Abstract
Template switching rates of Moloney murine leukemia virus reverse transcriptase mutants were tested using a retroviral vector-based direct-repeat deletion assay. The reverse transcriptase mutants contained alterations in residues that modeling of substrates into the catalytic core had suggested might affect interactions with primer and/or template strands. As assessed by the frequency of functional lacZ gene generation from vectors in which lacZ was disrupted by insertion of a sequence duplication, the frequency of template switching varied more than threefold among fully replication-competent mutants. Some mutants displayed deletion rates that were lower and others displayed rates that were higher than that of wild-type virus. Replication for the mutants with the most significant alterations in template switching frequencies was similar to that of the wild type. These data suggest that reverse transcriptase template switching rates can be altered significantly without destroying normal replication functions.
Reverse transcriptase (RT) must perform two template switches—the first and second strong-stop template switches—to complete integration-competent cDNA synthesis (13). It has been proposed that the requirement to perform these two replicative switches confers onto RT the tendency to make additional, nonrequired template switches that can result in genetic recombination (5, 41).
Most DNA polymerases do not switch templates as frequently as RT does, which suggests that this process may require some structural or biochemical properties of RT. RT has been mutagenized extensively to identify features important for polymerization, RNase H activity, fidelity, drug resistance, and processivity (39). It is thought that RT pauses before switching templates; therefore, mutants with altered pausing and/or processivity may be affected in template switching (20, 27, 43, 44).
In this report, a panel of RT mutants was constructed based on the crystal structure of a catalytic fragment of Moloney murine leukemia virus (MLV) RT (12). Although the biochemical properties of the mutants were not tested here, the basis of these mutants' design was the hypothesis that by limiting interactions with the primer-template, RT template switching rates might be altered. These mutants contained alterations in residues proximal to the primer and/or template strands in a model of nucleic acid bound in the polymerase active site. Experiments presented in this report examined the replication of Moloney MLV mutants harboring these mutations and tested these mutants for effects on template switching during viral replication.
MATERIALS AND METHODS
Plasmids.
RT mutations were introduced by PCR and standard cloning procedures into the infectious clone pMLV-neo (34) and pMLV Ψ−, a packaging-defective clone (30), and the entire PCR-generated region for each mutant was confirmed by dideoxynucleotide sequencing.
Cells.
NIH 3T3 cells and rat2 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% calf serum (Gibco). 293T- and ET-based cell lines were grown in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum (HyClone). ET is a 293T-based line that expresses ecotropic env (30). Puromycin-resistant 3T3 cells were selected in 6 μg of puromycin (Sigma) per ml.
Replication assays.
pMLV-neo-based DNA and pCH110 lacZ reporter (14) were cotransfected into 50%-confluent 293T cells using Lipofectamine (Gibco) according to the manufacturer's instructions. Two days posttransfection, virus was harvested and stored at −70°C, and the cells were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (30) to confirm that at least 20% stained blue for each cotransfection. For Fig. 2, 20%-confluent 3T3 cells were infected with equal volumes of thawed virus plus 0.8 μg of hexadimethrine bromide/ml (Polybrene; Sigma). Two days postinfection, media were harvested and cells were passaged 1:10. Cells were passed and medium was sampled every 2 to 4 days thereafter. Spread of virus was monitored by assaying RT activity (37). Note that even noninfectious mutants (D114N, R116L, and N119A) yielded robust signals when examined as purified enzymes (25); hence this assay was an appropriate method for monitoring virus spread. To detect subtle differences in replication, virus was quantified by the amount of viral RNA by an RNase protection assay (35). 3T3 cells were infected as described above with an amount of virus containing the equivalent of 10, 1, or 0.1 U of viral RNA normalized to an amount of wild-type RNA arbitrarily assigned the value of 1 U.
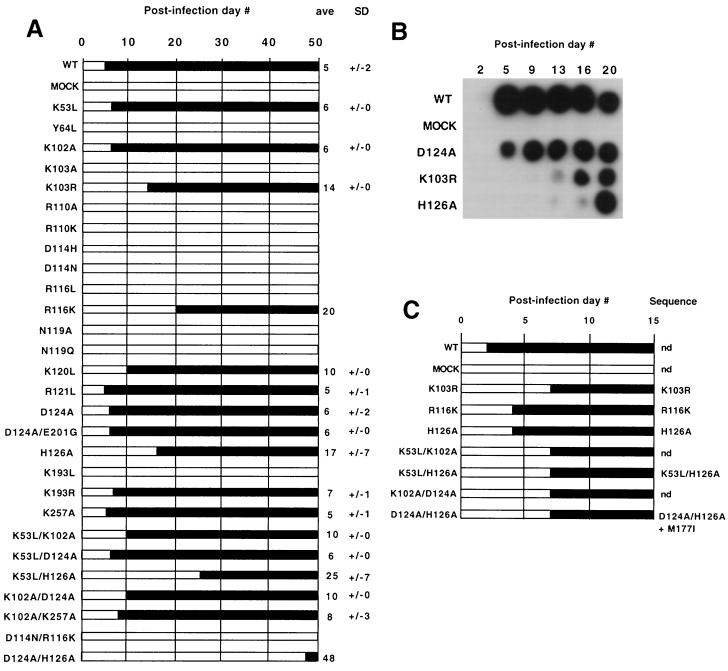
FIG. 2.
Mutant virus replication. (A) Replication time course. Results are averages from at least two independent transfection experiments for each mutant. Black bars represent the day postinfection (indicated at the top) when spread was first detected, and a lack of detectable spread is represented by clear bars. The average first day of detection and the standard deviation (in days) are at right. Averages without standard deviations indicate cultures that tested positive in one experiment (possibly due to compensatory mutations or reversion) but not in experimental repetitions. (B) Sample viability (RT activity) assay. Culture medium was harvested from confluent cells infected with the virus stocks indicated at the left on the days shown at the top and was assayed for RT activity as described in Materials and Methods. Note that this assay was used to determine the presence or absence of detectable virus: whether variations in signal intensity reflected altered enzymatic activity or other parameters, such as cell growth rate-dependent differences in virus concentration, was not determined. (C) Reversion analysis. Virus-containing media, from the first time points shown in panel A at which spread was detectable, were used to infect 3T3 cells.
Reversion analysis.
Fresh 3T3 cells were infected with wild-type or putative revertant viruses (those with variable time courses or replication delays), and spread was monitored as described above. Low-molecular-weight DNA was isolated from rat2 cells infected with these viruses (17). The RT region was PCR amplified, subcloned into pUC19, and sequenced. Note that only ∼200 bp near the mutation site were sequenced for the reversion analysis.
Direct-repeat deletion and error rate assays.
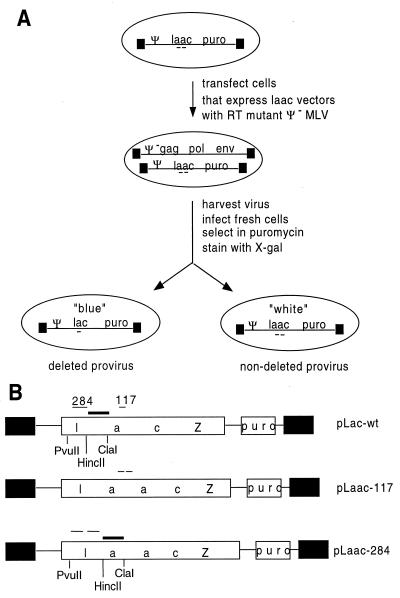
ET pLaac 117, ET pLaac 284, or ET pLacPuro cells were transfected with pMLV Ψ−-based plasmids, virus was harvested and used to infect 3T3 cells, and drug-resistant colonies were stained with X-Gal and counted as described previously (30).
Southern blots.
At least 100 colonies were pooled for genomic DNA for each virus type. DNA was isolated using Wizard Prep kits (Promega) and was digested with PvuII and ClaI, separated on 5% polyacrylamide–8M urea gels, electrophoretically transferred to a membrane (Hybond N; Amersham Pharmacia), and hybridized with a probe (42) that was 32P-radiolabeled using the Rediprime II kit (Amersham Pharmacia). Products were visualized by autoradiography and quantified by PhosphorImager (Molecular Dynamics).
Endogenous RT reactions.
Virus was harvested from cells grown in DMEM + 10% Nu serum (Collaborative Research), filtered, and concentrated as described previously (37). Twenty microliters of 70-fold-concentrated virus and 50 μl of a solution (50 mM Tris [pH 8.3], 50 mM NaCl, 0.01% NP-40, 1 mM dithiothreitol, 2 mM dATP, 2 mM dGTP, 2 mM dCTP, 0.5 mM [α-32P]TTP at 1.2 Ci/mmol) were incubated 1 h at 37°C. Two microliters of 0.5 M EDTA, 7 μl of 10% sodium dodecyl sulfate, and 1.6 μl of 2.5-μg/μl proteinase K were added. After 30 min at 37°C, the products were phenol extracted and ethanol precipitated. The pellets were resuspended in 20 μl of 0.33 M NaOH, incubated at 55°C for 20 min, and ethanol precipitated. Products were separated on 5% polyacrylamide–8M urea gels or 0.8% denaturing agarose gels and visualized by autoradiography (35).
RESULTS
RT mutant design.
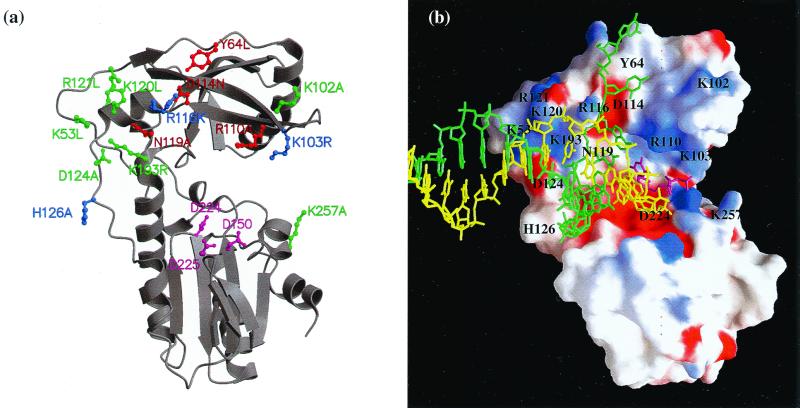
The design of the mutants described here was based on an analysis of the crystal structure of a catalytic fragment, including the fingers and palm domains of Moloney MLV reverse transcriptase reported at a 1.8 Å resolution, and on subsequent substrate modeling studies (12). In an initial model, an idealized A-form DNA primer-template and an incoming nucleotide were modeled into the active site of the fragment structure based on superpositional studies with the rat polymerase β ternary complex and the human immunodeficiency virus type 1 (HIV-1) RT:Fab:DNA complex (19, 29). We previously proposed that residues in a conserved, positively charged surface in the fingers domain within 4 to 5 Å of the primer-template might play a role in processivity (12). Thus, charged residues and others in close proximity to this positively charged surface were selected for mutational analysis in the present study. Additional residues that might interact with the single-stranded template overhang or incoming nucleotide were also altered. The goal was to introduce changes in the ability of the enzyme to interact with the template and/or primer that might lead to altered elongation properties without perturbing the enzyme structure enough to render virus noninfectious.
The targeted amino acid residues were located primarily in the fingers domain, and their putative interactions as implicated by structural modeling are listed in Table 1. The residues were classified structurally based on our initial model for MLV RT and positions of analogous residues in the more recent HIV-1 RT:DNA:TTP complex (18, 19) as follows (Fig. 1). The basic residues K53, R116, K120, R121, and K193 contribute to the positively charged patch on the surface of the fingers domain, which was proposed to interact with the template. Additional residues that were altered because they may affect these same interactions were D114, N119, D124, and H126. Residues proposed to interact with the single-stranded template overhang or nucleotide include K102, K103, and R110, which are part of the β4-β5 loop (β3-β4 loop in HIV-1 RT), and Y64. The remaining residue examined was K257, which may interact with the incoming nucleotide.
TABLE 1.
Locations of RT residues and putative interactions
| Amino acid for:
|
Locationa | Proposed interaction | |
|---|---|---|---|
| MLV | HIV | ||
| K53 | K13 | Behind | Template |
| Y64 | W24 | Ahead | ss templateb |
| K102 | K64 | Ahead | dNTP |
| K103 | K65 | Ahead | dNTPb |
| R110 | R72 | Ahead | dNTPb |
| D114 | D76 | Behind | Templateb |
| R116 | R78 | Behind | Templateb |
| N119 | N81 | Behind | Templateb |
| K120 | K82 | Behind | Template |
| R121 | R83 | Behind | Template |
| D124 | D86 | Behind | Template |
| H126 | Q91 | Behind | Template |
| K193 | K154 | Behind | Template |
| K257 | K219 | Ahead | dNTP |
Location relative to the active site.
Interactions are predicted to be within 4 Å based on analysis of the HIV-1 RT:DNA:TTP crystal structure (28). D114, R116, and N119 are also involved in interactions with the template primer in crystal structures of an N-terminal fragment from MLV RT complexed with nucleic acid (13). Assignment of equivalent residues in HIV RT is based on a structural sequence alignment (12). ss, single stranded.
FIG. 1.
RT structure and mutant design. (a) Ribbon diagram (23, 24) of the fingers and palm domains of MLV RT. Positions of mutagenized residues and of active site residues D150, D224, and D225 (magenta) are shown as ball and stick models. Mutant viability is represented by color coding, with green indicating viable, blue indicating delayed but viable, and red indicating not viable. (b) Positions of specific residues (black labels) relative to a primer-template model on an electrostatic potential surface rendering (26) of MLV RT fingers and palm domains. The primer, template, and incoming nucleotide are shown as stick models in yellow, green, and magenta, respectively. The view is similar to that in panel a. The position of the DNA, shown in a stick model, results from superpositioning the fingers and palm domains of MLV with HIV-1 RT from the HIV-1 RT:DNA:TTP structure (Protein Data Bank accession code 1rtd).
Initially, the wild-type amino acids, which are primarily basic residues, were changed to either alanine or leucine. After the viability of these mutants was tested (see below), more conservative amino acid changes were made for some nonviable mutants. The first sequenced candidate for one mutant, D124A, was found to contain an additional mutation in codon 201 (E201G). Replication properties of both this fortuitous double mutant (denoted D124A/E201G) and the intended single mutant, D124A, were examined.
Mutant virus viability.
Viability was examined by infecting cells and monitoring virus spread by determining the time point at which virus was first detectable in the culture medium. Averaged results from two independent experiments are presented in Fig. 2A, and sample data are displayed in Fig. 2B. Three patterns of replication were observed: mutants that spread at approximately the same rate as the wild type (e.g., K53L and K102A), mutants with delayed replication (e.g., K120L and H126A), and mutants for which replication was never detected over 2 months of passage (e.g., K103A and K193L). Whether the principal defects of mutants that failed to spread were in reverse transcription or some other aspect of viral replication was not determined.
For several noninfectious mutants, more conservative amino acid changes were tested (Fig. 2A). Of these, K193R replicated like the wild type, suggesting that side chain charge and/or size is important for this residue. Two other conservative change mutants, K103R and R116K, spread with delays.
Assays were performed to assess possible subtle differences among some mutants (K53L, R121L, and D124A/E201G) which appeared to replicate like the wild type. To accomplish this, the amounts of virus in each stock and in a replication-delayed control (H126A) were quantified by RNase protection assays of encapsidated genomic RNA. Separate plates were infected with the same amount of each virus stock (as determined by virion RNA content) or by serial dilutions, and virus spread was monitored. The delay of the replication-impaired mutant, H126A, was accentuated by serial dilution, but no differences in spread were detectable among the wild type and the tested mutants, K53L, R121L, and D124A/E201G (data not shown). Hence, the replication time courses of the latter mutants were indistinguishable from that of the wild type.
Fresh cells were infected with post-passage virus for mutants with delayed or inconsistent time courses to test for mutant reversion. As shown in Fig. 2C, some viruses, such as the D124A/H126A mutant, appeared to have reverted because they spread far more rapidly on secondary passage than in the initial experiment. DNA from these viruses was isolated, and the vicinity of the original mutations was sequenced. As shown in Fig. 2C, all sequenced mutants (K103R, R116K, H126A, K53L/H126A, and D124A/H126A) retained the original RT substitutions. Only one mutant, D124A/H126A, contained an additional change—M177I—within the ∼200 bases sequenced. Whether or not M177I was responsible for the revertant phenotype or if additional, more distal mutations accumulated in D124A/H126A or other mutants was not determined.
It is important to note that whereas some mutants were subject to reversion during passage, the template switching assays reported below involved single rounds of replication. All viral proteins in the following experiments were translation products of transfected plasmid-encoded mRNAs and not products of viral replication. Thus the findings below reflect properties of the original mutants without any contribution from revertants that accumulate during replication.
Template switching and error rates of RT mutants.
Template switching frequencies were tested with a tandem repeat deletion assay (30). On templates with direct repeats, RT template switching frequently results in the deletion of one repeat (21, 22, 27, 32, 33, 45). Repeat deletion rates are generally roughly proportional to the lengths of the repeats (1, 20, 21, 27, 30, 46). We previously determined that MLV vectors with repeats of 117, 284, and 971 bases yield deleted products 5%, 27%, and 60% of the time, respectively (30).
The tandem repeat deletion assay is described schematically in Fig. 3A (30). This assay relies on retroviral vectors that contain lacZ genes with internal direct repeats (denoted laacZ) (Fig. 3B). If the direct repeat within laacZ remains undeleted during reverse transcription, cells transduced by the resulting provirus remain unstained when incubated with X-Gal. However, if precise repeat deletion occurs, transduced cells stain blue. Packaging-defective RT mutant proviruses were transiently transfected into cells that expressed the 117-base repeat vector (ET pLaac 117 cells), virus was harvested, fresh 3T3 cells were infected, and provirus-containing colonies were stained with X-Gal and counted. The results, which are normalized to the deletion value (5.1%) for the wild type, are shown in Fig. 4A. Deletion frequencies ranged from 40% (for D124A/E201G) to 128% (for K102A/K257A) of the wild-type value.
FIG. 3.
Template switching assay. (A) Experimental overview: pMLV Ψ− was transiently transfected into stable clonal transfectants expressing each vector to produce virus used to infect 3T3 cells. Puromycin-resistant colonies were stained with X-Gal to determine deletion frequencies. (B) MLV vectors containing direct repeats within lacZ. The parental vector, pLac-wt, contains the puromycin resistance gene transcribed from the SV40 promoter and lacZ driven by the upstream long terminal repeat. pLaac-117 and pLaac-284 contain 117- and 284-base repeats within lacZ, respectively. Long terminal repeats are represented by black boxes, and direct-repeat locations are shown. PvuII and ClaI were used to digest genomic DNA, and a HincII-ClaI fragment was used as the probe (represented by a thick bar) for Southern analysis (see Fig. 5).
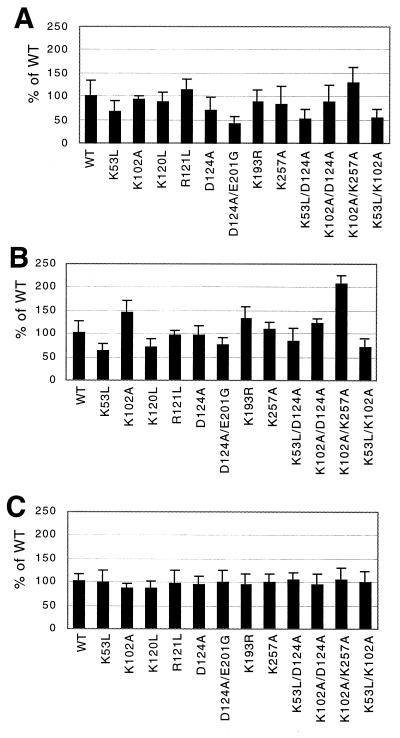
FIG. 4.
Template switch and error rates. Error bars represent standard deviations. (A) Deletion rates (117-base) for wild-type and mutant RTs. The data shown for the mutants selected for extensive study (WT RT, K53L, D124A, D124A/E201G, K53L/D124A, and K102A/K257A) are from at least 15 plates from two or more independent transfections and at least three independent infections for each of these mutants. Other mutants (K102A, K120L, R121L, K193R, K257A, K102A/D124A, and K53L/K102A) were included in a subset of these experiments. Data presented from the latter mutants were obtained from at least six plates from at least two independent infection experiments for each virus. (B) Deletion rates (284-base). The mutants listed above were chosen for extensive analysis, and for these, results are from at least 12 plates from two or more independent transfections and at least three independent infections. (C) lacZ inactivation rates. For these experiments, WT RT, K53L, D124A, K53L/D124A, and K102A/K257A were extensively studied (results are from at least nine plates from two or more independent transfections and at least three independent infections).
Mutants were also tested using a 284-base duplication in a different region of lacZ (Fig. 4B). The average wild-type deletion rate for this repeat was 27.2%. Deletion frequencies for most mutants differed less from the wild-type frequency on this template than on the 117-base repeat, but some mutants (K53L, R120L, D124A/E201G, and K53L/K102A) displayed consistent decreases. In contrast, K102A/K257A showed a twofold increase in template switching compared to the wild type.
To address whether apparent differences in template switching might instead be due to differences in RT fidelity, lacZ inactivation rates were compared, as has previously been described (15, 30). A vector carrying uninterrupted lacZ (pLac-wt) (Fig. 3B) was encapsidated into virions harboring either wild-type or mutant RT, and the fraction of product colonies that stained blue with X-Gal was determined. Results shown in Fig. 4C are normalized to the average rate of lacZ inactivation for the wild type (9.1%) and demonstrate that whereas template switching rates among mutants varied more than threefold, lacZ inactivation rates for all mutants were within 20% of the wild-type value. This suggests that differences in rates of lacZ inactivation did not contribute significantly to apparent template switching rates.
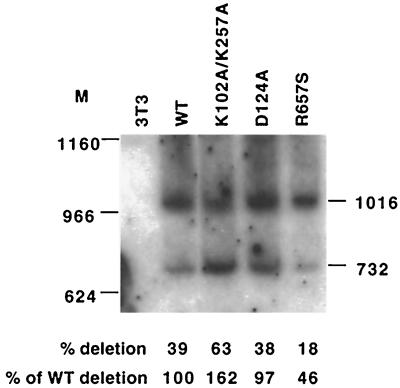
The assay described above could not rule out the possibility that apparent differences in the switching frequency reflect altered rates of template switch-associated errors. Thus, to address whether or not deletion rates determined by blue-white screening correlated with deletion frequencies, proviral DNAs were examined by Southern analysis to address the question of what portion of Laac 284-templated proviruses contained repeat deletions. A typical blot, which analyzed deletion rates of Laac 284 among proviral DNAs from pooled puromycin-resistant infected cells, is shown in Fig. 5. Here, the wild type was compared to R657S, an RNase H mutant that appears to be particularly defective in template switching (a rate more than threefold reduced by blue-white screening; J. K. Pfeiffer, unpublished data) as well as to K102A/K257A, which appears to delete 284-base repeats 2.1-fold more than in the wild type, and to D124A, which showed approximately the same deletion rate as the wild type (Fig. 4B). DNA from pools of integrated proviruses was digested with restriction enzymes whose sites flanked the repeat, and deletion rates were estimated by comparing ratios of fast (deleted) and slow (undeleted) migrating bands by PhosphorImager. Quantification of bands in Fig. 5 suggested that deletion rates were higher for the K102A/K257A mutant (a 1.6-fold increase over that of the wild type), lower for R657S (a 2.2-fold decrease from that of the wild type), and essentially the same as the wild-type rate for D124A. Due to low product signals and uneven background values, our PhosphorImager-determined magnitudes of deletion rates varied somewhat from blot to blot and even among repeated quantifications of individual blots when different filter regions were used to calculate background values (data not shown). However, results with several blots consistently demonstrated the highest 284-base duplication deletion rates for K102A/K257A, followed by, in descending order, the wild type, D124A, K53L, and R657S. Thus in each instance, trends in observed ratios of deleted and undeleted bands correlated well with deletion rates assigned based on generation of intact LacZ.
FIG. 5.
Southern analysis of PvuII/ClaI-digested genomic DNA from 3T3 cells infected with a 284-base repeat vector. The 1,016-nucleotide band represents undeleted laacZ, and the 732-nt band represents deleted products. Marker band locations are indicated to the left. See Fig. 3B for a map of restriction sites and the probe. Bands were quantified by PhosphorImager, and deletion rates were calculated (bottom).
Endogenous reverse transcription reactions.
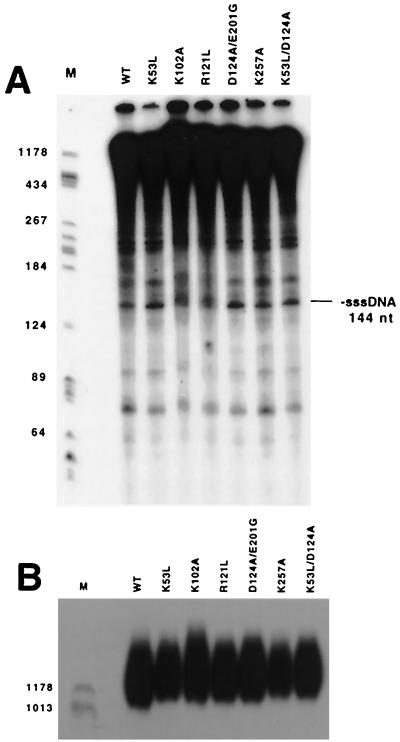
Studying the properties of purified mutant enzymes was beyond the scope of this study. However, previous studies have shown that at least in some cases, RT mutants that differ in processivity display readily detectable differences when DNA synthesis on the native viral genome is examined by permeabilizing purified virions and performing endogenous reaction assays (38, 40). Thus, as an initial examination of the mutant RTs' elongation properties, endogenous reverse transcription products were compared for wild-type RT and for mutants that displayed altered template switching rates. Some of these analyses of endogenous reaction products separated on denaturing polyacrylamide and agarose gels, respectively, are shown in Fig. 6A and B. As is evident from the similarity of banding patterns in all lanes, pausing patterns were indistinguishable at this level of resolution, and all tested mutants yielded DNA products similar to the wild type's, including minus-strand strong-stop DNA and weak-stop products (Fig. 6A). The ratios of the amount of minus-strand strong-stop products to the amount of products longer than minus-strand strong stop for the wild type and all tested mutants were comparable (Fig. 6A), suggesting that minus-strand strong-stop transfer was not grossly altered for the mutants. Additionally, endogenous reaction products longer than 1,000 bases (Fig. 6B) were similar in quantity and in length for all tested RTs.
FIG. 6.
Endogenous reactions. (A) Denaturing polyacrylamide gel. Numbers at left indicate lengths, in bases, of size standards. Mobility of minus-strand strong-stop DNA (−sssDNA) is indicated at right. (B) Denaturing agarose gel to visualize longer endogenous reaction products. Size standards are indicated as for panel A.
DISCUSSION
Template switching frequencies were tested for a panel of targeted RT mutants using an intracellular tandem repeat deletion assay. The frequency of direct-repeat deletion was decreased for most affected mutants (such as K53L and D124A/E201G) but was significantly increased for others (e.g., K102A/K257A) (Fig. 4).
The present work examined replication and functional properties of viral mutants, and the biochemical properties of the mutant enzymes were not tested directly. However, the basis of the mutants' designs was the hypothesis that weakening interactions between RT and its primer-template might affect rates of template switching. Thus most mutants studied here contained alterations in residues that were predicted to interact with the primer-template or incoming nucleotide. K103, R110, D114, R116, N119, and H126 are equivalent to residues in HIV-1 RT that are within 4 Å of nucleic acid in the HIV-1 RT:DNA:TTP structure (Table 1) (18). K103, R110, D114, R116, and N119 are highly conserved. Predictions suggest that K103 and R110 form hydrogen bonds with the incoming nucleotide (2, 3, 18, 25). D114, R116, and N119 are involved in critical interactions of the primer-template with the fingers domain in crystal structures of the N-terminal fragment of MLV RT complexed with DNA. A mechanistic role in processive synthesis has been proposed for interactions of the primer-template with the fingers domain binding site (25). Y64 was predicted to interact with the single-stranded template overhang. In the experiments reported here, mutations in several of these residues resulted in a severe delay or lack of detectable replication (Fig. 2). Because the assays that were subsequently used to assess deletion frequencies relied on good viability, switching effects could not be assessed for this group of replication-impaired mutants.
Some of these same mutant enzymes have previously been purified and characterized in several assays, including those that use polyriboadenylate-oligodeoxyribosylthymine template-primers (25). The studied enzymes included K103A, R110A, D114N, R116K, D114N/R116K, R116L, and N119A, all of which failed to support replication or led to a delayed phenotype when tested in viruses in the experiments described here. As previously reported, K103A and R110A enzymes retain less than 10% of wild-type activity (2, 3), while the remaining enzymes retain 40 to 70% of wild-type activity in the polyriboadenylate-oligodeoxyribosylthymine assay. However, despite their relatively high levels of activity on homopolymeric templates, all of the enzymes with substitutions for D114, R116, or N119 synthesized significantly less full-length product than the wild-type enzyme when assayed on an mRNA template, suggesting that they have processivity defects (25). In light of these findings regarding defects detectable in reconstituted reactions, it is not surprising that replication of viruses harboring these alterations was impaired.
Other residues that were mutated in the present study that could potentially interact with nucleic acid were not within 4 Å of the primer-template or incoming nucleotide in the model. Of these, the viable mutants K102A and K257A are positioned ahead of the polymerase active site. Interestingly, the K102A/K257A double mutant displayed the highest template switching rates measured, while most other mutants showed rates of deletion that were the same as or lower than those of the wild type. This may suggest that the template switching properties affected for some classes of mutants differed from those affected for others. Some mutants that replicated well (K53L, K120L, R121L, D124A, and K193R) contained substitutions in or near a conserved, positively charged surface in the fingers domain. Residues altered in the single mutants which displayed the greatest decreases in template switching—D124 and K53—were surface exposed and are proposed to bind nucleic acids.
Alterations to RT regions other than those targeted here may also affect template switching. For example, D124A/E201G, a PCR-created mutant that contained an unintended change in a somewhat conserved palm domain α-helix residue in addition to a targeted mutation, displayed the lowest rate of 117-base repeat deletion. Evidence that RT mutations other than those studied here may affect template switching includes preliminary observations with RNase H mutants such as R657S (Fig. 5), which display exceptionally low deletion rates (Pfeiffer, unpublished data). The observation that some RNase H alterations affect switching rates raises the possibility that some of the studied DNA polymerase domain mutations may have exerted their effects on template switching by modifying RNase H activity. Both MLV and HIV-1 RT DNA polymerase mutants altered in RNase H activity have been described (11, 31, 36).
Like the wild type, all mutants displayed increased rates of deletion when tested on a longer repeat, but mutant-specific effects were generally more modest with the 284-base repeat than with the 117-base repeat. In comparisons of the 117- and 284-base repeats, switching rates relative to the wild type's were similar for some mutants, such as K53L, but quite different for others, such as K102A (Fig. 4A and B). The cause of these differences is not clear, but mutants may differ in how they respond to specific sequences. In purified reactions, HIV-1 RT displays increased pausing and strand transfers in A- or AU-rich sequences (7, 8, 16). The 117-base repeat included some AU-rich regions, but the 284-base repeat contained proportionally more. Because mutants may differ in their responses to sequences that signal pausing or promote template switching, it would be interesting to examine the effects on deletion rates of altering AU-rich content. Alternately, some models for retroviral recombination postulate that interactions behind the growing point are important for template switching (4, 6, 28, 42). Because fewer such interactions would be possible for the 117-base repeat, template-switching deficiencies for mutants defective in this interaction might be particularly apparent on the shorter repeat.
This study's results indicate that effects on template switching did not correlate with detectable defects in virus replication or gross defects in DNA synthesis. This is interesting in light of previous suggestions that RT evolved its tendency to make nonrequired template switches as a result of the requirement for it to perform strong-stop template switches (5, 41). Differences in replicative (strong-stop) switching were not detectable in the endogenous reactions performed here, but these assays were crude enough that fairly substantial differences could have been overlooked. Nonetheless, relatively small changes in virus fitness should be magnified during repeated cycles of viral replication, and the mutations that conferred the greatest effects on template switching (D124 and K53) did not detectably impair replication when assayed over several replication cycles. Thus, it is tempting to speculate that either strong-stop template switching is not a rate-limiting step during virus replication or the nucleic acid binding functions affected by these mutations may be more important to recombinogenic switching than to aspects of primer-template recognition required during normal DNA synthesis. For example, portions of the enzyme that are altered for some mutants may actively contribute to template switching by promoting acceptor template binding or by stabilizing a kinetic intermediate from which recombination can occur.
More sensitive assays will be required to examine mutants for subtle differences that might account for the observed template switching differences. Alternately, it is possible that retroviral replication and recombination require distinct sets of RT structural properties, as has previously been suggested for the replicase of the plant RNA virus, brome mosaic virus (BMV) (9, 10). Based on findings in the BMV system that template switching phenotypes did not correlate with replication defects, the authors of those studies proposed that the regions of BMV replicase which are important to replication and to recombination properties may differ.
ACKNOWLEDGMENTS
We thank John Moran and James Peliska for critical reading of the manuscript and Kelly Cuttle and Bert Topping for assistance in early stages of the study.
This work was supported by American Cancer Society grant RPG-95-058-04-MBC to A.T., NIH grant 5R01GM55026 to M.M.G., and the Nancy Lewton Loeb Fund and NIH grant T32 GM 07544 to J.K.P.
REFERENCES
- 1.Anderson J A, Bowman E H, Hu W-S. Retroviral recombination rates do not increase linearly with marker distance and are limited by the size of the recombining subpopulation. J Virol. 1998;72:1195–1202. doi: 10.1128/jvi.72.2.1195-1202.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basu A, Basu S, Modak M J. Site-directed mutagenesis of Moloney murine leukemia virus reverse transcriptase: demonstration of lysine 103 in the nucleotide binding site. J Biol Chem. 1990;265:17162–17166. [PubMed] [Google Scholar]
- 3.Chowdhury K, Kaushik N, Pandey V, Modak M J. Elucidation of the role of Arg 110 of murine leukemia virus reverse transcriptase in the catalytic mechanism: biochemical characterization of its mutant enzymes. Biochemistry. 1996;35:16610–16620. doi: 10.1021/bi961462l. [DOI] [PubMed] [Google Scholar]
- 4.Coffin J. Retroviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fundamental virology. 3rd ed. Philadelphia, Pa: Lippincott; 1996. pp. 763–843. [Google Scholar]
- 5.Coffin J M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979;42:1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- 6.Delviks K A, Pathak V K. Effect of distance between homologous sequences and 3′ homology on the frequency of retroviral reverse transcriptase template switching. J Virol. 1999;73:7923–7932. doi: 10.1128/jvi.73.10.7923-7932.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeStefano J J, Buiser R G, Mallaber L M, Fay P J, Bambara R A. Parameters that influence processive synthesis and site-specific termination by human immunodeficiency virus reverse transcriptase on RNA and DNA templates. Biochem Biophys Acta. 1992;1131:270–280. doi: 10.1016/0167-4781(92)90025-u. [DOI] [PubMed] [Google Scholar]
- 8.DeStefano J J, Mallaber L M, Rodriguez-Rodriguez L, Fay P J, Bambara R A. Requirements for strand transfer between internal regions of heteropolymer templates by human immunodeficiency virus reverse transcriptase. J Virol. 1992;66:6370–6378. doi: 10.1128/jvi.66.11.6370-6378.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figlerowicz M, Nagy P D, Bujarski J J. A mutation in the putative RNA polymerase gene inhibits nonhomologous, but not homologous, genetic recombination in an RNA virus. Proc Natl Acad Sci USA. 1997;94:2073–2078. doi: 10.1073/pnas.94.5.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figlerowicz M, Nagy P D, Tang N, Kao C C, Bujarski J J. Mutations in the N terminus of the Brome mosaic virus polymerase affect genetic RNA-RNA recombination. J Virol. 1998;72:9192–9200. doi: 10.1128/jvi.72.11.9192-9200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao H-Q, Boyer P L, Arnold E, Hughes S H. Effects of mutations in the polymerase domain on the polymerase, RNase H and strand transfer activities of human immunodeficiency virus type 1 reverse transcriptase. J Mol Biol. 1998;277:559–572. doi: 10.1006/jmbi.1998.1624. [DOI] [PubMed] [Google Scholar]
- 12.Georgiadis M M, Jessen S M, Ogata C M, Telesnitsky A, Goff S P, Hendrickson W A. Mechanistic implications from the structure of a catalytic fragment of Moloney murine leukemia virus reverse transcriptase. Structure. 1995;3:879–892. doi: 10.1016/S0969-2126(01)00223-4. [DOI] [PubMed] [Google Scholar]
- 13.Gilboa E, Mitra S W, Goff S, Baltimore D. A detailed model of reverse transcription and tests of crucial aspects. Cell. 1979;18:93–100. doi: 10.1016/0092-8674(79)90357-x. [DOI] [PubMed] [Google Scholar]
- 14.Hall C V, Jacob P E, Ringold G M, Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2:101–109. [PubMed] [Google Scholar]
- 15.Halvas E K, Svarovskaia E S, Pathak V K. Development of an in vivo assay to identify structural determinants in murine leukemia virus reverse transcriptase important for fidelity. J Virol. 2000;74:312–319. [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison G P, Mayo M S, Hunter E, Lever A M L. Pausing of reverse transcriptase on retroviral RNA templates is influenced by secondary structure both 5′ and 3′ of the catalytic site. Nucleic Acids Res. 1998;26:3433–3442. doi: 10.1093/nar/26.14.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 18.Huang H, Chopra R, Verdine G L, Harrison S C. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science. 1998;282:1669–1675. doi: 10.1126/science.282.5394.1669. [DOI] [PubMed] [Google Scholar]
- 19.Jacobo-Molina A, Ding J, Nanni R G, Clarke A D J, Lu X, Tantillo C, Williams R L, Kamer G, Ferris A L, Clark P, Hizi A, Hughes S H, Arnold E. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 A resolution shows bent DNA. Proc Natl Acad Sci USA. 1993;90:6320–6324. doi: 10.1073/pnas.90.13.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones J S, Allan R W, Temin H M. One retroviral RNA is sufficient for synthesis of viral DNA. J Virol. 1994;68:207–216. doi: 10.1128/jvi.68.1.207-216.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Julias J G, Hash D, Pathak V K. E-vectors: development of novel self-inactivating and self-activating vectors for safer gene therapy. J Virol. 1995;69:6839–6846. doi: 10.1128/jvi.69.11.6839-6846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katz R A, Skalka A M. Generation of diversity in retroviruses. Annu Rev Genet. 1990;24:409–445. doi: 10.1146/annurev.ge.24.120190.002205. [DOI] [PubMed] [Google Scholar]
- 23.Kraulis P J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J Appl Cryst. 1991;24:946–950. [Google Scholar]
- 24.Merritt E A, Bacon D J. Raster3d: photorealistic molecular graphics. Methods Enzymol. 1997;277:505–524. doi: 10.1016/s0076-6879(97)77028-9. [DOI] [PubMed] [Google Scholar]
- 25.Najmudin S, Cote M L, Sun D, Yohannan S, Montano S, Gu J, Georgiadis M M. Crystal structures of an N-terminal fragment from Moloney murine leukemia virus reverse transcriptase complexed with nucleic acid: functional implications for template-primer binding to the fingers domain. J Mol Biol. 2000;296:613–632. doi: 10.1006/jmbi.1999.3477. [DOI] [PubMed] [Google Scholar]
- 26.Nicholls A, Sharp K, Honig B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins Struct Funct. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- 27.Pathak V K, Temin H M. Broad spectrum of in vivo forward mutations, hypermutations, and mutational hotspots in a retroviral shuttle vector after a single replication cycle: deletions and deletions with insertions. Proc Natl Acad Sci USA. 1990;87:6024–6028. doi: 10.1073/pnas.87.16.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peliska J A, Benkovic S J. Mechanism of DNA strand transfer reactions catalyzed by HIV-1 reverse transcriptase. Science. 1992;258:1112–1118. doi: 10.1126/science.1279806. [DOI] [PubMed] [Google Scholar]
- 29.Pelletier H, Sawaya M R, Kuman A, Wilson S H, Kraut J. Structures of ternary complexes of rat DNA polymerase β, a DNA template-primer, and ddCTP. Science. 1994;264:1891–1903. [PubMed] [Google Scholar]
- 30.Pfeiffer J, Topping R S, Shin N-H, Telesnitsky A. Altering the intracellular environment increases the frequency of tandem repeat deletion during Moloney murine leukemia virus reverse transcription. J Virol. 1999;73:8441–8447. doi: 10.1128/jvi.73.10.8441-8447.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasad V R, Goff S P. Linker insertion mutagenesis of human immunodeficiency virus reverse transcriptase expressed in bacteria: definition of the minimal polymerase domain. Proc Natl Acad Sci USA. 1989;86:3104–3108. doi: 10.1073/pnas.86.9.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pulsinelli G A, Temin H M. Characterization of large deletions occurring during a single round of retrovirus replication: novel deletion mechanism involving errors in strand transfer. J Virol. 1991;65:4786–4797. doi: 10.1128/jvi.65.9.4786-4797.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhode B W, Emerman M, Temin H M. Instability of large direct repeats in retrovirus vectors. J Virol. 1987;61:925–927. doi: 10.1128/jvi.61.3.925-927.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robson N D, Telesnitsky A. Effects of 3′ untranslated region mutations on plus-strand priming during Moloney murine leukemia virus replication. J Virol. 1998;73:948–957. doi: 10.1128/jvi.73.2.948-957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin N H, Hartigan-O'Connor D, Pfeiffer J K, Telesnitsky A. Replication of lengthened Moloney murine leukemia virus genomes is impaired at multiple stages. J Virol. 2000;74:2694–2702. doi: 10.1128/jvi.74.6.2694-2702.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanese N, Goff S P. Domain structure of the Moloney MuLV reverse transcriptase: mutational analysis and separate expression of the polymerase and RNAse H activities. Proc Natl Acad Sci USA. 1988;85:1777–1781. doi: 10.1073/pnas.85.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Telesnitsky A, Blain S, Goff S P. Assays for retroviral reverse transcriptase. Methods Enzymol. 1995;262:347–362. doi: 10.1016/0076-6879(95)62029-x. [DOI] [PubMed] [Google Scholar]
- 38.Telesnitsky A, Blain S W, Goff S P. Defects in Moloney murine leukemia virus replication caused by a reverse transcriptase mutation modeled on the structure of Escherichia coli ribonuclease H. J Virol. 1992;66:615–622. doi: 10.1128/jvi.66.2.615-622.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Telesnitsky A, Goff S P. Reverse transcriptase and the generation of retroviral DNA. In: Coffin J, Hughes S, Varmus H, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1997. pp. 121–160. [PubMed] [Google Scholar]
- 40.Telesnitsky A, Goff S P. Strong-stop strand transfer during reverse transcription. In: Skalka A M, Goff S P, editors. Reverse transcriptase. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1993. pp. 49–83. [Google Scholar]
- 41.Temin H M. Retrovirus variation and reverse transcription: abnormal strand transfers result in retrovirus genetic variation. Proc Natl Acad Sci USA. 1993;90:6900–6903. doi: 10.1073/pnas.90.15.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Topping R, Demoitie M-A, Shin N H, Telesnitsky A. Cis-acting elements required for strong stop acceptor template selection during Moloney murine leukemia virus reverse transcription. J Mol Biol. 1998;281:1–15. doi: 10.1006/jmbi.1998.1929. [DOI] [PubMed] [Google Scholar]
- 43.Wooley D P, Bircher L A, Smith R A. Retroviral recombination is nonrandom and sequence dependent. Virology. 1998;243:229–234. doi: 10.1006/viro.1998.9052. [DOI] [PubMed] [Google Scholar]
- 44.Wu V, Blumberg B M, Fay P J, Bambara R A. Strand transfer mediated by human immunodeficiency virus reverse transcriptase in vitro is promoted by pausing and results in misincorporation. J Biol Chem. 1995;270:325–332. doi: 10.1074/jbc.270.1.325. [DOI] [PubMed] [Google Scholar]
- 45.Xu H, Boeke J D. High-frequency deletion between homologous sequences during retrotransposition of Ty elements in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1987;84:8553–8557. doi: 10.1073/pnas.84.23.8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Temin H M. Retrovirus recombination depends on the length of sequence identity and is not error prone. J Virol. 1994;68:2409–2414. doi: 10.1128/jvi.68.4.2409-2414.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]