Abstract
Background:
Babesia ovis, an intraerythrocytic parasite carried by ticks and one of the most common subclinical ovine illnesses, was studied to ascertain its seroprevalence and endemic status in ram and ewe populations in East Azerbaijan Province, Iran, in lambs, yearlings, and adults of over two years of age.
Methods:
A total of 960 sheep from 10 cities were selected from Jan 2018 to Nov 2019. Blood samples were collected from each animal and tested for the presence of B. ovis antibodies by applying a developed enzyme-linked immunosorbent assay (ELISA) technique. Checkerboard titrations were used to determine the optimal dilution of the antigen using negative and positive control sera. To determine whether the disease is endemically stable, inoculation rates for each age group were also calculated. Correlation coefficients were calculated between age and infection rates and also between age and inoculation rates.
Results:
The results revealed an average infection rate of 49.4% in East Azerbaijan Province. There was a positive correlation between the age of animals and susceptibility to infection except for lambs and yearlings, whereas there was no meaningful difference in exposure to B. ovis between rams and ewes. The negative correlation between age and inoculation rates indicates increased disease instability with age. Inoculation rate results revealed the endemically instable status of B. ovis in the studied area.
Conclusion:
High prevalence rates and endemically instable status of the disease suggest demand for vaccine development and implementation of appropriate control measures for ovine babesiosis to mitigate the associated economic losses.
Keywords: Babesia ovis, Endemic status, Seroepidemiology, ELISA, Iran
Introduction
Babesia ovis is a species of protozoan parasite that is transmitted by ticks and infects red blood cells. It belongs to the Babesiidae family and the genus Babesia, causing ovine babesiosis, a widely distributed zoonotic infectious disease of the small ruminants taking place in tropical and subtropical regions (1, 2).
The main transmitting vectors of Babesia ovis are the tick genera Ixodes, Rhipicephalus, and Hyalomma (3, 4). In Iran and the north-west region of the country, Babesia ovis transmission primarily occurs through various tick species and genera, notably R. bursa (5, 6), Hyalomma (7) R. turanicus and R. sanguineus (6).
Chronic cases of infection are usually asymptomatic, except for parasitemia and unthriftiness. Severe infection cases are characterized by hyperthermia, progressive hemolytic anemia, hemoglobinuria, icterus, and weakness (8), with a mortality rate of 30–50% (9, 10). The high prevalence rate of ovine babesiosis, a major parasitic disease of small ruminants in Iran, followed by substantial economic losses, decreased production, and cost of treatment and control programs, emphasizes the critical importance of epidemiological studies in the mentioned areas (11–13).
To conduct epidemiological investigations for efficient disease control, a valid diagnosis of the infection and a road map of the disease are required (14). The diagnosis of babesiosis is based on clinical symptoms and microscopic inspection of Giemsa-stained blood films for merozoites (15). Recovered animals from acute infection often sustain subclinical infections, being undetectable by microscopic methods. These animals are considered a source of infection for the disease for the potential vector, causing the disease’s natural transmission (16).
Livestock herds that operate under pastoral and small mixed farming systems increase the likelihood of interspecies transmission of infections, often facilitated by asymptomatic carrier animals (17). Therefore, serological techniques are commonly applied for the detection of subclinical infections (18, 19), among which the enzyme-linked immunosorbent assay (ELISA) is widely used as a sensitive diagnostic test for population screenings (20, 21).
Considerably different seroprevalence rates of B. ovis have been reported in East Azerbaijan and the surrounding regions in Iran; A prevalence of 23.6%, 24.5%, and 86.4% for the disease among sheep have been reported for East Azerbaijan, Baneh, and Ardabil, respectively by PCR method (22–24). No previous reports are available for the endemic status of the disease for the mentioned region. The endemic status of a disease, which serves as an indicative factor for understanding the immunity of a population, should be thoroughly studied to determine the necessity of developing a vaccine. The stability or instability of a population’s immunity can be determined by the inoculation rate of the disease, a factor often measured by serological methods (25).
Limited information exists regarding the seroprevalence, spatial distribution, and the endemic status of the condition in various areas of the country. Thus, the present study was accomplished to investigate the seroprevalence of B. ovis in sheep by ELISA technique in East Azerbaijan Province, northwest of Iran.
Materials and Methods
Study area and Sampling
East Azerbaijan Province has a four-season climate affected by Mediterranean and arid to semi-arid subtropical climate, covering about 45,650 km2 at 37.9036° N, 46.2682° E. In the period between January 2018 and November 2019, 960 sera were collected from 10 different cities. Randomly selected sheep in each city were in three age groups of 6–12, 12–24, and more than 24 months old, with 32 sheep in each group (a total of 96 sheep from 8 herds in each city, 12 samples from each herd). There were equal numbers of samples selected from each age and sex strata. The collected blood samples were kept in ethylenediaminetetraacetic acid (EDTA) tubes and were maintained cold until they were delivered to the laboratory. No babesiosis mortality was recorded throughout the study. In this study, stratified sampling was employed, with samples randomly selected from distinct age and gender strata. A comprehensive approach was adopted, resulting in a large sample size to enhance the study’s robustness.
Serological assay
An ELISA technique previously described by Hashemzadeh et al. (26) with slight modifications was used to detect B. ovis-specific antibodies from the collected sera.
Preparation of Babesia ovis ELISA antigen
To prepare the ELISA antigen, a blood sample was previously collected in the presence of EDTA from a heavily infected lamb with a 5% parasitemia. The parasites were then rinsed in phosphate-buffered saline (PBS) and pelleted by centrifugation after being lysed in cold (four °C) distilled water. Next, the lysate was centrifuged (12000 ×g for 30 min), and parasites were pelleted and then washed in PBS as before. Following sonication in the proper volumes for 75 seconds, the parasites were centrifuged with an ultra-high force of 105000 g for 60 minutes at a temperature of four °C. The supernatant liquid was then kept and combined with glycerol for storage in aliquots at −70 °C until use in the ELISA. Additionally, negative control sera were earlier obtained from newborn lambs before the initial intake of colostrum, while positive control sera were obtained from severely diseased sheep.
ELISA procedure
Multiple dilutions of the ELISA antigen were prepared, and 100 μl per well of the coating solution (0.1 M carbonate buffer with pH= 9.6) was applied for coating the ELISA plates (Greiner Bio-One, Germany) and incubated at four °C overnight. Initially, blocking was carried out using 100 μl per well of a 10% solution of bovine serum albumin (BSA) in the coating buffer at a temperature of 25 °C for three hours. The wells were then rinsed with 300 μl per well of a washing buffer containing tween (PBST, PBS pH= 7.2 with 0.1% Tween 20). Next, 100 μl of the serum samples (diluted 1:100 in PBST with 10% BSA) were applied to duplicate wells and left to incubate at 25 °C for one hour. Following three subsequent washes, 100 μl of peroxidase-conjugated anti-sheep IgG (Sigma-Aldrich, USA), diluted 1: 1400 in PBST with 10% BSA, were added to each well. The plates were then incubated at 25 °C for an additional hour and washed three more times. Subsequently, 100 μl of ABTS ELISA peroxidase substrate (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)) (Roche Diagnostics, Mannheim, Germany) were added to each well and allowed to react for 15 minutes. To stop the substrate reaction, 100 μl/well of a 1% solution of sodium dodecyl sulphate (SDS) was introduced. The absorbance was measured at 405 nm using an ELISA microplate reader. The threshold for classifying sera as positive was determined as 1.5 times the average value obtained from the negative control sera.
Calculation of the endemic status
The probability of occurrence of babesiosis sheep population in the province was determined by calculating the inoculation rate (25, 27) formulated as follows:
where ‘h’ expresses the inoculation rate. A stable endemic status of a population would demonstrate an h value of 0.005 up to 0.05, while an instable status would express an h value of 0.0005 up to 0.005. For h values lower than 0.0005, disease outbreaks are less likely to occur in populations.
Statistical analysis
Data analyses were performed using Minitab software (version 18.1, 2017). Tukey’s test followed by one-way ANOVA was performed to assess differences in infection rates in the three age strata. An Independent t-test was also performed to assess differences in infection rates between rams and ewes. Spearman’s correlation coefficient test was used to determine the correlation between the three age ranges as ordinal ranked variables and the seropositivity to B. ovis. Pearson’s correlation coefficient test was also utilized to evaluate the correlation between age and inoculation rates. Significant differences were declared at P< 0.05.
Results
Standardization of ELISA
The checkerboard titration revealed that a dilution ratio of 1:300 was determined to be the optimal dilution for the ELISA antigen. The average absorbance value of the negative control sera was 0.150±0.01, which led to a cutoff value of 0.225.
Seroprevalence of Babesia ovis infection
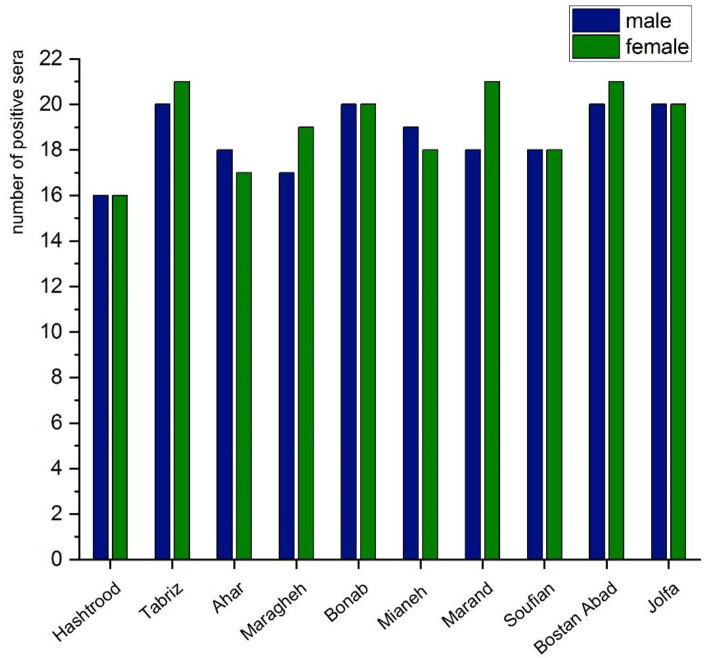
Of the 960 sera, 475 were seropositive to B. ovis, revealing that the total seroprevalence of the infection in the province is 49.4%, with the highest rate (42.7%) in Tabriz and Bostan Abad and the lowest rate (33.3%) in Hashtrood.
There was a significant difference in the prevalence of the infection between over two years old sheep and yearlings (p= 0.042) and also between over two years old sheep and lambs (p< 0.001); however, there was no significant difference between lams and yearlings (p= 0.095). The highest seroprevalence was observed in adult sheep over two years old (49%), followed by yearlings (38.7%) and lambs (30%). No significant difference was found between rams and ewes infection rates (p= 0.499). The prevalence rate significantly correlated (rs=0.659) with the animal’s age according to the results obtained from Spearman’s correlation test (p< 0.001). The results revealed the mean seropositivity of 38.7% in rams and 39.8% in ewes (Figs. 1 and 2).
Fig. 1.
The prevalence of Babesia ovis infection in blood sheep specimens determined by ELISA and categorized by gender in various locations of East Azerbaijan Province, 2018–2019
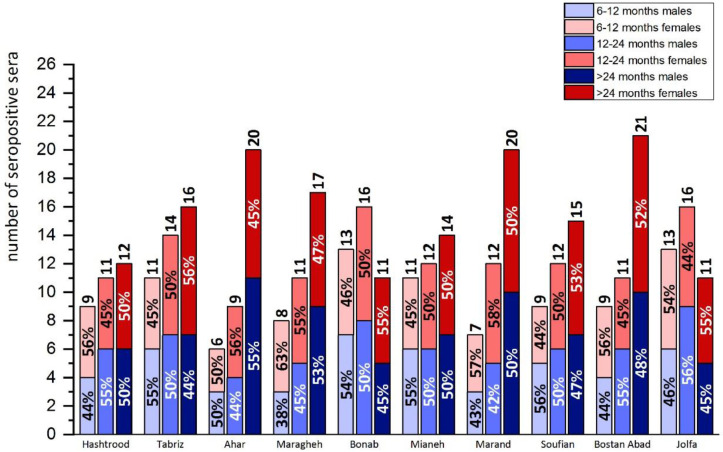
Fig. 2.
The distribution of Babesia ovis infection in sheep blood specimens determined by ELISA and categorized by age range and gender in various locations of East Azerbaijan Province, 2018–2019
Endemic status of Babesia ovis
To assess the endemic status of the disease, the inoculation rates for age groups for each city were determined using the ELISA data (Table 1).
Table 1.
Sampling regions, proportion of seropositive sheep blood specimens to Babesia ovis determined by ELISA, and the calculated inoculation rates for different cities and age groups in East Azerbaijan Province, 2018–2019
| Sampling region | Animal age (month) | Mean of age range (day) | No. of tested sera | No. of positive sera | Proportion of positive sera | Calculated inoculation rate (h)* |
|---|---|---|---|---|---|---|
| Hashtrood | 6–12 | 270 | 32 | 9 | 0.28 | 0.0012 |
| 12–24 | 540 | 32 | 11 | 0.34 | 0.0008 | |
| >24 | 1440 | 32 | 12 | 0.38 | 0.0003 | |
| Tabriz | 6–12 | 270 | 32 | 11 | 0.34 | 0.0016 |
| 12–24 | 540 | 32 | 14 | 0.44 | 0.0011 | |
| >24 | 1440 | 32 | 16 | 0.50 | 0.0005 | |
| Ahar | 6–12 | 270 | 32 | 6 | 0.19 | 0.0008 |
| 12–24 | 540 | 32 | 9 | 0.28 | 0.0006 | |
| >24 | 1440 | 32 | 20 | 0.63 | 0.0007 | |
| Maragheh | 6–12 | 270 | 32 | 8 | 0.25 | 0.0011 |
| 12–24 | 540 | 32 | 11 | 0.34 | 0.0008 | |
| >24 | 1440 | 32 | 17 | 0.53 | 0.0005 | |
| Bonab | 6–12 | 270 | 32 | 13 | 0.41 | 0.0019 |
| 12–24 | 540 | 32 | 16 | 0.50 | 0.0013 | |
| >24 | 1440 | 32 | 11 | 0.34 | 0.0003 | |
| Mianeh | 6–12 | 270 | 32 | 11 | 0.34 | 0.0016 |
| 12–24 | 540 | 32 | 12 | 0.38 | 0.0009 | |
| >24 | 1440 | 32 | 14 | 0.44 | 0.0004 | |
| Marand | 6–12 | 270 | 32 | 7 | 0.22 | 0.0009 |
| 12–24 | 540 | 32 | 12 | 0.38 | 0.0009 | |
| >24 | 1440 | 32 | 20 | 0.63 | 0.0007 | |
| Soufian | 6–12 | 270 | 32 | 9 | 0.28 | 0.0012 |
| 12–24 | 540 | 32 | 12 | 0.38 | 0.0009 | |
| >24 | 1440 | 32 | 15 | 0.47 | 0.0004 | |
| Bostan Abad | 6–12 | 270 | 32 | 9 | 0.28 | 0.0012 |
| 12–24 | 540 | 32 | 11 | 0.34 | 0.0008 | |
| >24 | 1440 | 32 | 21 | 0.66 | 0.0007 | |
| Jolfa | 6–12 | 270 | 32 | 13 | 0.41 | 0.0019 |
| 12–24 | 540 | 32 | 16 | 0.50 | 0.0013 | |
| >24 | 1440 | 32 | 11 | 0.34 | 0.0003 | |
| All regions | 6–12 | 270 | 320 | 96 | 0.30 | 0.0013 |
| 12–24 | 540 | 320 | 124 | 0.39 | 0.0009 | |
| >24 | 1440 | 320 | 157 | 0.49 | 0.0005 |
’h’ indicates the inoculation rate: Stable endemic (0.005–0.05); Unstable (0.0005–0.005); ‘h’ below 0.0005 implies a lower likelihood of outbreaks.
The inoculation rates in all the age ranges were almost lower than 0.005 in most of the regions suggesting an endemic instability. Furthermore, the results of Pearson’s correlation coefficient test indicate a strong negative correlation (r= −0.761) between age and inoculation rates signifying the increasing endemic instability of B. ovis with age (P< 0.001). In addition, the inoculation rates for more than two years old sheep in Hashtrood, Bonab, Mianeh, Soufian, and Jolfa were lower than 0.0005, suggesting a low probability of occurrence of an outbreak for the mentioned age group.
Discussion
The widely employed diagnostic method for identifying clinical babesiosis in sheep and goats involves Giemsa staining, a technique where blood smears are stained with Giemsa dye to visualize the intraerythrocytic parasites. This staining method facilitates the microscopic examination of blood samples for the presence of characteristic Babesia species, aiding in the diagnosis of the disease (28). However, despite its common usage, Giemsa staining has limitations in terms of both specificity and sensitivity, particularly when it comes to detecting various species of Babesia. This method may not reliably identify subtle cases of subclinical babesiosis in small ruminants, potentially leading to an underestimation of the true prevalence of the infection. Therefore, alternative diagnostic approaches are often necessary to complement and enhance the accuracy of babesiosis detection in diverse clinical and subclinical cases (10). The disease commonly manifests with mild symptoms in older animals, attributed to the development of high protective immunity over time. Consequently, the prevalence rate of clinically apparent illness tends to decrease in older animals, underscoring the role of acquired immunity in mitigating the severity of Babesia infection in aging populations (29). Among the various serological assays, ELISA is recognized as a versatile and valuable tool, particularly suitable for investigating large populations owing to its high sensitivity and scalability (30).
In our investigation, we identified a seroprevalence of 49.4% for ovine babesiosis, surpassing the previously documented rate in the East Azerbaijan Province of Iran reported by Bazmani et al. (22), who utilized a semi-nested PCR method and reported a seroprevalence of 23.6% in a sample size of 123 sheep. Diverse prevalence rates of ovine babesiosis are reported in adjacent regions of Iran; Habibi et al. (23) detected seropositivity of 86.4% in the sheep population in Baneh, Iran, using semi-nested PCR and competitive PCR methods. Shahbazi et al. (24) reported a seropositivity of 24.5% in 200 sheep from Ardabil, northwest Iran, using PCR. Ovine babesiosis has also been a country-wide epidemiological issue in Turkey, with the infection rate ranging from 0% to 67.38%, with the endemic status being instable across all age groups (29).
Age emerges as a pivotal factor influencing the susceptibility rate to ovine babesiosis. Lambs younger than three months exhibit high resistance to acute B. ovis infections in areas where the disease is prevalent, owing to their non-specific inherent resistance and the protective immunity they acquire from colostrum. This immunity remains effective for three months following birth, potentially contributing to the endemic stability of the disease in sheep populations by elevating antibody titers and neutralizing sporozoites. Contrastingly, the resistance to infection in non-exposed lambs to transmitter ticks decreases before three months of age (27). The current study revealed that the prevalence rates of B. ovis among different sexes were not statistically different.
Furthermore, comprehending the age-related dynamics of ovine babesiosis susceptibility highlights the critical need for robust disease surveillance in resource-limited regions. A structured surveillance system not only aids in discerning disease epidemiology before vaccine introduction but also enables the evaluation of intervention impacts, providing valuable insights into the effectiveness of vaccination programs. Many low- and middle-income countries have not fully benefited from such surveillance systems, emphasizing the urgent importance of implementing them to better understand disease dynamics and assess the impact of interventions on reducing disease burden (31, 32).
In this study, Pearson’s correlation test results further revealed a negative association between age and inoculation rates of B. ovis, supporting the hypothesis of heightened endemic instability in older individuals (29, 33). Despite the high prevalence of the disease within a population, clinical symptoms are infrequent in cases of long-standing stable endemic diseases. This situation occurs when functional immunity is achieved at a young age in most of the population due to the sufficient power of the infection. Older animals possess high protective immunity causing mild symptoms and low prevalence of clinical disease (29). In the context of diseases exhibiting instability within a population, vaccination is recommended as a preventive measure. However, vaccination is not suggested as a control measure for diseases that are already stable within an endemic region. Despite the low occurrence of clinical diseases in stable endemic populations, the use of acaricides for the management of vector tick populations is not commonly employed (34, 35). This comprehensive understanding of disease dynamics and immunity status informs strategic decisions for effective disease control and management.
Based on the findings from this study on inoculation rates of B. ovis across different cities and age groups (Table 1), it can be inferred that the endemic status of the disease is almost instable among all age groups within the province. As a result, it is recommended to implement a vaccination program for this disease. Although specific proteins of B. ovis, such as rBoSA2, BoSPD, rBoSA1, and ovipain-2, have been proposed for potential vaccine development using recombinant DNA technologies, there is currently no existing vaccine available for ovine babesiosis (29).
In light of our prior study (26) utilizing a similar ELISA procedure, wherein an 85% concordance was observed between the results of ELISA and immunofluorescent tests, we acknowledge the inherent limitations of the in-house ELISA technique in definitively distinguishing Babesia species due to potential cross-reactivity. We recommend a cautious interpretation of the observed seroprevalence, recognizing that the high rates reported in our study may be influenced by the method’s reduced specificity in differentiating B. ovis from other species. Due to the large sample size of the study, the feasibility of microscopic examination of blood samples for the identification of B. ovis was logistically impractical. Future research should explore additional diagnostic tools to enhance species identification.
Conclusion
This study uncovers a significantly higher rate of ovine babesiosis seroprevalence in the East Azerbaijan Province of Iran, surpassing previous reports. The findings underscore the substantial impact of age on susceptibility to the disease, with a notable prevalence among older animals. In contrast, no gender-based susceptibility differences to the disease were observed. The endemic instability of the disease in the studied area suggests the necessity for implementing a vaccination program. However, further studies are needed to assess the endemic status and devise a comprehensive strategy, as well as identify additional recombinant proteins for vaccine development, to effectively control ovine babesiosis and mitigate its associated economic losses.
Acknowledgements
We would like to acknowledge the research community as a whole for their commitment to expanding knowledge across diverse fields.
Footnotes
Ethical considerations
Owners of the herds and farms that were chosen for this study were notified about the survey and sampling of their animals, and ethical standards were considered in the conduction of the study. This study was conducted based on Islamic Azad University of Tabriz (IAUT) ethical committee guidelines.
Conflict of interest statement
The authors declare there is no conflict of interests.
References
- 1.Mehlhorn H, Shein E. (1984) The piroplasms: life cycle and sexual stages. Adv Parasitol. 23: 37–103. [DOI] [PubMed] [Google Scholar]
- 2.Altay K, Dumanli N, Aktas M. (2007) Molecular identification, genetic diversity and distribution of Theileria and Babesia species infecting small ruminants. Vet Parasitol. 147(1–2): 161–165. [DOI] [PubMed] [Google Scholar]
- 3.Song R, Wang Q, Guo F, Liu X, Song S, Chen C, Tu C, Wureli H, Wang Y. (2018) Detection of Babesia spp., Theileria spp. and Anaplasma ovis in Border Regions, northwestern China. Transbound Emerg Dis. 65(6): 1537–1544. [DOI] [PubMed] [Google Scholar]
- 4.Spotin A, Dalir F, Hazratian T, Shekarchi AA, Mahami-Oskouei M, Farmani M, Dolatkhah A, Ahmadpour E. (2023) Global haplotype distribution of Babesia ovis inferred by 18S rRNA sequences; a phylogeographical systematic review. Microb Pathog. 181: 106179. [DOI] [PubMed] [Google Scholar]
- 5.Esmaeilnejad B, Tavassoli M, Asri-Rezaei S, Dalir-Naghadeh B, Mardani K, Jalilzadeh- Amin G, Golabi M, Arjmand J. (2014) PCR-based detection of Babesia ovis in Rhipicephalus bursa and small ruminants. J Parasitol Res. 2014: 294704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shayan P, Hooshmand E, Rahbari S, Nabian S. (2007) Determination of Rhipicephalus spp. as vectors for Babesia ovis in Iran. Parasitol Res. 101(4): 1029–1033. [DOI] [PubMed] [Google Scholar]
- 7.Razmi G, Pourhosseini M, Yaghfouri S, Rashidi A, Seidabadi M. (2013) Molecular detection of Theileria spp. and Babesia spp. in sheep and ixodid ticks from the northeast of Iran. J Parasitol. 99(1): 77–81. [DOI] [PubMed] [Google Scholar]
- 8.Hurtado A, Barandika JF, Oporto B, Minguijón E, Povedano I, García-Pérez AL. (2015) Risks of suffering tick-borne diseases in sheep translocated to a tick infested area: A laboratory approach for the investigation of an outbreak. Ticks Tick Borne Dis. 6(1): 31–37. [DOI] [PubMed] [Google Scholar]
- 9.Hashemi-Fesharki R. (1997) Tick-borne diseases of sheep and goats and their related vectors in Iran. Parassitologia. 39(2): 115–117. [PubMed] [Google Scholar]
- 10.Friedhoff KT. (1997) Tick-borne diseases of sheep and goats caused by Babesia, Theileria or Anaplasma spp. Parassitologia. 39(2): 99–109. [PubMed] [Google Scholar]
- 11.Esmaeilnejad B, Tavassoli M, Asri-Rezaei S, Dalir-Naghadeh B, Mardani K, Golabi M, Arjmand J, Kazemnia A, Jalilzadeh G. (2015) Determination of prevalence and risk factors of infection with Babesia ovis in small ruminants from West Azerbaijan Province, Iran by polymerase chain reaction. J Arthropod Borne Dis. 9(2): 246–252. [PMC free article] [PubMed] [Google Scholar]
- 12.Hasheminasab SS, Moradi P, Wright I. (2018) A four year epidemiological and chemotherapy survey of babesiosis and theileriosis, and tick vectors in sheep, cattle and goats in Dehgolan, Iran. Ann Parasitol. 64(1): 43–48. [DOI] [PubMed] [Google Scholar]
- 13.Ocaido M, Muwazi RT, Opuda JA. (2009) Economic impact of ticks and tick-borne diseases on cattle production systems around Lake Mburo National Park in South Western Uganda. Trop Anim Health Prod. 41(5): 731–739. [DOI] [PubMed] [Google Scholar]
- 14.Yadufashije C, Habyarimana T. (2019) Epidemiological Studies and Validity of Diagnostic Test. Am J Biomed Sci. 11(1): 1–14. [Google Scholar]
- 15.Guo S, Yuan Z, Wu G, Wang W, Ma D, Du H. (2002) Epidemiology of ovine theileriosis in Ganan region, Gansu Province, China. Parasitol Res. 88(Suppl 1): S36–37. [DOI] [PubMed] [Google Scholar]
- 16.Calder JA, Reddy GR, Chieves L, Courtney CH, Littell R, Livengood JR, Norval RA, Smith C, Dame JB. (1996) Monitoring Babesia bovis infections in cattle by using PCR-based tests. J Clin Microbiol. 34(11): 2748–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tumwebaze MA, Byamukama B, Tayebwa DS, Byaruhanga J, Angwe MK, Galon EM, Liu M, Lee SH, Ringo AE, Adjou Moumouni PF, Li J. (2020) First molecular detection of Babesia ovis, Theileria spp., Anaplasma spp., and Ehrlichia ruminantium in Goats from Western Uganda. Pathogens. 9(11): 895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aktaş M, Altay K, Dumanli N. (2005) Development of a polymerase chain reaction method for diagnosis of Babesia ovis infection in sheep and goats. Vet Parasitol. 133(4): 277–281. [DOI] [PubMed] [Google Scholar]
- 19.Passos LM, Bell-Sakyi L, Brown CG. (1998) Immunochemical characterization of in vitro culture-derived antigens of Babesia bovis and Babesia bigemina. Vet Parasitol. 76(4): 239–249. [DOI] [PubMed] [Google Scholar]
- 20.Waltisbuhl DJ, Goodger BV, Wright IG, Commins MA, Mahoney DF. (1987) An enzyme linked immunosorbent assay to diagnose Babesia bovis infection in cattle. Parasitol Res. 73(2): 126–131. [DOI] [PubMed] [Google Scholar]
- 21.Furuta PI, Oliveira TMFDS, Teixeira MCA, Rocha AGR, Machado RZ, Tinucci-Costa M. (2009) Comparison between a soluble antigen-based ELISA and IFAT in detecting antibodies against Babesia canis in dogs. Rev Bras Parasitol Vet. 18(03): 41–45. [PubMed] [Google Scholar]
- 22.Bazmani A, Abolhooshyar A, Imani-Baran A, Akbari H. (2018) Semi-nested polymerase chain reaction-based detection of Babesia spp. in small ruminants from northwest of Iran. Vet World. 11(3): 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habibi G, Sepahvand-Mohammadi E, Afshari A, Bozorgi S. (2020) Molecular detection of Theileria spp. and Babesia ovis infection in sheep in Baneh, Iran. Arch Razi Inst. 75(2): 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gholamreza S, Somaieh M, Roya S, Alireza B, Ghazale A, Yasin B. (2017) First detection of Babesia ovis in Dermacentor spp in Ardabil area, northwest of Iran. J Vector Borne Dis. 54(3): 277–281. [DOI] [PubMed] [Google Scholar]
- 25.Mahoney D, Ross D. (1972) Epizootiological factors in the control of bovine babesiosis. Aust Vet J. 48(5): 292–298. [DOI] [PubMed] [Google Scholar]
- 26.Hashemzadeh Farhang H, Nabavi L, Seyfiabad Shapouri MR, Rahbari S, Azizi F. (2006) Development of an ELISA technique for the detection of Babesia ovis and serological survey of the parasite in Khouzestan Province, southern Iran. Iran J Vet Res. 7(2): 53–58. [Google Scholar]
- 27.Ekici OD, Sevinc F, Isik N. (2012) Instability of ovine babesiosis in an endemic area in Turkey. Vet Parasitol. 188(3–4): 372–375. [DOI] [PubMed] [Google Scholar]
- 28.Razmi GR, Naghibi A, Aslani MR, Fathivand M, Dastjerdi K. (2002) An epidemiological study on ovine babesiosis in the Mashhad suburb area, province of Khorasan, Iran. Vet Parasitol. 108(2): 109–115. [DOI] [PubMed] [Google Scholar]
- 29.Ceylan O, Sevinc F. (2020) Endemic instability of ovine babesiosis in Turkey: A country-wide sero-epidemiological study. Vet Parasitol. 278: 109034. [DOI] [PubMed] [Google Scholar]
- 30.Böse R, Jorgensen WK, Dalgliesh RJ, Friedhoff KT, de Vos AJ. (1995) Current state and future trends in the diagnosis of babesiosis. Vet Parasitol. 57(1–3): 61–74. [DOI] [PubMed] [Google Scholar]
- 31.Lahariya C. (2016) Vaccine epidemiology: A review. J Family Med Prim Care. 5(1): 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO (2009) State of the World’s Vaccines and Immunization. World Health Organization, UNICEF, World Bank. 3rd edition. Available at: https://apps.who.int/iris/handle/10665/44169 [Google Scholar]
- 33.Sevinc F, Sevinc M, Ekici OD, Yildiz R, Isik N, Aydogdu U. (2013) Babesia ovis infections: Detailed clinical and laboratory observations in the pre-and post-treatment periods of 97 field cases. Vet Parasitol. 191(1–2): 35–43. [DOI] [PubMed] [Google Scholar]
- 34.Hay SI. (2001) The paradox of endemic stability. Trends Parasitol. 17(7): 310–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonsson NN, Bock RE, Jorgensen WK, Morton JM, Stear MJ. (2012) Is endemic stability of tick-borne disease in cattle a useful concept? Trends Parasitol. 28(3): 85–89. [DOI] [PubMed] [Google Scholar]