Abstract
Sedentary behavior (SB) or sitting is associated with multiple unfavorable health outcomes. Bone tissue responds to imposed gravitational and muscular strain with there being some evidence suggesting a causal link between SB and poor bone health. However, there are no population-based data on the longitudinal relationship between SB, bone change, and incidence of fragility fractures. This study aimed to examine the associations of sitting/SB (defined as daily sitting time), areal BMD (by DXA), and incident low trauma (fragility) osteoporotic fractures (excluding hands, feet, face, and head). We measured baseline (1995-7) and 10-yr self-reported SB, femoral neck (FN), total hip (TH), and lumbar spine (L1–L4) BMD in 5708 women and 2564 men aged 25 to 80+ yr from the population-based, nationwide, 9-center Canadian Multicentre Osteoporosis Study. Incident 10-yr fragility fracture data were obtained from 4624 participants; >80% of fractures were objectively confirmed by medical records or radiology reports. Vertebral fractures were confirmed by qualitative morphological methods. All analyses were stratified by sex. Multivariable regression models assessed SB-BMD relationships; Cox proportional models were fit for fracture risk. Models were adjusted for age, height, BMI, physical activity, and sex-specific covariates. Women in third/fourth quartiles had lower adjusted FN BMD versus women with the least SB (first quartile); women in the SB third quartile had lower adjusted TH BMD. Men in the SB third quartile had lower adjusted FN BMD than those in SB first quartile. Neither baseline nor stable 10-yr SB was related to BMD change nor to incident fragility fractures. Increased sitting (SB) in this large, population-based cohort was associated with lower baseline FN BMD. Stable SB was not associated with 10-yr BMD loss nor increased fragility fracture. In conclusion, habitual adult SB was not associated with subsequent loss of BMD nor increased risk of fracture.
Keywords: sedentary behavior, areal BMD, population-based cohort, CaMos, incident fragility fractures
Lay Summary
The number of hours of sitting in a day (often called “sedentary behavior”) is currently understood to be “bad for bone health” both because of increased bone loss and a higher risk for fractures.
Very few studies in randomly sampled men and women from a whole population have consistently asked about hours of sitting and examined baseline bone density. Fewer still have compared hours of sitting and its changes over 10 yr with changes in bone density and the number of new fractures that occurred.
The Canadian Multicentre Osteoporosis Study obtained sitting hours from 5708 women and 2564 men aged 25 to 80+ yr and compared it with the spine, total hip (TH), and femoral neck (FN) bone density values. The average sitting at 7.4 h in men was associated with slightly lower adjusted femoral neck bone density; in women, sitting 6.7 h/d was associated with slightly lower adjusted FN and TH bone density.
Ten-year follow-up data (now in about 5000 people) showed no relationship between the slightly longer sitting (an increase of 18% in men and 22% in women) and bone loss or new bone fractures.
In this large country-wide population-based study, hours of sitting each day were not associated with 10-yr BMD loss in women or men nor did sitting more associate with new bone fractures. These data are reassuring; women and men who walk regularly and have some moderate-vigorous physical activity each day, despite more sitting, do not seem to be at greater risk for osteoporosis.
Introduction
Strong evidence links daytime, nonsleep sitting, or sedentary behavior (SB) to unfavorable health outcomes such as all-cause mortality,1 fatal2 and nonfatal cardiovascular disease,3 type 2 diabetes (T2DM),2 and certain cancers.4 Bone tissue responds to imposed gravitational and muscular strain,5 thus negative effects of a sedentary lifestyle on areal BMD and fragility fracture also seem probable. Quick and continuous loss of bone mass is observed in studies of bed rest, space flight, or spinal injury in which inactivity is imposed.6 However, the effects of a habitual, long-term sedentary lifestyle on bone health are not well known. Only two previous SB and BMD investigations were population-based, but accelerometry data were collected from low percentages of participants and for short durations of time; the results of these studies provided conflicting results.7,8
The World Health Organization (WHO) recently highlighted the important association between SB and bone health in their 2020 guidelines on physical activity (PA) and SB.9 There is, however, a lack of good quality studies on the issue, as pointed out in a paper summarizing the scientific evidence behind the SB guidelines.10 A 2021 systematic review further highlighted the scarcity of studies on this issue in elderly populations.11
The importance of PA is well established for the maintenance of BMD and minimizing or preventing osteoporotic fractures.12-16 Alternatively, few studies of SB related to bone health have been performed in adult and elderly populations.17 Cross-sectional studies of SB have yielded inconsistent results in any observed associations.7,18-20 Few longitudinal population-based studies of SB and adult bone health are published7,8; results are heterogeneous both for measurement site and for sex/gender.
Results also vary for longitudinal analyses of SB and fragility fractures. In menopausal women, increased SB hours were associated with overall risk of any fracture.21 In elderly men, each 90-min increase in SB/d was associated with a 20% increase in fractures.22 SB has not yet been studied related to incident fragility fractures in randomly sampled, large cohorts prospectively documented. The Canadian Multicentre Osteoporosis Study (CaMos) was designed to study BMD and fracture risk in a population-based sample from across Canada.23
The aims of the current study were first to explore the cross-sectional relationship between SB and BMD in the population-based CaMos cohort, and second to examine whether SB at baseline and SB change during the 10-yr CaMos follow-up predicted 10-yr BMD changes and/or fracture incidence.
Materials and methods
Study design and participants
We used CaMos data of noninstitutionalized adults (69% women), aged 25 yr and older, randomly selected from the general population living within 50 km of 9 Canadian cities. Detailed study methods have been published previously,23 and further information is available at www.camos.org.
Data collection
At baseline (1995–1997), participants completed a standardized interviewer-administered questionnaire (CaMos questionnaire ©1995) including demographics; family, reproductive, and health history; lifestyle; and any previous fracture. Baseline clinical assessment included height, weight, and BMD as well as vertebral radiographs if ≥50 yr old. Participants attended follow-up visits and completed the CaMos questionnaire again at year 10 (2005–2007). Postal questionnaires queried fractures annually.
A total of 9423 individuals agreed to full participation. Information on baseline SB and BMD was available for 8272 participants. At the 10-yr follow-up, information on SB and BMD was available for 4624 participants as summarized in Figure 1. Ethical approval was granted through McGill University and ethics review boards for each of the 9 local institutions. All participants provided signed informed consent in accordance with the Helsinki Declaration.
Figure 1.
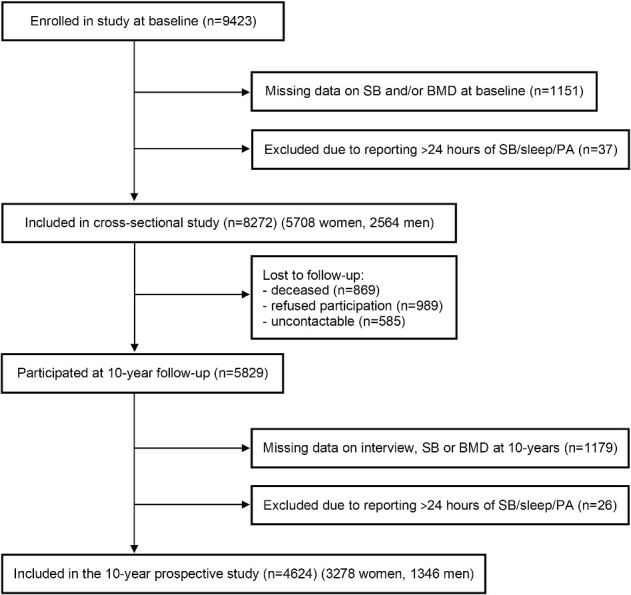
Flow chart showing included Canadian Multicentre Osteoporosis Study participants aged ≥25 yr in cross-sectional and longitudinal analyses of sitting time (sedentary behavior), BMD, and incident fragility fracture.
Sedentary Behavior
Reported as hours/day of sitting, SB was assessed by self-report questions regarding average time spent sitting (h/d) during transit (car, bus, etc.), at work, watching television, at meals, and in other sitting activities such as reading, playing cards, and sewing, over the preceding 12 mo. Computer screen time (for leisure) was added at the 10-yr follow-up. Response options were “never,” “<1,” “1 to 2,” “3 to 4,” “5 to 6,” “7 to 10,” and “11 h or more.” The mid-point of the ranges for each response option was used to summarize specific sitting times into a “total sitting time.”24 All sitting activities were summed to generate the total h/d spent in SB. Ten-year change in SB (absolute change in total hours) was calculated for each participant.
Baseline covariates
Information collected at baseline included date of birth, sex and ethnicity, h/wk of walking, and h/wk of combined moderate, vigorous, and strenuous PA. Average duration (h/d) of sleep, including naps was also reported. Education was classified as the highest grade completed of <12 yr, high school diploma, postsecondary education; smoking was classified as current, prior, or never smoker. Daily total calcium (mg/d) and vitamin D (IU/d) intakes were obtained from a semiquantitative food frequency questionnaire of calcium-rich foods25 plus reported regularly taken supplements (total calcium and total vitamin D). Alcohol consumption was classified as none, moderate (<1 drink/d in women, <2 drink/d in men), or high (≥1 drink/d in women, ≥2 drinks/d in men); caffeine consumption was reported in mg/d.
Participants provided information on previous fragility fractures (ie, without trauma or caused by a fall from standing height or less), use of menopausal hormone therapy (never, former, current), bisphosphonate therapy (current), and weight cycling (ever lost/regained ≥10 pounds) and immobilization (ie, confined to a bed, wheelchair, or by a cast for more than 1 mo). Women also answered questions regarding the use of combined hormonal contraceptives (CHC, ever) and menopausal (1-yr without flow) status. Comorbidities (including heart disease, hypertension, T2DM, cancer) were recorded and classified as none, 1 to 2, ≥3. From measured height and weight, BMI was calculated as kg/m2.
BMD and its changes over time
Areal BMD (g/cm2) was measured at the lumbar spine (L1–L4), total hip (TH), and femoral neck (FN) by DXA. All centers performed recommended daily and weekly DXA quality assurance tests, and all DXA scans were read centrally by the same two technologists. Details on BMD quality control and national data integration are published elsewhere.26
Fragility fractures
Participants reported any incident fractures at all scheduled interviews (baseline and years 3, 5, and 10) and in all other years by annual postal questionnaire. These fractures are referred to as clinical fractures. Confirmation and further information regarding clinical fractures (eg, fracture site, date and circumstances leading to fracture and its medical treatment) were collected in a structured in-person (years 3, 5, and 10) or telephone interview. Radiology or hospital reports were obtained where possible (with participants’ written consent); over 80% of fractures were objectively confirmed. We included all fragility clinical fractures (excluding the skull, face, hands, and feet) and examined major osteoporosis fractures (hip, clinical spine, humerus, and forearm/wrist) and fragility hip fracture.
As part of study protocol, participants aged 50 yr and older underwent thoracic and lumbar spine radiographs at baseline and year 10 follow-up. Morphologic vertebral fractures (MVFs) were obtained from assessment of these radiographs using a morphologic method as previously documented.27,28 Incident MVFs also included an increase in the severity or grade of a given MVF within-person from baseline to year 10; no MVF means 0 severity change.
Statistical analysis
Statistical analyses were completed using SAS Studio release 3.8 (2012–2018, SAS Institute Inc.,). All statistical analyses were a priori stratified by sex, based on known sex-related differences in SB, BMD, and fracture occurrence. As our study is an exploratory, descriptive study, we analyzed our data without multiplicity adjustment.
Descriptive statistics are reported as mean (±SD) or number (%). At baseline, univariate linear regression models of SB by BMD suggested a nonlinear relationship. We therefore analyzed baseline SB according to quartiles, based on the sex-specific distribution. Univariate linear regression models were fit for each baseline covariable with each baseline BMD site. Those variables reaching statistical significance (CI excluding zero) were included in the linear multivariable regression model used to assess the relationship between SB (quartiles) and BMD. Due to the known nonlinear relationship between BMD and age, we considered age as a polynomial of up to 3 degrees. Age, height, BMI, and time spent in walking and in PA (moderate, vigorous, strenuous) were included, regardless of their statistical significance, at the univariate step.
Multivariable regression models were fit in several steps. First, we reported the association between SB and BMD (or change thereof) in unadjusted models. Second, we adjusted for age. Third, we further adjusted for significant covariates, plus height, walking time, and hours of moderate, vigorous, or strenuous PA. Finally, we further adjusted for BMI. Interactions with SB of age, PA, and BMI were considered, but none were statistically significant.
For the longitudinal analysis, baseline SB (quartiles) was the main independent variable and change in BMD in each measured site the dependent variable. We adopted a modeling strategy similar to that for cross-sectional analyses. We first fitted unadjusted models, second adjusted for age, and third adjusted for each of the baseline covariates specific for BMD change at each measurement site based on univariate regressions, and finally further adjusted for baseline BMI. To study the influence of 10-yr change in SB on 10-yr change in BMD, we then regressed the SB change (independent variable) on the residuals of the previous model (dependent variable; model including baseline SB, age, baseline covariates and BMI) and further adjusted for change in walking time and moderate, vigorous, or strenuous PA as well as changes in BMI.
To examine the relationship between SB and incident clinical fragility fractures (excluding MVF), person-time (years) included the time from study enrolment to exit (earliest date of: incident fracture, death, loss to follow up, 10-yr follow-up interview) for participants aged ≥50 yr at baseline (n = 6712 participants; 1917 men, 4795 women). We used Cox models to adjust for known important baseline covariables such as age, height, BMI, walking and moderate to vigorous PA time, FN BMD, and prevalent fragility clinical fractures. The number of covariates included in the models for hip fractures was limited by the number of events.
Since incident MVF were ascertained from radiographs every 5 yr, we used logistic regression to examine the association of baseline SB with 10-yr incident fragility fractures including incident MVF.
Finally, to explore the potential impact of participant immobilization on our findings, we conducted sensitivity analyses after excluding participants who reported a period of immobilization of 1 mo or longer in the year prior to baseline.
Results
Characteristics of all study participants with baseline measures are presented in Table 1, and the flow of study participants through both the cross-sectional and prospective analyses is in Figure 1. Mean baseline age of the whole sample (n = 8272) was 61.1 (±13.0) yr. Mean BMI was 27.0 kg/m2, and about half of the sample reported at least one weight-cycling episode (10 pounds loss and regain). At baseline, 72.4% of women were menopausal (≥1 yr since final menstruation). Current or former use of menopausal hormone therapy was reported by, respectively, 25.1% and 18.4% of women; 49.0% had ever used CHC. Moderate, vigorous, and strenuous PA averaged 15.0 h/wk. Participants reported 4.2 h/wk of walking, on average. More than a third reported a parental history of fractures, and 18.5% of all participants reported one or more prevalent or past clinical fragility fractures. Thirteen women and 7 men were immobilized for more than 1 mo in the year prior to baseline: 18 for <3 mon, 1 for 4 mon, and 1 for 6 mon.
Table 1.
Characteristics for all CaMos participants ≥ 25 yr with sedentary time data (sitting h/d) at baseline.
| All (n = 8272) | Men (n = 2564) | Women (n = 5708) | |
|---|---|---|---|
| Age (yr) | 61.1 (13.0) | 59.0 (14.1) | 62.0 (12.4) |
| White, n (%) | 7856 (95.0) | 2392 (93.3) | 5464 (95.7) |
| Weight (kg) | 72.8 (14.7) | 81.7 (13.3) | 68.8 (13.5) |
| Height (cm) | 164.1 (9.2) | 173.6 (7.1) | 159.8 (6.4) |
| BMI (kg/m2) | 27.0 (4.7) | 27.1 (3.9) | 26.9 (5.0) |
| Weight cycling >10 pounds (%) | 4253 (51.4) | 1226 (47.8) | 3027 (53.0) |
| Education (% within sex) | |||
| <12 yr | 2884 (34.9) | 798 (30.8) | 2095 (36.7) |
| High school | 1214 (14.7) | 332 (13.0) | 882 (15.5) |
| ≥Postsecondary diploma | 4174 (50.5) | 1443 (56.3) | 2731 (47.9) |
| Smoking (%) | |||
| Never | 3888 (47.0) | 860 (33.6) | 3028 (53.1) |
| Former | 3118 (37.7) | 1238 (48.3) | 1880 (32.9) |
| Current | 1265 (15.3) | 465 (18.1) | 800 (14.0) |
| Caffeine consumption (mg/d) | 295.2 (274.2) | 345.2 (322.8) | 272.7 (246.0) |
| Alcohol consumptiona | |||
| Nondrinker | 3065 (37.1) | 643 (25.1) | 2422 (42.5) |
| Moderate | 4060 (49.2) | 1536 (60.0) | 2524 (44.3) |
| High | 1132 (13.7) | 380 (14.9) | 752 (13.2) |
| Total calcium intake (mg/d) | 1018.3 (614.7) | 928.3 (590.9) | 1058.6 (620.9) |
| Total vitamin D intake (μg/d) | 6.9 (22.5) | 5.4 (14.0) | 7.5 (25.4) |
| Osteoporosis diagnosis (self-report) (%) | 579 (7.1) | 27 (1.1) | 552 (9.9) |
| History of clinical fragility fracture (%) | 1526 (18.5) | 412 (16.1) | 114 (19.6) |
| Parental history of fractures (%) | 2680 (35.2) | 769 (33.4) | 1911 (36.0) |
| Current use of antiresorptive medication (%) | 1475 (17.8) | 3 (0.1) | 1472 (25.8) |
| Current use of corticosteroids (%) | 99 (1.2) | 27 (1.1) | 72 (1.3) |
| Comorbidities | |||
| 0 | 4514 (54.6) | 1454 (56.7) | 3060 (53.6) |
| 1–2 | 3418 (41.3) | 1002 (39.1) | 2416 (42.3) |
| ≥3 | 340 (4.1) | 108 (4.2) | 232 (4.1) |
| Reproductive status (women only) (%) | N/A | N/A | |
| Premenopausal | 1001 (17.5) | ||
| Menopausal | 4133 (72.4) | ||
| Bilateral oophorectomy | 573 (10.0) | ||
| Menopausal hormone therapy (%) | N/A | N/A | |
| Never | 3225 (56.5) | ||
| Former | 1050 (18.4) | ||
| Current | 1433 (25.1) | ||
| Combined hormonal contraceptives, ever (%) | N/A | N/A | 2796 (49.0) |
| Sleep (h/night) | 7.1 (1.4) | 7.1 (1.4) | 7.1 (1.5) |
| Moderate, vigorous, strenuous physical activity (h/wk) | 15.0 (10.8) | 14.3 (11.3) | 15.3 (10.6) |
| Walking (h/wk) | 4.2 (4.4) | 4.1 (4.6) | 4.2 (4.2) |
| Study center | |||
| Calgary | 1002 (12.1) | 300 (11.7) | 702 (12.3) |
| Hamilton | 937 (11.3) | 301 (11.7) | 636 (11.1) |
| Halifax | 920 (11.1) | 281 (11.0) | 639 (11.2) |
| Kingston | 821 (9.9) | 251 (9.8) | 570 (10.0) |
| Quebec | 924 (11.2) | 281 (11.0) | 643 (11.3) |
| Saskatoon | 1017 (12.3) | 314 (12.3) | 703 (12.3) |
| St-John’s | 860 (10.4) | 259 (10.1) | 601 (10.5) |
| Toronto | 767 (9.3) | 257 (10.0) | 510 (8.9) |
| Vancouver | 1024 (12.4) | 320 (12.5) | 704 (12.3) |
| Sedentary behavior (sitting h/d) | 6.9 (2.7) | 7.4 (2.8) | 6.7 (2.6) |
| Lumbar spine L1–L4 BMD (g/cm2) | 0.971 (0.178) | 1.047 (0.169) | 0.937 (0.172) |
| Femoral neck BMD (g/cm2) | 0.725 (0.124) | 0.789 (0.122) | 0.699 (0.115) |
| Total hip BMD (g/cm2) | 0.903 (0.162) | 1.009 (0.149) | 0.855 (0.144) |
Results presented as mean (SD) for continuous variables and n (% of all or within sex) for categorical variables.
aModerate alcohol consumption was defined as <1 drink/d in women, <2 drink/d in men and high consumption as ≥1 drink/d in women, ≥2 drinks/d in men.
N/A, not applicable.
For women, SB was categorized into quartiles with ≤4.5 h/d being the first quartile, 5.0 to 6.0 h/d (second quartile), 6.5 to 8.0 h/d (third quartile), and 8.5 to 19.5 h/d (fourth quartile). In men, SB quartiles were ≤5.0 h/d (first quartile), 5.5 to 7.0 h/d (second quartile), 7.5 to 9.0 h/d (third quartile), and 9.5 to 19.0 h/d (fourth quartile).
The cross-sectional analysis of the baseline association between SB and BMD is shown in Table 2. Significant differences were observed in crude models across categories of sedentary behavior for all BMD measurement sites in women and TH and FN in men. However, most were no longer present after adjusting for relevant factors in the analysis, such as PA. (Additional models for analysis of Table 2 are in Supplementary Table S1.) In adjusted analyses, FN BMD was lower in women in the third and fourth quartiles versus the first quartile. In addition, lower TH BMD was observed in women in the third quartile of SB compared with the first quartile. Men in the third quartile of SB had significantly lower adjusted FN BMD than those in the first quartile of SB.
Table 2.
Parameter estimates (95% CIs) for baseline and 10-yr change in lumbar spine, femoral neck, and total hip BMD with baseline sedentary behavior (in quartiles of sitting time, h/d; reference = first quartile, ie, lowest sitting time).
| Outcome | Variables in models | Women | Men | ||||
|---|---|---|---|---|---|---|---|
| Second quartile | Third quartile | Fourth quartile | Second quartile | Third quartile | Fourth quartile | ||
| 5.0–6.0 h/d | 6.5–8.0 h/d | >8.5 h/d | 5.5–7.0 h/d | 7.–9.0 h/d | >9.5 h/d | ||
| Cross-sectional analyses with baseline BMD (g/cm 2 ) | |||||||
| Lumbar spine BMD | 1: Unadjusted | −0.018 (−0.030 to −0.005) | −0.004 (−0.017 to 0.009) | 0.024 (0.011 to 0.038) | 0.010 (−0.008 to 0.028) | 0.001 (−0.018 to 0.020) | 0.007 (−0.012 to 0.025) |
| 2: + age + covariatesa | −0.001 (−0.012 to 0.011) | 0.007 (−0.005 to 0.019) | 0.008 (−0.004 to 0.020) | 0.002 (−0.017 to 0.020) | 0.005 (−0.014 to 0.025) | 0.009 (−0.009 to 0.031) | |
| 3: + BMI | −0.007 (−0.018 to 0.004) | −0.003 (−0.015 to 0.008) | −0.005 (−0.017 to 0.007) | −0.002 (−0.020 to 0.016) | −0.001 (−0.020 to 0.018) | 0.003 (−0.016 to 0.023) | |
| Femoral neck BMD | 1: Unadjusted | −0.017 (−0.026 to −0.008) | −0.015 (−0.024 to −0.005) | 0.014 (0.004 to 0.024) | 0.002 (−0.012 to 0.015) | −0.002 (−0.016 to 0.012) | 0.026 (0.012–0.040) |
| 2: + age + covariatesb | 0.000 (−0.008 to 0.008) | −0.001 (−0.009 to 0.007) | 0.000 (−0.008 to 0.009) | 0.001 (−0.012 to 0.014) | −0.010 (−0.023 to 0.003) | 0.000 (−0.013 to 0.014) | |
| 3: + BMI | −0.005 (−0.012 to 0.002) | −0.009 (−0.016 to −0.002) | −0.010 (−0.018 to −0.002) | −0.002 (−0.014 to 0.010) | −0.015 (−0.027 to −0.002) | −0.007 (−0.020 to 0.006) | |
| Total hip BMD | 1: Unadjusted | −0.016 (−0.027 to −0.006) | −0.013 (−0.024 to −0.003) | 0.019 (0.008–0.030) | 0.006 (−0.010 to 0.022) | 0.002 (−0.015 to 0.019) | 0.023 (0.007–0.040) |
| 2: + age + covariatesc | 0.003 (−0.006 to 0.012) | 0.003 (−0.006 to 0.013) | 0.008 (−0.002 to 0.018) | 0.006 (−0.009 to 0.021) | −0.003 (−0.019 to 0.013) | 0.006 (−0.011 to 0.022) | |
| 3: + BMI | −0.005 (−0.013 to 0.003) | −0.009 (−0.017 to 0.000) | −0.007 (−0.016 to 0.002) | 0.001 (−0.013 to 0.015) | −0.010 (−0.025 to 0.005) | −0.005 (−0.020 to 0.010) | |
| Longitudinal analyses with 10-yr change in BMD (g/cm 2 ) | |||||||
| Lumbar spine BMD | 1: Unadjusted | 0.016 (0.007 to 0.025) | 0.017 (0.008 to 0.025) | −0.005 (−0.014 to 0.004) | −0.001 (−0.013 to 0.010) | −0.008 (−0.020 to 0.004) | −0.013 (−0.025 to −0.002) |
| 2: + age + covariatesd | 0.006 (−0.002 to 0.013) | 0.006 (−0.002 to 0.013) | 0.002 (−0.007 to 0.011) | −0.004 (−0.015 to 0.007) | −0.008 (−0.019 to 0.004) | −0.006 (−0.018 to 0.005) | |
| 3: + BMI | 0.005 (−0.003 to 0.013) | 0.005 (−0.003 to 0.013) | 0.001 (−0.007 to 0.009) | −0.005 (−0.016 to 0.006) | −0.009 (−0.020 to 0.003) | −0.007 (−0.019 to 0.004) | |
| Femoral neck BMD | 1: Unadjusted | 0.003 (−0.002 to 0.009) | 0.003 (−0.002 to 0.009) | 0.002 (−0.004 to 0.007) | −0.000 (−0.009 to 0.008) | 0.003 (−0.006 to 0.011) | −0.005 (−0.013 to 0.003) |
| 2: + age + covariatese | 0.001 (−0.005 to 0.006) | 0.001 (−0.004 to 0.007) | 0.004 (−0.002 to 0.010) | 0.001 (−0.008 to 0.009) | 0.003 (−0.005 to 0.012) | −0.005 (−0.013 to 0.004) | |
| 3: + BMI | 0.002 (−0.004 to 0.007) | 0.002 (−0.003 to 0.008) | 0.005 (−0.001 to 0.011) | 0.001 (−0.007 to 0.009) | 0.004 (−0.005 to 0.013) | −0.004 (−0.012 to 0.005) | |
| Total hip BMD | 1: Unadjusted | 0.002 (−0.004 to 0.008) | 0.005 (−0.001 to 0.011) | 0.005 (−0.001 to 0.011) | −0.008 (−0.018 to 0.001) | 0.000 (−0.10 to 0.010) | −0.005 (−0.014 to 0.005) |
| 2: + age + covariatesf | 0.002 (−0.004 to 0.008) | 0.004 (−0.002 to 0.010) | 0.004 (−0.002 to 0.010) | −0.005 (−0.014 to 0.004) | −0.001 (−0.010 to 0.008) | −0.006 (−0.015 to 0.003) | |
| 3: + BMI | 0.003 (−0.003 to 0.009) | 0.005 (−0.001 to 0.011) | 0.006 (−0.000 to 0.012) | −0.004 (−0.013 to 0.004) | 0.000 (−0.009 to 0.009) | −0.005 (−0.014 to 0.004) | |
aCovariates for L1–L4 BMD: For women: height, education, center, smoking, alcohol, physical activity, walking, weight cycling, caffeine intake, vitamin D intake, osteoporosis diagnosis, prior clinical fragility fracture, antiresorptive use, corticosteroid use, combined hormonal contraceptives (CHC); for men: height, White, center, smoking, alcohol, physical activity, walking, weight cycling, calcium intake, osteoporosis diagnosis, prior clinical fragility fracture, parental fracture, corticosteroid use, comorbidities.
bCovariates for femoral neck BMD: For women: height, education, center, smoking, alcohol, physical activity, walking, weight cycling, caffeine intake, vitamin D intake, osteoporosis diagnosis, prior clinical fragility fracture, antiresorptive use, corticosteroid use, CHC, comorbidities; For men: education, height, center, smoking, alcohol, physical activity, walking, weight cycling, calcium intake, osteoporosis diagnosis, prior clinical fragility fracture, corticosteroid use, comorbidities, hours of sleep.
cCovariates for total hip BMD: For women: height, education, center, alcohol, physical activity, walking, weight cycling, vitamin D intake, osteoporosis diagnosis, prior clinical fragility fracture, antiresorptive use, corticosteroids, CHC, comorbidities; For men: education, height, center, smoking, alcohol, physical activity, walking, weight cycling, calcium intake, osteoporosis diagnosis, prior clinical fragility fracture, corticosteroid use, hours of sleep.
dCovariates for L1–L4 BMD: For women: height, education, center, alcohol, physical activity, walking, caffeine intake, vitamin D intake, calcium intake, osteoporosis diagnosis, prior clinical fragility fracture, comorbidities, CHC; For men: height, center, smoking, physical activity, walking, vitamin D intake, antiresorptive use.
eCovariates for femoral neck BMD: For women: height, education, center, physical activity, walking, weight cycling, calcium intake, osteoporosis diagnosis, prior clinical fragility fracture, antiresorptive use, corticosteroid use, comorbidities, CHC; For men: height, center, physical activity, walking, weight cycling.
fCovariates for total hip BMD: For women: height, White, physical activity, walking, weight cycling, antiresorptive use, comorbidities; For men: height, White, center, physical activity, walking, weight cycling, antiresorptive use, comorbidities.
Univariate linear regression models were fit for each baseline covariable with each baseline BMD site. Variables reaching statistical significance (P < .05) were included in the respective model.
Those who participated in both baseline and year 10 (n = 4624) were younger than those who died or were lost to follow-up (n = 2443) (Supplementary Table S2). Among participants with measures of BMD at both baseline and year 10 (n = 4624), BMD decreased at all measurement sites in both sexes except for the lumbar spine in men (Supplemental Table S3). Mean SB increased over 10 yr by 21.7% in women and 17.5% in men.
In longitudinal analyses (Table 2, Supplementary Table S1), unadjusted models indicated a significant difference in change in lumbar spine BMD in both women and men. However, baseline SB did not predict change in BMD after adjustment for age, PA, and other covariates. Linear regression of the residuals of those models with 10-yr change in SB (per 1 SD of change) showed no associations with change in BMD at any site in women (L1–L4: 0.000 g/cm2 [−0.003; 0.002], FN: 0.000 g/cm2 [−0.002; 0.002], TH: 0.000 g/cm2 [−0.002; 0.002]). Likewise, in men, there was no association of change in SB with BMD change (L1–L4: −0.001 g/cm2 [−0.004; 0.003], FB: 0.001 g/cm2 [−0.002; 0.004], TH: 0.001 g/cm2 [−0.002; 0.004]).
Survival analyses for incident clinical fragility fractures (not including MVFs) were performed on participants aged ≥50 yr at baseline (n = 6712 participants; 1917 men, 4795 women). Women’s person-years were 37 895 to 40 689 for the various fracture sites. Incident clinical fragility fractures were observed in 673 women, 17.8 (95% CI, 16.5–19.2) per 1000 person-years. In men, this was 14 917 to 15 419 person-years with 128 having incident clinical fragility fractures, a rate of 8.6 (95% CI, 7.2–10.2) per 1000 person-years. Incident major osteoporotic fractures (including only clinical fractures) occurred in 420 women with a rate of 10.7 (9.8–11.8) per 1000 person-years and in 68 men, with a rate of 4.5 (3.5–5.7) per 1000 person-years. Hip fractures were documented in 95 women, a rate of 2.3 (1.9–2.9) per 1000 person-years versus 29 men with a rate of 1.9 (1.3–2.7) per 1000 person-years.
Table 3 presents hazard ratios for clinical fragility fractures during the 10-yr follow-up. Fracture risk did not differ across quartiles of SB for either women or men. In logistic regression models (Supplementary Table S4), fracture risk did not differ across quartiles of SB for any incident fragility fractures (including incident morphometric vertebral fractures) after adjustment for confounding variables.
Table 3.
Hazard ratios (95% CIs) for 10-yr incident clinical fragility fracture, MOF, and hip fracture by sedentary behavior in the CaMos cohort ≥50 yr old.
| Hazard ratios (ref = First quartile) | Women a | Men b | ||||
|---|---|---|---|---|---|---|
| Second quartile | Third quartile | Fourth quartile | Second quartile | Third quartile | Fourth quartile | |
| 5.0–6.0 h/d | 6.5–8.0 h/d | >8.5 h/d | 5.5–7.0 h/d | 7.5–9.0 h/d | >9.5 h/d | |
| Any clinical fragility fracture | ||||||
| Unadjusted | 1.01 (0.81–1.25) | 1.12 (0.90–1.39) | 0.97 (0.77–1.23) | 1.08 (0.69–1.68) | 1.08 (0.67–1.75) | 0.75 (0.43–1.29) |
| Fully adjustedc,d | 0.92 (0.74–1.15) | 0.98 (0.79–1.23) | 0.98 (0.77–1.25) | 1.13 (0.72–1.77) | 1.14 (0.70–1.86) | 0.99 (0.57–1.75) |
| MOF | ||||||
| Unadjusted | 1.03 (0.78–1.35) | 1.22 (0.93–1.60) | 1.01 (0.74; 1.36) | 1.11 (0.61–2.04) | 1.07 (0.55–2.09) | 0.71 (0.33–1.52) |
| Fully adjustede,f | 0.93 (0.70–1.23) | 1.06 (0.80–1.41) | 1.05 (0.77; 1.43) | 1.09 (0.59–2.02) | 1.06 (0.54–2.07) | 1.01 (0.46–2.21) |
| Fragility hip fracture | ||||||
| Unadjusted | 0.80 (0.45–1.40) | 1.05 (0.61–1.82) | 0.86 (0.47–1.60) | 0.91 (0.36–2.30) | 0.99 (0.37–2.66) | 0.59 (0.18–1.91) |
| Fully adjustedg,h | 0.64 (0.36–1.14) | 0.83 (0.47–1.45) | 0.93 (0.50–1.73) | 0.97 (0.39–2.45) | 1.07 (0.40–2.88) | 1.05 (0.32–3.42) |
aQuartile 1 women: <4.5 h/d of sitting.
bQuartile 1 men: <5.0 h/d.
Covariates:
cWomen: age, height, BMI, White, education, center, smoking, physical activity, walking, osteoporosis diagnosis, prior clinical fragility fracture, antiresorptives use, corticosteroids use, CHC, comorbidities.
dMen: age, height, BMI, physical activity, walking, comorbidities.
eWomen: age, height, BMI, smoking, physical activity, walking, osteoporosis diagnosis, prior clinical fragility fracture, antiresorptives use, corticosteroids use, hours of sleep, smoking, comorbidities, CHC.
fMen: age, height, BMI, smoking, physical activity, walking, alcohol, hours of sleep, comorbidities.
gWomen: age, height, BMI, physical activity, osteoporosis diagnosis, prior clinical fragility fracture, comorbidities, CHC.
hMen: age, BMI.
MOF, major osteoporotic fracture; CHC, combined hormonal contraceptives.
Finally, excluding the 20 participants who were immobilized for 1 to 6 mo in the year prior to baseline did not modify any of our results, whether they were from cross-sectional or longitudinal models for BMD or from Cox or logistic models for incident fractures (with or without MVF).
Discussion
This is the largest and longest study of SB and bone health in population-based data to our knowledge. These robust data are reassuring. Our results provide no evidence for negative effects of SB on 10-yr BMD change or incident fractures. Cross-sectional analyses did show higher adjusted FN BMD in women and men with the lowest self-reported sitting time (<4.5 and < 5 h/d, respectively), a proxy for SB, versus women and men with higher (third quartile) sitting times. In longitudinal analyses, however, SB that increased very little over time was not associated with the nonsignificant 10-yr BMD change nor with 10-yr incident fragility fractures. It is worth noting that these data do not necessarily apply to individuals who experience a marked increase in SB, such as becoming bedridden or wheelchair bound.
Our proxy for SB, mean time per day spent sitting, was slightly lower in the CaMos cohort than is often reported for adults of this age range. Most studies find that healthy individuals around the age of 60 yr spend 7–8 h/d being sedentary.18,29 Ideally, studies should combine the use of objective and subjective methods in assessment of SB, since participants may underestimate their sedentary time when responding to a questionnaire. At the same time, questionnaires can capture domain-specific SB, information that cannot be retrieved from objective measures.30 However, CaMos data collection occurred before accelerometers became a readily available research tool. Therefore, this study is limited to subjective SB assessment, although similar questions on a Workforce Sitting Questionnaire had acceptable criterion validity against accelerometry in women (r = 0.22–0.46) and men (r = 0.18–0.29).31 The new WHO guidelines for PA and SB stated that, “There are no standardized measures or analytical protocols for SB.”9 Our questionnaire included several subcategories of sitting thus covering a wide range of sitting behaviors. Our cross-sectional findings indicate that higher amounts of baseline SB may be associated with lower FN BMD values in both women and men. This aligns, in part, with previously published research on middle-aged and elderly women.19,32,33 No association between SB and FN BMD had previously been observed in men.19,32,33 However, the significant association we found may not be of clinical importance. In addition, the trend we observed toward lower BMD with more SB was not observed in the highest quartile of SB.
Although we have done our best to mitigate any confounding in our analyses, some confounding factors that were not included in our study may contribute to this observation. Genetics, hormonal changes (other than menopause), socioeconomic status, body composition, and differences in medication use are some potential factors. Additional prospective studies using objective measures of SB and PA, coupled with questionnaire data and including an even wider range of potential confounders such as activity intensity may help to clarify this relationship. These data might then be suitable for compositional analyses.8
Although questionnaire data resulting in self-reported SB lack objective confirmation, the evidence that accelerometer data, of necessity over shorter time frames and often in fewer participants, are more reflective of actual sitting are currently equivocal. Two previous population-based cross-sectional studies that used accelerometers to measure SB found contradictory results.7,20 McMillan et al. studied 209 community-dwelling men and women with a mean age of 64.4 yr and found lower BMC with more SB in women but not in men.7 In contrast, Rodríguez-Gómez et al. found that, in 776 participants aged 65 yr and older, men with more SB had lower arm and pelvic BMC; in women, more SB was associated with higher BMC in the leg and whole body.20
Quartiles of baseline SB were not associated with 10-yr change in BMD nor with clinical fragility fracture or MVF risk in our 10-yr longitudinal analysis. The small changes we observed are also likely not of clinical importance. Other population-based longitudinal studies with smaller cohorts and of shorter durations found no associations between SB and BMD change for men7,8 and contradictory results for women in which TH BMD increased in participants with more versus fewer sedentary hours.7 However, a slower rate of BMD loss was observed in women who reduced their sedentary time.8 Shorter follow-up, older participants having higher BMI and lower education compared with our participants may explain the differences in our results as well as the varying methods of accounting for participants’ activity levels. Our observed association between SB and the FN BMD was independent of participants’ time spent walking and doing moderate to vigorous PA. However, PA and increases therein may have counteracted negative effects of SB in our cohort. In the fourth quartile of SB, 99.5% of men and 99.3% of women were engaged in some level of walking, strenuous, vigorous, or moderate PA. Specific light activities such as very light household activities may not have been picked up by the questionnaires used in the study and thus a change in those may not have been identified and/or controlled for. The overall PA participation rates for any activity were 99.5% for men and 99.6% for women. However, the absence of change in the estimated relationship between SB and BMD after including PA in the models suggests that PA does not mediate the relationship between SB and BMD. This could also be explained by the fact that the model adequately controls for potential confounding factors related to both SB and PA such as age. However, the absence of change in the estimated relationship between SB and BMD after including PA in the models suggests that PA and SB are concurrent but independent variables associated with BMD after adjusting for other common confounders. Consequently, the estimates for SB’s impact on BMD remain stable despite the inclusion of PA. Finally, it cannot be ruled out that a more diverse or varied sample might yield different results. To confirm these results, the hypothesis that SB does (or does not) affect bone health should be tested in future confirmatory studies.
As suggested in a very recent systematic review on the association between SB and BMD,11 the role of BMI needs careful consideration. In our cohort, the cross-sectional association between SB and BMD at the FN in women and men and at the TH in women became significant after BMI was added to statistical models, indicating that BMI is of key importance. Individuals with very high or very low BMI may be either more or less likely to be sedentary. However, as SB could also affect BMI and, secondarily, BMD, we did not include BMI as an independent variable in the primary (a priori) analyses. The slight overweight (a mean characteristic of our participants) may serve as a protective factor for loss of BMD.
In the cross-sectional analysis, we included all participants with available data on our key outcomes, SB and BMD, including those individuals who, over the next 10 yr, died or were lost to follow-up. Thus, increased SB and lower BMD may, in some cases, have been proxy measures for declining health and functionality. We found that individuals who died during follow-up were older and had lower BMD at baseline than did continuing participants, implying a “healthy cohort” effect. The majority of those who moved to institutional care did not continue to participate, so some of the most frail may have been lost to follow-up. As opposed to many prior studies,11 we did not exclude any participants with comorbidities, but assessed the potential confounding effects of their inclusion. Thus, our findings have good generalizability to the Canadian elderly population.
True population effect sizes are difficult to reach in a diverse population, and this is reflected in quite wide CIs in some of our estimates such as for hip fragility fractures. We carefully considered a variety of confounders in this study including sex, age, weight, menopausal status, and prevalent clinical fragility fractures. We also adjusted for several modifiable risk factors, such as high alcohol consumption, total vitamin D and calcium intakes, and smoking. Thus, we have attempted to avoid bias related to unmeasured or not considered variables. Additional confounding, however, cannot be ruled out.
One additional strength of this study, besides its random sampling, large numbers, and 10-yr documentation, is our low number of nonresponders to questions on SB. In studies using accelerometers to assess SB and bone health, 37%, 40%, and 76% of participants, respectively, were excluded due to invalid or missing data.7,18,19 We note 3 key limitations of our study: (1) data from only 56% of the baseline cohort were available for study at year 10; (2) we could not identify participants who were immobilized in the year prior to the year 10 follow-up interview; and (3) we had only self-report data on h/d of sitting via questionnaire rather than accelerometer measurements. However, questionnaires are useful for ranking sitting time in large observational studies. As the same questionnaire was used at baseline and year 10, systematic bias unlikely affected results for change in sitting time during follow-up. The hypotheses generated in this exploratory epidemiological investigation need to be tested in further studies.
Public health implications
In this large country-wide population-based study, SB was not associated with 10-yr BMD loss in women and men nor did it associate with incident fragility fractures. These data are reassuring. Women and men who walk regularly and have some moderate-vigorous PA per day, despite more daily sitting time, seem to be at no greater long-term osteoporosis risk compared with those with fewer sitting hours.
The CaMos Research Group
David Goltzman (co-principal investigator, McGill University), Nancy Kreiger (co-principal investigator, University of Toronto), Alan Tenenhouse (principal investigator emeritus, Toronto).
McGill University, Montreal, Quebec: Elham Rahme (biostatistician), J. Brent Richards (investigator), Suzanne N. Morin (investigator); Data Analysis Centre: Claudie Berger (study statistician).
Memorial University, St. John’s Newfoundland: Carol Joyce (director), Christopher S. Kovacs (co-director).
Dalhousie University, Halifax, Nova Scotia: Susan Kirkland, Stephanie M. Kaiser (co-directors).
Laval University, Quebec City, Quebec: Jacques P. Brown (director), Louis Bessette (co-director), GRMO.
Queen’s University, Kingston, Ontario: Tassos P. Anastassiades (director), Tanveer Towheed (co-director), Wilma M. Hopman (investigator).
University of Toronto, Toronto, Ontario: Angela M. Cheung (director), Robert G. Josse (co-director), Andy Kin On Wong (co-director).
McMaster University, Hamilton, Ontario: Jonathan D. Adachi (director), Alexandra Papaioannou (co-director).
University of Saskatchewan, Saskatoon, Saskatchewan: Wojciech P. Olszynski (director), K. Shawn Davison (co-director).
University of Calgary, Calgary, Alberta: David A. Hanley (director), Steven K. Boyd (co-director).
University of British Columbia, Vancouver, British Columbia: Jerilynn C. Prior (director), Shirin Kalyan (co-director), Brian Lentle (investigator/radiologist), Millan S. Patel (investigator).
University of Alberta, Edmonton, Alberta: Stuart D. Jackson (medical physicist).
University of Manitoba, Winnipeg, Manitoba: William D. Leslie (investigator/nuclear medicine physician).
Supplementary Material
Acknowledgments
We thank Dr Brian Lentle for his extensive, conscientious work on vertebral fractures using the modified algorithm-based qualitative (morphologic) method that assesses actual breaks in vertebral cortical surfaces. We deeply appreciate the commitment of the randomly sampled Canadians from coast to coast in participating and continuing to add their experiences to our valuable CaMos prospective database.
Contributor Information
Sigríður Lára Guðmundsdóttir, School of Education, Department of Health Promotion, Sport and Leisure Studies, University of Iceland, 101 Reykjavik, Iceland.
Claudie Berger, Centre for Outcomes Research and Evaluation (CORE), Research Institute of the McGill University Health Centre, Montreal, QC H3H 2R9, Canada.
Heather Macdonald, Department of Family Practice, Faculty of Medicine, University of British Columbia, Vancouver, BC V6T 2A1, Canada; Active Aging Research Team, Faculty of Medicine, University of British Columbia, Vancouver, BC V5Z 1M9, Canada.
Jonathan D Adachi, Department of Medicine, McMaster University, Hamilton, ON L8N 3Z5, Canada.
Wilma M Hopman, Kingston General Hospital Research Institute, Kingston Health Sciences Centre, Kingston, ON K7L 2V7, Canada; Department of Public Health Sciences, Queen’s University, Kingston, ON K7L 3N6, Canada.
Stephanie M Kaiser, Department of Medicine, Dalhousie University, Halifax, NS B3H 2Y9, Canada.
Christopher S Kovacs, Discipline of Medicine/Endocrinology, Faculty of Medicine, Memorial University of Newfoundland, St. John's, NL A1B 3V6, Canada.
Kenneth Shawn Davison, A Priori Medical Sciences Inc., Vancouver, BC, Canada.
Suzanne N Morin, Centre for Outcomes Research and Evaluation (CORE), Research Institute of the McGill University Health Centre, Montreal, QC H3H 2R9, Canada; Department of Medicine, McGill University Health Center, Montreal, QC H4A 3J1, Canada.
David Goltzman, Centre for Outcomes Research and Evaluation (CORE), Research Institute of the McGill University Health Centre, Montreal, QC H3H 2R9, Canada; Department of Medicine, McGill University Health Center, Montreal, QC H4A 3J1, Canada.
Jerilynn C Prior, Division of Endocrinology, Department of Medicine, Centre for Menstrual Cycle and Ovulation Research, University of British Columbia, Vancouver, BC V5Z 1M9, Canada; School of Population and Public Health, Faculty of Medicine, University of British Columbia, Vancouver, BC V6T 1Z3, Canada.
CaMos Research Group:
David Goltzman, Nancy Kreiger, Alan Tenenhouse, Elham Rahme, J Brent Richards, Suzanne N Morin, Claudie Berger, Carol Joyce, Christopher S Kovacs, Susan Kirkland, Stephanie M Kaiser, Jacques P Brown, Louis Bessette, Tassos P Anastassiades, Tanveer Towheed, Wilma M Hopman, Angela M Cheung, Robert G Josse, Andy Kin On Wong, Jonathan D Adachi, Alexandra Papaioannou, Wojciech P Olszynski, K Shawn Davison, David A Hanley, Steven K Boyd, Jerilynn C Prior, Shirin Kalyan, Brian Lentle, Millan S Patel, Stuart D Jackson, and William D Leslie
Author contributions
Sigríður Lára Guðmundsdóttir (Formal analysis, Writing—original draft, Writing—review & editing), Claudie Berger (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing—review & editing), Heather Macdonald (Investigation, Project administration, Writing—review & editing), Jonathan D. Adachi (Data curation), Wilma M. Hopman (Investigation, Project administration), Stephanie M. Kaiser (Data curation), Christopher S. Kovacs (Data curation), Kenneth Shawn Davison (Data curation), Suzanne N. Morin (Investigation, Project administration), David Goltzman (Investigation, Project administration), and Jerilynn C. Prior (Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Writing—review & editing).
Funding
The Canadian Multicentre Osteoporosis Study (CaMos) was funded by the Canadian Institutes of Health Research (CIHR); Amgen Canada Inc; Actavis Pharma Inc (previously Warner Chilcott Canada Co); Dairy Farmers of Canada; Eli Lilly Canada Inc: Eli Lilly and Company; GE Lunar; Hologic Inc; Merck Frosst Canada Ltd; Novartis Pharmaceuticals Canada Inc; P&G Pharmaceuticals Canada Inc; Pfizer Canada Inc; Roche (F. Hoffmann-La Roche Ltd); Sanofi-Aventis Canada Inc (previously Aventis Pharma Inc); Servier Canada Inc; and The Arthritis Society.
Conflicts of interest
None of the authors have commercial or other conflicts of interest related to these data.
The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
S.L.G. has a position with Sidekick Health, Iceland.
D.G. has been a consultant for Amgen, Eli Lilly, Merck, and Novartis.
C.S.K/. has received honoraria for advisory boards, consultancies, or speaker’s fee from Amgen, Abbott, Danone, Eli Lilly, Merck, and Novartis.
K.S.D. has served as advisory board member/received honoraria from Amgen, Merck, Proctor & Gamble, Sanofi-Aventis, and Servier.
S.M.K. has served as advisory board member/received honoraria from Amgen, Eli Lilly, Novartis, and Warner-Chilcott. Eli Lilly, Novartis, Proctor & Gamble, Sanofi-Aventis, Servier, and Wyeth-Ayerst.
S.N.M. has received research grants from Amgen, Eli Lilly, Merck, and Novartis.
J.D.A. has received research grants and/or personal fees from Amgen, Eli Lilly, Merck, Actavis, and AgNovos.
J.C.P., C.B., H.M., and W.M.H. report no conflicts of interest.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Biswas A, Oh PI, Faulkner GE, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162(2):123–132. 10.7326/M14-1651. [DOI] [PubMed] [Google Scholar]
- 2.Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. 2018;33(9):811–829. 10.1007/s10654-018-0380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wijndaele K, Brage S, Besson H, et al. Television viewing and incident cardiovascular disease: prospective associations and mediation analysis in the EPIC Norfolk Study. PLoS One. 2011;6(5):e20058. 10.1371/journal.pone.0020058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomark Prev. 2010;19(11):2691–2709. 10.1158/1055-9965.EPI-10-0815. [DOI] [PubMed] [Google Scholar]
- 5.Frost HM. Perspective: genetic and hormonal roles in bone disorders: insights of an updated bone physiology. J Musculoskelet Neuronal Interact. 2003;3(2):118–135. [PubMed] [Google Scholar]
- 6.Zerwekh JE, Ruml LA, Gottschalk F, Pak CY. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J Bone Miner Res. 1998;13(10):1594–1601. 10.1359/jbmr.1998.13.10.1594. [DOI] [PubMed] [Google Scholar]
- 7.McMillan LB, Aitken D, Ebeling P, Jones G, Scott D. The relationship between objectively assessed physical activity and bone health in older adults differs by sex and is mediated by lean mass. Osteoporos Int. 2018;29(6):1379–1388. 10.1007/s00198-018-4446-4. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Gomez I, Manas A, Losa-Reyna J, et al. Compositional influence of movement behaviors on bone health during aging. Med Sci Sports Exerc. 2019;51(8):1736–1744. 10.1249/MSS.0000000000001972. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . WHO Guidelines on Physical Activity and Sedentary Behaviour. Geneva: World Health Organization.; 2020. [PubMed] [Google Scholar]
- 10.Dempsey PC, Biddle SJH, Buman MP, et al. New global guidelines on sedentary behaviour and health for adults: broadening the behavioural targets. Int J Behav Nutr Phys Act. 2020;17(1):151. 10.1186/s12966-020-01044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMichan L, Dick M, Skelton DA, et al. Sedentary behaviour and bone health in older adults: a systematic review. Osteoporos Int. 2021;32(8):1487–1497. 10.1007/s00198-021-05918-2. [DOI] [PubMed] [Google Scholar]
- 12.Marin-Cascales E, Alcaraz PE, Ramos-Campo DJ, Rubio-Arias JA. Effects of multicomponent training on lean and bone mass in postmenopausal and older women: a systematic review. Menopause. 2018;25(3):346–356. 10.1097/GME.0000000000000975. [DOI] [PubMed] [Google Scholar]
- 13.Petit MA, Prior JC, Barr SI. Running and ovulation positively change cancellous bone in premenopausal women. Med Sci Sports Exerc. 1999;31(6):780–787. 10.1097/00005768-199906000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Kelley GA, Kelley KS, Tran ZV. Exercise and bone mineral density in men: a meta-analysis. J Appl Physiol. 2000;88(5):1730–1736. 10.1152/jappl.2000.88.5.1730. [DOI] [PubMed] [Google Scholar]
- 15.Langsetmo L, Hitchcock CL, Kingwell EJ, et al. Physical activity, body mass index and bone mineral density—associations in a prospective population-based cohort of women and men: The Canadian Multicentre Osteoporosis Study (CaMos). Bone. 2012;50(1):401–408. 10.1016/j.bone.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann I, Kohl M, von Stengel S, et al. Exercise and the prevention of major osteoporotic fractures in adults: a systematic review and meta-analysis with special emphasis on intensity progression and study duration. Osteoporos Int. 2023;34(1):15–28. 10.1007/s00198-022-06592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katzmarzyk PT, Powell KE, Jakicic JM, et al. Sedentary behavior and health: update from the 2018 Physical Activity Guidelines Advisory Committee. Med Sci Sports Exerc. 2019;51(6):1227–1241. 10.1249/MSS.0000000000001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hind K, Hayes L, Basterfield L, Pearce MS, Birrell F. Objectively-measured sedentary time, habitual physical activity and bone strength in adults aged 62 years: the Newcastle Thousand Families Study. J Public Health. 2020;42(2):325–332. 10.1093/pubmed/fdz029. [DOI] [PubMed] [Google Scholar]
- 19.Chastin SFM, Mandrichenko O, Helbostadt JL, Skelton DA. Associations between objectively-measured sedentary behaviour and physical activity with bone mineral density in adults and older adults, the NHANES study. Bone. 2014;64:254–262. 10.1016/j.bone.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Gomez I, Manas A, Losa-Reyna J, et al. Associations between sedentary time, physical activity and bone health among older people using compositional data analysis. PLoS One. 2018;13(10):e0206013. 10.1371/journal.pone.0206013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaMonte MJ, Wactawski-Wende J, Larson JC, et al. Association of physical activity and fracture risk among postmenopausal women. JAMA Netw Open. 2019;2(10):e1914084. 10.1001/jamanetworkopen.2019.14084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cauley JA, Harrison SL, Cawthon PM, et al. Objective measures of physical activity, fractures and falls: the osteoporotic fractures in men study. J Am Geriatr Soc. 2013;61(7):1080–1088. 10.1111/jgs.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreiger N, Tenenhouse A, Joseph L, et al. The Canadian Multicentre Osteoporosis Study (CaMos): background, rationale, methods. Can J Aging. 1999;18(3):376–387. 10.1017/S0714980800009934. [DOI] [Google Scholar]
- 24.Armitage P, Berry J, Matthews J. Statistical Methods in Medical Research. 4th ed. Hoboken: Wiley-Blackwell; 2001. [Google Scholar]
- 25.Poliquin S, Joseph L, Gray-Donald K. Calcium and vitamin D intakes in an adult Canadian population. Can J Diet Pract Res. 2009;70(1):21–27. 10.3148/70.1.2009.21. [DOI] [PubMed] [Google Scholar]
- 26.Berger C, Goltzman D, Langsetmo L, et al. Peak bone mass from longitudinal data: implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J Bone Miner Res. 2010;25(9):1948–1957. 10.1002/jbmr.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prior JC, Oei EHG, Brown JP, Oei L, Koromani F, Lentle BC. Where's the break? Critique of radiographic vertebral fracture diagnostic methods. Osteoporos Int. 2021;32(12):2391–2395. 10.1007/s00198-021-06207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lentle BC, Berger C, Probyn L, et al. Comparative analysis of the radiology of osteoporotic vertebral fractures in women and men: cross-sectional and longitudinal observations from the Canadian Multicentre Osteoporosis Study (CaMos). J Bone Miner Res. 2018;33(4):569–579. 10.1002/jbmr.3222. [DOI] [PubMed] [Google Scholar]
- 29.Loyen A, Chey T, Engelen L, et al. Recent trends in population levels and correlates of occupational and leisure sitting time in full-time employed Australian adults. PLoS One. 2018;13(4):e0195177. 10.1371/journal.pone.0195177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Healy GN, Clark BK, Winkler EA, Gardiner PA, Brown WJ, Matthews CE. Measurement of adults' sedentary time in population-based studies. Am J Prev Med. 2011;41(2):216–227. 10.1016/j.amepre.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chau JY, van der Ploeg HP, Dunn S, Kurko J, Bauman AE. A tool for measuring workers' sitting time by domain: the Workforce Sitting Questionnaire. Brit J Sport Med. 2011;45(15):1216–1222. 10.1136/bjsports-2011-090214. [DOI] [PubMed] [Google Scholar]
- 32.Wu F, Wills K, Laslett LL, Oldenburg B, Jones G, Winzenberg T. Moderate-to-vigorous physical activity but not sedentary time is associated with musculoskeletal health outcomes in a cohort of Australian middle-aged women. J Bone Miner Res. 2017;32(4):708–715. 10.1002/jbmr.3028. [DOI] [PubMed] [Google Scholar]
- 33.Braun SI, Kim Y, Jetton AE, Kang M, Morgan DW. Sedentary behavior, physical activity, and bone health in postmenopausal women. J Aging Phys Act. 2017;25(2):173–181. 10.1123/japa.2016-0046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


