Abstract
This study examines the biological effects of palbociclib and ribociclib in hormone receptor-positive breast cancer, pivotal to the HARMONIA prospective phase III clinical trial. We explore the downstream impacts of these CDK4/6 inhibitors, focusing on cell lines and patient-derived tumor samples. We treated HR+ breast cancer cell lines (T47D, MCF7, and BT474) with palbociclib or ribociclib (100 nM or 500 nM), alone or combined with fulvestrant (1 nM), over periods of 24, 72, or 144 h. Our assessments included PAM50 gene expression, RB1 phosphorylation, Lamin-B1 protein levels, and senescence-associated β-galactosidase activity. We further analyzed PAM50 gene signatures from the CORALLEEN and NeoPalAna phase II trials. Both CDK4/6 inhibitors similarly inhibited proliferation across the cell lines. At 100 nM, both drugs partially reduced p-RB1, with further decreases at 500 nM over 144 h. Treatment led to reduced Lamin-B1 expression and increased senescence-associated β-galactosidase activity. Both drugs enhanced Luminal A and reduced Luminal B and proliferation signatures at both doses. However, the HER2-enriched signature significantly diminished only at the higher dose of 500 nM. Corresponding changes were observed in tumor samples from the CORALLEEN and NeoPalAna studies. At 2 weeks of treatment, both drugs significantly reduced the HER2-enriched signature, but at surgery, this reduction was consistent only with ribociclib. Our findings suggest that while both CDK4/6 inhibitors effectively modulate key biological pathways in HR+/HER2- breast cancer, nuances in their impact, particularly on the HER2-enriched signature, are dose-dependent, influenced by the addition of fulvestrant and warrant further investigation.
Subject terms: Breast cancer, Cell biology
Introduction
The addition of cyclin-dependent kinase 4/6 (CDK4/6) inhibitors to endocrine therapy in the treatment of patients with advanced hormone receptor-positive and HER2-negative (HR+/HER2-) breast cancer has significantly improved survival outcomes becoming the first-line standard of care therapy in this group of patients1–11. However, not all patients benefit to the same extent and efforts to identify biomarkers of sensitivity and resistance are ongoing.
HR+/HER2- breast cancer can be classified into four main molecular subtypes using gene expression profiling (PAM50) (i.e., Luminal A, Luminal B, HER2-enriched, and Basal-like)12. Of note, 5%–20% of HR+/HER2- tumors do not fall into the Luminal A or B subtypes but rather fall into the HER2-enriched phenotype13,14. Moreover, a higher proportion of the HER2-enriched subtype is detected in metastases compared to primary HR+/HER2- tumors, while the proportion of the Luminal A subtype is lower in metastases and the proportion of Luminal B and Basal-like subtypes is similar in metastases and primary tumors15.
The ability of the molecular subtypes to predict benefit from CDK4/6 inhibitors in breast cancer has been evaluated in samples from the PALOMA-216, the NeoPalAna17, and the MONALEESA-2,-3, and -718 studies. A retrospective analysis of the PALOMA-2 trial, which randomized patients with HR+/HER2‒ advanced breast cancer to letrozole +/− palbociclib, analyzed the molecular subtype of 455 tumors. Whilst Luminal A (50%) and Luminal B (30%) subtypes benefited from the addition of palbociclib to letrozole, the HER2-enriched (18.7%) and Basal-like (0.5%) subtypes were associated with worse progression-free survival (PFS) in both treatment arms compared to the Luminal A group16. In the NeoPalAna study, which evaluated the effects of palbociclib plus anastrazole in patients with primary breast cancer, PAM50 subtype was determined in 32 tumors at baseline. Of note, two tumors with non-luminal subtypes were identified, both of which were resistant to palbociclib17. Additionally, the SOLTI-1303 PATRICIA study of palbociclib and trastuzumab in HR+/HER2 + advanced breast cancer showed that the Luminal A and B subtypes benefited substantially from palbociclib, while the HER2-enriched group had very small absolute benefit19. In contrast, a retrospective pooled analysis of the MONALEESA-2, -3, and -7 pivotal trials with ribociclib and endocrine therapy evaluated the PAM50 subtype of 1,160 tumor samples. Except for Basal-like tumors (2.6%), all other intrinsic subtypes showed a consistent PFS and overall survival (OS) benefit from the combination of endocrine therapy and ribociclib over endocrine therapy alone, with the HER2-enriched subtype (12.7%) exhibiting the highest relative and absolute benefit18,20.
In retinoblastoma (RB1)-competent cells, CDK4/6 inhibitors trigger cell cycle arrest by reducing the phosphorylation of downstream RB1 tumor suppressor protein and can also induce cellular senescence. Palbociclib and ribociclib are CDK4/6 inhibitors of similar structure that selectively bind to the ATP-binding pocket of CDK4 (palbociclib IC50 = 9–11 nM, ribociclib IC50 = 10 nM) and CDK6 (palbociclib IC50 = 15 nM, ribociclib IC50 = 39 nM)21,22. One could argue that despite both being CDK4/6 inhibitors their slightly different chemical structures23,24, mechanisms of action25 and pharmacokinetics24–26 might lead to dissimilarities in efficacy. On the other hand, in clinical practice palbociclib is given at a lower dose than ribociclib (125 mg daily vs 600 mg daily, respectively)27, which could indicate a dose-dependent efficacy of CDK4/6 inhibitors in this biologically aggressive subtype. These differences may have relevant clinical implications as ribociclib, but not palbociclib, has shown clinical benefit in other clinical scenarios such as high-risk early breast cancer28–31.
Here, we assess the biological changes that occur upon CDK4/6 inhibition in breast cancer cell lines and clinical samples of patients who participated in two neoadjuvant phase II studies of ribociclib or palbociclib in combination with endocrine therapy.
Results
Phenotypic changes in breast cancer cell lines during CDK4/6 inhibition
The biological changes that occur during CDK4/6 inhibition were assessed in T47D (HR+/HER2-/HER2-enriched), MCF7 (HR+/HER2-/Luminal B), and BT474 (HR+/HER2+ /HER2-enriched) breast cancer cell lines. Despite being HER2+ , the inclusion of the BT474 cell line to our study could be of relevance since it belongs to the HER2-enriched subtype by PAM50, and palbociclib has been shown to be less efficient in HR+/HER2+/HER2-enriched breast cancer19. Moreover, it has been shown that, except for the amplification and RNA/protein overexpression of ERBB2 in HER2+ tumors, very minor biological differences exist at DNA, RNA, and protein levels between HER2+/HER2-enriched and HER2-/HER2-enriched tumors32.
First, we analyzed the proliferation inhibitory effect of palbociclib and ribociclib +/− fulvestrant against all three cell lines and found that both CDK4/6 inhibitors had very similar effects (Fig. 1A, Supplementary Fig. 1A). In T47D and MCF7 cells, the combination of CDK4/6 inhibitors plus fulvestrant was superior than palbociclib or ribociclib alone, while in BT474 no significant differences were observed between the combination of CDK4/6 inhibitors with fulvestrant and palbociclib or ribociclib alone (Supplementary Fig. 2A).
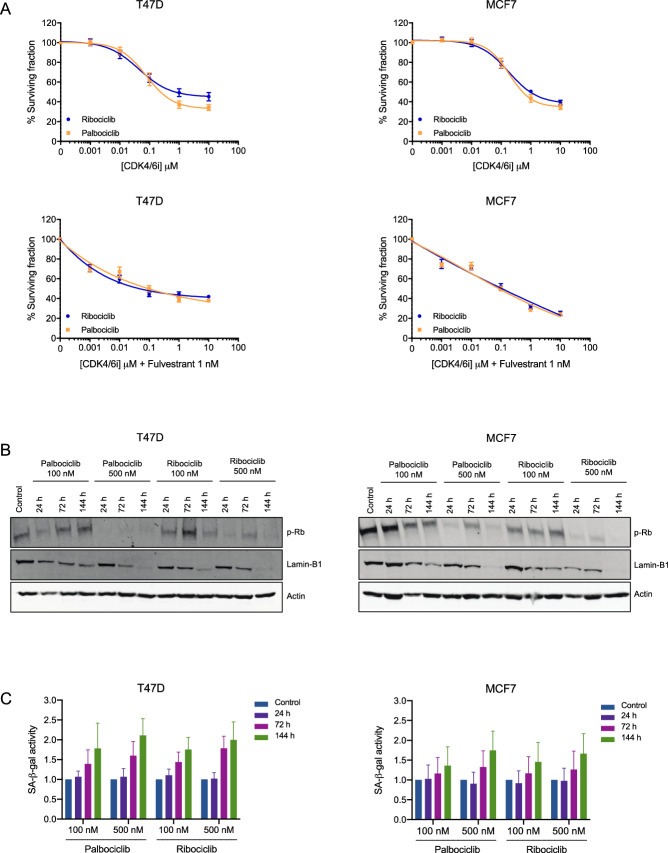
Figure 1.
Biological changes during CDK4/6 inhibition in vitro. (A) T47D and MCF7 cells were treated with increasing doses of palbociclib or ribociclib +/− fulvestrant (1 nM) for 72 h. Shown are representative graphs of cell viability redouts determined by Hoechst 33342. Data was normalised to untreated cells and three independent experiments were performed. Mean values ± SEM are shown. (B) T47D and MCF7 cells were treated with palbociclib or ribociclib (100 or 500 nM) for 24, 72 or 144 h and expression of p-RB1 and Lamin-B1 was assessed by western blot. Actin was used as a loading control. (C) T47D and MCF7 cells were treated with palbociclib or ribociclib (100 or 500 nM) for 24, 72 or 144 h and SA-β-gal activity was determined by flow cytometry. Data was normalised to untreated cells and three independent experiments were performed. Mean values ± SEM are shown.
RB1-competent T47D and MCF7 cell lines were treated with two different doses of palbociclib or ribociclib (i.e., 100 or 500 nM) for different periods of time (i.e., 24, 72, or 144 h) in order to assess changes in the phosphorylation of RB1 (p-RB1). In T47D and MCF7 cells treatment with 100 nM CDK4/6 inhibitors partially reduced p-RB1 with a further decrease in cells treated with 500 nM palbociclib or ribociclib regardless of treatment duration (Fig. 1B). Total RB1 mRNA expression did not change after CDK4/6 inhibition (Supplementary Fig. 3). The cytotoxic effect of palbociclib and ribociclib was also assessed in the RB1-mutated MDA-MB-468 cell line to ensure that no off-target effects were given with the chosen doses (i.e., 100 or 500 nM) (Supplementary Fig. 2B).
Next, the effect on cellular senescence was assessed upon treatment with CDK4/6 inhibitors by determining protein expression levels of Lamin-B1, a structural component of the nucleus whose loss has been associated with senescence33,34. In T47D and MCF7 cells, CDK4/6 inhibitors decreased the expression of Lamin-B1 in a time and dose-dependent manner, with the lowest expression levels corresponding to those treated with 500 nM palbociclib or ribociclib for 144 h (Fig. 1B). Additionally, we assessed the senescence-associated β-galactosidase (SA-β-gal) activity. Both palbociclib and ribociclib (100 or 500 nM) increased β-galactosidase staining in T47D and MCF7 cell lines after 72 or 144 h treatments compared to non-treated controls indicating induction of senescence35 (Fig. 1C). Although no significant differences were observed between treatment conditions, a dose- and time-related tendency was noticeable, with the highest β-galactosidase staining levels corresponding to cells treated with 500 nM CDK4/6 inhibitors for 144 h. Similar changes were observed in cells treated with palbociclib or ribociclib for all treatment conditions (Fig. 1C).
As for the RB1-competent BT474 cell line, a reduction of p-RB1 was also observed upon 24 or 72 h treatments with CDK4/6 inhibitors (100 or 500 nM), although p-RB1 levels were reestablished 144 h from treatment (Supplementary Fig. 1B). Lower levels of β-galactosidase activity were detected generally and the highest activity was detected after 72 h treatments with either CDK4/6 inhibitor, followed by a decrease in cellular senescence 144 h from treatments (Supplementary Fig. 1C). These observations indicate that the BT474 cell line may harbor an intrinsic resistance to CDK4/6 inhibition +/− fulvestrant compared to the T47D and MCF7 cell lines.
Effects of CDK4/6 inhibition on gene expression in breast cancer cell lines
Gene expression profiling was performed in untreated cells and upon treatment in order to identify changes in the PAM50 biology induced by CDK4/6 inhibitors in T47D, MCF7, and BT474 cell lines treated with different doses of palbociclib and ribociclib (100 or 500 nM) +/− fulvestrant (1 nM). The expression of the 50 genes of the PAM50 intrinsic subtype predictor and 6 signatures (Basal-like, HER2-enriched, Luminal A, Luminal B, Normal-like, and the 11-gene proliferation score) were explored at both treatment conditions. Paired t-tests and multiclass SAM showed that both CDK4/6 inhibitors (100 or 500 nM) +/− fulvestrant (1 nM) significantly increased (FDR < 5%) the Luminal A and Normal-like signatures and significantly decreased (FDR < 5%) the Basal-like and proliferation signatures (Fig. 2A, Supplementary Fig. 4). Interestingly, the HER2-enriched signature was only significantly reduced when the CDK4/6 inhibitors were given at 500 nM either alone (palbociclib p = 0.045, ribociclib p = 0.041) or in combination with fulvestrant (palbociclib p = 0.002, ribociclib p = 0.012), while no significant changes were observed with 100 nM CDK4/6 inhibitor monotherapy (palbociclib p = 0.275, ribociclib p = 0.596) or in combination with fulvestrant (palbociclib p = 0.466, ribociclib p = 0.613) (Fig. 2A,B). Treatment with fulvestrant alone significantly increased the HER2-enriched signature (p < 0.001) (Fig. 2A,B).
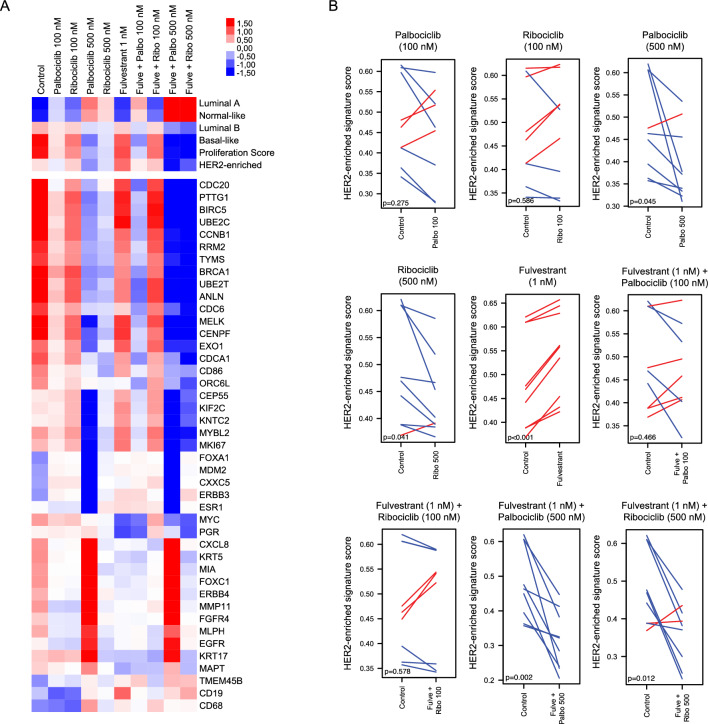
Figure 2.
Changes in the HER2-enriched signature upon treatment with CDK4/6 inhibitors +/− fulvestrant in vitro. (A) Heatmap of a multiclass SAM representing the PAM50 molecular subtypes, proliferation score and genes that are differentially expressed (FDR < 5%) in T47D, MCF7, and BT474 cells treated with CDK4/6 inhibitors (100 or 500 nM) +/− fulvestrant (1 nM). Three independent mRNA extractions per cell line were performed. (B) Paired samples t-test analyses showing changes in the HER2-enriched signature following treatment of T47D, MCF7, and BT474 cells with CDK4/6 inhibitors (100 or 500 nM) +/− fulvestrant (1 nM). Three independent mRNA extractions and gene expression analyses were performed for each cell line.
Next, we assessed individual gene expression across treatments using multiclass SAM. Forty-three (64.2%) genes were differentially expressed across treatment groups (FDR < 5%). Notably, both inhibitors, especially at 500 nM, led to a lower expression of proliferative genes (e.g.: CDC20, UBE2C, KNTC2, MKI67, BIRC5, CDCA1, PTTG1, CEP55, TYMS, and RRM2). Interestingly, in lower doses of CDK4/6 inhibitors (100 nM) the combination of fulvestrant and palbociclib had a stronger inhibitory effect over cell proliferation than the combination of fulvestrant and ribociclib. Additionally, we performed a paired SAM analysis to check the differences between 500 nM of palbociclib vs 500 nM ribociclib with or without fulvestrant. A proportion of 19.4% and 25.3% of genes were differentially expressed after treatment with 500 nM of palbociclib vs 500 nM ribociclib with or without fulvestrant, respectively (Supplementary Table 1). Interestingly, a higher expression of the HER2-enriched genes FGFR4 and TMEM45B was observed in cells treated with 500 nM palbociclib compared to those treated with 500 nM ribociclib with and without fulvestrant (Fig. 2A).
Early in vivo biological changes during CDK4/6 inhibitor in tumor samples from CORALLEEN and NeoPalAna phase II studies
To identify molecular changes induced by CDK4/6 inhibitors, we performed gene expression analyses in baseline, day 15, and surgery tumor samples of patients treated with ribociclib plus letrozole in the CORALLEEN trial (Fig. 3A) as well as in baseline, day 15, and surgery samples of patients treated with palbociclib plus anastrazole in the NeoPalAna trial (Fig. 3B).
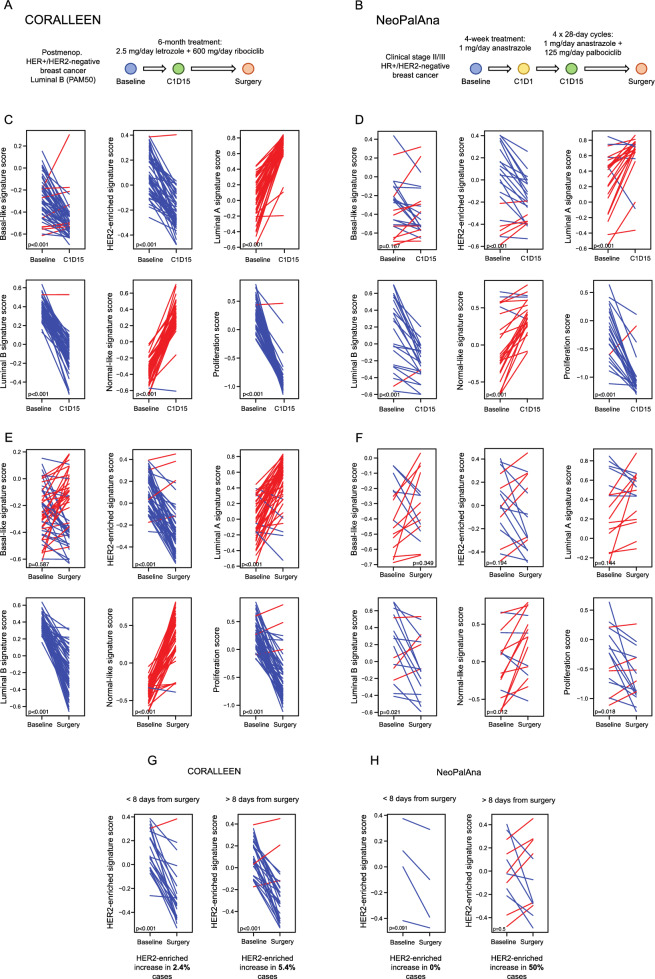
Figure 3.
Changes in the PAM50 signatures in the CORALLEEN and NeoPalAna studies. Schematic summaries of the samples analyzed from (A) the CORALLEEN trial design and (B) the NeoPalAna trial design. (C) Paired samples t-test analyses showing changes in the PAM50 signatures at cycle 1 day 15 (C1D15) in tumor samples from CORALLEEN and (D) NeoPalAna phase II studies. (E) Changes in the PAM50 signatures at surgery in tumor samples from CORALLEEN and (F) NeoPalAna. (G) Changes in the HER2-enriched signature in tumor samples from CORALLEEN and (H) NeoPalAna from patients who underwent surgery ≤ 8 or > 8 days from the last dose of CDK4/6 inhibitors + endocrine therapy.
First, we assessed early changes in 49 paired baseline and day 15 tumor samples from the CORALLEEN trial (Fig. 3C) and 23 paired baseline and day 15 tumor samples from the NeoPalAna trial (Fig. 3D). Treatment with ribociclib and endocrine therapy led to a significant increase in Luminal A (p < 0.001) and Normal-like (p < 0.001) signatures and a significant decrease in Basal-like (p < 0.001), HER2-enriched (p < 0.001), Luminal B (p < 0.001) and proliferation (p < 0.001) signatures (Fig. 3C). Similarly, treatment with palbociclib plus endocrine therapy led to a significant increase in Luminal A (p < 0.001) and Normal-like (p < 0.001) signatures and a significant decrease in HER2-enriched (p < 0.001), Luminal B (p < 0.001) and proliferation (p < 0.001) signatures (Fig. 3D).
Biological changes after CDK4/6 inhibitor in tumor samples from CORALLEEN and NeoPalAna phase II studies
Next, we assessed changes in 49 paired baseline and surgery tumor samples from the CORALLEEN (Fig. 3E) and 16 paired baseline and surgery tumor samples from the NeoPalAna (Fig. 3F).
Treatment with ribociclib and endocrine therapy led to a significant increase in Luminal A (p < 0.001) and Normal-like (p < 0.001) signatures and a significant decrease in HER2-enriched (p < 0.001), Luminal B (p < 0.001), and proliferation (p < 0.001) signatures (Fig. 3E). Treatment with palbociclib plus endocrine therapy led to a significant increase in the Normal-like (p = 0.012) signature and a significant decrease in the Luminal B (p = 0.021) and proliferation signature (p = 0.018) (Fig. 3F). Importantly, the HER2-enriched signature did not decrease in surgical samples of patients treated with palbociclib (p = 0.194), although a difference in sample size could explain this result (Figs. 3F).
In CORALLEEN, the median number of days between the last dose of ribociclib and surgery was 13.1 days (range: 1–78)36, whereas in NeoPalAna the median number of days between the last dose of palbociclib and surgery was 29 days (range: 8–49), except for 8 patients who received additional 10–12 days of palbociclib immediately before surgery17. In patients from CORALLEEN, the HER2-enriched signature was significantly decreased in patients who underwent surgery at 8 days from the last dose of ribociclib or before (p < 0.001), as well as in those who underwent surgery after > 8 days from the last dose of ribociclib (p < 0.001) (Fig. 3G). In 4 patients from NeoPalAna who underwent surgery at 8 days from the last dose of palbociclib or before, a tendency of reduction in the HER2-enriched signature was also observed. However, in patients who underwent surgery after > 8 days from the last dose of palbociclib, the HER2-enriched signature increased in 50% of the cases (Fig. 3H).
Discussion
In the last few years, three CDK4/6 inhibitors (i.e., palbociclib, ribociclib, abemaciclib) have been approved for the treatment of patients with metastatic HR+/HER2- breast cancer in combination with endocrine therapy1–11. While the three inhibitors are theoretically considered to provide a similar class effect, they have some chemical and pharmacological differences and are given at different doses22,25. Currently, no specific biomarkers are used to select the first-line CDK4/6 inhibitor37. On one side, the first-line trials using the CDK4/6 inhibitors ribociclib and palbociclib, despite demonstrating identical primary endpoint PFS results, have recently reported different OS results, with palbociclib not showing an OS benefit38. It is unknown if this is due to differences in the type of inhibitor, trial, patient population, or other features.
On the other side, it has been demonstrated that the PAM50 molecular subtypes are prognostic in patients treated with CDK4/6 inhibitors16,18,19 and accumulated evidence suggests that the combination of endocrine therapy with palbociclib might be less effective than combination with ribociclib in patients with advanced HR+/HER2- and HER2-enriched breast cancer. Indeed, retrospective analyses on samples of the MONALEESA-2,-3, and -718 trials showed that patients that harbored HER2-enriched tumors exhibited a PFS and OS benefit from the combination of endocrine therapy and ribociclib, whereas those treated with endocrine therapy and palbociclib in the PALOMA-216 did not benefit from the combination, even though the retrospective analysis of PALOMA-2 was not powered to study the effect across PAM50 subtypes. Nevertheless, this hypothesis has not been formally tested head-to-head, and the SOLTI-2101 HARMONIA39 prospective phase III trial (NCT05207709) is currently evaluating if the combination of ribociclib with endocrine therapy is superior to the combination with palbociclib in prolonging PFS in this particular subset of patients.
In order to better understand the molecular effects of palbociclib and ribociclib, we analyzed both cell lines and clinical samples treated with CDK4/6 inhibitors. Our main observations in breast cancer cell lines were that palbociclib and ribociclib had identical dose-dependent proliferation inhibition and that in CDK4/6 inhibitor-sensitive cell lines, both inhibitors reduced the levels of p-RB1 (marker of cell cycle inhibition21) and Lamin-B1 (marker of senescence40–42) in a similar manner, where treatment duration played a part but changes mostly relied on the administered doses.
Importantly, gene expression analyses revealed that palbociclib and ribociclib significantly increased the Luminal A and Normal-like signatures and decreased the Luminal B, Basal-like, and proliferation signatures with both doses. However, the HER2-enriched signature was only significantly reduced in cells treated with 500 nM of CDK4/6 inhibitors +/− fulvestrant. Interestingly, treatment with fulvestrant alone significantly increased the HER2-enriched signature, but the addition of 500 nM palbociclib or ribociclib was still capable of significantly decreasing its levels. Assessment of individual gene expression suggested that in lower doses palbociclib might be more potent CDK4/6 inhibitor than ribociclib, and that co-treatment with fulvestrant further enhances these changes in gene expression.
In patient tumor samples from the CORALLEEN and NeoPalAna phase II studies a similar change in PAM50 biology was observed with both drugs namely an increase in Luminal A and Normal-like signatures and a decrease in Luminal B and proliferation signatures after 2 weeks of treatment and at surgery. At 2 weeks of treatment the HER2-enriched signature was significantly decreased in both studies. However, the decrease in the HER2-enriched signature was only observed in surgical samples of patients treated with ribociclib, but not palbociclib. Interestingly, in patients from NeoPalAna who underwent surgery at 8 days from the last dose of palbociclib or before, a reduction of the HER2-enriched signature was observed, although it was not of statistical significance possibly due to sample size. This result is consistent with the results of the NeoPalAna trial, where a Ki67 rebound at surgery following palbociclib was observed in patients where palbociclib treatment was finalized > 8 days before surgery, while this washout was suppressed if patients received a cycle 5 of palbociclib17. If palbociclib was given until surgery, the effect could be as good as the effect of ribociclib. However, sample size in NeoPalAna was much smaller compared to CORALLEEN and this represents a limitation on the interpretation of the results.
Our study acknowledges several other limitations. Firstly, our analysis was limited to early-stage breast cancer tumor samples, as obtaining paired biopsies in a metastatic setting is challenging. This may restrict the applicability of our findings to more advanced disease stages. Secondly, while most of these samples were not HER2-enriched, we attempted to mitigate this by analyzing each PAM50 intrinsic subtype score as a continuous variable, since these scores are strictly related to the biological information provided by the PAM50 genes characterizing each breast cancer intrinsic subtype43. Nonetheless, we acknowledge this may not fully capture the complexities of HER2-enriched biology. Thirdly, there is an acknowledged gap in our understanding of the actual concentration of palbociclib and ribociclib that reaches the tumor in patients, which may differ from the prescribed doses and preclinical models, adding a layer of uncertainty to the direct translatability of our results to clinical practice. Fourthly, the specificity of the CORALLEEN trial to patients with PAM50 Luminal B disease narrows the breadth of our findings, potentially limiting their generalizability to other breast cancer subtypes. Lastly, our study did not include abemaciclib, which has been proposed to target additional CDKs in addition to CDK4/644,45. These limitations highlight the need for further research in order to fully understand the implications of CDK4/6 inhibitors in varying contexts of breast cancer treatment.
In conclusion, our results show that biological responses to palbociclib and ribociclib are primarily dose-dependent and influenced by the addition of fulvestrant. Our findings suggest that while both CDK4/6 inhibitors effectively modulate key biological pathways in HR+/HER2- breast cancer, nuances in their impact, particularly on the HER2-enriched signature, warrant further investigation. The ongoing SOLTI-2101 HARMONIA trial39 will ultimately test which CDK4/6 inhibitor is best for continued response and survival benefit in patients with HER2-enriched breast cancer.
Methods
Cell lines and drugs
MCF7, T47D, BT474, and MDA-MB-468 cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Dulbecco’s Modified Eagle Medium (DMEM)/nutrient mixture F-12 supplemented with 10% v/v heat-inactivated fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific Inc., Waltham, MA, USA), 1% GlutaMAX (Gibco; Thermo Fisher Scientific Inc.), and 1% Penicillin/Streptomycin (Sigma-Aldrich, Saint Louis, MO, USA) in a 37 °C, 5% CO2 humidified incubator. Cells were detached from flasks by incubation with 0.25% Trypsin–EDTA (1X) (Gibco; Thermo Fisher Scientific Inc.). Palbociclib, ribociclib, and fulvestrant were purchased from Selleckchem (Houston, TX, USA).
Clinical samples
The SOLTI-1402 CORALLEEN phase II study (NCT03248427) randomized 106 postmenopausal women with stage I–IIIA HR+/HER2- breast cancer and Luminal B by PAM50 with histologically confirmed, operable primary tumour size of at least 2 cm in diameter as measured by magnetic resonance imaging (MRI). Patients were randomly assigned (1:1) to receive either six 28-days cycles of ribociclib (oral 600 mg once daily for 3 weeks on, 1 week off) plus daily letrozole (oral 2.5 mg/day) or four cycles of doxorubicin (intravenous 60 mg/m2) and cyclophosphamide (intravenous 600 mg/m2) every 21 days followed by weekly paclitaxel (intravenous 80 mg/m2) for 12 weeks36. Here, we analyzed formalin-fixed, paraffin-embedded (FFPE) tumor samples of the ribociclib plus letrozole arm, including 49 paired baseline versus cycle 1 day 15 (C1D15) samples and 49 paired baseline versus surgery samples.
Additionally, gene expression data of the NeoPalAna phase II trial (NCT01723774), which treated 50 patients with HR+/HER2- early breast cancer with anastrazole (1 mg daily) for 4 weeks, followed by four 28-day cycles of palbociclib (125 mg daily) plus anastrazole (1 mg daily)17, was downloaded from the Gene Expression Omnibus (GSE93204). We analyzed 23 paired baseline versus C1D15 samples and 16 paired baseline versus surgery samples.
Ethics approval and consent to participate
This study was approved by the Ethics Committee at Hospital Clinic of Barcelona (HCB.2022.0086) and all methods were carried out in accordance with relevant guidelines and regulations. This study involves the use of tissue samples of patients that have received treatment with CDK4/6 inhibitors within the context of the CORALLEEN trial. These samples are stored in the biorepository of the Translational genomics and targeted therapies in solid tumors group at IDIBAPS as long as patients sign the specific informed consent of the collection.
RNA extraction
RNA of tumor samples from the CORALLEEN study was extracted using the High Pure FFPET RNA isolation kit (Roche, Indianapolis, IN, USA) following manufacturer’s protocol. At least 1–5 10 μm FFPE slides were used for each tumor specimen and macrodissection was performed to avoid contamination with normal breast tissue if needed. MCF7, T47D, and BT474 cells were seeded in a 6-well plate at 150,000 cells per well and after overnight incubation medium was replaced with two different dose levels of palbociclib or ribociclib (i.e., 100 nM or 500 nM) +/− fulvestrant (1 nM) for 72 h (h). mRNA was extracted using QIAGEN’s RNeasy extraction kit (QIAGEN, Hilden, Germany) following manufacturer’s instructions.
Gene expression analysis
The nCounter platform (NanoString Technologies, Seattle, WA, USA) analyzed RNA samples from tumor samples and cell lines. A minimum of 100 ng of total RNA was used to measure the expression of 50 genes of the PAM50 intrinsic subtype predictor assay and 5 housekeeping genes (ACTB, MRPL19, PSMC4, RPLP0 and SF3A1). Expression counts were then normalized and the PAM50 signature scores (Basal-like, HER2-enriched, Luminal A and B, Normal-like) and the proliferation signature score were calculated using customized R scripts43. PAM50 molecular subtypes were calculated in the publicly available gene expression data from the NeoPalAna including 23 baseline samples, 23 week-2 samples and 16 surgery samples.
In vitro cell growth assay
MCF7, T47D, BT474, and MDA-MB-468 cells were seeded in triplicate at 5,000 cells per well in 96-well plates. Following overnight incubation, cells were treated with five 1:10 serial dilutions of palbociclib or ribociclib starting at 10 µM. Cell viability was assessed after 72 h with Hoechst 33342 staining solution (Invitrogen, Thermo Fisher Scientific Inc.) and quantified using SynergyHT microplate reader and Windows based Gen5 software.
Western blotting
MCF7, T47D, and BT474 cells were seeded in 6-well plates at 150,000 cells per well and after overnight incubation medium was replaced with palbociclib or ribociclib (100 or 500 nM). After 24, 72, or 144 h, cell lysates were obtained using radioimmunoprecipitation (RIPA) lysis and extraction buffer (Thermo Fisher Scientific Inc.) supplemented with protease inhibitors: 5 mM sodium fluoride, 1 mg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 1 mM benzamidine, 1 mM leupeptin, and 1 mM dithiothreitol. Total protein extracts were quantified using the DC Protein Assay (BioRad Laboratories, Hercules, CA, USA) and 50 μg of proteins were separated in reducing conditions (2.5% β-mercaptoethanol) by SDS–PAGE and transferred to nitrocellulose membranes (BioRad Laboratories) for further processing, following standard western blotting procedures. The primary antibodies used in this study were phospho-RB1 (Ser807/811) (D20B12) and Lamin-B1 (D9V6H) from Cell Signaling Technologies (Danvers, MA, USA) and anti-actin (A2066) from Sigma-Aldrich. The secondary fluorescent antibody used was the IRDye 800CW Donkey anti-Rabbit IgG (LI-COR Biosciences, Lincoln, NE, USA). Fluorescent signal was acquired by the Odyssey Imaging System (LI-COR Biosciences).
Senescence-associated β-galactosidase activity
MCF7, T47D, and BT474 cells were seeded in 24-well plates at 35,000 cells per well and after overnight incubation medium was replaced with palbociclib or ribociclib (100 or 500 nM). Following 24, 72, or 144 h treatments, senescence dye from the Senescence Assay Kit (Abcam, Cambridge, UK) was added to wells. Cells were incubated for 1–2 h in a 37 °C, 5% CO2 humidified incubator and the mean fluorescence was analysed by flow cytometry for the detection of β-galactosidase activity. Propidium iodide (Invitrogen, Thermo Fisher Scientific Inc.) was used as a viability marker. Untreated controls were added, as well as unstained controls for the evaluation of potential auto-fluorescence.
Statistical analysis
Changes in gene expression and PAM50 signatures upon CDK4/6 inhibition were determined by paired t-tests and paired and multiclass significant analysis of microarray (SAM) with a false discovery rate (FDR) < 5%. These analyses were performed using R software. All statistical tests were two sided and the statistical significance level was set to p < 0.05. For in vitro cell growth, determination of half maximal inhibitory concentrations (IC50s), and senescence assays, GraphPad Prism was used for statistics.
Supplementary Information
Acknowledgements
A.P. received funding from Fundació La Marató TV3 201935-30, Fundación CRIS contra el cáncer PR_EX_2021-14, Agència de Gestó d'Ajuts Universitaris i de Recerca 2021 SGR 01156, Fundación Fero BECA ONCOXXI21, Instituto de Salud Carlos III PI22/01017, Asociación Cáncer de Mama Metastásico IV Premios M. Chiara Giorgetti, Breast Cancer Research Foundation BCRF-22-198 and BCRF-23-198, and RESCUER, funded by European Union's Horizon 2020 Research and Innovation Programme under Grant Agreement No. 847912. F.B-M. received funding from Fundación científica AECC Ayudas Investigador AECC 2021 (INVES21943BRAS). N.C. is supported by Fundación SEOM, Becas FSEOM para Formación en Investigación en Centros de Referencia en el Extranjero 2021.
Author contributions
The authors confirm contribution to the paper as follows: study conception and design: F.B-M., A.P; data collection: N.L-C, P.G., P.B., O.C., O.G-M., A.F-M., N.C., I.G-F., A.R., R.G-B., B.A., M.V., M.M., F.S., T.P., C.M.P., J.G.; analysis and interpretation of results: N.L-C, F.B-M., A.P., O.G-M., A.F-M., N.C., F.S., T.P., M.M; draft manuscript preparation: F.B-M., A.P., N.L-C. All authors reviewed the results and approved the final version of the manuscript.
Data availability
Investigators interested in data access and collaboration should contact the corresponding author. Access can be obtained for academic use only under a data transfer agreement and upon Ethics Committee approval.
Competing interests
A.P. reports advisory and consulting fees from Roche, Pfizer, Novartis, Amgen, BMS, Puma, Oncolytics Biotech, MSD, Guardan Health, Peptomyc and Lilly, lecture fees from Roche, Pfizer, Novartis, Amgen, BMS, Nanostring Technologies and Daiichi Sankyo, institutional financial interests from Boehringer, Novartis, Roche, Nanostring, Sysmex Europa GmbH, Medica Scientia inno. Research, SL, Celgene, Astellas and Pfizer; stockholder and consultant of Reveal Genomics, SL; patents filed PCT/EP2016/080056, PCT/EP2022/086493, PCT/EP2023/060810, EP23382703 and EP23383369. F.B-M. has patents filed: PCT/EP2022/086493, PCT/EP2023/060810, EP23382703 and EP23383369 and part-time employment with Reveal Genomics, SL. J.G. reports scientific advisory board fees from Novartis, Pfizer and Lilly, consultancy training fees from Roche, Novartis and MSD, honoraria fees from Roche, Novartis and Pfizer. I.G-F. reports financing of courses and talks from Novartis. The remaining authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-67126-2.
References
- 1.Cristofanilli, M. et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phas. Lancet Oncol.17(4), 425–439 (2016). 10.1016/S1470-2045(15)00613-0 [DOI] [PubMed] [Google Scholar]
- 2.Finn, R. S. et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med.375(20), 1925–1936 (2016). 10.1056/NEJMoa1607303 [DOI] [PubMed] [Google Scholar]
- 3.Turner, N. C. et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N. Engl. J. Med.379(20), 1926–1936 (2018). 10.1056/NEJMoa1810527 [DOI] [PubMed] [Google Scholar]
- 4.Hortobagyi, G. N. et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol.29(7), 1541–1547 (2018). 10.1093/annonc/mdy155 [DOI] [PubMed] [Google Scholar]
- 5.Im, S.-A. et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N. Engl. J. Med.381(4), 307–316 (2019). 10.1056/NEJMoa1903765 [DOI] [PubMed] [Google Scholar]
- 6.Slamon, D. J. et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J. Clin. Oncol.36(24), 2465–2472 (2018). 10.1200/JCO.2018.78.9909 [DOI] [PubMed] [Google Scholar]
- 7.Tripathy, D. et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol.19(7), 904–915 (2018). 10.1016/S1470-2045(18)30292-4 [DOI] [PubMed] [Google Scholar]
- 8.Goetz, M. P. et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J. Clin. Oncol.35(32), 3638–3646 (2017). 10.1200/JCO.2017.75.6155 [DOI] [PubMed] [Google Scholar]
- 9.Johnston, S. et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer.5(1), 1–8 (2019). 10.1038/s41523-018-0097-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sledge, G. W. et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol.35(25), 2875–2884 (2017). 10.1200/JCO.2017.73.7585 [DOI] [PubMed] [Google Scholar]
- 11.Sledge, G. W. et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2: A randomized clinical trial. JAMA Oncol.6(1), 116–124 (2020). 10.1001/jamaoncol.2019.4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schettini, F., Brasó-Maristany, F., Kuderer, N. M. & Prat, A. A perspective on the development and lack of interchangeability of the breast cancer intrinsic subtypes. NPJ Breast Cancer.10.1038/s41523-022-00451-9 (2022). 10.1038/s41523-022-00451-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cejalvo, J. M. et al. Clinical implications of the non-luminal intrinsic subtypes in hormone receptor-positive breast cancer. Cancer Treat. Rev.67, 63–70 (2018). 10.1016/j.ctrv.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 14.Aftimos, P. et al. Genomic and transcriptomic analyses of breast cancer primaries and matched metastases in Aurora, the breast international group (Big) molecular screening initiative. Cancer Discov.11, 2796–2811 (2021). 10.1158/2159-8290.CD-20-1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falato, C., Schettini, F., Pascual, T., Brasó-Maristany, F. & Prat, A. Clinical implications of the intrinsic molecular subtypes in hormone receptor-positive and HER2-negative metastatic breast cancer. Cancer Treat. Rev.112, 102496 (2023). 10.1016/j.ctrv.2022.102496 [DOI] [PubMed] [Google Scholar]
- 16.Finn, R. S. et al. Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naïve metastatic breast cancer. Clin. Cancer Res.26(1), 110–121 (2020). 10.1158/1078-0432.CCR-19-0751 [DOI] [PubMed] [Google Scholar]
- 17.Ma, C. X. et al. NeoPalAna: Neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor–positive breast cancer. Clin. Cancer Res.23(15), 4055–4065 (2017). 10.1158/1078-0432.CCR-16-3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prat, A. et al. Correlative biomarker analysis of intrinsic subtypes and efficacy across the MONALEESA phase III studies. J. Clin. Oncol.39(13), 148–1467 (2021). 10.1200/JCO.20.02977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciruelos, E. et al. Palbociclib and trastuzumab in HER2-positive advanced breast cancer: Results from the Phase II SOLTI-1303 PATRICIA Trial. Clin. Cancer Res.10.1158/1078-0432.ccr-20-0844 (2020). 10.1158/1078-0432.ccr-20-0844 [DOI] [PubMed] [Google Scholar]
- 20.Prat, A. et al. Intrinsic subtype and overall survival of patients with advanced HR+/HER2− breast cancer treated with ribociclib and ET: Correlative analysis of MONALEESA-2, -3, -7. Clin. Cancer Res.10.1158/1078-0432.ccr-23-0561 (2023). 10.1158/1078-0432.ccr-23-0561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asghar, U., Witkiewicz, A. K., Turner, N. C. & Knudsen, E. S. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov.14(2), 130–146 (2015). 10.1038/nrd4504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schettini, F. et al. CDK 4/6 inhibitors as single agent in advanced solid tumors. Front Oncol.8, 608 (2018). 10.3389/fonc.2018.00608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabt, A. et al. Discovery of 3,6-disubstituted pyridazines as a novel class of anticancer agents targeting cyclin-dependent kinase 2: Synthesis, biological evaluation and in silico insights. J. Enzyme Inhib. Med. Chem.35(1), 1616–1630 (2020). 10.1080/14756366.2020.1806259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marra, A. & Curigliano, G. Are all cyclin-dependent kinases 4/6 inhibitors created equal?. NPJ Breast Cancer.5(1), 1–9 (2019). 10.1038/s41523-019-0121-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George, M. A., Qureshi, S., Omene, C., Toppmeyer, D. L. & Ganesan, S. Clinical and pharmacologic differences of CDK4/6 inhibitors in breast cancer. Front Oncol.11, 693104 (2021). 10.3389/fonc.2021.693104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braal, C. L. et al. Inhibiting CDK4/6 in breast cancer with palbociclib, ribociclib, and abemaciclib: Similarities and differences. Drugs.81(3), 317–331 (2021). 10.1007/s40265-020-01461-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwapisz, D. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: palbociclib, ribociclib, and abemaciclib. Breast Cancer Res. Treat.166(1), 41–54 (2017). 10.1007/s10549-017-4385-3 [DOI] [PubMed] [Google Scholar]
- 28.Schäffler, H. et al. The clinical relevance of the NATALEE study: Application of the NATALEE criteria to a real-world cohort from two large German breast cancer centers. Int. J. Mol. Sci.24(22), 1–12 (2023). 10.3390/ijms242216366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slamon, D. J., Fasching, P. A., Hurvitz, S., Chia, S. & Hortobagyi, G. N. Rationale and trial design of NATALEE: A Phase III trial of adjuvant ribociclib + endocrine therapy versus endocrine therapy alone in patients with HR+/HER2- early breat cancer. Ther. Adv. Med. Oncol.15, 1–16 (2023). 10.1177/17588359231178125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loibl, S. et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer-the penelope-B trial. J. Clin. Oncol.39(14), 1518–1530 (2021). 10.1200/JCO.20.03639 [DOI] [PubMed] [Google Scholar]
- 31.Gnant, M. et al. Adjuvant palbociclib for early breast cancer: The PALLAS trial results (ABCSG-42/AFT-05/BIG-14-03). J. Clin. Oncol.40(3), 282–293 (2021). 10.1200/JCO.21.02554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prat, A. et al. Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. J. Natl. Cancer Inst.106(8), 1–8 (2014). 10.1093/jnci/dju152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freund, A., Laberge, R. M., Demaria, M. & Campisi, J. Lamin B1 loss is a senescence-associated biomarker. Mol. Biol. Cell.23(11), 2066–2075 (2012). 10.1091/mbc.e11-10-0884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimi, T. et al. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev.25(24), 2579–2593 (2011). 10.1101/gad.179515.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA.92(20), 9363–9367 (1995). 10.1073/pnas.92.20.9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prat, A. et al. Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol.21(1), 33–43 (2020). 10.1016/S1470-2045(19)30786-7 [DOI] [PubMed] [Google Scholar]
- 37.Grinshpun, A., Tolaney, S. M., Burstein, H. J., Jeselsohn, R. & Mayer, E. L. The dilemma of selecting a first line CDK4/6 inhibitor for hormone receptor-positive/HER2-negative metastatic breast cancer. NPJ Breast Cancer.9(1), 1–4 (2023). 10.1038/s41523-023-00520-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finn, R. S., Rugo, H. S., Dieras, V. C., Harbeck, N., Im, S.-A., Gelmon, K. A., et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): Analyses. 2022;40(17_suppl):LBA1003. 10.1200/JCO20224017_supplLBA1003
- 39.Pascual, T., Stover, D. G., Thuerigen, A., Sanchez-Bayona, R., Perou, C. M., Ciruelos, E. M., et al. Ribociclib (RIB) vs. palbociclib (PAL) in patients (pts) with hormone receptor-positive/HER2-negative/HER2-enriched (HR+/HER2-/HER2-E) advanced breast cancer (ABC): A head-to-head phase III study—HARMONIA SOLTI-2101/AFT-58. 2023;41(16_suppl): TPS1125. 10.1200/JCO20234116_supplTPS1125.
- 40.Saleh, T. et al. The expression of the senescence-associated biomarker Lamin B1 in human breast cancer. Diagnostics.12, 609 (2022). 10.3390/diagnostics12030609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matias, I. et al. Loss of lamin-B1 and defective nuclear morphology are hallmarks of astrocyte senescence in vitro and in the aging human hippocampus. Aging Cell.21, e13521 (2021). 10.1111/acel.13521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, A. S., Ong, P. F., Choj, A. & Clavel, C. Loss of lamin B1 is a biomarker to quantify cellular senescence in photoaged skin. Sci. Rep.7, 15678 (2017). 10.1038/s41598-017-15901-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parker, J. S. et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J. Clin. Oncol.27(8), 1160–1167 (2009). 10.1200/JCO.2008.18.1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wells, C. I. et al. Quantifying CDK inhibitor selectivity in live cells. Nat. Commun.11, 1–11 (2020). 10.1038/s41467-020-16559-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hafner, M. et al.Clin. Activity.26(8), 1067–1080 (2019). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Investigators interested in data access and collaboration should contact the corresponding author. Access can be obtained for academic use only under a data transfer agreement and upon Ethics Committee approval.