseling, including PrEP use provided by a fully automated AI-assisted chatbot RCT 528 Chinese-speaking MSM Hong Kong |
Recruitment and enrollment of participants started in April 2023. |
| PrEPBot | Braddock (2023) | Study protocol | Describe the iterative and community-engaged process that was used to develop PrEPBot | SMS text messaging-based AI-assisted chatbot tailored to SGM AYAs that would support navigator functions and disseminate PrEP-related information Unspecified study design and sample Louisiana |
Development of SMS text messaging or rule-based chatbot with the assistance of commercially available tools is feasible. |
| Telemedicine Experience for PrEP Care | Zhang (2022) | Qualitative | Assess the telemedicine experience in PrEP care | Cross-sectional 18 PCPs and 29 PrEP-eligible women New York |
Nearly quarter of PrEP-eligible women preferred chatbot while no data for PCPs’ opinions on chatbot were available. |
| MyTestBot | Ni, Z. | Ongoing, unpublished | Evaluate the efficacy and implementation outcome relative to treatment as usual | AI-assisted chatbot-based mobile health intervention to promote HIV testing RCT 296 MSM (estimated) Malaysia |
Not yet recruiting. Primary completion (estimated) date: July 15, 2025 |
| TestBot | Wickersham, J. A. | Ongoing, unpublished | Assess the feasibility by comparing it to treatment as usual | AI-assisted chatbot to promote HIV testing RCT 80 MSM (estimated) Malaysia |
Not yet recruiting. Primary completion (estimated) date: December 31, 2024 |
| Chatbot in Southern US | NCT05968755 | Ongoing, unpublished | Assess the acceptability and feasibility relative to treatment as usual | Chatbot to promote PrEP awareness and uptake RCT 145 black MSM (estimated) Southern US |
Not yet recruiting. Primary completion (estimated) date: June 1, 2025 |
| Natural Language Processing (n=1) | |||||
| Ontology-based Conversational Agent for PrEP | Amith (2020) | Intervention description | Describe the development of the conversational agent and assess its functionality on PrEP and PEP | Automated conversation with ontology-based medication conversational agent for PrEP and PrEP through a computer-based agent Unspecified study design/sample/place |
High functionality of automated conversations, but further studies needed for foresee the possibility. |
| Machine Learning (n=1) | |||||
| PCP’s Perspective on using ML algorithm | Van den Berg (2021) | Qualitative | Assess PCPs’ perspectives on using automated HIV risk predictors generated by ML in electronical health record | Automated HIV risk prediction models generated with ML algorithm driven by electronic health record data to identify PrEP candidates Cross-sectional 42 PCPs Massachusetts |
PCPs’ perspective on using ML algorithm |
AI: artificial intelligence; CDC’s PRS EBI: Centers for Disease Control and Prevention's Prevention Research Synthesis Evidence-Based Intervention, HIVST: Home-based HIV self-testing; ML: machine learning; MSM: men who have sex with men; PCP: primacy care provider; PEP: post-exposure prophylaxis; PrEP: pre-exposure prophylaxis; RCT: randomized controlled trial; SGM AYA: sexual and gender minority adolescents and young adults; SMS: short message service.
HIV Self-Testing (HIVST)-chatbot was a fully automated HIVST service with HIV risk assessment provided by an AI chatbot for gay, bisexual, and other men who have sex with men (collectively referred to as MSM) in Hong Kong.23 Their previous project, HIVST with online instruction and counseling (HIVST-OIC),35 increased HIV testing and has been included in CDC’s PRS Evidence-Based Intervention (EBI) compendium.36 However, HIVST-OIC had an implementation issue: it required intensive resources and capacity development (e.g., online real-time HIV testing instruction and counseling by a nurse, a 15-minute motivational interviewing phone call, and immediate online psychological support).23,35 Thus, the proposed study would explore whether a fully automated HIVST-chatbot is as efficacious, if not more efficacious, as HIVST-OIC in increasing HIVST uptake and the proportion of HIVST users receiving counseling, including HIV risk assessment, and increasing PrEP use. The study was anticipated to start recruitment in April 2023 and enroll 528 Chinese-speaking MSM in Hong Kong (264 in HIVST-chatbot group, 264 in HIVST-OIC group).23
Developing an AI chatbot intervention can be labor intensive and expensive, but PrEPBot showed that AI chatbot development does not need to be labor intensive nor expensive.24 PrEPBot was a low-cost, short message service (SMS) text messaging-based chatbot. The chatbot was tailored to sexual and gender minority adolescents and young adults in Louisiana to support patient navigation and disseminate PrEP-related information as part of their TelePrEP program.24 The intervention developer chose a commercial, readily available, easily deployable chatbot platform instead of an independently designed app. Additionally, PrEPBot used SMS text messaging with rule-based conversations that were scripted but had limited capacity for recognizing free text input. This study suggested the possibility of implementing an AI chatbot intervention with a limited budget by using a commercially available tool that can be programmed by researchers with no programming experience. Acceptability and usability of PrEPBot will be evaluated in their pilot trial.24
Amith (2020) assessed functionality of a computer-based ontology-driven approach (an automated method to group responses and tailor follow-up questions) to manage conversations on PrEP and post-exposure prophylaxis (PEP).32 The dialogue could provide automated counseling and improve patient-provider communication by using NLP to develop the ontology-based method for handling dialogue and automating the communication of PrEP. The study showed that high functionality of automated conversations may provide real-time counseling and high availability to inform patients, but more studies are needed to explore further possibilities.
While the above 3 studies were about developing AI tools, Zhang (2022) and van den Berg (2021) assessed perceptions or preferences of using AI chatbots or ML among PrEP-eligible women27 and primary care providers (PCPs).31 Almost a quarter of PrEP-eligible women participants (7 out of 29 women) in New York expressed enthusiasm about chatbots in PrEP care; however, about one-fifth of the women were strongly opposed to using chatbots.27 A sample of 42 PCPs in Massachusetts reported high acceptance of using automated HIV risk prediction models to identify PrEP candidates. The models used ML algorithms to detect patterns in electronic health records (EHR) data indicative of risk for HIV acquisition.31 Moreover, PCPs stated that automated HIV risk prediction models might help patient-provider communication in general primary care settings by making HIV risk assessment more routine, reducing stigma, and empowering PCPs to prescribe PrEP instead of referring to HIV specialists.31 However, PCPs reported skepticism about using ML without knowing more about how the models worked, and some PCPs worried that patients might react negatively if they discovered that their HIV risk was predicted by using automated computer algorithms.31
The three ongoing chatbot studies are focused on MSM. Two studies (MyTestBot and TestBot) would be implemented in Malaysia,29,30 and the other (Chatbot in Southern US) would focus on Black or African American (hereafter referred to as Black) MSM in the Southern US.28 Both MyTestBot and TestBot promote HIV testing, and Chatbot in Southern US promotes PrEP awareness and uptake. While the two studies in Malaysia would either develop an AI chatbot or assess feasibility of the chatbot, Chatbot in Southern US would develop and pilot test to assess acceptability and feasibility of the chatbot (possibly not AI-driven).
Behavioral Interventions With AI/Automation Evaluation Outcomes (n=4)
Four intervention studies reported outcomes data that were used to evaluate the AI/automation interventions. Types of AI/automation used in these interventions were either chatbot (n=2)25,26 or other AI (n=2).33,34 Two studies evaluated the same intervention, Directory-observed therapy (DOT) Diary, thus this review actually looked at three, and not four, unique AI/automation interventions (Table 2). DOT Diary was US-based33,34 and the other two interventions were non-US-based (Brazil25 and Zambia26). Only one of the DOT Diary33 studies was RCT. A majority of the studies (n=3)25,33,34 had low risk of bias.
Table 2:
Study and intervention characteristics of behavioral interventions reporting evaluation outcomes (N=4)
| Study Name | First Author (publication year) |
Study Objectives | Study Design and Samples |
Intervention Characteristics | Relevant Outcomes, Limitations | Risk of Bias* |
|---|---|---|---|---|---|---|
| Chatbot (n=2) | ||||||
| Amanda Selfie | Massa (2023) | Develop an AI-assisted chatbot and evaluate acceptability, functionality, and usability and its results on demand creation for PrEP among AMSM and ATGW | Cross-sectional 122 AMSM and ATGW aged 15-19 (trial) 1,288 AMSM and ATGW aged 15-19 (final) Brazil |
AI-assisted chatbot conceived as a Black transgender woman and to function as a virtual peer educator. Chose Facebook Messenger platform, which allowed the use of more elaborate conversation flows and offered free mobile data packages in Brazil. |
Well accepted as a peer educator, clearly and objectively communicating on topics such as gender identity, sexual experiences, HIV and PrEP. Mainly accessed via users’ smartphones. Limitation includes inaccurate answers, did not give sufficient time for users to read through information, and using technical terms when describing STIs. |
Low |
| Waiting-Area Chatbot | Yam (2022) | Develop and test a waiting-area non-AI-assisted chatbot for dual HIV and pregnancy prevention | Cross-sectional 30 Women aged 15-49 Zambia |
Non-AI-assisted chatbot on a touch-screen tablet in waiting areas in FP clinics to provide information on dual protection against both HIV and pregnancy. Chose Microsoft Azure Bot Service application, which was not driven by AI but relatively simple, geographically available, low cost, and not much time and human resources needed. |
High feasibility, acceptability, and effective on knowledge and provider interaction were reported. Provided users with tablets in waiting areas. Unclear if clients would engage with the chat in future implementation if tablets were not provided and clients had to use their own mobile devices. |
High |
| Other AI (n=2) | ||||||
| DOT Diary | Liu (2021) | Development and refinement of an automated DOT (aDOT) platform for monitoring and supporting PrEP use and evaluating acceptability and ease of use of the app among YMSM | Cross-sectional followed by cohort 54 YMSM (focus group) 20 YMSM (8-weeks optimization pilot) San Francisco, Atlanta |
Captured data through the device's front-facing camera, processes, and analyzes those data using computer vision and deep learning algorithms. Based on the focus groups, aDOT was combined with electronic sexual diary to provide feedback on level of protection during sex Received daily dosing reminder alarms prompting them to go into the application and visually confirm taking their medication. |
The app was highly accepted, and the use was high, with median PrEP adherence of 91% based on a DOT-confirmed dosing. Some reported using aDOT application was time consuming. |
Low |
| Buchbinder (2023) | Test the accuracy of aDOT to measure PrEP adherence and ability of DOT Diary (a smart phone app that combines aDOT with a PrEP adherence visualization tool kit) to increase adherence among a diverse group of YMSM | RCT (CO: standard of care) 100 YMSM (IV: 34, CO: 66) San Francisco, Atlanta |
Used computer vision and neural networks to confirm that the correct participant is presenting the correct medication and ingesting the correct medication. Used ML to optimize the computer vision and algorithms. |
No significant difference in PrEP adherence between study arms. High level of concordance of DOT diary adherence measures and dried blood spots to test for TFV-DP and FTC-TP. Significant and monotonic decline in the number of PrEP doses taken using the app by study month while the decline was not seen in tenofovir-diphosphate (TFV-DP) levels in dried blood spots (DBS) over time, suggesting underuse of the app over time |
Low | |
AI: artificial intelligence; AMSM: adolescent men who have sex with men; ATGW: adolescent transgender women; CO: control group; DOT: directly-observed therapy; FP: family planning; FTC-TP: emtricitabine triphosphate; HIVST: HIV self-testing; IV: intervention group; ML: machine learning; MSM: men who have sex with men; PrEP: pre-exposure prophylaxis; RCT: randomized controlled trial; STI: sexually transmitted infection; TFV-DP: tenofovir diphosphate; YMSM: young men who have sex with men.
Risk of bias was assessed by using either a revised Cochrane risk-of-bias tool for randomized trials (RoB 2) or for randomized controlled trials (RCT) or an adapted Newcastle-Ottawa Quality Assessment Scale (NOS) for non-randomized studies.
Both Amanda Selfie25 and Waiting-area Chatbot26 were developed and tested for acceptability and usefulness. Amanda Selfie was a culturally tailored, AI chatbot tested among 1,288 adolescent MSM (AMSM) and adolescent transgender women (ATGW) aged 15-19 in Brazil.25 The target population was chosen because of concerns about their behavioral changes (e.g., increased unprotected anal intercourse, reduced HIV risk perceptions) and high usage and values toward technologies including AI and chatbot. Participants were recruited from the demonstration cohort study which explored acceptability, use, and PrEP persistence.37 Eligible participants interacted with Amanda Selfie, transgender chatbot using AI, who provided sex education and enabled them to link up to the PrEP clinics or other HIV testing and care services as needed. All interactions were monitored and assessed to analyze the consistency and accuracy of Amanda Selfie’s. The qualitative evaluation demonstrated that Amanda Selfie was highly usable, functionable, and acceptable, especially among ATGW. While some participants reported feeling comfortable talking to Amanda Selfie about their sexuality and emphasized that they felt safer and less exposed to judgment talking to a chatbot than to humans, others reported the importance of migrating to a dialog with a “real” person on topics that required greater depth (e.g., family problems, doubts about PrEP medications). Limitations reported by participants include inaccurate answers, moving too quickly or taking too long to read through all information, and using medical terms that were hard to understand.
The other chatbot intervention, Waiting-area Chatbot, was developed to provide information on dual protections against HIV and pregnancy while patients were in waiting areas in family planning (FP) clinics in Zambia.26 This chatbot was web-based and the chat content was scripted as a series of closed-ended questions, so AI was not required. The non-AI chatbot was chosen because of its relative simplicity, geographic availability, cost, and available human resources. Because FP providers may not be able to spend enough time with each patient to address their concerns or patients may feel too uncomfortable to discuss HIV vulnerability, the Waiting-area Chatbot enabled women to engage in digital conversations about such topics as PrEP while waiting for their appointments.26 High feasibility, acceptability, and positive effect on knowledge and provider interactions were reported among the sample of 30 women. Limitations of the study include providing a tablet with chatbot and mobile data for participants to use. Further studies are needed to determine if participants would use their own mobile devices and their own mobile data plan to proactively engage with the chat.
DOT Diary used AI to improve adherence by correctly capturing PrEP ingestion.33,34 The DOT Diary app contained an automated DOT (aDOT) monitor and supported PrEP adherence by capturing data through the front-facing camera of the mobile device and analyzing the visual data using ML. High feasibility, acceptability, and usability of the app, as well as high PrEP adherence, were reported during the 8-week pilot among young, Black and Hispanic/Latino MSM in San Francisco and Atlanta.34 However, a separate study of an RCT among 100 young MSM in these cities showed no significant difference in PrEP adherence between groups (i.e., the intervention [Dot Diary] and control [standard of care]), even though the accuracy of aDOT to measure PrEP ingestion was established.33 In the pilot study, some participants reported that using the aDOT app was time consuming.34 In the RCT, the number of PrEP pills recorded in the app showed a monotonic decline over time, but such a decline was not observed in tenofovir-diphosphate levels in dried blood spots of the participants. This discrepancy may suggest that the use of aDOT while taking PrEP decreases over time.
DISCUSSION
This review identified 12 intervention studies that used AI/automation to promote PrEP care. The evaluated interventions showed AI/automation was feasible and acceptable in PrEP care while improving PrEP-related outcomes (i.e., uptake,25 knowledge and discussions with providers,26 and adherence34). This review also found how AI/automation has been incorporated in PrEP care, advantages and disadvantages of the implementation, and what is to come.
AI has been implemented to reduce time-consuming and labor-intensive tasks, such as DOT,33,34 and to provide real-time, 24/7 instructions and counseling.23 DOT has been used for decades in public health to ensure adherence to tuberculosis infection treatment38 and has successfully improved ART adherence.39,40 HIVST-chatbot, an AI-driven version of HIVST-OIC, which is CDC’s PRS EBI, could meet the increasing demand for integrating real-time HIV testing instruction and counseling with home-based HIVST. AI may help provide high-quality information, respond to requests rapidly, and provide round-the-clock support. However, if the use of any AI tool proves time consuming, the usage may decline over time.33,34 Moreover, rapid response with a large quantity of information can be a disadvantage. Responding too rapidly or with too much information may make users realize that they are talking to a robot, not a human, and they may feel disconnected.24,25 Additionally, although AI technology may accurately monitor behaviors (e.g., PrEP ingestion), product using AI may not necessarily improve or encourage behavior change (e.g., PrEP adherence).33 More research to develop AI tools that more closely mimic the natural flow of human conversations and actions, as well as to evaluate AI’s ability to improve PrEP care outcomes and the sustainability of the tools, may be needed in future studies.
Second, AI/automation may help integrate PrEP care into primary care settings. CDC’s PRS has identified interventions that integrate PrEP care into primary care41 and women’s clinics42 as PrEP Evidence-Informed interventions.43,44 “Normalizing” PrEP care, a practice model in which healthcare providers who have access to PrEP candidates prescribe PrEP routinely as a standard of care, may be desirable.45 AI-generated, clinical decision-support tools, such as automated HIV risk prediction,31 could lead to more routine patient-provider HIV conversations, and risk assessments initiated by a chatbot26 may be important, particularly for providers with little experience with PrEP, to assist PCPs with confidently identifying PrEP candidates, which could empower PCPs to prescribe PrEP in general primary care settings instead of referring PrEP patients to HIV specialists. However, additional education or technical assistance for PCPs may still be necessary.
Finally, although developing a chatbot seems labor intensive and to require an experienced programmer or IT technicians, a chatbot can be developed with a limited budget and by staff with minimal coding experience. Waiting-area Chatbot, a non-AI chatbot, was found to have effects on knowledge about PrEP and provider interaction despite its relative simplicity.26 Moreover, PrEPBot, a commercially available, easily deployable chatbot and SMS text messaging platform, was promising because of its low cost, wide accessibility, and possibility of being programmed with minimal coding experience. PrEPBot may increase accessibility and feasibility of PrEP services compared to an independently designed app because it is not labor intensive nor expensive.24 Future study may compare the differences between AI and non-AI chatbot interventions and the benefits (e.g., cost, effectiveness, accessibility, maintenance) of using each tool.
Because AI/automation is a new area in PrEP research, limitations of this review include publication bias, small sample sizes within each study, and a limited number of studies with a rigorous design, such as RCT. Another limitation was some PrEP-related studies that did not have PrEP terms, or AI-related studies that did not have AI terms, in title, abstract and indexing terms may be missed. On the other hand, this review may be overinclusive because unpublished studies found with PrEP keywords in study registries were considered.
In conclusion, AI/automation have high potential to improve HIV PrEP care. They cannot replace medical providers but could be used to efficiently and accurately support providers’ work. Furthermore, generative AI, like ChatGPT, is still a very new area, and we do not know how it can impact PrEP care. If we take advantage of technology development and use it appropriately and wisely, we may be able to accelerate PrEP use to prevent new HIV infections. Further research is needed to study which aspects of PrEP support (e.g., education, uptake, adherence) would benefit most from the integration of AI/automation technology and the adaptability and sustainability of the new strategy.
Supplementary Material
FUNDING
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention. This work was entirely funded by the US Government. All authors are federal government employees, and this report is not subject to copyright in the US.
Footnotes
Disclosure: There are no conflicts of interest to report or financial disclosures.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control.
REFERENCES
- 1.Centers for Disease Control and Prevention. HIV Treatment as Prevention. Updated August 9. Accessed October 13, 2023. https://www.cdc.gov/hiv/risk/art/index.html [Google Scholar]
- 2.Centers for Disease Control and Prevention. Pre-Exposure Prophylaxis (PrEP). October 13, 2023. Updated July 5, 2022. Accessed October 13, 2023. https://www.cdc.gov/hiv/risk/prep/index.html [Google Scholar]
- 3.Centers for Disease Control and Prevention. Diagnoses of HIV Infection in the United States and Dependent Areas, 2021. HIV Surveillance Report, 2021. 2023;34. May 2023. Accessed August 21, 2023. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html [Google Scholar]
- 4.Centers for Disease Control and Prevention. Core indicators for monitoring the Ending the HIV Epidemic initiative (preliminary data): National HIV Surveillance System data reported through June 2023; and preexposure prophylaxis (PrEP) data reported through March 2023. HIV Surveillance Data Tables 2023;4(3). October Accessed November 1, 2023. https://www.cdc.gov/hiv/library/reports/surveillance-data-tables/ [Google Scholar]
- 5.Centers for Disease Control and Prevention. Ending the HIV Epidemic in the U.S. Progress. February 20, 2024. Updated June 9, 2023. Accessed February 2, 2024. https://www.cdc.gov/endhiv/ehe-progress/index.html [Google Scholar]
- 6.Forbes. ChatGPT: Everything You Really Need To Know (In Simple Terms). Updated December 21. Accessed November 22, 2023. https://www.forbes.com/sites/bernardmarr/2022/12/21/chatgpt-everything-you-really-need-to-know-in-simple-terms/?sh=4e0ded95cbca [Google Scholar]
- 7.Gupta A. Introduction to AI Chatbots. International Journal of Engineering Research and. July/11 2020;V9doi: 10.17577/IJERTV9IS070143 [DOI] [Google Scholar]
- 8.Piccialli F, di Cola VS, Giampaolo F, Cuomo S The Role of Artificial Intelligence in Fighting the COVID-19 Pandemic. Inf Syst Front. 2021;23(6):1467–1497. doi: 10.1007/s10796-021-10131-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Zhang Y, Wang D, et al. Artificial Intelligence for COVID-19: A Systematic Review. Front Med (Lausanne). 2021;8:704256. doi: 10.3389/fmed.2021.704256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UNESCO. UNESCO IITE and VK.com Create a Chatbot for Teens to Answer Questions about Adolescence, Relationships, and Health. Updated November 25, 2020. Accessed September 15, 2023. https://iite.unesco.org/highlights/unesco-vkontakte-chat-bot-eli-2/ [Google Scholar]
- 11.HIV.gov. HIV.GOV Launches New Chatbot Tool. Updated June 2, 2021. Accessed September 15, 2023. https://www.hiv.gov/blog/hivgov-launches-new-chatbot-tool/ [Google Scholar]
- 12.Garett R, Young SD. Potential application of conversational agents in HIV testing uptake among high-risk populations. J Public Health (Oxf). 2023/March// 2023;45(1):189–192. doi: 10.1093/pubmed/fdac020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The White House. FACT SHEET: President Biden Issues Executive Order on Safe, Secure, and Trustworthy Artificial Intelligence. Updated October 30th, 2023. Accessed October 30th, 2023. https://www.whitehouse.gov/briefing-room/statements-releases/2023/10/30/fact-sheet-president-biden-issues-executive-order-on-safe-secure-and-trustworthy-artificial-intelligence/ [Google Scholar]
- 14.Kamitani E, Mizuno Y, Mishra N, Khalil G, Viguerie A, DeLuca JB. Artificial Intelligence Used in Interventions or Programs to Promote HIV Pre-exposure Prophylaxis (PrEP) Clinical Care: A Systematic Review. PROSPERO. 2023;CRD42023458870. https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=458870 [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. Mar 29 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyles CM, Crepaz N, Herbst JH, Kay LS. Evidence-based HIV behavioral prevention from the perspective of the CDC's HIV/AIDS Prevention Research Synthesis Team. AIDS Educ Prev. Aug 2006;18(4 Suppl A):21–31. doi: 10.1521/aeap.2006.18.supp.21 [DOI] [PubMed] [Google Scholar]
- 17.DeLuca JB, Mullins MM, Lyles CM, Crepaz N, Kay L, Thadiparthi S Developing a Comprehensive Search Strategy for Evidence Based Systematic Reviews. Evidence Based Library and Information Practice. March/17 2008;3(1):3–32. doi: 10.18438/B8KP66 [DOI] [Google Scholar]
- 18.Cochrane. Revised Cochrane Risk-of-bias Tool for Randomized Trials (RoB 2). August 22, 2019. Accessed September 15, 2023. https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2 [Google Scholar]
- 19.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 20.Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital. Accessed September 16, 2023. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 21.McPheeters ML, Kripalani S, Peterson NB, et al. Quality Improvement Interventions To Address Health Disparities. Closing the Quality Gap: Revisiting the State of the Science. Evidence Report No. 208. (Prepared by the Vanderbilt University evidence-based Practice Center under Contract No. 90-2007-10065.). AHRQ Publication No. 12-E009-EF. Rockville, MD. Agency for Healthcare Research and Quality; 2012. Accessed September 16, 2023. https://www.ncbi.nlm.nih.gov/books/NBK107315/pdf/Bookshelf_NBK107315.pdf [Google Scholar]
- 22.Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? a systematic review. BMC Public Health. 2013/February/19 2013;13(1):154. doi: 10.1186/1471-2458-13-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S, Zhang Q, Chan CK, et al. Evaluating an Innovative HIV Self-Testing Service With Web-Based, Real-Time Counseling Provided by an Artificial Intelligence Chatbot (HIVST-Chatbot) in Increasing HIV Self-Testing Use Among Chinese Men Who Have Sex With Men: Protocol for a Noninferiority Randomized Controlled Trial. JMIR Res Protoc. Jun 30 2023;12:e48447. doi: 10.2196/48447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braddock WRT, Ocasio MA, Comulada WS, Mandani J, Fernandez MI. Increasing Participation in a TelePrEP Program for Sexual and Gender Minority Adolescents and Young Adults in Louisiana: Protocol for an SMS Text Messaging–Based Chatbot. JMIR Res Protoc. 2023/May/31 2023;12:e42983. doi: 10.2196/42983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massa P, de Souza Ferraz DA, Magno L, et al. A Transgender Chatbot (Amanda Selfie) to Create Pre-exposure Prophylaxis Demand Among Adolescents in Brazil: Assessment of Acceptability, Functionality, Usability, and Results. J Med Internet Res. Jun 23 2023;25:e41881. doi: 10.2196/41881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yam EA, Namukonda E, McClair T, et al. Developing and Testing a Chatbot to Integrate HIV Education Into Family Planning Clinic Waiting Areas in Lusaka, Zambia. Glob Health Sci Pract. Oct 31 2022;10(5)doi: 10.9745/ghsp-d-21-00721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang C, Fiscella K, Przybylek S, Chang W, Liu Y Telemedicine Experience for PrEP Care among PrEP-Eligible Women and Their Primary Care Providers during the First Year of the COVID-19 Pandemic in the United States. Trop Med Infect Dis. Oct 2 2022;7(10)doi: 10.3390/tropicalmed7100280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leveraging Chatbot to Improve PrEP in the Southern United States. US: ClinigalTrials.gov ID: NCT05968755; 2023. Accessed September 13, 2023. https://clinicaltrials.gov/study/NCT05968755?cond=HIV&term=PrEP&intr=automatic&rank=2.
- 29.Ni Z. Developing an artificial intelligence-based mHealth intervention to increase HIV testing in Malaysia. Malaysia: NIH; Project Number: 1R21TW011663-01; 2020. Accessed September 13, 2023. https://reporter.nih.gov/search/JYCTubUmoE25vTMokj161g/project-details/10064898. [Google Scholar]
- 30.Wickersham JA. Developing an Artificial Intelligence Chatbot to Promote HIV Testing. NIH Project Number: 1R21AI152927-01A1 2020. Accessed September 13, 2023. https://reporter.nih.gov/search/KSVrKX1cL0K6XMA_aZxvCQ/project-details/10082768. [Google Scholar]
- 31.van den Berg P, Powell VE, Wilson IB, Klompas M, Mayer K, Krakower DS. Primary Care Providers' Perspectives on Using Automated HIV Risk Prediction Models to Identify Potential Candidates for Pre-exposure Prophylaxis. AIDS Behav. Nov 2021;25(11):3651–3657. doi: 10.1007/s10461-021-03252-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amith MT, Cui L, Roberts K, Tao C Towards an ontology-based medication conversational agent for PrEP and PEP. Proc Conf Assoc Comput Linguist Meet. Jul 2020;2020:31–40. doi: 10.18653/v1/2020.nlpmc-1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchbinder SP, Siegler AJ, Coleman K, et al. Randomized Controlled Trial of Automated Directly Observed Therapy for Measurement and Support of PrEP Adherence Among Young Men Who have Sex with Men. AIDS Behav. Feb 2023;27(2):719–732. doi: 10.1007/s10461-022-03805-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu AY, Laborde ND, Coleman K, et al. DOT Diary: Developing a Novel Mobile App Using Artificial Intelligence and an Electronic Sexual Diary to Measure and Support PrEP Adherence Among Young Men Who Have Sex with Men. AIDS Behav. Apr 2021;25(4):1001–1012. doi: 10.1007/s10461-020-03054-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Lau JTF, Ip M, et al. A Randomized Controlled Trial Evaluating Efficacy of Promoting a Home-Based HIV Self-Testing with Online Counseling on Increasing HIV Testing Among Men Who Have Sex with Men. AIDS and Behavior. 2018/01/January 2018;22(1):190–201. doi: 10.1007/s10461-017-1887-2 [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Home-Based HIV Self-Testing with Online Instruction and Conseling (HIVST-OIC). Compendium of Evidence-based Interventions adn Best Practices for HIV Prevention. Centers for Disease Control and Prevention,; 2020. https://www.cdc.gov/hiv/pdf/research/interventionresearch/compendium/si/cdc-hiv-Home_Based_HIV_Self_Testing_Online_SI_EBI.pdf [Google Scholar]
- 37.Dourado I, Magno L, Soares F, et al. Adapting to the COVID-19 Pandemic: Continuing HIV Prevention Services for Adolescents Through Telemonitoring, Brazil. AIDS and behavior. 2020/July// 2020;24(7):1994–1999. doi: 10.1007/s10461-020-02927-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaisson RE, Barnes GL, Hackman J, et al. A randomized, controlled trial of interventions to improve adherence to isoniazid therapy to prevent tuberculosis in injection drug users. Am J Med. Jun 1 2001;110(8):610–5. doi: 10.1016/s0002-9343(01)00695-7 [DOI] [PubMed] [Google Scholar]
- 39.Hart JE, Jeon CY, Ivers LC, et al. Effect of directly observed therapy for highly active antiretroviral therapy on virologic, immunologic, and adherence outcomes: a meta-analysis and systematic review. J Acquir Immune Defic Syndr. Jun 2010;54(2):167–79. doi: 10.1097/QAI.0b013e3181d9a330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma M, Brown BR, Coleman M, Kibler JL, Loewenthal H, Mitty JA. The feasibility of modified directly observed therapy for HIV-seropositive African American substance users. AIDS Patient Care STDS. Feb 2008;22(2):139–46. doi: 10.1089/apc.2007.0063 [DOI] [PubMed] [Google Scholar]
- 41.Lumsden J, Dave AJ, Johnson C, Blackmore C Improving access to pre-exposure prophylaxis for HIV prescribing in a primary care setting. BMJ Open Quality. 2022;11(2):e001749. doi: 10.1136/bmjoq-2021-001749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wahnich A, Gandhi AD, Cleghorn E, et al. Public Health Detailing to Promote HIV Pre- and Postexposure Prophylaxis Among Women's Healthcare Providers in New York City. American Journal of Preventive Medicine. Nov 2021;61(5 Suppl 1):S98–s107. doi: 10.1016/j.amepre.2021.05.032 [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. PrEP and PEP Public Health Detailing Campaign for Cisgender and Transgender Women: Evidence-Informed for the Pre-Exposure Prophylaxis Chapter, Evidence-Informed for the Structural Interventions Chapter. Compendium of Evidence-Based Interventions and Best Practices for HIV Prevention. 2023. February 22, 2023. Accessed September 14th, 2023. https://www.cdc.gov/hiv/pdf/research/interventionresearch/compendium/prep/cdc-hiv-PrEP_PEP_Public_Health_Detailing_Campaign_EI_PrEP.pdf [Google Scholar]
- 44.Centers for Disease Control and Prevention. PrEP for Primary Care: Evidence-Informed for the Pre-Exposure Prophylaxis Chapter, Evidence-Informed for the Structural Interventions Chapter. Compendium of Evidence-Based Interventions and Best Practices for HIV Prevention. 2023. Novebmer 27th, 2023. Accessed November 16th, 2023. https://www.cdc.gov/hiv/pdf/research/interventionresearch/compendium/prep/PrEP_Primary_Care_EI_PrEP.pdf [Google Scholar]
- 45.Kamitani E, Mizuno Y, Koenig LJ. Strategies to Eliminate Inequity in PrEP Services in the US South and Rural Communities. J Assoc Nurses AIDS Care. Nov 14 2023;doi: 10.1097/jnc.0000000000000437 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.