Abstract
Objective
To evaluate the feasibility and preliminary efficacy of the transition of an outpatient center-based rehabilitation program for middle and older aged Veterans with mobility limitations to a tele-health platform.
Design
Non-randomized non-controlled pilot study including 10 treatment sessions over 8 weeks and assessments at baseline, 8, 16, and 24 weeks.
Setting
VA Boston Healthcare System ambulatory care between August 2020 and March 2021.
Participants
Veterans aged 50 years and older (n=178) were contacted via letter to participate, and 21 enrolled in the study.
Intervention
Participants had virtual intervention sessions with a physical therapist who addressed impairments linked to mobility decline and a coaching program promoting exercise adherence.
Main Outcome Measures
Ambulatory Measure for Post-Acute Care (AM-PAC), Phone-FITT, and Self-Efficacy for Exercise (SEE) scale.
Results
Completers (n=14, mean age 74.9 years, 86% men) averaged 9.8 out of 10 visits. Changes in the Ambulatory Measure for Post-Acute Care (AM-PAC) exceeded clinically meaningful change after 8 and 24 weeks of treatment, at 4.1 units and 4.3 units respectively. Statistically significant improvements from baseline in AM-PAC and Phone-FITT were observed after 8 weeks of treatment and at 24 weeks. No significant changes were observed in exercise self-efficacy.
Conclusions
In this group of veterans, telerehab was feasible and demonstrated preliminary efficacy in both mobility and physical activity, thus justifying further investigation in a larger scale clinical trial.
KEYWORDS: Aged, Mobility, Physical therapy, Rehabilitation, Telehealth, Veterans
Mobility limitations, which include difficulty with tasks such as rising from a chair, climbing stairs, or walking, affect more than 25% of community dwelling older adults.1 These limitations are directly predictive of adverse health outcomes including hospitalization, nursing home admission, and death.2,3 Military Veterans are particularly vulnerable because they demonstrate greater severity of impairment and limitation when compared with age matched civilians.4
In response to the COVID-19 pandemic, rehabilitation services were forced to adapt care delivery to comply with restrictions aimed at minimizing physical contact between providers and patients.5 While the rapid transition to telehealth delivery of care was necessary, the feasibility and efficacy of tele-rehabilitation care was unknown. Exercise intervention studies have delivered telehealth care in various modalities (telephone, web-based applications, asynchronous video, synchronous video, and hybrid approaches) making comparisons across studies difficult.6, 7, 8 Furthermore, few telehealth studies have addressed care to increase physical activity and improve mobility for older adults, specifically those with multi-morbidity.9,10
The Veterans Administration (VA) had an established telehealth mechanism prior to the COVID-19 pandemic. The VA Video Connect (VVC) platform allows Veterans and health care providers to conduct remote synchronous visits. The VA supplied tablets that were equipped with VA applications only and were supported by both local and national telehealth help centers. Technicians were available to assist and train Veterans for device use.
In 2020, a study designed to evaluate an innovative mobility rehabilitation program targeting the prevention of functional decline among Veterans (Live Long Walk Strong [LLWS]) was halted due to pandemic restrictions.11 LLWS is a model of in-person physical therapy care that demonstrates preliminary effectiveness in enhancing mobility among older adults who either had a 6-month history of falls or reported modification to mobility tasks.12 LLWS intervention targets important physiological impairments that are associated with mobility decline, administers behavior change and motivation strategies, and assists with connecting patients into maintenance programs. The LLWS care program demonstrated preliminary clinical effectiveness with improving mobility performance above clinically meaningful thresholds.12 However, the feasibility and efficacy of telehealth delivered LLWS (T-LLWS) was not known. As a result, our research team adapted a telerehabilitation version of this care program.
Therefore, the focus of this pilot study was to address these knowledge gaps investigating the feasibility and benefit of T-LLWS using the VVC platform. The first aim was to determine the feasibility of providing T-LLWS and the second aim was to examine the preliminary efficacy of T-LLWS on mobility, physical activity, and self-efficacy. We hypothesized that the delivery of the telerehabilitation program would be feasible and acceptable (Aim 1) and that it would produce clinically meaningful changes in mobility, physical activity, and self-efficacy informing the design of a future larger scale clinical trial (Aim 2).
Methods
Study overview
This 24-week single-arm pilot trial was conducted within the New England Geriatric Research, Education and Clinical Center at the VA Boston Healthcare System (VABHS). This study was approved by the Institutional Review Board at VABHS. The study, as an adaptation of the parent study, was registered with Clinical Trials Registry (NCT04026503). To assess feasibility and acceptability (Aim 1), recruitment and retention metrics were evaluated. The length of each session, number of sessions attended, and reasons for withdrawal from the study were assessed. To examine technological feasibility, we recorded the number and type of technology issues along with the resolution to the issue. To evaluate Aim 2, we used patient reported measures corresponding to mobility using the Boston University Ambulatory Measure for Post-Acute Care (AM-PAC), self-reported physical activity (Phone-FITT), and self-efficacy for mobility skills (Activities-specific Balance Confidence Scale–ABC scale) as well as exercise (Self-efficacy for Exercise scale–SEE scale). Assessments were completed at baseline, treatment conclusion (8 weeks), and 16 weeks and 24 weeks post baseline.
Recruitment and enrollment
In late summer of 2020, community-dwelling Veterans aged 50 years and older were contacted via letter from ambulatory care clinic rosters at VABHS to participate in the study. Recruitment methods mimicked the target goals of our parent study, which was to recruit an equal number of participants into 3 age categories 50-65 years, 65-75 years, and over 75 years of age.11 A postage paid opt-out card was provided with the letter. If Veterans were not interested, the opt out card was returned. Those interested in the study could reach out to staff to verbalize interest. Those who did not express interest or return an opt out card were contacted by telephone 2-3 weeks after the mailing to inquire about participation.
All interested Veterans were screened in a 2-step process. Step 1 involved a phone screen to assess initial eligibility for the study. Veterans were included if they reported difficulty or modification to walking ½ mile (5-6 blocks) or climbing 1 flight of stairs. Modifications may include use of an assistive device, avoidance of the activity due to its difficulty, or requiring increased time to complete the task. Veterans were excluded if they reported use of supplemental oxygen, had a surgery within the past 3 months, on hospice (<2 years left to live), blind, could not speak or understand English, had planned major surgery in the upcoming year, had unstable medical conditions (eg, uncontrolled high blood pressure), or were participating in another clinical trial.
Those who met eligibility criteria from the phone screen were scheduled for consenting. Informed consent documents and study materials were mailed out prior to the consenting visit. Informed consent was obtained via VVC using an approved Institutional Review Board process. All study assessments were conducted via VVC. After obtaining consent, all participants underwent a second screen to assess final study eligibility. The Mini-Montreal Cognitive Assessment (mini-MoCA)13 was administered and those manifesting a score <10 were excluded. Those reporting no difficulty to the following questions on the AM-PAC were excluded as these responses reflect no significant difficulty with mobility tasks: taking a 1-mile brisk walk, without stopping to rest, running for 5 minutes on even surfaces, making sharp turns when running fast, and taking part in strenuous activities (eg, running 3 miles, swimming half-mile, etc).14 Additionally, participants reporting that an inability to stand up from an armless straight chair (dining room chair) and an inability to move up in bed on the AM-PAC were also excluded as these responses corresponded to a severity of disability limiting the ability to participate safely.
Instruments
Eligible participants then underwent a baseline evaluation. Demographic and health information were collected using the Katz self-administered comorbidity questionnaire,15 ABC scale,16 SEE scale,17 Brief Pain Inventory (BPI),18 Phone-FITT questionnaire,19 and the Patient Health Questionnaire-9.20 The AM-PAC is a valid and sensitive patient reported measure designed to assess activity limitations across the continuum of care.14 Respondents report the level of difficulty on various household and community based mobility activities from none to be unable to complete. Raw scores on the mobility self-report range from 18 to 72, with higher scores indicating higher mobility and less difficulty completing the tasks. The ABC scale is a well-established and validated scale measuring confidence with mobility and balance on 16 different items with scores ranging from 0 to 100.16 The SEE scale is an established, reliable, and valid scale to measure the confidence to engage in exercise.17 The scale ranges from 0 to 90, with a higher score indicating greater confidence to participate in exercise. The BPI is a rapid way to assess pain severity and its effect on functioning, with ratings ranging from 0 to 10 and a higher score indicating greater severity. The Phone-FITT is a brief physical activity interview that has demonstrated reliability and validity.19 Total physical activity scores are calculated from average engagement in household and recreational activities incorporating components of frequency and intensity. Higher scores indicate greater confidence in balance. The Patient Health Questionnaire-9 is a brief tool that can assess the severity of depressive symptoms, with scores ranging from 0 to 27, a higher score indicating greater severity of symptoms. These measures were evaluated again after completing the 8-week intervention, at week 16 (8 weeks post treatment), and at week 24 (16 weeks post treatment). Monthly calendars to assess falls, days of exercise, emergency department visits, and hospitalizations were also collected.
Intervention
Participants had 10 1:1 treatments via real-time synchronous 2-way video with a physical therapist (PT) over an 8-week period, with 2 visits per week for the first 2 weeks and then reduced to 1 session per week for the remaining weeks. If a gap in timing occurred, we did allow up to 2 sessions to be made up within a 2-week time frame consistent with LLWS care, treatment focused on addressing impairments linked to mobility decline including stepping patterns,21 power training (emphasizing both strength and speed of movement), and flexibility as well as a coaching program promoting the adoption of exercise behaviors.22 To ensure safety throughout the televisit sessions, the physical location of the participant and emergency contact were reviewed and recorded before each session. Each session began with a warm-up and 10-15 minutes of stepping patterns followed by 20-25 minutes of power/strength training. A brief review of exercises for the home exercise program and cool-down completed the exercise portion of the visit. Sessions ended with a coaching protocol including goal setting, identifying facilitators and barriers to exercise, and addressing the barriers in ways the participant identified as meaningful.22 The stepping patterns are task oriented movements to promote weight shifting, limb loading, and to break down the gait cycle into smaller components. For example, a stepping pattern may be to step the right leg backward fully shifting weight posterior and lateral, to promote hip extension and loading of the leg, and then stepping back to the starting position. The stepping patterns were selected and progressed by the PT to provide a challenge to the participant and varying levels (2-hand support, 1-hand support, no hands, etc) were employed to find the appropriate level at each session. The stepping patterns were progressed first on speed and accuracy and then based on complexity as skill improved. The goal was to complete 10 repetitions of a step with little effort before progressing to more complex patterns. The goal of the power training was to achieve a moderate to vigorous level of intensity (5-8/10 on modified Rate of Perceived Exertion scale23). Exercises were modified to target participant's range of motion and physical ability. The exercises were performed in sets of 8-12 repetitions with progression from 2 to 3 sets as tolerated. The goal was to exercise without causing excessive pain while achieving the desired training effect. Relevant exercises included performing the concentric component of muscle action as quickly as possible to optimize muscle power generation. Veterans were provided with a home exercise program that reinforced concepts that were relevant to their intervention session to complete on days in between the study visits.
Cognitive behavioral skill coaching was implemented at each intervention session as part of the intervention. A program based on the specific, measurable, actionable, realistic and time-oriented (SMART) goal approach was developed by the PT with the participant.22 Each intervention session built off the previous session's content and focused on creating, tracking, and monitoring exercise goals. Addressing internal and external barriers to exercise was also integrated into each session through motivational interviewing techniques. The manual which contained written information about goal setting and worksheet pages was mailed to the participant prior to initiating the intervention so the content would be available to the participant for ease of use.
Statistical analyses
Descriptive statistics were used to generate distributions for all variables. Only participants who were exposed to treatment were evaluated within multivariate models. Change in self-reported mobility using the AM-PAC and self-efficacy using the SEE were examined from baseline through follow-up assessments using linear mixed models with a fixed effect of time. Iterative models were evaluated using important covariates, including age and sex, 1 at a time to ensure model stability. Age and sex were considered as covariates due to the differences that may arise in function in both middle and older aged Veterans as well as differences that may occur across sexes with self-reported measures. Where possible, mean differences from baseline were inspected relative to published clinically meaningful thresholds of difference.
Results
A total of 178 Veterans were contacted via letter about the pilot study between September 2020 and May 2021. We received 122 returned opt out cards. Twenty-seven interested participants were screened for eligibility and 4 were excluded due to reporting no mobility issues and 2 were excluded due to a mini-MoCA score <10. A total of 21 participants were enrolled into our virtual pilot. There were 14 who completed the program. Reasons for dropout after enrollment included simultaneously enrolled in another clinical trial (n=2) medical complications not related to the study (chronic obstructive pulmonary disease flare up, atrial fibrillation, foot fracture) (n=3), undisclosed progressive neurologic disease (n=1), and death of immediate family member (n=1). None of these participants were exposed to treatment and thus were excluded from the analysis. Those who dropped out from the study were older (74.9 years±12) but had similar body mass index (30.8±6.6 kg/m2), race (83.3% white), and similar health rating (excellent/very good/good=67%, fair=33%).
Baseline characteristics are reported in table 1 and summarized as follows. The average age of those who completed the intervention was 70.3 years (±8.6, range 56–81 years), with 12/14 being men and 10/14 being white. Among the participants, 58% (n=8) completed college or graduate school with 29% (n=4) partially completing college and 14% (n=2) completing high school. The average mini-MoCA score was 12.7 (±1.4) out of a maximum score of 15. Half self-reported receiving assistance with activities of daily living (ADL)s or instrumental ADLs and 4/14 reported living alone. At baseline, 8/14 reported their current health as very good or good while 6/14 report their current health as fair. Less than half (6/14) reported that their overall health is the same compared with 1 year ago, while 5/14 report their health is somewhat worse. Over 10/14 of the participants had high blood pressure and a similar number manifested arthritis. Greater than half of the participants reported back pain (8/14), depression (8/14), or any fall in the past year (9/14). 5/9 falls were reported as injurious falls. Almost half (6/14) of the participants were diabetic.
Table 1.
Baseline characteristics (n=14)
| Age, years | 70.3 (8.6) |
| Sex (female), n (%) | 2 (14) |
| Body mass index | 31.9 (5.5) |
| Race, n (%) | |
| White | 10 (71) |
| Black | 1 (7) |
| Other | 3 (21) |
| Hispanic, Spanish, Latino Ethnicity | 2 (14) |
| Education, n (%) | |
| Graduate School | 4 (29) |
| College | 4 (29) |
| <4 years of College | 4 (29) |
| HS/GED | 2 (14) |
| Mini-MoCA score (/15) | 12.7 (1.4) |
| Live Alone, n (%) | 4 (29) |
| Receive assistance with ADLs/instrumental ADLs, n (%) | 7 (50) |
| Current health rating, n (%) | |
| Very Good/Good | 8 (57) |
| Fair | 6 (43) |
| Health rating compared with 1 year ago, n (%) | |
| Much better/Somewhat better | 3 (21) |
| Same | 6 (43) |
| Somewhat worse | 5 (36) |
| History of fall in past year, n (%) | 9 (64) |
| Injurious fall, n (%) | 5 (36) |
| Hospitalized, n (%) | 0 |
| Co-morbid conditions, n (%) | |
| History of heart disease | 4 (29) |
| History of high blood pressure | 10 (71) |
| History of diabetes | 6 (43) |
| History of depression | 8 (57) |
| History of OA or RA | 10 (71) |
| History of back pain | 8 (57) |
| History of neurologic disease | 4 (29) |
NOTE. Mean (standard deviation) unless noted.
Technological issues
Participants completed on average of 9.8 intervention visits out of 10 (range 8-10). There were on average 2.3 technology issues experienced by participants across their 10 intervention visits (range 0-6). Types of technology issues included connectivity and set up. Connectivity problems included delay between the audio and video, video freezing, the VVC link not facilitating connection, and national malfunction of the VVC platform. For most problems, the PT was able to solve the issue without involving the VA Telehealth Help Desk. Solutions included using the phone for audio to correct the delays or video freezing coupled with use of the exercise handbook. Only twice were visits re-scheduled due to connectivity related problems. Issues related to set up included positioning of the camera to maximize view of the participant during treatment and uncharged devices. The PT was able to spend time reviewing positioning during the first session and would remind participants to have devices charged. An uncharged device did result in 1 rescheduled visit. Most problems were solved in under 5 minutes of time. Use of the technology helpdesk required increased time. No adverse events occurred during study intervention visits.
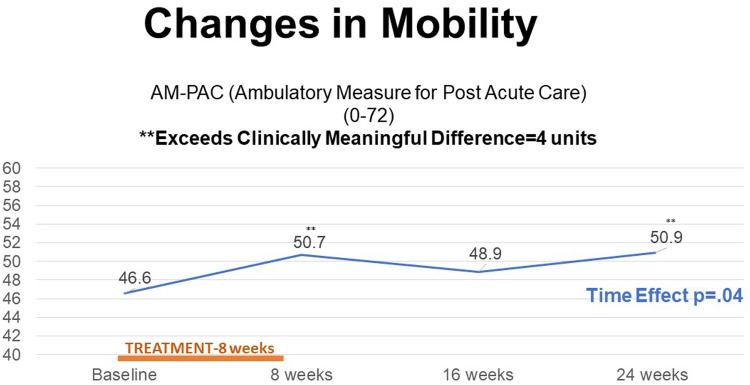
Inspection of repeated mean scores of the AM-PAC outpatient mobility scale revealed a significant increase in scores over time (P<.05). Additionally, improvements of >4 units from baseline exceeded a clinically meaningful threshold [25] at 8 and 24 weeks (see fig 1). When compared with an average baseline scaled value of 46.6, participants saw a 4.1 unit (5.6 SD) increase at 8 weeks, a 2.3 unit (8.1 SD) increase at 16 weeks, and a 4.3 unit (7.2 SD) increase at 24 weeks (for a final average scaled value of 50.9). Physical activity as measured by the Phone FITT increased significantly over time (P=.02) with a mean improvement of 12.6 units (15.9 SD), 4.8 units (20.3 SD), and 5.4 units (16.2 SD) at 8, 16, and 24 weeks, respectively. Clinically meaningful changes are not defined for this measure. No significant changes were observed for either measure of self-efficacy. Also, clinically meaningful increments of these measures are not defined. Complete results are summarized in table 2.
Fig 1.
Change in mobility.
Table 2.
Mean Assessment Scores over time (n=14)
| Baseline (0 weeks) | 8 weeks | 16 weeks | 24 weeks | P Value for Time | |
|---|---|---|---|---|---|
| AM-PAC | 46.6 (8.7) | 50.7† (5.7) | 48.9 (8.1) | 50.9† (7.2) | 0.04* |
| Phone-Fitt | 36.2 (14.5) | 48.6 (15.9) | 41.0 (20.3) | 41.6 (16.2) | 0.02* |
| SEE | 65.7 (16.7) | 66.6 (15.4) | 63.1 (18.9) | 67.7 (19.4) | 0.75 |
| ABC | 70.0 (21.2) | 72.2 (20.1) | 78.8 (17.8) | 76.2 (12.6) | 0.49 |
NOTE. Higher scores indicate better performance on each measure.
Statistical significance.
Exceeding a clinically meaningful difference from baseline.
Discussion
The major findings of this pilot study were that the T-LLWS program demonstrated both feasibility and acceptability among mildly to moderately mobility limited Veterans and that it may have the potential to improve both short- and long-term mobility and physical activity. We were also able to successfully employ measures of self-efficacy in this population; however, we were not able to demonstrate improvement within this construct after exposure to our intervention.
Use of the VA VVC platform to conduct 1:1 rehabilitative training was technologically feasible. Participants found this form of delivery to be acceptable with a completion rate of 98% for the intervention sessions. This high completion rate was achieved despite the occurrence of periodic technical problems. A cross-sectional survey done within the VA among 404 Veterans reported that 72% had never used telehealth for physical care and that 82% found this mode of care valuable and helpful.24 We can't generalize this rate of problems and success to other more remote VA settings where Wi-Fi and cellular coverage might vary; however, since completion of this study, the VA has improved the efficiency and performance of its VVC delivery systems. This virtual rehabilitation program was delivered at a moderate-high intensity for each participant to maximize training potential.25 Although not reported in our results section, the overall intensity of the intervention visits ranged from 5.2 to 6.3 on a modified 10-point rate of perceived exertion scale. The average time our participants spent actively engaged in the exercise program was approximately 39 minutes across all visits. It was well tolerated by participants with minimal physical complaints even though this was a sample with relatively high comorbidity (see table 1).
We also observed robust improvements in mobility 8 weeks and 24 weeks post baseline. These are valuable preliminary findings confirming that virtual rehabilitative care can not only improve function at the end of treatment, but in the case of T-LLWS care that there is the potential for sustainment of these improvements in physical function longer term. Sustained improvement in functioning is critical for care of mobility limited older patients given that the shorter-term benefits of standard PT care commonly necessitate repeated courses of treatment which increases the burden for patients and costs for payers.26 A systematic review and meta-analysis from Jirasakulsuk et al examined real-time telerehabilitation for musculoskeletal conditions in older adults with a goal to improve physical performance before surgery. They reported that real-time telerehabilitation via a specialized application demonstrated similar or better effects compared with usual in-person care for balance ability, range of motion, and leg strength.7 Seron et al reported in a rapid overview that that telerehabilitation was comparable with in-person rehabilitation for conditions such as low back pain, osteoarthritis, lower extremity joint replacement, multiple sclerosis, and within cardiac and pulmonary rehabilitation; however, majority of studies within the review used telephone calls and messaging or video gaming as their primary means of telerehabilitation.6 In a younger cohort of adults with chronic back pain over an 8-week physical therapy protocol, about 70% initiated the telehealth rehabilitation, which is comparable with in-person rehabilitation for low back pain. Participants also reported improvements in physical function at 24-week follow-up.27 Rehabilitation was provided via multiple modes (telephone calls, videos, or a specialized application). We captured information on feasibility of using real-time synchronous technology including number and type of issues that arose, which has not been reported previously. Our study was also composed of middle and older aged Veterans with multiple chronic conditions. However, among other studies and our own, further confirmation of efficacy within studies including control groups is warranted.
A focus on exercise behavior change is included in LLWS care and is theorized to enhance physical activity and self-efficacy. We did observe an increase in physical activity by the Phone-FITT that not only occurred during the initial 8 weeks of treatment but also was sustained though the 24 weeks of follow-up. So, it is plausible that this component of care facilitated the sustained improvements in physical functioning. We have observed this effect within larger related clinical trials conducted by members of this research team.28,29 However, we fully acknowledge that we did not observe significant changes in either measure of self-efficacy. Also, neither measure has clinically meaningful increments defined for similar populations making it more challenging to interpret the observed effect sizes. This is consistent with other studies in which self-efficacy improvements were not associated with observed changes in physical activity.30 So, we must interpret these preliminary findings with caution and view them as confirmation that further investigation of T-LLWS is scientifically warranted.
Study limitations
Our study has other limitations. This is a single-arm pilot study within a small, mostly white, men, well-educated, localized sample of middle and older aged Veterans. Thus, we can't generalize our findings to other regions and demographic groups. We also acknowledge that only a small number of Veterans expressed interest in the study during this time period which was marked by the most restrictive times of the COVID pandemic. By necessity, all assessments were based on self-report, which may be challenging for those with cognitive impairment. We used the VVC platform to conduct our assessments and intervention visits, which is not available outside of the Veteran's Health Administration. Technology issues and frequencies may not mirror other platforms.
There are some important strengths of this pilot study. We designed a rehabilitation program to target mobility limitations that fits within the VA model of care and is also consistent with the duration, intensity and focus of PT care delivered within ambulatory care settings. Care was designed to be delivered within a telehealth format and to be safe and effective when use of telehealth for health care was relatively new to many Veterans. With the widespread use of telerehabilitation programs during the pandemic, it is important to have empirical evidence supporting this mode of care. We conducted this study during the early stages of the pandemic, when patients were dealing with heightened stress. It would be important to replicate these findings under different circumstances. The importance of this approach extends beyond a health care system's ability to respond to a pandemic. It also addresses the needs and wants of older adults with mobility problems. It is recognized that physical function is the number 1 priority of older patients seeking care.31 Also, by circumstance, patients with mobility problems are challenged in their ability to travel and attend ambulatory care visits. This point was exemplified in the original clinical demonstration project of LLWS when it was provided as a model of outpatient rehabilitation.12 Of patients referred to that clinical demonstration project, 40% did not engage with care, largely due to their underlying mobility problems.12 We intend to evaluate the effectiveness of T-LLWS within larger samples of veterans and especially among those residing in rural settings, not residing in proximity to health care facilities and with more limited access to Wi-Fi and technical resources. Therefore, for health care systems such as the VA that care for large numbers of older mobility limited patients, many of whom who reside in rural settings, this line of research and our corresponding findings have great significance.
Conclusions
This pilot study demonstrated feasibility, acceptability, and preliminary efficacy of the T-LLWS care program among middle and older aged Veterans. This line of research should be continued within larger scale clinical trials powered to evaluate efficacy, effectiveness, and cost/benefit at the patient and health care system levels.
Footnotes
Methods and results from this pilot were presented at the Annual Conference of the American Congress of Rehabilitation Medicine 2022, Chicago, Illinois.
Staff conducting this work had effort supported through funding from VA Rehabilitation Research and Development Service RX003095-01, 1 I50 RX003430-01, and 1 RX003636 (Ogawa) as well as NIH K24 AG069176-06 (Bean) and AG057728-04 (Brach).
Clinical trial: NCT04026503.
De-identified data available through requests following all VA policies.
Disclosures: No conflict of interest to report.
References
- 1.Chen Y, Sloan FA. Explaining disability trends in the U.S. elderly and near-elderly population. Health Serv Res. 2015;50:1528–1549. doi: 10.1111/1475-6773.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 3.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 4.Selim AJ, Berlowitz D, Kazis LE, et al. Comparison of health outcomes for male seniors in the Veterans Health Administration and Medicare Advantage plans. Health Serv Res. 2010;45:376–396. doi: 10.1111/j.1475-6773.2009.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lugo-Agudelo LH, Cruz Sarmiento KM, Spir Brunal MA, et al. Adaptations for rehabilitation services during the COVID-19 pandemic proposed by scientific organizations and rehabilitation professionals. J Rehabil Med. 2021;53:jrm00228. doi: 10.2340/16501977-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seron P, Oliveros MJ, Gutierrez-Arias R, et al. Effectiveness of telerehabilitation in physical therapy: a rapid overview. Phys Ther. 2021;101:pzab053. doi: 10.1093/ptj/pzab053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jirasakulsuk N, Saengpromma P, Khruakhorn S. Real-time telerehabilitation in older adults with musculoskeletal conditions: systematic review and meta-analysis. JMIR Rehabil Assist Technol. 2022;9:e36028. doi: 10.2196/36028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonathan CR, Nicholas G, Artur D, Christina B, Ralph M. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart. 2016;102:1183. doi: 10.1136/heartjnl-2015-308966. [DOI] [PubMed] [Google Scholar]
- 9.Elavsky S, Knapova L, Klocek A, Smahel D. Mobile health interventions for physical activity, sedentary behavior, and sleep in adults aged 50 years and older: a systematic literature review. J Aging Phys Act. 2019;27:565–593. doi: 10.1123/japa.2017-0410. [DOI] [PubMed] [Google Scholar]
- 10.Alexander NB, Phillips K, Wagner-Felkey J, et al. Team VA Video Connect (VVC) to optimize mobility and physical activity in post-hospital discharge older veterans: baseline assessment. BMC Geriatr. 2021;21:502. doi: 10.1186/s12877-021-02454-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris R, Brach JS, Moye J, et al. The Live Long Walk Strong Rehabilitation Program Study: design and methods. Arch Rehabil Res Clin Transl. 2022;4 doi: 10.1016/j.arrct.2022.100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown LG, Ni M, Schmidt CT, Bean JF. Evaluation of an outpatient rehabilitative program to address mobility limitations among older adults. Am J Phys Med Rehabil. 2017;96:600–606. doi: 10.1097/PHM.0000000000000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 14.Jette AM, Haley SM, Ni P, Moed R. Adaptive short forms for outpatient rehabilitation outcome assessment. Am J Phys Med Rehabil. 2008;87:842–852. doi: 10.1097/PHM.0b013e318186b7ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 16.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A:M28–M34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 17.Resnick B, Jenkins LS. Testing the reliability and validity of the self-efficacy for exercise scale. Nurs Res. 2000;49:154–159. doi: 10.1097/00006199-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Poquet N, Lin C. The Brief Pain Inventory (BPI) J Physiother. 2016;62:52. doi: 10.1016/j.jphys.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Gill DP, Jones GR, Zou GY, Speechley M. The Phone-FITT: a brief physical activity interview for older adults. J Aging Phys Act. 2008;16:292–315. doi: 10.1123/japa.16.3.292. [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brach JS, Berthold R, Craik R, VanSwearingen JM, Newman AB. Gait variability in community-dwelling older adults. J Am Geriatr Soc. 2001;49:1646–1650. doi: 10.1046/j.1532-5415.2001.t01-1-49274.x. [DOI] [PubMed] [Google Scholar]
- 22.Bamonti PM, Moye J, Harris R, et al. Development of a coaching protocol to enhance self-efficacy within outpatient physical therapy. Arch Rehabil Res Clin Transl. 2022;4 doi: 10.1016/j.arrct.2022.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 24.Kintzle S, Rivas WA, Castro CA. Satisfaction of the use of Telehealth and access to care for veterans during the COVID-19 pandemic. Telemed J E Health. 2022;28:706–711. doi: 10.1089/tmj.2021.0262. [DOI] [PubMed] [Google Scholar]
- 25.White NT, Delitto A, Manal TJ, Miller S. The American Physical Therapy Association's top five choosing wisely recommendations. Phys Ther. 2015;95:9–24. doi: 10.2522/ptj.20140287. [DOI] [PubMed] [Google Scholar]
- 26.Musich S, Wang SS, Ruiz J, Hawkins K, Wicker E. The impact of mobility limitations on health outcomes among older adults. Geriatr Nurs. 2018;39:162–169. doi: 10.1016/j.gerinurse.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Fritz JM, Minick KI, Brennan GP, et al. Outcomes of Telehealth physical therapy provided using real-time, videoconferencing for patients with chronic low back pain: a longitudinal observational study. Arch Phys Med Rehabil. 2022;103:1924–1934. doi: 10.1016/j.apmr.2022.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Latham NK, Harris BA, Bean JF, et al. Effect of a home-based exercise program on functional recovery following rehabilitation after hip fracture: a randomized clinical trial. JAMA. 2014;311:700–708. doi: 10.1001/jama.2014.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bean JF, Brown L, DeAngelis TR, et al. The Rehabilitation Enhancing Aging through Connected Health (REACH) prehabilitation trial. Arch Phys Med Rehabil. 2019;100:1999–2005. doi: 10.1016/j.apmr.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Chang FH, Latham NK, Ni P, Jette AM. Does self-efficacy mediate functional change in older adults participating in an exercise program after hip fracture? A randomized controlled trial. Arch Phys Med Rehabil. 2015;96:1014-20.e1. doi: 10.1016/j.apmr.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fried TR, Tinetti ME, Iannone L, O'Leary JR, Towle V, Van Ness PH. Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med. 2011;171:1854–1856. doi: 10.1001/archinternmed.2011.424. [DOI] [PMC free article] [PubMed] [Google Scholar]