Highlights
-
•
Organ motion and volumetric changes in locally advanced cervical cancer (LACC) pose challenges in radiotherapy (RT).
-
•
Magnetic Resonance-guided Radiotherapy (MRgRT) with adaptive plans addresses these challenges effectively.
-
•
Mobility of cervical uterine structures varies during RT, suggesting reduced PTV margins after the third week.
-
•
Adaptive MRgRT optimizes dose delivery, highlighting streamlined IGRT with reduced PTV margins using a tailored MRgRT workflow.
Keywords: Locally advanced cervical cancer, Magnetic resonance guided radiation therapy, Organ motion, Adaptive radiotherapy
Abstract
Introduction
Organ motion (OM) and volumetric changes pose challenges in radiotherapy (RT) for locally advanced cervical cancer (LACC). Magnetic Resonance-guided Radiotherapy (MRgRT) combines improved MRI contrast with adaptive RT plans for daily anatomical changes. Our goal was to analyze cervico-uterine structure (CUS) changes during RT to develop strategies for managing OM.
Materials and methods
LACC patients received chemoradiation by MRIdian system with a simultaneous integrated boost (SIB) protocol. Prescription doses of 55–50.6 Gy at PTV1 and 45–39.6 Gy at PTV2 were given in 22 and 25 fractions. Daily MRI scans were co-registered with planning scans and CUS changes were assessed.
Six PTVs were created by adding 0.5, 0.7, 1, 1.3, 1.5, and 2 cm margins to the CUS, based on the simulation MRI. Adequate margins were determined to include 95 % of the CUSs throughout the entire treatment in 95 % of patients.
Results
Analysis of 15 LACC patients and 372 MR scans showed a 31 % median CUS volume decrease. Asymmetric margins of 2 cm cranially, 0.5 cm caudally, 1.5 cm posteriorly, 2 cm anteriorly, and 1.5 cm on both sides were optimal for PTV, adapting to CUS variations. Post-14th fraction, smaller margins of 0.7 cm cranially, 0.5 cm caudally, 1.3 cm posteriorly, 1.3 cm anteriorly, and 1.3 cm on both sides sufficed.
Conclusion
CUS mobility varies during RT, suggesting reduced PTV margins after the third week. MRgRT with adaptive strategies optimizes dose delivery, emphasizing the importance of streamlined IGRT with reduced PTV margins using a tailored MRgRT workflow with hybrid MRI-guided systems.
1. Introduction
Cervical cancer (CC) is the fourth most common cancer in women worldwide [1], [2].
The recommended treatment for patients with locally advanced cervical cancer (LACC) is represented by chemoradiotherapy (CRT), administered with weekly cisplatin, followed by magnetic resonance imaging (MRI) −guided brachytherapy (BRT) which resulted in improved disease control rates and overall survival [3], [4], [5].
The introduction of intensity-modulated radiotherapy (IMRT) allows for high-precision conformal dose distributions that provide adequate dose coverage to the clinical target volume (CTV) while sparing normal tissue [6].
However, highly conformal irradiation techniques are susceptible to intra- and inter-fraction organ motion, requiring accurate image guidance technique (IGRT) and an adequate planning target volume (PTV) margin depending on the anatomical site [7], [8], [9].
As it is well known that movement of cervix and uterus can occur in all directions in particular in the superior-inferior and anterior-posterior directions and can be influenced by rectal and bladder filling and tumour regression or shrinkage during CRT, which can significantly influence the position of the target and surrounding organs at risk (OARs) [8], [10], [11], [12], [13].
Therefore, the intra-fraction motion management during radiotherapy (RT) still remains a challenge as it may result in both under-dosing the clinical target volume (CTV) and an unnecessary dose to OARs [14].
In this scenario, it is essential to establish adequate margins from CTV to PTV and adopt effective IGRT strategies is mandatory to ensure adequate target coverage and to avoid early and late toxicity resulting from unnecessary OARs irradiation.
Previous consensus guidelines recommend a CTV to PTV expansion of 1.5–2.0 cm [15] but different margin expansions have been investigated in literature, depending on IGRT adopted and institutional quality assurance.
The cone beam computed tomography (CBCT) is the most widely used method of IGRT; the use of daily CBCT has made possible the reduction of the CTV to PTV margins because it allows online corrections of the patient’s set-up and offline image-guided adaptive RT (ART) strategies, such as library plan selection or replanning, to overcome uncertainty due to changing volumes of irradiated structures [16], [17], [18], [19], [20].
For the diagnosis and optimisation of RT for gynaecological cancers [21], MRI is becoming increasingly important. Due to its superior soft tissue contrast compared to computed tomography (CT), MRI has become the imaging modality of choice for the management of cervical cancer patients [22].
The introduction of magnetic resonance-guided RT (MRgRT) for LACC represents a real breakthrough in the treatment of gynaecological malignancies. It allows physicians to deliver online adaptive RT (ART), based on the anatomy of the day, and to monitor anatomical changes during treatment. Furthermore, online ART permits CTV to PTV margins reduction [23] thanks to the possibility to visualize daily the position of the CTV and OARs [24].
The primary aim of this study was to retrospectively assess the amount of the cervical uterine structure regression and inter-fraction motion, and how this might help to define appropriate margins for the treatment of LACC.
2. Methods and materials
2.1. Clinical and treatment characteristics
Women diagnosed with LACC undergoing MRgRT were retrospectively evaluated. Inclusion criteria were histological confirmation, age over 18 years, eligibility for MRgRT CRT treatment, and ability to sign a specific informed consent.
All patients underwent a clinical gynaecological examination, pelvic MRI and total body contrasted enhanced CT scan as staging imaging. 18F-FDG PET-CT was considered and performed only in selected cases.
Therapeutic indication for each patient was defined by the Institutional multidisciplinary tumour board (MTB), composed of radiation oncologists, gynaecologist oncologists, surgeons, radiologists, and pathologists. Patients were treated with radical or, in a selected case, neoadjuvant intent, given the extensive surgical expertise at our institution[25].
All patients underwent a 0.35 T MRI simulation on the hybrid Linac MRIdian system (ViewRay Inc, Mountain View, US). Patients were immobilized in supine position, using the Fluxboard device (MacroMedics, the Netherlands)[26]. An MR scan was acquired using true fast imaging (TRUFI) with steady-state procession sequences, with an acquisition time of 175 s and an image contrast balanced in T2*/T1. After approximately 15 min, a simulation CT was acquired in the same position to provide electron densities for dose calculation. To minimize organ motion, the patients were instructed to perform an enema two hours before each treatment and to fill their bladder by drinking 500 mL of water 30 min before the procedure [27].
Patients were treated with a simultaneous integrated boost (SIB) 2 volumes protocol [25], [28]: 22 fractions with a total dose of 50.6 Gy to PTV1 and 39.6 Gy to PTV2 or in 25 fractions with a total dose of 55 Gy to PTV1 and 45 Gy to PTV2.
CTV1 was obtained by delineating the GTV based on diagnostic MRI without adding margins. CTV2 included the CTV1, the entire cervical-uterine structure, parametria, the vagina (the upper half, the upper two/thirds, or entirely, depending on vaginal involvement) [15], the internal, external, obturator, presacral iliac lymph nodes to the third sacral vertebra, and common lymph nodes, if indicated based on the stage of disease.
PTV1 and PTV2 were obtained by adding a 0.5-cm isotropic margin to CTV1 and CTV2, respectively, to account for set-up uncertainties [26].
OARs considered were the femoral heads, bladder, bowel bag, rectum, bone marrow and anal canal.
IMRT plans with step-and-shoot technique were generated using the MRIdian treatment planning system (TPS). In accordance with ICRU 83 recommendations, at least 95 % of both PTVs receive 95 % of the prescribed dose and with less than 5 % of PTV1 receiving 105 % of the dose, QUANTEC constraints were used to assess dose limits to OARs [29].
The treatment was performed by aligning the patient on the daily bone anatomy with a 25-second TRUFI scan. Furthermore, intrafraction motion was managed using the uterus with a 5 mm isotropic boundary as a tracking structure on the patient's daily online cine-MRI (temporal resolution: 4 frames/s). Online adaptive radiation therapy (oART) strategies were applied in some cases depending on changes in the position or volume of the target or OARs during treatment.
2.2. Cervix regression and motion analysis
Daily TRUFI MR images used for alignment were retrospectively collected and used to perform interfractional motion analysis of the cervical uterine structure and vagina (CUS). All positioning MRI scans of all treatment fractions were co-registered with the planning MRI scan (pMR) rigidly aligning the bone anatomy.
An experienced radiation oncologist retrospectively delineated the CUS, bladder and rectum structures of all treatment fractions, including the pMR.
This resulted in the different CUSs, named CUS_sim, CUS_Fx1, CUS_Fx2…CUS_Fx25, as well as for bladder and rectum, following the same nomenclature, for each patient.
The CUS delineation as a region of interest for motion analysis includes the entire uterus, the entire cervix if not already included in the GTV and the vagina depending on its involvement [15].
The volume of each structure was calculated and reported in cubic centimetres (cc) and the inter-fraction volume changes compared to the volume of the pMR were assessed.
The TPS MRIdian was used to place a geometric centroid (GC) in each CUS volume to analyse the variation of the CUS position as a single point in each dimension, superior-inferior (SI), anterior-posterior (AP), and lateral right-left (RL), during the entire course of CRT.
Two different strategies were used to describe the intrapelvic motion of the CUS.
In the first, the coordinates of each CUS-GC for a given patient were compared with the coordinates of the CUS-GC from the pMR (ΔGC-S). These values allow the measurement of the inter-fraction shifts of the CUS with respect to the planning phase.
In the second strategy, CUS changes were described by measuring the displacement of the CUS-GC from the GC position of the previous fraction (ΔGC-FX). These values make it possible to assess the movement of the CUS during RT and to define the phase of treatment in which the greatest changes in position occur.
Pearson’s correlation coefficients were calculated for the relationship between changes in bladder and rectal filling with GC-CUS point displacement (Supplementary material Fig. S1).
All the statistical analyses were performed using R software (version 3.6.1, R Core Team, Austria) and Microsoft® Excel (Version 2212 Build 16.0.15928.20196).
2.3. Planning target volume analysis
To assess the extent to which CUS motion and regression influence the PTV margins. For each patient, different PTVs were obtained by adding isotropic margins of 0.5 cm, 0.7 cm, 1 cm, 1.3 cm, 1.5 cm, and 2 cm to CUS_sim, respectively. These volumes were named progressively PTV_sim, PTV_0.7, PTV_1…, PTV_2.
For all patients, the overlap of the CUS of all fractions and each of the 6 PTV volumes obtained as described above was assessed. The interfractional CUS movement was studied counting the number of fractions in which the 95 % of the CUS volume was included in the different margins of the PTVs.
MRgRT treatments were considered adequate when the minimum margins included 95 % of the CUS structures throughout treatment in at least 90 % of the patients treated. Given that the study was performed in 15 patients treated with 22 or 25 fractions, the optimal margin was one that could include 21/22 fractions (95,4%) or 24/25 fractions (96 %) in 14/15 patients (93.3 %).
Additionally, in order to assess the margin required to ensure PTV-CUS coverage over time, the average value of the CTV-PTV isotropic margins needed to completely cover the CUS was evaluated per week, for all patients.
2.4. Correlation analysis
In order to identify possible causes of inter-fractional variability in CUS, the correlation between CUS and the surrounding OARs (bladder and rectum) was studied on a patient-by-patient basis, using the same approach as a previously performed motion analysis. Variations in GC-S and GC-FX during RT treatment were correlated with changes in CUS volume using Pearson correlation coefficient (R). Parameters with a R greater than 0.7 were considered highly correlated. For each couple, to investigate whether some correlations were common among different patients, the percentage of patients with high correlations was calculated.[30].
3. Results
3.1. Patient characteristic
Fifteen patients with LACC treated with MRgRT from July 2019 to November 2020 were retrospectively included in this analysis. According to the FIGO (International Federation of Gynaecology and Obstretics) stage, patient and tumour characteristics were reported in Table 1.
Table 1.
Patients characteristics.
| N (%) | ||
|---|---|---|
| Median age at diagnosis years (range) | 48 (33–66) | |
| FIGO stage | ||
| IIA | 1 (6,7) | |
| IIB | 4 (26,6) | |
| IIIC1 | 10 (66,7) | |
| Tumor size | ||
| < 2 cm 2–4 cm > 4 cm |
0 (0) 7 (46,7) 8 (53,3) |
|
| RT dose | ||
| PTV1 50,6 Gy (2,3 Gy/fraction) + PTV2 39,6 Gy (1,8 Gy/fraction) | 6 (40) | |
| PTV1 55 Gy (2,2 Gy/fraction) + PTV2 45 Gy (1,8 Gy/fraction) | 4 (26,7) | |
| PTV 45 Gy (1,8 Gy/fraction) | 5 (33,3) | |
| Adaptive RT | ||
| No | 8 (53,3) | |
| Online | 4 (26,7) | |
| Offline | 3 (20) | |
FIGO: International Federation of Gynaecology and Obstretics; RT: Radiation therapy; PTV: planning target volume.
The median age of the patients was 48 years (33–66).
CRT was administered with concomitant weekly cisplatin chemotherapy (40 mg/m2, intravenous infusion).
A total of 372 TRUFI MR scans were used to contour the structures whose displacement and volume variations were assessed.
3.2. Cervix regression and motion analysis
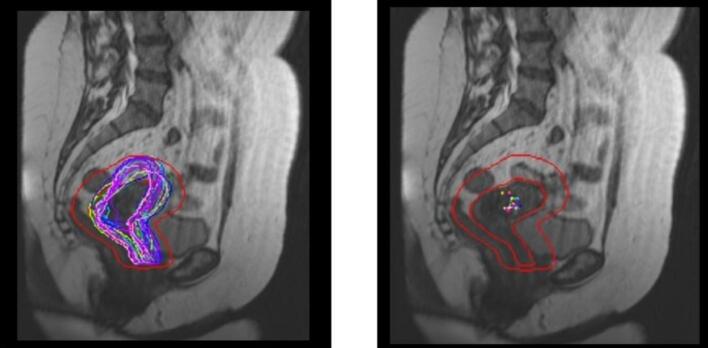
Visual assessment and analysis of uterine movement and regression was performed as shown in Fig. 1, where the case of one patient is shown with CUS segmentations in all fractions and the PTV obtained taking into account volume and position changes.
Fig. 1.
MRI scans of the cervico-uterine structure (CUS) were taken in the sagittal plane. On the left side, the contours of the CUS are displayed on the baseline image. On the right side, all the GCs of the CUS are projected onto the same sagittal slice. The PTV, which accounted for interfractional movement by applying asymmetric margins to the CUS in the planning fraction, is shown with a red line. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
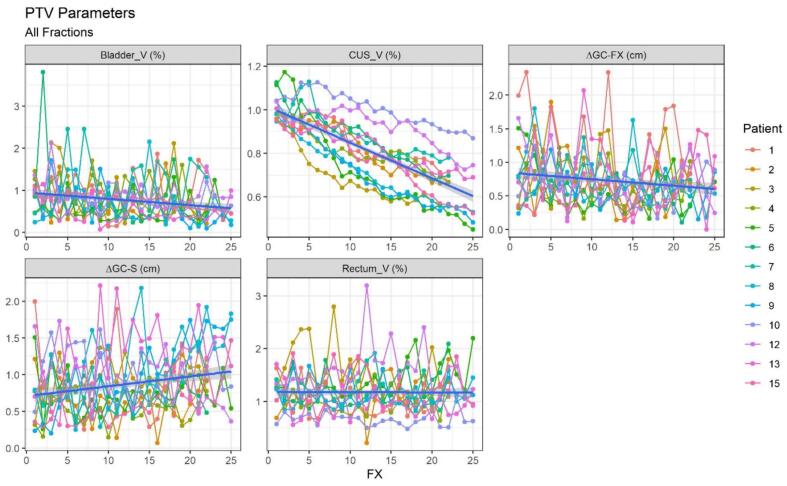
Gradual tumour regression and significant changes in CUS position were observed by visual assessment of CUS contours and measurements of CUS interfractional variation during treatment. Fig. 2 shows the trend over the weeks of CUS-GC displacement compared to pMR-CUS (ΔGC-S) and the previous fraction (ΔGC-FX) and the CUS, bladder, and rectal volume variaton for all patients.
Fig. 2.
Trend over the weeks of the CUS-GC shift compared to the previous fraction (ΔGC-FX) and the pMR-CUS (ΔGC-S) of the bladder (Bladder_V) CUS (CUS_V) and rectum (Rectum_V) volume changes for all patients. The X-axis shows the number of the fraction to which the value refers, all shifts are reported in centimetres, all volumes are normalized to the simulation volume and therefore expressed as a percentage. CUS-GC: cervical uterine structure-geometric centroid; pMR-CUS: planning MRI scan- cervical uterine structure.
Overall, a median decrease in CUS volume of −31 % (range −0.3 % / −51.7 %) was observed (Supplementary Table S1). The mean CUS volume was 115.6 cc (range, 41.1–224.1 cc) and 74.1 cc (range, 27.5–135.2 cc) at the start and the end of the treatment, respectively.
The detailed data on overall bladder volume observed during the treatment period are provided in the supplementary materials (see Supplementary Table S2).
Table 2 describes the values of the average weekly shifts in each direction of the CUS-GC compared to the pMR-CUS (ΔGC-S).
Table 2.
Weekly CUS shifts.
| Week | Min | 1st | Median | Mean | 3rd | Max | SD |
|---|---|---|---|---|---|---|---|
| Left-Right direction (X) | |||||||
| 1 | −0.79 | −0.2 | 0.01 | 0 | 0.2 | 0.8 | 0.29 |
| 2 | −0.85 | −0.22 | 0.1 | 0.04 | 0.30 | 0.99 | 0.39 |
| 3 | −0.81 | −0.24 | 0.08 | 0.05 | 0.41 | 0.87 | 0.40 |
| 4 | −0.8 | −0.30 | 0.05 | 0.03 | 0.31 | 0.91 | 0.41 |
| 5 | −0.87 | −0.34 | −0.01 | 0.03 | 0.42 | 1.03 | 0.44 |
| Cranio-Caudal direction (Y) | |||||||
| 1 | −0.86 | −0.17 | 0.22 | 0.25 | 0.58 | 1.65 | 0.60 |
| 2 | −0.77 | −0.23 | 0.01 | 0.07 | 0.31 | 1.18 | 0.45 |
| 3 | −1.27 | −0.16 | 0.11 | 0.15 | 0.49 | 1.26 | 0.47 |
| 4 | −1.63 | −0.25 | −0.01 | 0 | 0.38 | 1.37 | 0.55 |
| 5 | −1.61 | −0.41 | 0.05 | −0.05 | 0.29 | 1.09 | 0.63 |
| Antero-Posterior direction (Z) | |||||||
| 1 | −1.83 | −0.19 | 0.12 | 0.07 | 0.48 | 1.46 | 0.57 |
| 2 | −0.92 | −0.08 | 0.25 | 0.31 | 0.69 | 2.12 | 0.61 |
| 3 | −1.27 | −0.16 | 0.24 | 0.30 | 0.63 | 2.1 | 0.66 |
| 4 | −1 | −0.29 | 0.19 | 0.23 | 0.64 | 1.71 | 0.63 |
| 5 | −1.37 | −0.37 | 0.22 | 0.24 | 0.78 | 1.81 | 0.77 |
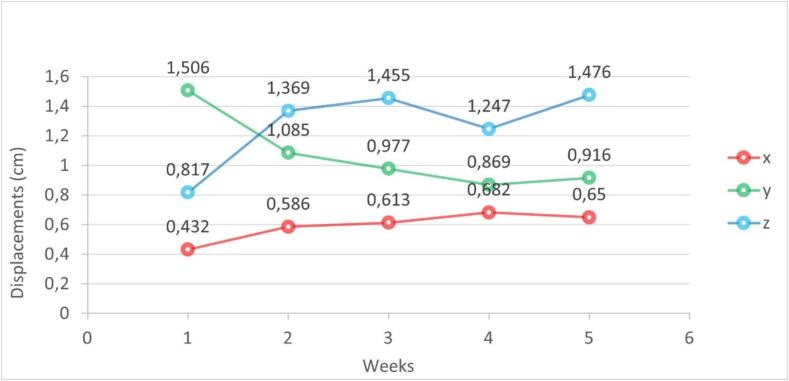
The mean maximum shift in the CUS position was 1,08 cm in the superior-inferior dimension (range, 0,56– 1,65 cm), 1.26 cm in the anterior-posterior dimension (range, 0.72–2.12 cm), and 0.7 cm in the right-left lateral dimension (range, 0.43–1.03 cm).
Fig. 3 shows the distribution of CUS-GC shifts (ΔGC-S) during RT of all patients evaluated weekly.
Fig. 3.
95th percentile of displacement by direction over treatment weeks. x: left–right direction; y: cranio-caudal direction; z: antero-posterior direction.
Analysing the CUS displacement compared to the previous fraction (ΔGC-FX), we observed a decreasing trend: in the first 5 fractions the mean ΔGC-FX was 0.96 cm, whereas in the last week was 0.6 cm. The mean CUS displacement in relation to the previous fraction was 0.74 cm (range, 0.53–1.15 cm). The data of the weekly average reduction of ΔGC-FX are summarised in Supplementary Table S3.
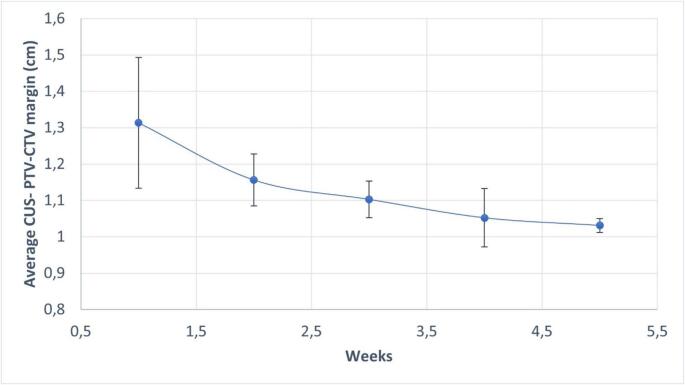
Fig. 4 shows the trend, by week, of the mean value of the CUS CTV-PTV isotropic margins needed to ensure coverage. This analysis shows a progressive reduction of median margins required over the weeks, with a median delta isotropic margins of 0,08 cm from the 3° to 5° week. (1° week 1,3cm, 2° week 1,17 cm, 3° week 1,1 cm, 4° week 1,05 cm and finally 5° week 1,02 cm).
Fig. 4.
Trend in average isotropic PTV expansion for CUS coverage (Weekly). CUS: cervico-uterine structure; CTV: clinical target volume; PTV: planning target volume.
In addition, we reported how the CUS displacement in all direction (anterior/posterior, right and left, and cranio/caudal), during the course of the entire treatment, influence PTV margins, without considering oART or replanning strategies.
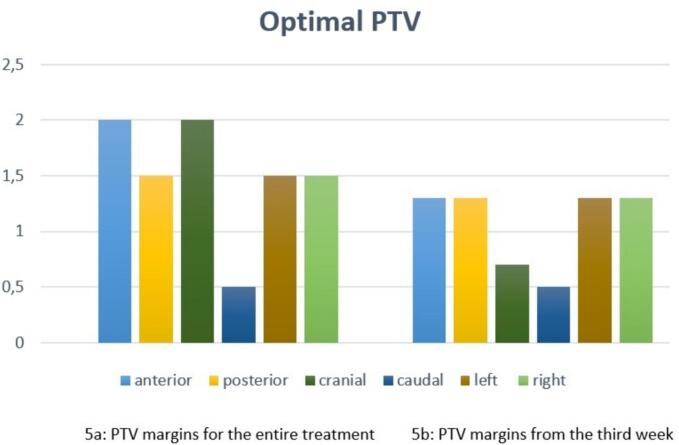
Fig. 5 summarizes the optimal PTV margins obtained for the CUS regions based on the analysis performed on the 15 patients.
Fig. 5.
Optimal planning target volume (PTV) margins (cm) for the cervical uterine structure (CUS) during the entire treatment (5a) and from the third week of radiotherapy (5b).
Fig. 5a shows optimal PTV margins for the CUS that enclose all fraction displacement during the entire treatment. In particular, according to this result, asymmetric margins (more precisely 2 cm cranially, 0.5 cm caudally, 1.5 cm posteriorly, 2 cm anteriorly and 1.5 cm both left and right) are required to obtain the optimal PTV taking into account the interfractional CUS.
Fig. 5b shows margin reduction if we take in consideration only the displacement of the CUS from the third week of RT. In particular, asymmetric margins (more precisely 0.7 cm cranially, 0.5 cm caudally, 1.3 cm posteriorly, 1.3 cm anteriorly and 1.3 cm both left and right) are required to obtain the optimal PTV taking into account the interfractional CUS volume reduction after the 14th fraction. Considering all analysed patients, all analysed fractions and considering a margin of 1.5 cm, it can be said that the CUS structure exceeded the aforementioned margin in 81 % of the cases anteriorly, posteriorly in 38 % of the cases, cranially in 44 % of the cases, on the right in 63 % of the cases and on the left in 44 % of the cases. A displacement exceeding 1.5 cm caudally was never found.
3.3. Correlation
Interestingly, the analysis of the displacement of the CUS evaluated using both ΔGC-S and ΔGC-FX, does not seem to be significantly correlated with the change in CUS volume. Six patients showed a non-significative correlation between ΔGC-S and CUS volume (|R| < 0.3), 2 patients showed a significative correlation (|R| > 0.7). In six patients a moderate correlation (|R| > 0.3) between CUS volume reduction and CUS mobility grade (ΔGC-FX) was found. The CUS displacement analysis assessed using the GC-S and GC-FX does not appear to be significantly correlated with the change in bladder or rectal volume. Complete analysis is shown in Supplementary Fig. S2.
4. Discussion
This study analyses retrospectively the inter-fraction motion of the CUS on MR images acquired with a 0.35 T MR hybrid linear accelerator. The availability of daily MRI sequences used for positioning made it possible to perform a displacement-based analysis to monitor its evolution during therapy. It is known that the movement of the uterus and its volumetric reduction during radiotherapy for cervical cancer can be considerable and patient-specific.
Several studies using different technique have attempted to quantify CUS movement during RT: Jadon et al. reported a mean variation in inter-fraction cervical motion between 2.3 and 16 mm in the anterior-posterior direction, 2.7 and 8 mm in the superior-inferior direction and between 0.3 and 10 mm in the lateral direction [7]. Furthermore, Raj et al. report mean-maximal cervical displacements of 1.4 mm anteriorly, 5.1 mm posteriorly, 3.9 mm superiorly, and 2.9 mm inferiorly by acquiring weekly MR scan, in healthy volunteers [31]. A recent study described the potential to reduce CTV to PTV margins from 1.5-2.0 cm to 5 mm in patients undergoing daily ART [32].
The peculiarity of this work is related to the availability of daily MRI images using MRIgRT, which allows the contours of the CUS to be visualised on the daily MRI. This has made it possible to define the gradual regression of the tumor and significant changes in the position of the CUS over the course of treatment.
The uterine fundus seems to move more and independently from the cervix due to the anatomical characteristics of the organ. Greater intra-factional mobility is related to the rotational movement: the upper portion of the uterus is free to rotate around a pivot consisting of the cervix or the isthmus, a more fixed part anchored to vaginal canal [31], [33].
Chan et al. analyzed mean displacements and trends between pre-RT MR and weekly MR performed weekly during CRT and reported that an isotropic internal target margin is required to encompass 90 % of the interscan motion were 4 cm at the fundus and 1.5 cm at the cervix. [34].
In our analysis, especially in the first 3 weeks, a distribution of uterine displacement is assessed for each patient, mainly in the fundus, which rotates around a fixed center of rotation represented by the cervix and the GTV.
Correlation with OAR, bladder and rectal filling was also evaluated.
Previous reports have shown that changes in bladder filling may result in CUS movement of up to 10.8 mm superior, 1.5 mm inferior, 3.19 mm anterior, 3.43 mm posterior, 2.74 mm left and 2.48 mm right [35]. Overall, we report a mean maximum CUS movement of 10.3 mm superior, 0.77 mm inferior, 9.7 mm anterior, 6.2 mm posterior, 4 mm left and 5.5 mm right. In our experience, we do not report any particular correlation between rectal and bladder filling. This is probably related to our sample size and the specific bladder protocol prior to each individual fraction. All patients performed bladder preparation by drinking 500 cc of water before the simulation and before each therapy session. We try to maintain this standard.
Currently, to compensate for CUS movement, the strategy used is to add a sufficiently large margin to the clinical target volume [9], [10], [3]. It is currently standard practice to add 1–2 cm of margin to the designated clinical target volume to account for CUS variation.
More specifically we attempt to study the CUS displacement in a different direction, as we reported in Fig. 3.
To achieve optimal PTV coverage while considering the CUS motion, asymmetric margins were found to be necessary. Specifically, our results indicate that cranially a margin of 1 cm is required, while caudally, a margin of 0.5 cm is sufficient. Posteriorly, a margin of 1.5 cm is required, while a margin of 2 cm is required anteriorly. Finally, both left and right directions necessitate a margin of 1.5 cm. However, this comes at the cost of increased exposure to critical structures.
The analysis of our data revealed that the interfractional CUS displacement significantly affects the required PTV margins to cover all uterine positions during the treatment without oART or replanning strategies.
The implementation of adaptive techniques could potentially further optimize treatment accuracy to avoid inadequate tumor coverage or increased radiation exposure to healthy tissues [36].
A promising approach to increase efficiency and streamline the online adaptive process is the development of a plan library that includes different uterine positions.
Further research and the development of standardized protocols are needed to validate and optimize the integration of adaptive strategies into clinical practice to improve treatment outcomes.
Additionally, in our study we noted that the progressive reduction in uterine volume affects the movement of the CUS, which appears to be greater in the SI and AP directions, especially in the first 3 weeks of treatment, then becomes stable in the last two weeks. The LR direction, on the other hand, is less affected by the movement and appears relatively stable throughout the overall treatment time.
In particular, we have noticed that in some patients there is a slight correlation between the reduction in CUS and the movement of the CUS among the fractions (ΔGC-FX). Reduced CUS volume is therefore associated with reduced CUS mobility.
At this stage, offline replanning to reduce margins would be optimal given the settled CUS position.
The assessment of the tumor shrinkage, adaptive RT methods, both online and offline, can be suggested to provide tailored approaches, especially during the first week and then in the third week when the CUS volume becomes more stable.
However, this study suffers from some limitations. The sample size of fifteen patients may not fully represent the diversity of the population, warranting future studies with larger cohorts to validate our results.
Aware of the limitations of this retrospective study, we acknowledge its interesting results regarding the analysis of uterine motion/reduction, which already allow us to create institutional offline/online adaptive protocols using MRgRT and 0.5 mm CTV-PTV margin with the aim of reducing potential urinary and gastrointestinal toxicities.
Such a reduced CTV-PTV should clearly be used in the context of MRgRT within the above protocols. Considering the confirmation of studies already published in the literature on the use of asymmetric CTV-PTV margins and the evidence of the trend towards CUS motion stability shown from the third week of treatment onwards, further research with larger numbers of patients may help to further customise also CBCT-gRT.
Further research is needed to explore the efficacy of oART and re-planning strategies in mitigating the impact of CUS displacement and improving RT treatment outcomes.
5. Conclusion
Our MRI study shows that CUS mobility is not regular throughout the treatment.
The suggested margins to take into account the overall CUS movement are 1.5 cm in the right-left direction, 1.5 cm posteriorly, 0.5 cm caudally, and 1 cm cranially. The widest displacement occurs in the anterior direction, whereby 2 cm of margin should be considered.
From the third week of therapy onwards, it would appear to be reasonable to consider a re-planning with reduced PTV margins.
Implementation of MRgRT for LACC and the use of offline and online adaptive strategies may allow to tailor individualised margins, offering potential solutions to optimizing dose delivery, maximizing CTV coverage while minimising dose to surrounding OARs.
In the perspective of future research, considering the enhanced soft tissue resolution of MRgRT, one option could be excluding the entire uterus from the CTV. Instead, a narrower volume based on the GTV could be considered. This approach has shown no compromise in locoregional control and is likely to reduce doses to the bowel [37].
However, the additional steps required for oART can significantly prolong the treatment time, and for that reason new IGRT strategies should be investigated to reduce the burdensome daily oART workflow while maintaining the use of reduced PTV margin. LACC patient benefits can be enhanced through an innovative and fully tailored MRgRT workflow by taking full advantage of the capabilities of hybrid MRI-guided systems.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2024.100808.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/CAAC.21660. [DOI] [PubMed] [Google Scholar]
- 2.Arbyn M., Weiderpass E., Bruni L., de Sanjosé S., Saraiya M., Ferlay J., et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan MBBS, MRCP, FRCR, MD LT, Tanderup PhD K, Kirisits PhD C, de Leeuw PhD A, Nout MD, PhD R, Duke MBBS, FRCR S, et al. Image-guided Adaptive Radiotherapy in Cervical Cancer. Seminars in Radiation Oncology 2019;29:284–98. https://doi.org/10.1016/J.SEMRADONC.2019.02.010. [DOI] [PubMed]
- 4.Pötter R., Tanderup K., Kirisits C., de Leeuw A., Kirchheiner K., Nout R., et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clinical and Translational Radiation Oncology. 2018;9:48–60. doi: 10.1016/j.ctro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pötter R., Tanderup K., Schmid M.P., Jürgenliemk-Schulz I., Haie-Meder C., Fokdal L.U., et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): a multicentre prospective cohort study. Lancet Oncol. 2021;22:538–547. doi: 10.1016/S1470-2045(20)30753-1. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi A.K., Sharma D.N., Rath G.K., Julka P.K., Subramani V., Sharma S., et al. Early clinical outcomes and toxicity of intensity modulated versus conventional pelvic radiation therapy for locally advanced cervix carcinoma: a prospective randomized study. Int J Radiat Oncol Biol Phys. 2013;87:542–548. doi: 10.1016/J.IJROBP.2013.06.2059. [DOI] [PubMed] [Google Scholar]
- 7.Jadon R., Pembroke C.A., Hanna C.L., Palaniappan N., Evans M., Cleves A.E., et al. A Systematic Review of Organ Motion and Image-guided Strategies in External Beam Radiotherapy for Cervical Cancer. Clin Oncol. 2014;26:185–196. doi: 10.1016/j.clon.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Eminowicz G., Motlib J., Khan S., Perna C., McCormack M. Pelvic Organ Motion during Radiotherapy for Cervical Cancer: Understanding Patterns and Recommended Patient Preparation. Clin Oncol. 2016;28:e85–e91. doi: 10.1016/J.CLON.2016.04.044. [DOI] [PubMed] [Google Scholar]
- 9.van de Bunt L., Jürgenliemk-Schulz I.M., de Kort G.A.P., Roesink J.M., Tersteeg R.J.H.A., van der Heide U.A. Motion and deformation of the target volumes during IMRT for cervical cancer: What margins do we need? Radiother Oncol. 2008;88:233–240. doi: 10.1016/j.radonc.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Mahantshetty U., Naga P., Nachankar A., Ghadi Y., Dheera A., Scaria L., et al. Set-Up Errors, Organ Motion, Tumour Regression and its Implications on Internal Target Volume-Planning Target Volume During Cervical Cancer Radiotherapy: Results From a Prospective Study. Clin Oncol. 2022;34:189–197. doi: 10.1016/j.clon.2021.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Niyoteka S., Berger T., Fokdal L.U., Petersen J.B.B., Zolnay A., Hoogeman M., et al. Impact of interfractional target motion in locally advanced cervical cancer patients treated with spot scanning proton therapy using an internal target volume strategy. Physics and Imaging in Radiation Oncology. 2021;17:84–90. doi: 10.1016/j.phro.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eminowicz G., Rompokos V., Stacey C., Hall L., McCormack M. Understanding the impact of pelvic organ motion on dose delivered to target volumes during IMRT for cervical cancer. Radiother Oncol. 2017;122:116–121. doi: 10.1016/j.radonc.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Lim K., Chan P., Dinniwell R., Fyles A., Haider M., Bin C.Y., et al. Cervical Cancer Regression Measured Using Weekly Magnetic Resonance Imaging During Fractionated Radiotherapy: Radiobiologic Modeling and Correlation With Tumor Hypoxia. Int J Radiat Oncol Biol Phys. 2008;70:126–133. doi: 10.1016/j.ijrobp.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Dutta S., Dewan A., Mitra S., Sharma M.K., Aggarwal S., Barik S., et al. Dosimetric impact of variable bladder filling on IMRT planning for locally advanced carcinoma cervix. J Egypt Natl Canc Inst. 2020;32 doi: 10.1186/S43046-020-00033-5. [DOI] [PubMed] [Google Scholar]
- 15.Lim K., Small W., Portelance L., Creutzberg C., Jürgenliemk-Schulz I.M., Mundt A., et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer. Int J Radiat Oncol Biol Phys. 2011;79:348–355. doi: 10.1016/J.IJROBP.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 16.Rigaud B., Simon A., Gobeli M., Lafond C., Leseur J., Barateau A., et al. CBCT-guided evolutive library for cervical adaptive IMRT. Med Phys. 2018;45:1379–1390. doi: 10.1002/MP.12818. [DOI] [PubMed] [Google Scholar]
- 17.Jensen N.B.K., Assenholt M.S., Fokdal L.U., Vestergaard A., Schouboe A., Kjaersgaard E.B., et al. Cone beam computed tomography-based monitoring and management of target and organ motion during external beam radiotherapy in cervical cancer. Physics and Imaging in Radiation Oncology. 2018;9:14–20. doi: 10.1016/J.PHRO.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Schoot A.J.A.J., de Boer P., Visser J., Stalpers L.J.A., Rasch C.R.N., Bel A. Dosimetric advantages of a clinical daily adaptive plan selection strategy compared with a non-adaptive strategy in cervical cancer radiation therapy. Acta Oncologica (stockholm, Sweden) 2017;56:667–674. doi: 10.1080/0284186X.2017.1287949. [DOI] [PubMed] [Google Scholar]
- 19.Sharfo A.W.M., Breedveld S., Voet P.W.J., Heijkoop S.T., Mens J.W.M., Hoogeman M.S., et al. Validation of Fully Automated VMAT Plan Generation for Library-Based Plan-of-the-Day Cervical Cancer Radiotherapy. PLoS One. 2016;11 doi: 10.1371/JOURNAL.PONE.0169202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ríos I., Vásquez I., Cuervo E., Garzón Ó., Burbano J. Problems and solutions in IGRT for cervical cancer. Reports of Practical Oncology and Radiotherapy : Journal of Greatpoland Cancer Center in Poznan and Polish Society of Radiation Oncology. 2018;23:517–527. doi: 10.1016/J.RPOR.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fields EC, Weiss E. A practical review of magnetic resonance imaging for the evaluation and management of cervical cancer. Radiation Oncology (London, England) 2016;11. https://doi.org/10.1186/S13014-016-0591-0. [DOI] [PMC free article] [PubMed]
- 22.Lo R.G., Cucinella G., Zaccaria G., Crapanzano A., Salerno S., Pinto A., et al. Role of MRI in the Assessment of Cervical Cancer. Seminars in Ultrasound, CT and MRI. 2023;44:228–237. doi: 10.1053/J.SULT.2023.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Klüter S. Technical design and concept of a 0.35 T MR-Linac. Clin Transl. Radiat Oncol. 2019;18:98–101. doi: 10.1016/j.ctro.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Portelance L., Corradini S., Erickson B., Lalondrelle S., Padgett K., van der Leij F., et al. Online Magnetic Resonance-Guided Radiotherapy (oMRgRT) for Gynecological Cancers. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.628131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrandina G., Gambacorta A., Gallotta V., Smaniotto D., Fagotti A., Tagliaferri L., et al. Chemoradiation With Concomitant Boosts Followed by Radical Surgery in Locally Advanced Cervical Cancer: Long-term Results of the ROMA-2 Prospective Phase 2 Study. Int J Radiat Oncol Biol Phys. 2014;90:778–785. doi: 10.1016/J.IJROBP.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 26.Boldrini L., Piras A., Chiloiro G., Autorino R., Cellini F., Cusumano D., et al. Low Tesla magnetic resonance guided radiotherapy for locally advanced cervical cancer: first clinical experience. Tumori. 2020;106:497–505. doi: 10.1177/0300891620901752. [DOI] [PubMed] [Google Scholar]
- 27.Chiloiro G., Boldrini L., Meldolesi E., Re A., Cellini F., Cusumano D., et al. MR-guided radiotherapy in rectal cancer: First clinical experience of an innovative technology. Clinical and Translational Radiation Oncology. 2019 doi: 10.1016/j.ctro.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrandina G., Palluzzi E., Gallotta V., Gambacorta M.A., Autorino R., Turco L.C., et al. Neo-adjuvant platinum-based chemotherapy followed by chemoradiation and radical surgery in locally advanced cervical cancer (Lacc) patients: A phase II study. European Journal of Surgical Oncology : the Journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2018;44:1062–1068. doi: 10.1016/J.EJSO.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 29.Bentzen S.M., Constine L.S., Deasy J.O., Eisbruch A., Jackson A., Marks L.B., et al. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): An Introduction to the Scientific Issues. Int J Radiat Oncol Biol Phys. 2010;76:S3. doi: 10.1016/J.IJROBP.2009.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boldrini L., Chiloiro G., Cusumano D., Romano A., Placidi L., Turco G., et al. Mesorectal motion evaluation in rectal cancer MR-guided radiotherapy: an exploratory study to quantify treatment margins. Radiation Oncology (london, England) 2023;18:4. doi: 10.1186/S13014-022-02193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raj KA, Guo P, Jones E et al. Intrafraction organ motion of the normal cervix [Abstract]. Int J Radiat Oncol Biol Phys 2005;63(Suppl.):S220. Int J Radiat Oncol Biol Phys 2005;63(Suppl):S220 n.d.
- 32.Yen A., Choi B., Inam E., Yeh A., Lin M.H., Park C., et al. Spare the Bowel, Don’t Spoil the Target: Optimal Margin Assessment for Online Cone Beam Adaptive Radiation Therapy (OnC-ART) of the Cervix. Pract Radiat Oncol. 2023;13 doi: 10.1016/J.PRRO.2022.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Huh S.J., Park W., Han Y. Interfractional variation in position of the uterus during radical radiotherapy for cervical cancer. Radiother Oncol. 2004;71:73–79. doi: 10.1016/j.radonc.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Chan P., Dinniwell R., Haider M.A., Bin C.Y., Jaffray D., Lockwood G., et al. Inter- and Intrafractional Tumor and Organ Movement in Patients With Cervical Cancer Undergoing Radiotherapy: A Cinematic-MRI Point-of-Interest Study. International Journal of Radiation Oncology*biology*physics. 2008;70:1507–1515. doi: 10.1016/J.IJROBP.2007.08.055. [DOI] [PubMed] [Google Scholar]
- 35.Li X., Wang L., Cui Z., Li Y., Liu P., Wang Y., et al. Online MR evaluation of inter- and intra-fraction uterus motions and bladder volume changes during cervical cancer external beam radiotherapy. Radiat Oncol. 2021;16 doi: 10.1186/s13014-021-01907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boldrini L., Chiloiro G., Pesce A., Romano A., Teodoli S., Placidi L., et al. Hybrid MRI guided radiotherapy in locally advanced cervical cancer: Case report of an innovative personalized therapeutic approach. Clin Transl Radiat Oncol. 2019;20:27–29. doi: 10.1016/j.ctro.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozak M.M., Koenig J.L., von Eyben R., Kidd E.A. Less Than Whole Uterus Irradiation for Locally Advanced Cervical Cancer Maintains Locoregional Control and Decreases Radiation Dose to Bowel. Pract Radiat Oncol. 2019;9:e164–e171. doi: 10.1016/j.prro.2018.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.