Abstract
Whether in performing arts, sporting, or everyday contexts, when we watch others move, we tend to enjoy bodies moving in synchrony. Our enjoyment of body movements is further enhanced by our own prior experience with performing those movements, or our ‘embodied experience’. The relationships between movement synchrony and enjoyment, as well as embodied experience and movement enjoyment, are well known. The interaction between enjoyment of movements, synchrony, and embodiment is less well understood, and may be central for developing new approaches for enriching social interaction. To examine the interplay between movement enjoyment, synchrony, and embodiment, we asked participants to copy another person's movements as accurately as possible, thereby gaining embodied experience of movement sequences. Participants then viewed other dyads performing the same or different sequences synchronously, and we assessed participants' recognition of having performed these sequences, as well as their enjoyment of each movement sequence. We used functional near‐infrared spectroscopy to measure cortical activation over frontotemporal sensorimotor regions while participants performed and viewed movements. We found that enjoyment was greatest when participants had mirrored the sequence and recognised it, suggesting that awareness of embodiment may be central to enjoyment of synchronous movements. Exploratory analyses of relationships between cortical activation and enjoyment and recognition implicated the sensorimotor cortices, which subserve action observation and aesthetic processing. These findings hold implications for clinical research and therapies seeking to foster successful social interaction.
Keywords: action observation network, embodiment, fNIRS, mirror game, motor synchrony, neuroaesthetics
We examine how embodiment gained through physically mirroring another person's movements can modulate observers' enjoyment of synchronous movements performed by dyads. The findings demonstrate how observers' ability to recognize movement sequences they have mirrored (i.e., embodied), predicts their enjoyment of the same movements performed synchronously by others, and further predicts activation of sensorimotor brain areas associated movement prediction and matching (i.e., imitation).
1. INTRODUCTION
An important component that contributes to the beauty of a corps de ballet's dancing, a synchronised swimming performance, or even a marching band in a parade, is the skilful execution of highly synchronous body movements. This observation has been theoretically formalised in two avenues of empirical aesthetics. In a first more general conceptualisation, gestalt theory states that objects (or bodies) moving smoothly and symmetrically are preferred over jerky and asymmetrical movements (Arnheim, 1974; Orgs et al., 2018). The second conceptualisation focuses more specifically on human movement in visual and performing arts: Freedberg and Gallese (2007) proposed an embodied simulation account of aesthetics, wherein viewing a visual artwork can evoke in us the movements required to produce that piece of art. Other researchers have argued that this account can also be applied to performing arts such as dance, where judgements of body movements may be influenced by the relative difference between the observed performer's motor abilities (i.e., embodiment) and our own abilities (Calvo‐Merino et al., 2005; Cross et al., 2006; Cross & Orlandi, 2020; Kirsch et al., 2015), with a number of studies documenting that a closer match between our embodied knowledge and observed movements tends to elicit more enjoyment (Calvo‐Merino et al., 2005; Kirsch et al., 2013, 2015; Kirsch & Cross, 2018). It seems plausible that this relationship between embodiment and enjoyment would remain the same when watching a dyad, or even a dozen people, move synchronously, though this remains empirically untested. In the present study, we set out to explore the extent to which embodiment influences aesthetic perceptions, particularly enjoyment, of synchronous movements, as well as the cortical mechanisms underpinning these perceptions.
1.1. Neuroaesthetics of synchronous movement
Early work examining the aesthetics of body movements (i.e., dance) demonstrated the aesthetic dimension of ‘liking’ or enjoyment of movements to have specific, verifiable neural correlates (Calvo‐Merino et al., 2008), while the aesthetic dimensions of complexity, interest, tension, and power were not (Berlyne, 1974; Calvo‐Merino et al., 2008). As a result, many ensuing studies have isolated the dimension of enjoyment as the sole element of interest. Indeed, recent work has demonstrated that ratings of enjoyment are positively correlated with the level of movement synchrony, whether among dyads or larger groups of performers (Moffat & Cross, 2024; Vicary et al., 2017). Moreover, we recently reported that enjoyment of dyadic synchrony is contingent on kinematic factors and inter‐individual differences, both at the trait‐level and in terms of perception (Moffat & Cross, 2024). In this prior work, we found that dyadic movements indexed as more synchronous and more predictable using objective measures were associated with greater subjective enjoyment. Individuals with greater empathy scores reported greater levels of enjoyment, as did those who were more adept at quantifying the degree of synchrony within a dyad's movements. Closer inspection revealed that variability in individuals' abilities to quantify synchrony was linked to individual differences in indirect measures of embodiment, including ratings of movement reproducibility and scores on a body competence scale (Moffat & Cross, 2024). These findings from our prior work shed light on the intersection of aesthetic perceptions of synchrony, kinematic features of dyadic movement, and individual differences in embodiment, but fall short of directly addressing the influence of embodiment on aesthetic appreciation of synchrony.
To test the role of embodiment on aesthetic perception, and perception more generally, some studies have compared the behavioural and neural responses of expert versus novice dancers (Calvo‐Merino et al., 2005; Christensen et al., 2016; Kirsch et al., 2016), while others have manipulated participants' degree of embodiment experimentally by teaching participants a selection of movements and subsequently comparing responses to learned and unlearned movements (Casale et al., 2023; Kirsch et al., 2013, 2015; Kirsch & Cross, 2018). In the present study, we implement the latter technique to examine how embodiment influences aesthetic perceptions of dyadic movements and the neural mechanisms underpinning these perceptions. That is, our participants engaged in mirror‐game movements (i.e., matched a partner's movements as closely as possible) and subsequently made aesthetic judgements about the mirrored dyadic movements, as well as dyadic movement sequences they had never seen before.
Our aim was to explore the link between enjoyment of synchrony and embodiment, while also considering the role of empathy. We take as a starting point our prior findings that empathy scores correlate with enjoyment of synchrony (Moffat & Cross, 2024) and that a dyad's shared level of embodiment influences the dyad's average degree of synchrony while playing the mirror game (Moffat, Roos, et al., 2024), that is, when one person moves their arms while the other tries to copy the movements as accurately as possible. Recent findings from Reiss et al. (2019) further suggest that the degree of synchrony achieved during a mirroring activity may relate to participants' empathy scores. Whereas Moffat and Cross (2024) measured ‘general’ empathy scores, calculated by taking the sum of all Interpersonal Reactivity Index (IRI) subscales (Davis, 1980), Reiss et al. (2019) measured ‘cognitive’ empathy using the sum of the perspective taking and fantasy subscales of the IRI. Considered together, these studies suggest that performance and perception of synchrony are intertwined with both embodiment and empathy. Further evidence bolstering the plausibility of this complex relationship comes from studies suggesting that individual differences in empathy may modulate neural responses in brain areas involved in observing and executing actions (Bekkali et al., 2021; Iacoboni, 2009a).
To disentangle the influences of embodiment and empathy on enjoyment of movement synchrony, we first consider the influence of observers' empathy scores on enjoyment. We then go one step further and explore whether observers might exhibit a form of embodied empathy: Might bodily experience mirroring a movement sequence with the aim of maintaining perfect synchrony influence how observers perceive another person's ability to achieve synchrony? For example, if an individual found a specific sequence of movements challenging to mirror, might that individual be more generous in their subjective rating of another person's ability to mirror the same sequence? In light of meta‐analytic evidence suggesting the inferior parietal lobule (IPL) and the inferior frontal gyrus (IFG) are loci of the most robust relationships between empathy and observing and executing actions (Bekkali et al., 2021), we explore whether this form of embodied empathy might have similar neural correlates.
1.2. Functional near‐infrared spectroscopy for measuring neuroaesthetics and embodied empathy during mirroring
To measure changes in brain activation, we employ functional near‐infrared spectroscopy (fNIRS). fNIRS is a non‐invasive brain imaging technique that can be used while participants move freely due to its relatively high robustness to motion artefacts (Balardin et al., 2017; Pinti et al., 2015). fNIRS uses light in the near‐infrared range to record changes in relative concentrations of oxygenated (HbO) and deoxygenated (HbR) haemoglobin (Ayaz et al., 2022; Huppert et al., 2006). One limitation of fNIRS is the penetration depth of the near‐infrared light: The most reliable measurements of activation are recorded from cortical regions approximately 1.5 cm below the scalp (Pinti et al., 2020). As we cannot assess the subcortical correlates of engaging in, observing, or evaluating synchronous movements with fNIRS, we focus on cortical regions of interest (ROIs) belonging to the networks involved in processing movement (executed, observed, and imagined) and formation of aesthetic evaluations.
Moving our bodies, as well as observing or imagining body movements, involves the aptly‐named action‐observation network (AON; Caspers et al., 2010; Cross et al., 2009; Hardwick et al., 2018). The AON comprises the ventral premotor cortex (PMC), inferior and middle frontal gyri (IFG/MFG), as well as the superior temporal and inferior parietal cortices. This network has been shown to respond more strongly to execution, relative to observation of movements (Bhat et al., 2017; Crivelli et al., 2018; Holper et al., 2010; Koehler et al., 2012), as well as to observation of embodied movements, relative to unknown movements (Calvo‐Merino et al., 2005; Cross et al., 2006; Kirsch et al., 2015). Within the AON, the STG encodes visual or other sensory information and projects it to IFG, PMC, and IPL (Iacoboni & Dapretto, 2006; Rizzolatti & Fogassi, 2014; Rizzolatti & Sinigaglia, 2010, 2016). Imitation, or mirroring, of movements is believed to rely most heavily on pathways between IFG, STG, and IPL. The IFG and IPL are involved in encoding the motor commands, as well as the imitative goal of the movement, while STG is thought to check the outcomes against the higher‐level sensory information it has encoded (Caspers et al., 2010; Iacoboni, 2005, 2009b; Keysers et al., 2018; Mengotti et al., 2012; Molnar‐Szakacs et al., 2005).
When we form aesthetic judgements about dynamic body stimuli, such as body movements, we rely on a network comprising visual cortices, primary motor cortex (MC), and somatosensory cortex, as well as the orbitofrontal and ventromedial frontal brain regions involved in reward processing (Kirsch et al., 2015). These evaluative processes also involve subcortical structures including the anterior insulae, amygdalae, the anterior cingulate, and the parahippocampal place area (Kirsch et al., 2015), all of which lie beyond the penetration depth of fNIRS. Moreover, a ventral pathway links the parietal regions, including IPL, to the ventral attentional network, which includes IFG and MFG (Doricchi et al., 2022). This pathway may play an important role in orienting to relevant kinematic information and cues for aesthetic judgements. These cortical components of the AON, ventral attentional network and network for aesthetic judgments overlap to a large degree, such that we can measure from all three using 12 cortical ROIs: Bilateral IFG, MFG, PMC, MC, STG, and IPL.
1.3. The present study
We set out to explore the relationship between embodiment, empathy, and enjoyment of movement synchrony at the level of behaviour and the brain. We preregistered our hypotheses and analysis plan on the Open Science Framework (OSF; https://osf.io/6xfjd), and our hypotheses were as follows: With respect to behavioural measures of action aesthetics, we hypothesised that enjoyment would increase with increasing synchrony (Hypothesis 1), and that participants would rate movements that they had mirrored as more enjoyable than unknown movements (Hypothesis 2). We also expected that participants' enjoyment would be positively correlated with their rating of another person's ability to achieve synchrony, particularly for sequences participants had mirrored relative to unknown sequences (Hypothesis 3, exploratory). We also implemented additional exploratory assessments of the relationship between participants' empathy scores and enjoyment, which were not preregistered.
In terms of the cortical activation evoked by embodiment of movement sequences, we anticipated that mirroring movement sequences and observing dyadic sequences (whether previously mirrored or unknown) will evoke increased cortical oxygenation in AON brain regions (Hypothesis 4) and that mirroring would evoke greater activation than observation across all ROIs. Additionally, we hypothesised that embodiment, measured by contrasting movements participants had mirrored versus unknown movements, would result in reduced cortical activation in PMC and MC while observing synchronous movements (Hypothesis 6).
Our final aim was to explore the relationship between action aesthetics, embodiment, and cortical activation. As such, we preregistered exploratory hypotheses without specifying ROIs or the directions of the effects. We predicted that ratings of enjoyment (Hypothesis 7) and ratings of another person's ability to synchronise (Hypothesis 8) might correlate with cortical activation. We subsequently explored a possible link to participants' empathy scores in an un‐preregistered analysis. Finally, we hypothesised that cortical activation during mirroring might be associated with enjoyment of the performed movement sequences (Hypothesis 9).
2. MATERIALS AND METHODS
2.1. Participants
Forty‐five participants were recruited from Macquarie University. As per our preregistration, this sample size was selected with the aim to triple the sample size used by Bhat et al. (2017), assess cortical activation in six ROIs during three condition (observation, execution, and observation + execution) using fNIRS. Our decision to triple the sample size exceeds Nelson et al.'s (2018) suggestion to increase the sample size by a factor of 2.5 when replicating existing studies. Moreover, this larger sample size was selected to maximise the reliability of our exploratory analyses of the links between cortical activation and behaviour (e.g., the relationship between cortical activation in each ROI during observation of performed movements and recognition of performed movements).
All participants were aged 18–40 years, had no history of head injury, neurological, or psychiatric diagnoses, and were not currently taking a psycho‐pharmaceutical medication (e.g., SSRIs or Ritalin). Following König et al. (2021), we included further inclusion criteria that participants must report no alcohol consumption within the 12 h prior or tetrahydrocannabinol use/exposure within the 24 h prior to the study. Two participants were excluded due to the session being unexpectedly interrupted, yielding a final sample of 43 participants (mean age = 23.40 years ±5.53, 21 female, 21 male, 1 preferred not to say).
Ethical approval for this study was obtained from the Macquarie University Human Research Ethics Committee (Ref: 520231287146898). Participants received course credit or a cash honorarium (AUD $30) for their involvement.
2.2. Stimuli
Stimuli comprised 40 videos of two stick figures moving in synchrony generated from videos of real people playing the mirror game in the context of a previous experiment (Moffat, Caruana, & Cross, 2024). During the mirror game, both people were seated facing each other. Each person took a 2.5‐min turn moving their arms while the other person attempted to copy the arm movements as identically as possible. See Moffat, Caruana, and Cross (2024) for additional details and visualisations, including the procedure for computing the objectively measured level of movement synchrony between stick figures.
We chose to represent the people as stick figures to mitigate possible influences of attributes such as gender, skin tone, and facial expressions on participants' responses (Macpherson et al., 2020). The stimulus videos were generated in four steps. First, we used OpenPose software (Cao et al., 2021) to estimate the positions of each persons' body parts per frame. Second, the extracted coordinates were arranged as time‐series data using code adapted from de Jonge‐Hoekstra (https://osf.io/6s73d/), including smoothing with a Savitzky–Golay filter (window length = 13 frames, polynomial order = 2) implemented with the signal R package (version 0.7‐7; signal developers, 2014). Third, stick figures were rendered using the Pillow python package (Clark, 2015) to plot dots and lines in a body shape on a black background per frame. The individual frames were appended into videos using python package OpenCV (version 4.5.5.62; Bradski, 2000). Finally, we extracted 16‐s segments using ffmpeg (Tomar, 2006). The 16‐s videos were screened for motion‐capture artefacts by R.M. and a junior lab member, yielding a set of 40 videos (Figure 1a). In all videos, the stick figure leading the mirror game was blue and on the left side of the screen, and the ‘follower’ stick‐figure was red and on the right side. This fixed colour–position relationship of the leader and follower roles was selected to maximise the predictability of stimulus format, allowing participants to focus on forming aesthetic judgements.
FIGURE 1.
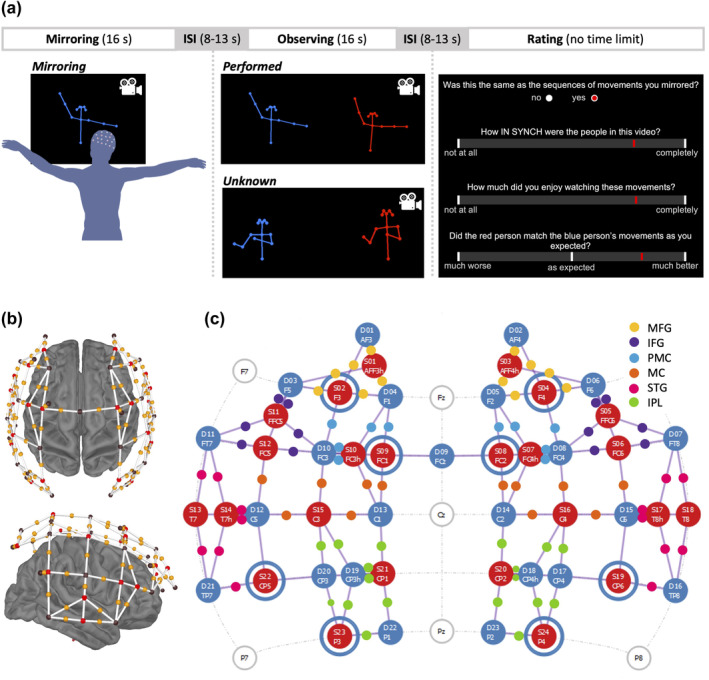
(a) Schematic of one trial. (b) Visualisation of montage over brain. Red spheres are sources, black spheres are detectors, yellow spheres indicate the point of measurement, and white bars show channels between sources and detectors. (c) 2D visualisation of montage showing sources (red circles), detectors (blue filled circles), short‐detector channels (blue rings around red circles), and channels (purple lines). Source and detector circles indicate optode number and position on scalp according to 10‐5 system (Oostenveld & Praamstra, 2001). Color‐coded dots indicate the ROI, to which channels are assigned. MFG = middle frontal gyrus, IFG = inferior frontal gyrus, PMC = premotor cortex, MC = motor cortex, STG = superior temporal gyrus, IPL = inferior parietal lobule.
Our next step was to create an identical copy of 50% of the videos, which only showed the leader. To do so, we randomly assigned the forty 16‐s videos to two sets of 20, creating the stimuli for the performed and unknown conditions (conditions described below). We duplicated the videos from the performed set and cropped the video to show only the blue leader of the mirror game (Figure 1a) to be shown during the mirroring condition.
When presented on the 28″ computer screen, stick figure's torsos measured approximately 9 cm, outstretched arms reaching approximate 12 cm across and arms stretched up reaching approximately 3 cm above the stick figure's head (slight variation due to difference in body size). Videos of one stick figure showed the stick figure's torso centred on the screen. Videos of two stick figures showed each stick figures' torsos 10 cm (left and right) from the centre of the screen. Participants viewed the screen from a distance of approximately 60 cm.
2.3. Procedure
Participants provided written informed consent then completed a demographic questionnaire including measures of extraversion (Goldberg, 1992), self‐esteem (Rosenberg, 1965), body perception (Cabrera et al., 2018), body competence (Miller et al., 1981), empathy (Davis, 1980), and autistic traits (English et al., 2021). Next, the researcher positioned the fNIRS cap and optodes on the participant's head. Subsequently, participants practiced moving their arms without moving their heads while seated in front of a desktop computer. This step was taken as a precaution to minimise head movements during the experiment.
During the main experiment, participants were seated and viewed a series of 60 videos (16 s each), presented in blocks of three. The first video showed one blue stick figure moving its arms and participants were instructed to match the figure's arm movements as closely as possible in time and space (mirroring condition). Next, participants were instructed to watch two more videos without moving and subsequently to provide ratings. These showed the blue stick figure completing the same sequence of arm movements (performed condition) or a different sequence (unknown condition) while being mirrored by a red stick figure and were presented in a randomised order. This sequence was repeated 20 times with unique videos. Participants rated their enjoyment of the movements (sliding scale from ‘not at all’ [0] to ‘completely’ [100]) and indicated whether the sequence was the same one they had mirrored in the mirroring condition (response options ‘yes’ and ‘no’), at the beginning of the block. Participants also rated how in synch they perceived the movements to be (sliding scale from ‘not at all’ [0] to ‘completely’ [100]) – these data are not analysed in the present work. Finally, participants rated how well they felt the red stick figure had followed the blue stick figure, yielding a proxy for empathy (sliding scale from ‘much worse’ [−100] to ‘as expected’ [0] to ‘much better’ [100]). No numbers were shown on any sliding scale (Figure 1a), and participants responded using the computer mouse. The duration of the interstimulus intervals between videos and questions was jittered between 8 and 13 s (Figure 1a), meaning that participants responded to the question querying their recognition of mirrored movement sequences approximately 30 s to 1 min after having performed the movements. The experiment lasted between 30 and 40 min.
2.4. fNIRS equipment
fNIRS recordings were made with a NIRScoutX (NIRx Medical Technologies LLC) with 24 LED sources and 32 avalanche photodiode detectors and NIRStar software (version 15.3). Sources emitted wavelengths of 760 and 850 nm with a sampling rate of 2.6 Hz. We mounted optodes onto mesh caps marked with International 10/10 positions maintaining 30‐mm separations in positions determined using the AAL2 atlas in the fOLD toolbox (Rolls et al., 2015; Tzourio‐Mazoyer et al., 2002; Zimeo Morais et al., 2018). We positioned optodes over bilateral PFC, IFG, STG, and IPL. Our montage comprised 24 sources, 23 detectors, and 8 short detectors (Figure 1b,c), totalling 78 long channels (source‐detector pairs ~30 mm apart) and 8 short channels (source‐detector pairs 8 mm apart). We distributed short channels across the scalp, maximum one per ROI, in light of the spatial heterogeneity in the extracerebral signals (Brigadoi & Cooper, 2015; Gagnon et al., 2012; Zhang et al., 2015).
2.5. Data analysis
As per our preregistration (https://osf.io/e7w4v/), we fit Bayesian multilevel models using the brms package (version 2.20.1; Bürkner, 2017) in the R language (version 4.3.1; R Core Team, 2022) within the RStudio IDE (version 2023.06.1; RStudio Team, 2020). For ease of interpretation and comparison of parameter estimates, we homogenised the scale of predictors using z‐scores. For our models, we set weakly informed priors to impose a constrained distribution on our expected results, as a means of acknowledging our existing knowledge regarding the expected outcomes, allowing for plausible large effects, and allowing the data to dominate the structure of the posterior distribution (Gelman, 2006; Lemoine, 2019). We constructed our models incrementally beginning with varying intercepts per participant (ID), then adding one parameter at a time (Barr, 2013). To compare models, we employed leave‐one‐out cross‐validation (LOO; Vehtari et al., 2017) to estimate and compare the out‐of‐sample accuracy of increasingly complex models. LOO is a useful metric for comparing increasingly complex models and assessing the accuracy of models' predictions. For our maximal models, we report and interpret the posterior distributions of parameters with a 95% credible interval. Credible intervals are computed using the highest posterior density region (HPD) method (McElreath, 2020). HPD values are reported in the same units as the parameter estimates. Finally, we use the emmeans package (version 1.8.8; Lenth, 2021) to compare parameter estimates extracted from our models. For models, model comparison, and visualisation of all model parameters please refer to Supplementary Materials B.
2.5.1. Haemodynamic response amplitude: First level
Analyses were performed using MNE (version 1.4.2; Gramfort et al., 2013), MNE‐NIRS (version 0.5.0; Luke et al., 2021), NiLearn (version 10.1; Abraham et al., 2014), and statsmodels (version 0.14.0; Seabold & Perktold, 2010). The generalised linear model (GLM) approach was taken to quantify the amplitude of evoked haemodynamic responses per ROI and condition (Huppert, 2016). Waveforms for visual inspection are presented in the Supplementary Materials A (Figure S3). The signal was converted from raw intensity to optical density. Motion artefacts were corrected using the temporal derivative distribution repair algorithm (Santosa et al., 2019). Next, the signal was converted to concentrations of HbO and HbR using the Modified Beer–Lambert law (Delpy et al., 1988; Kocsis et al., 2006) with a partial pathlength factor of 0.1, which accounts for the differential pathlength factor (DPF) and partial volume correction (PVC), where (DPF = 6)/(PVC = 60) is equal to 0.1 (Santosa et al., 2018; Strangman et al., 2003). The GLM was fit to the long‐channel data—isolated by rejecting channels <20 mm or >40 mm. The design matrix for the GLM was generated by convolving a 16‐s boxcar function at each event‐onset‐time with the canonical haemodynamic response function (Glover, 1999; Santosa et al., 2019). The GLM also included all principal components of short‐detector channels to account for extracerebral and physiological signal components. Further, drift orders accounting for signal components up to 0.01 Hz were included as regression factors (Huppert, 2016). The GLM was performed with a lag‐1 autoregressive noise model, to account for the correlated nature of the fNIRS signal components. Individual participants' estimates were then averaged within each ROI, weighted by the standard error of the estimates for that ROI.
2.5.2. Haemodynamic response amplitude: Second‐level
To address our group‐level hypotheses regarding cortical activation, we employed Bayesian multilevel Gaussian models as described above. As per our preregistration, we fit models which predicted HbO and HbR values simultaneously. Fitting models to HbO and HbR in tandem serves to capitalise on the correlated natures of the HbO and HbR response amplitudes to inform the model fit and minimise the risk of multicollinearity, which may arise from handling chromophores (HbO, HbR) as a categorical variable. We also preregistered that we would fit models to HbO–HbR difference values derived by subtracting HbR from HbO estimates per participant, ROI, and Condition. This measure is commonly used in fNIRS studies examining clinical and cognitive phenomena (Kaynezhad et al., 2019; Kolyva et al., 2014; Moffat et al., 2023; Moffat, Caruana, & Cross, 2024) and is well‐placed to enhance modelling of complicated relationships between cortical activation and behaviour. A major advantage of the HbO–HbR difference measure is that the sign (+/−) indicates whether the observed response corresponds to a canonical (+) or inverted (−) haemodynamic response. The latter may also be referred to as a negative BOLD response. Moreover, each HbO–HbR difference value can be categorised by the relationship between the original estimates: Taking a conservative stance, positively correlated HbO–HbR pairs may result from systemic phenomena such as blood‐pressure or extracerebral changes (Yücel et al., 2021; Zimeo Morais et al., 2017), whereas negatively correlated HbO–HbR pairs are more likely to reflect true cortical activation (Wolf et al., 2002). By retaining HbO–HbR values from negatively‐correlated pairs only, one can take a conservative approach to analyses (Moffat et al., 2023; Moffat, Caruana, & Cross, 2024). We present results from models fit to negatively correlated HbO–HbR pairs below and models fit to HbO and HbR values simultaneously, and to all HbO–HbR difference values (positively and negatively correlated) in Supplementary Materials A (Figure S2).
3. RESULTS
Our analyses address all nine of our preregistered hypotheses. To focus on the most meaningful findings, we report our behavioural and cortical findings, as well as the relationships between cortical activation and each ratings of enjoyment and recognition of performed movements in the manuscript. Our findings pertaining to relationships between cortical activation and each ratings of others' abilities to synchrony, empathy, and ratings of enjoyment during mirroring are reported in the Supplementary Materials A.
3.1. Behaviour
We first assessed the relationship between ratings of enjoyment and objectively measured movement synchrony. In line with our hypotheses (1 and 2), we observed a positive association between enjoyment and measured synchrony (ß = 4.33, HPD = [3.58, 5.08]; Figure 2a). We also found that observers rated performed compared to unknown movements as more enjoyable to watch (ß = 3.14, HPD = [0.82, 5.36]; Figure 2b).
FIGURE 2.
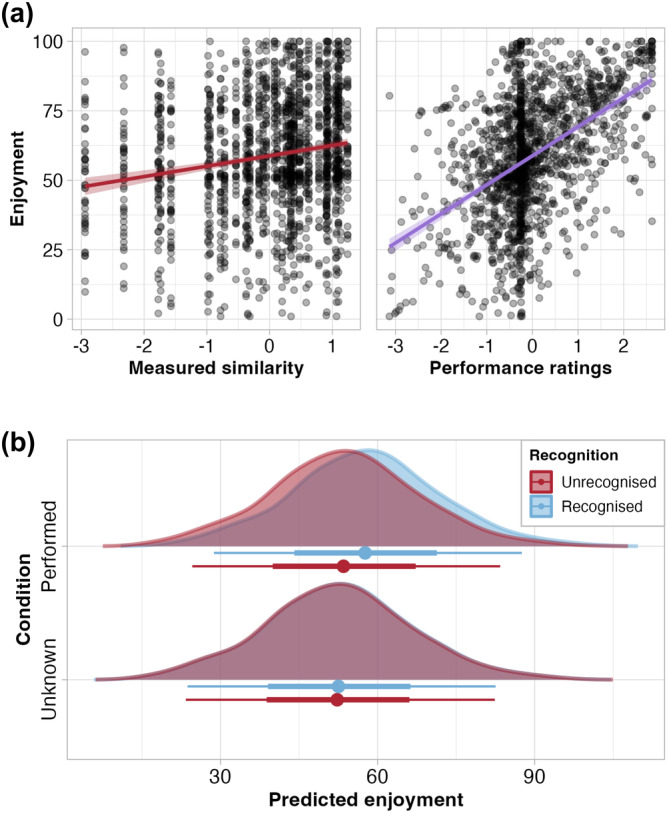
(a) Relationship between enjoyment ratings and measured movement synchrony between stick figures, and between enjoyment ratings and ratings of another person's ability to synchronise. (b) Posterior distributions of enjoyment ratings predicted by model, by condition (performed and unknown) and recognition (unrecognised and recognised). Summary points show median, and bars show interval covering 66 and 95% of the distribution. Descriptive statistics for recognition in Table S1a and recognition outcomes according to signal detection theory in Table S1b of Supplementary Materials A.
Next, we considered participants' abilities to recognise movements they had mirrored with their own bodies. Recognition of performed movements, that is, ‘hits’ according to signal detection theory, was high (mean = 0.85, SD = 0.13). Recognition of movements as unknown, that is, ‘correct rejections’, was poor for unknown movements (mean = 0.05, SD = 0.11; Tables S1a and S1b in Supplementary Materials A). This suggests that participants struggled to differentiate between movements they had mirrored and those they had not, responding disproportionately often that sequences matched the movements they mirrored.
We used pairwise comparisons to explore whether a hierarchical interaction between embodiment (performed, unknown) and recognition (recognised, unrecognised) might exist. Enjoyment was substantially greater for performed + recognised than performed + unrecognised (ß = 4.16, HPD = [7.04, 1.40]), unknown + unrecognised (ß = 5.17, [9.20, 1.15]), and unknown + recognised (ß = 5.25, HPD = [3.73, 6.75]) movements. In other words, we found evidence that performed + known movements were enjoyed above all other movements and found no evidence for any further hierarchy between embodiment and recognition (Table S1c in Supplementary Materials A).
Next, we took the same approach in analysing ratings of another person's ability to synchronise. Our rationale was that higher ratings for performed, or embodied, movements could be indicative of ‘embodied empathy’. Instead, we observed no substantial difference between ratings of another person's ability to synchronise for performed and unknown movements (ß = 0.08, HPD = [−0.05, 0.22]), meaning that our data did not show support for embodied empathy in this task. Exploratory pairwise comparisons revealed a similar pattern as for movement synchrony and enjoyment, where ratings of another person's ability to synchronise were highest for movement sequences that were both performed and recognised. Ratings were substantially higher for performed + recognised than performed + unrecognised (ß = 0.16, HPD = [0.00, 0.30]) and unknown + recognised (ß = 0.16, HPD = [0.08, 0.23]) movements, with a trend toward higher ratings for performed + recognised than unknown + unrecognised (ß = 0.16, HPD = [−0.06, 0.38]).
As per our third hypothesis, we examined the relationship between ratings of enjoyment and ratings of another person's ability to synchronise, while controlling for movement synchrony and complexity. The data revealed a positive association (ß = 9.32, HPD = [8.55, 10.10]; Figure 2a), which was not influenced by movement condition or recognition of the movement sequence, suggesting that aesthetic judgements, but not embodiment or recognition, shape perceptions of others' abilities to achieve synchrony, or vice versa (Table S4 of Supplementary Materials A). We then examined the relationship between enjoyment and each general and cognitive empathy. No associations were observed between general empathy, indexed as the sum of all IRI subscales (Table S2 in Supplementary Materials A). Cognitive empathy, as indexed by the IRI subscales of perspective taking and fantasy, was negatively associated with enjoyment (ß = −2.62, HPD = [−5.49, 0.01]), with a trend for unknown movements to amplify this negative relationship, relative to performed movements (Table S3 of Supplementary Materials A). We interpret this to mean that embodiment interacts with cognitive empathy in shaping aesthetic judgements. Put differently, if we first think of greater embodiment as being linked to greater enjoyment, we can then imagine how this positive effect might be less strong for individuals with higher cognitive empathy scores.
3.2. Cortical imaging
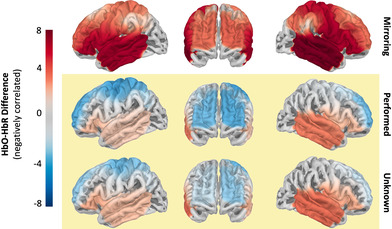
We next examined changes in cortical oxygenation in the AON while mirroring and observing movements to address our hypothesis (4) that the brain regions in the AON would respond to mirroring, as well as viewing performed and unknown, movements. We observed that during mirroring, all ROIs showed increased activation, as indexed by increasing HbO–HbR differences (Figure 3a). Observation of performed movements evoked positive HbO–HbR differences in right IFG and bilateral STG, as well as negative HbO–HbR differences in bilateral MFG, PMC, MC, and left IPL. Negative HbO–HbR differences reflect inverted BOLD responses, the origin and function of which remain debated (He et al., 2022; Maggioni et al., 2016). Observation of unknown movements evoked positive HbO–HbR differences in right IFG and bilateral STG, as well as negative HbO–HbR differences, reflecting inverted BOLD responses, in bilateral MFG, PMC, as well as left MC and IPL (Figure 3a). Parameter estimates are presented in Table S6 of Supplementary Materials A.
FIGURE 3.
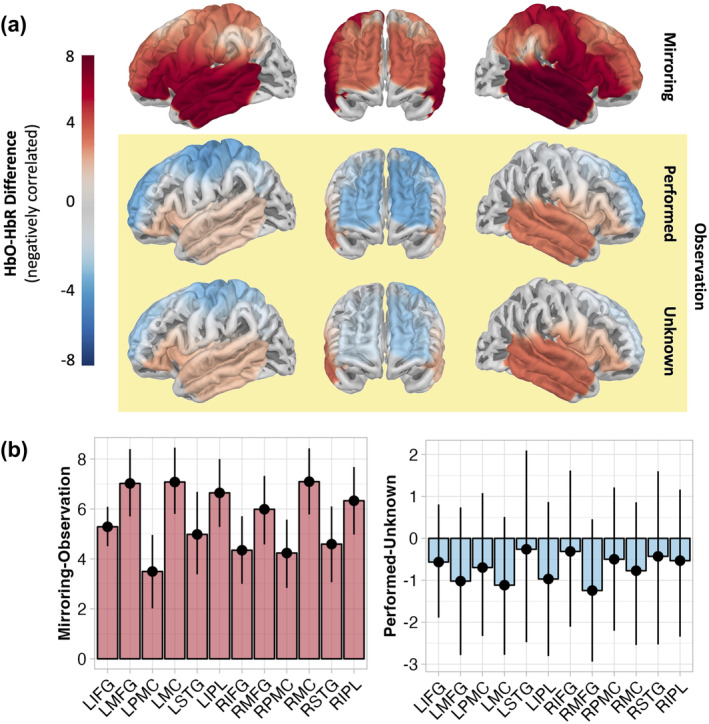
(a) Projection of HbO–HbR difference estimates to surface of the brain per condition. (b) Contrasts per ROI between mirroring and observation of movement (performed and unknown aggregated) and between observation or performed and unknown movements. Points show difference and error bars show lower and upper HPD. Differences can be considered substantial where the HPD does not overlap with zero (all ROIs for Mirroring‐Observation), or only overlaps very minimally (LIFG, LSTG, and RSTG for Performed‐Unknown). Percentage of negatively correlated HbO–HbR difference values per ROI shown in Table S5 of Supplementary Materials A.
Contrasts between the mirroring and observation conditions (i.e., performed and unknown aggregated), per ROI confirmed our fifth hypothesis that mirroring would evoke greater cortical activation across all ROIs (Figure 3b; Table S7 in Supplementary Materials A). We also hypothesised that comparing performed versus unknown movements would yield reduced activation in bilateral MC and PMC (Hypothesis 6). Comparisons per ROI revealed no differences in MC or PMC, or in any other ROI (Figure 3b). Parameter estimates from these ROI analyses can be found in Table S8 in Supplementary Materials A.
3.3. Brain—behaviour
Our next step was to explore the relationship between cortical activation, as indexed by negatively correlated HbO–HbR difference values, and participants' ratings of enjoyment and their recognition of having performed sequences (Hypothesis 7). With respect to enjoyment and cortical activation, increasing enjoyment was associated with greater activation in left STG for performed and unknown sequences (Figure 4). Participants' recognition of having performed movements correlated positively with activation in bilateral STG, left IFG, and right IPL during performed movements–corresponding to less strongly inverted responses and greater activation. During unknown movements, we observed no substantial associations between recognition and cortical activation in any ROI (Figure 4). Parameter estimates for these analyses are presented in Supplementary Materials A (Tables S9 and S10 and Figures S4 and S5). Figure 4 also summarises the relationships between brain activation and aspects of behaviour that we describe in more detail in Supplementary Materials A (i.e., Performance ratings–ratings of another person's ability to synchronise, cognitive empathy scores, and ratings of enjoyment as reflected in brain activation during mirroring; Tables S11–S13 and Figures S6–S8).
FIGURE 4.
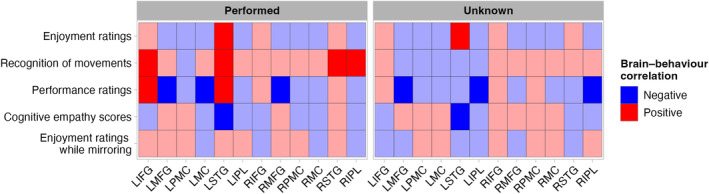
Relationships between behavioural measures and cortical activation. Colour indicates direction of the correlation (red squares = positive correlation between cortical activation and behaviour, blue squares = negative correlation between cortical activation and behaviour). Darker squares indicate substantial relationships, where HPD does not include zero and trends where <10% of HPD overlaps with zero. Light squares show relationships where >10% of HPD overlaps with zero. The cortical activation values in this figure are negatively correlated HbO–HbR difference values. In Supplementary Materials A, Tables S9–S13 and Figures S4–S8 provide additional numeric detail about these relationships.
4. DISCUSSION
In the present study, we set out to examine the influence of embodying movements on enjoyment of dyadic synchronised movement. We draw five main conclusions from our analyses. First, we replicated previous work demonstrating that greater objectively measured movement synchrony corresponds with greater enjoyment reported by observers. We further established that synchronous movements are most appreciated when the observer recognises the movements as a sequence they have performed themselves. Second, we found that enjoyment is positively associated with observers' ratings of how well other people achieve synchrony and negatively associated with cognitive empathy scores–yet neither of these effects are differentially sensitive to embodiment or recognition of having performed the movements. Third, recordings of cortical activation revealed that observing dyads move in synchrony evoked greater cortical activation in right IFG and bilateral STG, with no substantial difference observed between performed and unknown movements. Finally, we found preliminary evidence for the cortical mechanisms underpinning enjoyment of synchronous movements (left STG), and awareness of embodiment (left IFG and STG, right STG and IPL).
4.1. Behaviour: Enjoyment, embodiment, empathy, and recognition
4.1.1. Awareness of embodiment can enhance enjoyment of synchrony
Our analyses replicate previous findings that observers' enjoyment of dyadic movements increases in concert with the level of synchrony of dyadic movements (Moffat & Cross, 2024; Vicary et al., 2017). We also found that participants reported the greatest enjoyment of movement sequences when they were aware of having mirrored the same movements themselves (Figure 2b). This finding is consistent with previous studies demonstrating that embodiment has a positive influence on enjoyment of observed movement (Kirsch et al., 2013, 2015; Kirsch & Cross, 2018). Furthermore, this finding provides new insight into the influence of awareness of embodiment on action aesthetics. Specifically, awareness of embodiment shapes enjoyment of synchronous movements. A potential reason for this is that our approach of assessing embodiment through recall may have prompted observers to evaluate movement sequences analytically. Analytical evaluation involves tracking distinctive features rather than evaluating the movements holistically (Whittlesea & Price, 2001), which may boost familiarity with stimuli, in this case movement sequences, thereby enhancing enjoyment.
The relationship between movement familiarity and enjoyment has been assessed by previous work, which has typically employed ratings of ‘familiarity’ with the stimulus movements to assess the influence of visual fluency on aesthetic judgements (Cross & Orlandi, 2020; Orgs et al., 2013; Orlandi et al., 2020). This approach provides a valuable proxy for the extent to which a participant embodies one movement more than another. However, the drawback is that participants may interpret ‘familiarity’ to refer to movements that they have learned elsewhere, such as from daily life or social media dance trends (Casale et al., 2023). By contrast, we queried explicit recall of specific movement sequences and demonstrated that awareness of embodiment shapes enjoyment of synchronous movements on behavioural and cortical levels. Additional investigation could further disentangle the relative contributions of recognition and embodiment in enhancing enjoyment of synchronous movements. Specifically, the inclusion of a condition assessing participants' recognition of viewed (but not physically mirrored) movement sequences to compare embodiment and visual familiarity would clarify the role of embodiment per se on aesthetic judgements of synchronous movements.
Active mirroring techniques and practicing the recall of these movements may be useful in clinical and therapeutic settings, where the aim is to foster successful social interaction between two or more individuals (Salazar Kämpf et al., 2021; Salazar Kämpf & Kanske, 2023). Salazar Kämpf and colleagues' work details the degree to which clinicians adapt their level of synchrony to their individual patients and suggests that patients' mirroring tendencies should be a focus of the therapy–a sentiment echoed in similar work (Asher et al., 2020; Dean et al., 2021; Paulick et al., 2018). Offering concrete starting points for the future research required to understand the relationship between mirroring movements and clinical outcomes better, we propose that this combination of physical movement and perceptual cueing could be used, as a first step, to facilitate social initiation and prosocial behaviours (Bellini et al., 2007; Corbett et al., 2014; Kubzansky et al., 2023; Munandar et al., 2020). This approach would complement existing evidence suggesting that video‐based learning is a promising strategy for improving social skills (Munandar et al., 2020).
We would further like to address the fact that observers in the present study may have relied on social information to aid recall of movement sequences. Social (nonverbal) components of interactions, such as gesture, can prompt successful recall (Fedorov et al., 2018; Manera et al., 2011; Thornton & Conway, 2013), as can ‘social chunking’. Social chunking refers to the process of encoding interacting people as a single unit (Ding et al., 2017). We propose that the embodiment acquired through mirroring another person is inherently social and likely facilitates action recognition. However, in suggesting so, we assume that video representations of self‐other dyads are socially chunked in the same way as other‐other dyads. This assumption remains to be empirically investigated, perhaps using the mirror game.
4.1.2. No evidence for embodied empathy
We explored whether observers might perceive others' abilities to achieve synchrony differently when they themselves had mirrored the same movements, as compared to when they had not. Our proposal was that if embodiment enhanced ratings of another person's ability to achieve synchrony, this might reflect ‘embodied empathy’. However, the data show that embodiment does not shape judgements of others' ability to mirror, suggesting that embodied empathy, as we proposed it, did not emerge in our study.
Despite this lack of evidence that embodiment influences ratings of others' ability to achieve synchrony, observers' ratings followed the same hierarchical pattern as their ratings of enjoyment: Observers gave highest ratings to sequences they had performed and recognised as performed. Moreover, we found a strong association between ratings of enjoyment and ratings of another person's ability to synchronise (Figure 2a). It is possible that greater enjoyment results in more generous assessments of others' synchronising abilities, or that those with a tendency to rate other's abilities to synchronise more generously experience greater enjoyment of the dyadic movement. We were not able to discern the directionality of this relationship in the present study. Nonetheless, we ensured that the association between ratings of another person's ability to synchronise and enjoyment was not confounded by kinematic features such as movement synchrony and predictability, as these may drive ratings of enjoyment (Moffat & Cross, 2024) and cortical activation (Molnar‐Szakacs et al., 2005). It remains possible, however, that other kinematic features (speed or overall amount of movement) may further contribute to this relationship (Orlandi et al., 2020). In sum, our analyses showed that ratings of another person's ability to achieve synchrony are not sensitive to embodiment, but rather are related to ratings of enjoyment of synchronous movements. Practically, these findings suggest that promoting enjoyment of human movements, whether through one‐on‐one mirroring activities or other community activities involving synchronous group movements (i.e., yoga classes, rowing, ice skating, etc.) may result in more generous judgements of other people's physical efforts (Reddish et al., 2016). In other words, social physical activity bears promise of enhancing how we see others, opening the possibility to strengthen relationships with familiar people and to facilitate their initiation with unfamiliar people (Patterson et al., 2021; Zumeta et al., 2016).
4.1.3. Cognitive empathy, but not general empathy, dampens enjoyment of dyadic synchrony
We were surprised to find empathy scores (all subscales of IRI) did not associate with ratings of enjoyment of synchronous movements. As such, the present study failed to replicate our previous findings (Moffat & Cross, 2024). This is likely explained by the difference in sample sizes (current study = 43, previous study = 322) and the requirement for substantial sample sizes when considering individual differences (Gignac & Szodorai, 2016). We acknowledge that our (exploratory and not‐preregistered) analyses including general and cognitive empathy in the present study would benefit from greater sample sizes. Our rationale for including these analyses is that they provide context to our exploration of observers' ratings of other people's abilities to achieve synchrony, and that our Bayesian approach is suited to small samples (McNeish, 2016). Bearing the small sample size in mind, we interpret our findings pertaining to general and cognitive empathy tentatively. Alternatively, task differences could contribute to the discrepancy in the empathy–enjoyment association between our past and present study. In Moffat, Caruana, and Cross (2024); Moffat, Roos, et al. (2024), participants viewed 100 videos in a web‐based experiment, with no requirement for imitation or mirroring, and continuous presentation of videos after each response. We propose that the jittered time delay between videos involved in the present study (beneficial for extracting haemodynamic responses from fNIRS signal) may have made the overall experience less enjoyable, thereby attenuating participants' enjoyment ratings. Participants' enjoyment may also have been dampened by the experience of wearing the fNIRS cap and completing the experiment in the laboratory, as opposed to the comfort a web‐based experiment in a space of one's choosing.
Cognitive empathy was negatively associated with ratings of enjoyment, trending to be more strongly negative for unknown than performed movements. The existing literature suggests a positive association to be more intuitive: For example, cognitive empathy enhances participants' abilities to synchronise with others (Reiss et al., 2019), and the extent of synchrony is generally associated with positive affect (Mogan et al., 2017), and interpersonal liking (Ravreby et al., 2022). With respect to the overall negative relationship between enjoyment and cognitive empathy we observed in the present study, we speculate that individuals with greater cognitive empathy scores (i.e., greater metalizing and perspective taking tendencies) might have enjoyed the videos less due to the relative lack of human social features in the stick figures, such as gaze or facial expression (Cowan et al., 2014; Jospe et al., 2018). For the performed relative to unknown movements, this source of low enjoyment may have been slightly attenuated by embodiment gained through mirroring. In other words, greater embodiment may lead to a weaker relationship between enjoyment and cognitive empathy for performed movements. As mentioned above, these findings draw on individual differences in cognitive empathy from a relatively small number of participants and should be interpreted with care.
4.2. Cortical responses and correlates of behaviour
4.2.1. Execution versus observation in AON
In a recent review, Condy et al. (2021) commented on the dearth of statistical comparisons between execution and observation in AON research. In this study, we provide evidence of substantial differences across all ROIs, with the greatest differences observed in bilateral MFG, MC, and IPL (Figure 3b). The studies considered in Condy et al.'s review, mainly required participants to make small manual movements with one hand at a time, and as a result show more focal patterns of activation (Bhat et al., 2017; Holper et al., 2010; Koehler et al., 2012; Shimada & Abe, 2010). In contrast, our experiment required participants to mirror a series of complex bilateral upper body movements, none of which were previously known or familiar to the participant. This task involves an entire upper body motor component, as well as continuous prediction of upcoming movements, resulting in greater differences in activation between mirroring and observation conditions across all ROIs. Movement artefacts are also an obvious consideration, which we did not take lightly. Before the experiment, we trained participants to keep their head still while moving their arms, to reduce head motion during recording. Furthermore, we corrected the signal for motion artefacts algorithmically. Moreover, the GLM analysis approach involves autoregressive components that help minimise the influence of motion artefacts (Huppert, 2016), as evidenced by the success of brain–computer interface implementations relying on this analysis method to decode specific movements (von Lühmann et al., 2020). Nonetheless, future research would benefit from the incorporation of head movements, recorded with accelerometers, in the GLM (von Lühmann et al., 2020).
Comparisons of cortical responses to performed and unknown movements revealed no differences in activation in any ROI (Figure 3b). We were initially surprised that motor areas did not show our hypothesised reduction in activation during observation of performed, relative to unknown, movements (Gazzola & Keysers, 2009; Li et al., 2020; Vigneswaran et al., 2013) or the opposite, greater activation (Nishitani & Hari, 2000; Raos et al., 2004; Stefan et al., 2005). However, observers' recognition scores indicated that observers were unable to distinguish reliably between performed and unknown movements and believed the majority of sequences to be movements they had performed. We attribute this to the relative complexity of the movements that participants performed and observed, as well as the prolonged intervals between mirroring and recalling movement sequences. This could be confirmed in future work by comparing cortical activation in MC and PMC for performed and unknown movement sequences that are shorter in length, and presented in an event‐related design (i.e., with shorter intervals between mirroring and recall).
4.2.2. Enjoyment
We found enjoyment to be positively associated with activation in left STG for performed and unknown movements (Figure 4; top row). The parameter estimates are identical for performed and unknown movements, with a negligibly smaller 95% HPD for performed than unknown movements (Table S9 in Supplementary Materials A). In other words, embodiment induced by mirroring sequences did not modulate the relationship between enjoyment and cortical activation in left STG.
Our findings differ from those reported by Kirsch et al. (2015), though our study design is quite similar. Kirsch et al. recorded brain activation using functional magnetic resonance imaging (fMRI) before and after a four‐day full‐body movement training intervention using a dance video‐game. Kirsch et al. report that prior to training (comparable to unknown movements in this study), enjoyment of observed movements correlated with activation in subcortical regions, including the subthalamus and nucleus accumbens. After training (comparable to performed movements in this study), they observed that activation in bilateral STG and right middle temporal gyrus correlated with enjoyment. Finally, when contrasting post versus pre training, they found the left STG to be specifically sensitive to enjoyment after participants had gained experience with movements. Here, we found that left STG activation was contingent on enjoyment but not embodiment. This difference may be related to an underlying difference in the ‘strength’ of the embodiment of movements.
In Kirsch et al.'s study, participants aimed to learn dance movements and were scored on their performance by a computer algorithm implemented in a video game context. Our participants did not aim to learn movements, rather to mirror them as precisely as possible, and where possible to recognise the sequence. The present study thus does not tap into the same link between strongly embodied experience and enjoyment, but rather the link between minimally or briefly embodied experience and enjoyment. Future research could explore the extent to which attentional mechanisms play a role in behavioural and cortical measures of enjoyment of movements, with particular scrutiny of the trajectory from minimally to more strongly embodied movements, as well as on movements performed in an imitation for imitation's sake context, and those performed in a longer‐term learning/retention context.
4.2.3. Recognition of performed movements
Better recognition of performed movements was substantially associated with increased activation in bilateral STG, as well as left IFG and right IPL (Figure 4). No ROIs were associated with recognition for unknown movements, highlighting how embodiment may invoke differential processing of movements. Considering these findings together, increased activation in left IFG and bilateral STG may reflect intensified prediction of movements and matching of observed movements (Iacoboni, 2005, 2009b; Keysers et al., 2018). Both prediction and matching processes would likely draw on episodic memory. Activation of the right IPL aligns with activation in parietal regions reported for recall from episodic memory (Piccoli et al., 2015; Sestieri et al., 2011; Urgolites & Wood, 2013), spatial attention including orienting to salient or new perceptual input (Cabeza et al., 2012; Corbetta & Shulman, 2002), and motor imagery (Hanakawa et al., 2008; Koehler et al., 2012; Wriessnegger et al., 2008). Moreover, the right lateralisation of IPL activation has previously been associated with robust self‐other discrimination processes (Blanke & Arzy, 2005; Brass et al., 2009; Jeannerod, 2007; Wurm & Schubotz, 2018). These are all relevant to recognition of performed movement sequences. Greater recognition rates could reflect greater reliance on these mechanisms, which evoke activity in right IPL–but only with embodiment. In other words, embodiment might boost spatial, attentional, or motor imagery processes supported by right IPL. Further investigations involving a less challenging movement recall task, allowing greater balance in recognition scores between performed and unknown movements, are needed to confirm and disentangle the role of these processes.
5. IMPLICATIONS AND CONCLUSIONS
We examined the extent to which embodiment influences enjoyment of synchronous movements, as well as the cortical mechanisms underpinning these judgements. We found that correct recognition of having mirrored a movement sequence (i.e., a form of awareness of embodiment) had the greatest positive impact on enjoyment of synchronous movements. Our results also revealed that embodiment does not impact how we assess others' abilities to achieve synchrony, but that one's assessments of others' abilities to synchronise and one's own cognitive empathy are associated with enjoyment. Finally, we demonstrate that cortical activation in AON regions and the network subserving aesthetic processing underpin these behaviours. The main implications of our findings are that directing attention to the synchrony that we engage in has the potential to improve our enjoyment of synchronous movements, such as those that emerge in performing arts, athletic endeavours, and our daily lives.
AUTHOR CONTRIBUTIONS
RM and ESC both contributed to the conceptualisation of this study and reviewed the manuscript. RM created the experiment, collected, and analysed the data, and drafted the original manuscript. ESC acquired funding for this project.
FUNDING INFORMATION
This project was supported by a Leverhulme grant to ESC (PLP‐2018‐152) and the Professorship for Social Brain Sciences at ETH Zurich.
CONFLICT OF INTEREST STATEMENT
The authors declare no potential conflict of interest.
Supporting information
Data S1: Supporting information.
ACKNOWLEDGEMENTS
The authors thank Annika Richter for her help transforming the stick figures from simple times series to video stimuli and Luca Naudszus for proofreading the manuscript.
Moffat, R. , & Cross, E. S. (2024). Awareness of embodiment enhances enjoyment and engages sensorimotor cortices. Human Brain Mapping, 45(10), e26786. 10.1002/hbm.26786
Contributor Information
Ryssa Moffat, Email: ryssa.moffat@gess.ethz.ch.
Emily S. Cross, Email: emily.cross@gess.ethz.ch.
DATA AVAILABILITY STATEMENT
The datasets collect and analysed for this study can be found our repository entitled ‘Embodying aesthetic movement preferences’ on OSF: https://osf.io/e7w4v/.
REFERENCES
- Abraham, A., Pedregosa, F., Eickenberg, M., Gervais, P., Mueller, A., Kossaifi, J., Gramfort, A., Thirion, B., & Varoquaux, G. (2014). Machine learning for neuroimaging with scikit‐learn. Frontiers in neuroinformatics, 8, 14. 10.3389/fninf.2014.00014 [DOI] [PMC free article] [PubMed]
- Arnheim, R. (1974). Art and visual perception: A psychology of the creative eye (new version; expanded and revised edition of the 1954 original). University of California Press. [Google Scholar]
- Asher, M. , Kauffmann, A. , & Aderka, I. M. (2020). Out of sync: Nonverbal synchrony in social anxiety disorder. Clinical Psychological Science, 8(2), 280–294. 10.1177/2167702619894566 [DOI] [Google Scholar]
- Ayaz, H. , Baker, W. B. , Blaney, G. , Boas, D. A. , Bortfeld, H. , Brady, K. , Brake, J. , Brigadoi, S. , Buckley, E. M. , Carp, S. A. , Cooper, R. J. , Cowdrick, K. R. , Culver, J. P. , Dan, I. , Dehghani, H. , Devor, A. , Durduran, T. , Eggebrecht, A. T. , Emberson, L. L. , … Zhou, W. (2022). Optical imaging and spectroscopy for the study of the human brain: Status report. Neurophotonics, 9(S2), S24001. 10.1117/1.NPh.9.S2.S24001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balardin, J. B. , Zimeo Morais, G. A. , Furucho, R. A. , Trambaiolli, L. , Vanzella, P. , Biazoli, C. , & Sato, J. R. (2017). Imaging brain function with functional near‐infrared spectroscopy in unconstrained environments. Frontiers in Human Neuroscience, 11, 258. 10.3389/fnhum.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr, D. J. (2013). Random effects structure for testing interactions in linear mixed‐effects models. Frontiers in Psychology, 4(June), 3–4. 10.3389/fpsyg.2013.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkali, S. , Youssef, G. J. , Donaldson, P. H. , Albein‐Urios, N. , Hyde, C. , & Enticott, P. G. (2021). Is the putative mirror neuron system associated with empathy? A systematic review and meta‐analysis. Neuropsychology Review, 31(1), 14–57. 10.1007/s11065-020-09452-6 [DOI] [PubMed] [Google Scholar]
- Bellini, S. , Akullian, J. , & Hopf, A. (2007). Increasing social engagement in young children with autism spectrum disorders using video self‐modeling. School Psychology Review, 36(1), 80–90. 10.1080/02796015.2007.12087953 [DOI] [Google Scholar]
- Berlyne, D. (Ed.). (1974). Studies in the new experimental aesthetics: Steps toward an objective psychology of aesthetic appreciation. Hemisphere. [Google Scholar]
- Bhat, A. N. , Hoffman, M. D. , Trost, S. L. , Culotta, M. L. , Eilbott, J. , Tsuzuki, D. , & Pelphrey, K. A. (2017). Cortical activation during action observation, action execution, and interpersonal synchrony in adults: A functional near‐infrared spectroscopy (fNIRS) study. Frontiers in Human Neuroscience, 11, 431. 10.3389/fnhum.2017.00431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke, O. , & Arzy, S. (2005). The out‐of‐body experience: Disturbed self‐processing at the temporo‐parietal junction. The Neuroscientist, 11(1), 16–24. 10.1177/1073858404270885 [DOI] [PubMed] [Google Scholar]
- Bradski, G. (2000). The OpenCV library [Computer software].
- Brass, M. , Ruby, P. , & Spengler, S. (2009). Inhibition of imitative behaviour and social cognition. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1528), 2359–2367. 10.1098/rstb.2009.0066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigadoi, S. , & Cooper, R. J. (2015). How short is short? Optimum source–detector distance for short‐separation channels in functional near‐infrared spectroscopy. Neurophotonics, 2(2), 025005. 10.1117/1.nph.2.2.025005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürkner, P.‐C. (2017). Brms: An R package for Bayesian multilevel models using Stan. Journal of Statistical Software, 80(1), 1–28. 10.18637/jss.v080.i01 [DOI] [Google Scholar]
- Cabeza, R. , Ciaramelli, E. , & Moscovitch, M. (2012). Cognitive contributions of the ventral parietal cortex: An integrative theoretical account. Trends in Cognitive Sciences, 16(6), 338–352. 10.1016/j.tics.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera, A. , Kolacz, J. , Pailhez, G. , Bulbena‐Cabre, A. , Bulbena, A. , & Porges, S. W. (2018). Assessing body awareness and autonomic reactivity: Factor structure and psychometric properties of the Body Perception Questionnaire‐Short Form (BPQ‐SF). International Journal of Methods in Psychiatric Research, 27(2), e1596. 10.1002/mpr.1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo‐Merino, B. , Glaser, D. E. , Grèzes, J. , Passingham, R. E. , & Haggard, P. (2005). Action observation and acquired motor skills: An fMRI study with expert dancers. Cerebral Cortex, 15(8), 1243–1249. 10.1093/cercor/bhi007 [DOI] [PubMed] [Google Scholar]
- Calvo‐Merino, B. , Jola, C. , Glaser, D. E. , & Haggard, P. (2008). Towards a sensorimotor aesthetics of performing art. Consciousness and Cognition, 17(3), 911–922. 10.1016/j.concog.2007.11.003 [DOI] [PubMed] [Google Scholar]
- Cao, Z. , Hidalgo, G. , Simon, T. , Wei, S.‐E. , & Sheikh, Y. (2021). Openpose: Realtime multi‐person 2d pose estimation using part affinity fields. IEEE Transactions on Pattern Analysis and Machine Intelligence, 43(1), 172–186. 10.1109/TPAMI.2019.2929257 [DOI] [PubMed] [Google Scholar]
- Casale, C. E. , Moffat, R. , & Cross, E. S. (2023). Aesthetic evaluation of body movements shaped by embodiment and arts experience: Insights from behaviour and fNIRS. PsyArXiv. 10.31234/osf.io/n24kc [DOI]
- Caspers, S. , Zilles, K. , Laird, A. R. , & Eickhoff, S. B. (2010). ALE meta‐analysis of action observation and imitation in the human brain. NeuroImage, 50(3), 1148–1167. 10.1016/j.neuroimage.2009.12.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, J. F. , Gomila, A. , Gaigg, S. B. , Sivarajah, N. , & Calvo‐Merino, B. (2016). Dance expertise modulates behavioral and psychophysiological responses to affective body movement. Journal of Experimental Psychology: Human Perception and Performance, 42(8), 1139–1147. 10.1037/xhp0000176 [DOI] [PubMed] [Google Scholar]
- Clark, A. (2015). Pillow (PIL fork) documentation [Computer software]. Retrieved from https://buildmedia.readthedocs.org/media/pdf/pillow/latest/pillow.pdf
- Condy, E. E. , Miguel, H. O. , Millerhagen, J. , Harrison, D. , Khaksari, K. , Fox, N. , & Gandjbakhche, A. (2021). Characterizing the action‐observation network through functional near‐infrared spectroscopy: A review. Frontiers in Human Neuroscience, 15, 627983. 10.3389/fnhum.2021.627983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett, B. A. , Swain, D. M. , Coke, C. , Simon, D. , Newsom, C. , Houchins‐Juarez, N. , Jenson, A. , Wang, L. , & Song, Y. (2014). Improvement in social deficits in autism spectrum disorders using a theatre‐based, peer‐mediated intervention. Autism Research, 7(1), 4–16. 10.1002/aur.1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta, M. , & Shulman, G. L. (2002). Control of goal‐directed and stimulus‐driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Cowan, D. G. , Vanman, E. J. , & Nielsen, M. (2014). Motivated empathy: The mechanics of the empathic gaze. Cognition and Emotion, 28(8), 1522–1530. 10.1080/02699931.2014.890563 [DOI] [PubMed] [Google Scholar]
- Crivelli, D. , Sabogal Rueda, M. D. , & Balconi, M. (2018). Linguistic and motor representations of everyday complex actions: An fNIRS investigation. Brain Structure and Function, 223(6), 2989–2997. 10.1007/s00429-018-1646-9 [DOI] [PubMed] [Google Scholar]
- Cross, E. S. , & Orlandi, A. (2020). The aesthetics of action and movement. In Nadal M. & Vartanian O. (Eds.), The Oxford handbook of empirical aesthetics (1st ed., pp. 605–622). Oxford University Press. Retrieved from https://academic.oup.com/edited-volume/35428/chapter/303191180 [Google Scholar]
- Cross, E. S. , Hamilton, A. F. D. C. , & Grafton, S. T. (2006). Building a motor simulation de novo: Observation of dance by dancers. NeuroImage, 31(3), 1257–1267. 10.1016/j.neuroimage.2006.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross, E. S. , Kraemer, D. J. M. , Hamilton, A. F. D. C. , Kelley, W. M. , & Grafton, S. T. (2009). Sensitivity of the action observation network to physical and observational learning. Cerebral Cortex, 19(2), 315–326. 10.1093/cercor/bhn083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M. (1980). A multidimensional approach to individual differences in empathy. Journal of Personality and Social Psychology, 10, 85. [Google Scholar]
- Dean, D. J. , Scott, J. , & Park, S. (2021). Interpersonal coordination in schizophrenia: A scoping review of the literature. Schizophrenia Bulletin, 47(6), 1544–1556. 10.1093/schbul/sbab072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpy, D. T. , Cope, M. , Van Der Zee, P. , Arridge, S. , Wray, S. , & Wyatt, J. (1988). Estimation of optical pathlength through tissue from direct time of flight measurement. Physics in Medicine and Biology, 33(12), 1433–1442. 10.1088/0031-9155/33/12/008 [DOI] [PubMed] [Google Scholar]
- Ding, X. , Gao, Z. , & Shen, M. (2017). Two equals one: Two human actions during social interaction are grouped as one unit in working memory. Psychological Science, 28(9), 1311–1320. 10.1177/0956797617707318 [DOI] [PubMed] [Google Scholar]
- Doricchi, F. , Lasaponara, S. , Pazzaglia, M. , & Silvetti, M. (2022). Left and right temporal‐parietal junctions (TPJs) as “match/mismatch” hedonic machines: A unifying account of TPJ function. Physics of Life Reviews, 42, 56–92. 10.1016/j.plrev.2022.07.001 [DOI] [PubMed] [Google Scholar]
- English, M. C. W. , Gignac, G. E. , Visser, T. A. W. , Whitehouse, A. J. O. , Enns, J. T. , & Maybery, M. T. (2021). The comprehensive autistic trait inventory (CATI): Development and validation of a new measure of autistic traits in the general population. Molecular Autism, 12(1), 37. 10.1186/s13229-021-00445-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov, L. A. , Chang, D.‐S. , Giese, M. A. , Bülthoff, H. H. , & De La Rosa, S. (2018). Adaptation aftereffects reveal representations for encoding of contingent social actions. Proceedings of the National Academy of Sciences, 115(29), 7515–7520. 10.1073/pnas.1801364115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedberg, D. , & Gallese, V. (2007). Motion, emotion and empathy in esthetic experience. Trends in Cognitive Sciences, 11(5), 197–203. 10.1016/j.tics.2007.02.003 [DOI] [PubMed] [Google Scholar]
- Gagnon, L. , Cooper, R. J. , Yücel, M. A. , Perdue, K. L. , Greve, D. N. , & Boas, D. A. (2012). Short separation channel location impacts the performance of short channel regression in NIRS. NeuroImage, 59(3), 2518–2528. 10.1016/j.neuroimage.2011.08.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzola, V. , & Keysers, C. (2009). The observation and execution of actions share motor and somatosensory voxels in all tested subjects: Single‐subject analyses of unsmoothed fMRI data. Cerebral Cortex, 19(6), 1239–1255. 10.1093/cercor/bhn181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman, A. (2006). Prior distributions for variance parameters in hierarchical models (comment on article by Browne and Draper). Bayesian . Analysis, 1(3), 515–534. 10.1214/06-BA117A [DOI] [Google Scholar]
- Gignac, G. E. , & Szodorai, E. T. (2016). Effect size guidelines for individual differences researchers. Personality and Individual Differences, 102, 74–78. 10.1016/j.paid.2016.06.069 [DOI] [Google Scholar]
- Glover, G. H. (1999). Deconvolution of impulse response in event‐related BOLD fMRI. NeuroImage, 9(4), 416–429. 10.1006/nimg.1998.0419 [DOI] [PubMed] [Google Scholar]
- Goldberg, L. (1992). The development of markers for the Big‐Five factor structure. Psychological Assessment, 4(1), 26–42. 10.1037/1040-3590.4.1.26 [DOI] [Google Scholar]
- Gramfort, A., Luessi, M., Larson, E., Engemann, D. A., Strohmeier, D., Brodbeck, C., Goj, R., Jas, M., Brooks, T., Parkkonen, L., & Hämäläinen, M. (2013). MEG and EEG data analysis with MNE‐Python. Frontiers in neuroscience, 7, 267. 10.3389/fnins.2013.00267 [DOI] [PMC free article] [PubMed]
- Hanakawa, T. , Dimyan, M. A. , & Hallett, M. (2008). Motor planning, imagery, and execution in the distributed motor network: A time‐course study with functional MRI. Cerebral Cortex, 18(12), 2775–2788. 10.1093/cercor/bhn036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick, R. M. , Caspers, S. , Eickhoff, S. B. , & Swinnen, S. P. (2018). Neural correlates of action: Comparing meta‐analyses of imagery, observation, and execution. Neuroscience & Biobehavioral Reviews, 94, 31–44. 10.1016/j.neubiorev.2018.08.003 [DOI] [PubMed] [Google Scholar]
- He, H. , Ettehadi, N. , Shmuel, A. , & Razlighi, Q. R. (2022). Evidence suggesting common mechanisms underlie contralateral and ipsilateral negative BOLD responses in the human visual cortex. NeuroImage, 262, 119440. 10.1016/j.neuroimage.2022.119440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holper, L. , Muehlemann, T. , Scholkmann, F. , Eng, K. , Kiper, D. , & Wolf, M. (2010). Testing the potential of a virtual reality neurorehabilitation system during performance of observation, imagery and imitation of motor actions recorded by wireless functional near‐infrared spectroscopy (fNIRS). Journal of Neuroengineering and Rehabilitation, 7(1), 57. 10.1186/1743-0003-7-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert, T. J. (2016). Commentary on the statistical properties of noise and its implication on general linear models in functional near‐infrared spectroscopy. Neurophotonics, 3(1), 010401. 10.1117/1.nph.3.1.010401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert, T. J. , Hoge, R. D. , Diamond, S. G. , Franceschini, M. A. , & Boas, D. A. (2006). A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. NeuroImage, 29(2), 368–382. 10.1016/j.neuroimage.2005.08.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni, M. (2005). Neural mechanisms of imitation. Current Opinion in Neurobiology, 15(6), 632–637. 10.1016/j.conb.2005.10.010 [DOI] [PubMed] [Google Scholar]
- Iacoboni, M. (2009a). Imitation, empathy, and Mirror neurons. Annual Review of Psychology, 60(1), 653–670. 10.1146/annurev.psych.60.110707.163604 [DOI] [PubMed] [Google Scholar]
- Iacoboni, M. (2009b). Neurobiology of imitation. Current Opinion in Neurobiology, 19(6), 661–665. 10.1016/j.conb.2009.09.008 [DOI] [PubMed] [Google Scholar]
- Iacoboni, M. , & Dapretto, M. (2006). The mirror neuron system and the consequences of its dysfunction. Nature Reviews Neuroscience, 7(12), 942–951. 10.1038/nrn2024 [DOI] [PubMed] [Google Scholar]
- Jeannerod, M. (2007). Being oneself. Journal of Physiology‐Paris, 101(4–6), 161–168. 10.1016/j.jphysparis.2007.11.005 [DOI] [PubMed] [Google Scholar]
- Jospe, K. , Flöel, A. , & Lavidor, M. (2018). The interaction between embodiment and empathy in facial expression recognition. Social Cognitive and Affective Neuroscience, 13(2), 203–215. 10.1093/scan/nsy005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaynezhad, P. , Mitra, S. , Bale, G. , Bauer, C. , Lingam, I. , Meehan, C. , Avdic‐Belltheus, A. , Martinello, K. A. , Bainbridge, A. , Robertson, N. J. , & Tachtsidis, I. (2019). Quantification of the severity of hypoxic‐ischemic brain injury in a neonatal preclinical model using measurements of cytochrome‐c‐oxidase from a miniature broadband‐near‐infrared spectroscopy system. Neurophotonics, 6(4), 1. 10.1117/1.NPh.6.4.045009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers, C. , Paracampo, R. , & Gazzola, V. (2018). What neuromodulation and lesion studies tell us about the function of the mirror neuron system and embodied cognition. Current Opinion in Psychology, 24, 35–40. 10.1016/j.copsyc.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch, L. P. , & Cross, E. S. (2018). The influence of sensorimotor experience on the aesthetic evaluation of dance across the life span. In Progress in brain research (Vol. 237, pp. 291–316). Elsevier. 10.1016/bs.pbr.2018.03.012 [DOI] [PubMed] [Google Scholar]
- Kirsch, L. P. , Dawson, K. , & Cross, E. S. (2015). Dance experience sculpts aesthetic perception and related brain circuits. Annals of the New York Academy of Sciences, 1337(1), 130–139. 10.1111/nyas.12634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch, L. P. , Drommelschmidt, K. A. , & Cross, E. S. (2013). The impact of sensorimotor experience on affective evaluation of dance. Frontiers in Human Neuroscience, 7, 521. 10.3389/fnhum.2013.00521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch, L. P. , Snagg, A. , Heerey, E. , & Cross, E. S. (2016). The impact of experience on affective responses during action observation. PLoS One, 11(5), e0154681. 10.1371/journal.pone.0154681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis, L. , Herman, P. , & Eke, A. (2006). The modified Beer‐Lambert law revisited. Physics in Medicine and Biology, 51(5), N91–N98. 10.1088/0031-9155/51/5/N02 [DOI] [PubMed] [Google Scholar]
- Koehler, S. , Egetemeir, J. , Stenneken, P. , Koch, S. P. , Pauli, P. , Fallgatter, A. J. , & Herrmann, M. J. (2012). The human execution/observation matching system investigated with a complex everyday task: A functional near‐infrared spectroscopy (fNIRS) study. Neuroscience Letters, 508(2), 73–77. 10.1016/j.neulet.2011.12.021 [DOI] [PubMed] [Google Scholar]
- Kolyva, C. , Ghosh, A. , Tachtsidis, I. , Highton, D. , Cooper, C. E. , Smith, M. , & Elwell, C. E. (2014). Cytochrome c oxidase response to changes in cerebral oxygen delivery in the adult brain shows higher brain‐specificity than haemoglobin. NeuroImage, 85, 234–244. 10.1016/j.neuroimage.2013.05.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky, L. D. , Epel, E. S. , & Davidson, R. J. (2023). Prosociality should be a public health priority. Nature Human Behaviour, 7, 2051–2053. 10.1038/s41562-023-01717-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König, N. , Steber, S. , Borowski, A. , Bliem, H. , & Rossi, S. (2021). Neural processing of cognitive control in an emotionally neutral context in anxiety patients. Brain Sciences, 11(5), 543. 10.3390/brainsci11050543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine, N. P. (2019). Moving beyond noninformative priors: Why and how to choose weakly informative priors in Bayesian analyses. Oikos, 128(7), 912–928. 10.1111/oik.05985 [DOI] [Google Scholar]
- Lenth, R. V. (2021). emmeans: Estimated marginal means, aka least‐squares means [Computer software].
- Li, X. , Krol, M. A. , Jahani, S. , Boas, D. A. , Tager‐Flusberg, H. , & Yücel, M. A. (2020). Brain correlates of motor complexity during observed and executed actions. Scientific Reports, 10(1), 10965. 10.1038/s41598-020-67327-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke, R. , Larson, E. , Shader, M. J. , Innes‐Brown, H. , Van Yper, L. , Lee, A. K. C. , Sowman, P. F. , & McAlpine, D. (2021). Analysis methods for measuring passive auditory fNIRS responses generated by a block‐design paradigm. Neurophotonics, 8(2), 025008. 10.1117/1.nph.8.2.025008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson, M. C. , Fay, N. , & Miles, L. K. (2020). Seeing synchrony: A replication of the effects of task‐irrelevant social information on perceptions of interpersonal coordination. Acta Psychologica, 209, 103140. 10.1016/j.actpsy.2020.103140 [DOI] [PubMed] [Google Scholar]
- Maggioni, E. , Zucca, C. , Reni, G. , Cerutti, S. , Triulzi, F. M. , Bianchi, A. M. , & Arrigoni, F. (2016). Investigation of the electrophysiological correlates of negative BOLD response during intermittent photic stimulation: An EEG‐fMRI study. Human Brain Mapping, 37(6), 2247–2262. 10.1002/hbm.23170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manera, V. , Del Giudice, M. , Bara, B. G. , Verfaillie, K. , & Becchio, C. (2011). The second‐agent effect: Communicative gestures increase the likelihood of perceiving a second agent. PLoS One, 6(7), e22650. 10.1371/journal.pone.0022650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElreath, R. (2020). Statistical rethinking; a Bayesian course with examples in R and Stan (2nd ed.). Chapman and Hall/CRC. [Google Scholar]
- McNeish, D. (2016). On using bayesian methods to address small sample problems. Structural Equation Modeling: A Multidisciplinary Journal, 23(5), 750–773. 10.1080/10705511.2016.1186549 [DOI] [Google Scholar]
- Mengotti, P. , Corradi‐Dell'Acqua, C. , & Rumiati, R. I. (2012). Imitation components in the human brain: An fMRI study. NeuroImage, 59(2), 1622–1630. 10.1016/j.neuroimage.2011.09.004 [DOI] [PubMed] [Google Scholar]
- Miller, L. C. , Murphy, R. , & Buss, A. H. (1981). Consciousness of body: Private and public. Journal of Personality and Social Psychology, 41(2), 397–406. 10.1037/0022-3514.41.2.397 [DOI] [Google Scholar]
- Moffat, R. , & Cross, E. S. (2024). Evaluations of dyadic synchrony: Observers' traits influence estimation and enjoyment of synchrony in mirror‐game movements. Scientific Reports, 14, 2904. 10.1038/s41598-024-53191-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat, R. , Başkent, D. , Luke, R. , McAlpine, D. , & Van Yper, L. (2023). Cortical haemodynamic responses predict individual ability to recognise vocal emotions with uninformative pitch cues but do not distinguish different emotions. Human Brain Mapping, 44(9), 3684–3705. 10.1002/hbm.26305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat, R. , Caruana, N. , & Cross, E. S. (2024). Inhibiting responses under the watch of a recently synchronized peer increases self‐monitoring: Evidence from functional near‐infrared spectroscopy. Open Biology, 14, 230382. 10.1098/rsob.230382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat, R. , Roos, L. , Casale, C. , & Cross, E. S. (2024). Dyadic body competence predicts movement synchrony during the mirror game. Frontiers in Human Neuroscience, 18, 1401494. 10.3389/fnhum.2024.1401494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogan, R. , Fischer, R. , & Bulbulia, J. A. (2017). To be in synchrony or not? A meta‐analysis of synchrony's effects on behavior, perception, cognition and affect. Journal of Experimental Social Psychology, 72, 13–20. 10.1016/j.jesp.2017.03.009 [DOI] [Google Scholar]
- Molnar‐Szakacs, I. , Iacoboni, M. , Koski, L. , & Mazziotta, J. C. (2005). Functional segregation within pars opercularis of the inferior frontal gyrus: Evidence from fMRI studies of imitation and action observation. Cerebral Cortex, 15(7), 986–994. 10.1093/cercor/bhh199 [DOI] [PubMed] [Google Scholar]
- Munandar, V. D. , Morningstar, M. E. , & Carlson, S. R. (2020). A systematic literature review of video‐based interventions to improve integrated competitive employment skills among youth and adults with autism spectrum disorder. Journal of Vocational Rehabilitation, 53(1), 29–41. 10.3233/JVR-201083 [DOI] [Google Scholar]
- Nelson, L. D. , Simmons, J. , & Simonsohn, U. (2018). Psychology's renaissance. Annual Review of Psychology, 69(1), 511–534. 10.1146/annurev-psych-122216-011836 [DOI] [PubMed] [Google Scholar]
- Nishitani, N. , & Hari, R. (2000). Temporal dynamics of cortical representation for action. Proceedings of the National Academy of Sciences, 97(2), 913–918. 10.1073/pnas.97.2.913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld, R. , & Praamstra, P. (2001). The five percent electrode system for high‐resolution EEG and ERP measurements. Clinical Neurophysiology, 112(4), 713–719. 10.1016/S1388-2457(00)00527-7 [DOI] [PubMed] [Google Scholar]
- Orgs, G. , Calvo‐Merino, B. , & Cross, E. S. (2018). Knowing dance or knowing how to dance? Sources of expertise in aesthetic appreciation of human movement. In The neurocognition of dance: Mind, movement and motor (2nd ed.). Routledge. [Google Scholar]
- Orgs, G. , Hagura, N. , & Haggard, P. (2013). Learning to like it: Aesthetic perception of bodies, movements and choreographic structure. Consciousness and Cognition, 22(2), 603–612. 10.1016/j.concog.2013.03.010 [DOI] [PubMed] [Google Scholar]
- Orlandi, A. , Cross, E. S. , & Orgs, G. (2020). Timing is everything: Dance aesthetics depend on the complexity of movement kinematics. Cognition, 205, 104446. 10.1016/j.cognition.2020.104446 [DOI] [PubMed] [Google Scholar]
- Patterson, M. S. , Gagnon, L. R. , Vukelich, A. , Brown, S. E. , Nelon, J. L. , & Prochnow, T. (2021). Social networks, group exercise, and anxiety among college students. Journal of American College Health, 69(4), 361–369. 10.1080/07448481.2019.1679150 [DOI] [PubMed] [Google Scholar]
- Paulick, J. , Deisenhofer, A.‐K. , Ramseyer, F. , Tschacher, W. , Boyle, K. , Rubel, J. , & Lutz, W. (2018). Nonverbal synchrony: A new approach to better understand psychotherapeutic processes and drop‐out. Journal of Psychotherapy Integration, 28(3), 367–384. 10.1037/int0000099 [DOI] [Google Scholar]
- Piccoli, T. , Valente, G. , Linden, D. E. J. , Re, M. , Esposito, F. , Sack, A. T. , & Salle, F. D. (2015). The default mode network and the working memory network are not anti‐correlated during all phases of a working memory task. PLoS One, 10(4), e0123354. 10.1371/journal.pone.0123354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinti, P. , Aichelburg, C. , Lind, F. , Power, S. , Swingler, E. , Merla, A. , Hamilton, A. , Gilbert, S. , Burgess, P. , & Tachtsidis, I. (2015). Using fiberless, wearable fNIRS to monitor brain activity in real‐world cognitive tasks. Journal of Visualized Experiments, 106, 53336. 10.3791/53336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinti, P. , Tachtsidis, I. , Hamilton, A. , Hirsch, J. , Aichelburg, C. , Gilbert, S. , & Burgess, P. W. (2020). The present and future use of functional near‐infrared spectroscopy (fNIRS) for cognitive neuroscience. Annals of the new York Academy of Sciences, 1464, 5–29. 10.1111/nyas.13948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2022). R: A language and environment for statistical computing [Computer software]. R Foundation for Statistical Computing. Retrieved from http://www.r-project.org/ [Google Scholar]
- Raos, V. , Evangeliou, M. N. , & Savaki, H. E. (2004). Observation of action: Grasping with the mind's hand. NeuroImage, 23(1), 193–201. 10.1016/j.neuroimage.2004.04.024 [DOI] [PubMed] [Google Scholar]
- Ravreby, I. , Shilat, Y. , & Yeshurun, Y. (2022). Liking as a balance between synchronization, complexity and novelty. Scientific Reports, 12(1), 3181. 10.1038/s41598-022-06610-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddish, P. , Tong, E. M. W. , Jong, J. , Lanman, J. A. , & Whitehouse, H. (2016). Collective synchrony increases prosociality towards non‐performers and outgroup members. British Journal of Social Psychology, 55(4), 722–738. 10.1111/bjso.12165 [DOI] [PubMed] [Google Scholar]
- Reiss, P. T. , Gvirts, H. Z. , Bennet, R. , Kanterman, A. , Fahoum, N. , & Shamay‐Tsoory, S. G. (2019). A time‐varying measure of dyadic synchrony for three‐dimensional motion. Multivariate Behavioral Research, 54(4), 530–541. 10.1080/00273171.2018.1547874 [DOI] [PubMed] [Google Scholar]
- Rizzolatti, G. , & Fogassi, L. (2014). The mirror mechanism: Recent findings and perspectives. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1644), 20130420. 10.1098/rstb.2013.0420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti, G. , & Sinigaglia, C. (2010). The functional role of the parieto‐frontal mirror circuit: Interpretations and misinterpretations. Nature Reviews Neuroscience, 11(4), 264–274. 10.1038/nrn2805 [DOI] [PubMed] [Google Scholar]
- Rizzolatti, G. , & Sinigaglia, C. (2016). The mirror mechanism: A basic principle of brain function. Nature Reviews Neuroscience, 17(12), 757–765. 10.1038/nrn.2016.135 [DOI] [PubMed] [Google Scholar]
- Rolls, E. T. , Joliot, M. , & Tzourio‐Mazoyer, N. (2015). Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. NeuroImage, 122, 1–5. 10.1016/j.neuroimage.2015.07.075 [DOI] [PubMed] [Google Scholar]
- Rosenberg, M. (1965). Society and the adolescent self‐image. Princeton University Press. [Google Scholar]
- RStudio Team . (2020). RStudio: Integrated development for R [Computer software]. RStudio. Retrieved from http://www.rstudio.com/ [Google Scholar]
- Salazar Kämpf, M. , & Kanske, P. (2023). Mimicry and affective disorders. Frontiers in Psychiatry, 13, 1105503. 10.3389/fpsyt.2022.1105503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar Kämpf, M. , Nestler, S. , Hansmeier, J. , Glombiewski, J. , & Exner, C. (2021). Mimicry in psychotherapy—An actor partner model of therapists' and patients' non‐verbal behavior and its effects on the working alliance. Psychotherapy Research, 31(6), 752–764. 10.1080/10503307.2020.1849849 [DOI] [PubMed] [Google Scholar]
- Santosa, H. , Huppert, T. J. , Fishburn, F. , & Zhai, X. (2019). Investigation of the sensitivity‐specificity of canonical‐ and deconvolution‐based linear models in evoked functional near‐infrared spectroscopy. Neurophotonics, 6(2), 1–10. 10.1117/1.NPh.6.2.025009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosa, H. , Zhai, X. , Fishburn, F. , & Huppert, T. J. (2018). The NIRS brain AnalyzIR toolbox. Algorithms, 11(5), 73. 10.3390/a11050073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabold, S. , & Perktold, J. (2010). Statsmodels: Econometric and statistical modeling with Python (pp. 92–96). SciPy Organizers. 10.25080/Majora-92bf1922-011 [DOI] [Google Scholar]
- Sestieri, C. , Corbetta, M. , Romani, G. L. , & Shulman, G. L. (2011). Episodic memory retrieval, parietal cortex, and the default mode network: Functional and topographic analyses. The Journal of Neuroscience, 31(12), 4407–4420. 10.1523/JNEUROSCI.3335-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada, S. , & Abe, R. (2010). Outcome and view of the player modulate motor area activity during observation of a competitive game. Neuropsychologia, 48(7), 1930–1934. 10.1016/j.neuropsychologia.2010.03.012 [DOI] [PubMed] [Google Scholar]
- Signal Developers . (2014). signal: Signal processing [Computer software]. Retrieved from http://r-forge.r-project.org/projects/signal/
- Stefan, K. , Cohen, L. G. , Duque, J. , Mazzocchio, R. , Celnik, P. , Sawaki, L. , Ungerleider, L. , & Classen, J. (2005). Formation of a motor memory by action observation. The Journal of Neuroscience, 25(41), 9339–9346. 10.1523/JNEUROSCI.2282-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strangman, G. E. , Franceschini, M. A. , & Boas, D. A. (2003). Factors affecting the accuracy of near‐infrared spectroscopy concentration calculations for focal changes in oxygenation parameters. NeuroImage, 18(4), 865–879. 10.1016/S1053-8119(03)00021-1 [DOI] [PubMed] [Google Scholar]
- Thornton, M. A. , & Conway, A. R. A. (2013). Working memory for social information: Chunking or domain‐specific buffer? NeuroImage, 70, 233–239. 10.1016/j.neuroimage.2012.12.063 [DOI] [PubMed] [Google Scholar]
- Tomar, S. (2006). Converting video formats with FFmpeg [Computer software].
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , Mazoyer, B. , & Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15(1), 273–289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Urgolites, Z. J. , & Wood, J. N. (2013). Binding actions and scenes in visual long‐term memory. Psychonomic Bulletin & Review, 20(6), 1246–1252. 10.3758/s13423-013-0440-1 [DOI] [PubMed] [Google Scholar]
- Vehtari, A. , Gelman, A. , & Gabry, J. (2017). Practical Bayesian model evaluation using leave‐one‐out cross‐validation and WAIC. Statistics and Computing, 27(5), 1413–1432. 10.1007/s11222-016-9696-4 [DOI] [Google Scholar]
- Vicary, S. , Sperling, M. , von Zimmermann, J. , Richardson, D. C. , & Orgs, G. (2017). Joint action aesthetics. PLoS One, 12(7), e0180101. 10.1371/journal.pone.0180101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneswaran, G. , Philipp, R. , Lemon, R. N. , & Kraskov, A. (2013). M1 corticospinal mirror neurons and their role in movement suppression during action observation. Current Biology, 23(3), 236–243. 10.1016/j.cub.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lühmann, A. , Ortega‐Martinez, A. , Boas, D. A. , Yücel, M. A. , Lühmann, A. V. , Ortega‐Martinez, A. , & Boas, D. A. (2020). Using the general linear model to improve performance in fNIRS single trial analysis and classification: A perspective. Frontiers in Human Neuroscience, 14(February), 30. 10.3389/fnhum.2020.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittlesea, B. W. A. , & Price, J. R. (2001). Implicit /explicit memory versus analytic/nonanalytic processing: Rethinking the mere exposure effect. Memory & Cognition, 29(2), 234–246. 10.3758/BF03194917 [DOI] [PubMed] [Google Scholar]
- Wolf, M. , Wolf, U. , Toronov, V. , Michalos, A. , Paunescu, L. A. , Choi, J. H. , & Gratton, E. (2002). Different time evolution of oxyhemoglobin and deoxyhemoglobin concentration changes in the visual and motor cortices during functional stimulation: A near‐infrared spectroscopy study. NeuroImage, 16(3), 704–712. 10.1006/nimg.2002.1128 [DOI] [PubMed] [Google Scholar]
- Wriessnegger, S. , Kurzmann, J. , & Neuper, C. (2008). Spatio‐temporal differences in brain oxygenation between movement execution and imagery: A multichannel near‐infrared spectroscopy study. International Journal of Psychophysiology, 67(1), 54–63. 10.1016/j.ijpsycho.2007.10.004 [DOI] [PubMed] [Google Scholar]
- Wurm, M. F. , & Schubotz, R. I. (2018). The role of the temporoparietal junction (TPJ) in action observation: Agent detection rather than visuospatial transformation. NeuroImage, 165, 48–55. 10.1016/j.neuroimage.2017.09.064 [DOI] [PubMed] [Google Scholar]
- Yücel, M. A. , Lühmann, A. V. , Scholkmann, F. , Gervain, J. , Dan, I. , Ayaz, H. , Boas, D. , Cooper, R. J. , Culver, J. , Elwell, C. E. , Eggebrecht, A. , Franceschini, M. A. , Grova, C. , Homae, F. , Lesage, F. , Obrig, H. , Tachtsidis, I. , Tak, S. , Tong, Y. , … Wolf, M. (2021). Best practices for fNIRS publications. Neurophotonics, 8(1), 1–34. 10.1117/1.nph.8.1.012101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Tan, F. , Xu, X. , Duan, L. , Liu, H. , Tian, F. , & Zhu, C.‐Z. (2015). Multiregional functional near‐infrared spectroscopy reveals globally symmetrical and frequency‐specific patterns of superficial interference. Biomedical Optics Express, 6(8), 2786–2802. 10.1364/boe.6.002786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimeo Morais, G. A. , Balardin, J. B. , & Sato, J. R. (2018). FNIRS optodes' location decider (fOLD): A toolbox for probe arrangement guided by brain regions‐of‐interest. Scientific Reports, 8, 1–11. 10.1038/s41598-018-21716-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimeo Morais, G. A. , Scholkmann, F. , Balardin, J. B. , Furucho, R. A. , de Paula, R. C. V. , Biazoli, C. E. , & Sato, J. R. (2017). Non‐neuronal evoked and spontaneous hemodynamic changes in the anterior temporal region of the human head may lead to misinterpretations of functional near‐infrared spectroscopy signals. Neurophotonics, 5(1), 1. 10.1117/1.NPh.5.1.011002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumeta, L. N. , Oriol, X. , Telletxea, S. , Amutio, A. , & Basabe, N. (2016). Collective efficacy in sports and physical activities: Perceived emotional synchrony and shared flow. Frontiers in Psychology, 6, 1960. 10.3389/fpsyg.2015.01960 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Supporting information.
Data Availability Statement
The datasets collect and analysed for this study can be found our repository entitled ‘Embodying aesthetic movement preferences’ on OSF: https://osf.io/e7w4v/.


