Abstract
Filamentous fungi are important cell factories for the production of high-value enzymes and chemicals for the food, chemical, and pharmaceutical industries. Under submerged fermentation, filamentous fungi exhibit diverse fungal morphologies that are influenced by environmental factors, which in turn affect the rheological properties and mass transfer of the fermentation system, and ultimately the synthesis of products. In this review, we first summarize the mechanisms of mycelial morphogenesis and then provide an overview of current developments in methods and strategies for morphological regulation, including physicochemical and metabolic engineering approaches. We also anticipate that rapid developments in synthetic biology and genetic manipulation tools will accelerate morphological engineering in the future.
Keywords: Filamentous fungi, morphological engineering, mycelial morphogenesis
Introduction
Filamentous fungi are a group of microorganisms with a highly developed mycelium, characterized by diverse morphology, multinucleation, multicellularity, and high chromosome number compared to bacteria and yeast. Most of them belong to the taxonomic divisions Ascomycota and Basidiomycota, which are found in different natural habitats, but the taxonomic discussion is still ongoing [1, 2]. Currently, filamentous fungi are one of the important expression systems for the production of microbial fermentation products [3], which are widely used in the food, textile, medical, and chemical industries and have great commercial application value [4, 5]. For example, filamentous fungi, especially Ascomycetes and Basidiomycetes, are known to produce an extraordinary range of colors, including several chemical classes of pigments, such as melanins, azaphilones, flavins, phenazines, and quinines [6]. Compared to other conventional sources, industry is more focused on filamentous fungi, which can be easily grown in the laboratory and provide the possibility of large-scale production [7]. Filamentous fungi can secrete a wide variety and large amounts of enzymes, including glycosylases, proteases, phytases, pectinases, and peroxidases [8-10], which can degrade polymers in organic wastes, such as (hemi)cellulose and starch, into small molecules as energy and carbon sources. Thus, filamentous fungi also play an important role in nature by degrading organic wastes [11, 12].
The enormous secretion capacity of fungi has already been used by industry for decades to produce organic acids and polymers, represented by citric acid, polylactic acid, gluconic acid, itaconic acid and lactic acid, as potential alternatives to petroleum-based plastics or raw materials [13]. Of these, citric acid has been commercially produced using Aspergillus niger for almost a century, generating significant economic value [14]. In addition, filamentous fungi are productive strains of some important antibiotics, such as penicillin [15]. Thus, filamentous fungi have wide applications in the food, chemical, agriculture, and pharmaceutical industries, and research on them plays a catalytic role in the emerging global bio-economy, circular economy, and healthcare.
Despite the obvious advantages of working with filamentous fungi, it can sometimes seem difficult to obtain higher production levels. In traditional food fermentation processes, such as wine making, filamentous fungi tend to carry out their metabolic activities in the form of mycelia [16]. In large-scale industrial production, submerged fermentation is typically used to efficiently produce large quantities of product using aerated-stirred bioreactors [17]. However, in submerged fermentation, filamentous fungi are often associated with highly non-Newtonian properties and variations in fungal morphology. Bacterial and yeast growth can be easily described by the duplication of single cells, whereas filamentous fungi undergo a complex morphological evolution from conidia to hyphae to dispersed mycelia or distinct particles [18]. Variations in fungal morphology are not only difficult to detect and control, but are often accompanied by changes in the rheological, mass transfer, and mixing properties of the fermentation broth [19]. This makes the fermentation process complex and difficult to control, which in turn affects the synthesis of the target product and the efficiency of the fermentation process. The dispersed hyphae increase the viscosity of the medium, which affects mass transfer, and nutrient distribution is extremely uneven. The hyphae will wrap around the impellers of the fermentation tank, causing clogging and diffusion into the sampling and overflow lines [20]. However, compared to other microorganisms, apart from the greater difficulty of gene editing and transformation, the main problem seems to be the cultivation process itself. The existence of these problems affects the growth and development as well as the metabolism and product production of filamentous fungi. Therefore, studies on the morphology of filamentous fungi under submerged fermentation are of great importance. This paper first reviews the mechanism of mycelial development in filamentous fungi, and then summarizes the methods for regulating mycelial morphology, in which the importance of morphometabolic breeding for regulating mycelial morphology is outlined in detail.
Mycelial Morphogenesis of Filamentous Fungi
Filamentous fungi are characterized by their ability to form highly polarized hyphae; no other cell type, with the exception of neurons and pollen tubes, exhibits such an extreme degree of polarized growth [21]. Due to their multicellular nature, filamentous fungi maintain a more complex morphology and characteristic chemoattractant mechanisms than unicellular fungi [22]. In recent years, new insights have been gained into the mechanisms of cell growth and morphological development of filamentous fungi, but they are still not well understood [23].
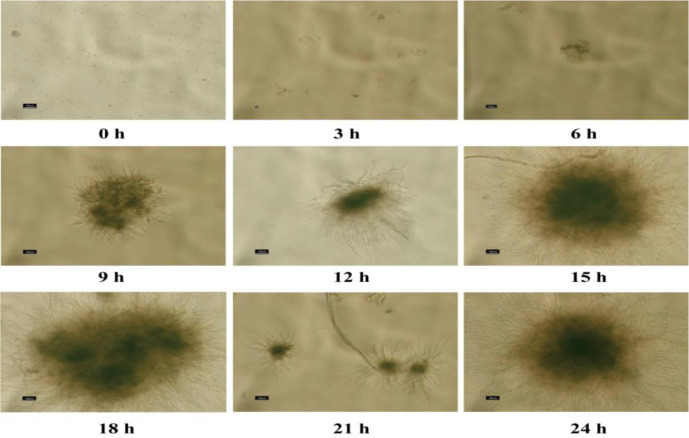
Filamentous fungi reproduce by means of spores, the first stage being spore germination [21]. Under suitable nutrient conditions, the spores break metabolic dormancy, swell, and gradually synthesize cell walls as water enters the cells. Next, myosins and morphogens recruit the actin cytoskeleton at specific sites to establish polarity, which is continuously maintained to produce a highly polarized germ tube that grows into a hypha [24]. The hallmark of hyphae is the growth of mycelial cells by apical extension [25], when proteins associated with the cell membrane, extracellular hydrolases, and cell wall synthesis are 'packaged' into Golgi vesicles and transported along microtubules and the actin cytoskeleton to the tip. The vesicles accumulate at the mid-apical position and bind to the exocyst protein complex, anchoring them to the plasma membrane [26]. Mycelial polarity is mediated by cell-end-marking proteins on the plasma membrane, with the tip inserting into the new membrane by exocytosis and taking up membrane binders and soluble material into the cell by endocytosis. Polar cells extend in an oscillatory manner, with pulses of Ca2+ injection coordinating the cycle of actin polymerization, cytosolic exocytosis, and tip extension. The process of mycelial extension exerts pressure on the newly synthesized cell wall, and this internal pressure is essential for the continued growth of the mycelium [27]. Thus, control of cell wall integrity is a fundamental aspect of mycelial growth and viability. On the other hand, the delivery of cell wall synthetase from the vesicle to the tip must be balanced with the secretion of enzymes for extracellular nutrient acquisition. As growth continues, the mycelium divides across the wall by forming septa. Once a mature hypha has formed, mycelial growth enters the second-phase branching [21]. The mycelium forms branches at the apical or lower insertion zone, and different mycelia are able to fuse together, eventually forming a network of cells called the mycelium. As the mycelium matures, the secondary cell wall gradually thickens and the mycelium reproduces asexually by producing conidia. The process of dispersed spores germinating and gradually forming a pellet under submerged fermentation conditions is shown in Fig. 1.
Fig. 1. The process of spore germination under submerged culture conditions.
While traditional solid-state cultivation has clear benefits in some types of processes, there are many technical limitations when considering industrial applications [28]. On the other hand, deep liquid fermentation is more widely used in industry, and a great deal of research in this area has already been conducted. Compared with solid-state fermentation, submerged fermentation has significant advantages, such as easy scale-up, simple parameter control and processing, short fermentation cycle, and low labor intensity and cost savings. Moreover, this culturing process is becoming a major form of industrial biotechnology for producing target fungal metabolites, such as industrial enzymes, antibiotics, organic acids, and pigments [29, 30]. However, the variable morphology of filamentous fungi under submerged fermentation conditions is one of the main constraints to their productivity. During submerged cultivation in bioreactors, the morphology of filamentous fungi can be one of three morphological forms, namely dispersed mycelia, clumped aggregates, or mycelial pellets (including rough and smooth pellets) [18, 19, 31]. Fungal morphology can be strongly and intricately determined by environmental conditions and inherent molecular or genetic biology. After inoculation of conidia into the liquid medium, they aggregate due to electrostatic, salt bridge or hydrophobic interactions between surface polysaccharides or proteins, resulting in the aggregation of multiple single spores to form a mycosphere [32]. Apparently, pellets can reduce the viscosity of fermentation broth and improve mechanical strength, but slow growth and metabolism in the interior regions of large particles may limit the formation of the target product due to poor oxygen diffusion. In contrast, dispersed mycelium grows rapidly and has no limitations in nutrient transport. Some disadvantages of the dispersed growth state compared to pellets include higher medium viscosity, limited mass heat and momentum transfer between gas and liquid, and increased tolerance to shear stress, which has affected the yield and productivity of target products in bioreactors [33].
Research Progress on Morphological Control of Filamentous Fungi
Physicochemical Methods for Control of the Morphology
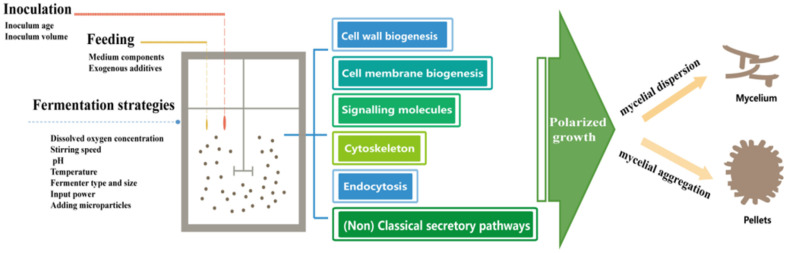
Fungal morphology has been shown to vary with changes in nutrients and environmental conditions during fermentation. Factors that have been reported to influence the morphology of filamentous fungi include inoculum age, inoculum volume, medium components, exogenous additives such as surfactants, dissolved oxygen concentration, stirring speed and stirring paddle shape, pH, temperature, fermenter type and size, and input power (Fig. 2) [5, 34].
Fig. 2. Mechanisms of influence on morphology during submerged fermentation of filamentous fungi.
The results of many studies have shown that morphology is one of the important factors influencing the ability to synthesize products, and the traditional means of regulating morphology include changing the variables in the fermentation environment or adding particle enhancers (Table 1) [35, 36]. Bioreactor configuration also plays a crucial role in fungal morphology, as it affects solid-liquid mixing, aeration, agitation, shear stress, etc. [28]. The design of bioreactors is usually based on the mechanisms of mass transfer and shear stress, as they determine the availability of oxygen, diffusion of nutrients, cellular integrity, etc. [37, 38]. However, the targeted design and processing of equipment is more time-consuming and labor-intensive than engineering the fungal morphology to match the required conditions. Furthermore, bioprocesses that can be more easily adapted to existing infrastructures are more likely to be implemented in large-scale industrial production [28].
Table 1.
Advances in physicochemical methods to regulate the morphology of filamentous fungi.
| Strains | Products | Regulatory parameters | Optimal morphology | Reference |
|---|---|---|---|---|
| A. oryzae | a-Amylase | pH | Filamentous | [39] |
| A. niger | Citric acid | Agitation | Little pellets | [40] |
| A. niger | Protease | Supplement carboxymethylcellulose | Filamentous | [41] |
| A. terreus | Lovastatin | Agitation and aeration | Smaller pellets | [42] |
| A. niger | Glucosamine | Spore inoculum level | Pellets | [35] |
| A. oryzae | Recombinant lipase | Feeding with maltose | Increase in hyphal diameter | [43] |
| A. sojae | Polygalacturonase | Concentrations of maltrin and corn steep liquor, agitation speed and inoculation ratio | The diameter of pellets between 0.05 and 0.76 cm | [44] |
| Rhizopus oryzae | Lactic acid | Addition potato dextrose broth and calcium carbonate | Small smooth pellets | [45] |
| Caldariomyces fumago | Chloroperoxidase | Microparticle-enhanced cultivation | Particles of 42 mm in diameter | [46] |
| Aspergillus sp. | Glucosamine | Pellet size, working volume, agitation rate and stimulating factor | pellet | [47] |
| A. niger | Glucoamylase and fructofuranosidase | Addition of microparticles | Freely dispersed mycelium | [48] |
| A. niger | Glucoamylase | Aeration and agitation intensities | Less compact surface structure of the pellets | [49] |
| A. niger | Fructofuranosidase | Osmolality | Dimensionless morphology | [50] |
| Trichoderma reesei | Cellulase | Agitation | Pellet | [51] |
| A. niger | Fructofuranosidase | Titanate microparticles | Loose inner pellets | [36] |
| A. niger | β-Fructofuranosidase | Addition of talc micro particles | Freely dispersed mycelium | [52] |
| Mortierella isabellina | Lipid | Additions of different concentrations of magnesium silicate microparticles | Free dispersed mycelia | [53] |
| R. oryzae | Fumaric acid | Soybean meal hydrolysate as the nitrogen source | Uniformly dispersed mycelial clumps | [54] |
| A. sojae | β-Mannanase | Addition of talcum and aluminum oxide | Pellet/mycelium mixture | [55] |
| A. niger | Enniatin B | Addition of talcum microparticles | Diameter 50–150 μm | [23] |
| Mucor rouxii | Chitosan | Replacing supplementary nutrients with fungal extract | Purely filamentous | [56] |
| A. terreus | Lovastatin | Addition of magnesium silicate | Small dense pellets | [57] |
| A. niger | Lipase | N:C ratio and FeCl3 | Dispersed fungal morphology | [58] |
A. niger is an excellent host for protease and amylase production, and the effect of morphology on enzyme production has been studied to some extent [5]. The addition of silicate and alumina particles of different sizes and concentrations can also result in many different morphologies of A. niger. Analysis of morphogenetic images during culture showed that the addition of particles disrupted the aggregation of conidia and formed loose mycelium more conducive to enzyme secretion [48]. The addition of talc microparticles at concentrations of 1 g/l, 3 g/l, and 10 g/l prior to inoculation reproducibly produced a variety of morphological structures. The most relaxed morphology was obtained with the addition of 10 g/l talc microparticles and the highest β-fructofuranosidase activity was observed [50]. Different morphologies of the oleaginous fungus Mortierella isabellina were observed by addition of different concentrations of magnesium silicate microparticles. Significantly higher lipid content (0.75 g lipid/g cell biomass) and lipid yield (0.18 g lipid/g glucose consumed) were achieved in freely dispersed mycelia than in pellets, and the by-product malate was suppressed [53]. In addition to the addition of particles, there was also a significant increase in protease activity released by A. niger when the mycelial morphology was broken down from pellet to filamentous during fermentation using carboxymethylcellulose [41]. In addition to the loose morphology being more conducive to the production of some enzymes, oxygen appears to be necessary for enzyme production, but pellets are known to limit the diffusivity of oxygen, substrates, and products. The pure filamentous morphology was found to be more favorable for the production of chitosan during the fermentation of Trichoderma reesei, although the change in morphology did not have a significant effect on the synthesis of ethanol [56].
Compared with the filamentous or flocculent form, the aggregation of spores into pellets during submerged fermentation can effectively reduce the viscosity of the fermentation broth and facilitate separation, which is advantageous for the production of products such as organic acids, fatty acids, and secondary metabolites [32]. Currently, 99% of the world's citric acid is derived from the fermentation of A. niger pellets [5]. A study of the relationship between the morphological development of A. niger and the level of spore inoculation found that at a spore inoculum concentration of 104-105 particles/ml, A. niger can easily form pellets under liquid fermentation conditions, which is more favorable for citric acid production. As the spore concentration increases, the dissolved oxygen concentration in the fermentation broth decreases rapidly, the length and branching frequency of the mycelium increases significantly, the development continues in a dispersed mycelial form, and the production of citric acid decreases significantly [35]. The study found that some nutrients such as soy peptone and calcium carbonate favored the formation of smooth pellets during the culture, whereas the addition of metal ions had a significant negative effect on pellet formation. Adding potato dextrose broth (PDB) and calcium carbonate can significantly increase the yield of organic acids produced by Rhizopus oryzae, as its production of lactic acid and fumaric acid was increased from 32.0 g/l and 21.5 g/l to 65.0 g/l and 31.0 g/l, respectively [45]. Pellet diameter was also found to have a significant effect on cell productivity. The diameter of pellets in fermenters can be up to several millimeters, while dissolved oxygen and other nutrients in the fermentation broth can only penetrate about 200 μm into the surface of the mycelial spheres [20], causing activity within the pellets to decrease dramatically, or even become inactive [35]. In polygalacturonase production by A. sojae, polygalacturonase activity was increased by 74% and biomass by 40% when the diameter of the pellets was between 0.05 and 0.76 cm by controlling the nutrient conditions, stirring speed, and spore concentration [44]. Aspergillus sp. showed the highest yield of biomass and glucosamine at a diameter of 2.15 mm, and an increase or decrease in diameter was detrimental to the synthesis of products [47]. The titanate particles (TiSiO4, 8 mm) could bind tightly to the cells to form core-shell particles. The smaller particles and core-shell structure reduced the thickness of the biomass layer, and the resulting loose internal particle structure also allowed for higher mass transfer and penetration depth, resulting in a 3.7- and 9.5-fold increase in the activity of furanosidase and glucoamylase, respectively, in the growth medium of A. niger with increasing titanate content [36].
Although optimization of fermentation conditions can achieve some effective control of morphology, it is not yet possible to accurately predict the most favorable state for the production of the target product, and therefore requires considerable effort in process design.
Metabolic Engineering of Morphology
In addition to physicochemical conditions, the morphology of filamentous fungi during fermentation is regulated by several genes encoding proteins that alter morphology by controlling cell size, mycelial polarity, or the spacing of intercellular septa (Table 2) [59]. In 2001, Professor Jens Nielsen, an international authority on metabolic engineering, pioneered metabolic engineering of morphology by studying the effect of mycelial aggregation on metabolic pathways and target product synthesis at the molecular level [59, 60]. Comparative genomics is a powerful tool for uncovering gene-level information on superior variants, and with advances in DNA sequencing technology, more fungal genetic information is being annotated and published [61]. This achievement combined with advances in gene editing and RNA technology has accelerated metabolic engineering-based breeding in fungi [62].
Table 2.
Overview of development of metabolic engineering morphology of filamentous fungi.
| Strains | Products | Genotype | Morphology | Reference |
|---|---|---|---|---|
| A. oryzae | Amylase | Disrupt chsB and csmA | higher branch | [60] |
| A. oryzae | -- | Overexpress sclR | extremely branched aerial hyphae | [63] |
| Penicillium chrysogenum | Penicillin | Class III chitin synthase gene silencing | shorter and more branched hyphae | [64] |
| A. niger | Protein | Lacks the gene related with the initiation of asexual sporulation | propagation through hyphae | [65] |
| A. glaucus | Aspergiolide A | ΔAgkipA mutant | pellets | [66] |
| A. niger | Citric acid | chsC gene silencing | pellets | [67] |
| A. oryzae | L-malate | Optimizing the expression of CDC14, CDC20 and CDC45. | pellets | [68] |
| Aspergillus species | -- | Deficient in α-1,3-glucan and galactosaminogalactan | dispersed hyphae | [20] |
In 1999, Bocking et al. used UV and nitrite mutagenesis to obtain a highly branched mycelial mutant strain of A. oryzae with reduced fermentation broth viscosity and increased glycosylase production [69]. Traditionally, fungal morphogenesis and secondary metabolism were thought to be independent, but genome sequencing revealed mutations in the gene encoding the methyltransferase (LaeA) of the low-viscosity Penicillium chrysogenum mutant, which is involved in the synthesis of the heterotrimeric velvet complex in filamentous fungi [70]. Currently, LaeA mutations have been applied to modify various filamentous fungi, which can simultaneously activate natural product synthesis and regulate cell morphology [71]. Through genomic comparative analysis, the high-yield protein variant of A. niger was found to have differences in gene sequences encoding cell wall synthesis compared to the original strain, including cell signaling, chitin synthesis, and β-1,3-glucan synthesis.
Chitin is a major structural component of the cell wall of filamentous fungi, and the final reaction in the chitin biosynthetic pathway is catalyzed by chitin synthase (chs), which has become a high-profile target for the investigation of the factors that influence the morphology, yield, and productivity of the fungus in submerged fermentation [60, 72]. Based on amino acid sequence homology, the chs enzyme family can be divided into seven classes (I to VIl), with different fungal species expressing different numbers of chs genes [73]. By blocking the expression of the gene for chitin synthase B (chsB) in A. oryzae, the apical growth rate of A. oryzae mycelium was 88% slower than that of the wild type, while the frequency of mycelial divergence increased by 188%. In addition, the modified A. oryzae mycelium did not aggregate into clumps during submerged fermentation, which facilitated overall control of the fermentation process, although α-amylase production did not increase [60]. Using RNAi technology to reduce the expression levels of chsC and chs4, genes encoding chitin synthase in A. niger and P. chrysogenum, respectively, to obtain dense hyphae and highly branched morphology, citric acid and penicillin yields were increased by 40% and 27-41%, respectively [64, 67].
In addition to the direct effects of the chs genes, the regulation of chitin biosynthesis is also determined to varying degrees by a number of other factors, including transcription factors, mitogen-activated protein kinase (MAPK), and calcium [74]. Transcriptomics revealed a sophisticated defense system in A. niger that employs at least three transcription factors, including RlmA, MsnA, and CrzA, to protect against cell wall stress [75]. Of these, CrzA is a calcium-signaling transcription factor that can bind to the promoter region of the chs gene, thereby regulating chitin biosynthesis and ultimately affecting mycelial aggregation patterns [76]. Thus, the calcium/calcineurin signaling pathway and the transcription factor CrzA are promising targets for biotechnological manipulation of fungal growth, development, and stress resistance. In T. reesei, deletion of the crzA gene resulted in a hyperbranched phenotype, which was accompanied by increased secretion of haemicellulases [77]. In addition to the calcium signaling pathway, the cAMP/PKA signaling pathway also influences growth and metabolism in filamentous fungi [5]. In this pathway, activation of a GPCR causes an adenylate cyclase to catalyze the conversion of ATP to cAMP, which then activates cAMP-dependent protein kinase A (PKA). Activated PKA phosphorylates various target proteins, including transcription factors, resulting in their entry into the nucleus and modification of gene expression [78]. In A. niger, overexpression of the PKA signaling subunit PkaC resulted in a more compact colony morphology [79]. In addition to overexpression, the ACY1 and PKAC1 genes coordinate light, filamentous growth, and cellulase gene expression in T. reesei, providing a way to simultaneously titrate morphology and cellulase expression [80].
Transcriptomic sequencing techniques (RNA-seq) and microarray gene expression profiling have revealed potential candidate genes for optimizing fungal morphology in different industrial processes. Moreover, comparative analysis of transcriptomic information between high-yielding strains and wild fungi during fermentation revealed significant differences in transcript levels of genes involved in morphology and cell growth, including typical and atypical secretion pathways, cytoskeletal components, endocytosis, cytosolic action, and cell wall and cell membrane biosynthesis [81]. In addition, proteins encoded by more than 2000 genes, including various signaling pathways that drive and control the aforementioned subcellular processes, are involved to some extent in the growth and development of filamentous fungi [82]. These studies suggest that the altered morphology of the fungus may be a result of cellular differentiation, accompanied by a response of the fungus to changes in the cellular microenvironment. Chen et al. controlled the optimal range of the total volume of pellets in a unit volume of fermentation broth (V value) by expressing the cytokinin-related proteins CDC14, CDC20, and CDC45, and controlled the expression intensity of CDC14 for L-malic acid production to 120-130 mm3/ml, so that the yield of L-malic acid was increased to 142.5 g/l in the fermenter [68]. The gul1 gene encodes a putative mRNA-binding protein, and transcriptome analysis revealed that a number of genes encoding cell wall remodeling enzymes and hydrophobins were differentially expressed in the Δgul1 strain [83]. Smaller clumps of mycelium and a significantly lower viscosity of the fermentation broth were observed when gul1 was disrupted in Trichoderma reesei, and cellulase production was improved by 22% compared to the parental strain [83]. A titer of 235.8 g/l L-malic acid was produced by T. reesei Δgul1, which was a significant increase compared to the titer of 170 g/l of the original strain [84].
Perspectives
Filamentous fungi in submerged fermentation conditions are diverse and sensitive to changes in environmental conditions, thus affecting the synthesis of target products and the efficiency of the fermentation process, which is one of the important factors affecting economic efficiency. Therefore, the study of the formation and control of fungal morphology is important for the stable control of the fermentation process and the realization of large-scale production.
The traditional means of controlling morphology, including physicochemical measures such as altering fermentation environment variables or adding particulate enhancers, can control morphological stability to some extent. However, the study of the fermentation process is hampered by the inability to accurately predict the optimal state of cell growth and target product production. While optimized bioreactor design is one way to achieve higher production targets, it is also time-consuming and labor-intensive, so strains and fermentation processes that can be more easily adapted to existing infrastructure are more likely to be implemented in large-scale industrial production. With the advancement of genomics and transcriptomics, the formation mechanism of filamentous fungal morphology under specific fermentation conditions has been intensively studied, and morphology optimization and design at the genetic level has become possible [85]. The genome size of filamentous fungi is several times larger than that of bacteria, the trophic cells are mostly diploid, and the mechanism of nonhomologous recombination makes gene targeting much more difficult, with low transformation efficiency and few available screening markers [86]. It is therefore more difficult to manipulate genes in filamentous fungi than in prokaryotes. The development of synthetic biology over the last few years is expected to overcome existing research bottlenecks [87]. For example, the CRISPR/Cas9 and CRISPRa systems have recently been introduced into filamentous fungi to explore the potential of target genes [88]. From A. oryzae and T. reesei to A. niger and A. nidulans, CRISPR/Cas9-based systems have become versatile platforms for precise genome editing, and great progress has already been made in the production of valuable metabolic products. In addition, fundamental tools for genome minimization have now been developed and are expected to reduce the genome complexity of filamentous fungi [89-91]. The combination of processes such as promoter sequence optimization, multi-copy expression of genes, transcription factors and post-transcriptional modifications gives filamentous fungi great potential in the sustainable bioeconomy.
Although it is becoming possible to control fungal morphology at the molecular level, there are still some issues that need to be considered and resolved in order to take full advantage of the excellent cell factories of filamentous fungi. For example, when using cheap substrate as a carbon source, a loose morphology is more favorable for hydrolytic enzyme secretion, but not necessarily for product synthesis, so how to achieve a balance between the two, or dynamic regulation of morphology? As fermentation conditions change, morphology-related genes are selectively expressed, so how can precise control be achieved at different stages? Also, there are many types of filamentous fungi, including septate and non-septate mycelium, mononuclear and multinuclear cells, so can a universal solution be applied to different types of industrial strains? With more researchers focusing on basic and applied fungal research, these challenges will be overcome and more excellent filamentous fungi will be applied to industrial production.
Acknowledgments
This work was supported by the Youth Foundation of Shandong Natural Science Foundation of China (Grant No. ZR2020QC007); the Innovation Project for Major Application Technology in the Agricultural Sector in Shandong Province (Grant No. SD2019ZZ005); and Special Funds for the Central Management of Scientific and Technological Development at the Local Level (Grant No. YDZX2021070).
Footnotes
Conflict of Interest
The authors have no financial conflicts of interest to declare.
References
- 1.Abarca ML, Accensi F, Cano J, Cabañes FJ. Taxonomy and significance of black aspergilli. Antonie Van Leeuwenhoek. 2004;86:33–49. doi: 10.1023/B:ANTO.0000024907.85688.05. [DOI] [PubMed] [Google Scholar]
- 2.Grimm LH, Kelly S, Krull R, Hempel DC. Morphology and productivity of filamentous fungi. Appl. Microbiol. Biotechnol. 2005;69:375–384. doi: 10.1007/s00253-005-0213-5. [DOI] [PubMed] [Google Scholar]
- 3.Troiano D, Orsat V, Dumont MJ. Status of filamentous fungi in integrated biorefineries. Renew. Sustain. Energ. Rev. 2020;117:109472. doi: 10.1016/j.rser.2019.109472. [DOI] [Google Scholar]
- 4.Meyer V, Andersen MR, Brakhage AA, Braus GH, Caddick MX, Cairns TC, et al. Current challenges of research on filamentous fungi in relation to human welfare and a sustainable bio-economy: a white paper. Fungal Biol. Biotechnol. 2016;3:6. doi: 10.1186/s40694-016-0024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairns TC, Zheng X, Zheng P, Sun J, Meyer V. Moulding the mould: understanding and reprogramming filamentous fungal growth and morphogenesis for next generation cell factories. Biotechnol. Biofuels. 2019;12:77. doi: 10.1186/s13068-019-1400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palacio-Barrera AM, Areiza D, Zapata P, Atehortúa L, Correa C, Peñuela-Vásquez M. Induction of pigment production through media composition, abiotic and biotic factors in two filamentous fungi. Biotechnol. Rep. (Amst) 2019;21:e00308. doi: 10.1016/j.btre.2019.e00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalra R, Conlan XA, Goel M. Fungi as a potential source of pigments: Harnessing filamentous fungi. Front. Chem. 2020;8:00369. doi: 10.3389/fchem.2020.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang T, Liu H, Lv B, Li C. Regulating strategies for producing carbohydrate active enzymes by filamentous fungal cell factories. Front. Bioeng. Biotechnol. 2020;8:691. doi: 10.3389/fbioe.2020.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward OP. Production of recombinant proteins by filamentous fungi. Biotechnol. Adv. 2012;30:1119–1139. doi: 10.1016/j.biotechadv.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Liu G, Qu Y. Engineering of filamentous fungi for efficient conversion of lignocellulose: tools, recent advances and prospects. Biotechnol. Adv. 2019;37:519–529. doi: 10.1016/j.biotechadv.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Wösten HAB. Filamentous fungi for the production of enzymes, chemicals and materials. Curr. Opin. Biotechnol. 2019;59:65–70. doi: 10.1016/j.copbio.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Baron NC, Rigobelo EC, Zied DC. Filamentous fungi in biological control: current status and future perspectives. Chilean J. Agric. Res. 2019;79:307–315. doi: 10.4067/S0718-58392019000200307. [DOI] [Google Scholar]
- 13.Alberti F, Foster GD, Bailey AM. Natural products from filamentous fungi and production by heterologous expression. Appl. Microbiol. Biotechnol. 2017;101:493–500. doi: 10.1007/s00253-016-8034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cairns TC, Nai C, Meyer V. How a fungus shapes biotechnology: 100 years of Aspergillus niger research. Fungal Biol. Biotechnol. 2018;5:13. doi: 10.1186/s40694-018-0054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu JH, Keller N. Regulation of secondary metabolism in filamentous fungi. Ann. Rev. Phytopathol. 2005;43:437–458. doi: 10.1146/annurev.phyto.43.040204.140214. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa M, Bisson LF, García-Martínez T, Mauricio JC, Moreno-García J. New insights on yeast and filamentous fungus adhesion in a natural co-immobilization system: proposed advances and applications in wine industry. Appl. Microbiol. Biotechnol. 2019;103:4723–4731. doi: 10.1007/s00253-019-09870-4. [DOI] [PubMed] [Google Scholar]
- 17.González-Sáiz JM, Garrido-Vidal D, Pizarro C. Scale up and design of processes in aerated-stirred fermenters for the industrial production of vinegar. J. Food Eng. 2009;93:89–100. doi: 10.1016/j.jfoodeng.2009.01.002. [DOI] [Google Scholar]
- 18.Kossen NWF. The Morphology of Filamentous Fungi, In History of Modern Biotechnology II. Ed. Springer Berlin Heidelberg; 2000. pp. 1–33. [Google Scholar]
- 19.Cairns TC, Feurstein C, Zheng X, Zheng P, Sun J, Meyer V. A quantitative image analysis pipeline for the characterization of filamentous fungal morphologies as a tool to uncover targets for morphology engineering: a case study using aplD in Aspergillus niger. Biotechnol. Biofuels. 2019;12:149. doi: 10.1186/s13068-019-1473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazawa K, Yoshimi A, Abe K. The mechanisms of hyphal pellet formation mediated by polysaccharides, α-1,3-glucan and galactosaminogalactan, in Aspergillus species. Fungal Biol. Biotechnol. 2020;7:10. doi: 10.1186/s40694-020-00101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris SD. Cell Polarity in Filamentous Fungi: Shaping the Mold, International Review of Cytology. Ed. Academic Press; 2006. pp. 41–77. [DOI] [PubMed] [Google Scholar]
- 22.Lichius A, Lord KM. Chemoattractive Mechanisms in Filamentous Fungi. Open Mycol. J.. 2014;8:28–57. doi: 10.2174/1874437001408010028. [DOI] [Google Scholar]
- 23.Kurt T, Marbà-Ardébol AM, Turan Z, Neubauer P, Junne S, Meyer V. Rocking Aspergillus: morphology-controlled cultivation of Aspergillus niger in a wave-mixed bioreactor for the production of secondary metabolites. Microb. Cell Fact. 2018;17:128. doi: 10.1186/s12934-018-0975-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riquelme M, Aguirre J, Bartnicki-García S, Braus GH, Feldbrügge M, Fleig U, et al. Fungal morphogenesis, from the polarized growth of hyphae to complex reproduction and infection structures. Microbiol. Mol. Biol. Rev. 2018;82:00068–00017. doi: 10.1128/MMBR.00068-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riquelme M. Tip growth in filamentous fungi: A road trip to the Apex. Ann. Rev. Microbiol. 2013;67:587–609. doi: 10.1146/annurev-micro-092412-155652. [DOI] [PubMed] [Google Scholar]
- 26.Steinberg G, Peñalva MA, Riquelme M, Wösten HA, Harris SD. Cell biology of hyphal growth. Microbiol. Spectrum. 2017;5:0034. doi: 10.1128/microbiolspec.FUNK-0034-2016. [DOI] [PubMed] [Google Scholar]
- 27.Meyer V, Arentshorst M, Flitter SJ, Nitsche BM, Kwon MJ, Reynaga-Peña CG, et al. Reconstruction of signaling networks regulating fungal morphogenesis by transcriptomics. Eukaryot. Cell. 2009;8:1677–1691. doi: 10.1128/EC.00050-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomes DG, Coelho E, Silva R, Domingues L, Teixeira JA. In: Current Developments in Biotechnology and Bioengineering. Taherzadeh MJ, Ferreira JA, Pandey A, editors. Ed. Elsevier; 2023. 8 - Bioreactors and engineering of filamentous fungi cultivation; pp. 219–250. [Google Scholar]
- 29.Liu J, Guo T, Luo Y, Chai X, Wu J, Zhao W, et al. Enhancement of monascus pigment productivity via a simultaneous fermentation process and separation system using immobilized-cell fermentation. Bioresour. Technol. 2019;272:552–560. doi: 10.1016/j.biortech.2018.10.072. [DOI] [PubMed] [Google Scholar]
- 30.Gong Z, Zhang S, Liu J. Recent advances in chitin biosynthesis associated with the morphology and secondary metabolite synthesis of filamentous fungi in submerged fermentation. J. Fungi. 2023;9:205. doi: 10.3390/jof9020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papagianni M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv. 2004;22:189–259. doi: 10.1016/j.biotechadv.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Zhang J. The filamentous fungal pellet and forces driving its formation. Crit. Rev. Biotechnol. 2016;36:1066–1077. doi: 10.3109/07388551.2015.1084262. [DOI] [PubMed] [Google Scholar]
- 33.Veiter L, Rajamanickam V, Herwig C. The filamentous fungal pellet-relationship between morphology and productivity. Appl. Microbiol. Biotechnol. 2018;102:2997–3006. doi: 10.1007/s00253-018-8818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krull R, Wucherpfennig T, Esfandabadi ME, Walisko R, Melzer G, Hempel DC, et al. Characterization and control of fungal morphology for improved production performance in biotechnology. J. Biotechnol. 2013;163:112–123. doi: 10.1016/j.jbiotec.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 35.Papagianni M, Mattey M. Morphological development of Aspergillus niger in submerged citric acid fermentation as a function of the spore inoculum level. Application of neural network and cluster analysis for characterization of mycelial morphology. Microb. Cell Fact. 2006;5:3. doi: 10.1186/1475-2859-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Driouch H, Hänsch R, Wucherpfennig T, Krull R, Wittmann C. Improved enzyme production by bio-pellets of Aspergillus niger: targeted morphology engineering using titanate microparticles. Biotechnol. Bioeng. 2012;109:462–471. doi: 10.1002/bit.23313. [DOI] [PubMed] [Google Scholar]
- 37.Mantzouridou F, Roukas T, Kotzekidou P. Effect of the aeration rate and agitation speed on β-carotene production and morphology of Blakeslea trispora in a stirred tank reactor: mathematical modeling. Biochem. Eng. J. 2002;10:123–135. doi: 10.1016/S1369-703X(01)00166-8. [DOI] [Google Scholar]
- 38.Lan T-Q, Wei D, Yang S-T, Liu X. Enhanced cellulase production by Trichoderma viride in a rotating fibrous bed bioreactor. Bioresour. Technol. 2013;133:175–182. doi: 10.1016/j.biortech.2013.01.088. [DOI] [PubMed] [Google Scholar]
- 39.Carlsen M, Spohr AB, Nielsen J, Villadsen J. Morphology and physiology of an α-amylase producing strain of Aspergillus oryzae during batch cultivations. Biotechnol. Bioeng. 1996;49:266–276. doi: 10.1002/(SICI)1097-0290(19960205)49:3<266::AID-BIT4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 40.Paul GC, Priede MA, Thomas CR. Relationship between morphology and citric acid production in submerged Aspergillus niger fermentations. Biochem. Eng. J. 1999;3:121–129. doi: 10.1016/S1369-703X(99)00012-1. [DOI] [Google Scholar]
- 41.Ahamed A, Singh A, Ward OP. Culture-based strategies for reduction of protease activity in filtrates from Aspergillus niger NRRL-3. World J. Microbiol. Biotechnol. 2005;21:1577–1583. doi: 10.1007/s11274-005-8121-5. [DOI] [Google Scholar]
- 42.Casas López JL, Sánchez Pérez JA, Fernández Sevilla JM, Rodríguez Porcel EM, Chisti Y. Pellet morphology, culture rheology and lovastatin production in cultures of Aspergillus terreus. J. Biotechnol. 2005;116:61–77. doi: 10.1016/j.jbiotec.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Haack MB, Olsson L, Hansen K, Eliasson Lantz A. Change in hyphal morphology of Aspergillus oryzae during fed-batch cultivation. Appl. Microbiol. Biotechnol. 2006;70:482–487. doi: 10.1007/s00253-005-0085-8. [DOI] [PubMed] [Google Scholar]
- 44.Tari C, Gögus N, Tokatli F. Optimization of biomass, pellet size and polygalacturonase production by Aspergillus sojae ATCC 20235 using response surface methodology. Enzyme Microb. Technol. 2007;40:1108–1116. doi: 10.1016/j.enzmictec.2006.08.016. [DOI] [Google Scholar]
- 45.Liao W, Liu Y, Chen S. Studying pellet formation of a filamentous fungus Rhizopus oryzae to enhance organic acid production. Appl. Biochem. Biotechnol. 2007;137:689–701. doi: 10.1007/978-1-60327-181-3_56. [DOI] [PubMed] [Google Scholar]
- 46.Kaup BA, Ehrich K, Pescheck M, Schrader J. Microparticle-enhanced cultivation of filamentous microorganisms: Increased chloroperoxidase formation by Caldariomyces fumago as an example. Biotechnol. Bioeng. 2008;99:491–498. doi: 10.1002/bit.21713. [DOI] [PubMed] [Google Scholar]
- 47.Sitanggang AB, Wu HS, Wang SS, Ho YC. Effect of pellet size and stimulating factor on the glucosamine production using Aspergillus sp. BCRC 31742. Bioresour. Technol. 2010;101:3595–3601. doi: 10.1016/j.biortech.2009.12.084. [DOI] [PubMed] [Google Scholar]
- 48.Driouch H, Sommer B, Wittmann C. Morphology engineering of Aspergillus niger for improved enzyme production. Biotechnol. Bioeng. 2010;105:1058–1068. doi: 10.1002/bit.22614. [DOI] [PubMed] [Google Scholar]
- 49.Lin PJ, Scholz A, Krull R. Effect of volumetric power input by aeration and agitation on pellet morphology and product formation of Aspergillus niger. Biochem. Eng. J. 2010;49:213–220. doi: 10.1016/j.bej.2009.12.016. [DOI] [Google Scholar]
- 50.Wucherpfennig T, Hestler T, Krull R. Morphology engineering - Osmolality and its effect on Aspergillus niger morphology and productivity. Microb. Cell Fact. 2011;10:58. doi: 10.1186/1475-2859-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu L, Chao Y, Wensel P, Chen S. Hydrodynamic and kinetic study of cellulase production by Trichoderma reesei with pellet morphology. Biotechnol. Bioeng. 2012;109:1755–1768. doi: 10.1002/bit.24433. [DOI] [PubMed] [Google Scholar]
- 52.Wucherpfennig T, Lakowitz A, Driouch H, Krull R, Wittmann C. Customization of Aspergillus niger morphology through ddition of Talc micro particles. J. Vis. Exp. 2012;15:4023. doi: 10.3791/4023-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao D, Zeng J, Yu X, Dong T, Chen S. Improved lipid accumulation by morphology engineering of oleaginous fungus Mortierella isabellina. Biotechnol. Bioeng. 2014;111:1758–1766. doi: 10.1002/bit.25242. [DOI] [PubMed] [Google Scholar]
- 54.Zhang K, Yu C, Yang ST. Effects of soybean meal hydrolysate as the nitrogen source on seed culture morphology and fumaric acid production by Rhizopus oryzae. Process Biochem. 2015;50:173–179. doi: 10.1016/j.procbio.2014.12.015. [DOI] [Google Scholar]
- 55.Yatmaz E, Karahalil E, Germec M, Ilgin M, Turhan İ. Controlling filamentous fungi morphology with microparticles to enhanced β-mannanase production. Bioprocess Biosyst. Eng. 2016;39:1391–1399. doi: 10.1007/s00449-016-1615-8. [DOI] [PubMed] [Google Scholar]
- 56.Abasian L, Shafiei Alavijeh R, Satari B, Karimi K. Sustainable and effective chitosan production by dimorphic fungus Mucor rouxii via replacing yeast extract with fungal extract. Appl. Biochem. Biotechnol. 2020;191:666–678. doi: 10.1007/s12010-019-03220-w. [DOI] [PubMed] [Google Scholar]
- 57.Saberi A, Jalili H, Nikfarjam A, Koohsorkhi J, Jarmoshti J, Bizukojc M. Monitoring of Aspergillus terreus morphology for the lovastatin production in submerge culture by impedimetry. Biochem. Eng. J. 2020;159:107615. doi: 10.1016/j.bej.2020.107615. [DOI] [Google Scholar]
- 58.Salvatierra HN, Regner EL, Baigorí MD, Pera LM. Orchestration an extracellular lipase production from Aspergillus niger MYA 135: biomass morphology and fungal physiology. AMB Express. 2021;11:42. doi: 10.1186/s13568-021-01202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McIntyre M, Müller C, Dynesen J, Nielsen J. In Metabolic Engineering. Ed. Springer Berlin Heidelberg; Berlin, Germany: 2001. Metabolic engineering of the morphology of Aspergillus; pp. 103–128. [DOI] [PubMed] [Google Scholar]
- 60.Müller C, McIntyre M, Hansen K, Nielsen J. Metabolic engineering of the morphology of Aspergillus oryzae by altering chitin synthesis. Appl. Environ. Microbiol. 2002;68:1827–1836. doi: 10.1128/AEM.68.4.1827-1836.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weld RJ, Plummer KM, Carpenter MA, Ridgway HJ. Approaches to functional genomics in filamentous fungi. Cell Res. 2006;16:31–44. doi: 10.1038/sj.cr.7310006. [DOI] [PubMed] [Google Scholar]
- 62.Wang S, Chen H, Tang X, Zhang H, Chen W, Chen YQ. Molecular tools for gene manipulation in filamentous fungi. Appl. Microbiol. Biotechnol. 2017;101:8063–8075. doi: 10.1007/s00253-017-8486-z. [DOI] [PubMed] [Google Scholar]
- 63.Jin FJ, Takahashi T, Matsushima K-i, Hara S, Shinohara Y, Maruyama J-i, et al. SclR, a basic helix-loop-helix transcription factor, regulates hyphal morphology and promotes sclerotial formation in Aspergillus oryzae. Eukaryot. Cell. 2011;10:945–955. doi: 10.1128/EC.00013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu H, Zheng Z, Wang P, Gong G, Wang L, Zhao G. Morphological changes induced by class III chitin synthase gene silencing could enhance penicillin production of Penicillium chrysogenum. Appl. Microbiol. Biotechnol. 2013;97:3363–3372. doi: 10.1007/s00253-012-4581-3. [DOI] [PubMed] [Google Scholar]
- 65.Yin C, Wang B, He P, Lin Y, Pan L. Genomic analysis of the aconidial and high-performance protein producer, industrially relevant Aspergillus niger SH2 strain. Gene. 2014;541:107–114. doi: 10.1016/j.gene.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 66.Cai M, Zhang Y, Hu W, Shen W, Yu Z, Zhou W, et al. Genetically shaping morphology of the filamentous fungus Aspergillus glaucus for production of antitumor polyketide aspergiolide A. Microb. Cell Fact. 2014;13:73. doi: 10.1186/1475-2859-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun X, Wu H, Zhao G, Li Z, Wu X, Liu H, et al. Morphological regulation of Aspergillus niger to improve citric acid production by chsC gene silencing. Bioprocess Biosyst. Eng. 2018;41:1029–1038. doi: 10.1007/s00449-018-1932-1. [DOI] [PubMed] [Google Scholar]
- 68.Chen X, Zhou J, Ding Q, Luo Q, Liu L. Morphology engineering of Aspergillus oryzae for l-malate production. Biotechnol. Bioeng. 2019;116:2662–2673. doi: 10.1002/bit.27089. [DOI] [PubMed] [Google Scholar]
- 69.Bocking SP, Wiebe MG, Robson GD, Hansen K, Christiansen LH, Trinci APJ. Effect of branch frequency in Aspergillus oryzae on protein secretion and culture viscosity. Biotechnol. Bioeng. 1999;65:638–648. doi: 10.1002/(SICI)1097-0290(19991220)65:6<638::AID-BIT4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 70.Bayram Ö, Krappmann S, Ni M, Bok JW, Helmstaedt K, Valerius O, et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science. 2008;320:1504–1506. doi: 10.1126/science.1155888. [DOI] [PubMed] [Google Scholar]
- 71.Terfehr D, Dahlmann TA, Kück U. Transcriptome analysis of the two unrelated fungal β-lactam producers Acremonium chrysogenum and Penicillium chrysogenum: Velvet-regulated genes are major targets during conventional strain improvement programs. BMC Genomics. 2017;18:272. doi: 10.1186/s12864-017-3663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gong Z, Zhang S, Liu J. Recent advances in chitin biosynthesis associated with the morphology and secondary metabolite synthesis of filamentous fungi in submerged fermentation. J. Fungi. 2023;9:205. doi: 10.3390/jof9020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang J, Jiang H, Du Y, Keyhani NO, Xia Y, Jin K. Members of chitin synthase family in Metarhizium acridum differentially affect fungal growth, stress tolerances, cell wall integrity and virulence. PLoS Pathog. 2019;15:e1007964. doi: 10.1371/journal.ppat.1007964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takeshita N. Control of actin and calcium for chitin synthase delivery to the hyphal tip of Aspergillus. Fungal Cell Wall. 2019;193:113–129. doi: 10.1007/82_2019_193. [DOI] [PubMed] [Google Scholar]
- 75.Fiedler MRM, Lorenz A, Nitsche BM, van den Hondel CA, Ram AFJ, Meyer V. The capacity of Aspergillus niger to sense and respond to cell wall stress requires at least three transcription factors: RlmA, MsnA and CrzA. Fungal Biol. Biotechnol. 2014;1:5. doi: 10.1186/s40694-014-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spielvogel A, Findon H, Arst Herbert N, Jr, Araújo-Bazán L, Hernández-Ortíz P, Stahl U, et al. Two zinc finger transcription factors, CrzA and SltA, are involved in cation homoeostasis and detoxification in Aspergillus nidulans. Biochem. J. 2008;414:419–429. doi: 10.1042/BJ20080344. [DOI] [PubMed] [Google Scholar]
- 77.Chen L, Zou G, Wang J, Wang J, Liu R, Jiang Y, et al. Characterization of the Ca2+-responsive signaling pathway in regulating the expression and secretion of cellulases in Trichoderma reesei Rut-C30. Mol. Microbiol. 2016;100:560–575. doi: 10.1111/mmi.13334. [DOI] [PubMed] [Google Scholar]
- 78.Tag A, Hicks J, Garifullina G, Ake C, Jr, Phillips TD, Beremand M, et al. G-protein signalling mediates differential production of toxic secondary metabolites. Mol. Microbiol. 2000;38:658–665. doi: 10.1046/j.1365-2958.2000.02166.x. [DOI] [PubMed] [Google Scholar]
- 79.Bencina M, Panneman H, Ruijter GJG, Legiša M, Visser J. Characterization and overexpression of the Aspergillus niger gene encoding the cAMP-dependent protein kinase catalytic subunit. Microbiology (Reading, England) 1997;143(Pt 4):1211–1220. doi: 10.1099/00221287-143-4-1211. [DOI] [PubMed] [Google Scholar]
- 80.Schuster A, Tisch D, Seidl-Seiboth V, Kubicek CP, Schmoll M. Roles of protein kinase A and adenylate cyclase in lightmodulated cellulase regulation in Trichoderma reesei. Appl. Environ. Microbiol. 2012;78:2168–2178. doi: 10.1128/AEM.06959-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yin X, Shin H-d, Li J, Du G, Liu L, Chen J. Comparative genomics and transcriptome analysis of Aspergillus niger and metabolic engineering for citrate production. Sci. Rep. 2017;7:41040. doi: 10.1038/srep41040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van den Berg MA, Albang R, Albermann K, Badger JH, Daran J-M, M Driessen AJ, et al. Genome sequencing and analysis of the filamentous fungus Penicillium chrysogenum. Nat. Biotechnol. 2008;26:1161–1168. doi: 10.1038/nbt.1498. [DOI] [PubMed] [Google Scholar]
- 83.Zhao Q, Liu Q, Wang Q, Qin Y, Zhong Y, Gao L, Liu G, Qu Y. Disruption of the Trichoderma reesei gul1 gene stimulates hyphal branching and reduces broth viscosity in cellulase production. J. Ind. Microbiol. Biotechnol. 2021;48:kuab012. doi: 10.1093/jimb/kuab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen Y, Wang J, Wang M, Han A, Zhao X, Wang W, Wei D. Engineering the metabolism and morphology of the filamentous fungus Trichoderma reesei for efficient L-malic acid production. Bioresour. Technol. 2023;387:129629. doi: 10.1016/j.biortech.2023.129629. [DOI] [PubMed] [Google Scholar]
- 85.Carrillo AJ, Cabrera IE, Spasojevic MJ, Schacht P, Stajich JE, Borkovich KA. Clustering analysis of large-scale phenotypic data in the model filamentous fungus Neurospora crassa. BMC Genomics. 2020;21:755. doi: 10.1186/s12864-020-07131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, Wang L, Wu F, Liu F, Wang Q, Zhang X, et al. A Consensus ochratoxin A biosynthetic pathway: Insights from the genome sequence of Aspergillus ochraceus and a comparative genomic analysis. Appl. Environ. Microbiol. 2018;84:e01009–01018. doi: 10.1128/AEM.01009-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mózsik L, Pohl C, Meyer V, Bovenberg RAL, Nygård Y, Driessen AJM. Modular synthetic biology toolkit for filamentous fungi. ACS Synt. Biol. 2021;10:2850–2861. doi: 10.1021/acssynbio.1c00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang C, Lv G, Tu Y, Cheng X, Duan Y, Zeng B, et al. Applications of CRISPR/Cas9 in the synthesis of secondary metabolites in filamentous fungi. Front. Microbiol. 2021;12:638096. doi: 10.3389/fmicb.2021.638096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shao Y, Lu N, Wu Z, Cai C, Wang S, Zhang L-L, et al. Creating a functional single-chromosome yeast. Nature. 2018;560:331–335. doi: 10.1038/s41586-018-0382-x. [DOI] [PubMed] [Google Scholar]
- 90.Luo J, Sun X, Cormack BP, Boeke JD. Karyotype engineering by chromosome fusion leads to reproductive isolation in yeast. Nature. 2018;560:392–396. doi: 10.1038/s41586-018-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng X, Zheng P, Zhang K, Cairns TC, Meyer V, Sun J, et al. 5S rRNA Promoter for guide RNA expression enabled highly efficient CRISPR/Cas9 genome editing in Aspergillus niger. ACS Synt. Biol. 2019;8:1568–1574. doi: 10.1021/acssynbio.7b00456. [DOI] [PubMed] [Google Scholar]