Abstract
The EFSA Panel on Animal Health and Welfare (AHAW) was asked to deliver a scientific opinion on the use of high‐expansion foam for stunning and killing pigs and poultry. A dossier was provided by the applicant as the basis for an assessment of the extent to which the method is able to provide a level of animal welfare at least equivalent to that ensured by the currently allowed methods for pigs and poultry. According to legislation, to be approved in the EU, new stunning methods must ensure (1) the absence of pain, distress or suffering until the onset of unconsciousness, and (2) that the animal remains unconscious until death. An ad hoc Working Group set up by EFSA performed the assessment as follows: (1) The data provided were checked against the criteria laid down in the EFSA Guidance (EFSA, 2018), and was found to partially fulfil those criteria; (2) extensive literature search; (3) data extraction for quantitative assessment; (4) qualitative exercise based on non‐formal expert elicitation. The assessment led to conclude that it is more likely than not (certainty > 50%–100%) that high‐expansion foam for stunning and killing pigs and poultry, named NEFS in container (Nitrogen Expansion Foam Stunning in container), provides a level of welfare at least equivalent to one or more of the currently allowed methods listed in Annex I of Council Regulation (EC) No 1099/2009. The overall assessment of EFSA is valid only under the technical conditions described in this Opinion for laying hens, broiler chickens of all age and pigs weighing 15–41 kg in situations other than slaughter. The overall assessment of EFSA is that NEFS can be suitable for depopulation using containers for pig and poultry farms respecting the technical conditions and the categories and types of animals defined in this Scientific Opinion.
Keywords: animal welfare, container, nitrogen expansion foam, on farm killing, pigs, poultry, stunning
SUMMARY
Council Regulation (EC) No 1099/2009 on the protection of animals at the time of killing lists in its Annex I the stunning applications currently allowed in the European Union (EU), together with the conditions under which those applications can be implemented. With the aim of constantly improving animal welfare, the Commission can amend the list of the approved methods in Annex I, considering scientific and technical progress. However, for a new or modified stunning application to be listed as an approved method in Annex 1, evidence that it ensures a level of animal welfare at least equivalent to that ensured by the currently approved methods must be provided.
The European Food Safety Authority (EFSA) was requested to perform such assessment regarding high expansion foam using nitrogen for on‐farm killing of pigs and poultry. Killing of poultry and pigs using inert gases, i.e. nitrogen and argon, is an approved method but the use of high expansion foam as a vehicle to carry nitrogen and displace atmospheric air in a container and the use of nitrogen jet stream to destroy the foam and release its content to create anoxia are considered a modification of the approved method. The modified method is named Nitrogen Expansion Foam Stunning in container (NEFS in container), which is an irreversible stunning system. According to the description of the method, high expansion refers to an expansion ratio of 1:> 250 where the expansion ratio is defined as the volume of foaming solution (volume of foaming agent plus water) to the volume of foam produced, while anoxia refers to an atmosphere containing less than 2% residual oxygen. The maintenance of unconsciousness until death is ensured by maintenance of less than 2% residual oxygen in nitrogen for 5 min in poultry and 7 min in pigs.
An ad hoc working group (WG) was set up by EFSA to address the terms of reference of the mandate received by the Commission. The welfare risk assessment was focused on the hazards associated with the foam, rather than anoxia per se. In this regard, the animals will come in contact with the foam and remain so until the foam is burst, and nitrogen gas is released to establish less than 2% by volume of residual oxygen in nitrogen, which could take up to 45 s, and until loss of consciousness occurs in the animals. For assessment phase 1, which is check of data for risk assessment, the WG assessed the scientific papers and the related annexes provided by the applicant in a dossier, following the procedure of EFSA Guidance on the assessment criteria for applications for new or modified stunning methods regarding animal protection at the time of killing (EFSA AHAW Panel, 2018). The outcome of the assessment was that the scientific papers contained information to partially fulfil the requirements for assessment phase one. The wording ‘partial’ was added, since the dossier, for example, failed to correlate adequately neurological measures with the behavioural measures. The lack of specific scientific data did not allow the WG to conclude on the occurrence and prevalence of the hazards, as well as on the time during which the animals could be subjected to these hazards. The method was assessed only for animal categories for which data were provided. The dossier also contained field data, i.e. number of animals killed belonging to different species, to contribute to the determination of the external validity of the assessment, but no experimental details or the animal welfare outcomes (failures) were disclosed. Therefore, the dossier submitted only partially fulfilled the eligibility criteria of EFSA Guidance, but there was still sufficient information provided by the applicant to perform phase two of the assessment, which is the risk assessment of the proposed stunning method, particularly about pain, distress or suffering caused to certain types of pigs and poultry exposed to the NEFS.
The most critical stage of assessment phase 2 was to compare the NEFS method with the existing stunning applications in terms of impact on animal welfare using quantitative and qualitative approaches. The quantitative assessment of the already allowed stunning/killing methods is difficult to perform because of the insufficient quantitative data available. In addition, differences in protocols and measurement methods can lead to significant variability in results. Therefore, it was agreed to use a qualitative method based on non‐formal expert elicitation to assess the equivalence of NEFS in containers with the other killing methods approved in the legislation. As a first step, the WG experts (n = 4) identified the main hazards related to each stunning method, i.e. mechanical and electrical stunning methods, controlled atmosphere stunning methods and NEFS. The experts were asked to rank these hazards in terms of impact on animal welfare.
It is worth noting that the conclusions provided in the dossier and as described in Section 3.3.2.3 of this Scientific Opinion are the applicant's conclusions and do not represent the views of EFSA, and that the independent assessment performed by EFSA is presented in Section 4.2, while the conclusions separately are presented in Section 5. However, EFSA and the applicant reached the same conclusions for some points.
The AHAW Panel concluded with > 50%–100% certainty (more likely than not) that:
exposure of pigs to anoxia (< 2% by volume of residual oxygen) created using high expansion foam (expansion ratio 1:> 250) filled with nitrogen (in less than a minute) provides a level of animal welfare at least equivalent to that ensured by exposure to carbon dioxide (CO2) at high concentration.
exposure of poultry to anoxia (< 2% by volume of residual oxygen) created using high expansion foam (expansion ratio 1:> 250) filled with nitrogen provides a level of animal welfare at least equivalent to that ensured by exposure to carbon dioxide (CO2) at high concentration, especially during whole house gassing, as well as by electrocution using electrical water bath.
These conclusions are valid only under the key parameters described in this Scientific Opinion and the additional conditions of operation of the method as described in the dossier, i.e. (i) residual oxygen concentration is achieved and maintained at less than 2% by volume until the animals are dead; (ii) exposure time should be minimum 5 min for poultry and 7 min for pigs; (iii) foam expansion ratio is maintained at 1:> 250; (iv) diameter of the bubbles in 95% of the foam is at least 10 mm and foam homogeneity is maintained; (v) foam production rate is adequate to fill the container in less than 45 s; (vi) uniform distribution of the foam without air pockets; (vii) stocking density/space allowance is adequate to establish uniform and simultaneous exposure of animals to anoxia; (viii) temperature of the water supply is lukewarm and nitrogen is fully vapourised prior to reaching the foam generator; (ix) foaming agent is not harmful to animals; (x) foam is transparent and observation of animals is not obscured. Deviation from the conditions might have consequences for animal welfare which were not assessed in this exercise and will need a dedicated assessment.
The emergency procedures associated with system failures and backup killing method appropriate to the species and category of animals should be included by the manufacturer in the manufacturer's instructions for the effective use of the equipment and the end user of the method should follow the manufacturer's instructions and include them in the standard operating procedures.
Finally, based on the current assessment, the method can be suitable for situations other than slaughter and depopulation using containers for pig and poultry farms and respecting the technical conditions and the categories and types of animals defined in this Scientific Opinion.
1. INTRODUCTION
The Commission received a request from a business operator to allow the use of a high‐expansion foam for stunning and killing animals. With this application, the Commission has received a series of publications and technical information in order to obtain a full assessment of the method. This information is attached to the present request.
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Regulation (EC) No 1099/2009 on the protection of animals at the time of killing (later referred as ‘the killing Regulation’) defines stunning in Article 2(f) as any intentionally induced process which causes loss of consciousness and sensibility without pain including any process resulting in instantaneous death.
Article 3(1) requires that animals shall be spared any avoidable pain, distress or suffering during their killing and related operations.
Article 4(1) requires that animals shall only be killed after stunning in accordance with the methods and specific requirements related to the application of those methods set out in Annex I of the Regulation and that the loss of consciousness and sensibility shall be maintained until the death of the animal.
Annex I to the killing Regulation lists the stunning methods and related specifications.
Article 4(2) allows the Commission to amend Annex I as to take into account scientific and technical progress on the basis of an opinion of the EFSA. Any such amendments shall ensure a level of animal welfare at least equivalent to that ensured by the existing methods.
At present, the use of high‐expansion foam is not listed in Annex I and therefore not allowed for stunning animals.
1.1.2. Terms of Reference
In accordance with Article 29 (1) (a) of Regulation (EC) No 178/2004, 1 EFSA is requested to evaluate the use of high‐expansion foam for stunning and killing animals as proposed in the application (later called ‘the proposed method’).
In particular, EFSA shall assess:
Whether the proposed method and information provided with the application meets the eligibility criteria of EFSA's guidelines. 2
- Whether the proposed method can provide a level of animal welfare at least equivalent to that ensured by the existing methods in the legislation and specially:
- whether the proposed method ensures that pigs and poultry are spared of avoidable pain, distress or suffering during the killing as referred to in paragraph 1 of Article 3 of the killing Regulation and,
- whether the proposed method maintains the loss of consciousness and sensibility until death of pigs and poultry as referred to in paragraph 1 of Article 4 of the killing Regulation.
1.2. Interpretation of the Terms of Reference
Considering the background and the Terms of Reference (ToR) as provided by the European Commission, this Scientific Opinion (SO) of EFSA:
Based its assessment on the most recent updated (2nd update) dossier submitted to EFSA, which included all the files and documents provided by the applicant. The main document of the dossier and the additional necessary files for the method are available under the Supporting Information section on the online version of the scientific output in Annex C. The applicant specifically requested an evaluation of the NEFS method: Nitrogen Expansion Foam Stunning in container, for stunning and killing pigs and poultry and for situations other than slaughter. Additionally,
According to the dossier (page 14), the target species and animal categories for the application of NEFS include day‐old chicks, laying hens of all ages, broiler chickens, turkeys, doves (pigeons), ducks and geese and pigs from birth (neonates) until 80 kg. However, according to the scientific information provided in the dossier, there is no evidence that NEFS has been scientifically evaluated, using EEGs or other appropriate ABMs, for ducks, pigeons and turkeys and for pigs outside the weight range of 15–41 kg. Peer‐reviewed scientific publications are required as evidence.
Therefore, the conclusions of the assessment of this modified killing method regarding poultry will be for laying hens and broiler chickens of all age and concerning pigs for weight of 15–41 kg (for more information see Sections 3.1.4 and 3.1.5).
It is inferred that the NEFS method proposed by the applicant is the same as ‘nitrogen filled high expansion foam’ or ‘dry foam created using nitrogen’ reported in the scientific literature (e.g. Raj et al., 2008) and/or EFSA opinions on killing of pigs and poultry on farm for purposes other than slaughter (EFSA AHAW Panel, 2019, 2020a, 2020b).
-
2
Assesses whether the proposed method and information provided with the application meets the eligibility criteria of EFSA's guidelines (ToR1), which is the ‘Guidance on the assessment criteria for applications for new or modified stunning methods regarding animal protection at the time of killing’ (EFSA AHAW Panel, 2018) and this guidance is also the protocol that is followed for the SO.
-
3
Assesses whether the proposed method can provide a level of animal welfare (AW) at least equivalent to that ensured by the methods that exist in Annex I of Council Regulation (EC) 1099/2009 for pigs and poultry for situations other than slaughter (ToR2), i.e.:
the methods that exist in Annex I of Council Regulation (EC) 1099/2009 for pigs and poultry for situations other than slaughter,
whether the proposed method ensures that pigs and poultry are spared of avoidable pain, distress or suffering during the killing as referred to in paragraph 1 of Article 3 of the killing Regulation (above mentioned Regulation) (ToR2i).
whether the proposed method maintains the loss of consciousness and sensibility until death of pigs and poultry as referred to in paragraph 1 of Article 4 of the killing Regulation, that is from the onset on unconsciousness until death (ToR2ii).
-
4
Uses ‘pain, distress and/or suffering’ together, because this is how it is mentioned in EFSA Guidance 2018 and also in the killing Regulation.
-
5
Bases its evaluation on two published scientific papers provided by the applicant as well as other published scientific papers after extensive literature search on high expansion foam stunning method. For more information, see Section 2.
-
6
Will conclude if the use of the modified proposed method is valid under commercial conditions, stating to what extent the method proposed for stunning and killing pigs and poultry is able to provide a level of AW in compliance with the above‐mentioned legislation and, if yes, under which conditions.
1.3. Additional information
During the assessment process, as defined in the EFSA guidance, it became apparent that some of the information submitted by the applicant lacked sufficient detail to clearly outline the potential welfare concerns.
For this reason, and with the specific aim of gathering all necessary information and data to assess the exact sequence of the events during the high expansion foam process, EFSA asked the applicant for additional data and information twice, and specifically on 07 February 2023 and on 20 July 2023. See Section 2.1 for more information.
2. DATA AND METHODOLOGY
2.1. Data
The applicant provided EFSA with two scientific publications (McKeegan, Reimert, et al., 2013 and Lindahl et al., 2020; for poultry and for pigs, respectively) considered in this assessment. Following the request of additional information, the applicant added, as part of the dossier, ongoing confidential research and unpublished field trial data. However, all non‐published research included only basic summary results and no individual data or detailed methodology such as time of exposure to less than 2% O2 in containers. Therefore, a full assessment of these data was not possible, and these data have been excluded from the assessment, with the assessment regarding the applicant's provision solely relying on the two published scientific papers.
As stated in Section 1.3, EFSA asked the applicant for additional material. More specifically, EFSA requested additional information and/or clarifications:
On 07.02.2023 regarding the 1. Description of the stunning method, 2. Description of the individual studies submitted, 3. Overall integration of findings from all studies. For more information, see in the following link of OpenEFSA https://open.efsa.europa.eu/questions/EFSA‐Q‐2022‐00344?search=foam, in the Supporting documents on page 5, the file ‘RFI’. The reply along with the updated dossier of the applicant was received on 08.05.2023.
On 20.07.2023 concerning the 1. Description of the stunning method: Description of the method including potential sources of pain, distress and suffering, Key parameters of the effective use of the method, Scientific basis of induction and maintenance of unconsciousness for this method, Potential causes of system failure and chances of occurrence. For more information, see in the following link of OpenEFSA https://open.efsa.europa.eu/questions/EFSA‐Q‐2022‐00344?search=foam, in the Supporting documents on page 5, the file ‘Additional Data Request’. The reply along with the latest updated dossier of the applicant was received on 03.10.2023.
2.2. Methodology
This SO follows the protocol detailed in the methodological guidance that was developed by the EFSA AHAW Panel to deal with all the mandates on the assessment criteria for applications for new or modified stunning methods regarding animal protection at the time of killing (EFSA AHAW Panel, 2018).
2.2.1. Literature search
An extensive literature search was conducted on the use of high‐expansion foam in poultry and pigs utilising the Web of Science (WoS) and Scopus databases. The search included the period from January 1975 until June 2023 and English language.
The query to the WoS database used the following search statement:
“TS = (foam AND killing) and TS = (poultry OR pigs) NOT TS = (occlusion or suffocation or drowning or asphyxia or firefighting) NOT TS = “water‐based”.
From a total of 10 retrieved scientific publications only four met the following criteria: use of high‐expansion foam, application for stunning or killing and used in poultry or pigs. Publications describing the use of water‐based foam, low and medium‐expansion foam or firefighting foam were not considered, because these were not representative of the method used and the cause of death was different (i.e. suffocation rather than anoxia), which may have different implications in terms of animal welfare, as in water‐based foam, the means of death is reported to be occlusion of the trachea (Korenyi‐Both et al., 2022). Any method designed to cause occlusion of the trachea would be equivalent to death by drowning or suffocation, which is not recognised as humane killing method in Council Regulation (EC) No 1099/2009 on the protection of animals at the time of killing.
Additionally, the query to the Scopus database used the following search statement:
“TITLE‐ABS‐KEY ((foam AND killing) AND (poultry OR pigs)) AND (LIMIT‐TO (DOCTYPE, “ar”))”.
From this query, four scientific publications were retrieved following exclusion of water‐based, low and medium expansion foam methods. These publications were the same as those retrieved from the first query in WoS and three out of four were for poultry. The final four publications are listed in Table 2.
TABLE 2.
List of publication retrieved after literature search.
| Paper | Authors | Title | Journal | Year | Doi |
|---|---|---|---|---|---|
| P1 | Raj, A. B. M., Smith, C., Hickman, G. | Novel method for killing poultry in houses with dry foam created using nitrogen | Veterinary record | 2008 | https://doi.org/10.1136/vr.162.22.722 |
| P2 | Gerritzen, M. A., Sparrey, J. | A pilot study to assess whether high expansion CO2‐enriched foam is acceptable for on‐farm emergency killing of poultry | Animal welfare | 2008 | https://doi.org/10.1017/S0962728600032206 |
| P3 | McKeegan, D. E. F., Reimert, H. G. M., Hindle, V. A., Boulcott, P., Sparrey, J. M., Wathes, C. M., Demmers, T. G. M., Gerritzen, M. A. | Physiological and behavioural responses of poultry exposed to gas‐filled high expansion foam | Poultry science | 2013 | https://doi.org/10.3382/ps.2012‐02587 |
| P4 | Lindahl, C., Sindhoj, E., Hellgren, R. B., Berg, C. Wallenbeck, A. | Responses of Pigs to Stunning with Nitrogen Filled High‐Expansion Foam | Animals | 2020 | https://doi.org/10.3390/ani10122210 |
In addition, articles, review articles and grey literature identified in the literature search or cited in identified publications were checked, irrespective of the year of publication and included if considered relevant by the working group.
Literature used for the comparison of different killing methods (Section 4) in pigs and poultry was retrieved by an extensive literature search following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) methodology. This framework identifies evidence in a non‐biased and independent review of the literature and follows a three‐phase approach: identify scientific publications pertinent to the defined aim through eligibility criteria (Phase 1), assessment and quality (Phase 2) and finally ranking the publications in relation to the quality and relevance (Phase 3). The final search terms were identified following extensive piloting and a total of 12 permutations. In the final iteration, relevant literature was identified using the same search terms in all four key search engines (1) PubMed; (2) Science Direct; (3) Scopus; and (4) Web of Knowledge. The search terms used (including all titles, abstracts and keywords) included: TITLE: (killing* OR slaughter* OR depopulation* OR euthanasia*) AND TOPIC: (animal welfare* OR physiology* OR behaviour* OR indicator* OR stress) AND TITLE: (poultry* OR chick* OR turkey* OR broiler* OR layer* OR duck* OR bird* OR pullet* OR hen* OR pig* OR hog* OR swine* OR sow* OR piglet*) NOT TOPIC: (water‐based) NOT TOPIC: (firefighting).
Following collation of publications identified from the finalised search terms, publications were scrutinised based on a series of eligibility inclusion criteria: open access availability (including ‘green access’); full publication available in English language (unless translations were readily accessible); peer‐reviewed; species specified; topic specified; and full papers only (e.g. Conference proceedings excluded). Reviews of the literature and guidance documents were identified and excluded from the systematic review assessment, but not removed from the reference database, as they provided informative benchmarks to the current review narrative. Phase 1 eligibility screening was conducted with assessors blinded to authors and their affiliations to minimise bias. Any duplications of publications were also omitted. Two additional exclusion criteria were adopted, which excluded publications with no outcomes relevant to animal welfare or methods of killing which are not approved for use in Council Regulation (EC) 1099/2009. Finally, critical reported data were extracted from each publication to aid final quality assessment (Phase 3), where papers were scored according to the quality specifications outlined in Table 3. Papers identified with a quality score of 1 were excluded from evaluation and review of the final literature selection. The period and language of the search were the same as above.
TABLE 3.
Phase 3 quality scoring criteria for literature used for the comparison of different killing methods (Section 4) in pigs and poultry.
| Score 1 (Lowest quality ranking) | According to data extracted, the publication was identified as either one or a combination of the following:
|
| Score 2 (Medium quality ranking) |
According to data extracted, the publication was identified as either one or a combination of the following: Sufficient replication and sample size Welfare outcomes identified Mode of killing detail sufficient |
| Score 3 (Highest quality ranking) | According to data extracted, the publication was identified as either one or a combination of the following:
|
In total, 199 eligible publications were identified from the four key search engines. After the screening process, a final 42 publications were identified and quality assessed for inclusion for the comparison of NEFS to the various killing methods approved for use in pigs and poultry (see Section 4).
2.2.2. EFSA guidance on the assessment criteria for applications for new or modified stunning methods regarding animal protection at the time of killing (EFSA AHAW Panel, 2018)
The first part of the assessment process involved checking the submitted documentation against the criteria laid down in the EFSA guidance.
In case the criteria are not fulfilled, the assessment report has to highlight the shortcomings and indicate where improvements are required before the study/method can be further assessed. It should be noted that the EFSA Guidance is applicable to the individual studies. As mentioned in Section 2.1, the Working Group (WG) experts identified some lack of information in the submitted papers. As a consequence, considering these limitations with regard to at least one of the criteria laid down in the guidance (see following sections), none of them would have passed the first assessment phase regarding ToR1. However, the WG experts, the AHAW Panel and European Commission representatives agreed that, in order to address ToR2 as well as to promote development of potential improvements of existing methods or encourage innovation leading to the introduction of improved stunning methods, the WG would assess the entire information combined, originating from the combination of the information provided by the applicant, the submitted papers, other existing literature and expert knowledge elicitation/evaluation rather than applying the criteria to the individual and/or unpublished studies, which is still in agreement with EFSA Guidance. Additionally, the AHAW Panel and the WG experts decided to ask the applicant for additional data, in order to perform ad hoc analyses with the aim of improving clarity of understanding and to facilitate the assessment (see Section 2.1).
2.2.3. Assessment of the level of animal welfare provided by the high expansion foam (equivalence with other killing methods)
2.2.3.1. Quantitative assessment
To compare NEFS with other killing methods used for purposes other than slaughter, a scientific literature review (see Section 2.2.1) was conducted to gather data on neurological and behavioural indicators of onset of unconsciousness and death (see Section 3.3.1.1). When discrepancies were detected between studies in the methodology or criteria used to ascertain these states, the worst‐case scenario was always considered. This means that for Loss of Posture (LOP), Loss of Consciousness (LOC) and Motionless, the highest values were selected (considering the case in which this occurred the latest). In addition, the time to onset of LOP was considered as a behavioural indicator of the onset of unconsciousness. The same rule was applied for motionless and an isoelectric pattern in the EEG to ascertain onset of death. If isoelectric EEG occurred later than the time to become motionless, the time for motionless was not considered. Finally, for behaviours that indicated aversion, distress or discomfort, the lowest values were selected (considering then the case in which this occurred the earliest). For full explanation regarding the use of indicators and comparisons among the methods, see Section 4.2.1.
2.2.3.2. Qualitative assessment and hazard ranking
The second step (ToR2) involved assessing the equivalence of NEFS with other stunning methods outlined in the legislation. To address this, a comparison between the proposed modified method and existing ones in terms of animal welfare was necessary. Various methodologies could be employed, including a comparative analysis of welfare outcome measures (ABMs) reflecting animals' responses to the method or an assessment of welfare hazards associated with each method.
However, conducting a quantitative comparison based on welfare outcome measures was deemed unfeasible due to reasons outlined in Sections 2.2.2 and 4.2.1. Hence, the equivalence assessment was based on a non‐formal expert elicitation utilising a semi‐qualitative approach and expert opinions.
The exercise was structured in five steps repeated to be applicable to chickens and pigs:
Identification of comparable killing methods;
Identification of a list of hazards within each killing method, along with their associated welfare consequences;
Evaluation of the magnitude of these hazards by experts, specifically tailored for each killing method in relation to chickens and pigs;
Expert discussions on the outcome of the exercise and to refine individual assessments based on collective insights;
Identification of highly relevant hazards for each method and presentation of the results.
1. Identification of comparable killing methods
For the identification of comparable killing methods, descriptions provided in the more recent EFSA scientific opinions on the welfare aspects of the main systems for stunning and killing of pigs and poultry (EFSA AHAW Panel, 2019, 2020a, 2020b) were taken into consideration.
Regarding chickens, head‐to‐body electrical stunning, non‐penetrative captive bolt, maceration and firearm with free projectile were excluded as comparable killing methods. For both chickens and pigs, the killing method using carbon monoxide (CO) either of pure source or associated with other gases was also excluded. These methods were excluded because they are not used in practice, especially for on‐farm killing.
Regarding pigs, whole house gassing with carbon dioxide (CO2) was retained, because it could be utilised under certain conditions, albeit that its use is uncommon.
The remaining methods were included in comparison to NEFS. The final list of comparable killing methods in chickens and pigs is reported in Annexes A and B (Tables A.1, B.1).
2. Identification of hazards and associated welfare consequences
EFSA experts identified, for each of the killing methods, the list of hazards to be considered for the exercise based on the associated welfare consequences for the animal. Hazards were included considering all phases: pre‐stunning, stunning and the actual process of killing, which encompasses the induction of unconsciousness and the onset of death.
Hazards and associated welfare consequences related to NEFS in container, ABMs and associated welfare consequences were identified in this opinion as described in Section 4.2 (Tables 22, 23 and 24) and summarised in Annexes A and B (Table A.3 and B.3, respectively).
TABLE 22.
Hazards associated with NEFS common to both pigs and chickens.
| Hazard | Description |
|---|---|
| Rough handling | Catching animals for the purpose of crating (inserting into transport crates), restraining or moving them into the foaming container in an inappropriate way |
| Loud noise | Noise caused by the foam generator and injection of gas |
| Slipping | Losing balance and/or falling due to foaming solution on the slippery floor of container |
| Substance irritating the mucosa | Exposure to foam can irritate mucosa in the nostrils and eyes. |
| Surrounded by foam | Animals being encircled or enveloped by foam prior to bursting with the jet of nitrogen gas. |
| Inhalation of the foam | Animals inhale foaming liquid, i.e. foaming liquid entering upper respiratory tract prior to disruption of the foam using jet of nitrogen gas |
| Jet stream of gas at animal level | Administration of pressurised nitrogen gas pulse to break up the foam and release nitrogen contained in the foam |
| Hurting | As a result of wing flapping in chickens or slipping and falling in pigs due to foaming liquid on the floor of the container or escape attempts can lead to injury in pigs and poultry |
| Too short exposure | Exposure to anoxia is not long enough to kill leading to animals remaining alive or even regaining consciousness |
| Overloading | Stocking density is too high leading to inadequate exposure of some animals to anoxia |
| Too small bubble size | Bubble size is less than 10 mm in diameter leading to high water content in the foam with increased chances of animals being drowned |
| Low foam production rate | Foam production rate is not adequate to fill the container rapidly, leading to prolonged exposure to the foam rather than nitrogen gas |
| Too low gas concentration | Residual oxygen concentration in the container is above 2% by volume leading to persistence of or recovery of consciousness in animals |
| Too low temperature | Temperature of the water or nitrogen gas used to generate the foam is too low leading to hypothermia |
TABLE 23.
ABMs for assessment of welfare consequences during exposure of chickens to NEFS.
| ABMs | Description | Welfare consequences (WCs) | Hazards associated with WCs |
|---|---|---|---|
| Escape attempts | Birds try to escape by jumping (McKeegan, Reimert, et al., 2013) | Pain and fear | Handling, loud noise, substance irritating the mucosa, surrounded by foam |
| Vocalisation | Birds vocalise | Pain and fear | Handling, loud noise, substance irritating the mucosa, surround by foam |
| Wing flapping | Birds flap their wings (McKeegan, Reimert, et al., 2013) | Pain and fear | Handling, loud noise, substance irritating the mucosa, surround by foam |
| Head shaking | Birds shake their heads during contact with foam (McKeegan, Reimert, et al., 2013) | Pain, fear and respiratory distress | Substance irritating the mucosa, disruption to rhythmic breathing pattern, inhalation of foam |
| Frequent blinking | Birds make frequent movement of nictitating membrane or eyelids | Pain | Substance irritating the mucosa (because of foam entering the eyes) |
| Increased lacrimation | Birds produce abundant tears | Pain | Substance irritating the mucosa |
| Gasping | Open mouth breathing with extended neck | Pain, fear and respiratory distress | Inhalation of foam |
TABLE 24.
ABMs for assessment of welfare consequences during exposure of pigs to NEFS.
| ABMs | Description | Welfare consequences (WCs) | Hazards associated with WCs |
|---|---|---|---|
| Escape attempts | Pigs try to escape by climbing on the wall (Lindahl et al., 2020) | Pain and fear | Handling, loud noise, substance irritating the mucosa, surrounded by foam |
| Vocalisation | Pigs squeal with high pitch vocalisations (Lindahl et al., 2020) | Pain and fear | Handling, loud noise, substance irritating the mucosa, surround by foam |
| Head shaking/tilting | Pigs shaking or tilting their heads during contact with the foam | Pain, fear and respiratory distress | Substance irritating the mucosa, disruption to rhythmic breathing pattern, inhalation of foam |
| Frequent blinking | Pigs make frequent movement of eyelids | Pain | Substance irritating the mucosa (because of foam entering the eyes) |
| Increased lacrimation | Pigs produce abundant tears | Pain | Substance irritating the mucosa |
| Gasping | Open mouth breathing with extended neck (Lindahl et al., 2020) | Pain, fear and respiratory distress | Inhalation of foam |
| Sitting | Sitting, upright position with the hind legs resting on the ground and front legs straight with hooves on the floor (Lindahl et al., 2020) | Pain, fear and respiratory distress | Inhalation of foam |
The hazards and associated welfare consequences related to comparable killing methods have been identified and modified in previous EFSA opinions (EFSA AHAW Panel, 2019, 2020a, 2020b) and are summarised in Annexes A and B (Tables A.3, B.3, respectively).
3. Evaluation of the magnitude of the hazards by experts
For each of the hazards in relation to each killing method, four EFSA experts were asked to estimate the following:
Prevalence (%) of birds/pigs exposed to the hazard in each of the methods during routine operation. The experts were requested to provide an estimate of the prevalence of animals exposed to each hazard within each method by providing the minimum, the maximum and the mean estimated value.
Severity and duration of the exposure to stress and pain (welfare consequences) caused by each hazard, using the scoring system reported in Table 4. This scoring system is based on the Likert scale (Wikman et al., 2016)
TABLE 4.
Scoring method (Likert scale) used to estimate severity and duration of the exposure to stress and pain of animals (chicken and poultry) within each hazard in each of the identified killing methods.
| Score | Impact on animal welfare | Interpretation from the experts |
|---|---|---|
| 1 | No negative impact | No stress |
| 2 | Mild stress | |
| 3 | Moderate stress | |
| 4 | Mild pain and short duration (< 2 s) | |
| 5 | Moderate pain and short duration (< 2 s) or mild pain and long duration > 2 s | |
| 6 | Severe pain and short duration (< 2 s) or moderate pain and long duration > 2 s | |
| 7 | Very negative impact | Severe pain and long duration > 2 s |
Each expert provided individual judgements. Within each individual evaluation, for each killing method, a final score on each hazard was calculated by multiplying the mean prevalence by the score (indicating severity and duration) given.
4. Experts discussion on the outcome of the exercise
The individual judgements of hazards associated with each killing method were subjected to group discussion among the experts. Each expert was asked to provide supporting arguments for their evaluations. Subsequently, all experts were asked whether, in light of the reasoning that were brought up during the group discussion, they were inclined to reconsider their evaluation.
5. Identification of highly relevant hazards for each method and presentation of the results
The ranking of hazards was conducted individually by each expert. Initially, only the hazards that scored more than 4 on severity and duration, corresponding to the welfare consequence of pain, were selected. Next, considering the final score (obtained by multiplying prevalence by the score on severity and duration), the hazards ranked in the top five positions (to facilitate the comparison among the methods) with the highest values designated as ‘highly relevant’. The final list of highly relevant hazards comprised those hazards that ranked in the top five positions for at least one expert (Tables 29, 31 for chickens and Tables 30, 32 for pigs). The final objective of the exercise was to identify the top five relevant hazards and not to rank them. The results of the exercise including the final list of the highly relevant hazards are reported in Section 4.2.2.1. The obtained results were discussed through two main comparisons: firstly, between NEFS and other CAS methods, and secondly, between NEFS and the remain methods (Sections 4.2.2.1 and 4.2.2.2).
TABLE 29.
Selected highly relevant hazards related to NEFS and other CAS methods in chickens.
| Hazards | NEFS in container | Whole house gassing with CO2 | CO2 in two phases in containers | CO2 + inert gases in containers | Inert gases in containers | LAPS |
|---|---|---|---|---|---|---|
| Expansion of gases in the body cavity | x | |||||
| Handling | x | x | x | x | x | |
| Hurting | x | x | x | x | x | x |
| Inhalation of high CO 2 concentration | x | x | x | |||
| Inhalation of the foam | x | |||||
| Jet stream of gas at bird level | x | x | x | x | ||
| Loud noise | x | x | x | x | x | x |
| Low foam production rate | x | |||||
| Substance irritating the mucosa | x | x | x | x | ||
| Overloading | x | x | x | x | x | x |
| Surrounded by foam | x | |||||
| Too fast decompression | x | |||||
| Too low gas concentration | x | x | x | x | ||
| Too low temperature | x | x | x | x | x | x |
| Too short exposure time | x | x | x | x | x | x |
| Too small bubble size | x |
Notes: The selection of hazards has been made following non‐formal expert knowledge elicitation, which implies ranking of hazards. For each killing method, only hazards that were ranked in the first five positions by at least one of the experts involved are reported in this table.
TABLE 31.
Selected highly relevant hazards related to NEFS and remaining killing methods (non‐CAS) in chickens.
| Hazards | NEFS in container | Electrical waterbath | Head‐only electrical stunning | Captive bolt | Cervical dislocation | Percussive blow to the head | Lethal injection |
|---|---|---|---|---|---|---|---|
| Handling | x | x | X | x | x | x | x |
| Hurting | x | x | X | x | x | x | x |
| Inappropriate route of administration | x | ||||||
| Inappropriate electrical parameters | x | X | |||||
| Incorrect application of the manual method | x | x | |||||
| Incorrect captive bolt parameters | x | ||||||
| Incorrect shooting position | x | ||||||
| Inhalation of the foam | x | ||||||
| Inversion | X | x | x | x | |||
| Jet stream of gas at bird level | x | ||||||
| Loud noise | x | ||||||
| Low foam production rate | x | ||||||
| Manual restraint | X | x | x | x | x | ||
| Substance irritating the mucosa | x | ||||||
| Overloading | x | ||||||
| Poor electrical contact | x | X | |||||
| Pre‐stun shocks | x | ||||||
| Shackling | x | ||||||
| Sublethal dose | x | ||||||
| Surrounded by foam | x | ||||||
| Too low gas concentration | x | ||||||
| Too low temperature | x | ||||||
| Too short exposure time | x | x | X | ||||
| Too small bubble size | x |
Notes: The selection of hazards has been made following non‐formal expert knowledge elicitation, which implies ranking of hazards. For each killing method, only hazards that were ranked in the first five positions by at least one of the experts involved are reported in this table.
TABLE 30.
Selected highly relevant hazards related to NEFS and other CAS methods in pigs.
| Hazards | NEFS in containers | Whole house gassing CO2 | CO2 at high concentration in containers | CO2 + inert gases in containers | Inert gases in containers |
|---|---|---|---|---|---|
| Exposure to high CO 2 concentrations | x | x | x | ||
| Handling | x | x | x | x | |
| Hurting | x | x | x | x | x |
| Inhalation of foam | x | ||||
| Jet stream of gas at pigs' level | x | x | x | x | x |
| Loud noise | x | x | x | x | x |
| Low foam production rate | x | ||||
| Substance irritating the mucosa | x | x | x | x | |
| Overloading | x | x | x | x | x |
| Slipping | x | x | x | x | x |
| Surrounded by foam | x | ||||
| Too low gas concentration | x | x | x | x | x |
| Too low temperature of the gas | x | x | x | x | x |
| Too short exposure time | x | x | x | x | x |
| Too small bubble size | x |
Notes: The selection of hazards has been made following non‐formal expert knowledge elicitation, which implies ranking of hazards. For each killing method, only hazards that were ranked in the first five positions by at least one of the experts involved are reported in this table.
TABLE 32.
Selected highly relevant hazards related to NEFS and remaining killing methods (non‐CAS) in pigs.
| Hazards | NEFS in containers | Electrical killing | Captive bolt | Non‐penetrative captive bolt | Percussive blow to the head | Firearm | Lethal injection |
|---|---|---|---|---|---|---|---|
| Handling | x | x | x | x | x | X | x |
| Hurting | x | x | x | x | x | X | x |
| Inappropriate electrical parameters | x | ||||||
| Inappropriate power and calibre of the cartridge | x | ||||||
| Inappropriate route of administration | x | ||||||
| Inappropriate type of projectile | x | ||||||
| Incorrect application of the shot to the head | x | ||||||
| Incorrect captive bolt parameters | x | x | |||||
| Incorrect shooting position | x | x | x | ||||
| Inhalation of foam | x | ||||||
| Inversion | x | ||||||
| Jet stream of gas at pigs' level | x | ||||||
| Loud noise | x | ||||||
| Low foam production rate | x | ||||||
| Substance irritating the mucosa | x | ||||||
| Overloading | x | ||||||
| Poor electrical contact | x | ||||||
| Restraint | x | x | x | x | x | ||
| Slipping | x | ||||||
| Sublethal dose | x | ||||||
| Surrounded by foam | x | ||||||
| Too low gas concentration | x | ||||||
| Too low temperature of the gas | x | ||||||
| Too short exposure time | x | x | |||||
| Too small bubble size | x | ||||||
| Wrong placement of the electrodes | x |
Notes: The selection of hazards has been made following non‐formal expert knowledge elicitation, which implies ranking of hazards. For each killing method, only hazards that were ranked in the first five positions by at least one of the experts involved are reported in this table.
2.2.3.3. Limitations of the exercise and uncertainty analysis
Some limitations in the exercise were identified. Because the number of the hazards to be scored for each method was not the same, it could inadvertently lead to a potential bias. However, the number of hazards was not considered as a factor for the comparison. Comparison between group killing methods (CAS) and individual killing methods highlighted the difficulty in comparing the prevalence of animals affected by a certain hazard, tending to penalise hazards related to group methods. In addition, more uncertainty could be expected around certain methods for which limited information exists versus others with a lot of scientific evidence available. These limitations were discussed and accounted for as much as possible in the third phase of the exercise, the group discussion.
Regarding the uncertainty analysis, the method used was the EFSA Guidance on Uncertainty Analysis in Scientific Assessments (EFSA Scientific Committee, 2018a, 2018b). For conclusions reached conducting a non‐formal expert elicitation, WG experts provide their individual judgement on the certainty for a rephrased question referring to a well‐defined quantity of interest, according to the suggested ranges in Table 5 below.
TABLE 5.
Certainty range for performing the uncertainty judgement.
| Quantitative assessment | Certainty range | ||
|---|---|---|---|
| > 50%–100% | 66%–100% | 90%–100% | |
| Qualitative translation | More likely than not | From likely to almost certain | From very likely to almost certain |
For assessing the equivalence with an already approved method, the experts were presented with the hazards and their associated WCs identified for NEFS and each alternative method. Since the objective was to assess if NEFS was equivalent to or better than each method in terms of the overall welfare of animals subjected to it, experts were asked how certain they were that the proportion of animals experiencing the identified WCs associated with the hazards for NEFS was similar or lower to the ones experienced with each alternative method. They were asked to also take into consideration in their judgement the magnitude of the hazard (and associated welfare consequences) in an informal way (so that a low proportion of animals experiencing a highly severe WC could be indicative of poorer welfare than a higher proportion of animals experiencing a milder WC). A group discussion was held, and a consensus certainty range was agreed, which is reported in the body text of the Opinion in the assessment section and in the key conclusions.
From the results of the dossier, the experts were asked about their certainty on the three following points:
How certain are you that chicks below 7 days of age subjected to NEFS will experience the same WCs with a similar severity and affecting a similar proportion of animals as adult/slaughter weight chickens?
How certain are you that the foam is not irritative to any animal subjected to NEFS?
How certain are you that the foam is not entering the upper airways in any animal?
3. ASSESSMENT PHASE 1: CHECK OF DATA FOR RISK ASSESSMENT
3.1. Eligibility criteria of EFSA's guidelines (ToR 1)
3.1.1. Name of the method
NEFS in container: Nitrogen Expansion Foam Stunning in container.
The dossier (Annex C), submitted for approval, describes a method that involves using containers and a jet pulse of nitrogen gas at the end of filling with foam (method thereafter called NEFS by the applicant).
3.1.2. Description of the method including potential sources of pain, distress and suffering
In this assessment, poultry and pigs are presented under separate sections for the sake of clarity. The following section outlines the method and potential sources of pain, distress and/or suffering. In this proposed method, high‐expansion foam is used as an inert carrier (of nitrogen) to increase effectiveness and efficiency of nitrogen as an anoxic inert gas that is already permitted for stunning and killing of animals in Annex 1 of Council Regulation (EC) No 1099/2009.
Principle of the method
The method can be applied to an individual animal (an injured animal) or in small groups (depopulation) depending upon the users' requirement or the container size. Animals can be walked into the foaming container without any form of restraint, or animals may be placed in a transport crate (poultry) or a cage (pigs) which can be loaded into the foaming container for the purpose of killing.
The method is based on the rapid displacement of oxygen by purging the air from a container with foam filled with N2 and afterwards bursting the foam bubbles with a jet of nitrogen gas, resulting in an anoxic atmosphere with less than 2% residual oxygen in nitrogen and maintaining anoxia until the animals are dead. The high‐expansion foam is used to deliver and distribute the nitrogen throughout the chamber. It is reported in the dossier that the use of the foam may increase the effectiveness of the killing with nitrogen gas by significantly reducing the filling time of the container and gas consumption compared to flushing with nitrogen gas alone. The foam works as a carrier displacing the air without mixing nitrogen with it, as it fills the chamber from the bottom upwards and pushes the displaced air out through the ventilation holes (a flap door) in the ceiling of the container.
The submitted dossier solely reports the application of NEFS in containers for poultry and pigs, which therefore precludes its consideration and assessment for application in other contexts e.g. whole house gassing. Foam can be classified by the expansion ratio into three categories: low (1:20), medium (1:> 20–< 200) or high (1:> 200–2000). The expansion ratio is defined as the volume of foaming solution (volume of foaming agent plus water) to the volume of foam produced (Gerritzen & Sparrey, 2008).
High expansion foam is generated by spraying foam solution into a wire mesh (e.g. stainless steel screen) and blowing the solution through the screen with a nitrogen stream to produce the required volume of foam bubbles in the desired time (i.e. cubic metres/minute). Size of the holes in the mesh, the rate of spraying of the foaming solution and the rate of nitrogen injection would determine the size of bubbles in the foam. The high expansion foam can be administered from the bottom up or through the side(s) of the foaming container to completely displace atmospheric air. A burst of nitrogen gas can be administered to break the bubbles and release the nitrogen gas contained in the bubbles such as described in process flow. The bubbles are burst by a N2 jet stream. Additionally, they can be simply destroyed by the movements of the animals. The purpose is to create anoxia in the container, which is considered to occur when the volume of oxygen is < 2%.
The oxygen concentration in the container will be monitored continuously throughout the killing process. Additional foam will have to be administered in the event of the residual oxygen concentration exceeding 2% by volume in the container to re‐establish and maintain anoxia throughout the exposure time required for killing animals. The foaming container includes holes or a flap door in the roof‐lid to allow the displaced air to escape. It is envisaged that the high expansion foam generator required to produce large volumes of foam may also include an electric fan, especially when gas diffusers are used to minimise the noise, or nitrogen jet, to force the foaming solution through the mesh. It is stated in the dossier that the foam generator has sufficiently large output capacity to fill the container rapidly, e.g. in less than a minute.
The main parts of the system used are:
Container with foam generator, lid and closable air valves (flap door in the lid).
Control cabinet, for operational control.
Distribution cabinet containing pump, gas solenoid valve and preset regulator distributing gas and foaming liquid into the container.
These parts can be seen in Figure 1 below from left to right and a video describing the procedure of using the system can be found in the applicant's dossier.
FIGURE 1.
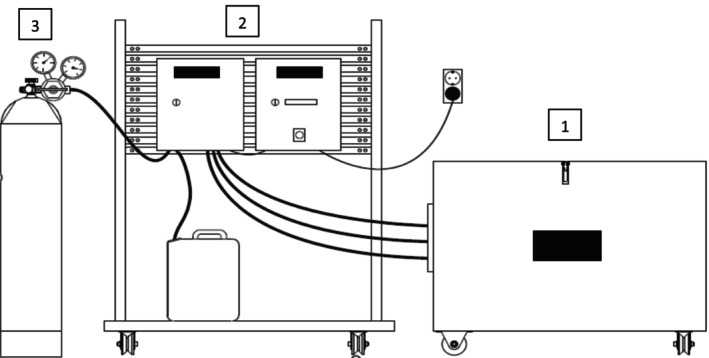
Main parts of the system (as provided by the applicant in their Standard Operating Procedure – Manual).
© HEFT AB.
Foaming agent
The composition of the foam agent as provided by the applicant is detailed in Table 6. The submitted dossier included the data sheet for the foam agent as well as information relating to chemical composition and the safety data sheet. No information was provided to demonstrate the absence of mucosal or skin irritation in animals exposed to foam.
TABLE 6.
Chemical composition of the foam mixture. Source: document ‘safety Sheet HEFT agent’.
| Product/substance | Identifiers | % w/w |
|---|---|---|
| Potassium oleate | CAS No.: 143‐18‐0 | 10%–15% |
| Fatty acids, coco, potassium salts | CAS No.: 61789‐30‐8 | < 5% |
Process flow
The process is described in the dossier as following: The process is initiated by pressing the button on the control cabinet. The pump is started and the foaming solution (mixture of foaming agent and water) is sprayed, covering the perforated plate on the foam generator. When the perforated plate is covered (~ 5 s), the button is pressed again and the nitrogen gas flow is started, initiating the foam production. Nitrogen, encapsulated in high‐expansion foam, fills the container and pushes out the air through the air valves (flap door in the lid). Once the container is filled completely, the air valves (flap door) are manually closed and the button is pressed again, stopping the foam production and triggering a pulse (1–2 s; ■■■■■) of nitrogen gas to burst the bubbles.
Potential sources of pain, distress and/or suffering
Recently, EFSA evaluated the risks of applying inert gas‐based foam in its scientific opinion on the welfare of poultry and pigs during killing for other purposes than slaughter (2019 and 2020, respectively). Whole house foaming with nitrogen gas made from liquid nitrogen was assessed in poultry, and both open and closed systems were discussed in pigs in 2020.
When stunning with gases, the displacement rate of air is critical regarding the welfare of the animal. This is determined by the time frame in which the desired oxygen concentration is reached and maintained. When stunning with gases only by flushing, a prolonged period of flushing is necessary to obtain the desired residual oxygen concentration of below 2% by volume in nitrogen, during which period animals may experience respiratory distress, due to the gradual induction and increasing hypoxia. The terms anoxia and hypoxia below were defined in the dossier, but were revised by EFSA's experts:
Hypoxia: when insufficient levels of oxygen are available, resulting in bodily tissues having inadequate oxygen supply, which disrupts normal function (i.e. disrupted homeostasis). In humans, hypoxic effects can be observed when the atmospheric oxygen is below 18% by volume, with symptoms becoming progressively worse as available oxygen further decreases.
Anoxia: The absence of available oxygen in the atmosphere or total depletion of oxygen within the bodily tissues, with an oxygen range in atmosphere from 0% to 2%.
Handling of animals
Handling of animals during exposure to NEFS is very similar to some other methods of on‐farm killing, for example, containerised gassing system.
The type and quality of animal handling is important in the overall efficacy of the killing process and to avoid unnecessary pain, distress and suffering. Operators must be competent in handling the animals and equipment utilised in the killing process must also be adequate and used properly.
The different sources of pain, distress and suffering related to handling of pigs, such as described in the dossier, are:
-
–
When pigs are moved from their home pens to the place of killing: (i) it should be done only when all preparations are complete, to ensure the animals are killed without undue delay; (ii) they should be moved calmly and allowed to progress freely in familiar groups at their own pace. When necessary, boards may be used to guide them. Small piglets can be carried by hand and placed into the container. If pigs are of a larger size and mobile, they can walk and enter the chamber through the door with as little human intervention as possible.
-
–
When pigs are individually restrained: They will experience the welfare consequence of stress isolation. An advantage of a gas killing method is that animals can be killed/stunned in groups and without individual restraint.
-
–
If unfamiliar pigs are mixed: Animals that are unfamiliar (e.g. from different pens) should not be mixed to avoid the potential of stress and pain associated with mixing (e.g. aggression).
-
–
When pigs are left waiting in the container: It is stated in the dossier that once in the container, if the animal is not subject to emergency killing as defined in Council Regulation (CE) 1099/2009, the animal should be left for a short period (1–2 min) to quietly settle (e.g. appearing calm) before the foaming starts. However, EFSA disputes that statement for animal welfare reasons, based on the fact that confining in a container is a novel experience for the animals and therefore could lead to animals experiencing fear and distress.
Poultry may be caught and loaded into transport crates or modules and taken to the point of killing. Manual handling and loading of birds into the foaming container directly can also be performed.
The different sources of pain, distress and suffering related to handling of birds such as described in the dossier are:
-
–
When the birds are handled wrongly: inverted and/or carried by one leg or wings or by the neck. Birds should be caught and loaded with care into the crates by trained staff such as described in EFSA AHAW Panel (2019) to prevent handling stress and soft tissue and bone damages.
-
–
When birds are crated automatically with catching machines, are operated incorrectly (EFSA AHAW Panel, 2022).
-
–
Heat stress when birds are left in the crates in summer months.
-
–
A hazard directly linked to the NEFS method is linked to the use of crates within the foaming container: If the crate bars are too narrow, it might be that the foam has difficulty to penetrate the crates and surround animals. The dossier stipulates that the crate bars should have a minimum width of 25 mm, for the foam to freely enter into the crate and reach the birds without obstruction. Ideally, the crates should have perforated floor and roof so that the foam can flow through the birds and displace air trapped between them.
-
–
When birds are left waiting in the container: There should be no delay between loading of crates into the container and foaming.
Contact with foam
The characteristics of the foam are determined by a combination of foam agent properties and foam generator properties. According to the dossier, the foaming agent contains no toxic materials, carries no safety warning labels even in concentrated form and can be classified as soap.
Inevitably, the animals will come in contact with the foam and remain so until the foam is burst, and nitrogen gas is fully released, so the novelty of being surrounded by the foam must be considered a potential hazard for the animals (when compared to gas only). Turkeys, broilers, hens and ducks have been reported to react to high‐expansion foam (either air‐filled, nitrogen‐filled or carbon dioxide‐filled) enveloping them (e.g. head shaking, escape attempts and transient increases in heart rate in McKeegan, Reimert, et al., 2013). Lindahl et al. (2020) reported that pigs seemed to avoid putting their heads and snouts into the foam, and the rate of escape attempts through the lid increased when foam levels became higher.
Aversion to the foam can be assessed by behavioural observations when animals are presented and/or exposed to direct contact with the foam as it was done by McKeegan, Reimert, et al. (2013) and Lindahl et al. (2020). The risk of aversion and the potential negative impacts on animal welfare are limited to the period that the animal is conscious and therefore may occur during foam delivery, filling of the container and until the onset of unconsciousness after the foam bubbles are burst during the process. Evidently, the duration of exposure to the foam before the release of the nitrogen jet should be kept as short as possible to minimise the duration of animals being exposed to a potentially aversive environment.
Additionally, other potential sources of pain, distress and suffering should be considered, such as mucosal irritation (of eyes and nasal mucosae) by the foaming agent and the foaming solution entering the upper respiratory tract. Literature reveals that stimulation (chemical, electrical or mechanical) of pharyngeal branch of glossopharyngeal nerve evokes a short‐duration spasmodic inspiratory effort termed sniff‐ or gasp‐like aspiration reflex (AR). Similar responses are elicited by stimulation of whole pharyngeal cavity, upper pharynx or nasal septum (Benacka & Tomori, 1995). Physiological studies revealed that this reflex is activated from the subepithelial free‐nerve endings. This reflex is a part of physiological mechanisms that help animals to re‐establish normal breathing. It is very likely that animals exposed to high expansion nitrogen foam will manifest gasp‐like aspiration reflex if the foam entered the upper respiratory tract during the induction of unconsciousness.
Terrestrial animals have mechanical and chemical receptors to detect and to expel (through cough reflex) foreign body (such as the foaming liquid or irritant gases) as a defence mechanism to survive. Therefore, it is very likely that animals will experience respiratory distress, pain and fear if the foaming liquid entered the upper respiratory tract and triggered these receptors. The act of coughing in birds is manifested as open mouth breathing with neck extended accompanied with vocalisation during expiration, as seen in birds infected from respiratory diseases or during exposure to high concentrations of CO2.
In addition, mechanical obstruction of airways can lead to drowning if the bubbles enter the trachea and associated respiratory distress if obstruction (even partial) did occur. The dossier did not provide sound scientific evidence to rule out this possibility. Physiologically, suffocation can be defined as complete separation of the atmospheric air (or gaseous environment in this case) and the upper respiratory tract, for example, water during drowning, chocking with solid food particles and crushing of the neck during strangulation (see EFSA, 2004). Therefore, entry of even small quantities of foaming solution into the upper respiratory tract would trigger cough reflex but may not be considered equivalent to drowning.
Use of nitrogen gas pulse to break bubbles
The use of a nitrogen gas pulse to destroy the foam and release the nitrogen gas is an integral part of the process to achieve anoxic conditions in the container, which suggests that the foam is not fragile enough to breakup spontaneously upon contact with the animals. However, bursting of bubbles improves the visibility of animals and facilitates monitoring of the killing process. From the description in the dossier, it remains unclear where the pulse of nitrogen gas is targeted within the container and how direct delivery into the vicinity of the animal's head is avoided. Indeed, this would have animal welfare consequences, as documented in scientific literature where puffs of air have been demonstrated as aversive to poultry (Paul et al., 2018) and pigs (Ede & Parsons, 2023; Brietzke et al., 2013). According to the submitted dossier, the likelihood of the nitrogen gas pulse reaching sensitive areas of the animals (e.g. eyes) is very low. The technical design of the gas nozzle and outlet pressure ■■■■■ at 5 cm from the nozzle acts as a refinement to reduce this risk. However, it is inferred from the ‘process flow’ provided in the dossier that the animals will be in contact with the foam until the container is completely filled with the foam and the bubbles are burst with the N2 jet which is less than a minute.
Nevertheless, use of gas diffusers has been stated in the dossier as a preventive measure to eliminating this hazard. Indeed, the possibility of animals being hit by jet stream of gas mixture was prevented by using gas diffusers in the containerised gassing system investigated for poultry by Raj et al. (2008). Sparks et al. (2010) prevented this hazard during whole house gassing of poultry by using a CO2 delivery lance pipe that had approximately 32 mm nominal bore pipe with a 30° chamfer to the lance end to introduce a vertical element to the flow of gas inside the building. This was to ensure that the plume of gas was directed towards the roof and above the level of the birds, preventing them being hit by the full force of the gas as it entered the building. The lance was inserted into the building at approximately 1.2 m above ground level.
3.1.3. Key parameters for the effective use of the method
According to the EFSA guidance (EFSA AHAW Panel, 2018), the table of key parameters should contain parameters necessary for effective use of the system, component and description as columns. These tables need to be revised and additional parameters should be included, as commented in the present assessment. Furthermore, conditions of use of each animal species should be specified as it is done in the Council Regulation (EC) No 1099/2009 on the protection of animals at the time of killing and stunning.
The dossier states that: ‘Animals that are stunned in a high expansion foam filled with an inert gas, are being stunned by the inert gas and not by the foam, as experiments with poultry in air‐filled high‐expansion foam have shown. Therefore, the key parameters are basically the same as those listed in Annex 1 of Council Regulation (EC) 1099/2009 for inert gases but with a different relevance or content. In addition, there are parameters that are essential for a proper functioning of the foam method, that are proposed in Table 1 [of the dossier]’.
TABLE 1.
Overview of translation of the mandate assessment questions into subquestions.
| Assessment question | Subquestions |
|---|---|
|
1. Describe the new method |
1.1 What is the primary objective of the method? |
| 1.2 How does the new method work and what are key steps to achieve its primary objective? | |
| 1.3 What materials and tools are required for the new method? | |
|
2. Does the proposed method and information provided with the application comply with the eligibility criteria of EFSA's guidelines? |
|
|
3. Can the proposed method provide a level of animal welfare at least equivalent to that ensured by the existing methods in the legislation |
3.1 Can the proposed method provide a level of AW at least equivalent with the methods that exist in Annex I of Council Regulation (EC) 1099/2009 for pigs and poultry for situations other than slaughter? |
| 3.2 Does the proposed method ensure that pigs and poultry are spared of avoidable pain, distress or suffering during the killing as referred to in paragraph 1 of Article 3 of the killing Regulation? | |
| 3.3 Does the proposed method maintain the loss of consciousness and sensibility until death of pigs and poultry, as referred to in paragraph 1 of Article 4 of Council Regulation (EC) 1099/2009? | |
|
4. To what extend the information provided by applicant is taken into account for the final assessment and if the method is evaluated by other sources of information. |
|
|
5. To what extent the method proposed for stunning and killing pigs and poultry is able to provide a level of AW in compliance with the above‐mentioned legislation and, if yes, under which conditions. |
Table 7 was produced by the WG based on the information provided in the dossier. The rationale of some key parameters indicates whether the parameter is ‘essential’ meaning that it will impact directly the efficiency of the method to provoke death: failure to achieve < 2% oxygen is one of them. The other key parameters are mainly impacting the welfare of the animals during the induction and maintenance of unconsciousness until death occurs.
TABLE 7.
Proposed key parameters for inert gas foam in poultry and pigs.
| Parameter | Rationale | Reference value |
|---|---|---|
| Oxygen concentration | Essential, < 2% should be reached and maintained | < 2% in the input gas and during the stunning process. The residual oxygen concentration should be continuously monitored at the bottom and beneath the roof of the container and maintained throughout exposure time |
| Purity of N 2 gas | Residual oxygen concentration in nitrogen is critical. With (membrane) nitrogen generators, the machine backpressure setting may cause a too high oxygen content in the excess of 2% by volume |
The purity of nitrogen should be at least 98% Enclosed environment (like container) necessary to guarantee oxygen concentration |
| Temperature |
In practice only relevant when liquid nitrogen is used. Gas from high‐pressure cylinders maintains ambient temperature even when the compressed gas is expanding Nitrogen generators have no temperature effect. If necessary, foam temperature can be adjusted by controlling water temperature |
10–30°C for animals; the foam generators will technically function from 5°C to 60°C |
| Exposure time | Essential to allow for irreversibility of stunning (and thus for killing) by inert gases | Minimum exposure time of 5 min for poultry and 7 min for pigs |
| Foam expansion ratio | The expansion ratio is defined as the volume of foaming liquid (volume of foaming agent plus water) to the volume of foam produced. Expansion ratio is a measure for the water content of the foam. This must be high expansion to prevent water uptake during breathing | High expansion foam with an expansion ratio of 250–350 to ensure that water content is low. Upper limit to ensure that the foam keeps flowing so that animals are readily being covered and the risk of air pockets is decreased |
| Bubble diameter | In a given volume of foam, the volume of liquid (volume of foaming agent plus water) is inversely proportional to the diameter of bubbles in the foam. Bubbles with small diameter contain more water than those with larger diameter, and hence, exposure of animals to foam containing smaller diameter is likely to increase the chances of foaming liquid entering the respiratory tract | Bubble diameter: 95% of the gas in bubbles > 10 mm diameter |
| Foam production rate | Foam generator should have the capacity to completely fill the container within seconds so that the animals are exposed to the gas rapidly. The production rate will vary according to the size of the container | The volumes of foaming solution and nitrogen gas supply should be adequate to ensure optimal production rate. In any case, a container should be filled within 45 s. When filling the container with foam from below to push out air, the foam speed governs air displacement. The animals are submersed into an anoxic environment once their heads are covered in foam |
| Foam transparency | Transparency and bubble diameter describe the foam structure and depend on gas flow in relation to foam agent flow. Transparency is a measure for the absence of persistent, very small bubbles (diameter < 2 mm) that might enter the trachea. A uniform distribution of mid‐size bubbles ensures that these burst when inhaled, and that no oxygen gradient is generated above the foam level. This maintains the immediate transition from air to anoxia for the animals | Transparency > 30 cm for check on the absence of very small bubbles and expansion rate, making observation of the stunning process possible |
| Foam agent | Essential to guarantee foam quality – must be shown to produce high expansion foam, preferably optimised for use other than firefighting |
Special attention to drainage retardants (potential environmental hazards and limits to rendering), biodegradability and influence on the methanogenic phase of manure digestion Optimum drainage halftime about 15 min to reduce carcass weight |
| Foam atmosphere homogeneity | Essential |
Filling the container from below to push out air (plug flow) and make sure that no air pockets remain This is assured by a gas pulse (with inert gas) to destroy the foam as soon as the foam has driven out the air from the container. The gas pulse also makes it possible to determine real‐time oxygen values as the sensors will not be covered with foam anymore, and to fully observe the stunning process |
| Uniform distribution of foam throughout the container | Furniture and fittings, including crates and modules, in the container should not impede flow and uniform distribution of foam. Crates and modules should have perforated floor and roof to facilitate distribution of foam. The crate bars should be sufficiently wide (> 25 mm) to allow the foam to penetrate without compromising foam integrity and quality (e.g. bubble size). This should be ensured to guarantee the establishment of an anoxic atmosphere in all crates throughout the process. Overcrowding/stocking of poultry within crates and modules would impede with the flow | The operator should inspect and remove obstructions and ensure uniform distribution of foam within the container. The operator should ensure the crates and modules and the stocking density is adequate to prevent the hazard |
| Visibility of the animals during the process | Important for checking the stunning process | Windows should allow visibility, nitrogen pulse destroying the foam improves visibility of the animals and facilitates monitoring |
| Stocking density/space allowance | Stocking density and the design and use of any crates used to hold animals within the container is important to avoid system failure (see causes of system failure, Section 3.1.5) | All the animals should have adequate space to lie down when they lose consciousness, i.e. animals should not be falling on top of each other |
Process control
Animals exposed to anoxia created using high expansion nitrogen foam should remain in the container until they are dead. Any animal that was found to be alive or conscious should be killed using an appropriate back up method without delay and the cause of system failure should be investigated and rectified before resuming to stun/kill next batch of animals. The lack of control over stocking density during NEFS may result in container overload and smothering of animals during the application of NEFS. In the dossier, the Standard Operating Procedure (SOP) recommends adhering to the stocking density guidelines outlined for transportation (Regulation EC 1/2005). It is recommended to provide a table containing this information for the staff's reference, with the goal of preventing overloading and any potential future amendment of the Transport Regulation should also be taken into account, guaranteeing uniform distribution of the foam and less than 2% residual oxygen in the container. Additionally, the dossier describes three containers with different technical specifications (see Table 8 for poultry and pigs). However, illumination, volume and flow rate of gas and foam solution, foam production capacity and surfactant source and quality are not reported. The container should also have a temperature monitoring and display throughout the cycle of operation.
TABLE 8.
Technical specifications for the containers used for NEFS in pigs* and poultry.
| Name product | Length (mm) | Width (mm) | Height (mm) | Volume (L) | Number of foam generators | Filling time (s) |
|---|---|---|---|---|---|---|
| H1 | 850 | 600 | 280 | 143 | 1 | 8 |
| C1 | 880 | 730 | 700 | 450 | 2 | 9 |
| C3 | 6100 | 2400 | 1200 | 17,568 | 24 | 35 |
Neonate piglets in H1.
The data provided in Table 8 above come from the first set of the dossier submitted by the applicant. However, the latest updated dossier states that these containers are not used anymore, and purpose‐built containers are used to accommodate different animal categories. The above table is presented here to highlight the fact that the total filling time varies according to the size of the container.
Stocking density in crates and related hazards
It may happen that poultry species may be caught and crated for the purpose of killing them with the nitrogen filled high expansion foam outside the rearing area. Under this situation, the operators should be given clear instructions regarding maximum acceptable stocking density in each crate or drawer of a module to prevent system failure for several reasons. For example, overstocking will impede the spread of foam, both laterally and vertically, when injected from the bottom of the container, leading to ineffective or prolonged filling time. Secondly, the air remaining between tightly packed poultry may not be effectively displaced by the foam leading to failure to achieve less than 2% residual oxygen and some birds may keep their heads under the wings of adjacent birds, leading to ineffective stunning/killing. More importantly, higher than effective residual oxygen concentrations remaining in these trapped air pockets may not be detected by the oxygen monitors supplied with samples from the bottom or top of the container. Thirdly, all the birds may not be exposed simultaneously to the anoxic conditions leading to uneven exposure of birds to anoxia, and consequently, birds that are exposed to anoxia will lose consciousness and start wing flapping (due to the onset of anoxic convulsions) against adjacent birds remaining conscious potentially causing injury to the latter. Evidently, stocking density is an important key parameter that needs to be monitored closely to prevent system failure.
3.1.4. Scientific basis of induction and maintenance of unconsciousness for this method
The NEFS method relies on purging the air from a closed container by replacing it with nitrogen gas through the novel delivery of foam and rapid release of the gas through bursting the foam bubbles with a gas pulse, creating anoxic atmosphere, i.e. less than 2% residual oxygen throughout the container, which is maintained until the animals are dead. Stunning and killing with inert gases have been extensively described in scientific literature for pigs and poultry at specific age/production stages as summarised in the SO on Welfare aspects of animal stunning and killing methods (EFSA, 2004) and included as an approved method for stunning under the Council Regulation (EC) 1099/2009.
Unconsciousness is induced by the rapid transition of the animal from air to the anoxic atmosphere, leading to hypoxia in the tissues, especially the brain. The maintenance of unconsciousness until death is ensured by maintenance of less than 2% residual oxygen in nitrogen for 5 min in poultry (EFSA, 2004) and 7 min in pigs (Raj, 1999), i.e. until the animals are dead. Due to behavioural observations being severely obscured by the presence of the foam (poor visibility of animals enveloped in foam), the utilisation of behavioural proxies for loss of consciousness are insufficient and yields limited information. In the videos provided, the clips recorded in the laboratory situation, showed the use of N2 jet to disrupt the bubbles which made the animals more visible, whereas some other video clips, especially those recorded to the field did not involve N2 jet and the animals were obscured by the foam until it was disrupted by the anoxic convulsions. Therefore, time to onset of anoxic convulsions (e.g. wing flapping) may be audible (and listened to) and used as an indicator of onset of unconsciousness.
3.1.5. Potential causes of system failure and chances of occurrence
Animal factors
The submitted information from the dossier lists the target species and animal categories for the application of NEFS as encompassing various poultry and swine categories: day‐old chicks, layer hens (in laying age), broilers (at slaughter age), turkeys, doves (commonly known as pigeons), ducks, geese and pigs from birth (neonates) until reaching 80 kg. However, the dossier lacks scientific evaluations, via EEGs or other proper ABMs allowing welfare assessment of NEFS for ducks, pigeons and turkeys. Therefore, this assessment will exclude turkeys, ducks, doves and geese. The dossier indicates that 4000 day‐old chicks (Gallus gallus) were killed using NEFS, yet fails to disclose data concerning the duration of unconsciousness induction and welfare consequences during this induction period. However, in the light of the scientific literature on the use of CAS methods using inert gases, the assessment on the use of NEFS will specifically focus on laying hens, broiler chickens as well as day‐old chicks. This decision is informed by research indicating that newly hatched chicks across various species exhibit a tolerance to hypoxia, as outlined in the paper of Burton and Carlisle (1969). These authors studied the concentration of oxygen needed for 50% of the chicks (aged from 0 to 31 days) to lose consciousness and found that concentration in O2 required was decreasing from 31 to 0 days. They modelled the tolerance to acute hypoxia and found an asymptotic decrease for chicks from 0 up to 8 days of age, requiring lower oxygen to lose consciousness compared to chicks after 8 days of age. In day‐old chickens, behavioural observations using different CAS methods indicated the presence of headshaking using N2 gas and gasping behaviours, however substantially lower compared to CO2 gas (Gurung et al., 2018; Wang et al., 2021). The research by Raj and Whittington (1995) encompasses three experiments assessing the feasibility of inducing anoxia in day‐old chicks using an argon environment with 2% residual oxygen. The first experiment revealed that chicks (n = 18) lost posture within an average time of 12 s (±0.5 SE). In the second experiment, a batch of 20 chicks placed in a perforated tray and exposed to anoxia for 2 min resulted in 10 survivors, who began to vocalise at 40 s and exhibited head‐righting reflexes at 50 s after reintroduction to air. The third experiment involved four batches of 20 chicks each, exposed to less than 2% residual oxygen for 3 min (where NEFS uses 5 min exposure); none recovered upon reintroduction to air. Notably, Raj and Whittington documented that the induction of unconsciousness in chicks occurred without gasping or head shaking. It is also worth noting that the average times to loss of posture recorded in chicks (12 s) is similar to that reported for adult layer hens (average 11s; Raj et al., 1991) and broiler chickens (13 s; Raj, 1997; Gent et al., 2020).
Considering the available data on young chicks exposed to anoxia through inert gases, it is plausible to extend the use of NEFS to chickens of other ages (than laying/slaughter age) including chicks up to 7 days old with a certainty > 50%–100% (more likely than not). However, it is important to note that this extrapolation only considers the welfare consequences imposed on chicks exposed to inert gases. There is no evidence submitted in the dossier or available in the published literature of the welfare impacts of exposure to high‐expansion foam on chickens other than layer hens (in laying age) and broilers (at slaughter age). Further analysis of data regarding young chicks exposed to NEFS during the induction of unconsciousness could help reduce uncertainties regarding the application of NEFS in chicks up to 7 days of age.
For pigs, the weight range of 15–41 kg was determined by reverse calculation from data extracted from the study by Lindahl et al. (2020). Consequently, the EFSA can only conduct welfare impact assessments for those pig weight categories where data on the effects during unconsciousness induction are available. For other weight categories in pigs – neonates, slaughter weight or sows – the submitted information from the dossier nor other scientific evidence on the use of NEFS do not provide sufficient results to enable EFSA to evaluate the welfare impacts during unconsciousness induction. These gaps highlight the necessity for further research to ascertain the duration and magnitude of potential welfare consequences across different species and developmental stages.
Physical feature of the system and poor maintenance
A full HACCP evaluation of the nitrogen foam process (according to the format of Table A6 in EFSA Guidelines 2018) is presented in Table 6 of the submitted dossier. Additionally, an unpublished report ■■■■■ was included, which evaluated field trials and failure notifications of Anoxia™ nitrogen foam stunning machines under commercial conditions in both pigs and poultry. ■■■■■ But, as the duration of the process was not reported, it is impossible to conclude on the basis of this report on the efficacy of the submitted method since we cannot exclude that in the report, the animals were left in the containers much more than 5 and 7 min for poultry and pigs, respectively.
Furthermore, several other potential causes of failure are not mentioned in the dossier, e.g. the functionality of oxygen meters may be compromised due to the presence of foam and high humidity, leading to potential inaccuracies in oxygen measurements. The disruption of the foam by a nitrogen gas pulse may also release trapped air from pockets which may occasionally form inside the container, especially with the use of poultry crates. Overloading (high stocking densities) of the crates can also lead to air pockets between birds and hinder uniform distribution of the foam and failure to achieve less than 2% residual oxygen in the container. Operational procedures should ensure that formation of such air pockets is minimised. However, how to minimise formation of such air pockets and how essential their influence can be is difficult to determine based on the limited evidence provided. So even if this event constitutes a potential source of failure of the system, its likelihood of appearance is unknown.
According to the dossier, the foaming container was compartmentalised using flexible materials to minimise volume of foam required to killing. When the animal lost consciousness and started exhibiting anoxic convulsions, it can result in the collapse of such partitions. This can lead to blanketing of animals with air pockets or mixing of air with N2 leading to animal regaining consciousness and a complete failure of the system.
There are two distinct hazards to the systems operation in relation to the modified atmosphere and gas concentrations: (1) too low nitrogen gas supply; and (2) failure to achieve < 2% oxygen. In relation to an insufficient nitrogen supply, continuation of foaming will not mitigate this hazard as foam generation will be impaired. Therefore, mitigation should include nitrogen and foaming agent supply checks/monitoring prior to operation to confirm adequate supply for two‐full cycles (twice the volume of the container), in order to allow immediate reapplication if necessary and back‐up supplies of nitrogen immediately available.
The water should be free of particles and suspended solids that may block the liquid spray nipple of the foam generator, which would lead to system failure. The water should be filtered before feeding into the foam generator to eliminate this problem.
It is stated in the dossier that failure in foam production may be caused by e.g. improper concentration of foam solution, incorrect connection of hoses, too low gas pressure or insufficient soaking of mesh with foaming solution before applying gas.
Although the submitted dossier states that exposure to anoxia will lead to death and therefore no backup methods are necessary, it is very likely that in the event of a system failure, as referenced in the field trial for pigs above, one would require a backup method appropriate to the species and the size/age of the animals, as a part of the standard operating procedures and to be compliant with Council Regulation (EC) 1099/2009.
Possible system failures include operator mistakes and technical problems. Possible operator mistakes are non‐closing of separator (entrance gate) before foam filling, as well as non‐checking of critical ingredients for foam formation (gas, soap). Possible technical problems can be oxygen sensor failure and electricity shortage.
It is stated in the dossier that field trial has indicated that the use of ‘temporary flexible partitions’ in the container to create restriction of space and minimise the volume of foam required has resulted in two compartments, one filled with air on one side and another containing the animal filled with the foam, has collapsed during the induction of unconsciousness in pigs resulting in either mixing of these two atmospheres leading to residual oxygen concentrations more than 2% by volume or animals hiding their heads under the blanket containing atmospheric air pockets, and, as a consequence, either animals not losing consciousness or surviving the recommended duration of exposure stated in the key parameters.
In addition, Annex 1 provided in the file ‘Potential causes of system failure and chances of occurrence’ as a part of the dossier, contains an additional source of system failure, freezing of solenoid valve used to control the nitrogen gas flow, which should be also taken into account.
3.2. Description of the individual studies submitted
3.2.1. Introduction
3.2.1.1. Background and rationale
Regulation (EC) 1099/2009 permits the use of CO2 at high concentration for depopulation of poultry and pigs on farm.
Currently, carbon dioxide (CO2) is commonly used for depopulation of farm animals in the EU, in accordance with Article 18 of Regulation (EC) 1099/2009 in response to public health, animal health, animal welfare or environmental reasons (e.g. outbreaks of infectious diseases or natural or man‐made disasters) (EURCAW‐Poultry‐SFA, 2023). In the European Union, the controlled atmosphere methods allowed for purposes other than slaughter in poultry and pigs are:
CO2 at high concentration (above 40% by volume) of CO2;
CO2 in two phases (i.e. successive exposure of conscious animals to a gas mixture containing up to 40% of CO2, and when animals have lost consciousness, followed by a higher concentration of CO2) (poultry only);
CO2 associated with inert gases (i.e. direct or progressive exposure of conscious animals to a gas mixture containing up to 40% of CO2 associated with inert gases leading to anoxia);
Inert gases (i.e. direct or progressive exposure of conscious animals to an inert gas mixture such as Argon or Nitrogen leading to anoxia);
Carbon monoxide (i.e. exposure of conscious animals to a gas mixture containing > 4% carbon monoxide) (for poultry and piglets only);
Carbon monoxide associated with other gases (i.e. exposure of conscious animals to a gas mixture containing > 1% carbon monoxide with other toxic gases) (for poultry and piglets only);
Low atmospheric pressure stunning (LAPS) (i.e. exposure of conscious animals to gradual decompression with reduction in available oxygen to < 5%) (Broiler chickens < 4 kg only).
Carbon dioxide is known to cause aversion, pain and respiratory distress (i.e. breathlessness) in both poultry and pigs but with different sensitivities (e.g. Conlee et al., 2005; Llonch et al., 2012; McKeegan et al., 2006; Raj & Gregory, 1995, 1996; Steiner et al., 2019; Velarde et al., 2007). Hence, the use of CO2 for killing represents a risk for poor welfare. The use of NEFS has been described as an alternative to high concentrations of CO2 for killing (McKeegan, Reimert, et al., 2013; Raj et al., 2008). N2 is an inert gas and studies have shown that it is less aversive than high concentrations of carbon dioxide when inhaled for both pigs (e.g. Dalmau et al., 2010; 85% nitrogen plus 15% CO2) and poultry (e.g. Sandilands, 2011). In the case of the proposed NEFS method, the foam serves as a carrier of the N2 gas, in the sense, the N2‐filled foam displaces the air within the container and creates an atmosphere to achieve anoxia when the foam is burst and nitrogen is released (Lindahl et al., 2020). The purpose of this exercise is to assess the welfare implications of N2 filled foam in the vicinity of the animals rather than anoxia per se induced with N2 gas, which is already authorised in the current Regulation (EC) 1099/2009.
3.2.1.2. Objective
Two scientific publications that were submitted by the applicant to EFSA were considered in this assessment.
The first one is about poultry killing. The aim of McKeegan, Reimert, et al. (2013) was to investigate the potential of N2‐filled and CO2‐filled high expansion foam as depopulation methods for some poultry species and their impact on the birds' welfare. Additionally, the study assessed the potential aversion to foam itself, by including air‐filled foam as a treatment group.
The second paper is about killing of pigs. The aim of Lindahl et al. (2020) was to use certain behavioural and physiological responses of pigs to NEFS to assess possible impact on the welfare of the pigs during induction phase, and to evaluate the stun‐kill efficiency. Furthermore, the study aimed to assess potential aversion due to the foam itself by including an air‐filled foam treatment.
3.2.2. Materials and methods (for each single study)
The information related to the material and methods from both studies McKeegan, Reimert, et al. (2013) and Lindahl et al. (2020) are described in Tables 10, 11.
TABLE 10.
Quality control and risk assessment of reported information and/or data in relation to the method applied to poultry and pigs exposed to NEFS according to EFSA Guidelines (EFSA AHAW Panel, 2018).
| Broiler chickens | Laying hens | Pigs (weaned, 10 weeks old [~ 27.8 kg]) | |
|---|---|---|---|
| Reference | McKeegan, Reimert, et al. (2013) | McKeegan, Reimert, et al. (2013) | Lindahl et al. (2020) |
| Method | |||
| Study population | Data provided | Data provided | Data provided |
| Sampling strategy | Data not provided | Data not provided | Data not provided |
| Experimental design | Data provided | Data provided | Data provided |
| Ethical considerations | Data provided | Data provided | Data provided |
| Randomisation and blinding | Data not provided | Data not provided | Data provided |
| Reporting the methods of analysis | Data provided | Data provided | Data provided |
TABLE 11.
Quality control and risk assessment of reported information and/or data in relation to the measurement of the outcomes of poultry and pigs exposed to NEFS, according to EFSA Guidelines (2018).
| Broiler chickens | Laying hens | Pigs (weaned, 10 weeks old [~ 27.8 kg]) | |
|---|---|---|---|
| Reference | McKeegan, Reimert, et al. (2013) | McKeegan, Reimert, et al. (2013) | Lindahl et al. (2020) |
| Measurement of the outcomes | |||
| Onset and duration of unconsciousness | Data provided | Data provided | Data provided |
| Time to death | Data provided | Data provided | Data provided |
| EEG – onset of unconsciousness, all parameters should be specified | Data provided | Data provided | Data not provided |
| ABMs Vocalisation | Data not provided | Data not provided | Data provided |
| ABMs postures and movements | Data provided | Data provided | Data provided |
| ABMs general behaviour | Data provided | Data provided | Data provided |
| ABMs hormone concentrations | Data not provided | Data not provided | Data not provided |
| ABMs blood metabolites | Data not provided | Data not provided | Data not provided |
| ABMs autonomic responses | Data provided | Data provided | Data provided |
| Pain magnitude (duration and severity) | Data not provided | Data not provided | Data not provided |
| Correlation neurological and other ABMs | Data not provided | Data not provided | Data not provided |
It is important to note that the proposed NEFS system being evaluated was only assessed in the Lindahl et al. (2020) study on pigs. The submitted study in poultry (McKeegan, Reimert, et al., 2013) was undertaken with high‐expansion foam but following a different methodology than the one described in the present submission. Indeed, while the principle of generating anoxic environment is the same, the application is notably different. In the McKeegan, Reimert, et al. (2013) study, the animal is placed into a container (1 m × 1 m × 1 m), with one side wall removable to allow the animal to be safely placed in the chamber and retrieved. The container had no lid and was top opened. The foam was introduced from the top and filled in approx. 60s, once filled the foam generation was halted. No nitrogen or gas pulse was then used to breakdown the foam bubbles, and the bird was left submerged in foam. The hypothesised process centred around bird movement, providing the necessary force to burst the bubbles directly around it, releasing the N2 gas. Despite the differences in method of application (e.g. no lid vs. lid; no crating of poultry vs. crating; no intervention to burst bubbles vs. bursting foam bubbles with N2 pulse), the animal welfare consequences of exposure to nitrogen filled high expansion foam reported by McKeegan, Reimert, et al. (2013) could be comparable to the NEFS proposed by the applicant.
3.2.2.1. Method
The information contained in the two scientific papers submitted by the dossier regarding the method are summarised in Table 9, as recommended in EFSA guidance (EFSA AHAW Panel, 2018).
TABLE 9.
Technical details of the methods applied in McKeegan, Reimert, et al. (2013) as P3 and Lindahl et al. (2020) as P4.
| Method | P3 | P4 |
|---|---|---|
| Study population |
20 broiler chickens (Ross 308), reared from 1‐day‐old in a single group under commercially relevant conditions until 5 weeks old. Sex or body weights not reported 20 adult hens (ISA Brown) were obtained from a commercial supplier at 42 weeks of age and housed in individual cages. They were older than 42 weeks. Females only. Body weights not reported |
60 pigs (mean weight 27.8 ± 4.4 kg) with an age of approximately 9 weeks were included in the study. The pigs were crossbreeds of Yorkshire*Dutch Yorkshire dams and Hampshire sires. The piglets were born at the research facility. They were weaned at 5 weeks of age and remained in the farrowing pens for an additional 5 weeks after weaning. Pigs from six different litters, were used in the experiments |
| Sampling strategy |
Individual birds were assigned randomly to N2 or air‐filled foam treatments 20 animals of each category were included to retrieve sufficient valid EEG, since some will be unusable |
The 60 pigs were divided into three treatments (Control (air), N2 or air‐filled foam) and randomly allocated for each treatment |
| Experimental design | A total of 12 hens were exposed to anoxic (N2‐filled) foam, whereas 8 were exposed to control (air‐filled) foam. Ten broilers were exposed to anoxic (N2‐filled) foam and 10 were exposed to control (air‐filled) foam. The variation in sample sizes was reported as compensating for loss of EEG data due to water ingress into equipment | The pigs were divided into three treatments (Control (air), N2 or air‐filled foam), with 20 pigs per treatment. Pigs within pen and sex were divided into groups of three, and within these groups, one pig was randomly selected for each treatment. Each treatment included 9 immuno‐castrated males and 11 females from the same pens |
| Ethical considerations | All the experiments conducted in the United Kingdom and were carried out at the Royal Veterinary College under Home Office Authority, which followed institutional ethical review and approval | Experimental work was conducted in Sweden at the Swedish Livestock Research Centre, Swedish University of Agricultural Sciences. Ethical approval for animal experimentation was received from the ethical committee in Uppsala, Sweden (ref. no. 5.8.18–09852/2018; approval date 27 July 2018) |
| Randomisation and blinding |
In the study, it is only mentioned that individual birds were assigned randomly to N2‐ or air‐filled foam treatments No specific consideration about blinding was reported |
The 60 pigs were divided into three treatments (Control, Air foam and Nitrogen foam), with 20 pigs per treatment. Pigs were divided in groups of three and one pig was randomly selected for each treatment and this is repeated 20 times. However, it is also mentioned that it was not possible to blind the observer to the treatments each pig was subjected to due to the visual differences between the treatments (e.g. presence of foam; convulsive activity in anoxic environment) |
| Reporting the methods of analysis |
The study reports these analyses: The logged data files were uploaded into a data acquisition and analysis program (Spike 2 Version 4.2, Cambridge Electronic Design, Cambridge, UK). A combination of automated and manual analysis techniques was used to produce dedicated event channels representing heartbeats per min (2‐s bins) from the raw traces during baseline and after foam application. Where clear waveforms were present, heart rate (ECG) was measured by telemetry every 5 s Visual inspection of the EEG traces allowed estimation of the timing of onset of different types of EEG activity: baseline, transitional, suppressed, and isoelectric Statistical analysis in the form of a GLM with bird type (broiler, hen) and gas (CO2, N2) as factors was performed for times to transitional, suppressed, and isoelectric EEG and time to ataxia, flapping onset, and cessation of movement. This was followed by post‐hoc paired t‐tests to highlight differences between bird types and gases. Visual obscuration by the foam limited the extent of detailed behavioural measurements. Nevertheless, several observations were carried out from the video recordings of each trial. As foam was introduced, counts of gasping, headshakes, foam avoidance, and escape attempts were noted. After submersion, time to ataxia, time to loss of posture, wing flapping (flapping onset, number of bouts, total flapping duration) and cessation. The behaviours of birds were recorded from the underside of the clear plastic box by using a video camera |
The study reports these analyses: Data (both behavioural and physiological) were divided into 10 s time intervals, where the first three intervals (intervals 1–3) were before the start of foam production into the box. The following 9 intervals (intervals 4–12) were with foam in the box for the two foam treatments. As the time to fill up the box differed depending on how much the pig moved inside the box, the gas pulse occurred in different intervals for different pigs. Due to measurement and observation errors, 102 (of which 24 from the Control, 24 from the Air foam and 54 from the Nitrogen foam treatment) of the total 720 intervals (60 pigs × 12 intervals) for heart rate measurements and 27 intervals (of which zero from the Control, 15 from the Air foam and 12 from the Nitrogen foam treatment) for the movement observations were excluded from the statistical analyses Differences between treatments were compared within each 10 s interval and change in each variable over time (i.e. differences within variables between intervals was assessed). Heart rate was recorded with telemetry and analysed as the difference between average heart rate during a baseline interval (60–30 s before the pigs were let into the stun box, i.e. when they were in the holding pen and had been there for acclimatisation for 1 min) and the average heart rate for the 10 s interval. According to the dossier, respiratory rate was not analysed due to unreliable data. Also, continuous variables were stated to be normally distributed heart rate difference rate and movement (i.e. number of lines crossed in the box) and were analysed with general linear model 1 in Proc Mixed. Binary variables (behaviours performed or not during the 10 s interval) were analysed with generalised linear model 2 in Proc Glimmix (with binomial distribution and logit link). The random effect of individual pigs was not included in Model 2 as analyses with such a model did not converge |
Information on studies about poultry and pigs
The content of the single published scientific paper on poultry and the content of the other related single published scientific paper on pigs were considered for risk assessment and quality control and are summarised in Table 10.
3.2.2.2. Measurement of the outcomes (Applicant's submission)
The applicant provided derived information related to the measurement of the outcomes from both studies McKeegan, Reimert, et al. (2013) (P3) and Lindahl et al. (2020) (P4) regarding both poultry and pigs.
Table 11 presents which measurements might be pertinent when assessing NEFS, in accordance with EFSA guidance. These measures can be neurological, physiological, behavioural or physical reflexes (EFSA AHAW Panel, 2018) and they are presented in the table together with the information if they are provided or not.
The applicant provided information related to the measurement of outcomes derived from McKeegan, Reimert, et al. (2013) (P3) and Lindahl et al. (2020) (P4). This information can be found in the dossier (Section 3.2.2, Table 4). The information provided is incomplete; therefore, it does not conform entirely with the EFSA Guidance on the assessment criteria for applications for new or modified stunning methods regarding animal protection at the time of killing (EFSA AHAW Panel, 2018).
3.2.3. Reporting the results
3.2.3.1. Reporting outcomes and estimations
Quality control and risk assessment for the report of estimation and outcomes according to EFSA Guidelines (2018) are presented in Tables 12, 13. The report of estimation and outcomes provided by the dossier is presented in Table 14. Information provided does not always conform with the EFSA Guidance on the assessment criteria for applications for new or modified stunning methods regarding animal protection at the time of killing (EFSA AHAW Panel, 2018). Information provided by the applicant only includes a selection of outcomes and estimations, omitting some data reported in the published studies.
TABLE 12.
Quality control and risk assessment of reported information and/or data in relation to details on outcomes and estimations of poultry exposed to NEFS, according to EFSA Guidelines (2018).
| Species | Broiler chickens | Laying hens |
|---|---|---|
| Reference | McKeegan, Reimert, et al. (2013) | McKeegan, Reimert, et al. (2013) |
| Reporting outcomes and estimations | ||
| Proportion of animals mis‐stunned | Data provided | Data provided |
| Time to onset of unconsciousness | Data provided | Data provided |
| Duration of pain, distress and suffering | Data not provided | Data not provided |
| Magnitude of pain, distress and suffering | Data not provided | Data not provided |
| Duration unconsciousness | Data provided | Data provided |
| Frequency of animals recovering consciousness before death | Data provided | Data provided |
| Time to death | Data provided | Data provided |
| Proportion of death animals | Data provided | Data provided |
| Adverse events | Data provided | Data provided |
TABLE 13.
Check of data for risk assessment of NEFS in relation to details on outcomes and estimations of pigs according to EFSA Guidelines (2018).
| Species | Pigs (weaned, 10 weeks old [~ 27.8 kg]) |
|---|---|
| Reference | Lindahl et al. (2020) |
| Reporting outcomes and estimations | |
| Proportion of animals mis‐stunned | Data provided |
| Time to onset of unconsciousness | Data provided |
| Duration of pain, distress and suffering | Data not provided |
| Magnitude of pain, distress and suffering | Data not provided |
| Duration unconsciousness | Data provided |
| Frequency of animals recovering consciousness before death | Data provided |
| Time to death | Data not provided |
| Proportion of death animals | Data not provided |
| Stun‐to‐stick interval | Data not provided |
| Adverse events | Data not provided |
TABLE 14.
The information provided in the dossier related to the report of outcomes and estimations derived from McKeegan, Reimert, et al. (2013) (P3) and Lindahl et al. (2020) (P4). Text enclosed in quotation marks has been quoted directly from the published study by the applicant.
| Outcomes | P3 | P4 |
|---|---|---|
| Proportion of mis‐stunned animals | No birds were mis‐stunned. All the birds exposed to the foam for additional 3 min following cessation of movements were confirmed dead | No pigs were mis‐stunned. All the pigs exposed to the foam for 5 min were reported to be dead. Death was confirmed as a non‐audible heartbeat via a stethoscope and a successful stun by lack of corneal or pain reflexes, no muscle contraction and motionless |
| Time to onset of unconsciousness |
Times are in relation to the full submersion in N2 foam LOP: Hens: 16 ± 1 (SE) s Broilers: 9 ± 1 s EEG suppressed: Hens: 30 ± 2 s Broilers: 18 ± 1 s If the time to onset of wing flapping, which occurs as spinal reflex due to the loss of control of the brain over the spinal cord (and hence a behavioural indicator of onset of unconsciousness) is considered, then the reported average time to onset of unconsciousness is 18 s for hens and 15 s for broilers |
Mean time elapsed from the onset of foam production to loss of posture was 57.0 ± 8.8 s (range 43–76 s, = 20) |
| Duration of pain, distress and suffering |
From the results provided through ‘time to onset of unconsciousness’, we can deduce the maximum time during which animals might be submitted to pain distress and suffering, but this information was not provided Absence of a treatment study where birds are administered analgesic makes it impossible to assess (via ABMs) if animals do feel pain, distress and suffering before being unconscious |
From the results provided through ‘time to onset of unconsciousness’, we can deduce the maximum time during which animals might be submitted to pain distress and suffering, but this information was not provided Absence of a treatment study where pigs are administered analgesic makes it impossible to assess (via ABMs) if animals do feel pain, distress and suffering before being unconscious |
| Magnitude of pain, distress and suffering |
Absence of analgesic treatment study does not allow direct assessment of magnitude of pain, distress or suffering through ABMs No escape attempts were reported in N2 foam. This is consistent with the fact that N2 by itself is not aversive, but foam might be considered aversive to birds. However, birds exposed to air‐filled high expansion foam exhibited headshakes and escape attempts. Headshaking was seen in both hens and broilers in response to initial foam delivery [mean number 2.1 ± 1.3 (SE) in hens and 2.5 ± 0.8 in broilers]. Similar mean counts of headshakes were observed in both broilers and hens in N2 foam [2 ± 1 for both]. Escape attempts (vertical jumps at box wall) were seen in one hen (2 attempts) in response to control air‐filled foam The authors consider that ‘initial aversion to the foam is not extreme, although submersion of conscious birds in air‐filled foam for up to 60 s appeared to be unpleasant.’ |
Percentage of pigs exhibiting ABMs were reported to be: Defaecation: 20% in N2 filled foam 25% in air filled foam, 5% in air without foam Shaking: 10% in N2 filled foam, 15% in air filled foam vs. 0% in air without foam Slipping: 20% in N2‐filled foam, 45% in air‐filled foam, 0% in air without foam Vocalisation scream: 95% in N2‐filled foam, 45% in air‐filled foam, 80% in air without foam Escape attempts: 80% in N2‐filled foam, 80% in air‐filled foam, 15% in air without foam. Therefore, these results are considered to indicate moderate aversion to N2 foam, without knowing exactly if the pigs felt pain, distress or suffering (no analgesic treatment). Duration (time to LOP): N2 57.0 s (SD ± 8.8, range 43–76 s, n = 20) |
| Duration unconsciousness | Not relevant, as the proposed method is a killing procedure | Not relevant, as the proposed method is a killing procedure |
| Frequency of animals recovering consciousness before death | Unconsciousness lasted until death occurred in all the birds during exposure to the foam | Unconsciousness lasted until death occurred in all the pigs during exposure to the foam |
| Time to death |
Indicated by both time to cessation of visible movement and isoelectric EEG being observed Time to cessation of movement: Hens: 65 ± 3 s Broilers: 51 ± 2 s EEG isoelectric: Hens: 65 ± 3 s Broilers: 47 ± 2 s |
The mean time between loss of posture and the last observed muscle contraction was 132.5 s (SD ± 16.3, n = 20). Therefore, approximate time to motionless was approx. 190 s (132.5 + 57 s) However, animals were exsanguinated after 5 min exposure to the foam |
| Proportion of dead animals | 100%* | 100% |
| Stun‐to‐stick interval | Not relevant as the proposed method is a killing process | Not relevant as the proposed method is a killing process |
| Adverse events |
‘In response to air‐filled foam exposure, 4 out of 8 birds swallowed foam so that it was visible in the mouth and oesophagus at the necropsy. A few birds regurgitated food during exposure to air‐filled foam. Many birds exposed to control foam also had small amounts of foam in the tracheal opening, but the trachea was never occluded. Birds exposed to N2‐filled foam also had foam in the mouth and oesophagus, and some regurgitated food. Birds exposed to N2‐filled foam regularly had foam present deeper in the trachea than controls (10/12 hens and 9/10 broilers) maybe due to deep inhalation In one hen, foam was present as far down as the syrinx, but foam was more usually observed 3–10 cm from the tracheal opening. The foam was visible as a few tiny bubbles clinging to the tracheal wall, and in no instance was the trachea even partially occluded by foam’ |
Events related to aversion and respiratory distress. The vocalisations before LOP suggest pain or fear. There is no information related to post‐mortem observations |
In N2 and CO2 foam trials, all measurements continued for 3 min after birds exhibited cessation of movement. In control (air foam) trials, all measures continued for 60s after submersion, after which the bird was rapidly retrieved and immediately euthanised (barbiturate overdose, administered intravenously).
3.2.3.2. Reporting uncertainty
In the submitted papers, P3 and P4 uncertainties are not discussed, but verbal expressions are used in the dossier to express probability of unwanted events (e.g. very likely) although these are not translated into specific probabilities.
Specifically, it is stated in the dossier that:
In terms of uncertainty analysis for poultry (broilers and laying hens):
It is beyond reasonable doubt that all birds will die due to prolonged exposure to anoxia.
It is very likely that all birds will be irreversibly stunned after 3 min in anoxic foam (or anoxic atmosphere).
It is very likely that the stunning and killing process is not influenced by species or size.
There is some likelihood that birds will show aversive reactions to the foam.
It is unlikely that foam will enter the trachea and cause irritation to mucous tissues.
It is most unlikely that birds will die due to occlusion.
In terms of uncertainty analysis for pigs:
It is beyond reasonable doubt that all pigs will die due to prolonged exposure to anoxia.
It is very likely that all pigs will be irreversibly stunned after 10 min in anoxic foam (or anoxic atmosphere)
It is likely that the stunning process is influenced by the respiratory health status of the pigs.
It is likely that the stunning and killing process is not influenced by weight.
There is some likelihood that pigs will show light till moderate aversive reaction to the foam.
It is unlikely that foam will enter the trachea and cause irritation to mucous tissues.
It is most unlikely that pigs will die due to occlusion.
The independent assessment of the WG on uncertainty is stated in Section 4.2.2.1.
3.2.4. Discussion and conclusions
3.2.4.1. Reporting interpretation of results
Interpretation and review of the submitted dossier
Poultry
The use of foam as a carrier for N2 may lead to pain, distress or suffering in poultry from the time they are placed in the closed container until they lose consciousness. Indeed, the foam itself has the potential to induce fear responses in poultry.
The use of N2‐filled foam was an effective method to quickly purge the air from the box to create and maintain stable anoxic conditions, resulting in the death of all birds exposed to it. McKeegan, Reimert, et al. (2013) reported that headshaking and escape attempts occurred in birds exposed to air‐filled foam. Head shaking could be considered as a behavioural indication of attempts by birds to expel foam from the upper respiratory tract. Escape attempts could be interpreted as an aversive reaction to being surrounded by foam, loss of visual contact with the environment or novelty.
Gasping behaviour was measured in this study, and importantly no gasping behaviour in birds exposed to N2‐filled foam was reported, potentially indicative of minimal respiratory distress. Absence of gasping in N2‐filled foam would also mean that either foam did not enter the upper respiratory tract during the induction of unconsciousness and/or minimal mucosal irritation either by N2 or the foam. Gasping was reported in CO2‐filled foam, suggesting expression of this behaviour is unlikely due to the foam alone, but rather to the CO2 present within the bubbles in the corresponding treatment. Since CO2 is an acidic gas and pungent to inhale, it is very likely that CO2 entering the respiratory tract acted as a chemical irritant and the birds exhibited gasping to expel the irritant. However, in the dossier, it is reported that the foam agent could induce irritation but stated that mucosal or skin irritation due to foam would take hours or days to appear. The dossier, however, provided little evidence to support this statement. There was no evidence to support the absence of irritation (e.g. skin, mucosal, eyes, etc.) from the foam agent during NEFS in the presented dossier.
In the necropsy of laying hens and broiler chickens exposed to air or N2 or CO2‐filled foam, McKeegan, Reimert, et al. (2013) reported small bubbles observed in the larynx and trachea in the majority of birds (air‐filled: 50% of birds; N2‐filled and CO2‐filled: ~ 90% of birds). Foam bubbles were noted in the oral cavity and airways (including the larynx, trachea and syrinx). However, the authors stated that there was no evidence of occlusion of airways by the foam. Combined with the behavioural outcomes (e.g. gasping), the welfare implications of the necropsy finding are unclear.
No histopathological examinations were conducted as part of this study (McKeegan, Reimert, et al., 2013), which could have been useful to inform on the presence of irritation due to foam. Such examinations could have been beneficial to identify reddening/inflammation of tissues which had come into contact with foam (e.g. eyes, mucosa, skin, etc.), identifying an acute inflammatory reaction immediately after exposure to the foam. As a precautionary principle, the substance irritating the mucosa alone should be considered as a hazard during the NEFS, which is supported by the occurrence of head shaking in chickens during exposure to air‐filled foam. The dossier presented limited information concerning the estimation of pain in laying hens and broiler chickens, and there is a lack of studies involving the use of analgesics. The use of analgesic and anxiolytic drugs can help to distinguish behavioural reactions due to pain and/or due to fear/anxiety. The measure of magnitude of fear, pain and distress is a challenge due to limited visibility of behaviour even when the foam is considered transparent. As evident from the visual footage presented by the applicant, the foam forms a white layer that impairs the behavioural assessment of the animals. Irrespective of this, according to the pathological findings in poultry (McKeegan, Reimert, et al., 2013), high expansion foam does not appear to pose a risk of suffocation due to occlusion of the airways. However, if the foam quality is poor (e.g. low expansion ratio, high water content or bubbles with a diameter below 15 mm), it could lead to suffocation after entering the trachea.
Pigs
The anoxia system using N2‐filled foam was an effective method to quickly purge the air from the box to create and maintain stable anoxic conditions.
The use of foam as a carrier for N2 may lead to pain, distress or suffering in pigs from the time they are placed in the closed container until they lose consciousness. Indeed, the foam itself has the potential to induce fear responses in pigs, along with respiratory distress and pain if the foam entered the respiratory tract in conscious animals, as explained previously for poultry.
It is reported that pigs explored the foam with their snout when the foam generator started; however, they seemed to avoid putting their heads and snouts into the foam when foam levels became high, at which point escape attempts through the lid increased, with 80% of pigs exposed to either air‐ or N2‐filled foam performing escape attempts (Lindahl et al., 2020). Lindahl et al. (2020) reported that escape attempts did not appear to be induced by panic, but instead by a response to the novel experience of being covered in foam. However, they also report that exploratory behaviours were more focussed upwards and towards the lid of the container in pigs exposed to N2‐filled foam compared to those exposed to air‐filled foam, who directed more exploratory behaviours to the floor. This might be indicative of further avoidance behaviour in pigs exposed to N2‐filled foam. Lindahl et al. (2020) concluded that pigs did not show any aversive behaviours to the N2‐filled foam in comparison to the air‐filled foam. However, although results showed 80% of pigs exposed to either air‐ or N2‐filled foam showed escape attempts, the average number of escape attempts was greater in air‐filled foam (4.9, range 0–11) than N2‐filled (1.9, range 0–4). The results highlight that the percentage of pigs that performed escape attempts was similar in intervals 8–9 (10s recording intervals), but escape attempts increased only in air‐filled foam in subsequent intervals (e.g. intervals 10–12), while on average most pigs in N2‐filled foam were now showing lying and convulsive behaviours as they succumbed to hypoxia. Therefore, the difference is likely due to the fact that when exposed to N2‐filled foams, pigs were losing consciousness and therefore were less able to perform escape attempts and other conscious behaviours. Importantly, pigs exposed to both air‐filled and N2‐filled foam performed more escape attempts compared to control pigs (pig held in container for 5 min with no foam), demonstrating additional aversion to foam as well as the novel environmental, potentially due to fear, pain or discomfort.
A limitation of this study is the reporting of behavioural measures in binary format (1/0‐sampling) within 10‐s intervals, where a pig was recorded as performing a behaviour at least once during a 10‐s interval (Lindahl et al., 2020). Therefore, the magnitude of aversion may be limited as individual total escape attempts (and other behaviours) per pig are not reported and latencies of behaviours are averaged based on these intervals, adding a greater level of inaccuracy in actual onsets of behaviours. Like in the study in poultry, there was no use of analgesic and anxiolytic drugs in Lindahl et al. (2020), which would have aided distinguishing between behavioural reactions due to pain and/or due to fear/anxiety.
The video footage provided by the applicant shows that, like in the poultry study, the foam forms a white layer, reducing visibility and making it difficult to observe the animals clearly. Based on the behavioural assessment reported in Lindahl et al. (2020), there is no evidence to support the applicant's claim that pigs do not experience pain, fear and respiratory distress prior to the onset of unconsciousness. Furthermore, the absence of postmortem/necropsy data available in Lindahl et al. (2020) provides no corroboration to the behavioural outcomes and potential welfare impacts.
3.2.5. Conflict of interest statement
Poultry
No conflicts of interest were declared by the study authors (McKeegan, Reimert, et al., 2013).
The WG notes that the proposers for the NEFS system were not associated in this study and did not provide equipment.
Pigs
The study authors declared no conflict of interest (Lindahl et al., 2020).
The WG notes that the funders (Tönnies Forschung) had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. But the proposers for the NEFS system were associated with the study and were acknowledged in the publication.
3.3. Overall integration of findings from all studies
3.3.1. Demonstration of equivalence with existing methods
Article 4(2) of Council Regulation (EC) No 1099/2009 requires that any new or modified stunning method ensures a level of animal welfare which is at least equivalent to that ensured by the existing methods. The method of killing by N2‐filled high expansion foam was submitted for evaluation by EFSA in order to kill all stages of production/ages for poultry (including broilers, laying hens, turkeys, ducks and pigeons) and pigs. The dossier provided the applicant's interpretation of equivalence with existing methods. However, the assessment was incomplete and/or did not follow EFSA guidelines (2018). Therefore, the WG summarised the information provided and included additional considerations, where considered necessary. The independent assessment was performed by EFSA in Chapter 4.2.
The dossier states: ‘Referring to the various papers summarised in Tables B.1, B.2 in the Technical Report. Obvious ABM like wing flapping or cessation of movement is more readily reported than the more subtle evasions (escape attempts) or headshakes. A general impression from Table B.1 is that a gradual creation of an anoxic atmosphere by flushing causes more escape attempts than N2 foaming (compare McKeegan et al., 2007, McKeegan, Reimert, et al., 2013). Gasping was reported in one study during exposure to CO2 and in prefilled Ar but not in N2 foam. Headshakes and wing flapping were reported in almost all treatments. A general conclusion is that from an animal welfare point of view, N2 foam seems equivalent to other gas methods, and better than exposure to high concentrations of CO2’.
3.3.1.1. Quantitative approaches
To ensure that the level of animal welfare when using NEFS is at least equivalent to that in existing stunning and killing methods, a quantitative approach should be adopted, following EFSA guidance (EFSA AHAW Panel, 2018). According to this guidance, the welfare outcome measures that are common to all existing stunning methods can be classified into neurological (e.g. EEG records), physiological (e.g. heart rate variability), behavioural (e.g. escape attempts) or physical reflexes (e.g. clonic/tonic convulsions).
This approach would necessitate that these kinds of data are presented and compared in the dossier for both the submitted method and the other methods already approved under Regulation (EC) 1099/2009. Welfare outcome measures of different classes were obtained from the NEFS dossier and extracted from scientific literature for killing methods for other purposes than slaughter for laying hens, broiler chickens and pigs (ANNEX 1 of Council Regulation (EC) 1099/2009). But not all welfare outcomes (neurological, physiological, behavioural and/or physical reflexes) were provided for the NEFS method for all species and types/production stages of animals.
Time to onset of unconsciousness in CAS methods
Regarding the neurological data, in the answers provided in the dossier to the question of how the visual analysis of EEG can confirm the loss of consciousness, it was stated: ‘The set of neurological indicators of loss of consciousness is described on Table 3 (of the dossier). Loss of posture can rather easily be observed, also under field circumstances, and as such is an important criterion for the stunning process’.
Tables 15, 16 were elaborated by EFSA on the basis of the ones provided in the dossier to summarise the time differences between behavioural loss of posture (LOP) and loss of consciousness (LOC) inferred from neurological data derived from visual inspection of EEG waveforms in poultry (Table 14) and pigs (Table 15), comparing high expansion N2‐filled foam (including NEFS) with controlled atmosphere stunning (CAS) gas methods. Information about turkeys provided as unpublished data was not included in these tables, because of absence of material and method description, too small sample size (n = 4) and absence of peer review.
TABLE 15.
Comparisons of behavioural loss of posture (LOP) and loss of consciousness (LOC) inferred from neurological EEG analysis for laying hens and broiler chickens exposed to high expansion N2‐filled foam (including the NEFS) or controlled atmosphere stunning (CAS) gas methods approved for use in Regulation EC 1099/2009, derived from Table 4 of subsection 3.3.1 of the dossier.
| Study | Subject animals | Killing method | Mean ± SE latency to LOP (s) | Mean ± SE latency to LOC (s) |
|---|---|---|---|---|
| McKeegan, Reimert, et al. ( 2013 ) | Laying hens (n = 12) | High expansion N2‐filled foam |
16 ± 1 a (66a1) |
30±2 a (80a1) (onset of EEG suppression) |
| Slaughter‐weight broilers (n = 10) | High expansion N2‐filled foam |
9 ± 1 a (34a1) |
18 ± 1 a (43a1) (onset of EEG suppression) |
|
| Raj et al. ( 1991 ) | Laying hens (n = 20) | CAS: 90% Argon (Ar) in air, prefilled (O2 2%) | 11 ± 0.4 b |
17 ± 0.9 b (onset of EEG suppression) 29 ± 1.8 b (loss of SEPs) |
| Raj, Wotton, et al. ( 1998 ) | Slaughter‐weight broilers (n = 16) | CAS: 90% Argon (Ar) in air, prefilled (O2 = 1.4%) | No data |
17 ± 1.9 b (onset of EEG suppression) 32 ± 3.1 b (loss of SEPs) |
| McKeegan et al. ( 2007 ) | Slaughter‐weight broilers (Ar n = 4; N2 n = 8) | CAS: 100% Argon (Ar) in air, rapid‐fill OR 100% N2 in air, rapid‐fill | No data |
Ar = 55.0 ± 10.8 b N2 = 45.0 ± 3.2 b (onset of EEG suppression) |
| Lambooij et al. ( 1999 ) | Slaughter‐weight broilers (n = 16) | CAS: 90% Argon (Ar) in air, prefilled | 15.6 ± 3.6 b | No data |
Abbreviation: SEPs, somatosensory evoked potentials in the brain.
Time elapsed is recorded from bird being fully immersed in foam (i.e. does not include fill‐time). Estimated latencies calculated to include the maximum time to foam submersion (i.e. fill‐time/start of process) for laying hens (50s) and broiler chickens (25s) as reported in the study, are within brackets and annotated (a1).
Time elapsed from the beginning of foam formation to the onset of the appearance of the ABM.
TABLE 16.
Comparisons of behavioural loss of posture (LOP) and loss of consciousness (LOC) inferred from neurological EEG analysis in pigs exposed to high expansion N2‐filled foam (including the NEFS) or controlled atmosphere stunning (CAS) gas methods approved for use in Regulation EC 1099/2009, derived from Table 4 of subsection 3.3.1 of the dossier.
| Study | Subject animals | Killing method | Mean ± SE latency to LOP (s) | Mean ± SE latency to LOC (s) |
|---|---|---|---|---|
| Verhoeven et al. ( 2016 ) | Slaughter‐weight pigs (~ 108 kg, n = 24) | CAS: 80% CO2, prefilled | 44 ± 5 a |
47 ± 6 a (onset of slow waves) |
| CAS: 95% CO2, prefilled | 26 ± 5 a |
33 ± 7 a (onset of slow waves) |
||
| Lindahl et al. ( 2020 ) | Grower pigs (~ 28 kg, n = 20) | NEFS | 57.0 ± 2.0 a | No data |
| Raj et al. ( 1997 ) | Grower pigs (~ 38 kg, n = 12) | CAS: 90% Argon (Ar) in air, prefilled (O2 = 1.4%) | No data |
15.2 ± 1.2 a (onset of slow waves) 15.3 ± 1.1 a (loss of SEPs) |
| Sadler et al. ( 2014 ) | Suckling piglets, moribund (n = 6) | CAS: 100% Argon (Ar) in air, prefilled (O2 = 5–7%) | 212 ± 32 a | No data |
| Sadler et al. ( 2014 ) | Suckling piglets, low viability (n = 6) | CAS: 100% Argon (Ar) in air, prefilled (O2 = 5–7%) | 77 ± 29 a | No data |
| Kells, Beausoleil, Johnson, and Sutherland ( 2018 ), Kells, Beausoleil, Sutherland, and Johnson ( 2018 ) | Suckling piglets (n = 5) | CAS: 100% Argon (Ar) in air, prefilled | 20.8 ± 1.6 a |
61.1 ± 4.5 a (onset of slow waves) |
Abbreviation: SEPs, somatosensory evoked potentials in the brain.
Time elapsed from the beginning of foam formation to the onset of the appearance of the ABM.
In general, the data from Tables 15, 16 indicate that behavioural LOP occurs prior to neurological indicators of LOC, and in some cases, the time of LOC can be twice that of LOP (e.g. laying hens exposed to nitrogen foam [McKeegan, Reimert, et al., 2013]). Critically, this highlights that behavioural LOP does not exactly correlate with electrical activity of the brain and therefore may not be a reliable indicator of loss of consciousness for methods which are designed to induce a gradual induction to unconsciousness with modified atmospheres (e.g. gas). Therefore, according to these data, animals could be exposed to negative affective states following LOP and until onset of supressed electrical brain activity (i.e. lose consciousness). In contrast, Raj et al. (1991) performed quantitative analysis of EEG signals from layer hens during exposure to argon‐induced anoxia and reported that the latency to onset of high amplitude and low frequency electrical activity (slow waves; 95% confidence interval 9–12 s) in the brain coincided with the latency to onset of LOP (95% confidence interval 10–12 s). The assessment of electrophysiological brain states (assessed by means of EEG) provides validation of a state of unconsciousness (i.e. LOC) or death (isoelectric) in corroboration with certain behavioural patterns and physical reflexes, therefore allowing behavioural indicators to be used as proxy indicators for states of unconsciousness and death in ‘field’ conditions where EEG recording is not feasible. For instance, the LOP is a behavioural proxy of the onset of unconsciousness and motionless a proxy for death. Loss of consciousness in controlled atmosphere methods is considered a gradual process; therefore, the use of neurophysiological data is beneficial to corroborate behaviour proxies. However, previous studies have reported correlation between behavioural and EEG indicators as occasionally unreliable and that behavioural indicators should be used with care. For example, LOP often occurs prior to EEG suppression or transition to slow‐wave activity in animals exposed to controlled atmosphere (gas) stunning methods (e.g. Martin, Christensen, Vizzier‐Thaxton, and McKeegan, 2016; Martin, Christensen, Vizzier‐Thaxton, Mitchell, and McKeegan, 2016; McKeegan, Reimert, et al., 2013; Verhoeven et al., 2016). Consequently, important consideration that LOP occurs prior to LOC during N2‐filled high expansion foam could therefore mean that animals are conscious for longer and highlights the need for neurological data to validate behavioural indicators.
Welfare consequences during the induction of unconsciousness in CAS methods
Behavioural measures are an informative tool to assess animal welfare. However, generalising findings across studies can be challenging due to heterogeneity in the terminology and descriptions/definitions used for both ethograms and neurological markers across the scientific literature. Consequently, for this Opinion, the terminology and their descriptions have been defined by EFSA for behavioural measures that occurred before LOC, and their associated welfare consequence(s) (Table 17), facilitating generalisation and comparison across studies where they may refer to different terminology, but descriptions are comparable.
TABLE 17.
EFSA defined animal behavioural measures that can be assessed during exposure to high‐expansion N2‐filled foam and NEFS in laying hens, broiler chickens and pigs and their associated welfare consequences. Cross‐referencing to terminology used in McKeegan, Reimert, et al. (2013) (P3) and Lindahl et al. (2020) (P4) are provided.
| Behavioural measure | Relevant animal categories | Definition/Behavioural manifestation | Animal state | References | Related terminology used in P3 and P4 |
|---|---|---|---|---|---|
| Loss of posture (LOP) |
Laying hens Broiler chickens Pigs |
Inability to maintain upright and controlled posture (e.g. standing or sitting) and loss of neck tension resulting in the animal's head resting against a surface (e.g. floor, wall of container or a conspecific) | Early indicator of onset of loss of consciousness, due to cerebral cortex no longer controlling posture | Gent et al. (2020), Gerritzen et al. (2004), Martin, Christensen, Vizzier‐Thaxton, and McKeegan (2016), Martin, Christensen, Vizzier‐Thaxton, Mitchell, and McKeegan (2016), Llonch et al. (2013), Raj and Gregory (1996), Raj et al. (1992); Raj (1999), Raj et al. (1991), Verhoeven et al. (2015) |
P3 – ‘Loss of posture/Ataxia’ P4 – ‘Loss of posture’ |
| Motionless |
Laying hens Broiler chickens Pigs |
No discernible body or breathing movements, and considered the last behaviour observed | Death | EFSA AHAW Panel (2013, 2017), Martin, Christensen, Vizzier‐Thaxton, and McKeegan (2016) |
P3 – ‘Time to cessation of movement’ P4 – Not recorded |
| Head shaking |
Laying hens Broiler chickens Pigs |
Purposeful quick, sudden rotational movements left and right (side to side) isolated to the head, while the animal maintains neck tone/tension. | Pain, fear and/or respiratory distress | Gerritzen et al. (2004), Martin, Christensen, Vizzier‐Thaxton, and McKeegan (2016), Martin, Christensen, Vizzier‐Thaxton, Mitchell, and McKeegan (2016), Raj (1999), Kells, Beausoleil, Johnson, and Sutherland (2018), Kells, Beausoleil, Sutherland, and Johnson (2018) |
P3 – ‘Headshakes’ P4 – Not recorded |
| Open‐mouth/beak breathing |
Laying hens Broiler chickens Pigs |
Deep rhythmic breathing, with mouth/beak open and often with or without neck extension | Respiratory distress | Gerritzen et al. (2004)), Martin, Christensen, Vizzier‐Thaxton, and McKeegan (2016) |
P3 – Not recorded P4 – Not recorded |
| Deep inhalation |
Laying hens Broiler chickens Pigs |
Deep non‐rhythmic inspiration from the mouth may be accompanied by extension of the neck (e.g. gasping) | Respiratory distress | Verhoeven et al. (2016), Martin, Christensen, Vizzier‐Thaxton, and McKeegan (2016), Martin, Christensen, Vizzier‐Thaxton, Mitchell, and McKeegan (2016) |
P3 – ‘Gasping’ P4 – ‘Gasping’ |
| High‐pitch vocalisation |
Laying hens Broiler chickens Pigs |
Single or repeated short and loud voluntary sound(s) at high frequencies (i.e. screaming, alarm call or shriek) | Fear, pain | Manteuffel et al. (2004), Verhoeven et al. (2015), Martin, Christensen, Vizzier‐Thaxton, and McKeegan (2016), Velarde and Dalmau (2012) |
P3 – Not recorded P4 – ‘Vocalisation scream’ |
| Escape attempt |
Laying hens Broiler chickens Pigs |
Purposeful and rapid locomotor behaviour which could include rearing up, climbing or jumping in an attempt to exit the situation | Fear, pain | Graml et al. (2008), Martin, Christensen, Vizzier‐Thaxton, and McKeegan (2016), Martin, Christensen, Vizzier‐Thaxton, Mitchell, and McKeegan (2016), Jongman et al. (2021), Verhoeven et al. (2016) |
P3 – ‘Escape attempt’ P4 – ‘Escape attempt’ |
| Clonic convulsions |
Laying hens Broiler chickens |
Involuntary rapid/vigorous movement of the wings and/or leg movements (i.e. leg paddling) and/or whole body which are associated with uncontrolled muscle contractions, occurring in sporadic bouts | Indicator of unconsciousness | EFSA AHAW Panel (2017), Martin, Christensen, Vizzier‐Thaxton, and McKeegan (2016), Martin, Christensen, Vizzier‐Thaxton, Mitchell, and McKeegan (2016), Erasmus et al. (2010), McKeegan et al. (2007) | P3 – ‘Wing flapping’ |
| Pigs | Involuntary rapid/vigorous movement of legs (i.e. leg paddling) and/or whole body which are associated with uncontrolled muscle contractions. Often occurs in sporadic bouts | Indicator of unconsciousness | EFSA AHAW Panel (2013), Verhoeven et al. (2015, 2016) | P4 – ‘Muscular excitation’ | |
| Tonic convulsions |
Laying hens Broiler chickens Pigs |
Involuntary muscular spasms or twitching accompanied by a rigid whole‐body posture | Indicator of unconsciousness | EFSA AHAW Panel (2017), Martin, Christensen, Vizzier‐Thaxton, and McKeegan (2016), Martin, Christensen, Vizzier‐Thaxton, Mitchell, and McKeegan (2016), Erasmus et al. (2010), McKeegan et al. (2007), Verhoeven et al. (2015, 2016) |
P3 – Not recorded P4 – Not recorded |
All birds exposed to high‐expansion N2‐filled foam in Gerritzen et al. (2010) and McKeegan, Reimert, et al. (2013) were experiencing unconsciousness followed by death. Similarly, all pigs exposed to NEFS as reported in Lindahl et al. (2020) were induced to unconsciousness followed by death. The time to onset of unconsciousness and death based on EEG and behavioural measures are summarised in Table 18 (birds) and Table 19 (pigs).
TABLE 18.
Time to the onset of unconsciousness and death according to the EEG (neurological) and animal behavioural measures (loss of posture and motionless) in laying hens (H) and broiler chickens (B) exposed to high‐expansion N2‐filled foam. Data extracted by EFSA from Gerritzen et al. (2010) and McKeegan, Reimert, et al. (2013).
| Category of animal‐based measure (ABM) | Defined ABM | Welfare consequence | Sample size (n) | Mean (± SE) latency time (s) |
|---|---|---|---|---|
| Neurological | EEG waveform activity (visual assessment only); latency to onset of suppressed EEG containing intermittent slow wave activity (i.e. burst suppression) | Loss of consciousness |
H: 12a B: 10a, 8 a |
H: 30 ± 2a |
| Behavioural | Loss of posture (latency time) | Early indicator of onset of loss of consciousness, due to cerebral cortex no longer controlling posture |
H: 12a B: 10a, 8 a |
H: 16 ± 1 b |
| Neurological | EEG waveform activity (visual assessment only); latency to isoelectric EEG |
Latency to onset of a suppressed EEG as an indicator of onset unconsciousness Brain death: characterised by lack of EEG activity/electrical activity of the brain |
H: 12a B: 10a, 8 a |
H: 65 ± 3 b |
| Behavioural | Motionless (latency time) | Death |
H: 12a B: 10a, 8 a |
H: 65 ± 3a B: 51 ± 2a, 106±5 a |
TABLE 19.
Time to the onset of loss of consciousness and death according to animal behavioural measures (loss of posture and motionless) in pigs exposed to NEFS. Data extracted from Lindahl et al. (2020) by EFSA.
| Category of animal‐based measure (ABM) | Defined ABM | Animal welfare consequence | Sample size (n) | Mean (±SE) latency time (s) |
|---|---|---|---|---|
| Behavioural | Loss of posture (latency) | Early indicator of onset of loss of consciousness, due to cerebral cortex no longer controlling posture | 20a | 57.0 ± 2.0a |
| Behavioural | Motionless (latency) | Death | 20a | $ a |
Note: The symbol ($) represents where data could be not extracted/was not reported in the studies but was included in the text description as being observed.
Data extracted from Lindahl et al. (2020).
Additional behavioural measures occurring prior to loss of consciousness (e.g. head shaking) are shown in Table 20 (birds) and Table 21 (pigs). It is important to note that the results reported in McKeegan, Reimert, et al. (2013) are latencies to ABMs following birds being fully submerged in foam and do not include the foam fill time (therefore, the time is under‐estimated). The study reports time ranges for foam submersion (i.e. fill‐time) for both laying hens (approx. 25–50s) and broiler chickens (approx. 10–25s). Therefore, the latencies reported from this study in Table 18 and Table 20 should be considered with the addition of these ranges to provide an accurate duration of time birds are exposed to foam (from foam production starting) and the potential for negative animal welfare outcomes. Furthermore, except for loss of posture, the behaviours observed from the Lindahl et al. (2020) study were recorded by 1/0‐sampling within 10‐s intervals, where a pig was recorded as performing a behaviour at least once during a 10‐s interval, with results reported as the proportion of pigs showing this behaviour within a 10‐s interval, therefore rendering the exact latency of events uncertain.
TABLE 20.
Proportion of laying hens (H) and broiler chickens (B) showing behavioural animal‐based measures (ABMs), including escape attempts, head shaking and deep inhalation, when exposed to high‐expansion N2‐filled foam. Data extracted by EFSA from Gerritzen et al. (2010) and McKeegan, Reimert, et al. (2013).
| Behavioural ABM | Proportion of birds displaying behaviour | Mean (±SE) latency (s) | ||
|---|---|---|---|---|
| N2‐filled foam | Air‐filled foam (control) | N2‐filled foam | Air‐filled foam (control) | |
| Escape attempts |
H: 2/12a B: 0/10a, $/8 a |
H: 1/8a B: 0/10a |
H: $a B: – b , $b |
H: $a B: – b |
| Head shaking |
H: $/12a B: $/10a, 0/8b |
H: $/8a B: $/10a |
H: 2 ± 1 b |
H: $a B: $a |
| Deep inhalation |
H: 0/12a B: 0/10a, 1/8b |
H: 0/8a B: 0/10a |
H: – b |
H: – b B: – b |
Note: The symbol ($) represents where data could not be extracted/was not reported in the published studies but was included in the text description as being observed; while a dash (−) represents where data are unavailable/not relevant as no animal performed this behaviour.
Data extracted from Gerritzen et al. (2010). Time elapsed from the beginning of foam formation to the onset of the appearance of the ABM.
Data extracted from McKeegan, Reimert, et al. (2013). Note: Time elapsed is recorded from bird being immersed in foam (does not include fill‐time).
TABLE 21.
Proportion of pigs showing behavioural animal‐based measures (ABMs), including escape attempts, deep inhalation, convulsions and high‐pitch vocalisations when exposed to NEFS. Data extracted from Lindahl et al. (2020) by EFSA.
| Behavioural ABM | Proportion of pigs displaying behaviour | |
|---|---|---|
| N2‐filled foam | Air‐filled foam (control) | |
| Escape attempts | 16/20 a | 16/20 a |
| Deep inhalation | 0/20 a | 0/20 a |
| Clonic convulsions | 20/20 a | 0/20 a |
| High‐pitch vocalisations | 19/20 a | 9/20 a |
Data extracted from Lindahl et al. (2020).
It should be noted that in Table 19, there is considerable data missing, which was not reported in the published studies, impairing the complete assessment of the welfare impact for animals during induction of loss of consciousness.
In summary, where possible based on ethogram descriptions provided in the published studies, EFSA have devised a comprehensive ethogram (Table 16), which allows for comparison across killing methods. However, it should be noted that there is variation in gas mixture composition and fill methodology (e.g. gradual vs. pre‐fill), which should be considered when making comparisons of ABMs reported (especially behavioural latencies). When no EEG data are available, LOP provides a reasonable behavioural indicator of the early onset of unconsciousness and a critical measure to compare high expansion N2‐filled foam or NEFS with other gas methods. However, in general, LOP occurs prior to LOC (derived from EEG assessment), particularly in the case of CAS methods. LOP is only feasible to accurately record and observe in freely moving/unrestrained animals. Given the marked number of ABMs which have not been reported or measured across multiple studies, a complete quantitative assessment was not possible. Factors contributing to this information gap include:
Approval of all currently available stunning methods by the European Commission pre‐dated the EFSA guidance, leading to inconsistent and non‐harmonised data recording methodology and presentation across studies about already approved methods.
The substantial procedural heterogeneity of existing killing methods. However, a conceptual comparison between NEFS/high expansion N2‐filled foam and gas stunning methods is considered valid due to their procedural similarities.
From an animal welfare perspective, the assessment of N2‐filled high expansion foam (including NEFS) is further challenged given the nature of foam (i.e. opaque), which greatly reduces visibility of the animals and therefore prevents detailed observation of the animals, and therefore, behavioural assessment is limited. Subtle behaviours which may be indicative of aversion (e.g. head shaking, blinking, deep inhalations, etc.) are difficult to assess, and therefore, lack of observation may not infer lack of aversion. The available video footage highlighted this concern and demonstrated that clear observation of animals is hindered due to foam opacity. Therefore, new procedures need to be developed to improve the possibility for monitoring of animals when exposed to N2‐filled high expansion foam (including NEFS).
There are only two peer‐reviewed published studies evaluating N2‐filled high expansion foam (including NEFS) in broilers, laying hens and pigs, which means there is also a lack of replication of the results, as well as no evidence to support this method's use in specific animal categories (e.g. age/production stage) or validation in field trials, unlike with other CAS methods. Furthermore, the presentation and reporting of results in McKeegan, Reimert, et al. (2013) and Lindahl et al. (2020) is incomplete, with many text statements not including the quantitative data to support them (e.g. % of birds performing escape attempts or deep inhalations).
3.3.1.2. Qualitative approaches
The dossier does not contain any qualitative approach.
3.3.2. Overall discussion and conclusions (outcome of dossier assessment)
The dossier provided summaries of the results from the two published studies submitted and synthesised the results with the wider scientific literature to draw conclusions. The independent assessment was performed by EFSA in Section 4.2.
3.3.2.1. Results regarding welfare impact
In assessing the impact of CAS methods (including high expansion foam) on animal welfare, the applicant considers the following three aspects:
the start‐up period for inserting gas (or foam) and the onset of stunning (as for example shown in a transient EEG)
the onset of loss of posture, where in an early stage it is difficult to discern between slipping and trying to stand up, loss of posture and convulsions after loss of consciousness
the time lapse between loss of posture and loss of consciousness, that is often difficult to ascertain because of artefact due to muscular activity.
An important point of discussion presented in the dossier is what happens between loss of posture and loss of consciousness. In the field, simple landmark behaviours (e.g. loss of posture (LOP)) can be observed, but it is unclear whether an animal experiences pain, distress and suffering in that period of time (prior to LOP). No matter how short this period may be (in an order of magnitude of 10 s), this may influence the conclusion regarding equivalence with other methods.
In pigs, Lindahl et al. (2020) concluded that: ‘The scientific evidence to support LOP as an indicator of unconsciousness in pigs is inconclusive. Verhoeven et al. showed that loss of posture was on average 10s before electroencephalography (EEG)‐based loss of consciousness in 80% and 95% CO2 concentrations. Both Rodriguez et al., 2008 and Verhoeven et al., 2016 found that muscle contractions occurred before significant changes in brain function appeared, which could possibly indicate that pigs were conscious during this period. Convulsions are not usually a cause for concern from a welfare perspective if they occur after unconsciousness, but since the onset of involuntary muscle contractions has been shown to start before, during and after the loss of consciousness in different studies, this is an issue that needs further attention in research. Studies have shown that stunning with inert gases induces more severe convulsions compared to CO2 stunning, but how this relates to loss of consciousness and animal welfare is not known’.
An earlier conclusion by Raj et al. (1997) also in pigs: ‘Based on the time to loss of Somatosensory Evoked Potentials (SEPs), it is concluded that during killing with a high concentration of carbon dioxide, pigs would have to endure a moderate to severe respiratory distress induced with this gas for a considerable period of time prior to the loss of brain responsiveness. Argon‐induced anoxia appears to be the first choice from a welfare point of view for killing pigs, based on its lack of aversive properties and its effectiveness in rapidly abolishing brain responsiveness’.
In laying hens stunned with CO2, transient EEG were found before loss of posture and such EEG patterns are likely to be found in pigs as well (after all, the behaviour LOP will be caused by transitions in brain events/cognitive impairment). Again Raj and Gregory (1990): ‘It is concluded that the birds had not lost the primary response in their SEPs by the time they started convulsing, but the reduction in the amplitude of the SEPs, changes in their spontaneous EEG and a negative response to comb pinch before the start of the convulsions indicated that the birds were unconscious when they convulsed’.
It remains to be seen however, whether this difference between LOP and LOC, as found by several authors, has any real meaning for animal welfare. It may also be an artefact wholly or partly caused by the fact that when an animal drops down at LOP, the shock may disturb the EEG signal. It will take seconds for the EEG signal to stabilise so that LOC can be concluded. Furthermore, EEG measurements cannot be used in practice/in the field, and LOP is unanimously accepted as the best approximation of LOC. Whatever is happening between loss of posture and loss of consciousness, available data do not suggest a difference between inert gases alone, or foam filled with either inert gases and carbon dioxide in this matter. As such, this issue does not influence the conclusion that inert foam stunning is at least equivalent to (pre‐filled) gas carbon dioxide stunning on a welfare ground. As illustrated by Lindahl et al. (2020), the use of foam has advantages as the desired oxygen concentration is reached quickly and efficiently (factor 3 quicker). Therefore, it seems advantageous in terms of animal welfare to use foam, if the foam in itself does not impact the welfare of the animal.
In summary, according to the dossier, the two core studies show:
Flushing a container with inert gases will result in a period of hypoxia and longer times to loss of posture and loss of consciousness than encapsulating nitrogen in foam, due to the rapid transition to an anoxic atmosphere.
Stunning with inert gases can only be done in humane way by an almost instantaneous transfer from atmospheric air to anoxic conditions. With argon, this can be done by immersion in a well, but this is not feasible for on‐farm killing of animals that will not be slaughtered. With nitrogen, an instantaneous transfer can only be obtained by filling a container with nitrogen foam to expel all air.
Inserting an animal in a container prefilled with high expansion foam will result in an air tunnel requiring additional foaming.
Pigs and poultry may show a slight aversion to foam, but their aversion seems less than the aversion against carbon dioxide.
Inert gases cause less respiratory distress than carbon dioxide as shown by the absence or occurrence of gasping before loss of consciousness.
The timescale of loss of posture and loss of consciousness in inert gases (prefill or foam) is like that in carbon dioxide (prefill). In both cases, loss of posture can be used as a practical approximation of loss of consciousness. The time lapse between loss of posture and loss of consciousness is similar between poultry and pigs and is not influenced by the type of gas used in stunning.
Commentary from the WG: These conclusions are provided in the dossier and do not represent the views of EFSA. The independent assessment was performed by EFSA in Section 4.2. The conclusions drawn are limited and include assumptions where in some incidents no evidence is provided. The reported time differences between LOP and LOC derived from EEG is highly dependent on the killing/stunning method and therefore equivalence between methods should not be assumed. Furthermore, the overreliance on LOP to form conclusions is questionable, as it does not provide consideration of welfare impacts associated with other behavioural indicators observed (e.g. escape attempts, animals slipping, ataxia and head shaking) which could be indicative that either the foam and/or the inert gas cause negative welfare consequences. Finally, published evidence has only been provided for slaughter‐weight broilers, laying hens and ~ 30 kg weaned pigs. Therefore, there is no evidence supporting the use of NEFS in other production stages of these animals or in other poultry species (e.g. turkeys). A potential risk for extrapolating results to these other animal groups could be that the foam bubble size may be inappropriate and could lead to occlusion (even partial) of airways and sways and severe respiratory distress. Further studies are required to validate its efficacy and animal welfare consequences in these animal groups, which should include behavioural and EEG derived indicators of consciousness as well as necropsy results including histopathology.
3.3.2.2. External validity
It is stated in the dossier that ‘practical demonstrations of stunning 3‐4 kg ducks were held in France in November 2021 to experts from public and private organisations in the framework of Avian Influenza emergency intervention’ and ‘practical experiences with N2 foam were obtained during emergency killings of doves, geese, ducks that all showed LOP and end of movement on the same time scale’. However, no methodological details of these field trials or data on the ABM outcomes were provided.
Additionally, it is stated in the dossier that the method has been successfully applied to neonate piglets and to finisher pigs of 140 kg, and to day‐old chicks and adult turkeys. No summary data and no detailed data are provided, and no description of the methodology and procedure are provided.
Field trials have been carried out in four European countries, involving piglets, adult pigs, layer hens, broilers, day‐old chicks and ducks, and reported in the submitted dossier to be successful (see also Section 4.1.3).
The dossier notes that field studies with moribund animals require large numbers because of the inherent heterogeneity of the population. Balzer (2017) found in moribund piglets (n = 41) that the latency from the time the animals were placed in the nitrogen gas filled foam until the end of the vocalisations was 21.67 (± 15.45) seconds and similar to the data collected by Sadler et al. (2014) for the displacement gas argon. This was 2–10 times longer compared to the use of CO2 in the studies by Sutherland et al. (2017) and Sadler et al. (2014). According to Balzer (2017), the use of 100% nitrogen in foam seems to prolong the phase of initiation associated with screaming vocalisations that can be evaluated aversively. The application of 100% foam‐bound nitrogen appears to result in similar latency to last movement as a 100% CO2 atmosphere, and to be similarly efficient in terms of the animal's movement response.
3.3.2.3. Discussion on equivalence with existing methods
The dossier concludes with the following summary:
Based on the time to loss of consciousness for the different gases and species of animals' exposure to nitrogen‐filled high expansion foam is equivalent to exposure to carbon dioxide.
There are some signs of aversion to the foam itself in both pigs and poultry demonstrated by behaviours interpreted as signs of aversion, e.g. escape attempts and/or vocalising. As gasping is absent before loss of consciousness in birds, there is a welfare benefit of using nitrogen‐filled high expansion foam over exposure to carbon dioxide.
The use of nitrogen‐filled high expansion foam to displace atmospheric air and create anoxic conditions necessary to kill animals is faster and more reliable than flushing a container with nitrogen gas.
Nitrogen foam stunning seems to be applicable to a range of weights in pigs, and to a range of species in poultry.
Commentary from the WG: These conclusions are provided in the dossier and at least some of them do not represent the views of EFSA. The independent assessment was performed by EFSA in Section 4.2.
4. ASSESSMENT PHASE 2: RISK ASSESSMENT OF THE HIGH EXPANSION FOAM STUNNING METHOD
In this phase, the AHAW Panel assessed the NEFS method. Two main aspects were characterised following the EFSA guidance (EFSA AHAW Panel, 2018): (i) the animal welfare risk assessment based on the analysis of the outcomes resulting from the killing method and (ii) the validation of the equivalence of the proposed stunning/killing methods with existing approved methods. A similar process was also followed for the assessment of the LAPS method (EFSA AHAW Panel, 2017).
Although the dossier provided only partially met the requirements under the EFSA Guidance 2018 (ToR1), EFSA considered the proposed method for further evaluation as per Council Regulation (EC) 1099/2009 to assess whether this method can provide a level of animal welfare at least equivalent with the existing killing methods (ToR2). Indeed, there was sufficient information in the dossier to perform assessment phase two, particularly about pain, distress or suffering caused to certain types of pigs and poultry exposed to the NEFS. Additionally, given the rising threat of diseases becoming endemic in Europe (e.g. Highly Pathogenic Avian Influenza, African Swine Fever) and the current state where existing methods are not always being feasible under certain farming conditions, new developed methods for killing entire flocks of animals are urgently needed.
4.1. Animal welfare risk assessment
The claim in the dossier (Annex C) to use NEFS in pigs from neonates till 80 kg pigs is not supported by the evidence provided (e.g. Lindahl et al., 2020). Therefore, assessment of the NEFS in comparison with other methods of killing on‐farm for pigs only concerns pigs between 15 and 41 kg of body weight, such as the ones used by Lindahl et al. (2020). This does not mean that the welfare consequences induced by the use of NEFS are different for heavier or lighter pigs, but it means that EFSA was not able to perform the assessment on other categories of pigs.
According to EFSA guidelines (2018), for the assessment of pain, distress and suffering and the onset and duration of unconsciousness or death, the measures chosen in the submitted dossier will be scrutinised in terms of validity and reliability. This will be done based on the justification provided by the applicant concerning the choice of the measures. The measures will be compared with the scientific state of the art, taking as far as possible species, animal category and breed/genetic lines into account.
4.1.1. Assessment of onset and duration of unconsciousness
The assessment of NEFS involves evaluation of the methodology and criteria used for determining unconsciousness and death. In addition, results of the welfare outcomes are scrutinised.
4.1.1.1. Methodological aspects
In this section, the methodologies used in the evaluation of NEFS are assessed for validity and reliability, including the criteria and the thresholds used for the determination of unconsciousness. Specifically, the brain mechanisms involved in inducing unconsciousness and the scientific rationale used in the selection of the neurological measures are examined. Furthermore, the use of the behavioural (e.g. loss of posture) and physical (lack of response to comb pitching in poultry or lack of response to nose prick in pigs) reflexes selected for assessment of unconsciousness is reviewed. Finally, the approach used to establish the association between neurological measures and other ABMs is evaluated.
Neurological measures
According to the EFSA guidelines (EFSA AHAW Panel, 2018), it is recommended that an EEG method is used to determine the onset and the duration of unconsciousness as well as the time to onset of death, because these have previously been accepted in internationally recognised and peer‐reviewed journals and can better justify the effectiveness of a stunning method. Even though EEG data are best to be provided, they are not mandatory for the assessment of the above and other ABMs can be used instead. Additionally, measures must be taken to eliminate potential bias. In this regard, the neurophysiological basis of induction of unconsciousness and death during exposure of pigs and poultry to inert gases, especially argon has been extensively reviewed (see EFSA, 2004). However, due to the fact that NEFS is a killing procedure intended to induce death in all animals and does not require neck‐cut and exsanguination to induce death, the duration of unconsciousness was not reported in the dossier, and the stun‐to‐stick interval is not relevant, since there is no simple stunning where the animals may recover consciousness.
Behavioural and physical reflexes for determination of unconsciousness
The time to LOP has been used as a behavioural indicator of unconsciousness during exposure of pigs and poultry to gas mixtures (EFSA, 2004). Similarly, the absence of muscle tone (e.g. tension in neck muscles or those controlling lower mandibles) or response to nociceptive stimulus (e.g. pin prick to snout in pigs or comb pinching in poultry) have been used as indicators of unconsciousness following the application of a stunning procedure in pigs and poultry (see EFSA, 2004). In the two scientific publications submitted as evidence to support the application, the time to LOP has been used to determine the time to onset of unconsciousness in poultry (McKeegan, Reimert, et al., 2013) and pigs (Lindahl et al., 2020).
Association between neurological measures and other ABMs
There is no evidence of a direct association between LOC determined by EEG and LOP in any study provided. McKeegan, Reimert, et al. (2013) recorded EEGs in hens and broilers to determine the time to onset of unconsciousness but did not correlate changes occurring in the EEG with the behavioural data, and Lindahl et al. (2020) did not record EEG. Nevertheless, scientific research on the use of CAS methods has concluded that when EEG cannot be quantified, LOP or onset of anoxic convulsions can be used as a behavioural indicator of the onset of unconsciousness, and therefore, LOP is still a valuable measure for comparing the speed of induction of unconsciousness between NEFS and other killing methods.
4.1.1.2. Results regarding onset and duration of unconsciousness and death
The assessment of the effectiveness of NEFS in relation to unconsciousness and death should consider the following variables: frequency of correctly stunned animals and time to onset of unconsciousness as well as time to death.
NEFS can be considered an effective method in poultry (i.e. broiler chickens and laying hens) and pigs (15–41 kg body weight), since all animals exposed to NEFS were rendered unconscious followed by death. Moreover, if all the necessary preventive measures are taken, any potential WCs can be avoided (see Section 4.3).
To ensure death of animals, exposure time should last a minimum of 5 min for poultry and 7 min for pigs from the time the animals are fully exposed to anoxia (i.e. less than 2% residual oxygen in nitrogen) after the destruction of the foam with nitrogen pulse. However, it is important to note that the risk of negative welfare consequences begins when the animals come in contact with the foam; therefore, it is crucial to consider the duration of exposure to the foam and the effects on animal welfare.
4.1.2. Assessment of pain, distress or suffering associated with the pre‐stunning process, during induction of unconsciousness and due to mis‐stunning
4.1.2.1. Methodological aspects
The method should be standardised and maximum thresholds set to minimise any delays. Submitted video footage (e.g. video 23) suggests that following the nitrogen gas pulse was initiated, it took ~ 20 s to establish an anoxic environment (< 2% oxygen). However, the accuracy of this estimate is limited based on technical challenges with the oxygen monitors and potential delays in recordings. Following the physical destruction of the foam, an anoxic environment (< 2% oxygen) should be established immediately after the administration of the nitrogen pulse for 2″.
The WG identified key hazards associated with NEFS (Table 22) as well as the welfare consequences and ABMs based on the background scientific literature (Table 23 for poultry and Table 24 for pigs).
The dossier provided by the applicant was scrutinised to assess the validity and reliability of data used to ascertain pain, fear, distress and/or suffering in broiler chickens and laying hens of all ages and pigs. The working group identified several inadequacies/inconsistencies in the scientific papers provided in the dossier in support of the method and the important ones related to the hazards are listed below:
The absence of control groups of animals given analgesic treatment in the design of experiments in these studies does not allow the determination of the duration and/or intensity of pain.
There is no information on the effect of the pre‐stunning process or the effect of NEFS during induction of unconsciousness on physiological measures of blood (e.g. cortisol, lactate, glucose). Therefore, no comparison between the methods of killing can be made on the basis of physiological responses known to be indicators of stress.
In addition, the fact that animals cannot be clearly observed during the induction phase to unconsciousness in NEFS, i.e. the animals are obscured by the surrounding foam, may impair the accuracy of behavioural assessment.
The use of a limited ethogram for occurrences of ABMs is leading to poor precision of observation.
Behavioural indicators of aversion (ABMs) could elucidate the assessment of the magnitude of pain, fear, distress or suffering. In this regard, both scientific papers reported escape attempts, indicating aversion to the presence of foam, however, the latency to onset and duration of occurrence of aversive reactions could not be estimated either due to the fact that the latency to envelop the animals with the foam varied considerably.
4.1.2.2. Evidence of pain, distress or suffering in high expansion foam method (ToR 2i)
The potential sources of pain, distress and suffering during exposure to NEFS are described in detail under Section 3. Consequently, the emphasis in this section will be on the evidence submitted by the applicant to ascertain these animal welfare consequences.
According to the EFSA guidelines (2018), two criteria/rules must be fulfilled before a stunning method is considered not to induce avoidable pain, distress or suffering prior to the onset of unconsciousness and insensibility:
The ABMs chosen in the submitted dossier should not indicate a greater magnitude of pain, fear, distress or suffering in the treatment group compared to the appropriate control group.
The response of animals exposed to the procedure/apparatus without the application of stunning (control or sham operation) should not be different from the response of the animals exposed to the procedure/apparatus with stunning (treatment).
In general, the outcomes of the different ABMs on an individual animal should point into the same direction, allowing for a clear interpretation about the consequence on animal welfare. If there is evidence that the method leads to pain, distress or suffering, the evaluation will be based on the proportion of animals affected as well as, where possible, the severity of the infliction and the duration of the negative experience. For this purpose, the existing literature and/or expert opinion will be used to aid in data interpretation.
McKeegan, Reimert, et al. (2013) reported that broilers (n = 10) and hens (n = 8) exposed to air‐filled high expansion foam exhibited head shaking (number of head shakes = 2 in total of each animal category). The authors also reported that 2 out of 12 hens exposed to nitrogen‐filled high expansion foam exhibited escape attempts and none of the broilers (n = 10). The difference between broilers and hens can hardly be considered as significant. The fact that layer hens had slower rates of exposure to the foam (prolonged fill time) compared to broilers could also explain the presence of escape attempts in hens. This implies that the foam production rate and container filling time are critical and therefore included as key parameters in the dossier submitted by the applicant. Head shaking behaviour occurring twice during induction of unconsciousness with the air‐filled high expansion foam by McKeegan, Reimert, et al. (2013) could be seen as an initial response to mucosal irritation and/or foam entering the nostrils and/or mouth. Since the behaviour occurred only twice during the induction of unconsciousness, it is unclear if the foam is really irritative for the mucosa, and therefore, the uncertainty associated with this interpretation is high. The fact that only few animals showed escape attempts and head shaking indicates a lack of strong evidence to infer the exposure to the foam causes pain, fear, distress or suffering.
The results reported by Lindahl et al. (2020) indicated that 3 of 20 piglets exposed to atmospheric air, and 16 of 20 piglets exposed to air‐filled high expansion foam and 16 of the 20 piglets exposed to nitrogen filled high expansion foam exhibited escape attempts. The authors stated that the escape attempts increased approximately 30–40 s after the start of foam production, indicating that the foam was most aversive to pigs when starting to cover their heads. It is not certain, due to the lack of a control group of animals given analgesic for example, whether exposure to the foam causes pain, but escape attempts indicate they are experiencing fear (same proportion of escape attempt for air and nitrogen filled foam). Another hazard reported in this study is slipping due to the foam on the plexiglass floor and these could lead to injury. Ideally, the floor should have been made of an effective anti‐slip material, but the video recording from the bottom required plexiglass. Sitting posture can occur in pigs affected by respiratory disease/distress. This behaviour was reported in 10%, 40% and 60% of the pigs exposed to air, air‐filled foam and nitrogen‐filled foam, respectively. These findings suggested more suspicions of respiratory distress in air‐filled foam and N2‐filled foam. However, since none of the pigs exposed to nitrogen‐filled foam exhibited gasping, the authors concluded that respiratory distress did not occur. It is also worth noting that foam, by obstructing the view, can also lead to fear, even in the absence of irritative characteristics of the foam itself.
4.1.3. Assessment of external validity
In this part of the assessment, it is foreseen to consider to which degree the findings from laboratory studies are consistent with those studies carried out under commercial conditions.
■■■■■ However, no experimental details or the animal welfare outcomes (failures) were disclosed.
■■■■■
4.2. Assessment of equivalence of the method with approved stunning methods (TOR 2ii)
To compare NEFS with other authorised killing methods used for purposes other than slaughter, a scientific literature review was conducted initially, to gather data on neurological and behavioural indicators of onset of unconsciousness and death in poultry and pigs.
4.2.1. Quantitative assessment
The methodology for quantitative assessment is described in Section 2.2.3.1. In poultry, a summary of the times to onset of unconsciousness and death measured by brain activity (EEG) and behaviour according to different authorised killing methods that were compared under this chapter is presented in Tables 25, 26. These tables, based on different published studies, also include a summary of the onset of behaviours suggestive of pain, distress or suffering. The negative welfare consequences will persist until loss of consciousness as indicated by the ABMs such as LOP or onset of anoxic convulsions on unconscious animals (e.g. wing flapping in chickens). If we focus on the methodology used in the studies, there is variability in the method used to analyse EEG and the terminology and description of the behavioural indicators assessed. Furthermore, the study referred to as whole house gassing (Gerritzen et al., 2004) was not done in commercial conditions (so not real whole house). Actually, the study took place as a laboratory simulation, in a room with 7.4 × 4.5 m of floor space and a height of 2.7 m where a cage of 3 × 1 × 1 m (length × width × height) was placed and divided into three sections of 1 × 1 m each with a stocking density of 20 broiler chickens/m2 to simulate the stocking densities typically found under commercial conditions. Additionally, comparison across studies to determine LOC is highly dependent on the rate of gas delivery and latency to achieving less than 2% O2 or at least 45% CO2 at the bird level, which is variable among studies.
TABLE 25.
Comparison of high expansion N2‐filled foam with other authorised controlled atmosphere stunning (CAS) and killing methods available for laying hens (H) and broiler chickens (B) for other purposes than slaughter in relation to the time appearing in different ABMs.
| ABMs | Mean ± SE latencies of ABMs in seconds (s) and percentage of birds performing the behavioural ABM are reported in brackets (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Bird type | High expansion N2‐filled foam | CO2 at high concentrations | CO2 in whole house gassing (WHG) | CO2 in two phases in containers | CO2 + Inert gases WHG or in containers | Inert gases in containers | LAPS | |
| Loss of posture | H | 16 ± 1 a , 66a1 (100%) |
35.8 ± $ (100%)e2 34.9 ± $ (100%)e3 |
170 ± $ (100%) g | NA |
31.9 ± $ (100%)e4 11 ± 0.6 (100%)q2 |
35.4 ± $ (100%)e5 11 ± 0.6 (100%) o |
NA |
| B |
9 ± 1 a ,34a1 (100%) 48 ± 7 b (100%) |
37.9 ± $ (100%)e2 35.4 ± $ (100%)e3 17 ± 5 (100%)h1 |
172 ± $ (100%)p1 |
NA |
28.5 ± $ (100%)e4 12 ± 2 (100%)h2 123.5 (100%)j2 16.7 ± 1.3 (100%)k2 669 ± $ (100%)p2 (WHG) |
30.7 ± $ (100%)e5 110.5 (100%)j1 15.6 ± 0.9 (100%)k1 |
86.6 ± 2.8 (100%) f 57.2 ± 0.9 (100%)m3 62.3 ± 1.1 (100%)m1 55.9 ± 1.2 (100%)m2 |
|
| Loss of consciousness (onset of EEG suppression) | H | 30 ± 2 a , 80a1 | 11 ± 1.3 q3 | 469.7 ± 156.6 l | NA | 11 ± 0.6q2 |
17 ± 0.9 o 17 ± 1.0q4 |
NA |
| B |
18 ± 1 a , 53a1 51 ± 12 b |
NA |
183 ± 39p1 |
60±23 i |
26.0 ± 1.9d3 23.2 ± 4.6d4 19 ± 1.9n2 730 ± 135 p2 (WHG) |
45.0 ± 3.2d2 55.0 ± 10.8d1 17 ± 1.9n1 |
53.6 ± 11.8m2 24.5 ± 3.0m1 |
|
| Motionless | H | 65 ± 3 a , 115a1 (100%) | NA |
934.1 ± 311.4 (100%) l |
NA | 60.0 ± $ (100%)q1 | NA | NA |
| B |
51 ± 2 a , 76a1 (100%) 106 ± 5 b (100%) |
NA |
700 ± $ (100%)p1 |
NA |
124.1 ± 62.1 (100%)j1 1090 ± $ (100%) p2 (WHG) |
194.3 ± 59.5 (100%)j1 |
141.2 ± 2.7 (100%) f 144.0 ± 2.9 (100%)m3 153.9 ± 2.6 (100%)m1 145.2 ± 3.3 (100%)m2 |
|
| Death (onset of isoelectric EEG) | H | 65 ± 3 a , 115a1 | 76 ± 1.4q3 | NA | NA |
58 ± $ q1 50 ± 2.2 (100%)q2 |
62 ± 1.7 o 62 ± 1.9q4 |
NA |
| B |
47 ± 2 a , 72a1 92 ± 6 b |
NA | NA | 247±64 i |
70.7 ± 6.5d3 67.8.0 ± 4.6d4 34.3 ± 12.1j1 41 ± 2.1n2 |
76.7 ± 4.4d2 74.7 ± 12.9d1 48.9 ± 10.4j1 58 ± 2.3n1 |
87.0 ± 9.2m1 101.6 ± 6.1m2 |
|
|
Ataxia |
H | NA |
17.8 ± $ (60%)e2 22.4 ± $ (50%)e3 |
125 ± $ ($%) g | NA | 23.6 ± $ (80%)e4 | $ (30%)e5 | NA |
| B | NA |
19.0 ± $ (80%)e2 15.4 ± $ (80%)e3 |
NA | NA | 19.5 ± $ (100%)e4 | 28.2 ± $ (90%)e5 |
41.8 ± 1.0 (100%)m3 43.3 ± 1.4 (100%)m1 38.3 ± 1.3 (100%)m2 |
|
| Head shaking | H | 2 ± 1 a , 52a1 ($%) |
11.2 ± $ (100%)e2 9.2 ± $ (100%)e3 |
70 ± $ ($%) g | NA | 11.5 ± $ (100%)e4 | 24.2 ± $ (10%)e5 | NA |
| B |
6.4 ± $ (90%)e2 6.7 ± $ (80%)e3 3 ± 3 (91.7%)h1 |
37 ± $ (100%)p1 | 18 ± 2 ($%) i |
$ (12.5%)d3 $ (75%)d4 15.4$ (100%)e4 3 ± 2 (93.3%)h2 87 ± $ (100%) p2 (WHG) |
22.9 ± $ (100%)e5 8 ± 1 (87.5%)k1 |
40.5 ± 3.4 (68%)m3 47.2 ± 4.2 (53.2%)m1 28.7 ± 7.5 (20%)m2 |
||
|
Clonic convulsions |
H | 18 ± 1 a , 68a1 ($%) | 28 ± 3.3 (100%)q3 | 188.7 ± 62.9 (100%) l |
17.1 ± $ (100%)q1 21 ± 1.4 (100%)q2 |
22 ± 1.2 (100%) o 22 ± 0.8 (100%)q4 |
NA |
|
| B | 15 ± 2 a , 40a1 ($%), 50 ± 24 b ($%) | 19 ± 7 (100%)h1 | 177 ± $ (100%)p1 | 304 ($%) i |
15 ± 2 (100%)h2 10.0 ($%)j2 359 ± $ (100%)p1 |
15.7 ± $(100%)d2, 17.5 ± $(100%)d1 12.0 ($%)j1 |
78.2 ± 3.3 (100%)m3 71.0 ± 2.6 (100%)m1 63.8 ± 1.4 (100%)m2 |
|
| Tonic convulsions | H | NA | NA | NA | NA |
35.0 ± $ (100%)q1 33 ± 1.3 (100%)q2 |
35 ± 1.4 (100%) o | NA |
| B | NA | NA | NA | NA |
119.1 ± 3.5 (79%)m3 115.7 ± 4.5 (70.5%)m1 105.0 ± 3.8 (100%)m2 |
|||
| Deep inhalation | H | $, a (0%) | NA |
189.1 ± 63.0 (100%) l |
NA | NA | NA | NA |
| B |
50 ± 24 b (12.5%) |
6 ± 3 (66.7%)h1 46 ± $ (100%)p1 |
46 ± $ (100%)p1 |
21 ± 1 ($%) i |
5 ± 2 (93.3%)h2 4 ± 1 (73.3%)k2 112 ± $ (100%) p2 (WHG) |
9 ± 0 (6.3%)k1 |
82.5 ± 7.1 (63%)m3 80.7 ± 9.0 (33.3%)m1 64.0 ± 3.9 (40%)m2 |
|
| Mandibulation | H | NA |
6.0 ± $ (100%)e2 6.1 ± $ (100%)e3 |
NA | NA |
8.8 ± $ (100%)e4 |
$ (10%)e5 | NA |
| B | NA |
4.2 ± $ (100%)e2 4.4 ± $ (100%)e3 |
NA | NA |
5.4 ± $ (100%)e4 |
9.3 ± $ (60%)e5 $ (0%)d2 $ (0%)d1 |
18.8 ± 3.7 (44%)m3 29.5 ± 3.2 (54.4%)m1 20.1 ± 2.3 (60%)m2 |
|
| Escape attempts | H | $, a (16.7%) | NA | NA | NA | NA | NA | NA |
| B | NA | NA | NA | NA | NA |
$ (0%)m3 $ (0%)m1 $ (0%)m2 |
||
| Open‐mouth/bill breathing | H | NA |
6.2 ± $ (100%)e2 7.0 ± $ (100%)e3 |
70 ± $ ($%) g | NA | 10.1 ± $ (100%)e4 | $ (10%)e5 | NA |
| B | NA |
5.8 ± $ (100%)e2 5.4 ± $ (100%)e3 |
NA | NA |
$ (100%)d3 $ (100%)d4 7.3 ± $ (100%)e4 |
$ (10%)d2 $ (100%)d1 $ (0%)e5 |
65.3 ± 1.6 (83%)m3 64.2 ± 2.8 (64.1%)m1 57.5 ± 2.5 (90%)m2 |
|
| High‐pitch vocalisation | H | NA | NA | NA | NA | NA | NA | NA |
| B | NA | NA | NA | NA | NA | NA |
66.6 ± 21.8 (12.8%)m1 50.7 ± 20.2 (15%)m2 |
|
Note: Mean ± SE latencies in seconds (s) are shown for the onset of listed ABMs, the percentage of birds performing the behaviour are reported in brackets ().
Abbreviation: NA, ABM not included in methodology for study and therefore no data available.
McKeegan, Reimert, et al. (2013). Note: Time elapsed is recorded from bird being fully immersed in foam (does not include fill‐time). Estimated latencies calculated to include the maximum time to foam submersion (i.e. fill‐time/start of process) for laying hens (50s) and broiler chickens (25 s) are reported alongside, italicised and annotated (a1).
Gerritzen et al. (2010). Time elapsed from the beginning of foam formation to the onset of the appearance of the ABM.
McKeegan et al. (2007). Broilers exposed to 100% Ar (< 2% residual O2)d1; 100% N2 (< 2% residual O2)d2; 30% CO2 in Ard3; or 40% CO2 in N2 d4.
Webster and Fletcher (2001). Broilers and laying hens, gradual‐fill containers: 30% CO2 in aire1; 40% CO2 in aire2; 60% CO2 in aire3; 70% Ar + 30% CO2 e4; 100% Are5.
Gent et al. (2020). Broiler breeders exposed to LAPS.
Berg et al. (2014). Laying hens, exposed to CO2 whole house gassing.
Gerritzen et al. (2010). Broilers, gradual‐fill containers, exposed to 60% CO2h1; and 70% Ar + 30% CO2 h2.
Gerritzen et al. (2013). Broilers, CO2 multistage gradual‐fill containers.
Coenen et al. (2009). Broilers, pre‐filled container, exposed to 100% N2 j1; 70% N2 + 30% CO2 j2. Behavioural latencies reported as medians only.
Lambooij et al. (1999). Broilers, pre‐filled container, exposed to 90% Ark1; 60% Ar + 30% CO2 k2.
McKeegan et al. (2011). Laying hens, exposed to CO2 whole house gassing.
Martin, Christensen, Vizzier‐Thaxton, and McKeegan (2016), Martin, Christensen, Vizzier‐Thaxton, Mitchell, and McKeegan (2016). Broilers exposed to LAPS in welfare assessment studym1; LAPS light trialm2; and LAPS analgesic trialm3.
Raj, Richardson, et al. (1998). Broilers, pre‐filled container, exposed to 90% Arn1; and 60% Ar + 30% CO2 n2.
Raj et al. (1991). Laying hens, pre‐filled container, exposed to 100% Ar.
Gerritzen et al. (2004). Broilers, whole house gassing, exposed to 100% CO2 p1; 50% N2 + 50% CO2 p2.
Raj et al. (1992). Laying hens, pre‐filled container, exposed to 30% CO2 in Ar (< 5% residual O2)q1; 31% CO2 in Ar (< 2% residual O2)q2; 49% CO2 q3; 100% Ar (< 2% residual O2)q4.
Data not available from literature/not reported in study, but ABM was included as measure in methodology.
$+one of the above letters (e.g. $a): Data not available from the specific literature that each letter represents, but ABM was included as measure in methodology.
TABLE 26.
Comparison of High expansion N2‐filled foam with other authorised methods available to kill laying hens (H), and broiler chickens (B) for purposes other than slaughter in relation to the time in appearing different ABMs.
| ABMs | Bird type | High expansion N2‐filled foam | Electrical waterbath | Captive bolt | Percussive blow to the head | Cervical dislocation | Maceration | Lethal injection |
|---|---|---|---|---|---|---|---|---|
| Loss of posture | H | 16 ± 1 a , 66a1 (100%) | NA | NA | NA | NA | NA | NA |
| B |
9 ± 1 a , 34a1 (100%) 48 ± 7 b (100%) |
NA | NA | NA | NA | NA | NA | |
| Loss of consciousness (EEG derived, onset of EEG suppression) | H | 30 ± 2 a , 80a1 | NA | NA | NA | 3.6 ± 0.6k1 (80%) | NA | NA |
| B |
18 ± 1 a , 53a1 51 ± 12 b |
NA | 7.3 ± 0.2 e (100%) | NA |
3.2 ± 0.3g1 (100%) 3.6 ± 0.6k1 (80%) |
NA | NA | |
| Motionless | H | 65 ± 3 a , 115a1 (100%) | NA |
164 ± 25d1(100%) 126 ± 22d2 (100%) 132 ± 22d3 (100%) |
NA |
154 ± 26i1 (100%) |
NA |
47 ± 38i2 (100%) |
| B |
51 ± 2 a , 76a1 (100%) 106 ± 5 b (100%) |
NA | NA | NA | NA | NA | ||
|
Death (EEG) |
H | 65 ± 3 a , 115a1 | NA | NA | NA |
207 ± 23i1 (100%) 36.4 ± 3.4k1 (100%) |
NA |
20 ± 23i2 (100%) |
| B |
47 ± 2 a , 72a1 92 ± 6 b |
NA | 15.0 ± 2.1 e (100%) | NA |
39.2 ± 5.2g1(100%) 36.4 ± 3.4k1 (100%) |
NA | NA | |
|
Head shaking |
H | 2 ± 1 a , 52a1 ($%) | NA | NA | NA | NA | NA | NA |
| B | NA | NA | NA | NA | NA | NA | ||
|
Clonic convulsions |
H | 18 ± 1 a , 68a1 ($%) | NA |
0.0 ± $ k2 (100%) |
NA |
0.0 ± 0.0 g (100%) 0.3 ± −0.2i1 (100%) 0.0 ± $ k1 (100%) |
NA | NA |
| B | NA |
0 ± $ f1 (100%) 124 ± $ h2 (100%) 0.0 ± $ k2 (100%) |
NA |
0 ± $ f2 (100%) 0.0 ± 0.0 g (100%) 93 ± $ h1 (100%) 0.0 ± $ k1 (100%) |
NA | NA | ||
|
Tonic convulsions |
H | NA | NA |
122 ± 22d1 (100%) 126 ± 22d2 (100%) 132 ± 22d3 (100%) |
NA |
187 ± 27i1 (78%) |
NA |
35 ± 33i2 (40%) |
| B | NA | NA | NA | NA | NA | NA | NA | |
|
Deep inhalation |
H | $, a ,$ a1 (0%) | NA | NA | NA | NA | NA | NA |
| B |
50 ± 24 b (12.5%) |
NA | NA | NA | NA | NA | NA | |
|
Mandibulation |
H | NA | NA | NA | NA | NA | NA | NA |
| B | NA | NA | NA | NA | NA | NA | NA | |
|
Escape attempts |
H | $, a , $ a1 (16.7%) | NA | NA | NA | NA | NA | NA |
| B | NA | NA | NA | NA | NA | NA | ||
|
Open‐mouth/bill breathing |
H | NA | NA | NA | NA | NA | NA | NA |
| B | NA | NA | NA | NA | NA | NA | NA | |
|
High‐pitch vocalisation |
H | NA | NA | NA | NA | NA | NA | NA |
| B | NA | NA | NA | NA | NA | NA | NA |
Note: Mean ± SE latencies in seconds (s) are shown for the onset of listed ABMs, the percentage of birds performing the behaviour are reported in brackets ().
Abbreviation: NA, ABM not included in methodology for study and therefore no data available.
McKeegan, Reimert, et al. (2013). Note: Time elapsed is recorded from bird being fully immersed in foam (does not include fill‐time). Estimated latencies calculated to include the maximum time to foam submersion (i.e. fill‐time/start of process) for laying hens (50s) and broiler chickens (25s) are reported alongside, italicised and annotated (a1).
Gerritzen et al. (2010). Time elapsed from the beginning of foam formation to the onset of the appearance of the ABM.
Bandara et al. (2019). Layer hens subjected to three captive bolts: Zephyr‐Ed1; Zephyr‐EXLd2 and Turkey Euthanasia Device (TED)d3.
Raj and O'Callaghan (2001). Broilers subjected to captive bolt.
Watteyn et al. (2022). Broilers subjected to cervical dislocationf1 and captive boltf2.
Martin et al. (2019). Broilers and laying hens exposed to cervical dislocation, combined broiler and layer averagesg1.
Baker‐Cook et al. (2021). Broilers exposed to cervical dislocationh1, and captive bolth2.
Hernandez et al. (2019a, 2019b). Laying hens subjected cervical disloxationi1 and lethal injection (Pentobarbital Sodium Injection)i2.
Jacobs et al. (2019). Broilers subjected to manual cervical dislocation.
McKeegan, Hopkins, et al. (2013). Broilers and laying hens subjected to cervical dislocationk1 and captive boltk2. Data averaged across bird type.
Data not available from literature/not reported in study, but ABM was included as measure in methodology.
To compare N2‐filled high expansion foam (including NEFS) with other modified atmosphere or not killing methods, authorised for purposes other than slaughter, a scientific literature review was conducted to gather data on animal‐based measures (ABMs) including neurological and behavioural indicators. The quantifiable ABMs related, for example, to mean latencies, standard errors and sample size, are presented in Tables 25, 26 for laying hens and broiler chickens, and in Tables 27, 28 for pigs, in order to compare NEFS with other CAS methods and then with other types of methods.
TABLE 27.
Comparison of high expansion N2‐filled foam with other authorised methods available to kill pigs for other purposes than slaughter in relation to the time in appearing different ABMs.
| ABMs | High expansion N2‐filled foam | CO2 at high concentrations | CO2 + inert gases | Inert gases at high concentrations | Head‐to‐body electrical killing | Head‐only electrical stunning |
|---|---|---|---|---|---|---|
| Loss of posture | 57.0 ± 2.0 (100%) a |
44 ± 1 (100%)c1 26 ± 1 (100%)c2 41.3 ± 4.4 (100%)d1 11.0 ± 0.4 (93%) e 17 ± 0.9 (100%)g3 40 ± 10 (100%) h 22.3 ± 0.5 (100%)i4 14.4 ± 1.6 (100%)k1 |
47.7 ± 8.4 (100%)d2 18 ± 1.3 (100%)g2 25.4 ± 0.7 (100%)i1 24.5 ± 0.8 (100%)i2 26.4 ± 20.0 (100%)i3 11.4 ± 1.6 (100%)k3 28.9 ± 1.0 ($%)l2 30.7 ± 1.0 ($%)l3 |
15 ± 0.8 (100%)g1 20.8 ± 1.6 (100%)k2 33.5 ± 1.3 ($%)l1 |
NA | NA |
| Loss of consciousness & (EEG derived) | NA |
47 ± 6 (83%)c1 33 ± 7 (75%)c2 21.2 ± 1.2 (100%)f3 40.7 ± 1.9 (100%)i4 32.9 ± 2.9 (100%)k1 |
16.6 ± 0.6 (100%)f2 40.7 ± 1.9 (100%)i1,i2,i3 48.9 ± 7.7 (100%)k3 |
15.3 ± 0.7 (100%)f1 61.1 ± 4.5 (100%)k2 |
< 1 (99%)m1 < 1 (93%) n < 1 (94%) o |
< 1 (98%)m2 < 1 (98–99%) p |
| Motionless | 189.5 ± 3.64 (100%) a | NA | NA | NA | NA | |
| Death (EEG derived) | NA |
75 ± 23 (83%)c1 64 ± 32 (75%)c2 31.9 ± 1.3 (100%)f3 46.2 ± 2.1 (100%)k1 |
39.0 ± 1.4 (100%)f2 67.1 ± 6.3 (100%)k3 |
53.7 ± 2.6 (100%)f1 69.0 ± 5.0 (100%)k2 |
NA | NA |
| Ataxia | NA |
NA |
NA | NA | NA | NA |
| Head shaking | NA |
24 ± 2 (33%)c1 14 ± 1 (50%)c2 |
NA | NA | NA | NA |
| Slipping | (20%) a | NA | NA | NA | NA | NA |
| Clonic/tonic convulsion | 57.0 ± 2.0 (100%) a |
14.4 ± 0.4 (97%) e 21 ± 1.2 (100%)g3 23.6 ± 0.9 ( $ %)i4 14.4 ± 1.6 (100%)k1 |
21 ± 1.6 (100%)g2 27.8 ± 1.2 ( $%)i1 26.4 ± 0.7 ( $%)i2 28.9 ± 1.9 ( $%)i3 11.4 ± 1.6 (100%)k3 |
21 ± 1.2 (100%)g1 20.8 ± 1.6 (100%)k2 |
NA |
NA |
| Deep inhalation | None |
23 ± 1 (100%)c1 9 ± 0 (100%)c2 12.9 ± 0.6 (93%) e (100%)f3 (80%)i4 33.2 ± 4.9 (100%)k1 |
(71%)i1 (54%)i2 (27%)i3 30.2 ± 5.7 (100%)k3 16.0 ± 2.1 ( $%)l2 12.4 ± 1.7 ( $%)l3 |
84.2 ± 20.0 (100%)k2 13.4 ± 2.1 (56%)l1 |
NA | NA |
| Escape attempt |
55 ± $ (80%)a |
34 ± 1 (50%)c1 14 ± 0 (46%)c2 6.3 ± 0.3 (91%) e 30 ± 5 (100%) h 3.9 ± 2.2 (100%)k1 |
8.0 ± 2.2 (100%)k3 28.9 ± 1.0 ( $%)l2 30.7 ± 1.0 ( $%)l3 |
19.0 ± 2.9 (100%)k2 6.0 ± 6.5 (11%)l1 |
NA | NA |
| Open‐mouth breathing | NA |
16.9 ± 1.6 (100%)d1 30 ± 10 (100%) h 5.8 ± 1.1 ( $%)k1 |
14.8 ± 0.9 (100%)d2 4.6 ± 1.1 ( $%)k3 |
21.2 ± 1.1 ( $%)k2 | NA | NA |
| High‐pitch vocalisation | 65 ± $ (95%) a |
27 ± 4.0 (50%)g3 34.1 ± 1.2 (40%)i4 0.0 ± 0.0 ( $%)k1 |
30 ± 1.6 (83%)g2 34.1 ± 1.2 (91–94%)i1,i2,i3 0.4 ± 0.6 ( $%)k3 |
27 ± 1.5 (75%)g1 1.9 ± 0.6 ( $%)k2 |
NA | NA |
Note: Mean ± SE latencies in seconds (s) are shown for the onset of listed ABMs, the percentage of pigs performing the behaviour are reported in brackets ().
Abbreviation: NA, ABM not included in methodology for study and therefore no data available.
Lindahl et al. (2020). Crossbred pigs (~ 30 kg) exposed to High expansion N2‐filled foam. Note: Estimated mean latencies for first occurrence of a behaviour were calculated and extrapolated from % histograms reported in the study.
Persson (2024). (https://stud.epsilon.slu.se/19719/). Crossbred 12‐week‐old pigs (~ 50 kg) exposed to high expansion N2‐filled foam.
Verhoeven et al. (2016). Crossbred pigs (~ 110 kg) exposed to 80%c1 or 90%c2 concentrations of carbon dioxide in a dip‐lift system. EEG derived loss of consciousness defined as high amplitude, low frequency dominated EEG traces by visual inspection and brain death by < 10% of baseline amplitude.
Terlouw et al. (2021). Crossbred pigs (~ 90 kg) exposed to 80% CO2/aird1; and 70% N2O/30% CO2d2 by being lowered into a gas‐filled box.
Atkinson et al. (2020). Crossbred pigs (~ 95 kg) exposed to 90% carbon dioxide in a dip‐lift system.
Raj et al. (1997). Crossbred pigs (~ 38 kg) exposed to 90% argon in air with 2% residual oxygenf1; a mixture of 30% carbon dioxide and 60% argon in air with 2% residual oxygenf2; or 80%–90% carbon dioxide in airf3 by being lowered into a gas‐filled box. EEG derived loss of consciousness defined by loss of somatosensory evoked potentials (SEPs); and brain death by onset of Isoelectric ECoG.
Raj (1999). Crossbred pigs (~50 kg) exposed to 90% argon in airg1; 30% carbon dioxide and 60% argon in airg2; and 80%–90% carbon dioxide in airg3 by being lowered into a gas‐filled box.
Sutherland et al. (2017). Crossbred 3‐week‐old pigs (~ 5 kg) were placed in a chamber pre‐filled with 90 + % CO2. Values estimated from Figure 2.
Llonch et al. (2012). Crossbred female pigs (~ 90 kg) were exposed to: 70% nitrogen (N2) and 30% carbon dioxide (CO2) (70N30C)i1; 80% N2 and 20% CO2 (80N20C)i2; and 85% N2 and 15% CO2 (85N15C)i3 compared with 90% CO2 in air (90C)i4 by being lowered into a gas‐filled box. EEG derived loss of consciousness defined through the use of the Index of Consciousness® and the electric suppression rate (ESR).
Lechner et al. (2021). Crossbred pigsj1 (~ 110 kg) and sowsj2 (~ 230 kg) were exposed to 80% carbon dioxide in a dip‐lift system. Values estimated from Figure 2.
Kells, Beausoleil, Johnson, and Sutherland (2018), Kells, Beausoleil, Sutherland, and Johnson (2018). Crossbred 2‐ to 3 week‐old piglets (~ 4 kg) were exposed to 100% carbon dioxidek1; 100% Ark2; or a mixture of 60% Ar and 40% carbon dioxidek3 by being lowered into a gas‐filled box.
Dalmau et al. (2010). Crossbred pigs (~ 100 kg) exposed to 90% Ar in airl1; 70% N2 and 30% CO2 (70N30C)l2; and 85% N2 and 15% CO2 (85N15C)l3 in a dip‐lift system.
Nodari et al. (2014). Crossbred heavy pigs (~ 160 kg) stunned via head‐to‐body (abattoir B)m1; or head‐only (abattoirs Ac & C)m2 electrical stunning.
May et al. (2022). Crossbred slaughter‐weight pigs (NA kg) stunned via head‐to‐body electrical stunning.
Von Wenzlawowicz et al. (2012). Crossbred pigs (~ 75 kg) stunned via head‐to‐body electrical stunning.
Velarde er al. (2001). Crossbred slaughter‐weight pigs (NA kg) stunned via head‐only electrical stunning.
For head‐to‐body electrical stunning, loss of consciousness is defined as an effective stun characterised by immediate collapse of the animal and tonic immobility during exposure to the stunning current. Immediately after exposure to the current, pigs show tonic seizure followed by clonic seizures (e.g. generalised epilepsy). Any rhythmic breathing, movement of the eyes, corneal reflex, etc., are indicative of an ineffective stun.
Data not available from literature/not reported in study, but ABM was included as measure in methodology.
TABLE 28.
Comparison of high expansion N2‐filled foam with other methods available to kill pigs for other purposes than slaughter in relation to the time in appearing different indicators.
| ABMs | High expansion N2‐filled foam | Captive bolt | Percussive blow to the head | Firearm with free projectile | Lethal injection |
|---|---|---|---|---|---|
| Loss of posture | 57.0 ± 2.0 (100%) a | < 1 (87%)i2 |
< 1 (100%) h < 1 (100%)i1 |
NA | NA |
| Loss of consciousness (EEG or behaviourally & derived) | NA |
< 1 (100%) c < 1 (100%) d < 1 (98.6%) e < 1 (100%) f < 1 (100%)g1‐5 < 1 (98%)j7 |
< 1 (100%)j8 |
< 1 (99.3%)j1 < 1 (99.5%)j2 < 1 (94.6%)j3 < 1 (97.2%)j4 < 1 (99.6%)j5 |
< 1 (97.9%)j6 |
| Motionless | 189.5 ± 3.64 (100%) a | NA | NA | NA | NA |
| Death (EEG or behaviourally & derived) | NA |
< 1 (98%)g1‐5 < 1 (98%)j7 |
64.3 ± 7.3 (100%) h < 1 (100%)j8 |
< 1 (99.3%)j1 < 1 (99.5%)j2 < 1 (94.6%)j3 < 1 (97.2%)j4 < 1 (99.6%)j5 |
< 1 (97.9%)j6 360.0 ± 32.4 (100%) k |
| Head shaking | NA | NA | NA | NA | NA |
| Slipping | (20%) a | NA | NA | NA | NA |
| Clonic/tonic convulsion | 57.0 ± 2.0 (100%) a | NA | NA | NA | NA |
| Deep inhalation | None | NA | NA | NA | NA |
| Escape attempt | NA | NA | NA | NA | |
| Open‐mouth breathing | NA | NA | NA | NA | NA |
| High‐pitch vocalisation | 65 ± $ (95%) a | NA | NA | NA | NA |
Note: Mean ± SE latencies in seconds (s) are shown for the onset of listed ABMs, the percentage of pigs performing the behaviour is reported in brackets () alongside the mean latencies.
Abbreviations: NA, ABM not included in methodology for study and therefore no data available.
Lindahl et al. (2020). Crossbred pigs (~ 30 kg) exposed to High expansion N2‐filled foam. Note: Estimated mean latencies for first occurrence of a behaviour were calculated and extrapolated from % histograms reported in the study.
Persson (2024). (https://stud.epsilon.slu.se/19719/). Crossbred 12‐week‐old pigs (~50 kg) exposed to high expansion N2‐filled foam.
Grist et al. (2018a). Crossbred piglets (~ 1 kg) stunned by captive bolt (Accles and Shelvoke CASH Small Animal Tool (CPK200)).
Casey‐Trott et al. (2013). Crossbred piglets (~ 1 kg) stunned by captive bolt (Zephyr‐Euthanasia (Zephyr‐E)).
Casey‐Trott et al. (2014). Crossbred pigs (~ 3–9 kg) stunned by captive bolt (Zephyr‐Euthanasia (Zephyr‐E)).
Grist et al. (2018b). Crossbred piglets (~ 2 kg) stunned by captive bolt (Zephyr EXL non‐penetrating captive bolt).
Grist et al. (2017). Crossbred piglets weighing 3 kgg1; 3–5 kgg2; 5–7 kgg3; 7–9 kgg4; and 9–11 kgg5 stunned by captive bolt (Zephyr EXL non‐penetrating captive bolt). Loss of consciousness was EEG derived by visual evoked potentials (VEPs) and brain death by complete loss of VEPs.
Costa et al. (2020). Crossbred piglets (~ 1 kg) stunned by manual blunt force trauma by a single operator holding the animal by its rear legs and striking the top of the head against the concrete floor. EEG derived death was classified as a trace with an amplitude of < 1/8 (12.25%) of that of normal pre‐stunning EEG with little or no low‐frequency components.
Widowski et al. (2008). Crossbred piglets (~ 1 kg) stunned by manual blunt force traumai1 (by a single operator holding the animal by its rear legs and striking the top of the head firmly and deliberately against a flat, hard surface); and Zephyr (ZE) non‐penetrating captive bolti2.
Whiting et al. (2011). Crossbred piglets (NA kg) killed by variations of a firearm with a free projectile (Short hollow point 1105 ft/s 27 grainj1; Ball round nose 710 ft/s 29 grainj2; Ball round nose 710 ft/s 29 grainj3; Lead round nose 710 ft/s 29 grainj4; and Subsonic HP 1050 ft/s 38 grainj5); lethal injectionj6 (by intra‐peritoneal injection of barbiturate, dosage > 600 mg/kg); captive boltj7 (Accles and Shelvoke CASH Small Animal Tool (CPK200)); and manual blunt force traumaj8 (by a single operator holding the animal by its rear legs and striking the top of the head firmly and deliberately against a flat, hard surface).
Kells, Beausoleil, Johnson, and Sutherland (2018), Kells, Beausoleil, Sutherland, and Johnson (2018). Crossbred piglets (~4 kg) killed by lethal injection (by intra‐peritoneal injection of sodium pentobarbital, dosage 250 mg/kg−1).
For captive bolt and Percussive blow to the head unless otherwise stated, loss of consciousness was defined as an effective stun characterised by immediate cessation of rhythmic breathing, corneal reflex, palpebral reflex and a cortical arc reflex. Death was defined as when all reflexes, convulsions and breathing were absent.
Data not available from literature/not reported in study, but ABM was included as measure in methodology.
These results were used to compare the NEFS system to other CAS methods based on ABMs. However, there was variability in the method application between the studies (e.g. whole house vs. container) which make a direct comparison inappropriate. From the results described in the different studies (Table 25), there is high variability in the results, when comparing the time to loss of posture in chickens exposed to NEFS against other CAS methods. For example, when applied in containers, NEFS results indicate a shorter latency to loss of posture (approx. 48–66s from the beginning of foam induction) than latencies in LAPS (approx. 55–86s) depending on protocols (e.g. container size and fill rate). If we consider onset of EEG suppression and including the fill time, NEFS results in up to 80s and 53s for hens and broilers, respectively, and therefore, it might be considered similar to LAPS (approx. 24–54s) and the use of inert gas only in containers (approx. 17–55s) or in combination with CO2 (approx. 19–26s). Nevertheless, direct comparison between methods is improper due to the high variation in ABMs measurement and results, likely due to differences in protocols (e.g. gas fill‐rate, gases used, pre‐fill vs. gradual fill, etc.). Overall, it was concluded that NEFS can be considered at least equivalent to LAPS in terms of unconsciousness induction. Both are, in general, less rapid in inducing unconsciousness than the others gas killing methods in containers.
For pigs, most of the data reported in the literature come from laboratory‐based studies rather than field data. In addition, the range of times reported for the onset of LOP (Table 27) varied depending upon the gases used (e.g. Ar or N2 or CO2), concentration of gases used, proportion of mixed gases, as well as fill rate. On average, the time to LOP ranged between 11 and 44s for CO2 in high concentration; 11–48s for CO2 plus inert gases; and 15–34s for inert gases only. For NEFS, the only available data presented in the dossier for pigs is 57 ± 2s for LOP. Similar to poultry, mechanical and electrical methods for pigs are designed to induce immediate unconsciousness and are therefore not comparable with NEFS for either species.
These data were systematically extracted from the published literature, but due to the variability of the method application, no formal meta‐analysis was undertaken.
Due to the limitations of quantitative comparisons with other methods, stemming from insufficient data within the current dossier and the constraints (e.g. missing data, difference in protocols, ABMs) of the methods already listed in Regulation (EC) No. 1099/2009, a qualitative assessment is considered essential to try to conclude on equivalence of the methods. This involved developing ranking of the hazards threatening animal welfare during application of approved methods and comparing these with the hazards linked to NEFS. The initial step entailed identifying potential hazards associated with each method, defined as any event or sequence of events related to the killing process that impacts animal welfare. These hazards were identified through a review of scientific literature (including data reported in Tables 25, 26, 27, 28 and EFSA AHAW Panel, 2019, 2020a, 2020b) and consultations with the working group experts, with the prerequisite that all killing processes comply fully with existing legislation. Therefore, hazards related to potential misuse or operator inefficiency were not considered in this analysis. The assessment assumes accurate execution of the procedure according to standard operating procedures (SOPs), absence of equipment defect or human errors. It also assumes that NEFS does not cause mechanical obstruction of the respiratory tract or suffocation.
4.2.2. Qualitative assessment
Data‐driven comparisons (quantitative assessment) of the killing methods are limited by the insufficient quantitative data available for most of them as well as because of important differences in protocols and methods for measurement leading also to important variability in results. Therefore, it was agreed to use a qualitative method based on non‐formal expert elicitation (EFSA, 2014) to assess the equivalence of NEFS in container with the other killing methods. This qualitative approach utilises a hazard list that details negative welfare impacts on chickens, hens and pigs when killed for purposes other than slaughter. A comprehensive explanation of the methodology used for this qualitative assessment can be found in Section 2.2.3.2 in the methodology.
For the exercise, NEFS in container was compared to 11 killing methods for chickens and 10 killing methods for pigs that were authorised under Council Regulation (EC) 1099/2009.
A total of 27 hazards for chickens and 28 for pigs were evaluated by four experts within EFSA's ad hoc working group. Information on killing methods and hazards can be found in Annexes A and B. From the total hazards, highly relevant hazards were selected (see Section 2.2.3.2). The welfare consequences for chickens and pigs associated with the selected highly relevant hazards were discussed to make comparison of NEFS in container with other authorised killing methods.
4.2.2.1. Results of the exercise
Final scores from experts for both chicken and pigs were very dispersed. Heterogeneity in expert hazards scoring was related to the range of values used by the experts when providing their judgement (with some experts scoring consistently higher in their overall elicitation, and others lower). Moreover, the first question was interpreted differently by a single expert, because the percentage (%) of failure of a killing method in relation to the occurrence of a single hazard was taken into consideration in the evaluation and other experts did not consider the % of failure of the method.
Firstly, for all CAS methods including NEFS, it is important to note that the physiological basis of induction to unconsciousness and death itself could be considered as a hazard, due to its non‐immediate nature and as it involves modifying the atmosphere the animal is exposed to reach hypoxia (with or without added CO2 leading to hypercapnic hypoxia). However, according to the scientific literature, animals exposed solely to hypoxia experience less aversion compared to those exposed to hypercapnia (EFSA, 2004; EFSA AHAW Panel, 2019).
When compared to CO2 methods, killing methods using inert gas can therefore be considered as resulting in less respiratory distress. There is no full consensus whether or not inert gases are generating minimal or even no respiratory distress when inducing hypoxia. It depends mainly on how respiratory distress is defined and how it is measured (e.g. is hyperventilation considered an ABM for respiratory distress). The ABMs themselves might not always refer to the same thing in between different publications (e.g. gasping may refer to deep inhalation but sometimes in unconscious and sometimes in conscious animals, that will not have the same welfare implication). As an example, in a recent review on air hunger (also referred to as dyspnoea, breathlessness or respiratory distress in this opinion) by Banzett et al. (2021), it is stated that ‘Air hunger may not even occur in response to hypoxia if the subject [human] is allowed to breathe freely. We now understand that humans can consciously sense hypoxia if PETCO2 (Pressure end‐tidal CO2) and ventilation are near normal, but that hypoxic hyperpnea increases mechanoreceptor feedback and lowers PETCO2, thus suppressing the air hunger signal prior to loss of consciousness’. Following this reasoning and considering the anatomical (with pigs) and physiological similarities between humans and other vertebrates, it could be likely that animals exposed to anoxia do not experience respiratory distress.
However, other publications have reported behavioural indicators of aversion when exposed to hypoxia alone (i.e. killing methods using inert gas). Dalmau et al. (2010) reported 37% of pigs gasping (while still conscious) and 3% showing escape attempts prior to LOC during exposure to Ar induced anoxia. The authors noted that ‘…the proportion of pigs that showed aversion (escape attempts and gasping) to 90% argon was lower than to the gas mixtures with N2 and CO2… Therefore, although it cannot be argued a lack of aversion exists to Ar, the aversion to high concentrations of this gas could be lower than when mixture contains 15% or 30% CO2’. Similar results were observed in Kells, Beausoleil, Johnson, and Sutherland (2018), Kells, Beausoleil, Sutherland, and Johnson (2018), with all piglets exposed to Ar exhibiting escape attempts, laboured breathing (two out of five) and gasping. The authors concluded that a more marked response in terms of escape behaviours and laboured breathing, suggests that piglets experience more stress or aversion prior to loss of consciousness when exposed to 100% CO2 than 100% Ar. However, Banzett et al. (2021) argued that the visible respiratory efforts shown in laboured breathing and gasping can be entirely generated by reflex pathways, thus do not necessarily indicate the presence of air hunger or respiratory distress.
Head shaking in poultry is thought to be an alerting response to novel or disturbing stimuli (Hughes, 1983) and has been used previously as an indicator of aversion (Lambooij et al., 1999; Webster & Fletcher, 2001) and respiratory distress (McKeegan et al., 2006; Webster & Fletcher, 2001). Gent et al. (2020) showed headshaking in broiler breeders was observed during exposure to LAPS, CO2 and N2 groups. All episodes were observed before LOP and were more frequent in the CO2 group. Unlike birds exposed to nitrogen, those exposed to LAPS and CO2 showed wing flapping prior to loss of posture, which is interpreted by the authors as escape attempts. Gerritzen et al. (2000) and Lambooij et al. (1999) also reported broilers exhibiting headshaking and gasping when exposed to Ar, but the frequency of occurrences was significantly lower than with gas mixtures inducing hypercapnic hypoxia. Therefore, it is a possibility that hypoxia alone does result in some behaviours indicative of aversion expressed in the dossier as respiratory distress, although much less than hypercapnic hypoxia.
In addition to this, other aspects discussed included, for example, that poultry reared in low lighting regimes will experience, at least, discomfort when exposed to bright day light during killing outside the house, regardless of the killing method. The animal welfare consequence of this would be worse when they are shackled prior to killing with electrical water baths. Similarly, animals' responses to novel environment may vary due to their genetics (e.g. Duroc vs. Large White pigs) and farming conditions (e.g. free‐range versus intensively reared animals). Additionally, there was high uncertainty around certain methods for which limited information exists compared to others with more evidence based on scientific publication. In the case of NEFS, lack of data (e.g. lack of replication, missing ABMs, etc.) led to high uncertainty.
Given these considerations, a detailed discussion was deemed essential for achieving a consensus whether NEFS is equivalent to other stunning/killing methods. Namely, it was discussed whether NEFS ensures that pigs and poultry are spared of avoidable pain, distress or suffering during the killing and whether it maintains the loss of consciousness and sensibility until death at least at the same level with at least one of the authorised methods and as described in the killing regulation.
All the experts agreed that comparing killing methods applied to individual animals and/or intended for immediate stunning (i.e. LOC) with methods applied to groups aiming for gradual LOC induction is challenging. However, they agreed that an evaluation starting from the welfare implication for the animals was still possible.
Selected highly relevant hazards are presented below in Tables 29, 31 for chickens/hens and Tables 30, 32 for pigs.
a) Comparison of NEFS with CAS methods
The results of the comparison of NEFS and CAS methods are displayed in Tables 29, 30.
Common hazards
NEFS and authorised CAS methods have at least five common hazards: handling, hurting, loud noise, overloading and too short exposure time.
When animals (chickens or pigs) are handled, they can all be subjected to handling stress in all the methods except whole house gassing. Rough handling can lead to hurting and/or tissue damage and negative affective states (e.g. pain and fear) (EFSA AHAW Panel, 2019, 2020a, 2020b). Loud noise can occur in all CAS methods during delivery of pressurised gases and in NEFS due to the noise generated by the nitrogen gas flowing through the foam generator. Overloading in crates (poultry only) or directly into the foaming containers or in containers used for gassing or NEFS will lead to (i) animals possibly being injured (e.g. by trying to climb/escape), resulting in bruising and abrasions; and (ii) decrease in method's efficiency leading to delay in onset of unconsciousness and death (EFSA AHAW Panel, 2019, 2020a, 2020b). This means that animals could experience pain, distress or suffering for longer periods. Too short exposure time will also lead to a delay in onset of unconsciousness or even total failure of the method, i.e. animals recovering consciousness or remaining alive at the end of exposure. Another hazard for pigs is the risk of slipping and falling due to rough handling and poor floor quality, leading to pigs experiencing pain and fear due to injury (EFSA AHAW Panel, 2019, 2020a, 2020b).
Clearly, existing methods applied to group of animals in containers or crates during killing with containerised gassing systems or NEFS have these five hazards in common and therefore no major difference can be established on the basis of these hazards.
Hazards specific to NEFS
Inhalation of foam, too small bubble size, surrounded by foam and too low foam production rates are hazards unique to NEFS.
When animals are surrounded by foam, they can experience fear (see Section 4.1.2). In addition, the possibility that animals inhale foam cannot be excluded (even in the absence of proof of total occlusion), which will lead to respiratory distress. If bubble size is too small (< 10 mm), it will increase the likelihood of higher water content in the foam (see Sections 3.1.3 and 4.1.1), which raises the animal welfare concern associated with foaming liquid entering the upper respiratory tract. The foam production rate should be appropriate to the container's size to be filled within 45 s, which is a key parameter, and the rate should be increased according to the container's volume to maintain consistent fill‐times. Otherwise, container filling time could be prolonged leading to welfare consequences.
Substance irritating the mucosa
Potential mucosal irritation occurring due to the foam or foaming liquid entering nostrils and eyes or entering the upper respiratory tract could act as physical irritation prior to loss of consciousness. For chickens, head shaking (possible false‐positive reaction) reported in the submitted dossier (McKeegan, Reimert, et al., 2013) could be considered as evidence of mucosal irritation due to foam entering the nostrils. However, birds did not display gasping (which is considered as equivalent to coughing reflex) during exposure to air‐filled or nitrogen‐filled high expansion foam and this is more in favour of indicating that foaming solution did not enter the upper respiratory tract in conscious birds. Still, small quantities of foam had been found in the larynx and upper part of the trachea during post‐mortem dissection (McKeegan, Reimert, et al., 2013; Raj et al., 2008).
It is well known that CO2 is an irritant to mucosae and inhalation of this gas, especially in high concentrations (40% or more) and induces respiratory distress prior to the onset of unconsciousness (EFSA, 2004). For example, studies in humans reported that hypercapnia caused by the inhalation of CO2 is a more potent respiratory stimulant than hypoxia (such as is caused by N2 or other inert gases). Exercise (e.g. running) induces breathlessness through the gradual increase in blood CO2 concentration. In contrast, inhalation of high concentrations of CO2 results in rapid increases in blood CO2 concentration, and this is more potent in producing respiratory distress (Raj, 2006; Steiner et al., 2019).
It was discussed that chickens exhibited escape attempts during exposure to NEFS (McKeegan, Reimert, et al., 2013) and these are signs of pain and fear caused by mucosal irritation due to foam. Chickens also exhibit escape attempts during exposure to high concentration of carbon dioxide (see EFSA, 2004), which are considered as evidence of mucosal irritation. The severity of respiratory sounds occurring during the induction of unconsciousness in pigs (until they lost posture) with gas mixtures was subjectively rated and used to ascertain severity of respiratory distress (Raj & Gregory, 1996). The results of this study showed that exposure to 90% argon induced minimal respiratory distress equivalent to that occurring during exposure to air, whereas exposure to 40–90% by volume of CO2 in air induced severe respiratory distress. Exposure to 30% by volume of CO2 in argon mixture induced moderate distress. Some pigs that were exposed to less than 70% CO2 in air showed escape attempts. By contrast, during exposure to argon‐induced anoxia, pigs lost posture without any evidence of behavioural arousal or escape attempts. For these reasons, NEFS can be considered at least equivalent (same or better in terms of welfare) to exposure to high concentrations of CO2 when considering the negative welfare consequences induced by the substance irritating the mucosa.
Exposure to too low temperatures
Exposure to too low temperatures was discussed as another hazard associated with the NEFS due to the use of cold water or low temperature of nitrogen gas. In general, inert gases such nitrogen (and argon) can exist in two physical states, i.e. liquid form when compressed in a cylinder and gaseous form when released into the atmosphere. Expansion of liquid into gaseous form occurs by using heat from the environment surrounding the delivery pipes and the environment inside the animal‐house or containers. Therefore, it is very likely that nitrogen gas used to create high expansion foam will expand and attain ambient temperature rapidly and may not be a hazard. In contrast, CO2 can exist in three states, liquid in compressed cylinder, solid (dry ice) when released without an external heat source (e.g. heated vaporisers) and gaseous form when fully expanded. In the absence of external heat source, extremely low ambient temperatures were recorded in a poultry house, i.e. below −80°C, during whole house gassing with liquid CO2 (Sparks et al., 2010) and such a low ambient temperature will lead to freezing of moisture content in the air. Consequently, animals will be forced to breathe cold and dry air plus CO2 mixture in the environment which would be worse, in terms of irritation of mucosae and/or respiratory distress (EFSA, 2004). It is worth noting that published scientific information concerning whole house gassing of pigs with CO2 is not available; however, the possibility of exposure to extremely low temperature when this method is applied could not be excluded.
A reduction in temperature of up to 5°C below the ambient temperature has also been recorded inside the LAPS apparatus during stunning of poultry (EFSA AHAW Panel, 2018), which is unlikely to be a hazard. On this basis, exposure to low temperatures during NEFS may be considered equivalent to LAPS and certainly better than whole house gassing with CO2 in chickens. In the case of pigs, based on the available evidence, NEFS would be considered comparable to exposure to high concentrations of CO2.
Delay in the time to onset of unconsciousness
Time to create the desired anoxic atmosphere (less than 2% oxygen by volume in nitrogen) in the foam container and, consequently, latency to induce unconsciousness was also discussed. This can be up to 45 s in NEFS according to the key parameters proposed in the dossier. On the other hand, it has been reported that during containerised gassing system developed in the United Kingdom, the containers could be filled with a gas mixture of 80% argon and 20% CO2 to achieve recommended oxygen concentration in 2 min when a transport module full of birds is placed inside the container and gas is supplied at 5 bar (500 kPa) (Raj et al., 2008). This latency to fill the container would vary according to the size of the container and gas flow rate. During whole house gassing of a poultry house (dimensions 30 m long and 12 m wide, 2.5 m at the eaves and 4.5 m to the roof apex), it took 5 min and 20 s for the target concentration of 45% CO2 to be reached at the rear of the building (i.e. furthest to CO2 inlets into the house) (Sparks et al., 2010). The time taken to achieve an atmosphere equivalent to less than 5% oxygen during exposure of broilers to LAPS is reported to be about 54 s (EFSA AHAW Panel, 2018).
Evidently, the latency to exposure of birds to anoxia during NEFS and hence the duration of animals experiencing negative welfare outcomes is similar, if not shorter, in NEFS when compared to other methods.
The two main causes of hurting occurring to animals during exposure to NEFS leading to pain were also discussed:
There is a risk of harm to animals during the injection of the puff of nitrogen gas to burst bubbles, albeit at a very low delivery pressure ■■■■■, if the jet stream hits sensitive parts such as eyes, it may cause pain and fear in animals and startle responses which could lead to injury. Importantly, the puff of nitrogen gas lasts only for a very short time (~ 3 s). The possibility of animals being hit by jet stream of gas also exists in the containerised gassing systems and whole house gassing methods (animals nearest to gas inlets into the house). It is worth noting that this hazard does not apply to LAPS, however, suction of air out of the container during decompression may have welfare consequences (e.g. aversion) and they have not been elucidated.
There is a risk that animals undergo anoxic convulsions (i.e. strong wing flapping and leg paddling), this may result in injury to the adjacent animals, which are not yet rendered unconscious. This will happen for all CAS methods, including LAPS (poultry only), during a time corresponding to the interval between the first and last animal losing consciousness. The severity of these convulsions is the same in all the CAS methods.
Considering these two sources of hurting to animals and the data available, the NEFS method can be considered equivalent to other CAS methods applied to groups of animals.
Hazards specific to LAPS (for chickens only)
Expansion of gas in the body cavities and too fast decompression rates are hazards unique to LAPS and only for chickens.
According to Boyle's law of gas, the volume of gas has an inverse relationship with the pressure at a given temperature. If the pressure decreases, the volume increases, and vice versa when the temperature is held constant. This implies that the expansion of gas in the body cavity during LAPS is independent of the rate of decompression. Therefore, it was not possible to rule out this hazard in LAPS (EFSA AHAW Panel, 2018) that will lead to potential pain if volume of air is expending in sinuses, gut or air sacs. This hazard does not apply to any other existing methods or NEFS.
Main result of comparison of NEFS with CAS methods
In summary, based on the argument developed above and from the elements brought in the dossier, it is more likely than not (certainty > 50%–100%) that for laying hens and broiler chickens of all age, NEFS is considered equivalent or better than exposure to whole house gassing with CO2 in terms of the proportion of animals experiencing the described WCs and the severity of these WCs. For pigs, it is more likely than not (certainty > 50%–100%) that NEFS is considered equivalent or better than exposure to high concentrations of CO2 in terms of the proportion of animals experiencing the described WCs and the severity of these WCs on the pigs.
b) Comparison between NEFS and other methods
NEFS was also compared with other authorised methods applied on individual animals. These methods have less in common with NEFS than CAS methods do, because they are applied to one single animal at a time (and not a batch), and these include mechanical and electrical methods or lethal injection. Therefore, there are fewer common hazards between NEFS and these individual methods to make any meaningful comparison. Since equivalence must be assessed between NEFS and other approved methods, different hazards and related welfare consequences were ascertained. The results of the comparison of NEFS and non‐CAS methods are displayed in Tables 31, 32.
Handling, manual restraint, inversion or shackling
All the methods applied to individual animals require handling. Electrical and mechanical methods as well as lethal injection require manual restraint of animals leading to pain and fear in all of them. Inversion of birds during the application of electrical and mechanical methods further exacerbates pain and fear in all animals. Shackling prior to electrical water bath stunning involves compression of the metatarsal bones between the metal shackles, which induces pain and fear in all the birds, with greater risk in heavier birds. The detailed welfare implication of these hazards can be found in the following EFSA published opinions: Killing for purposes other than slaughter: poultry (EFSA AHAW Panel, 2019); welfare of pigs during killing for purposes other than slaughter (EFSA AHAW Panel, 2020a, 2020b); and welfare aspects of the main systems of stunning and killing for the main commercial species of animals (EFSA, 2004).
NEFS, which is applied in a container, requires animals to be handled and either placed directly into the container or placed in a crate (for poultry) prior to placement in the container.
Pre‐stun shock (poultry only)
As shackling, pre‐stun shock is a hazard specific to electrical water bath stunning in poultry and does not apply to NEFS. Indeed, it is quite common that, at the entry of the water bath, birds touch the electrified water with their leading wings before the head is fully immersed to render them unconscious. This will induce acute pain to the birds concerned (EFSA, 2004).
c) Comparison of hazards applicable to all methods (NEFS, CAS and other methods)
Too short exposure time
The too short exposure time is a common issue in NEFS as well as in electrical and CAS methods, potentially leading to a decrease in the method efficacy or even the inability to induce unconsciousness and/or death, or recovery of consciousness (EFSA, 2004). Consequently, this particular hazard does not differentiate NEFS from electrical or other CAS methods.
Hazards leading to failure of the process
Each type of method has specific hazards leading to failure of induction of unconsciousness and/or death. This is the case for mechanical methods (e.g. incorrect application of manual method and incorrect shooting position), electrical methods (e.g. inappropriate electrical parameters and poor electrical contact) and lethal injection (e.g. sublethal dose and inappropriate route of administration). In CAS methods, including NEFS, there is usually a delay in the induction of unconsciousness and/or death or a risk of recovery of consciousness (see description in Section 4.1.1).
Delay in induction of unconsciousness or death
Unlike CAS methods (including NEFS), lethal injection, mechanical and electrical methods, when used correctly, have the advantage of inducing immediate onset of unconsciousness or death (EFSA, 2004). Therefore, the hazards identified in these methods are not comparable with the hazards present in CAS and NEFS methods.
4.2.2.2. Overall conclusion and sources of uncertainty
From the evidence submitted in the dossier, the working group concluded that NEFS is at least equivalent to water bath stunning/killing and whole‐house gassing with CO2 in chickens. In the case of pigs, it was concluded that NEFS is at least equivalent to exposure to high concentrations of CO2.
However, the lack of specific scientific data does not allow the working group to conclude on the occurrence and prevalence of the hazards: penetration of foam or foam liquid in the airways leading to irritation and/or mechanical stimulation. The time during which animals might be submitted to these hazards is also unknown. Finally, the lack of replication is also leading to uncertainty about the generalisation of results provided in the two scientific papers (Lindahl et al., 2020; McKeegan, Reimert, et al., 2013). Therefore, the assessment regarding the equivalence with existing methods determined by the EFSA experts is subjected to high uncertainty (> 50%–100%). To conclude with a higher degree of certainty, more scientific information is needed, such as:
replication of the studies and confirmation of reported results in the submitted dossier (external validity);
validation in other categories of pigs and poultry involving statistically sound experimental design and scientifically sound ABMs (internal validity);
use of control group treated with analgesics and/or anxiolytics (to assess pain and/or fear; internal validity);
use of additional ABMs, such as excessive lacrimation or frequent blinking due to irritation caused by the foam or use of reddening of the mucosae or increased mucosal secretions, as an indicator of acute inflammatory reaction, if any, due to foaming agent entering the respiratory tract;
determination of the exact time animals is exposed to each hazard identified in this SO and their prevalence to facilitate uncertainty assessment;
field data with more information to ascertain efficacy of NEFS and potential sources of system failure (e.g. more methodological details and ABMs outcomes);
EEG data to assess time to onset of unconsciousness and death in different animal categories (e.g. age and weight groups of pigs).
4.3. Identification of preventive and corrective measures
In total, 14 hazards associated with NEFS were identified, while preventive measures for 13 of these hazards and corrective measures for 9 are detailed in Table 33 below.
TABLE 33.
Preventive and corrective measures in relation to the hazards identified with the use of NEFS.
| Hazard | Welfare consequence(s) | Preventive measures | Corrective measures |
|---|---|---|---|
| Handling | Pain and fear |
|
|
| Loud noise | Fear |
|
Use of gas diffusers, if necessary |
| Slipping | Pain and fear | Use non‐slippery floor and keep it clean and dry | Clean the floor |
| Substance irritating the mucosa | Pain | Use non‐irritant foaming agent | None |
| Surrounded by foam | Fear | None |
|
| Inhalation of the foam | Pain and fear | Ensure the foam is fragile and breaks upon contact with the nostrils |
|
| Jet stream of gas at animal level | Pain and fear |
|
None |
| Hurting | Pain and fear | Achieve and maintain uniform distribution of 98% nitrogen throughout the container, so that all the animals are simultaneously exposed to hypoxia | Re‐establish less than 2% residual oxygen throughout the container, if necessary |
| Too short exposure | Pain and fear | Observe animals during exposure to the foam and maintain adequate duration (see key parameters) of exposure to less than 2% residual oxygen until they are dead (e.g. motionless or cessation of breathing). |
|
| Overloading | Pain and fear | Provide necessary training and clear instructions to staff regarding optimum stocking density (all animals should have enough space to lie down), necessary to allow uniform dispersal of foam and avoid air pockets |
None Retrain staff |
| Too small bubble size | Pain and fear |
|
Observe the foam bubble size during the initial part of filling and take appropriate measures (see process control) if the hazard is detected. |
| Low foam production | Pain and fear | Calculate the container volume and set the foam production rate to fill it within 45 s | None |
| Too low gas concentration | Pain and fear | Follow the SOPs to establish and maintain less than 2% by volume of oxygen in nitrogen throughout the container |
|
| Too low temperature | Pain |
|
None |
5. CONCLUSIONS
5.1. General summary results of the assessment of the high expansion foam procedure
-
The physiological basis of induction of unconsciousness and death with anoxia is well established, and the use of anoxia to kill animals is approved in the Council Regulation (EC) 1099/2009.
The innovation lies in employing high expansion foam filled with N2 to displace air in a container and using a nitrogen jet to burst the bubbles, thus creating anoxia—a process that necessitates a thorough risk assessment. Consequently, the welfare risk assessment primarily addressed potential hazards associated with the foam rather than anoxia itself, with appropriate preventive and corrective measures identified.
Considering the data available from the dossier and the available scientific literature, the present assessments are limited for poultry to laying hens and broiler chickens of all ages and for pigs to pigs weighing between 15 and 41 kg. Considering the available data on young chicks exposed to anoxia through inert gases, it is plausible to extend the use of NEFS to chickens of other ages (than laying/slaughter age) including chicks up to 7 days old with a certainty > 50%–100% (more likely than not).
The results provided do not indicate that mucosal irritation occurs due to exposure of animals to the foam. Therefore, it is concluded with a certainty > 50%–100% (more likely than not) that no mucosal irritation due to foam occurs.
As animals exposed to the foam did not show aspiration or cough reflex, thus it is considered with a certainty of > 50%–100% that no foam enters the upper respiratory tract of animals in conscious state subjected to NEFS.
The hazards associated with authorised methods applied to kill individual animals and groups of animals in containers or crates share similarities with NEFS allowing for the determination of equivalence through a qualitative assessment.
5.2. Extent to which the proposed method and information provided with the application meets the eligibility criteria of EFSA's guidelines (ToR 1)
The following eligibility criteria are met by the NEFS dossier:
The name of the method was provided.
Description of the method including potential sources of pain, distress and/or suffering was provided.
Key parameters required for effective use of the method were provided.
Scientific basis of induction and maintenance of unconsciousness for the method was provided.
Potential causes of system failure and chances of occurrence have been identified.
Individual studies submitted partially fulfil the eligibility criteria of EFSA's guidelines. Specifically, there were some deficiencies regarding the method (see Subsection 3.2.2.1), the measurement of the outcomes (see Subsection 3.2.2.2) and the report of outcomes and estimations (see Subsection 3.2.3.1).
Scientific publications provided by the applicant contained information to partially fulfil the requirements for assessment phase one (eligibility criteria of EFSA guidelines). Submitted papers had inadequacies, notably either failed to record EEGs or failed to correlate neurological measures with the behavioural measures (ABMs).
The submitted dossier lacked comprehensive welfare outcome data (neurological, physiological, behavioural, and/or physical reflexes) for the NEFS method across all types and production stages of pigs and poultry. Thus, the assessment was limited to the animal categories for which scientific data and literature were available.
The dossier contained field data, i.e. number of different species of animals killed, aiming in determining external validity. However, experimental details or specific animal welfare outcomes (failures) were not disclosed.
There was sufficient information to perform assessment phase two (risk assessment of the proposed method), particularly about pain, distress or suffering caused to certain types of pigs and poultry exposed to NEFS.
5.3. Extent to which high expansion foam can provide a level of animal welfare at least equivalent to that ensured by the existing methods in the legislation (ToR 2)
The AHAW Panel concluded with a certainty of > 50%–100% (more likely than not) that:
Exposure of pigs to high expansion foam (expansion ratio 1:> 250) filled with nitrogen leading to anoxia (< 2% by volume of residual oxygen) (achieved in less than a minute) ensures a level of animal welfare at least equivalent to that ensured by exposure to carbon dioxide (CO 2 ) at high concentration.
Exposure of poultry to high expansion foam (expansion ratio 1:> 250) filled with nitrogen leading to anoxia (< 2% by volume of residual oxygen) provides a level of animal welfare at least equivalent to that ensured by exposure to CO 2 at high concentration, especially during whole house gassing, as well as by electrocution using electrical water bath.
5.3.1. Whether the high expansion foam method ensures that pigs and poultry are spared of avoidable pain, distress or suffering during the killing (ToR 2i)
The procedures for animal handling, stunning and killing pigs and poultry using anoxia (< 2% by volume of residual oxygen) generated by using high expansion foam (expansion ratio 1:> 250) filled with nitrogen are at least equivalent to those procedures involved in containerised gas methods currently prescribed in the legislation.
Additionally, as with all methods, if the initial attempt to stun and kill the animals is unsuccessful, they may regain consciousness, which could lead to pain, distress or suffering.
5.3.1.1. To what extent and under which conditions
The applicant stated in the dossier that the NEFS equipment will be supplied to the end user along with SOPs and operating instructions. Deviation of the key parameters necessary to achieve killing by the end user, who may not be aware of the welfare consequences, places an additional risk.
Animals will be spared of avoidable pain, distress or suffering only when:
Animal handlers are adequately trained and certified, ensuring they possess the necessary knowledge and skills,
All the key parameters are strictly adhered to,
The equipment fit for the purpose is set up and used according to established standard operating procedures (SOPs),
The stocking density is appropriate to avoid air pockets and ensure that all the animals can lie down and are simultaneously exposed to anoxia.
Additionally, as with all methods, if animals are not successfully stunned and killed on the first attempt, they may regain consciousness, potentially leading to pain, distress or suffering. Therefore, it is crucial to:
Confirm death before proceeding with carcass disposal,
Have a backup killing method ready appropriate to the categories of animals to be used on any animal showing signs of consciousness or life.
5.3.2. Whether the high expansion foam method maintains the loss of consciousness and sensibility until the death of pigs and poultry, to what extent and under which conditions (ToR 2ii)
NEFS maintains the loss of consciousness and sensibility until the death of pigs and poultry, as referred to in paragraph 1 of Article 4 of the killing Regulation, provided that oxygen levels remain below 2% by volume in nitrogen throughout the entire process. The duration of exposure to anoxia is adequate and appropriate to kill the specified species and categories of animals specified above.
6. RECOMMENDATIONS
The procedure of stunning and killing should start only when information from all sensors of all critical parts of the system is appropriate. To minimise the probability of malfunction, fully automated control of the procedure including possibility of procedure start only with all critical parameters in their ranges is advised.
Animals should be placed in the foaming container only when the operator is ready to kill them without delay.
The operator should check the amount of N2 gas and the volume of foaming solution adequate to fill the container and achieve the target O2 level.
The nitrogen jet stream used to destroy the foam should be directed to the wall of the container, instead of directing towards the animals, such that it deflects and creates turbulence and lead to complete destruction of the foam and release the N2 within it. Such an arrangement will eliminate the hazard associated with jest stream of N2 hitting the animals.
Temporary partitions should not be used in the container to minimise the volume of foam required to killing the animal.
Ensure the container is not overloaded, i.e. all animals can lie down, to allow thorough foam percolation without trapping air pockets.
In case the system fails (animals survived), the source(s) of the problem should be investigated and rectified before resuming the operation.
The operator should be trained to have adequate knowledge, understanding and skills set required to operate the equipment.
A backup killing method appropriate to the categories of animals should be available to kill animals showing signs of consciousness or life.
Continuously monitor and maintain oxygen levels below 2% in the foaming container throughout the process.
Oxygen sensors should be calibrated regularly at the start of each shift and records maintained.
There should be provisions to avoid foaming solution entering gas monitoring system and interfere with the accurate measurement of residual oxygen in the container.
There should be a reliable monitoring system in the container to confirm death before opening the door.
The container should have a non‐slippery floor to ensure animals do not slip and fall leading to injury before the onset of unconsciousness.
The floor of the container should be clean and dry to avoid slipping and falling of animals, especially pigs.
The container itself may also be designed and constructed in a ‘dome’ shape, rather than rectangular, to avoid air pockets, especially in the corners.
The foaming container, irrespective of its size, should be filled with the foam in less than a minute to avoid or limit potential adverse welfare outcomes.
The nitrogen pulse delivered to destroy the foam and release its contents should begin without delay as soon as the container is filled.
There should be provisions to avoid system failure due to freezing of the gas delivery system (e.g. regulator or the solenoid valve) halfway through filling of the container with the foam, especially when the system is used in sub‐zero ambient temperatures.
Quality of the water, especially softness, should be assessed to ensure the source is fit for the purpose, as hard water is not conducive to making foam with uniform bubble size and, possibly, expansion ratio.
It should be ensured that the information provided in the SOPs and user manuals are up to date and provides adequate information to the end users to identify the source(s) of system failure, if any, and guide them to rectify the defect.
Potential methods to improve monitoring, in order for the foam not to hinder the clear observation of the animals could include: Implementation of transparent or semi‐transparent foam materials that allow for better visibility of the animals, the integration of surveillance technology, such as waterproof cameras, within the foam application area to provide real‐time monitoring capabilities or the use of sensors or other non‐visual monitoring tools to better track physiological and behavioural indicators, offering an alternative to visual observation.
Further research is necessary to validate results, evaluate pain for example using analgesic treatments, employ additional ABMs and replicate experiments across different categories of pigs and poultry.
The present version of the EFSA Guidance about the requirements needed both for a dossier submitted with a new or modified stunning/killing method and for the procedure of assessment should be revised.
7. DOCUMENTATION AS PROVIDED TO EFSA
High‐expansion foam. December 2022.
First additional data and updated dossier. May 2023.
Second additional data and updated dossier. October 2023. Submitted by Anoxia BV (RASA).
GLOSSARY
- Mandibulation
Repetitive and rapid opening and closing of the bill, not associated with inspiration or exhalation (McKeegan & Martin, 2020).
ABBREVIATIONS
- ABM
Animal Based Measure
- AHAW Panel
EFSA Panel on Animal Health and Welfare
- CAS
Controlled Atmosphere Stunning
- CO2
Carbon dioxide
- ECG
Electrocardiography
- EEG
Electroencephalography
- LAPS
Low Atmospheric Pressure Stunning
- LOC
Loss of Consciousness
- LOP
Loss of Posture
- N2
Nitrogen
- NEFS
Nitrogen Expansion Foam Stunning
- PETCO2
Pressure end‐tidal CO2
- SEP
Somatosensory Evoked Potential
- SO
Scientific Opinion
- SOP
Standard Operating Procedure
- ToR
Term of Reference
- WC
Welfare Consequence
- WG
Working Group
CONFLICT OF INTEREST
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
REQUESTOR
European Commission
QUESTION NUMBER
EFSA‐Q‐2022‐00344
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
PANEL MEMBERS
Søren Saxmose Nielsen, Julio Alvarez, Dominique Joseph Bicout, Paolo Calistri, Elisabetta Canali, Julian Ashley Drewe, Bruno Garin‐Bastuji, Jose Luis Gonzales Rojas, Christian Gortázar, Mette S. Herskin, Virginie Michel, Miguel Ángel Miranda Chueca, Barbara Padalino, Helen Clare Roberts, Hans Spoolder, Karl Stahl, Antonio Velarde, Christoph Winckler and Arvo Viltrop.
LEGAL NOTICE
Relevant information or parts of this scientific output have been blackened in accordance with the confidentiality requests formulated by the applicant pending a decision thereon by the European Food Safety Authority. The full output has been shared with the European Commission and the applicant. The blackening will be subject to review once the decision on the confidentiality requests is adopted by the European Food Safety Authority.
Supporting information
Dossier: Main document and necessary additional files for the method
ACKNOWLEDGEMENTS
The Panel wishes to thank the following for the support provided to this scientific output: the hearing experts Alexandra Contreras, Antoni Dalmau, Santiago Rucinque, Aranzazu Varvaro.
ANNEX A. List of compared killing methods and related hazards in chickens
A.1.
TABLE A.1.
List of killing methods compared with NEFS in container in chicken.
| Killing methods | Description of the method a |
|---|---|
| Whole house gassing with CO 2 | Section 3.4.3.1 |
| CO 2 in two phases in container | Section 3.4.3.3 |
| CO 2 + inert gases in containers | Section 3.4.3.3 |
| Inert gases in containers | Section 3.4.3.3 |
| LAPS | Section 3.4.3.4 |
| Electrical waterbath | Section 3.4.2.1 |
| Head‐only electrical stunning | Section 3.4.2.2 |
| Captive bolt | Section 3.4.4.1 |
| Percussive blow to the head | Section 3.4.4.2 |
| Cervical dislocation | Cervical dislocation following stunning – Section 3.4.4.4 |
| Lethal injection | Section 3.4.5 |
The sections refer to the Scientific opinion on methods for killing for purposes other than slaughter (poultry) (EFSA AHAW Panel, 2019. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2019.5850).
TABLE A.2 List of identified hazards in chickens for each respective killing method.
| Hazards | NEFS in containers | Whole house gassing with CO2 | CO2 in two phases in containers | CO2 + inert gases in containers | Inert gases in contain‐ners | LAPS | Electrical waterbath | Head‐only electri‐cal stunning | Captive bolt | Percussive blow to the head | Cervical dislocation | Lethal injection |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Handling | x | x | x | x | x | x | x | x | x | x | x | |
| Manual restraint | x | x | x | x | x | x | x | |||||
| Inversion | x | x | x | x | x | |||||||
| Shackling | x | |||||||||||
| Pre‐stun shocks | x | |||||||||||
| Loud noise | x | x | x | x | x | x | ||||||
| Substance irritating the mucosa | x | x | x | x | x | |||||||
| Surrounded by foam | x | |||||||||||
| Jet stream of gas at bird level | x | x | x | x | x | |||||||
| Hurting | x | x | x | x | x | x | x | x | x | x | x | x |
| Expansion of gases in the body cavity | x | x | ||||||||||
| Inhalation of the foam | x | x | ||||||||||
| Too short exposure time | x | x | x | x | x | x | x | x | ||||
| Too small bubble size | x | |||||||||||
| Low foam production rate | x | |||||||||||
| Too low gas concentration | x | x | x | |||||||||
| Too low temperature | x | x | x | x | x | x | ||||||
| Inhalation of high CO 2 concentra‐tion | x | x | x | |||||||||
| Overloading | x | x | x | x | x | x | ||||||
| Too fast decompres‐sion | x | x | ||||||||||
| Poor electrical contact | x | x | ||||||||||
| Inappropriate electrical parameters | x | x | ||||||||||
| Incorrect shooting position | x | |||||||||||
| Incorrect captive bolt parameters | x | |||||||||||
| Incorrect application of the manual method | x | x | ||||||||||
| Inappropriate route of administration | x | |||||||||||
| Sublethal dose | x |
TABLE A.3.
List of identified hazards in chickens along with their corresponding welfare consequences and references.
| Hazards | Welfare consequences a | Reference to the description of the hazard a |
|---|---|---|
|
1. Handling |
Pain, fear, distress | Section 3.6.1.2 |
|
2. Manual restraint |
Pain, fear | Section 3.6.1.5 |
|
3. Inversion |
Pain, fear | Section 3.6.1.4 |
|
4. Shackling |
Pain, fear | Section 3.6.1.6, 3.6.1.7 |
|
5. Pre‐stun shocks |
Pain | Section 3.6.1.8 |
|
6. Loud noise |
Fear | Unexpected loud noise ‐ Section 3.6.1.3 |
|
7. Substance irritating the mucosa |
Pain | |
|
8. Surrounded by foam |
Fear | |
|
9. Jet stream of gas at birds' level |
Pain, fear | Section 3.6.1.16 |
|
10. Hurting |
Pain | |
|
11. Expansion of gases in the body cavity |
Pain | Section 3.6.1.22 |
|
12. Inhalation of the foam |
Pain | Section 4.1.2.1 |
|
13. Too short exposure time |
Not dead, consciousness, pain, fear, respiratory distress (only for gas method) | Section 3.6.1.10 |
|
14. Too small bubble size |
Respiratory distress (suffocation), pain, fear | Section 3.6.1.17 |
|
15. Low foam production rate |
Not dead, consciousness, fear | Section 3.6.1.18 |
|
16. Too low gas concentration |
Not dead, consciousness, pain, fear, respiratory distress | Section 3.6.1.15 |
|
17. Too low temperature (of gas and/or water) |
Cold stress | Section 3.6.1.14 |
|
18. Inhalation of high CO 2 concentrations |
Pain, fear, respiratory distress | Section 3.6.1.19 |
|
19. Overloading |
Pain, fear, respiratory distress | Sections 3.6.1.20, 3.9.2 (only chicks) |
|
20. Too fast decompression |
Pain, respiratory distress | Section 3.6.1.21 |
|
21. Poor electrical contact |
Not dead, consciousness, pain, fear | Section 3.6.1.9 |
|
22. Inappropriate electrical parameters |
Not dead, consciousness, pain, fear | Section 3.6.1.11 |
|
23. Incorrect shooting position |
Not dead, consciousness, pain, fear | Section 3.6.1.24 |
|
24. Incorrect captive bolt parameters |
Not dead, consciousness, pain, fear | Section 3.6.1.26 |
|
25. Incorrect application of the manual method |
Not dead, consciousness, pain, fear | Section 3.6.1.25 |
|
26. Inappropriate route of administration |
Not dead, consciousness, pain, distress | Section 3.6.1.26 |
|
27. Sublethal dose |
Not dead, consciousness, pain, distress | Section 3.6.1.27 |
Scientific Opinion on methods for killing for purposes other than slaughter (poultry) (EFSA AHAW Panel, 2019. https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2019.5850).
ANNEX B. List of compared killing methods and related hazards in pigs
B.1.
TABLE B.1.
List of killing methods compared with NEFS in container for pigs.
| Killing methods | Description of the method (reference) a |
|---|---|
| Whole house gassing with CO 2 | |
| CO 2 at high concentration in containers | Section 3.3.7 |
| CO 2 + inert gases in containers | Section 3.3.7 |
| Inert gases in containers | Section 3.3.7 |
| Electrical killing | Section 3.3.2 |
| Captive bolt | Penetrative captive bolt stunning followed by a killing method ‐ Section 3.3.3 |
| Non‐penetrative captive bolt | Section 3.3.4 |
| Percussive blow to the head | Percussive blow to the head followed by a killing method in neonatal piglets ‐ Section 3.3.5 |
| Firearm | Firearm with free projectile killing method – Section 3.3.6 |
| Lethal injection | Section 3.3.9 |
The sections refer to the Scientific opinion on the welfare of pigs during killing for purposes other than slaughter (EFSA AHAW Panel, 2020a, 2020b. https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2020.6195).
TABLE B.2 List of identified hazards in pigs for each respective killing method.
| Hazards | NEFS in containers | Whole house gassing with CO2 | CO2 at high concentration in containers | CO2 + Inert gases in containers | Inert gases in containers | Electrical killing | Captive bolt | Non‐penetrative captive bolt | Percussive blow to the head | Firearm | Lethal injection |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Handling | x | x | x | x | x | x | x | x | x | x | |
| Restraint | x | x | x | x | x | ||||||
| Inversion | x | ||||||||||
| Loud noise | x | x | x | x | x | ||||||
| Slipping | x | x | x | x | x | ||||||
| Substance irritating the mucosa | x | x | x | x | |||||||
| Surrounded by foam | x | ||||||||||
| Jet stream of gas at pigs' level | x | x | x | x | x | ||||||
| Hurting | x | x | x | x | x | x | x | x | x | x | x |
| Too short exposure time | x | x | x | x | x | x | |||||
| Overloading | x | x | x | x | |||||||
| Too small bubble size | x | ||||||||||
| Low foam production rate | x | ||||||||||
| Inhalation of foam | x | ||||||||||
| Exposure to high CO 2 concentra‐tions | x | x | x | ||||||||
| Too low gas concentration | x | x | x | x | x | ||||||
| Too low temperature of the gas | x | x | x | x | x | ||||||
| Wrong placement of the electrodes | x | ||||||||||
| Poor electrical contact | x | ||||||||||
| Inappropriate electrical parameters | x | ||||||||||
| Incorrect shooting position | x | x | x | ||||||||
| Incorrect captive bolt parameters | x | x | |||||||||
| Sublethal dose of chemical | x | ||||||||||
| Incorrect application of the blow to the head | x | ||||||||||
| Inappropriate power and calibre of the cartridge | x | ||||||||||
| Inappropriate type of projectile | x | ||||||||||
| Inappropriate route of administra‐tion | x | ||||||||||
| Sublethal dose | x |
TABLE B.3.
List of identified hazards in pigs, along with their corresponding welfare consequences and references.
| Hazards | Welfare consequences a | Description of the hazard a |
|---|---|---|
|
1. Handling |
Pain, fear, impeded movement | Section 3.2 |
|
2. Restraint |
Pain, fear | Sections 3.3.3.1, 3.3.4.1, 3.3.9.4 |
|
3. Inversion |
Pain, fear | Section 3.3.5.1 |
|
4. Loud noise |
Fear | |
|
5. Slipping |
Pain and fear | |
|
6. Substance irritating the mucosa |
Pain | |
|
7. Surrounded by foam |
Fear | |
|
8. Jet stream of gas at pigs' level |
Pain and fear | |
|
9. Hurting |
Pain | |
|
10. Too short exposure time |
Pain, fear, respiratory distress | Sections 3.3.2.1, 3.3.7.1, 3.3.8.1 |
|
11. Overloading 1 |
Pain, fear | Sections 3.3.2.1, 3.3.3.1, 3.3.4.1, 3.3.5.1, 3.3.6.1, 3.3.7.1, 3.3.8.1, 3.3.9.1 |
|
12. Too small bubble size |
Pain, fear and respiratory distress | Sections 3.3.8.1 |
|
13. Low foam production rate |
Pain, fear and respiratory distress | Sections 3.3.8.1 |
|
14. Inhalation of the foam |
Respiratory distress | |
|
15. Exposure to high CO 2 concentrations |
Pain, fear, respiratory distress | Section 3.3.7.1 |
|
16. Too low gas concentration |
Respiratory distress | Section 3.3.7.1 |
|
17. Too low temperature of the gas |
Pain | Section 3.3.7.1 |
|
18. Wrong placement of the electrodes |
Pain, fear | Section 3.3.2.1 |
|
19. Poor electrical contact |
Pain, fear | Section 3.3.2.1 |
|
20. Inappropriate electrical parameters |
Pain, fear | Section 3.3.2.1 |
|
21. Incorrect shooting position |
Pain, fear | Sections 3.3.3.1, 3.3.4.1, 3.3.6.1 |
|
22. Incorrect captive bolt parameters |
Pain, fear | Section 3.3.3.1 |
|
23. Sublethal dose of chemical |
Pain, fear | Section 3.3.3.1 |
|
24. Incorrect application of the blow to the head |
Pain, fear | Section 3.3.5.1 |
|
25. Inappropriate power and calibre of the cartridge |
Pain, fear | Section 3.3.6.1 |
|
26. Inappropriate type of projectile |
Pain, fear | Section 3.3.6.1 |
|
27. Inappropriate route of administration |
Pain | Section 3.3.9.1 |
|
28. Sublethal dose |
Pain | Section 3.3.9.1 |
This includes also the hazard ‘disposal of pigs when alive’.
Scientific opinion on the welfare of pigs during killing for purposes other than slaughter (EFSA AHAW Panel, 2020a, 2020b. https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2020.6195).
ANNEX C. Dossier: Main document and necessary additional files for the method
C.1.
Annex C is available under the Supporting Information section on the online version of the scientific output.
EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen, S. S. , Alvarez, J. , Bicout, D. J. , Calistri, P. , Canali, E. , Drewe, J. A. , Garin‐Bastuji, B. , Gonzales Rojas, J. L. , Gortázar, C. , Herskin, M. S. , Miranda Chueca, M. Á. , Padalino, B. , Roberts, H. C. , Spoolder, H. , Stahl, K. , Velarde, A. , Winckler, C. , Viltrop, A. , … Michel, V. (2024). The use of high expansion foam for stunning and killing pigs and poultry. EFSA Journal, 22(7), e8855. 10.2903/j.efsa.2024.8855
Adopted: 3 June 2024
Annex C is available under the Supporting Information section
Note: A plain language summary of this scientific opinion is available at https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2024.p220702
Notes
Regulation 178/2002/EC, of 28 January 2002, laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002.
REFERENCES
- Atkinson, S. , Algers, B. , Pallisera, J. , Velarde, A. , & Llonch, P. (2020). Animal welfare and meat quality assessment in gas stunning during commercial slaughter of pigs using hypercapnic‐hypoxia (20% CO2 2% O2) compared to acute hypercapnia (90% CO2 in air). Animals, 10(12), 2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AVMA . (2020). Guidelines for the euthanasia of animals. 2020 edition, version 2020.01. (Chapters M1.2 and M1.5).
- Baker‐Cook, B. I. , Torrey, S. , Turner, P. V. , Knezacek, T. D. , Nicholds, J. , Gomis, S. , & Schwean‐Lardner, K. (2021). Assessing the effect of water deprivation on the efficacy of on‐farm euthanasia methods for broiler chickens. British Poultry Science, 62(2), 157–165. [DOI] [PubMed] [Google Scholar]
- Balzer, K. (2017). Tierschutzgerechte Betäubung und Tötung von nicht‐überlebensfähigen Ferkeln mit einem Stickstoff‐angereicherten Schaum im Erzeugerbetrieb. [Doctoral dissertation]. Hannover: Tierärztliche Hochschule Hannover.
- Bandara, R. M. A. S. , Torrey, S. , Turner, P. V. , Schwean‐Lardner, K. , & Widowski, T. M. (2019). Anatomical pathology, behavioral, and physiological responses induced by application of non‐penetrating captive bolt devices in layer chickens. Frontiers in Veterinary Science, 6. 10.3389/fvets.2019.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banzett, R. B. , Lansing, R. W. , & Binks, A. P. (2021). Air hunger: A primal sensation and a primary element of dyspnea. Comparative Physiology, 11(2), 1449–1483. 10.1002/cphy.c200001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benacka, R. , & Tomori, Z. (1995). The sniff‐like aspiration reflex evoked by electrical stimulation of the nasopharynx. Respiratory Physiology, 102, 163–174. [DOI] [PubMed] [Google Scholar]
- Berg, C. , Yngvesson, J. , Nimmermark, S. , Sandström, V. , & Algers, B. (2014). Killing of spent laying hens using CO2 in poultry barns. Animal Welfare, 23(4), 445–457. [Google Scholar]
- Brietzke, J. , Jaskulke, S. , Kanitz, E. , Tuchscherer, M. , Otten, W. , Schön, P. C. , Tuchscherer, A. , Manteuffel, G. , & Gimsa, U. (2013). 33. Neuroendocrine modulation of the immune system by repeated anticipation in domestic pigs. Brain, Behavior, and Immunity, 32, e10. [Google Scholar]
- Burton, R. R. , & Carlisle, J. C. (1969). Acute hypoxia tolerance of the chick. Poultry Science, 48(4), 1265–1268. 10.3382/ps.0481265 [DOI] [PubMed] [Google Scholar]
- Casey‐Trott, T. M. , Millman, S. T. , Turner, P. V. , Nykamp, S. G. , & Widowksi, T. M. (2013). Effectiveness of a non‐penetrating captive bolt for euthanasia of piglets less than 3 d of age. Journal of Animal Science, 91, 5477–5484. [DOI] [PubMed] [Google Scholar]
- Casey‐Trott, T. M. , Millman, S. T. , Turner, P. V. , Nykamp, S. G. , Lawlis, P. C. , & Widowksi, T. M. (2014). Effectiveness of a non‐pentrating captive bolt for euthanasia of 3 kg to 9 kg pigs. Journal of Animal Science, 92, 5166–5174. [DOI] [PubMed] [Google Scholar]
- Coenen, A. M. L. , Lankhaar, J. , Lowe, J. C. , & McKeegan, D. E. F. (2009). Remote monitoring of electroencephalogram, electrocardiogram, and behavior during controlled atmosphere stunning in broilers: implications for welfare. Poultry Science, 88(1), 10–19. [DOI] [PubMed] [Google Scholar]
- Conlee, K. M. , Stephens, M. L. , Rowan, A. N. , & King, L. A. (2005). Carbon dioxide for euthanasia: Concerns regarding pain and distress, with special reference to mice and rats. Laboratory Animals, 39(2), 137–161. 10.1258/0023677053739747 [DOI] [PubMed] [Google Scholar]
- Costa, F. A. D. , Gibson, T. J. , Oliveira, S. E. O. , Gregory, N. G. , Coldebella, A. , Faucitano, L. , Ludtke, C. B. , Buss, L. P. , & Dalla Costa, O. A. (2020). Evaluation of physical euthanasia for neonatal piglets on‐farm. Journal of Animal Science, 98(7). 10.1093/jas/skaa204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council Regulation (EC) . (2009). No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. Official Journal of the European Union, 303, 1–30. https://eur‐lex.europa.eu/legal‐content/EN/ALL/?uri=CELEX%3A32009R1099 [Google Scholar]
- Dalmau, A. , Rodríguez, P. , Llonch, P. , & Velarde, A. (2010). Stunning pigs with different gas mixtures: Aversion in pigs. Animal Welfare, 19(3), 325–333. 10.1017/S096272860000172X [DOI] [Google Scholar]
- Ede, T. , & Parsons, T. D. (2023). Cognitive tasks as measures of pig welfare: A systematic review. Frontiers in Veterinary Science, 10, 1251070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Saftey Authority) . (2004). Welfare aspects of animal stunning and killing methods. Scientific Report of the Scientific Panel for Animal Health and Welfare on a request from the Commission related to welfare aspects of animal stunning and killing methods. EFSA Journal, 45. 10.2903/j.efsa.2004.45 [DOI] [Google Scholar]
- EFSA (European Food Saftey Authority) . (2014). Guidance on Expert Knowledge Elicitation in Food and Feed Safety Risk Assessment. EFSA Journal, 12 (6), 3734. 10.2903/j.efsa.2014.3734 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) . (2012). Guidance on risk assessment for animal welfare. EFSA Journal, 10(1), 2513. 10.2903/j.efsa.2012.2513 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) . (2013). Scientific opinion on monitoring procedures at slaughterhouses for pigs. EFSA Journal, 11(12), 3523. 10.2903/j.efsa.2013.3523 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , More, S. , Bicout, D. , Bøtner, A. , Butterworth, A. , Calistri, P. , Depner, K. , Edwards, S. , Garin‐Bastuji, B. , Good, M. , Gortazar Schmidt, C. , Miranda, M. A. , Nielsen, S. S. , Sihvonen, L. , Spoolder, H. , Willeberg, P. , Raj, M. , Thulke, H. , Velarde, A. , … Michel, V. (2017). Low atmospheric pressure system for stunning broiler chickens. EFSA Journal, 15(12), 5056. 10.2903/j.efsa.2017.5056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Animal Welfare) , More, S. , Bicout, D. , Bøtner, A. , Butterworth, A. , Calistri, P. , Depner, K. , Edwards, S. , Garin‐Bastuji, B. , Good, M. , Gortazar Schmidt, C. , Miranda, M. A. , Nielsen, S. S. , Velarde, A. , Thulke, H. , Sihvonen, L. , Spoolder, H. , Stegeman, J. A. , Raj, M. , … Michel, V. (2018). Guidance on the assessment criteria for applications for new or modified stunning methods regarding animal protection at the time of killing. EFSA Journal, 16(7), 5343. 10.2903/j.efsa.2018.5343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) . (2019). Killing for purposes other than slaughter: Poultry. EFSA Journal, 17(11), 5850. 10.2903/j.efsa.2019.5850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen, S. S. , Alvarez, J. , Bicout, D. J. , Calistri, P. , Depner, K. , Drewe, J. A. , Garin‐Bastuji, B. , Gonzales Rojas, J. L. , Gortázar Schmidt, C. , Michel, V. , Miranda Chueca, M. Á. , Roberts, H. C. , Sihvonen, L. H. , Spoolder, H. , Stahl, K. , Viltrop, A. , Winckler, C. , Candiani, D. , … Velarde, A. (2020a). Welfare of pigs at slaughter. EFSA Journal, 18(6), 6148. 10.2903/j.efsa.2020.6148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Saxmose Nielsen, S. S. , Alvarez, J. , Bicout, D. J. , Calistri, P. , Depner, K. , Drewe, J. A. , Garin‐Bastuji, B. , Gonzales Rojas, J. L. , Gortázar Schmidt, C. , Michel, V. , Miranda Chueca, M. Á. , Roberts, H. C. , Sihvonen, L. H. , Spoolder, H. , Stahl, K. , Viltrop, A. , Winckler, C. , Candiani, D. , … Velarde, A. (2020b). Welfare of pigs during killing for purposes other than slaughter. EFSA Journal, 18(7), e06195. 10.2903/j.efsa.2020.6195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , Nielsen, S. S. , Alvarez, J. , Bicout, D. J. , Calistri, P. , Canali, E. , Drewe, J. A. , Garin‐Bastuji, B. , Gonzales Rojas, J. L. , Schmidt, G. , Herskin, M. , Michel, V. , Miranda Chueca, M. Á. , Mosbach‐Schulz, O. , Padalino, B. , Roberts, H. C. , Stahl, K. , Velarde, A. , Viltrop, A. , … Spoolder, H. (2022). Welfare of pigs on farm. EFSA Journal, 20(8), 7421. 10.2903/j.efsa.2022.7421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford, D. , Halldorsson, T. , Jeger, M. J. , Knutsen, H. K. , More, S. , Naegeli, H. , Noteborn, H. , Ockleford, C. , Ricci, A. , Rychen, G. , Schlatter, J. R. , Silano, V. , Solecki, R. , Turck, D. , Younes, M. , Craig, P. , Hart, A. , Von Goetz, N. , … Hardy, A. (2018a). Guidance on uncertainty analysis in scientific assessments. EFSA Journal, 16(1), 5123. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Benford, D. , Halldorsson, T. , Jeger, M. J. , Knutsen, H. K. , More, S. , Naegeli, H. , Noteborn, H. , Ockleford, C. , Ricci, A. , Rychen, G. , Schlatter, J. R. , Silano, V. , Solecki, R. , Turck, D. , Younes, M. , Craig, P. , Hart, A. , Von Goetz, N. , … Hardy, A. (2018b). Scientific Opinion on the principles and methods behind EFSA's guidance on uncertainty analysis in scientific assessment. EFSA Journal, 16(1), 5122. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erasmus, M. A. , Turner, P. V. , & Widowski, T. M. (2010). Measures of insensibility used to determine effective stunning and killing of poultry. Journal of Applied Poultry Research, 19(3), 288–298. [Google Scholar]
- EURCAW‐Poultry‐SFA . (2023). Answer_Q2E‐EURCAW‐Poultry‐SFA‐2021‐006: overview of the different on‐farm killing methods for turkeys and ducks, due to disease control situations (avian influenza). https://zenodo.org/records/10058017
- Gent, T. C. , Gebhardt‐Henrich, S. , Schild, S.‐L. A. , Rahman, A. A. , & Toscano, M. J. (2020). Evaluation of poultry stunning with low atmospheric pressure, carbon dioxide or nitrogen using a single aversion testing paradigm. Animals, 10(8), 1308. 10.3390/ani10081308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerritzen, M. , Reimert, H. , Hindle, V. , McKeegan, D. , & Sparrey, J. (2010). Welfare assessment of gas filled foam as an agent for killing poultry. https://research.wur.nl/en/publications/welfare‐assessment‐of‐gas‐filled‐foam‐as‐an‐agent‐for‐killing‐pou
- Gerritzen, M. A. , Lambooij, B. , Reimert, H. , Stegeman, A. , & Spruijt, B. (2004). On‐farm euthanasia of broiler chickens: Effects of different gas mixtures on behavior and brain activity. Poultry Science, 83, 1294–1301. 10.1093/ps/83.8.1294 [DOI] [PubMed] [Google Scholar]
- Gerritzen, M. A. , Lambooij, E. , Hillebrand, S. J. , Lankhaar, J. A. , & Pieterse, C. (2000). Behavioral responses of broilers to different gaseous atmospheres. Poultry Science, 79(6), 928–933. https://www.sciencedirect.com/science/article/pii/S0032579119415666 [DOI] [PubMed] [Google Scholar]
- Gerritzen, M. A. , Reimert, H. G. M. , Hindle, V. A. , Verhoeven, M. T. W. , & Veerkamp, W. B. (2013). Multistage carbon dioxide gas stunning of broilers. Poultry Science, 92(1), 41–50. 10.3382/ps.2012-02551 [DOI] [PubMed] [Google Scholar]
- Gerritzen, M. H. , & Sparrey, J. (2008). A pilot study to assess whether high expansion CO2‐enriched foam is acceptable for on‐farm emergency killing of poultry. Animal Welfare, 17, 285–288. [Google Scholar]
- Graml, C. , Niebuhr, K. , & Waiblinger, S. (2008). Reaction of laying hens to humans in the home or a novel environment. Applied Animal Behaviour Science, 113(1–3), 98–109. 10.1016/j.applanim.2007.10.004 [DOI] [Google Scholar]
- Grist, A. , Murrell, J. , McKinstry, J. , Knowles, T. , & Wotton, S. (2017). Humane euthanasia of neonates I: Validation of the effectiveness of the Zephyr EXL non‐penetrating captive‐bolt euthanasia system on neonate piglets up to 10.9 kg live‐weight. Animal Welfare, 26(1), 111–120. 10.7120/09627286.26.1.111 [DOI] [Google Scholar]
- Grist, A. , Lines, J. A. , Knowles, T. G. , Mason, C. W. , & Wotton, S. B. (2018a). The use of a non‐penetrating captive bolt for the euthanasia of neonate piglets. Animals, 8, 48. 10.3390/ani8040048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grist, A. , Knowles, T. G. , & Wotton, S. B. (2018b). Humane euthanasia of neonates II: field study of the effectiveness of the Zephyr EXL non‐penetrating captive‐bolt system for euthanasia of newborn piglets. Animal Welfare, 27, 319–326. 10.7120/09627286.27.4.319 [DOI] [Google Scholar]
- Gurung, S. , White, D. , Archer, G. , Zhao, D. , Farnell, Y. , Byrd, J. A. , Peebles, E. D. , & Farnell, M. (2018). Evaluation of alternative euthanasia methods of neonatal chickens. Animals, 8, 37. 10.3390/ani8030037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, E. , James, F. , Torrey, S. , Widowski, T. , Schwean‐Lardner, K. , Monteith, G. , & Turner, P. V. (2019a). Electroencephalographic, physiologic and behavioural responses during cervical dislocation euthanasia in turkeys. BMC Veterinary Research, 15(1), 1–14. 10.1186/s12917-019-1885-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez, E. , James, F. , Torrey, S. , Widowski, T. , Schwean‐Lardner, K. , Monteith, G. , & Turner, P. V. (2019b). Evaluation of brain death in laying hens during on‐farm killing by cervical dislocation methods or pentobarbital sodium injection. Frontiers in Veterinary Science, 6. 10.3389/fvets.2019.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, B. O. (1983). Headshaking in fowls: The effect of environmental stimuli. Applied Animal Ethology, 11(1), 45–53. [Google Scholar]
- Jacobs, L. , Bourassa, D. V. , Harris, C. E. , & Buhr, R. J. (2019). Euthanasia: manual versus mechanical cervical dislocation for broilers. Animals, 9(2), 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongman, E. C. , Woodhouse, R. , Rice, M. , & Rault, J. L. (2021). Pre‐slaughter factors linked to variation in responses to carbon dioxide gas stunning in pig abattoirs. Animal, 15(2), 100134. [DOI] [PubMed] [Google Scholar]
- Kells, N. , Beausoleil, N. , Johnson, C. , & Sutherland, M. (2018). Evaluation of different gases and gas combinations for on‐farm euthanasia of pre‐weaned pigs. Animals, 8(3), 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kells, N. , Beausoleil, N. , Sutherland, M. , & Johnson, C. (2018). Electroencephalographic responses of anaesthetised pigs to intraperitoneal injection of sodium pentobarbital. Animal Welfare, 27(3), 205–214. 10.7120/09627286.27.3.205 [DOI] [Google Scholar]
- Korenyi‐Both, J. , Vidaurre, J. , Held, T. , Campler, M. R. , Kieffer, J. , Cheng, T.‐Y. , Moeller, S. J. , Bowman, A. S. , & Arruda, A. G. (2022). Description of electroencephalographic data gathered using water‐based medium‐expansion foam as a depopulation method for nursery pigs. Scientific Reports, 12(1), 16798. 10.1038/s41598-022-21353-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambooij, E. , Gerritzen, M. A. , Engel, B. , Hillebrand, S. J. W. , Lankhaar, J. , & Pieterse, C. (1999). Behavioural responses during exposure of broiler chickens to different gas mixtures. Applied Animal Behaviour Science, 62(2–3), 255–265. 10.1016/S0168-1591(98)00214-7 [DOI] [Google Scholar]
- Lechner, I. , Léger, A. , Zimmermann, A. , Atkinson, S. , & Schuppers, M. (2021). Discomfort period of fattening pigs and sows stunned with CO2: duration and potential influencing factors in a commercial setting. Meat Science, 179, 108535. [DOI] [PubMed] [Google Scholar]
- Lindahl, C. , Sindhøj, E. , Hellgren, R. B. , Berg, C. , & Wallenbeck, A. (2020). Responses of pigs to stunning with nitrogen filled high‐expansion foam. Animals, 10(12), 1–16. 10.3390/ani10122210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llonch, P. , Dalmau, A. , Rodríguez, P. , Manteca, X. , & Velarde, A. (2012). Aversion to nitrogen and carbon dioxide mixtures for stunning pigs. Animal Welfare, 21(1), 33–39. 10.7120/096272812799129475 [DOI] [PubMed] [Google Scholar]
- Llonch, P. , Rodríguez, P. , Jospin, M. , Dalmau, A. , Manteca, X. , & Velarde, A. (2013). Assessment of unconsciousness in pigs during exposure to nitrogen and carbon dioxide mixtures. Animal, 7, 492–498. [DOI] [PubMed] [Google Scholar]
- Manteuffel, G. , Puppe, B. , & Schön, P. C. (2004). Vocalization of farm animals as a measure of welfare. Applied Animal Behaviour Science, 88(1–2), 163–182. 10.1016/j.applanim.2004.02.012 [DOI] [Google Scholar]
- Martin, J. E. , Christensen, K. , Vizzier‐Thaxton, Y. , & McKeegan, D. E. F. (2016). Effects of light on responses to low atmospheric pressure stunning in broilers. British Poultry Science, 57(5), 585–600. 10.1080/00071668.2016.1201200 [DOI] [PubMed] [Google Scholar]
- Martin, J. E. , Christensen, K. , Vizzier‐Thaxton, Y. , Mitchell, M. A. , & McKeegan, D. E. F. (2016). Behavioural, brain and cardiac responses to hypobaric hypoxia in broiler chickens. Physiology and Behavior, 163, 25–36. 10.1016/j.physbeh.2016.04.038 [DOI] [PubMed] [Google Scholar]
- Martin, J. E. , Sandilands, V. , Sparrey, J. , Baker, L. , Dixon, L. M. , & McKeegan, D. E. F. (2019). Welfare assessment of novel on‐farm killing methods for poultry. PLoS One, 14(2), 1–22. 10.1371/journal.pone.0212872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, K. , Hartmann, L. , von Wenzlawowicz, M. , Bühler, C. , & König, S. (2022). Key parameters of head‐heart electrical stunning need to be adapted to improve stunning effectiveness and meat quality in pigs of different genetic lines. Meat Science, 190, 108829. [DOI] [PubMed] [Google Scholar]
- McKeegan, D. E. , McIntyre, J. , Demmers, T. G. , Wathes, C. M. , & Jones, R. B. (2006). Behavioural responses of broiler chickens during acute exposure to gaseous stimulation. Applied Animal Behaviour Science, 99(3–4), 271–286. [Google Scholar]
- McKeegan, D. , McIntyre, J. , Demmers, T. , Lowe, J. , Wathes, C. , van den Broek, P. , Coenen, A. , & Gentle, M. (2007). Physiological and behavioural responses of broilers tocontrolled atmosphere stunning: implications for welfare. Animal Welfare, 16(4), 409–426. 10.1017/S0962728600027354 [DOI] [Google Scholar]
- McKeegan, D. E. F. , Hopkins, J. , Sparrey, J. , Sandercock, D. A. , Baker, L. , Sparks, N. H. C. , & Sandilands, V. (2013). Welfare costs and benefits of existing and novel on‐farm culling methods of poultry. Defra EVID4 Evidence Project Final Report (Rev. 06/11) (MH0145). https://sciencesearch.defra.gov.uk/ProjectDetails?ProjectId=17256
- McKeegan, D. E. F. , Reimert, H. G. M. , Hindle, V. A. , Boulcott, P. , Sparrey, J. M. , Wathes, C. M. , Demmers, T. G. M. , & Gerritzen, M. A. (2013). Physiological and behavioralresponses of poultry exposed to gas‐filled high expansion foam. Poultry Science, 92(5), 1145–1154. 10.3382/ps.2012-02587 [DOI] [PubMed] [Google Scholar]
- McKeegan, D. E. F. , Sparks, N. H. C. , Sandilands, V. , Demmers, T. G. M. , Boulcott, P. , & Wathes, C. M. (2011). Physiological responses of laying hens during whole‐house killing with carbon dioxide. British Poultry Science, 52(6), 645–657. [DOI] [PubMed] [Google Scholar]
- McKeegan, D. , & Martin, J. (2020). Improving welfare in poultry slaughter. In Understanding the behaviour and improving the welfare of chickens (pp. 459–508). Burleigh Dodds Science Publishing. [Google Scholar]
- Nodari, S. , Polloni, A. , Giacomelli, S. , Vezzoli, F. , & Galletti, G. (2014). Assessing pig welfare at stunning in Northern Italy commercial abattoirs using electrical method. Large Animal Review, 20, 87–91. [Google Scholar]
- Paul, E. S. , Edgar, J. L. , Caplen, G. , & Nicol, C. J. (2018). Examining affective structure in chickens: Valence, intensity, persistence and generalization measured using a conditioned place preference test. Applied Animal Behaviour Science, 207, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson, M. (2024). Behavioural response in pigs at gas stunning in foam. SLU. [Google Scholar]
- Raj, A. B. M. , & Gregory, N. G. (1990). Investigation into the batch stunning/killing of chickens using carbon dioxide or argon‐induced hypoxia. Research in Veterinary Science, 49, 364–366. [PubMed] [Google Scholar]
- Raj, A. B. , & Whittington, P. E. (1995). Euthanasia of day‐old chicks with carbon dioxide and argon. The Veterinary Record, 136, 292–294. [DOI] [PubMed] [Google Scholar]
- Raj, A. B. , Wotton, S. B. , McKinstry, J. L. , Hillebrand, S. J. , & Pieterse, C. (1998). Changes in the somatosensory evoked potentials and spontaneous electroencephalogram of broiler chickens during exposure to gas mixtures. British Poultry Science, 39(5), 686–695. 10.1080/00071669888584 [DOI] [PubMed] [Google Scholar]
- Raj, A. B. M. (1997). Gas stunning broilers: Welfare and product quality. In Lambooij E. (Ed.), Alternative stunning methods for poultry. ID‐DLO Institute for Animal Science and Health publication number 97.037. [Google Scholar]
- Raj, A. B. M. (1999). Behaviour of pigs exposed to mixtures of gases and the time required to stun and kill them: Welfare implications. Veterinary Record, 144, 165–168. [DOI] [PubMed] [Google Scholar]
- Raj, A. B. M. , & O'Callaghan, M. (2001). Evaluation of a pneumatically operated captive bolt for stunning/killing broiler chickens. British Poultry Science, 42(3), 295–299. 10.1080/00071660120055232 [DOI] [PubMed] [Google Scholar]
- Raj, A. B. M. (2006). Aversion to hypercapnoea or hypoxia in pigs: Behavioural responses to dyspnoea. Presented at the annual conference of the International Society for Advancement of respiratory psychophysics Newport, Rhode Island, USA, during October 21–23 2006.
- Raj, A. B. M. , & Gregory, N. G. (1995). Welfare implications of the gas stunning of pigs 1. Determination of aversion to the initial inhalation of carbon dioxide or argon. Animal Welfare, 4, 273–280. [Google Scholar]
- Raj, A. B. M. , & Gregory, N. G. (1996). Welfare implications of the gas stunning of pigs 2. Stress of induction of anaesthesia. Animal Welfare, 5, 71–78. [Google Scholar]
- Raj, A. B. M. , Gregory, N. G. , & Wotton, S. B. (1991). Changes in the somatosensory evoked potentials and spontaneous electroencephalogram of hens during stunning in argon‐induced anoxia. British Veterinary Journal, 147, 322–330. [DOI] [PubMed] [Google Scholar]
- Raj, A. B. M. , Johnson, S. P. , Wotton, S. B. , & McInstry, J. L. (1997). Welfare implications of gas stunning pigs: 3. The time to loss of somatosensory evoked potential and spontaneous electrocorticogram of pigs during exposure to gases. The Veterinary Journal, 153(3), 329–339. [DOI] [PubMed] [Google Scholar]
- Raj, A. B. M. , Richardson, R. I. , Wilkins, L. J. , & Wotton, S. B. (1998). Carcase and meat quality in ducks killed with either gas mixtures or an electric current under commercial processing conditions. British Poultry Science, 39(3), 404–407. 10.1080/00071669888962 [DOI] [PubMed] [Google Scholar]
- Raj, A. B. M. , Smith, C. , & Hickman, G. (2008). Novel method for killing poultry in houses with dry foam created using nitrogen. Veterinary Record, 162(22), 722–723. 10.1136/vr.162.22.722 [DOI] [PubMed] [Google Scholar]
- Raj, A. B. M. , Wotton, S. B. , & Gregory, N. G. (1992). Changes in the somatosensory evoked potentials and spontaneous electroencephalogram of hens during stunning with carbon dioxide and argon mixture. British Veterinary Journal, 147–156. 10.1016/0007-1935(92)90106-B [DOI] [PubMed] [Google Scholar]
- Sadler, L. J. , Karriker, L. A. , Schwartz, K. J. , Johnson, A. K. , Widowski, T. M. , Wang, C. , Sutherland, M. A. , & Millman, S. T. (2014). Are severely depressed suckling pigs resistant to gas euthanasia? Animal Welfare, 23(2), 145–155. [Google Scholar]
- Sandilands, V. (2011). The laying hen and bone fractures. The Veterinary Record, 169(2011), 411–412. [DOI] [PubMed] [Google Scholar]
- Sparks, N. H. C. , Sandilands, V. , Raj, A. B. M. , Turney, E. , Pennycott, T. , & Voas, A. (2010). Use of liquid carbon dioxide for whole‐house gassing of poultry and implications for the welfare of the birds. Veterinary Record, 167(11), 403–407. [DOI] [PubMed] [Google Scholar]
- Steiner, A. R. , Flammer, S. A. , Beausoleil, N. J. , Berg, C. , Bettschart‐Wolfensberger, R. , Pinillos, R. G. , Golledge, H. D. W. , Marahrens, M. , Meyer, R. , Schnitzer, T. , Toscano, M. J. , Turner, P. V. , Weary, D. M. , & Gent, T. C. (2019). Humanely ending the life of animals: Research priorities to identify alternatives to carbon dioxide. Animals, 9(11). 10.3390/ani9110911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland, M. A. , Bryer, P. J. , & Backus, B. L. (2017). The effect of age and method of gas delivery on carbon dioxide euthanasia of pigs. Animal Welfare, 26(3), 293–299. [Google Scholar]
- Terlouw, E. M. , Deiss, V. , & Astruc, T. (2021). Stunning of pigs with different gas mixtures: behavioural and physiological reactions. Meat Science, 175, 108452. 10.1016/j.meatsci.2021.108452 [DOI] [PubMed] [Google Scholar]
- Velarde, A. , Gispert, M. , Faucitano, L. , Alonso, P. , Manteca, X. , & Diestre, A. (2001). Effects of the stunning procedure and the halothane genotype on meat quality and incidence of haemorrhages in pigs. Meat Science, 58(3), 313–319. [DOI] [PubMed] [Google Scholar]
- Velarde, A. , Cruz, J. , Gispert, M. , Carrión, D. , Ruiz de la Torre, J. L. , Diestre, A. , & Manteca, X. (2007). Aversion to carbon dioxide stunning in pigs: Effect of carbon dioxide concentration and halothane genotype. Animal Welfare, 16(4), 513–522. 10.1017/S0962728600027445 [DOI] [Google Scholar]
- Velarde, A. , & Dalmau, A. (2012). Animal welfare assessment at slaughter in Europe: Moving from inputs to outputs. Meat Science, 92(3), 244–251. [DOI] [PubMed] [Google Scholar]
- Verhoeven, M. , Gerritzen, M. , Velarde, A. , Hellebrekers, L. , & Kemp, B. (2016). Time to loss of consciousness and its relation to behavior in slaughter pigs during stunning with 80 or 95% carbon dioxide. Frontiers in Veterinary Science, 3, 1–10. 10.3389/fvets.2016.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven, M. T. W. , Gerritzen, M. A. , Hellebrekers, L. J. , & Kemp, B. (2015). Indicators used in livestock to assess unconsciousness after stunning: A review. Animal, 9(2), 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Wenzlawowicz, M. , von Holleben, K. , & Eser, E. (2012). Identifying reasons for stun failures in slaughterhouses for cattle and pigs: a field study. Animal Welfare, 21(S2), 51–60. [Google Scholar]
- Wang, X. , Zhao, D. , Milby, A. C. , Archer, G. S. , Peebles, E. D. , Gurung, S. , & Farnell, M. B. (2021). Evaluation of euthanasia methods on behavioral and physiological responses of newly hatched male layer chicks. Animals, 2021, 11. 10.3390/ani11061802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watteyn, A. , Garmyn, A. , Ampe, B. , Jacobs, L. , Moons, C. P. , & Tuyttens, F. A. (2022). Comparison of methods for individual killing of broiler chickens: a matter of animal welfare and on‐farm feasibility. Frontiers in Animal Science, 3, 892186. [Google Scholar]
- Webster, A. B. , & Fletcher, D. L. (2001). Reactions of laying hens and broilers to different gases used for stunning poultry. Poultry Science, 80(9), 1371–1377. [DOI] [PubMed] [Google Scholar]
- Whiting, T. L. , Steele, G. G. , Wamnes, S. , & Green, C. (2011). Evaluation of methods of rapid mass killing of segregated early weaned piglets. The Canadian Veterinary Journal, 52(7), 753. [PMC free article] [PubMed] [Google Scholar]
- Wikman, I. , Hokkanen, A.‐H. , Pastell, M. , Kauppinen, T. , Valros, A. , & Hänninen, L. (2016). Attitudes of beef producers to disbudding and perception of pain in cattle. Animal Welfare, 25(4), 429–438. 10.7120/09627286.25.4.429 [DOI] [Google Scholar]
- Widowski, T. , Elgie, R. , & Lawlis, P. (2008) Assessing the effectiveness of a non‐penetrating captive bolt for euthanasia of newborn piglets. In Proceedings of the Allen D. Leman Swine Conference, St. Paul, MN, USA, 22 September 2008; Volume 107, p. 111.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dossier: Main document and necessary additional files for the method


