Abstract
Background:
The common bed bugs, Cimex lectularius, and tropical bed bugs, Cimex hemipterus are the primary species of public health importance in the family Cimicidae. This study aimed to determine the morphometric criteria and prevalent species of bed bugs in eastern Iran.
Methods:
Bed bugs were collected from March 2021 to June 2022 from infested residential buildings and apartments in eastern Iran, including North Khorasan, Khorasan Razavi, and Sistan and Baluchistan Provinces. The morphological and morphometrical characteristics were used to identify collected bed bugs at inter- and intra-specific levels.
Results:
A total of 34 isolates comprising 127 adult bed bugs were collected from Bojnord, Mashhad, Neishabur, Taibad, Sabzevar, Kashmer, Zahedan, Saravan, Rask, Pishin and Chabahar. Of these, 33 isolates (n=124) were found to be tropical bed bugs, C. hemipterus, and one population (n=3) was identified as C. lectularius. The index pronotal width/length ratio was calculated from 2.72 to 2.94 and 1.98 to 2.47 for C. lectularius and C. hemipterus, respectively. The length/width ratio of the hind femur was 3.365 in C. hemipterus and 4.267 in C. lectularius. The ratio of length/width of the third femur (F3 l/w) between populations of C. hemipterus was different, and this difference was statistically significant (P< 0.05).
Conclusion:
The results of this study indicated that C. hemipterus was the dominant bed bug species in the east of Iran and provided more morphometric criteria of C. hemipterus for researchers to identify the species and determine the intraspecific variations in the present and future.
Keywords: Bed bugs, Biology, Humans, Public health, Iran
Introduction
The common bed bug, Cimex lectularius, with a global distribution, and the tropical bed bug, Cimex hemipterus distributed chiefly in tropical countries, are the most important species of the family Cimicidae from the public health perspective. Infestation of human dwellings with this parasite can adversely affect human health and welfare through blood-sucking behavior during the night. However, the transmission of any pathogenic agents to humans through biting of bed bugs in natural conditions has not been reported (1). Its global extent, developing insecticide resistance, and difficulty in treatment have made this insect a critical problem in urban pest management. The distribution pattern of bed bugs is changing around the world. The presence of C. hemipterus in Russia and France was reported for the first time by Gapon (2) and Bérenger and Pluot-Sigwalt (3), respectively. In the past ten years, C. lectularius has given way to C. hemipterus in the cities of Iran that were studied, and C. hemipterus has become more dominant than before (4). Demographic dynamics and changes can occur for various reasons. However, the expansion of tourism, the development of domestic and international travel, and the phenomenon of global warming may be part of these reasons (5).
Leptocimex boueti is a less common species that can feed on human blood in limited areas, especially where people live near bat habitats or visit these places (6). Other genera and species of the family Cimicidae are less important than the abovementioned species from the point of view of human health. Accurate diagnosis of bed bug species based on morphological interspecific differences and attention to intraspecific morphological and morphometrical variations, along with tracking these variations, can be helpful for more accurate species identification and understanding of the speciation process.
Morphometrics is one of the best quantitative approaches to taxonomy, phenotypic, and genotypic evolution (7). This branch of biology has contributed to a better understanding of valuable phenomena such as phylogenetic and ecomorphological relationships, life histories, animal behavior, community structures, and ecological processes (8). Inter/intraspecific differences can be detectable with repeatable results by this approach if experts follow the same standards (9). Furthermore, morphometric findings are widely transferable and can become a reliable data source for alpha classification (10).
Following the initial report of the presence of C. hemipterus in Iran (4), subsequent researches have also confirmed the existence of tropical bed bugs in the western and central regions of the country (11, 12). Bahrami et al. (13) studied the phylogenetic and ultrastructure characterization of bed bugs in southwestern Iran and showed that C. hemipterus was the predominant species in that region. However, there is currently no available data regarding the dominant bed bug species in eastern Iran, nor any information on their morphometric characteristics. This study aimed to determine the morphometric criteria and prevalent species of bed bugs in eastern Iran.
Materials and Methods
This study was conducted from March 2021 to June 2022. The study area included four provinces North Khorasan, Khorasan Razavi, South Khorasan, and Sistan and Baluchistan, located in the east of Iran (Fig. 1). This area was selected due to a need for more concise scientific reports regarding bed bug infestations and related species in the cities of these provinces. Khorasan Razavi is the most crowded province in sampled areas with a population of 6434501, followed by Sistan and Baluchistan with 2775014, North Khorasan with 850,000, and South Khorasan with 768898 population. The study area is situated between latitudes 25°03′34.1″ N- 38°16′48.5″ N and longitudes 55°23′11.3″ E- 63°20′00.9″ E.
Fig. 1.
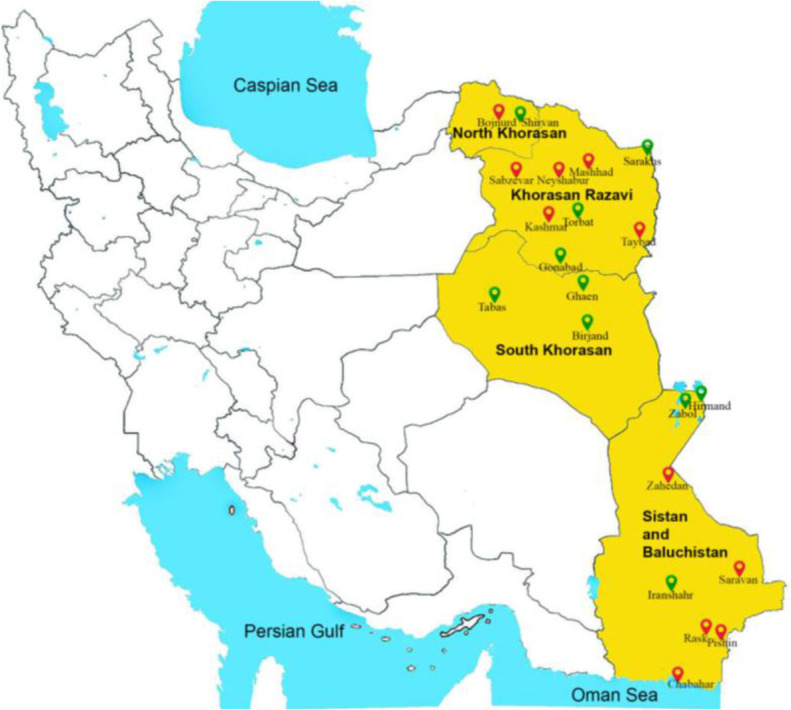
The map shows I. R. Iran and the yellow-colored study area, with red and green location points indicating respectively the cities with positive and negative bed bug infestation, 2021–2022
Adult bed bugs were collected from infested residential buildings and apartments reported by pest control companies in the study areas. Bed bugs were hand-collected using soft forceps transferred to plastic containers with tight lids and preserved in ethanol 96%. The specimens were taken to the parasitology laboratory, where each bed bug was placed between two slides and fixed by a pair of hair clips, then examined under a stereo microscope. The standard morphological keys were used to identify bed bugs based on their morphological features (14).
The methodology described by Balvin et al. (15) was used to assess the variation of different body parts of collected specimens. Morpho-metric characteristics were photographed by a digital camera (Vega 3146200 - Labomed, USA) under a stereomicroscope and measured using Image J software (version 1.52). Forty-six indices, including total body length (tl), width (tw), length of antennal segments (al1-al4), head length (hl) and width (hw), length (ed) and width (ew) of eyes, the intraocular space dorsally (is) and ventral (iv) surfaces, length of the longest hair between eyes and antennae (se), the width of clypeus (cw), length of hair on clypeus (sc), length of proboscis segments (rl1- rl3), length (pl) and width (pw) of pronotum, length of the middle part of pronotum (pm), depth of pronotal concavity (pc), length of setae on the pronotum (sp), length (sl) and width (sw) of scutellum, length (lh) and width (wh) of hemelytra, length of setae on hemelytra (sh), length (fl1-fl3) and the width (fw1-fw3) of the femurs and the length (tl1-tl3) and width (tw1-tw3) of the tibias, total antenna length (alt) and total rostellum length (rlt) were measured and recorded (Fig. 2). Some important ratios were also calculated, such as the ratio of pronotum width/length (pw/l) and hind femur length/width (f3l/w). The most extended distances were considered for the records; to measure the length of the setae, the longest was considered, and in measuring the width of the tibia, measurement was conducted almost in the middle of the tibia.
Fig. 2.
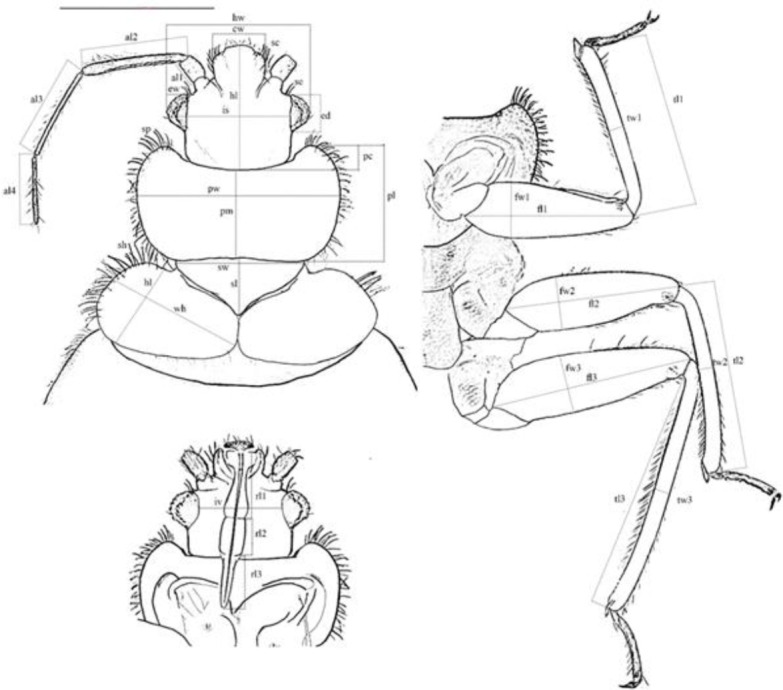
Measured indices used in the morphological analysis. Length of antennal segments (al1-al4), head length (hl) and width (hw), length (ed) and width (ew) of eyes, the intraocular space dorsally (is) and ventral (iv) surfaces, length of the most extended hair between eyes and antennae (se), the width of clypeus (cw), length of hair on clypeus (sc), length of probosci’s segments (rl1-rl3), length (pl) and width (pw) of pronotum, length of the middle part of pronotum (pm), depth of pronotal concavity (pc), length of setae on the pronotum (sp), length (sl) and width (sw) of scutellum, length (lh) and width (wh) of hemelytra, length of setae on hemlytra (sh), length (fl1-fl3) and the width (fw1-fw3) of the femurs and the length (tl1-tl3) and width (tw1-tw3) of the tibias (The figure is original and abbreviations adapted from Balvin et al. (12))
The data of indices obtained from males and females, as well as width/length of pronotum (pw/l), length/width of head, length/width of 3rd femur (f3 l/w), length of seta of pronotum (sp), head width/eye width (hw/ew), scutellum width/head width (sw/hw) and hemelytra length/hemelytra width of C. hemipterus specimens, were selected and submitted to SPSS software (Version 16). All data were assessed for normality by the Kolmogorov-Smirnov test. The data sets with non-normal distribution were compared using Kruskal-Wallis and Mann-Whitney tests. P values< 0.05 were considered statistically significant.
Results
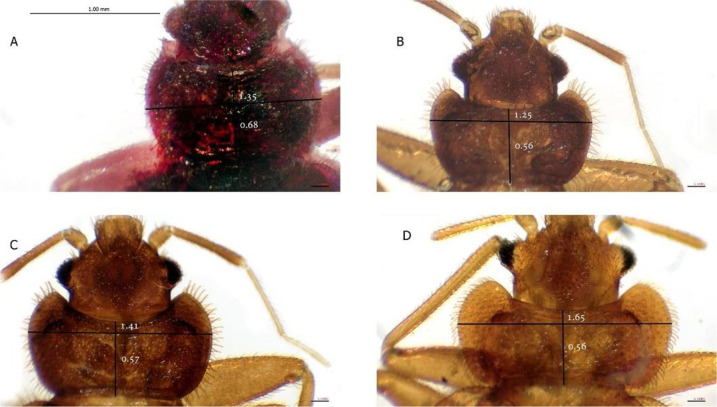
A total of 34 isolates comprising 127 adult bed bugs, including 61 females and 66 males, were collected from Bojnord, Mashhad, Neishabur, Taibad, Sabzevar, Kashmer, Zahedan, Saravan, Rask, Pishin and Chabahar. However, we could not find any bed bug infestation in the cities of South Khorasan Province. All collected specimens were identified based on morphometric characteristics to species level. Of these, 33 isolates (n=124) were found to be the tropical bed bugs, C. hemipterus, and one population (n=3) was identified as the common bed bug, C. lectularius (from an archived collection in Mashhad, Iran, collected in 2007). The index pronotal width/length ratio was calculated from 2.72 to 2.94 and 1.98 to 2.47 for C. lectularius and C. hemipterus, respectively (Fig. 3).
Fig. 3.
Pronotum and head of adult Cimex hemipterus (A, B, C) from three distinct populations and Cimex lectularius (D). The horizontal line on the pronotum has demonstrated intra/interspecific variation in the width size of the pronotum
In this study, the body length and width of C. hemipterus were 4.43–7.7 mm and 2.27–3.32 mm, respectively. The body length of C. lectularius was 5.3–5.4, and its width was measured at 2.7–3.4 mm. The mean size of the body width of females was significantly higher than that of males, whereas their difference in body length was insignificant. Detailed data of other measured indices and their significance between males and females can be found in Table 1.
Table 1.
Measured morphometric characteristics of male and female C. hemipterus collected from east of Iran (n=124)
| Index | Female | Male | P-Value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n | Mean (mm) | SD | n | Mean (mm) | SD | ||
| Total body length (tl) | 53 | 5.538 | 0.726 | 62 | 5.319 | 0.550 | 0.069 |
| Total body width (tw) | 53 | 2.955 | 0.163 | 62 | 2.687 | 0.158 | 0.000 |
| 1st antennal segment (al1) | 53 | 0.190 | 0.016 | 62 | 0.188 | 0.015 | 0.365 |
| 2nd antennal segment (al2) | 54 | 0.648 | 0.038 | 61 | 0.620 | 0.033 | 0.000 |
| 3rd antennal segment (al3) | 33 | 0.603 | 0.043 | 38 | 0.583 | 0.041 | 0.042 |
| 4th antennal segment (al4) | 27 | 0.449 | 0.036 | 37 | 0.435 | 0.032 | 0.166 |
| Total length of antenna (alt) | 26 | 1.877 | 0.109 | 35 | 1.821 | 0.089 | 0.035 |
| Head width (hw) | 58 | 0.995 | 0.034 | 65 | 0.955 | 0.037 | 0.000 |
| Head length (hl) | 57 | 0.813 | 0.051 | 64 | 0.788 | 0.052 | 0.014 |
| Eye width (ew) | 58 | 0.126 | 0.013 | 65 | 0.121 | 0.012 | 0.039 |
| Eye length (ed) | 58 | 0.237 | 0.019 | 65 | 0.227 | 0.018 | 0.002 |
| Intraocular space dorsally (is) | 58 | 0.742 | 0.031 | 65 | 0.714 | 0.037 | 0.000 |
| Intraocular space ventral (iv) | 55 | 0.767 | 0.048 | 62 | 0.730 | 0.041 | 0.000 |
| Seta between eyes and antennae (se) | 58 | 0.069 | 0.010 | 65 | 0.071 | 0.008 | 0.273 |
| Width of clypeus (cw) | 58 | 0.367 | 0.015 | 65 | 0.357 | 0.016 | 0.001 |
| Length of seta on clypeus (sc) | 56 | 0.072 | 0.008 | 65 | 0.072 | 0.007 | 0.966 |
| 1st segment of rostellum (rl1) | 49 | 0.444 | 0.040 | 51 | 0.430 | 0.038 | 0.088 |
| 2nd segment of rostellum (rl2) | 52 | 0.285 | 0.030 | 59 | 0.269 | 0.028 | 0.007 |
| 3rd segment of rostellum (rl3) | 53 | 0.329 | 0.026 | 60 | 0.320 | 0.024 | 0.028 |
| Total length of rostellum (rlt) | 48 | 1.060 | 0.049 | 50 | 1.022 | 0.048 | 0.000 |
| Pronotum width (pw) | 59 | 1.408 | 0.066 | 65 | 1.356 | 0.061 | 0.000 |
| Pronotum length (pl) | 58 | 0.821 | 0.044 | 64 | 0.796 | 0.048 | 0.012 |
| Pronotum length medially (pm) | 59 | 0.629 | 0.030 | 64 | 0.611 | 0.039 | 0.004 |
| Width to length of pronotum medially (pwl) | 59 | 2.240 | 0.086 | 64 | 2.227 | 0.088 | 0.647 |
| Depth of pronotal concavity (pc) | 58 | 0.185 | 0.027 | 64 | 0.178 | 0.024 | 0.109 |
| Length of setae on the pronotum (sp) | 58 | 0.099 | 0.021 | 64 | 0.098 | 0.022 | 0.790 |
| Scutellum width (sw) | 58 | 0.879 | 0.050 | 65 | 0.837 | 0.048 | 0.000 |
| Scutellum length (sl) | 58 | 0.395 | 0.035 | 65 | 0.382 | 0.033 | 0.028 |
| Hemlytra length (lh) | 58 | 0.608 | 0.042 | 64 | 0.578 | 0.045 | 0.000 |
| Hemlytra width (wh) | 58 | 0.931 | 0.059 | 64 | 0.880 | 0.065 | 0.000 |
| Setae on hemlytra (sh) | 58 | 0.104 | 0.029 | 64 | 0.109 | 0.028 | 0.238 |
| Length of 1st femur (fl1) | 54 | 1.121 | 0.048 | 62 | 1.100 | 0.066 | 0.040 |
| Width of 1st femur (fw1) | 54 | 0.369 | 0.022 | 62 | 0.353 | 0.020 | 0.000 |
| Length of 1st tibia (tl1) | 51 | 1.165 | 0.058 | 61 | 1.117 | 0.062 | 0.000 |
| Width of 1st tibia (tw1) | 53 | 0.114 | 0.006 | 61 | 0.110 | 0.007 | 0.000 |
| Length of 2nd femur (fl2) | 54 | 1.175 | 0.055 | 63 | 1.146 | 0.074 | 0.015 |
| Width of 2nd femur (fw2) | 54 | 0.382 | 0.021 | 63 | 0.362 | 0.021 | 0.000 |
| Length of 2nd tibia (tl2) | 50 | 1.256 | 0.077 | 63 | 1.200 | 0.066 | 0.000 |
| Width of 2nd tibia (tw2) | 51 | 0.116 | 0.009 | 63 | 0.110 | 0.007 | 0.000 |
| Length of 3rd femur (fl3) | 55 | 1.339 | 0.060 | 64 | 1.299 | 0.076 | 0.004 |
| Width of 3rd femur (fw3) | 55 | 0.405 | 0.023 | 64 | 0.379 | 0.024 | 0.000 |
| Length to width of 3rd femur (f3lw) | 55 | 3.308 | 0.155 | 64 | 3.428 | 0.162 | 0.000 |
| Length of 3rd tibia (tl3) | 51 | 1.731 | 0.114 | 62 | 1.638 | 0.101 | 0.000 |
| Width of 3rd tibia (tw3) | 51 | 0.122 | 0.009 | 62 | 0.113 | 0.007 | 0.000 |
| Length to width of the head (hl/hw) | 57 | 0.816 | 0.040 | 64 | 0.826 | 0.042 | 0.063 |
| Head width to eye width (hw/ew) | 58 | 7.992 | 0.847 | 65 | 7.941 | 0.738 | 0.859 |
The ratio of length/width of the third femur (F3 l/w) between populations of C. hemipterus was different, and this difference was statistically significant (P< 0.05).
Discussion
Since bed bugs are of medical importance, correct species identification is essential in managing their infestations. This study measured and recorded 46 morphometric indices of collected bed bugs from the east of Iran. Some of these indices were measured and presented in this study for the first time. Although the previous studies have tried to present some morphometric features, the present investigation attempted to provide the most. These data can improve our knowledge of morphological and morphometrical variations in different geographical regions. In addition, these data allow us to track the changes in these indices and speciation over time.
The morphometric studies showed that all bed bugs collected from the east of Iran belonged to the species C. hemipterus, and we found C. lectularius specimens in an archived collection from Mashhad. Cimex lectularius in Iran was reported from northern Iran for the first time by Lindberg in 1938 (16), and there was a record of this species in Tehran in 1950 (17). From then until 2019, C. lectularius was reported from different regions of Iran. In 2019, bed bug samples collected from Tehran and Khorramabad were identified as C. hemipterus based on morphological characteristics and confirmed by molecular assay (4). Since then, this species has become the dominant and prevalent species of the genus Cimex reported from Iran. Bahrami et al. (13) identified collected bed bugs from Ahvaz in southwestern Iran as C. hemipterus based on morphological characters and molecular phylogeny. Cimex hemipterus was reported from northwestern and the west of Iran based on unmentioned morphological criteria and molecular studies (11). Some reports from other regions of the country indicate the presence of C. hemipterus (12, 18). Cimex lectularius was reported from Mashhad in 2019 (19), and in the present study, we could not find this species in the study area, including Mashhad city. It seems that C. hemipterus has become the dominant bed bug species in the study area and areas that have even previously been home to C. lectularius. This phenomenon can be attributed to climatic conditions and tourism development based on more trips to neighboring countries and Southeast Asia, where this species is more common.
The identification of bed bugs at the species level is usually based on morphological characteristics such as pronotum, hind tibial, paragenital sinus, and paramer (12). The pronotum width-to-length ratio is the most influential factor for differentiating C. hemipterus and C. lectularius. Based on our findings C. hemipterus pronotum was less than 2.5 times as wide as long at the middle and more than 2.5 times for C. lectularius. Usinger (14) described the pronotum of C. hemipterus as slightly more than twice as wide as long but 2.5 times as wide as long in C. lectularius. The ratio of width to length of pronotum was reported as 2.1 and 2.1–2.37 in C. hemipterus collected from Malaysia and Russia, respectively (2, 20). This data showed intraspecific variations in pronotum width-to-length ratio among C. hemipterus populations and specimens collected from different locations. Although bed bugs can be identified based on other morphometric criteria or molecular characteristics, calculating the index of width to length of the pronotum is inexpensive, fast, and highly reliable for species identification.
We observed morphometrical variation between the two sexes, and females were the larger sex. According to Adams and Funk (21), sexual body size dimorphism is commonly observed in invertebrate taxa. This size difference is related to abdomen size as an adaptive advantage for greater fecundity in females (22). Blood in-take of adult females of C. hemipterus was reported to be 1.4 to 2.0 times more than adult males and this variation in the blood meal size can affect the body size of individuals (23). Although the body length is traditionally used by some researchers (13, 24, 25), high intra-specific variations were observed in the body length of collected populations. Therefore, it cannot serve as a reliable index for species identification. Body length is highly dependent on the size of the abdomen, and the latter is affected by gender and the amount of blood ingested. The correlation between collection sites and body size was not examined in the present study. However, some researchers concluded that a variation in size between populations can primarily be related to environmental conditions (26).
The fourth segment of the antenna was shorter, and the ratio of intraocular space to eye width was higher compared to C. hemipterus specimens studied by Usinger (14). Although these differences can reflect intraspecific variations in populations studied in two different geographic locations, they may be related to the conditions under which samples were preserved and fixed for morphometric studies. We preserved and fixed collected bed bugs in ethanol 96% while dried samples were used for morphometric analysis by Usinger (14). Considering that many samples were measured in both studies, the idea of intraspecies variation is more likely.
The length/width ratio of the hind femur was one of the important indices calculated in the current study. The length/width ratio of the hind femur in C. hemipterus from India was measured at 3.460 and 3.202 in two studied populations (27). Usinger (14) reported this value as 3.23 for C. hemipterus and 3.33 for C. lectularius. In the present study, this ratio was 3.365 in C. hemipterus, close to those reported in previous studies. It was 4.267 in C. lectularius, almost one unit more than the value reported by Usinger (14). This ratio was calculated at 2.71 in bat bugs by Usinger (14), which showed that the hind femur in C. adjunctus is wider compared to both bed bug species.
Conclusion
The results of this study indicated that the tropical bed bug, C. hemipterus was the dominant bed bug species in the east of Iran. Despite the predominance of C. lectularius in the recent past, this species was not found in the areas we sampled. The current study also provided more morphometric criteria for researchers to identify the two species and to determine the intraspecific variations in each species.
Acknowledgements
We thank Mr H Divangahi for his collaboration in sampling, Dr J Khedri for cooperation in collecting samples from Sistan and Baluchistan, and Dr M Azizzadeh for statistical analysis consultation.
Footnotes
Ethical considerations
Ethical approval is not applicable to this study.
Conflict of interest statement
The authors declare there is no conflict of interests.
References
- 1.Goddard J, Baker GT, Ferrari FG, Ferrari C. (2013) Bed Bugs (Cimex Lectularius) and Bat Bugs (several Cimex species): A Confusing Issue. Outlooks Pest Manag. 23(3): 125–127. [Google Scholar]
- 2.Gapon DA. (2016) First records of the tropical bed bug Cimex hemipterus (Heteroptera: Cimicidae) from Russia. Zoosystematica Ross. 25(2): 239–242. [Google Scholar]
- 3.Bérenger JM, Pluot-Sigwalt D. (2017) Présence en France de la Punaise de lit tropicale, Cimex hemipterus (Fabricius, 1803) (Hemiptera, Heteroptera, Cimicidae). Bull la Société Entomol Fr. 122(4): 423–427. [Google Scholar]
- 4.Hosseini-Chegeni A, Gidiglo G, Khedri J. (2019) The first record of the tropical bed bug, Cimex hemipterus (Hemiptera: Cimicidae) from Iran. Iran J Anim Biosyst. 15(1): 77–86. [Google Scholar]
- 5.Deku G, Combey R, Doggett SL. (2022) Morphometrics of the Tropical Bed Bug (Hemiptera: Cimicidae) From Cape Coast, Ghana. Rust M, editor.. J Med Entomol. 59(5): 1534–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullen GR, Durden LA. (2018) Medical and Veterinary Entomology. 3rd ed. Elsevier Science, London. [Google Scholar]
- 7.Dujardin JP, Slice DE. (2007) Contributions of Morphometrics to Medical Entomology. In: Encyclopedia of Infectious Diseases. John Wiley and Sons, Inc., Hoboken. [Google Scholar]
- 8.Yezerinac SM, Lougheed SC, Handford P. (1992) Measurement error and morphometric studies: Statistical power and observer experience. Syst Biol. 41(4): 471–482. [Google Scholar]
- 9.Cáceres M, Santo-Orihuela PL, Vassena CV. (2019) Evaluation of Resistance to Different Insecticides and Metabolic Detoxification Mechanism by Use of Synergist in the Common Bed Bug (Heteroptera: Cimicidae). J Med Entomol. 56(5): 1324–1330. [DOI] [PubMed] [Google Scholar]
- 10.Csősz S, Seifert B, Mikó I, Boudinot BE, Borowiec ML, Fisher BL, Prebus M, Puniamoorthy J, Rakotonirina JC, Rasoamanana N, Schultz R, Trietsch C, Ulmer JM, Elek Z. (2021) Insect morphometry is reproducible under average investigation standards. Ecol Evol. 11(1): 547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samiei A, Tavassoli M, Mardani K. (2020) Molecular analysis of pyrethroid resistance in Cimex hemipterus (Hemiptera: Cimicidae) collected from different parts of Iran. Vet Res Forum. 11(3): 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghavami MB, Ghahremani Z, Raeisi N, Taghiloo B. (2021) High levels of pyre-throid resistance and super-kdr mutations in the populations of tropical bed bug, Cimex hemipterus, in Iran. Parasit Vectors. 14(1): 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahrami S, Alizadeh I, Pazhoom F, Cork S, O Nzelu C, Alborzi AR. (2020) Phylogenetic and ultrastructural characterization of bed bugs in the southwest of Iran. Available at: www.researchsquare.com/article/rs-112151/v1 [Google Scholar]
- 14.Usinger RL. (1966) Monograph of Cimicidae (Hemiptera-Heteroptera). Entomological Society of America, Washington DC. [Google Scholar]
- 15.Balvín O, Munclinger P, Kratochvíl L, Vilímová J. (2012) Mitochondrial DNA and morphology show independent evolutionary histories of bed bug Cimex lectularius (Heteroptera: Cimicidae) on bats and humans. Parasitol Res. 111(1): 457–469. [DOI] [PubMed] [Google Scholar]
- 16.Ghahari H, Moulet P, Ostovan H. (2016) An annotated catalog of the Iranian Cimicidae and Largidae (Hemiptera: Heteroptera) and in memoriam Carl Walter Schaefer (1934–2015). Zootaxa. 4111(2): 194–200. [DOI] [PubMed] [Google Scholar]
- 17.Hoberlandt L. (1954) Hemiptera Heteroptera from Iran. I. Acta Entomol Musei Natl Pragae. 29: 121–148. [Google Scholar]
- 18.Tiotour M, Shaddel M, Aminianfar M, Mirahmadi H, Barzegar G, Solgi R, Darvishi M. (2022) Identification of Knockdown Resistance Mutations in the Cimex hemipterus (Hemiptera: Cimicidae) in Iran. Am J Trop Med Hyg. 107(1): 204–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berenji F, Moshaverinia A, Jadidoleslami A, Shamsian A, Doggett SL, Moghaddas E. (2019) Evaluation of the Common Bed Bug, Cimex lectularius (Insecta: Hemiptera: Cimicidae) Susceptibility to λ-Cyhalothrin, Malathion, and Diazinon in Northeastern Iran. J Med Entomol. 56(4): 903–906. [DOI] [PubMed] [Google Scholar]
- 20.Lim L, Ab Majid AH. (2021) Characterization of bacterial communities associated with blood-fed and starved tropical bed bugs, Cimex hemipterus (F.) (Hemiptera): a high throughput metabarcoding analysis. Sci Rep. 11(1): 8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams DC, Funk DJ. (1997) Morphometric inferences on sibling species and sexual dimorphism in Neochlamisus bebbianae leaf beetles: multivariate applications of the thin-plate spline. Syst Biol. 46(1): 180–194. [Google Scholar]
- 22.Benítez HA, Briones R, Jerez V. (2011) Intra and inter-population morphological variation of shape and size of the Chilean magnificent beetle, Ceroglossus chilensis in the Baker River Basin, Chilean Patagonia. J Insect Sci. 11: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanovski AD, Ogston CW. (1982) Sex differences in size of the blood meal in the bed bug Cimex hemipterus (Hemiptera: Cimicidae). J Med Entomol. 19(1): 45–47. [DOI] [PubMed] [Google Scholar]
- 24.Kettle DS. (1995) Blood-sucking hemiptera (bugs). In: Kettle DS. (ed): Medical and Veterinary Entomology. International CAB, Wallingford, pp. 344–359. [Google Scholar]
- 25.Golub VB, Aksenenko EV, Soboleva VA, Kornev II. (2020) New data on the distribution of the tropical bed bug Cimex hemipterus and the western conifer seed bug Leptoglossus occidentalis (Heteroptera: Cimicidae, Coreidae) in the European part of Russia. Russ J Biol Invasions. 11(2): 97–100. [Google Scholar]
- 26.Sukhodolskaya RA, Saveliev AA. (2017) Impact of environmental factors on the body shape variation and sexual shape dimorphism in Carabus granulatus L. (Coleoptera: Carabidae). Zool Syst. 42(1): 71–89. [Google Scholar]
- 27.Surendran A, Binoy CF. (2022) Morphological identification and DNA barcoding of the tropical bed bug Cimex hemipterus Fabricius (Hemiptera: Cimicidae) from Thrissur, Kerala, India. Int J Zool Investig. 08(02): 560–567. [Google Scholar]