Abstract
This paper aims to compare the cellular immune response to the SARS-CoV-2 BNT162b2 vaccine of pediatric patients with autoimmune inflammatory rheumatic disease (pAIIRD) and healthy controls. A prospective longitudinal study was conducted between April 2021 and December 2022 at the Tel Aviv Medical Center. Children <18 years, with pediatric-onset AIIRD and healthy controls, who have received at least two doses of the BNT162b2 vaccine, were included. Humoral response was evaluated by serum levels of anti-SARS-CoV-2 receptor-binding domain antibodies. Cellular response was evaluated by flow cytometry, measuring IFNγ and TNFα production by CD4+ T cells following stimulation with SARS-CoV-2 Spike peptide mix. The study included 20 pAIIRD patients and 11 controls. The mean age of participants was 12.6 ± 2.94 years, with 58.1% females. The cellular response to the BNT162b2 vaccine was statistically similar in both groups. However, the humoral response was statistically lower in pAIIRD compared with the healthy control group. There was no statistically significant correlation between the humoral response and cellular response. During the study period, 43.75% of AIIRD children and 72.7% of controls had a breakthrough COVID-19 infection (P = 0.48). Bivariate models examining the effect of the cellular response and presence of an AIIRD on breakthrough infections found no effect. Compared with healthy controls, pAIIRD demonstrated similar cellular responses. Patients showed reduced humoral response compared with healthy adolescents, but similar breakthrough infection rates. These findings may support the importance of the cellular response in protecting against COVID-19 infections.
Keywords: BNT162b2 vaccine, cellular immunity, rheumatic diseases, juvenile arthritis, humoral immunity, COVID-19
Pediatric rheumatic patients may be vulnerable to COVID-19 infections due to their disease and immunosuppressive medications. Compared with healthy controls, pediatric patients with autoimmune inflammatory rheumatic diseases demonstrated similar cellular responses and COVID-19 breakthrough infection rates, but reduced humoral responses. These findings may support the importance of the cellular response in protecting against COVID-19 infections.
Graphical Abstract
Graphical Abstract.
Introduction
Since 2019, COVID-19 has been a global health concern. This concern has been more prominent among patients with autoimmune inflammatory rheumatic diseases (AIIRD), mainly due to the use of immune-modulating or immunosuppressive medications. Unlike adults, children, including those with AIIRD (pAIIRD), are usually asymptomatic or present with a mild illness [1]. However, severe morbidity and mortality have also been described due to viral complications including multisystem inflammatory syndrome in children (MIS-C) [1–3]. Therefore, pAIIRD patients are recommended to receive anti-SARS-CoV-2 vaccines [4].
There is evidence showing that there is mostly an effective humoral immune response to the SARS-CoV-2 vaccine; however, absolute anti-SARS-CoV-2 antibody levels of AIIRD patients are mildly decreased compared to healthy controls, in both adults and adolescents. Nevertheless, this mildly decreased humoral response does not translate to a higher rate of breakthrough COVID-19 infections [5–12]. In general, studies show that medications targeting cellular components such as rituximab, abatacept, mycophenolate, and high-dose glucocorticoids have a larger impact on humoral immune response than anti-cytokine treatments [11, 13].
Viruses in general and SARS-CoV-2, in particular, are thought to be controlled mainly through cellular immune mechanisms and to a lesser extent through humoral mechanisms [14–16]. In adults with AIIRD, several studies found that the cellular immune response to anti-SARS-CoV-2 vaccines is dependent on age and the immunosuppressive medication used and may be diminished compared with healthy controls [17–19]. The data regarding the cellular immune response to the BNT162b2 mRNA vaccine in pAIIRD patients are scarce [12].
In this study, we aimed to examine the cellular response of children and adolescents with pAIIRD to the BNT162b2 vaccine and compare it to healthy controls.
Materials and methods
In this prospective longitudinal study, we evaluated the response of AIIRD patients to the SARS-CoV-2 BNT162b2 PfizerBioNTech mRNA vaccine [10, 11, 20]. The study was conducted between April 2021 and December 2022 at the Pediatric Rheumatology service of the Dana-Dwek Children’s Hospital of the Tel Aviv Sourasky Medical Center, Israel.
The primary endpoint was the cellular response to the vaccine, evaluated by the production of IFNγ and TNFα following stimulation with SARS-CoV-2 Spike peptide mix.
Secondary endpoints included the correlation between cellular and humoral responses to the vaccine, and the impact of cellular immunity on the clinical efficacy of the vaccine, as clinically evaluated by breakthrough COVID-19 infections.
Study population and procedures
The study included children under the age of 18 years, with pAIIRD (N = 20) and healthy controls (N = 11), who have received at least two doses of the BNT162b2 vaccine. The vaccine was given, as instructed by the company, in two doses, 3 weeks apart, by an intramuscular injection of a 10 or 30 μg dose for children < 11 years and those over 11 years, respectively.
Children with pAIIRD included those classified according to the relevant criteria or diagnosed clinically when there are no available criteria—juvenile idiopathic arthritis (JIA), inflammatory bowel disease (IBD) associated arthritis [21], systemic lupus erythematosus (SLE) [22], scleroderma, inflammatory myopathies [23], vasculitis, and uveitis associated with systemic diseases. Patients were instructed to continue their immunomodulatory medications without interruption during the vaccination period.
Exclusion criteria for healthy controls were a diagnosis of an autoimmune or autoinflammatory disease and an immunosuppressive or immunomodulatory treatment. Demographic and clinical data were collected from participants and medical records.
All participants and their parents gave written informed consent before entering the study. The study was approved by the institutional review board (TLV-21-0175).
Blood samples were drawn at least 2 weeks after the second vaccine dose, and up to a year after the first vaccine dose; for all 11 controls, and 14 of the pAIIRD patients samples were collected 2–8 weeks after the second dose of the BNT162b2 vaccine, for another 5 pAIIRD patients samples were collected 6 months after the first vaccine (3 of them had a breakthrough COVID-19 before the sample for cellular immunogenicity was taken), and for another patient, who also had a breakthrough COVID-19, a sample was collected 1 year after the first vaccine dose.
Cellular immune response
Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll gradient density. Following isolation, cells were stored at −80 °C for later flow cytometry-based peptide-induced intracellular cytokine staining.
T-cell response was assessed by stimulating donor PBMCs with pooled peptide mix in the presence of a protein transport inhibitor, followed by staining for the activation marker (CD40L) and intracellular cytokines (TNFα and IFNγ). For this purpose, we used SARS-CoV-2 T Cell Analysis Kits for human PBMCs (Cat# 130-128-156, Miltenyi Biotec, Germany), and the assay was performed according to manufacturer instructions. Briefly, donor PBMCs were plated in a 96-well plate at a concentration of 0.5–1 × 106 PBMCs/100 μl and incubated at 37°C and 5% CO2 with 2 μl of pooled Wuhan wild-type Spike glycoprotein (S)-peptide mix (Cat# 130-127-951, Miltenyi Biotec), covering the whole protein sequence of the S-protein. CytoStimTM reagent was used as positive control and 10% DMSO in sterile water as negative control. After 2 h, Brefeldin A was added to each well, and cells were incubated for an additional 4 h. Cells were then stained with viability dye, followed by fixation, permeabilization, and staining for surface markers (CD3, CD20, CD14, CD4, CD8, and CD154) and intracellular cytokines (TNFα and IFNγ). Following staining, samples were acquired using BD FACSCanto II, and 20 000 CD4+ events were collected for each sample.
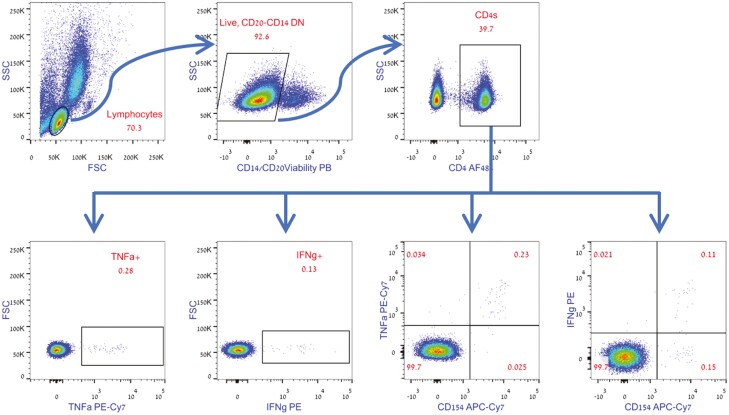
Analysis was performed on gated CD4+ T cells and the absolute number of activated CD154+, INFγ+, or TNFα+ cells was recorded and normalized for 1 × 106 CD4+ T cells (see Figure 1A for gating strategy). In order to calculate the actual response rate, the calculated number of positive events (per 1 × 106 CD4+ T cells) in the unstimulated negative control was deducted from the calculated number of events (per 1 × 106 CD4+ T-cells) in the peptide-stimulated samples, as shown in the following formula:
Figure 1.
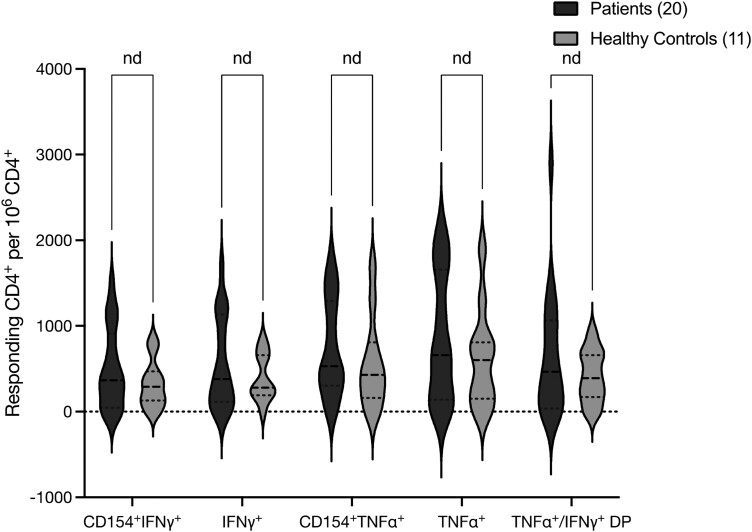
A: Representative gating strategy. The CD4+ population was gated based on positive staining for CD4, out of the live, CD14 negative, CD20 negative lymphocytes. Percent of intracellularly stained, cytokine-positive or cytokine-CD154 double-positive cells was gated out of the CD4+ population. The absolute numbers of the recorded TNFα, IFNγ, or cytokine-CD154 double-positive cells were used to calculate the number of positive events per 1 × 106 CD4+ T cells. B: Cellular immune responses of pediatric AIIRD patients compared with controls, as measured by IFNγ and TNFα production after stimulation of CD4+ T lymphocytes with SARS-CoV-2 spike-protein peptide mix.
In order to overcome possible variations between runs, the assay was performed at the same time on frozen cells collected at different time points.
Humoral immune response
Humoral response was evaluated by serum immunoglobulin G (IgG) levels against the SARS-CoV-2 spike receptor-binding domain (RBD). The assay used was the FDA emergency-authorized SARS-CoV-2 IgG II Quant assay (ARCHITECT, Abbott, IL, USA) that provided a qualitative and quantitative determination of anti-SARS-CoV-2 RBD IgG antibody levels (SARS-CoV-2 IgG II Quant, Cat#:6S60 Abbott, Ireland) which indicates the presence of neutralizing antibodies against SARS-CoV-2. The clinical sensitivity and specificity of this test are reported to be 98.1% and 99.6%, respectively [24]. Anti-RBD levels are reported in arbitrary units (AU)/ml, ranging between 0 and 40 000 AU/ml, and a level ≥ 50 AU/ml is considered positive by the manufacturer, while the Israeli Ministry of Health uses a cutoff of ≥ 150 AU/ml.
Breakthrough COVID-19 infections, defined by the Center for Disease Control as COVID-19 infections reported ≥ 14 days after the second vaccine dose [25], were reported by the participants following a PCR or an antigen test positive for SARS-CoV-2.
Statistical analysis
Cellular and humoral responses, as well as breakthrough COVID-19 infections, and the correlation between these variables were compared between pAIIRD patients (N = 20) and healthy controls (N = 11). To validate the results, we also analyzed the group of participants in which a sample was taken 2–8 weeks after the second BNT162b2 vaccine dose, which contained 14 pAIIRD children and all 11 controls, referred to in the results as the ‘2–8 weeks subgroup’. Importantly none of the participants in the subgroup had a breakthrough COVID-19 infection before the sample collection.
Categorical variables are presented as absolute and relative frequencies. Continuous variables are presented as mean and SD or median and range. Differences between continuous variables were tested for significance using the independent sample T-test. Differences between categorical variables were tested for significance using the Chi-square test or the Fisher exact test, as appropriate. Correlations between continuous variables were measured using the Pearson correlation. Survival models were built using the Cox regression method. Data were analyzed using R version 4.3.1 (R Development Core Team, Vienna, Austria).
Results
Study population
The study included 20 pAIIRD patients and 11 healthy controls. The mean age of participants was 12.05 ± 3.5 for AIIRD patients and 13.64 ± 1.21 years for controls, P = 0.075, with 65% (n = 13) vs. 45.5% (n = 5) females, respectively, P = 0.45.
The pAIIRD patients’ diagnoses included 8 (40%) with JIA, 3 (15%) each with SLE, scleroderma, and uveitis, and 1 (5%) each with either vasculitis, myositis, or inflammatory bowel disease (IBD) with arthritis. Immunomodulating treatments used included adalimumab in 8 patients (40%, 7 JIA, and one IBD-associated arthritis), rituximab in two patients (10%), both patients also received another immunosuppression—an ANCA-associated vasculitis patient was co-treated with azathioprine, and an SLE patient also received mycophenolate mofetil (MMF) and hydroxychloroquine (HCQ). In addition, there was one JIA patient treated with tocilizumab. Methotrexate was used in five patients (25%, one each with JIA, myositis, and scleroderma and 2 with uveitis); MMF in three patients (15%, including one SLE patient mentioned above, and two scleroderma patients, one of them also treated with abatacept). Two SLE patients received only HCQ (Supplementary Table S1).
Four of the pAIIRD participants had a breakthrough COVID-19 infection before the sample collection for the study was taken. The timing of the collection was 6 months after the vaccine for 3 participants and one year for one participant. These four patients were excluded from the ‘2–8 weeks subgroup’ analysis (see Supplementary Table S2 for their characteristics).
Cellular and humoral immune responses to the BNT162b2 vaccine
Cellular reactivity was evaluated using flow cytometry. Although samples of pAIIRD patients showed a higher average number of responding cells compared with controls, it did not reach statistical significance. As such, the calculated number of responding CD4+ T-cells staining positive for TNFα (median number per 1 × 106 CD4+ T-cells (range)) was 660 (0–2140) in pAIIRD patients vs. 600 (0–1890) in healthy controls (P = 0.36); for CD4+ IFNγ+, 380 (0–1700) vs. 280 (0–840) (P = 0.27), and for CD4+, TNFα+, and IFNγ+ double-positive 465 (0-2900) vs. 390 (0–920) (P = 0.22). The results were similar when comparing the calculated numbers of CD4+CD154+TNFα+ cells or CD4+ CD154+ IFNγ+ cells (Fig. 1B).
However, the humoral response, as measured by anti-RBD antibody levels, was statistically higher in the control group compared with pAIIRD (median (range)) 34 837.9 AU/ml (3716.7–40 000) vs. 6789.6 AU/ml (243–23 274.7), respectively, P < 0.001). The results were similar for the cellular and humoral responses when only ‘2–8 weeks subgroup’ patients were included (Table 1).
Table 1.
Cellular and humoral immune responses, 2–8 weeks after the second BNT162b2 vaccine dose, in AIIRD patients and healthy controlsa
| TNFα+IFNγ+b | TNFα+b | IFNγ+b | Anti-RBD Ab levels (AU/ml) | |
|---|---|---|---|---|
| AIIRD | 644.5 ± 718.0 | 870 ± 746.51 | 554 ± 527.82 | 7792.64 ± 6828.84 |
| AIIRD subgroup (n = 14) | 442.14 ± 14 | 795 ± 809.2 | 514.29 ± 563.16 | 8698.17 ± 7221.56 |
| Controls | 410 ± 307.02 | 625.46 ± 579.45 | 392.73 ± 272.55 | 28,141.16 ± 14,194.52 |
| P-value for all AIIRD | 0.216 | 0.355 | 0.271 | ≤0.001 |
| P-value for AIIRD subgroup | 0.853 | 0.564 | 0.487 | ≤0.001 |
aCellular immune response is measured by TNFα and IFNγ production by CD4+ T lymphocytes. The humoral immune response is measured by anti-RBD IgG antibody levels.
bNumbers represent the calculated absolute CD4+ T-cell staining positive for the suggested cytokines, per 1 × 106 CD4+ T cells.
Values are reported as mean ± SD. AIIRD, autoimmune inflammatory rheumatic disease patients; TNFα, tumor necrosis factor α; IFNγ, interferon γ; RBD, receptor-binding domain; AU, arbitrary units.
There was no statistically significant correlation between the humoral response and cellular responses: TNFα R = 0.097 (P = 0.65), IFNγ R = 0.275 (P = 0.19), TNFα and IFNγ co-production R = 0.328 (P = 0.12).
Although most participants showed a positive cellular response in all three parameters measured (IFNγ, TNFα, or co-production of INFγ and TNFα), one healthy control did not have any reaction to the stimulation, and another control had only an IFNγ response. One patient with uveitis treated with adalimumab did not respond by any parameter, another uveitis patient treated with methotrexate showed only a TNFα response, and a third patient, with SLE, treated with HCQ and eltrombopag for chronic thrombocytopenia, had only an IFNγ response.
Breakthrough COVID-19 infections
During the study period, 11 out of 20 (55%) pAIIRD patients and 8 of 11 (72.7%) controls had a breakthrough COVID-19 infection (P = 0.82; Supplementary Fig. S1). Bivariate models examining the effect of the cellular response and the presence of a pAIIRD on breakthrough COVID-19 infections found no effect of these variables in all participants and also in the 2–8 weeks subgroup (Table 2). Results were similar regarding breakthrough infections and their association with cellular response when only the 2–8 weeks subgroup was included.
Table 2.
Bivariate models for the effect of cellular immune responses on breakthrough COVID-19 infections, adjusted for study group
| Variablea | All AIIRD hazard ratio (95% CI) | P-value | Subgroup hazard ratio (95% CI) | P-value |
|---|---|---|---|---|
| TNFα | 1.0 (0.999–1.001) | 0.905 | 1.0 (0.999–1.001) | 0.808 |
| IFNγ | 1.0 (0.999–1.001) | 0.981 | 1.0 (0.999–1.001) | 0.835 |
| TNFα+IFNγ | 1.0 (1.000–1.001) | 0.32 | 0.999 (0.997–1.001) | 0.35 |
aRefers to the calculated of responding CD4+ cells per 1 × 106 CD4+ T cells.
Discussion
This prospective longitudinal study examined the cellular immune response of pediatric patients with AIIRD versus healthy controls following the BNT162b2 mRNA COVID-19 vaccine. Humoral response and breakthrough COVID-19 infections were recorded as well. Children and adolescents with AIIRD demonstrated a comparable cellular response to that of healthy adolescents, with even slightly higher response in the pAIIRD patients than controls, though not statistically significant.
However, the production of anti-RBD antibodies in response to the anti-SARS-COV-2 BNT162b2 mRNA vaccine was statistically significantly lower in pAIIRD. Despite that, the rate of breakthrough infections was similar between patients and controls, which supports the hypothesis that adaptive cellular immunity has an important role in the protection against viral infections, and may be more important or compensate for an impaired humoral response [15].
This is one of few studies to report on the cellular immune response to mRNA COVID-19 vaccines in AIIRD patients, and it is the first to include young rheumatic children under 12. In the only study to date to report on AIIRD adolescents by Udaondo et al., 40 patients aged 12–18 years with AIIRD showed no difference in the humoral and cellular response compared with 24 healthy controls. In that study, 40% of the patients and 53% of the controls had had COVID-19 infection before sample collection, and the recovered participants had a statistically significant higher IFNγ and IL2 response compared with naive patients [12]. In our study, we included participants who did not have COVID-19 before the initiation of the vaccination procedure. Only four participants (20% of the AIIRD) had COVID-19 infection before sample collection and thus we also included an additional analysis of a more homogeneous group of participants whose sample collection was in 2–8 weeks after the vaccine, with none of them being infected with COVID-19 before sample collection. Our results are also mostly in accordance with studies reporting on adult AIIRD, where the cellular, as well as humoral responses to anti-SARS-CoV-2 vaccines or COVID-19 infection, were dependent on the immunosuppressive medications used and the age of the patients. Nemeth et al. found that the cellular response in rheumatoid arthritis patients was lower than in healthy controls, but this was mainly driven by patients treated with CD20 depletion therapies, and older age was associated with a lower response [17]. Petrone et al. found a comparable IFNγ cellular response in patients with immune-mediated diseases, compared with healthy controls, but diminished response in patients treated with abatacept or TNFα inhibitors [18]. Filippini et al. found that AIIRD patients were able to mount a cellular response, while abatacept-treated patients had a lower humoral response [19]. In our study, two patients received rituximab and one received abatacept, and they all had a detectable cellular response. Concerning their humoral response, all three patients were seropositive with antibody levels of 956, 3890, and 18 520 AU/ml, and notably, the antibody levels were in the lower range for 2 out of these patients (one treated with rituximab and MMF and the other treated with abatacept and MMF).
The limitations of this study include the small number of participants, which is unfortunately not uncommon for studies in young patients with AIIRD. Due to the small number of participants, we could not assess the difference in cellular and humoral immunity in subsets of patients according to their AIIRD diagnosis or immunomodulating treatment.
Another limitation is that, unlike anti-SARS-CoV-2 antibody assays, there are no clinically validated cellular reactivity assays, or accepted cutoffs to define cellular response/ reactivity. For this reason, we used several parameters to evaluate cellular reactivity, including CD154 expression, as well as IFNγ and TNFα production, in an attempt to identify cellular response more accurately, specifically in AIIRD patients treated with medications that may affect or suppress cytokine production.
To summarize, even though patients with pAIIRD are being treated with immunomodulation and may also have an underlying immune dysregulation, they demonstrated an overall good cellular immune response following BNT162b2 mRNA COVID-19 vaccine. In addition, they did not show an increased breakthrough infection rate compared to healthy adolescents, despite reduced humoral response. Our data may support the importance of the cellular immune response in the protection against SARS-CoV-2 infections.
Supplementary data
Supplementary data is available at Clinical and Experimental Immunology online.
Contributor Information
Tali Eviatar, Rheumatology Department, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; School of Medicine, Faculty of Medical and Health Sciences, Tel Aviv University, Tel Aviv, Israel.
Adi Pappo, Pediatric Rheumatology Unit, Schneider Children’s Hospital, Petach Tikva, Israel.
Tal Freund, School of Medicine, Faculty of Medical and Health Sciences, Tel Aviv University, Tel Aviv, Israel; Department of Allergy and Clinical Immunology, Tel-Aviv Sourasky Medical Center, Tel Aviv, Israel.
Yishai Friedlander, StatistiX, Beer Tuvya, Israel.
Ori Elkayam, Rheumatology Department, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; School of Medicine, Faculty of Medical and Health Sciences, Tel Aviv University, Tel Aviv, Israel.
David Hagin, School of Medicine, Faculty of Medical and Health Sciences, Tel Aviv University, Tel Aviv, Israel; Department of Allergy and Clinical Immunology, Tel-Aviv Sourasky Medical Center, Tel Aviv, Israel.
Merav Heshin-Bekenstein, School of Medicine, Faculty of Medical and Health Sciences, Tel Aviv University, Tel Aviv, Israel; Pediatric Rheumatology Service, Dana Children’s Hospital of Tel Aviv Sourasky Medical Center, Tel Aviv, Israel.
Ethical approval
All participants and their parents gave written informed consent before entering the study. The study was approved by the institutional review board (TLV-21-0175).
Conflict of Interests
The authors have no conflict of interest to declare in regard to this study.
Funding
This study did not receive support in the form of funding or grants.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Kearsley-Fleet L, Chang M-L, Lawson-Tovey S, Costello R, Fingerhutová S, Švestková N, et al.; CARRA Registry Investigators. Outcomes of SARS-CoV-2 infection among children and young people with pre-existing rheumatic and musculoskeletal diseases. Ann Rheum Dis 2022, 81, 998–1005. doi: 10.1136/annrheumdis-2022-222241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, et al.; Overcoming COVID-19 Investigators. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021, 325, 1074–87. doi: 10.1001/jama.2021.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Son MBF, Murray N, Friedman K, Young CC, Newhams MM, Feldstein LR, et al.; Overcoming COVID-19 Investigators. Multisystem inflammatory syndrome in children - initial therapy and outcomes. N Engl J Med 2021, 385, 23–34. doi: 10.1056/NEJMoa2102605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jansen MHA, Rondaan C, Legger GE, Minden K, Uziel Y, Toplak N, et al. EULAR/PRES recommendations for vaccination of paediatric patients with autoimmune inflammatory rheumatic diseases: update 2021. Ann Rheum Dis 2023, 82, 35–47. doi: 10.1136/annrheumdis-2022-222574 [DOI] [PubMed] [Google Scholar]
- 5. Akgün O, Çakmak F, Guliyeva V, Demirkan FG, Tanatar A, Hançerli Torun S, et al. Humoral response and safety of BNT162b2 mRNA vaccine in children with rheumatic diseases. Rheumatology (Oxford) 2022, 61, 4482–90. doi: 10.1093/rheumatology/keac140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tzioufas AG, Bakasis A-D, Goules AV, Bitzogli K, Cinoku II, Chatzis LG, et al. A prospective multicenter study assessing humoral immunogenicity and safety of the mRNA SARS-CoV-2 vaccines in Greek patients with systemic autoimmune and autoinflammatory rheumatic diseases. J Autoimmun 2021, 125, 102743. doi: 10.1016/j.jaut.2021.102743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sieiro Santos C, Calleja Antolin S, Moriano Morales C, Garcia Herrero J, Diez Alvarez E, Ramos Ortega F, et al. Immune responses to mRNA vaccines against SARS-CoV-2 in patients with immune-mediated inflammatory rheumatic diseases. RMD Open 2022, 8, e001898. doi: 10.1136/rmdopen-2021-001898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deepak P, Kim W, Paley MA, Yang M, Carvidi AB, Demissie EG, et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a prospective cohort study. Ann Intern Med 2021, 174, 1572–85. doi: 10.7326/M21-1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dimopoulou D, Vartzelis G, Dasoula F, Tsolia M, Maritsi D.. Immunogenicity of the COVID-19 mRNA vaccine in adolescents with juvenile idiopathic arthritis on treatment with TNF inhibitors. Ann Rheum Dis 2022, 81, 592–3. doi: 10.1136/annrheumdis-2021-221607 [DOI] [PubMed] [Google Scholar]
- 10. Heshin-Bekenstein M, Ziv A, Toplak N, Lazauskas S, Kadishevich D, Ben-Nun Yaari E, et al. Safety and immunogenicity following the second and third doses of the BNT162b2 mRNA COVID-19 vaccine in adolescents with juvenile-onset autoimmune inflammatory rheumatic diseases: a prospective multicentre study. Vaccines (Basel) 2023, 11, 819. doi: 10.3390/vaccines11040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021, 80, 1330–1338. doi: 10.1136/annrheumdis-2021-220647 [DOI] [PubMed] [Google Scholar]
- 12. Udaondo C, Cámara C, Miguel Berenguel L, Alcobendas Rueda R, Muñoz Gómez C, Millán Longo C, et al. Humoral and cellular immune response to mRNA SARS-CoV-2 BNT162b2 vaccine in adolescents with rheumatic diseases. Pediatr Rheumatol Online J 2022, 20, 64. doi: 10.1186/s12969-022-00724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gianfrancesco MA, Hyrich KL, Gossec L, Strangfeld A, Carmona L, Mateus EF, et al.; COVID-19 Global Rheumatology Alliance Steering Committee. Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries. Lancet Rheumatol 2020, 2, e250–3. doi: 10.1016/S2665-9913(20)30095-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moga E, Lynton-Pons E, Domingo P.. The robustness of cellular immunity determines the fate of SARS-CoV-2 infection. Front Immunol 2022, 13, 904686. doi: 10.3389/fimmu.2022.904686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rouse BT, Mueller SN.. Host defenses to viruses. In: Rich RR, Fleisher TA, Shearer WT, Schroeder HW, Frew AJ, Weyand CM (eds), Clinical Immunology. Elsevier; 2019. p. 365–74.e1.doi:10.1016/B978-0-7020-6896-6.00025-9. [Google Scholar]
- 16. Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol 2022, 23, 186–93. doi: 10.1038/s41590-021-01122-w [DOI] [PubMed] [Google Scholar]
- 17. Nemeth D, Vago H, Tothfalusi L, Ulakcsai Z, Becker D, Szabo Z, et al. Factors influencing the SARS-CoV-2 infection and vaccination induced immune response in rheumatoid arthritis. Front Immunol 2022, 13, 960001. doi: 10.3389/fimmu.2022.960001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petrone L, Picchianti-Diamanti A, Sebastiani GD, Aiello A, Laganà B, Cuzzi G, et al. Humoral and cellular responses to spike of δ SARS-CoV-2 variant in vaccinated patients with immune-mediated inflammatory diseases. Int J Infect Dis 2022, 121, 24–30. doi: 10.1016/j.ijid.2022.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Filippini F, Giacomelli M, Bazzani C, Fredi M, Semeraro P, Tomasi C, et al. Efficacy of COVID-19 mRNA vaccination in patients with autoimmune disorders: humoral and cellular immune response. BMC Med 2023, 21, 210. doi: 10.1186/s12916-023-02868-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heshin-Bekenstein M, Ziv A, Toplak N, Hagin D, Kadishevich D, Butbul YA, et al. Safety and immunogenicity of BNT162b2 mRNA COVID-19 vaccine in adolescents with rheumatic diseases treated with immunomodulatory medications. Rheumatology (Oxford, England) 2022, 61, 4263–72. doi: 10.1093/rheumatology/keac103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al.; International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004, 31, 390–2. [PubMed] [Google Scholar]
- 22. Petri M, Orbai AM, Alarcõn GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012, 64, 2677–86. doi: 10.1002/art.34473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lundberg IE, Tjärnlund A, Bottai M, Werth VP, Pilkington C, Visser Marianne de, et al.; International Myositis Classification Criteria Project consortium, The Euromyositis register and The Juvenile Dermatomyositis Cohort Biomarker Study and Repository (JDRG) (UK and Ireland). EULAR/ACR classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 2017, 76, 1955–64. doi: 10.1136/annrheumdis-2017-211468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laboratories Inc A. AdviseDx SARS-CoV-2 IgG II - Healthcare Provider Fact Sheet. https://www.fda.gov/media/146369/download [Google Scholar]
- 25. Birhane M, Bressler S, Chang G, et al. COVID-19 Vaccine breakthrough infections reported to CDC – United States, January 1–April 30, 2021. MMWR Morb Mortal Wkly Rep 2021, 70, 792–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.