Abstract
Purpose
To compare tensile fatigue and strength measures of biocomposite and all-suture anchors in an ovine humerus-infraspinatus tendon model of rotator cuff repair.
Methods
Infraspinatus tendons on adult ovine humeri were sharply transected at the insertion. One of each pair was assigned randomly for fixation with 2 biocomposite or all-suture anchors. Constructs were tested with 200 cycles of 20 to 70 N tensile load, and gap formation was measured at the incised tendon end every 50 cycles. They were subsequently tested to failure. Outcome measures including fatigue stiffness, hysteresis, creep, and gap formation and tensile stiffness, and yield and failure displacement, load, and energy were compared between anchors.
Results
Biocomposite anchors had greater yield load (134.1 ± 6.5 N, P < .01) and energy (228.6 ± 85.7 J, P < .03) than all-suture anchors (104.7 ± 6.5 N, 169.8 ± 85.7 J). Fatigue properties were not different between anchors, but stiffness and gap formation increased and hysteresis and creep decreased significantly with increasing cycle number.
Conclusions
Although the yield displacement of both anchors was within the range of clinical failure, the tensile yield load and energy of ovine infraspinatus tendons secured to the humerus with 2 single-loaded all-suture anchors in a single row were significantly lower than those secured with 2 biocomposite anchors in the same configuration.
Clinical Relevance
It is important to understand the biomechanical properties for selecting anchors for rotator cuff repair. A direct comparison of fatigue testing followed by failure strength of infraspinatus tendon fixation with all-suture and biocomposite anchors could help guide anchor selection and postoperative mobility recommendations.
Rotator cuff disease accounts for more than 4.5 million physician visits per year in the United States.1, 2, 3, 4 Arthroscopic repair is the gold standard for surgical treatment of rotator cuff tears with persistent symptoms or tears larger than 1 to 1.5 cm5; repair of partial or complete tear has relatively good patient outcomes.6 Suture anchors are used to secure soft tissue to bone for repair of full-thickness tears, and metal, biodegradable, and biocomposite anchors are standard. Although there are both advantages and disadvantages to solid-anchor composition and design, they have comparable pull-out strength and perform equally well in terms of patient outcomes.7,8
One option for rotator cuff repair is all-suture anchors with high-strength suture strands attached to a stiff suture section that is deployed in a bone socket for subcortical fixation.9 All-suture anchors are reported to have advantages over solid anchors, including a smaller bone socket and the ability to accommodate multiple sutures.10, 11, 12 In addition, the material properties reduce the risk of articular damage from a dislodged anchor.7,13 There is a lack of consensus in the existing literature regarding the pull-out and fatigue properties of all-suture anchors compared with solid anchors, with substandard, comparable, and superior properties reported for all-suture anchors.10,13,14 Differences in testing methods, outcome measures, and species complicate comparisons among studies.10,12,13,15,16 Many of the studies do not include bone-tendon constructs but compare tensile strength and fatigue properties of individual implants in bone.12,15, 16, 17 Immediate postfixation bone-tendon construct properties are vital to the current paradigm of early postsurgical joint mobilization.18, 19, 20, 21 Human and ovine infraspinatus tendons are similar in size and shape, with an average thickness of 3.9 mm, and they have a comparable length and footprint.22,23 A direct comparison of fatigue testing followed by failure strength of infraspinatus tendon fixation with all-suture and biocomposite anchors could help guide anchor selection and postoperative mobility recommendations.
The purpose of this study was to compare tensile fatigue and strength measures of biocomposite and all-suture anchors in an ovine humerus-infraspinatus tendon model of rotator cuff repair.22, 23, 24, 25, 26, 27, 28 We hypothesized that there would be no significant difference in tensile fatigue and strength measures between the 2 infraspinatus fixation constructs.
Methods
Construct Preparation
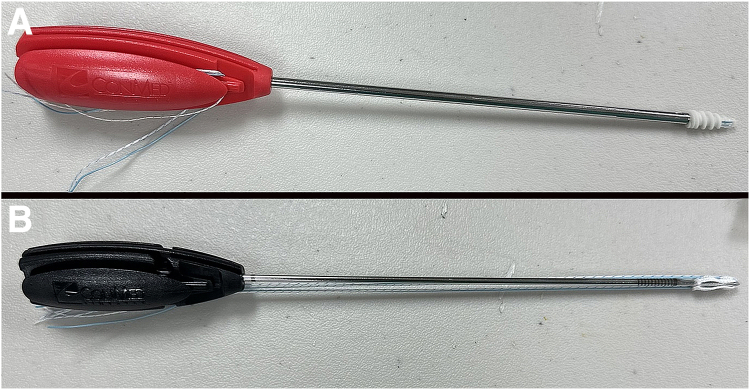
Five pairs of adult ovine shoulders were obtained immediately postmortem from an abattoir. Skin and subcutaneous tissue were removed, and the limbs were wrapped in saline-soaked towels, sealed in plastic bags, and maintained frozen at –20°C until use. They were thawed in room temperature saline (21°C) overnight. The scapula and all soft tissues were removed except the infraspinatus tendon, which was sharply detached from the muscle origin. The tendon was sharply transected from the humeral insertion. Specimens were assigned randomly to treatment cohorts with a random number generator (Excel, Microsoft 365; Microsoft Corp., Redmond, WA). Tendons were immediately reattached in the humeral footprint with either 2 single-loaded 5.5-mm biocomposite anchors (Fig 1A; CrossFT, CONMED Corp., Largo, FL) or two 2.8-mm all-suture anchors (Fig 1B; Y-Knot, CONMED Corp.) placed in a single row 3 to 4 mm from the tendon edge according to the manufacturer’s instructions. Simple square knots with 4 throws were used to secure the tendon to the anchor. All fixations were completed by the same investigator (L.L.). The solid bone anchors are stabilized by interdigitation of the anchor threads with bone. The all-suture anchors, in contrast, are held in place when they are deployed to expand to 5 mm under cortical bone after passing through a 2.8-mm bone tunnel. Therefore, the outer diameter of the all-suture anchors was 5.0 mm. One suture was removed from each anchor so that only one remained, and the suture size (#2 Hi-Fi; CONMED Corp.) was identical between the anchors.
Fig 1.
Photographs of a 5.5-mm-diameter biocomposite anchor (A) and a 2.8-mm all-suture anchor (B), each with two No. 2 sutures. For purposes of this study, one suture was removed from each anchor.
Biomechanical Testing
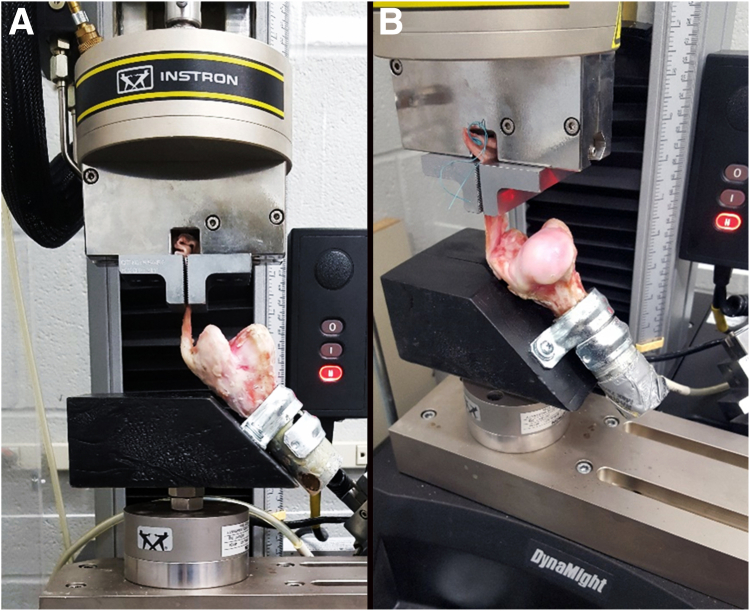
Humeri were transected in the mid-diaphysis with a sagittal saw (#DCS380B; Dewalt, Towson, MD) and stabilized within 2.3-cm (inner diameter) × 6-cm long electrical metallic tubing (Allied Tube and Conduit, Havey, IL) with fiberglass polyester resin (Bondo; 3M, Atlanta, GA). Constructs were affixed to the hypotenuse of an equilateral right-angle wedge fixture (12.7 long × 7.6 wide × 12.7 cm high) attached to the 1 × 103 N capacity load cell of a materials testing system (8841 Dynamite; Instron, Canton, MA) using 2 one-hole U-tube strap clamps (3.3 cm inner diameter, 2.5 cm high; W.W. Grainger, Inc., Lake County, IL) placed directly next to each other with their holes on opposite sides of the cylinder (Fig 2). The upper clamp was placed so that the highest edge of the cylinder and clamp were level. The wedge was attached to the load cell at the midpoint of its adjacent side. There was a small depression at the midpoint of the top edge of the wedge. The greater tuberosity of each specimen rested above the surface of the depression and the position of the specimens relative to the top edge of the wedge was adjusted such that the infraspinatus tendon was at an angle of 135° relative to the long axis of the humerus.16,29,30 The free end of the tendon was dipped in liquid nitrogen and then clamped in pneumatic versa grips with serrated faces (Instron) 10 mm from the transected edge of the tendon. Two 1-mm diameter circles of India ink were placed on the tendon next to each suture and on the bone directly beneath the transected edge with an 18-g needle. The distance between corresponding marks on the tendon and bone was measured with an electronic caliper (Mitutoyo #500-196; Mitutoyo Corp., Sakado, Japan) with 20 N applied and then with 70 N of tensile load after every 50 tensile load cycles described below. Four sequences of tensile loading cycles from 20 to 70 N were applied at a rate of 0.16 Hz to give a total of 200 cycles.16,29,31,32
Fig 2.
Ovine infraspinatus-humerus constructs stabilized in the testing fixture for tensile fatigue and single cycle to failure testing imaged from 2 perspectives, lateromedial (A) and caudocranial (B).
Outcome Data
Stiffness, hysteresis, and creep values were determined from load displacement curves of 3 cycles at the end of each testing sequence, 50, 100, 150, and 200 cycles. The gap formation, defined as the change in distance between the longitudinally aligned marks on the tendon and bone measured during the initial 20-N tensile load and a 70 N-load after each loading sequence, was calculated as Gapn = Gap70Nn – Gap20N0 where n = cycle number. After fatigue testing, specimens were tested in a single cycle to failure at a loading rate of 1 mm/s from a 20-N preload. Stiffness, yield and failure load, displacement, and energy were derived from load-displacement curves (10 Hz sampling rate). The failure mode was recorded during each test and confirmed on digital recordings of all tests.
Statistics
Data were analyzed using SAS/STAT, version 9.4 (SAS Institute Inc., Cary, NC). Failure outcomes were analyzed using MIXED models with anchor type as a fixed effect. Fatigue outcomes measured over cycles were analyzed as repeated measures using MIXED models with anchor type, cycle number, and their interaction as fixed effects. The testing session and the animal within the testing session were included as random effects in all above models. The dependency between fatigue observations within the animal was modeled using a compound symmetry covariance structure that provided the best fit based on the Bayesian information criterion. Residuals were independently identically normally distributed with homogenous variance. Significance was declared for P < .05.
Results
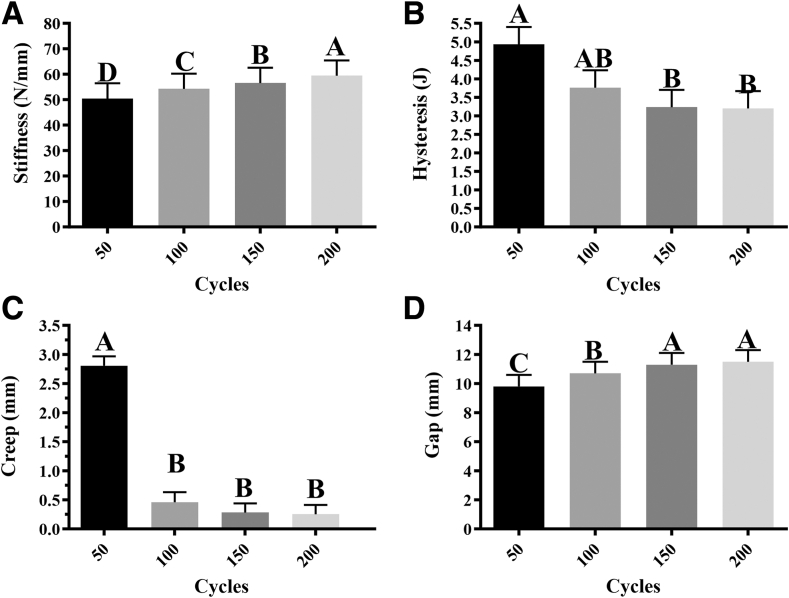
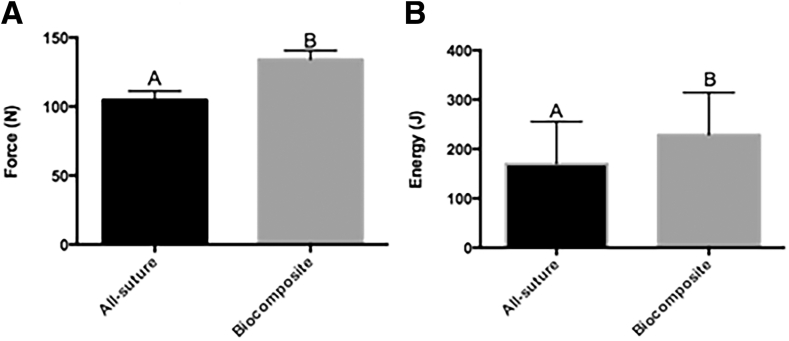
Data were available for 10 specimens (n = 5 biocomposite, n = 5 all-suture). There were no significant differences in fatigue outcomes between anchors, but all outcomes changed with increasing cycle numbers (Table 1). As such, results from both anchor types were combined to evaluate the cycle effect on fatigue properties. Significant differences included greater stiffness after each set of 50 cycles compared with the previous set, lower hysteresis after 150 and 200 cycles compared with after 50 cycles, and lower creep after 100, 150, and 200 cycles compared with after 50 cycles (Fig 3). In addition, the gap was greater after 100 versus 50 cycles and after 150 and 200 cycles compared with 50 and 100 cycles. Biocomposite anchors had greater yield load (134.1 ± 6.5 N) than all-suture anchors (104.7 ± 6.5 N, P < .011), and they also had a greater yield energy (228.6 ± 85.7 J) than the all-suture anchors (169.8 ± 85.7 J, P < .031) (Fig 4, Table 2). There were no differences in stiffness, yield displacement, or failure load, displacement, or energy between the 2 anchors. All constructs failed at the suture-tendon interface with suture pulling through tendon tissue (Fig 5).
Table 1.
Fatigue Testing Cycles Results (n = 5 Biocomposite, n = 5 All-Suture)
| Property | Least Square Mean ± SEM |
|||
|---|---|---|---|---|
| 50 C | 100 C | 150 C | 200 C | |
| Stiffness, Nmm | 50.4 ± 6.0a | 54.2 ± 6.0b | 56.5 ± 6.0c | 59.4 ± 6.0d |
| Hysteresis, J | 4.9 ± 0.5a | 3.8 ± 0.5a/b | 3.2 ± 0.5b | 3.2 ± 0.5b |
| Creep, mm | 2.81 ± 0.16a | 0.46 ± 0.17b | 0.28 ± 0.16b | 0.25 ± 0.16b |
| Gap, mm | 9.8 ± 0.8a | 10.7 ± 0.8b | 11.3 ± 0.8c | 11.5 ± 0.8c |
NOTE. Values with different superscripts within an outcome measure are significantly different between cycle numbers.
C, cycles; SEM, standard error of the mean.
Fig 3.
(A) Stiffness (A), hysteresis (B), creep (C), and gap (D) of all-suture and biocomposite anchors combined after 50, 100, 150, and 200 tensile cycles from 20-70 N (least square mean ± standard error of the mean). Columns with different superscripts within graphs are significantly different (P < .05).
Fig 4.
Yield load (A) and energy (B) from single cycle to failure tensile testing of all-suture and biocomposite anchors (least square mean ± standard error of the mean).
Table 2.
Failure Testing Results (n = 5 Biocomposite, n = 5 All-Suture)
| Property | Anchor Type |
Pr > F | |
|---|---|---|---|
| All-Suture |
Biocomposite |
||
| Least Square Mean ± SEM | |||
| Load, N | |||
| Yield | 104.7 ± 6.5a | 134.1 ± 6.5b | 0.011 |
| Failure | 196.8 ± 31.4 | 181.9 ± 31.4 | 0.647 |
| Displacement, mm | |||
| Yield | 2.5 ± 1.1 | 3.0 ± 1.1 | 0.087 |
| Failure | 8.8 ± 1.4 | 6.1 ± 1.4 | 0.255 |
| Energy, J | |||
| Yield | 169.8 ± 85.7a | 228.6 ± 85.7b | 0.031 |
| Failure | 1350 ± 390.5 | 716.8 ± 390.5 | 0.299 |
| Stiffness, N/mm | 42.1 ± 15.0 | 47.4 ± 15.0 | 0.366 |
NOTE. Values with different superscripts within an outcome measure are significantly different between anchor types.
Pr > F, p value of the F statistic; SEM, standard error of the mean.
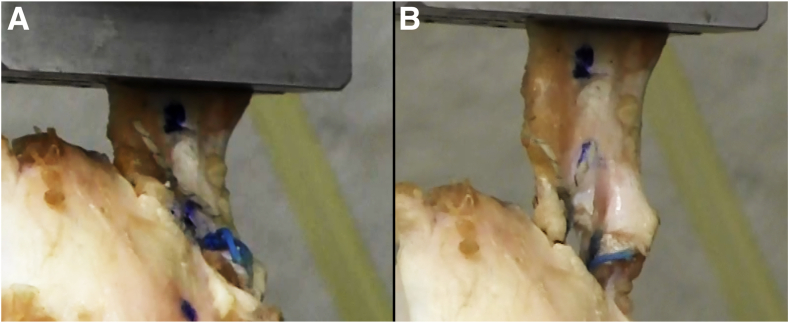
Fig 5.
Photographs showing a transected ovine infraspinatus tendon secured to the native humeral insertion site with 2 all-suture anchors before (left) and after (right) construct failure.
Discussion
On the basis of the results of the study, the hypothesis that there would be no significant difference in tensile fatigue and strength measures between the 2 infraspinatus fixation constructs was rejected. Specifically, the fatigue properties between the 2 anchors were similar, although the yield properties, load, and energy, were greater for the biocomposite anchor. Currently, there is no universally accepted gold standard construct for rotator cuff repair. All-suture anchors have advantages over solid like fewer loose body complications and reduced bone loss.10, 11, 12,33 They are reported to preserve more humeral bone surface area compared to biocomposite anchors, which is valuable for revision repairs.30 However, differences in mechanical properties of suture anchors continue to be an important focus of clinical investigation.7,34
Increasing stiffness and decreasing hysteresis and creep with increasing cycle number recorded in this study during fatigue testing is consistent with tissue cyclic stress relaxation.35 The number of cycles was sufficient to test the fatigue properties and supported uniform single cycle to failure testing as previously reported.15 An important contribution of this study that tends to be rarely reported is the yield energy, area under the load-displacement curve, that represents the energy absorbed by the construct before undergoing plastic deformation at the yield load. The lower yield properties can be explained by findings of a previous study comparing the pull-out behaviors of individual all-suture and biocomposite anchors in cadaveric human glenoids in which the all-suture anchor allowed more micromotion between the device and the bone because of greater compressibility.36 The yield displacements, about 2.5 mm for the all-suture and 3.0 mm for the biocomposite anchor, were consistent with clinical failure, considered to be 1 to 3 mm.37
Construct failure caused by tendon tissue tearing rather than anchor pull-out or anchor or suture breakage is typical of studies using bone-tendon constructs.25 The failure load for the all-suture and biocomposite anchors in this study, 196.8 ± 31.4 N and 181.9 ± 31.4 N, respectively, were comparable with the reported maximum tensile force of all-suture anchors in human humeral heads that ranged from 103.9 to 145.8 N and that of a plastic anchor that was 181.0 N.15 In the same study, the gap formation measured during fatigue testing tended to be similar to or greater than that quantified in this study. Specifically, fatigue gap formation ranged from 9.8 mm after 50 cycles to 11.5 mm after 200 cycles in this investigation. Among the all-suture anchors tested in the previous study, the gap formation range after 50 cycles was 6.9 to 10.3 mm, and it was 22.9 to 27.3 mm after 200 cycles, although 2 of the 4 anchors tested failed before 200 cycles. A distinct difference between the studies is that the gap was measured between the testing fixtures holding the suture and the bone while the gap between the bone and the end of the tendon was measured directly on the constructs for this investigation. In another study using cadaveric human labrums, the ultimate failure loads of the all-suture and biocomposite anchors were 146 N and 175 N, respectively.13 In contrast to most published studies, the constructs used in this study allowed comparison of soft-tissue fixation to bone with 2 different anchors. The tensile properties of bone-soft tissue constructs are distinct from those of anchors embedded in natural or synthetic bone, and bone-soft tissue constructs more closely represent clinical application. In this study, yield load and energy, consistent with clinical failure, were significantly different between the anchors tested in contrast to the lack of difference in failure properties when sutures pulled through tendon tissue. A recent systematic review and meta-analysis of tensile properties of single-loaded suture anchors in synthetic or human bone showed that load to failure was higher in all-suture versus biocomposite anchors.38 This further emphasizes that differences in methods and specimens between studies make comparisons among them difficult.
Although the results of this study indicate that all-suture anchors have a lower yield load and energy, recent noninferiority studies showed no differences in outcomes between arthroscopic repair of rotator cuff tears with solid or all-suture anchors.39,40 There were lower rates of retear, anchor displacement, and suture failure in the all-suture versus biocomposite anchor group in one study,39 and a nonsignificant, but greater retear rate at 12 months in the all-suture cohort compared with the titanium anchor in the other.40 In a study that included a comparison of the pull-out properties of all-suture anchors, the ultimate failure load of all suture anchors was directly correlated with the number of sutures.41 It is possible that the smaller footprint of the all-suture anchor and subsequent ability to place more anchors could compensate for the lower individual yield property and allow for greater overall repair strength. However, poor bone quality (osteopenia/osteoporosis) can negatively affect the purchase of all-suture anchors, particularly when the cortical integrity is compromised. Although outside the purview of this study, addition of sutures to the all-suture anchor or increasing the number of the anchors themselves could improve yield properties. Additional work is necessary to determine the clinical relevance of the study findings.
Limitations
The methods reported here were sufficient to test the stated hypothesis, but the results are limited to the single-loaded suture anchors evaluated within the constraints of an ex vivo mechanical testing study. The comparisons included only one commercial brand of both an all-suture and biocomposite anchor, and the mechanical distinctions identified between the anchors may not correspond to a clinical difference. Testing of additional constructs might have made it possible to identify other dissimilarities between the anchors. Although ovine and human infraspinatus tendons have similar biomechanical, anatomical, and histologic properties, there are inherent differences between species, and sharp tendon transections do not replicate traumatic tears.22, 23, 24 The configuration of single-loaded anchors used for comparative purposes in this study is not representative of the majority of clinical cases.
Conclusions
The results of this study contribute distinct, new information about the mechanical behavior of infraspinatus tendon fixation with single-loaded all-suture or biocomposite anchors. Although the yield displacement of both anchors was within the range of clinical failure, the tensile yield load and energy of ovine infraspinatus tendons secured to the humerus with 2 single-loaded all-suture anchors in a single row were significantly lower than those secured with 2 biocomposite anchors in the same configuration. It is possible that the lower yield properties can likely be overcome with additional sutures or suture anchors, but bone quality can also affect the effectiveness of all-suture anchors in the clinical setting. The mechanical behaviors quantified in this study may not directly translate to clinical differences. As such, further research is necessary to fully elucidate the clinical implications of the study outcomes and to address the limitations inherent to the study design.
Disclosures
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Financial support for this study was provided by the Department of Orthopaedics, Louisiana State University School of Medicine, New Orleans, Louisiana, and the Veterinary Clinical Sciences Department, Louisiana State University, Baton Rouge, Louisiana. All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
R.A.W. and L.L. are co–first authors.
A portion of the information was shared in a 10-minute presentation at the annual meeting of the Louisiana Orthopaedic Association on February 4, 2023, New Orleans, Louisiana, U.S.A.
Current affiliation of Robert A. Walton, M.D.: Louisiana State University Health Shreveport, 1501 Kings Hwy, Shreveport, Louisiana, 71103, U.S.A.
Current affiliation of Lindsey Liuzza Ochsner Health Center - Belle Meade, 605 Lapalco Boulevard, Suite 1B, Gretna, Louisiana, U.S.A. 70056, U.S.A.
Supplementary Data
References
- 1.Dang A., Davies M. Rotator cuff disease: Treatment options and considerations. Sports Med Arthrosc Rev. 2018;26:129–133. doi: 10.1097/JSA.0000000000000207. [DOI] [PubMed] [Google Scholar]
- 2.Edwards P., Ebert J., Joss B., et al. Exercise rehabilitation in the non-operative management of rotator cuff tears: A review of the literature. Int J Sports Phys Ther. 2016;11:279–301. [PMC free article] [PubMed] [Google Scholar]
- 3.Khan M., Warner J.J.P. Cochrane in CORR ((R)): Manual therapy and exercise for rotator cuff disease. Clin Orthop Relat Res. 2017;475:1779–1785. doi: 10.1007/s11999-017-5363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rashid M.S., Cooper C., Cook J., et al. Increasing age and tear size reduce rotator cuff repair healing rate at 1 year. Acta Orthop. 2017;88:606–611. doi: 10.1080/17453674.2017.1370844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dey Hazra R.O., Ernat J.J., Rakowski D.R., et al. The evolution of arthroscopic rotator cuff repair. Orthop J Sports Med. 2021;9 doi: 10.1177/23259671211050899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johannsen A.M., Arner J.W., Elrick B.P., et al. Minimum 10-year outcomes of primary arthroscopic transosseous-equivalent double-row rotator cuff repair. Am J Sports Med. 2021;49:2035–2041. doi: 10.1177/03635465211015419. [DOI] [PubMed] [Google Scholar]
- 7.Cho C.H., Bae K.C., Kim D.H. Biomaterials used for suture anchors in orthopedic surgery. Clin Orthop Surg. 2021;13:287–292. doi: 10.4055/cios20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longo U.G., Petrillo S., Loppini M., et al. Metallic versus biodegradable suture anchors for rotator cuff repair: A case control study. BMC Musculoskelet Disord. 2019;20:477. doi: 10.1186/s12891-019-2834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denard P.J., Burkhart S.S. The evolution of suture anchors in arthroscopic rotator cuff repair. Arthroscopy. 2013;29:1589–1595. doi: 10.1016/j.arthro.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Ntalos D., Sellenschloh K., Huber G., et al. Conventional rotator cuff versus all-suture anchors—A biomechanical study focusing on the insertion angle in an unlimited cyclic model. PLoS One. 2019;14 doi: 10.1371/journal.pone.0225648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thangarajah T., Lo I.K., Sabo M.T. Rotator cuff repair techniques: Current concepts. J Clin Orthop Trauma. 2021;17:149–156. doi: 10.1016/j.jcot.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dwyer T., Willett T.L., Dold A.P., et al. Maximum load to failure and tensile displacement of an all-suture glenoid anchor compared with a screw-in glenoid anchor. Knee Surg Sports Traumatol Arthrosc. 2016;24:357–364. doi: 10.1007/s00167-013-2760-0. [DOI] [PubMed] [Google Scholar]
- 13.Mazzocca A.D., Chowaniec D., Cote M.P., et al. Biomechanical evaluation of classic solid and novel all-soft suture anchors for glenoid labral repair. Arthroscopy. 2012;28:642–648. doi: 10.1016/j.arthro.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Goschka A.M., Hafer J.S., Reynolds K.A., et al. Biomechanical comparison of traditional anchors to all-suture anchors in a double-row rotator cuff repair cadaver model. Clin Biomech (Bristol, Avon) 2015;30:808–813. doi: 10.1016/j.clinbiomech.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Nagra N.S., Zargar N., Smith R.D., et al. Mechanical properties of all-suture anchors for rotator cuff repair. Bone Joint Res. 2017;6:82–89. doi: 10.1302/2046-3758.62.BJR-2016-0225.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietschmann M.F., Froehlich V., Ficklscherer A., et al. Biomechanical testing of a new knotless suture anchor compared with established anchors for rotator cuff repair. J Shoulder Elbow Surg. 2008;17:642–646. doi: 10.1016/j.jse.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Oh J.H., Jeong H.J., Yang S.H., et al. Pullout strength of all-suture anchors: Effect of the insertion and traction angle—a biomechanical study. Arthroscopy. 2018;34:2784–2795. doi: 10.1016/j.arthro.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 18.Kjaer B.H., Magnusson S.P., Henriksen M., et al. Effects of 12 weeks of progressive early active exercise therapy after surgical rotator cuff repair: 12 Weeks and 1-year results from the CUT-N-MOVE randomized controlled trial. Am J Sports Med. 2021;49:321–331. doi: 10.1177/0363546520983823. [DOI] [PubMed] [Google Scholar]
- 19.Matlak S., Andrews A., Looney A., et al. Postoperative rehabilitation of rotator cuff repair: A systematic review. Sports Med Arthrosc Rev. 2021;29:119–129. doi: 10.1097/JSA.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 20.Osborne J.D., Gowda A.L., Wiater B., et al. Rotator cuff rehabilitation: Current theories and practice. Phys Sportsmed. 2016;44:85–92. doi: 10.1080/00913847.2016.1108883. [DOI] [PubMed] [Google Scholar]
- 21.Thigpen C.A., Shaffer M.A., Gaunt B.W., et al. The American Society of Shoulder and Elbow Therapists' consensus statement on rehabilitation following arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2016;25:521–535. doi: 10.1016/j.jse.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 22.Gerber C., Schneeberger A.G., Beck M., et al. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg Br. 1994;76:371–380. [PubMed] [Google Scholar]
- 23.Turner A.S. Experiences with sheep as an animal model for shoulder surgery: Strengths and shortcomings. J Shoulder Elbow Surg. 2007;16:S158–S163. doi: 10.1016/j.jse.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad Z., Al-Wattar Z., Rushton N. Tissue engineering for the ovine rotator cuff: Surgical anatomy, approach, implantation and histology technique, along with review of literature. J Invest Surg. 2020;33:147–158. doi: 10.1080/08941939.2018.1483446. [DOI] [PubMed] [Google Scholar]
- 25.Cummins C.A., Appleyard R.C., Strickland S., et al. Rotator cuff repair: An ex vivo analysis of suture anchor repair techniques on initial load to failure. Arthroscopy. 2005;21:1236–1241. doi: 10.1016/j.arthro.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Demirhan M., Atalar A.C., Kilicoglu O. Primary fixation strength of rotator cuff repair techniques: A comparative study. Arthroscopy. 2003;19:572–576. doi: 10.1016/s0749-8063(03)00126-9. [DOI] [PubMed] [Google Scholar]
- 27.Nelson C.O., Sileo M.J., Grossman M.G., et al. Single-row modified Mason-Allen versus double-row arthroscopic rotator cuff repair: A biomechanical and surface area comparison. Arthroscopy. 2008;24:941–948. doi: 10.1016/j.arthro.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Sileo M.J., Ruotolo C.R., Nelson C.O., et al. A biomechanical comparison of the modified Mason-Allen stitch and massive cuff stitch in vitro. Arthroscopy. 2007;23:235–240. doi: 10.1016/j.arthro.2006.11.007. 240.e231-232. [DOI] [PubMed] [Google Scholar]
- 29.Schneeberger A.G., von Roll A., Kalberer F., et al. Mechanical strength of arthroscopic rotator cuff repair techniques: an in vitro study. J Bone Joint Surg Am. 2002;84:2152–2160. doi: 10.2106/00004623-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Aramberri-Gutierrez M., Martinez-Menduina A., Boyle S., et al. Biomechanical testing of trans-humeral all-suture anchors for rotator cuff repair. Shoulder Elbow. 2019;11:77–85. doi: 10.1177/1758573218779078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burkhart S.S., Diaz Pagan J.L., Wirth M.A., et al. Cyclic loading of anchor-based rotator cuff repairs: Confirmation of the tension overload phenomenon and comparison of suture anchor fixation with transosseous fixation. Arthroscopy. 1997;13:720–724. doi: 10.1016/s0749-8063(97)90006-2. [DOI] [PubMed] [Google Scholar]
- 32.De Carli A., Vadala A., Monaco E., et al. Effect of cyclic loading on new polyblend suture coupled with different anchors. Am J Sports Med. 2005;33:214–219. doi: 10.1177/0363546504267348. [DOI] [PubMed] [Google Scholar]
- 33.Dhawan A., Ghodadra N., Karas V., et al. Complications of bioabsorbable suture anchors in the shoulder. Am J Sports Med. 2012;40:1424–1430. doi: 10.1177/0363546511417573. [DOI] [PubMed] [Google Scholar]
- 34.Barber F.A., Herbert M.A. Cyclic loading biomechanical analysis of the pullout strengths of rotator cuff and glenoid anchors: 2013 update. Arthroscopy. 2013;29:832–844. doi: 10.1016/j.arthro.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 35.Schatzmann L., Brunner P., Staubli H.U. Effect of cyclic preconditioning on the tensile properties of human quadriceps tendons and patellar ligaments. Knee Surg Sports Traumatol Arthrosc. 1998;6(suppl 1):S56–S61. doi: 10.1007/s001670050224. [DOI] [PubMed] [Google Scholar]
- 36.Pfeiffer F.M., Smith M.J., Cook J.L., et al. The histologic and biomechanical response of two commercially available small glenoid anchors for use in labral repairs. J Shoulder Elbow Surg. 2014;23:1156–1161. doi: 10.1016/j.jse.2013.12.036. [DOI] [PubMed] [Google Scholar]
- 37.Provencher M.T., Verma N., Obopilwe E., et al. A biomechanical analysis of capsular plication versus anchor repair of the shoulder: Can the labrum be used as a suture anchor? Arthroscopy. 2008;24:210–216. doi: 10.1016/j.arthro.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y.S., Shih C.A., Fang C.J., et al. Biomechanical comparison of different suture anchors used in rotator cuff repair surgery-all-suture anchors are equivalent to other suture anchors: A systematic review and network meta-analysis. J Exp Orthop. 2023;10:45. doi: 10.1186/s40634-023-00608-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Gennaro S., Lecce D., Tarantino A., et al. Arthroscopic repair of rotator cuff injury with bioabsorbable suture anchor vs. all-suture anchor: A non-inferiority study. BMC Musculoskelet Disord. 2022;23:1098. doi: 10.1186/s12891-022-06061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan H., Zhao L., Wang J., et al. An all-suture anchor offers equivalent clinical performance to an established solid suture anchor in the arthroscopic repair of rotator cuff tears: A prospective, randomized, multicenter trial with 12-month follow-up. Arthroscopy. 2024;40:265–276. doi: 10.1016/j.arthro.2023.06.056. [DOI] [PubMed] [Google Scholar]
- 41.Barber F.A., Herbert M.A. All-suture anchors: Biomechanical analysis of pullout strength, displacement, and failure mode. Arthroscopy. 2017;33:1113–1121. doi: 10.1016/j.arthro.2016.09.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.