Abstract
Genome editing is a technology to make specific changes in the DNA of a cell or an organism. It has significantly altered the landscape of life sciences, facilitating the establishment of exceedingly customized genetic modifications. Among various genome editing technologies, the CRISPR/Cas9 system, a specific endonuclease induces a double stranded DNA break and enabling modifications to the genome, has surfaced as a formidable and adaptable instrument. Its significance cannot be overstated, as it not only allows for the manipulation of genomes in model organisms but also holds great potential for revolutionary advances in medicine, particularly in treating genetic diseases. This review paper explores the remarkable journey of CRISPR/Cas9, its natural function, mechanisms, and transformative impact on genome editing and finally the use of artificial intelligence and other intelligent manufacturing tools used. The introduction provides the background on genome editing, emphasizing the emergence and significance of CRISPR/Cas9. Subsequent sections comprehensively elucidate its natural function, disease modeling, agriculture, and biotechnology, address therapeutic applications, and ongoing clinical trials while also discussing prospects and ethical implications. We summarized the key findings, indicating that CRISPR/Cas9 has empowered the creation of disease-specific animal models. This provides invaluable insights into pathogenic mechanisms and opens new avenues for drug discovery, reaffirming the transformative impact of CRISPR/Cas9 on genome editing. Finally we discussed the importance of continued research and collaboration for comprehensive utilization of the inherent capabilities of this molecular precision tool in shaping forthcoming advancements.
Keywords: CRISPR/Cas9, genome editing, disease modeling, therapeutic applications
Graphical Abstract
Graphical Abstract.
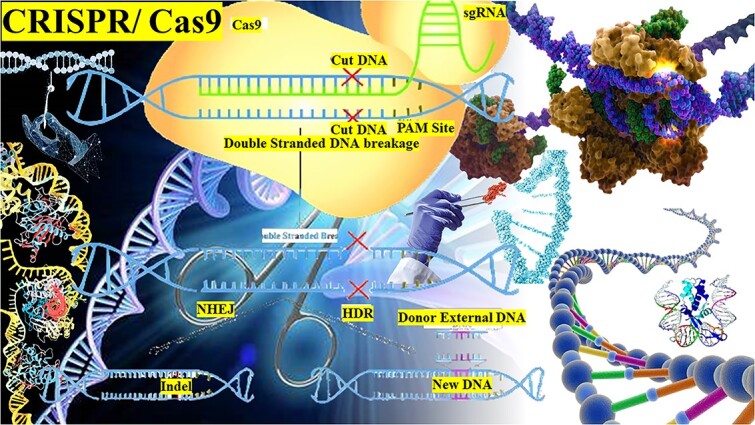
Introduction
Genome editing has erupted as a groundbreaking methodology that allows precise amendment of genetic material, revolutionizing the landscape of life sciences. 1 This field has witnessed significant advancements since the exploration of engineered nucleases, including zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALEN’s), which enabled targeted gene editing.2 However, it was the advancement of the CRISPR/Cas9 system that truly transformed genome editing with its simplicity, versatility, and efficiency.3,4 Jennifer Doudna and Emmanuelle Charpentier were awarded the Noble Prize in Chemistry in 2020 for their contributions to the development of this technique.
The CRISPR/Cas9 system is derived from the prokaryotic adaptive immune system found in bacteria and archaea, which provides defense against viral infections.5 Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) are DNA sequences interspersed with unique spacer sequences derived from viral or plasmid DNA. Adjacent to CRISPR loci are CRISPR-associated genes (Cas) that encode for Cas proteins, including Cas9.6 Cas9, the endonuclease effector protein of the CRISPR/Cas9 system, acts as a molecular scissors guided by a single-guide RNA (sgRNA) to recognize and cleave specific DNA sequences complementary to the sgRNA.7 This process initiates DNA repair mechanisms, such as non-homologous end joining (NHEJ) or homology-directed repair (HDR), leading to the introduction of targeted modifications or insertion.4
The transformative potential of the CRISPR/Cas9 system in genome editing is evident from its numerous successful applications. It allows researchers to perform gene knockouts, insertions, and precise mutations with relative ease and has been adopted as the method of choice in laboratories worldwide.8 CRISPR/Cas9’s versatility extends beyond model organisms to an extensive variety of species, including plants and animals, enabling researchers to explore gene functions and regulation in unprecedented ways.9 The significance of CRISPR/Cas9 in medical research and potential therapeutic applications is equally profound. It holds potential for treating genetic diseases by correcting pathogenic mutations, creating disease models for drug development, and potentially curing viral infections.4,10 CRISPR/Cas9 has also demonstrated its potential in precision medicine by enabling the creation of patient-specific therapies tailored to individual genetic profiles.11
Understanding CRISPR/Cas9 system
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and the associated Cas9 protein revolutionizes genetic engineering with unmatched precision. Originally part of the bacterial immune system, it’s now a powerful genome editing tool in various organisms, including humans. This technology utilizes CRISPR’s natural function for targeted DNA modifications. Our analysis covers CRISPR’s fundamentals, including its natural function and the intricate process of precise genome editing. Understanding these mechanisms is crucial for the broad applications of CRISPR/Cas9 in medicine, agriculture, and biotechnology. The discussion unfolds in two sections: an overview of CRISPR and its natural function, followed by a detailed examination of the genome editing mechanism.12–14
Overview of CRISPR and its natural function
The natural function of the CRISPR/Cas9 system involves three main stages: adaptation, expression, and interference (Fig. 1). First, adaptation occurs, which entails the insertion of novel genetic sequences known as spacers into the CRISPR locus. Subsequently, expression and processing of the CRISPR RNA take place. Lastly, interferences happen, wherein the CRISPR RNA and Cas proteins collaboratively detect and target specific genetic elements for neutralization.15 During the adaptation stage, the system acquires new spacers from foreign genetic elements, incorporating them into the CRISPR loci as a memory of previous infections. Subsequently, during the expression stage, the CRISPR arrays are transcribed into CRISPR RNAs (crRNAs) and pre-crRNAs. These pre-crRNAs undergo maturation to produce mature crRNAs, which consist of the repeat sequence and the spacer sequence.2 In the interference stage, the mature crRNAs guide the Cas effector proteins, such as Cas9, to recognize and target complementary DNA sequences of invading viruses or plasmids. The crRNA/Cas complex surveys the cell’s genetic material, and when it encounters a sequence complementary to the crRNA, Cas9 undergoes a conformational change and becomes activated.7 The activated Cas9 then cleaves the invading DNA, leading to the degradation of the viral or plasmid genetic material, effectively neutralizing the threat to the host cell.16
Fig. 1.
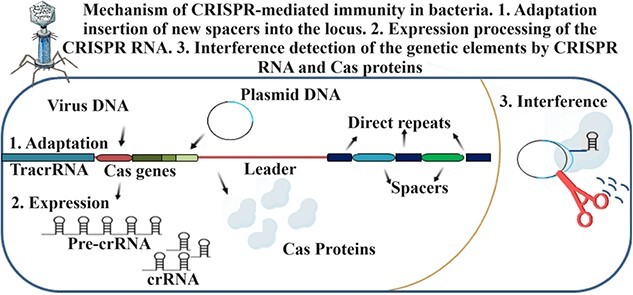
CRISPR-mediated immunity system.
Mechanism of CRISPR/Cas9 for genome editing
The mechanism of CRISPR/Cas9 facilitated genome editing has the subsequent stages: Formation of the crRNA/Cas9 complex, Recognition & Binding, DNA Cleavage, and DNA Repair. The sgRNA forms a complex with the Cas9 endonuclease, creating a ribonucleoprotein (RNP) complex. This complex searches the cellular DNA for a target sequence complementary to the RNA. Once the target DNA sequence is located, the sgRNA within the crRNA/Cas9 complex binds to the complementary DNA strand, forming a stable RNA–DNA hybrid structure.7 Upon binding to the target DNA, the Cas9 endonuclease generates double strand breaks (DSBs) at the target site, creating a blunt-end or staggered-end break, depending on the repair pathway engaged.2 Following the cleavage, the cell’s repair mechanisms, such as non-homologous end joining (NHEJ) or homology-directed repair (HDR), are activated. NHEJ leads to random insertions or deletions, often resulting in gene knockouts, while HDR can be harnessed for rigorous gene editing by providing an exogenous template.4
In fig. 2, the CRISPR/Cas9 framework consists of a gene featuring cleavage abilities, resulting in the creation of a double-strand break on the target DNA. Subsequently, the mending of these breaks takes place through two primary pathways: non-homologous end joining (NHEJ) and homology-directed repair (HDR). In HDR, Donor DNA is introduced as a prototype to guide careful restoration, while NHEJ results in repair without a template, potentially leading to insertions or deletions. This process enables precise genome editing and has garnered significant attention in genetic research and biotechnology.15
Fig. 2.
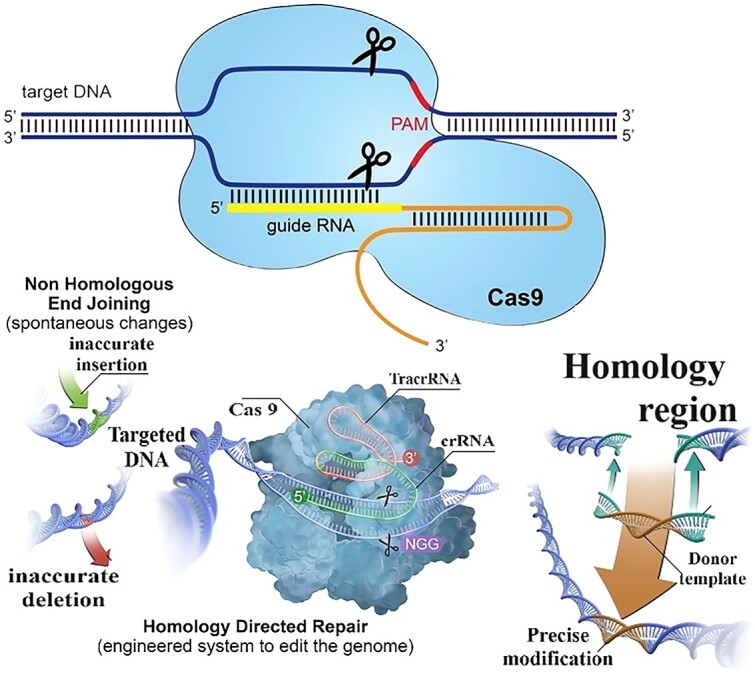
The CRISPR/Cas9 technique operates through a specific mechanism.
Applications of CRISPR/Cas9 Technology in Different Fields
This section highlights the extensive applications of CRISPR/Cas9 technology in diverse fields, and the discussion provides concise insights into the versatile use of CRISPR/Cas9, shedding light on its contributions to disease research, agricultural advancements, and the targeted therapeutic interventions.17
Applications of CRISPR/Cas9 in disease Modeling (biology)
The versatility of CRISPR/Cas9 in precise genome editing has redefined our approach to disease modeling. This subsection explores three key facets: Creating disease-specific biological models, unraveling pathogenic mechanisms for drug discovery, and assessing the potential for personalized medicine. From mimicking human genetic disorders in laboratory organisms to gaining insights into the molecular intricacies of diseases, CRISPR/Cas9 offers a transformative toolkit for advancing our understanding of diseases and catalyzing innovative therapeutic strategies.18
Creating a disease-specific biological models
A disease-specific biological models refers to an experimental system in which an animal or cellular framework is utilized to replicate and manifest the pathological processes inherent in a specific human or animal disease. CRISPR/Cas9 has transformed the creation of disease-specific biological models, enabling researchers to mimic human genetic disorders in laboratory organisms.9 This powerful gene editing tool allows the precise introduction of specific mutations associated with human diseases into the genomes of mice, rats, or other model organisms. Through the strategic manipulation of genes, researchers can study the pathogenesis and progression of these diseases in a controlled setting. These genetically engineered animal models provide invaluable realizations into the fundamental molecular processes of diseases and facilitate the development of novel rehabilitative strategies.19
Here, we’re outlining several commonly used methods for creating disease-specific animal models in Table 1.
Table 1.
Methods used in disease specific animal models.
| Methods | Description | Example |
|---|---|---|
| Microinjection | Direct injection of genetic material (e.g. DNA or RNA) into the nucleus of a cell using a micro-needle. | Creating a transgenic mouse model for Alzheimer’s disease by introducing a mutated human amyloid precursor protein gene. |
| Electroporation | Application of an electric field to cells, temporarily increasing membrane permeability for the uptake of exogenous genetic material (e.g. Plasmid DNA or RNA). | Introducing a cancer-specific mutation in the liver cells of mice by electroplating a CRISPR/Cas9 construct targeting a tumor suppressor gene. |
| GONAD (Genome-editing via Oviductal Nucleic Acid) | Direct delivery of CRISPR/Cas9 components into the oviducts of female mice to achieve efficient in vivo genome editing in developing embryos. | Introducing a mutation associated with cystic fibrosis in mouse embryos to create a model for studying the disease. |
These methods collectively illustrate the diversity of techniques used in creating disease-specific animal models, addressing different aspects of genetic manipulation and disease mechanisms.20
Pros and Cons of Creating Disease-Specific Animal Models:
-
■
Disease Mimicry: Genetically engineered animal models aid in understanding the pathogenesis and progression of human genetic disorders.
-
■
Fundamental Insights: These models provide invaluable insights into the molecular processes of diseases.
-
■
Ethical Considerations: The creation of animal models with human disease traits raises ethical questions about animal welfare.
-
■
Relevance to Humans: Some debates exist regarding the extent to which findings from these models can be extrapolated to human diseases.
Studying pathogenic mechanisms and drug discovery
CRISPR/Cas9 has significantly advanced our understanding of pathogenic mechanisms underlying various diseases.21 Its precision in modifying genes associated with disease development allows researchers to analyze the effects of these modifications on cellular processes and identification of critical molecular pathways involved in disease progression.17
In the context of studying pathogenic mechanisms, CRISPR/Cas9 serves as an invaluable tool. Pathogenic processes involve multi steps, from the initial infection and invasion to the manipulation of host cells or tissues. Virulence factors enhance the pathogens ability to cause disease, while dynamic host-pathogen interactions dictate the progression of the illness. CRISPR/Cas9 facilitates this exploration by enabling the selective knockout of specific genes in pathogens or host organisms. This selective gene modification allows scientists to discern the pivotal role of genes in pathogenicity. Functional genomics studies, employing genome-wide CRISPR screens, reveal genes critical for pathogen survival and shed light on essential facets of host-pathogen interactions. Moreover, the introduction of scientific mutations via CRISPR/Cas9 aids in dissecting the impact of genetic alterations on virulence factors, host responses, and overall disease progression.22
In this era of drug discovery, CRISPR/Cas9 continues to play a pivotal role. By accelerating high-throughput screening of potential drug targets, CRISPR/Cas9 expedites drug discovery efforts, paving the way for the development of robust and high efficient therapeutic modalities. The drug discovery process involves the identification and development of pharmaceutical compounds for disease treatment or preventions. CRISPR/Cas9’s involvement begins with the selective manipulation of genes to validate their potential as drug targets. Large-scale CRISPR screens unearth genes crucial for disease processes, offering a rich pool of novel targets for drug development. Additionally, CRISPR/Cas9 is employed in cell line engineering, creating modified cell lines that mimic disease conditions. These engineered cells serve as invaluable tools for screening potential drug candidates and unraveling drug mechanisms.23
Pros and Cons of Pathogenic Mechanisms and Drug Discovery:
-
■
Drug Development: High-throughput screening accelerates drug discovery efforts.
-
■
Off-Target Effects: Concerns exist about unintended genetic modification, potentially leading to unpredictable outcomes or new health issues.
-
■
Time and Cost: Despite expediting target identification, developing and approving drugs still require rigorous testing, clinical trials, and regulatory approval, contributing to substantial timelines and costs.
Potential for personalized medicine
CRISPR/Cas9 offers substantial promise for personalized medicine through enabling precise modification of the human genome to rectify mutations responsible for diseases.11 With the emergence of genetic manipulation techniques, the repair or replacement of faulty genes in patient-specific cells becomes feasible, paving the way for personalized gene therapies. This approach holds the capability to address a broad spectrum of hereditary disorders and could revolutionize the field of medicine, providing customized therapies anchored on an individual’s distinctive genetic composition.24
Pros and Cons of Personalized Medicine:
-
■
Targeted Therapy: Customized gene therapies can address hereditary disorders.
-
■
Medical Revolution: Personalized medicine has the potential to revolutionize healthcare.
-
■
Ethical Dilemmas: Balancing the promise of personalized medicine with ethical concerns surrounding germline editing remains a critical challenge.
-
■
Regulatory Frameworks: Developing clear regulations to ensure the safe and responsible use of CRISPR/Cas9 in personalized medicine is an ongoing challenge.
Applications of CRISPR/Cas9 in agriculture and biotechnology:
CRISPR/Cas9 emerges as a pliable instrument endowed with formidable potential for enhancing both agricultural practices and biotechnological advancements. Its flexibility opens doors to innovative solutions that can revolutionize crop traits, livestock breeding, and industrial applications, promising significant strides in the fields of agriculture and biotechnology.25,26
Improving crop traits and agricultural productivity
CRISPR/Cas9 has the capability to intensify crop peculiarities by introducing desirable genetic changes. Researchers can target specific genes responsible for characteristics such as pest and disease resistance, resilience to drought, reduced allergens, improved nutrient content, and enhanced output. By modifying these genes, scientists aim to create crops that are more resilient, nutritious, and productive.27
Here, we’ve highlighted three main methods (Table 2) for delivering CRISPR tools to plants. Each method has its benefits, limitations, and applications in enhancing crop traits and improving agricultural productivity.28
Table 2.
Methods of developing improved crops with CRISPR/Cas9.
| Delivery Methods | Description | Application/Benefits/Limitations |
|---|---|---|
| Agrobacteria Delivery | Introduces CRISPR components into plant cells using Agrobacterium tumefaciens, a bacterium that naturally transfers genes into plant cells. |
A = Introduction of foreign genes, gene knockout, and precise editing for disease resistant, pest resistant, and improved stress tolerance. B = Widely used; relatively simple and efficient for many plant species. L = Some plant species are less amenable to Agrobacterium-mediated transformation. |
| Protoplast Delivery | Protoplasts (Plant cells with cell walls removed) are isolated and treated with CRISPR components, allowing for direct uptake of genetic material. |
A = Editing plant cells that are difficult to transform using other methods, useful for certain recalcitrant species. B = Bypasses cell wall barriers, applicable to broader range of plant species. L = Protoplast isolation can be challenging, regeneration of whole plants from protoplasts can be species specific. |
| Biolistic Delivery or Gene Gun | Gold or tungsten particles coated with CRISPR components are shot into plant cells using a gene gun, allowing for the delivery of genetic material. |
A = Efficient transformation of a variety of plant tissues, including those with thick cell walls, useful for gene insertion and knockout. B = Versatile, applicable to a wide range of plant species, effective for tissues resistant to other methods. L = Potential for random integration of transgene, optimization of bombardment conditions can be required. |
These methods contribute to developing crops with improved traits, including resistance to pests, diseases, enhanced stress tolerance, and increased agricultural productivity. The targeted genetic modifications using CRISPR technologies hold significant promise for addressing global challenges in food security and sustainable agriculture.19
When contemplating the enhancement of crop traits and agricultural productivity through CRISPR/Cas9, a critical scientific question arises: What are the potential ecological impacts of genetically modified crops with enhanced characteristics, particularly in terms of interactions with native species and ecosystems? This question is unanswered, urging researchers to work into the broader ecological consequences of deploying genetically modified crops in various environments.29
The ecological impacts of genetically modified crops with enhanced traits are actively under scientific investigation. Researchers are studying how these modified crops interact with native plant and animal species, as well as the surrounding ecosystem. It’s crucial to ensure that the introduction of genetically modified crops doesn’t disrupt local biodiversity or ecological balance. By conducting comprehensive ecological risk assessments, scientists aim to minimize potential adverse effects and make informed decisions about the deployment of genetically modified crops.30,31
Enhancing livestock breeding programs
Livestock breeding refers to the controlled mating and reproduction of animals. In livestock breeding, CRISPR/Cas9 offers the opportunity to improve animal traits and accelerate the breeding process. By editing genes responsible for traits like disease resistance, meat quality, and fertility, scientists can create healthier and more productive livestock.32,33
Here, we’re summarizing the methods involved in livestock breeding using CRISPR/Cas9, specifically focusing on embryo genome editing, cloning and sperm genome editing.
Method involved in livestock breeding
Embryo genome editing
First Pronuclear microinjection has the steps like collection of eggs and sperm from parent animals, then fertilize eggs in vitro to form zygotes. Then microinject CRISPR/Cas9 component into the pro nuclei of zygotes. Then allow edited embryos to develop in vitro or in vivo. Finally implant edited embryos into surrogate mothers.
Cloning
The cloning method involves the somatic cell nuclear transfer (SCNT) technology. First obtain somatic cells from the donor animals. Then isolate the nucleus from the somatic cell. Remove the nucleus from the oocyte (egg cell). Introduce the somatic cell nucleus into the enucleated oocyte. Stimulate cell fusion and initiate embryonic development. Implant the cloned embryo into a surrogate mother for gestation. CRISPR/Cas9 Integration in Cloning. Use CRISPR/Cas9 to edit specific genes in somatic cells. Perform SCNT using the edited somatic cells. Generate cloned animals with desired genetic modification.34
Sperm genome editing
This method involves, in vitro genome editing of sperm. First collect sperms from the male animal. In vitro editing of sperms using CRISPR/Cas9. Artificial insemination with edited sperm. Gestation in surrogate mothers. Introduce CRISPR/Cas9 components onto the male reproductive system. Allow natural mating for gene-edited sperm to fertilize eggs. Gestation in surrogate mothers.35
It’s important to note that the success of these methods depends on factors such as the efficiency of CRISPR/Cas9 editing, the health of the resulting embryos, and the ethical and regulatory considerations associated with genetic modification in livestock.36
Pros and Cons of Livestock Breeding:
-
■
Healthier Livestock: Genetic editing can lead to healthier and more disease-resistant livestock, potentially reducing the need for antibiotics and improving animal welfare.
-
■
Increased Productivity: Improved traits can enhance livestock productivity, benefiting the agricultural industry and food production.
-
■
Potential Environmental Impact: The introduction of genetically edited livestock may have unforeseen consequences on ecosystem.
-
■
Ethical and Social Concerns: There may be ethical concerns surrounding the genetic modification of animals, and could impact social and cultural values.
Biotechnological applications in industrial settings
CRISPR/Cas9 has found applications in various biotechnological processes, particularly in industrial setting. It can be used to engineer microorganisms to produce valuable compounds such as biofuels, pharmaceuticals, and enzymes. By editing the genes responsible for specific metabolic pathways, researchers can optimize the micro-organisms ability to produce desired products efficiently.37 Likely, here’s a table outlining how CRISPR/Cas9 is used in various biotechnological applications within an industrial setting. Table 3 highlights the versatile applications of CRISPR/Cas9 in various biotechnological processes within an industrial setting, showcasing its role in optimizing micro-organisms for the efficient production of valuable compounds.38
Table 3.
CRISPR/Cas9 applications in biotechnology.
| Biotechnological Process | Application of CRISPR/Cas9 |
|---|---|
| Industrial Microbial Engineering |
|
| Food and Beverage Fermentation |
|
| Biopharmaceutical Production |
|
| Environmental Remediation |
|
| Biochemical and Enzyme Production |
|
Pros and Cons of Biotechnological Applications in Industrial Settings:
-
■
Industrial Efficiency: CRISPR/Cas9 offers the potential to enhance industrial processes by optimizing microorganisms for efficient production of valuable compounds.
-
■
Sustainable Solutions: Biotechnological applications of CRISPR/Cas9 can contribute to more sustainable practices in industries like biofuels, reducing reliance on fossil fuels.
-
■
Regulatory Oversights: The industrial use of CRISPR/Cas9 requires robust regulatory oversight to ensure safety and prevent unintended environmental consequences.
-
■
Ethical Considerations: Ethical concerns may arise in the context of biotechnological applications, particularly when it involves the production of pharmaceuticals and biofuels. Ethical guidelines and responsible practices are crucial.
Therapeutic applications of CRISPR/Cas9 in human health
The landscape of human health is undergoing a revolutionary shift with the advent of CRISPR/Cas9 technology. This transformative tool has paved the way for therapeutic breakthroughs, notably in the precise targeting genetic anomalies. Moreover, its application extends to combating infectious diseases at the genetic level and innovating cancer therapies. CRISPR/Cas9 is not just a scientific marvel, it’s a beacon of hope in redefining treatment strategies for genetic disorders, infectious disease, and various forms of cancer.39,40
Targeting genetic anomalies: Dilemmas and breakthroughs
CRISPR/Cas9 has shown great promise in therapeutic applications for targeting genetic disorders. It allows scientists to edit DNA sequences with high precision, offering potential treatments for various genetic conditions. However, there are both challenges and breakthroughs in this field.14
Dilemmas:
-
■
Delivery: One of the major challenges is efficiently delivering the CRISPR/Cas9 elements into the target units or tissues.
-
■
Off-target Effects: Ensuring that CRISPR/Cas9 doesn’t unintentionally edit other regions of the genome, leading to potential adverse effects.
-
■
Immune Response: The immune system might recognize CRISPR components as foreign and trigger an immune response, limiting its efficacy.
-
■
Ethical Concerns: The use of CRISPR for germline editing raises ethical questions about its long-term consequences.
Breakthroughs:
-
■
Clinical Trials: Despite the challenges, there have been significant breakthroughs in clinical trials using CRISPR/Cas9 to treat genetic maladies like Hemoglobinopathy S and Beta thalassemia syndrome.41
-
■
Base Editing: New techniques like base editing allow for more precise modifications, reducing the risk of unintended impacts.42
-
■
Disease Modeling: CRISPR facilitates the production of cellular and animal models, aiding in understanding affliction mechanisms and testing potential therapies.43
Genome editing for infectious disease treatment
CRISPR/Cas9 has potential in treating infectious diseases by targeting specific genetic elements of pathogens. The technology allows for precise modifications of the DNA of viruses and bacteria, leading to the disruption of their virulence factors or essential genes.44 For instance, researchers have triumphantly consumed CRISPR/Cas9 to target and inactivate the hereditary material responsible for the virulence of certain bacteria, such as Staphylococcus aureus and Streptococcus pyogenes. By accomplishing so, they were able to significantly reduce the bacteria’s ability to cause infections.45 In the context of viral infections, CRISPR/Cas9 has been explored as a potential treatment for viral diseases like HIV. Studies have investigated using CRISPR/Cas9 to target and excise the viral DNA from infected cells, potentially leading to a functional cure for the disease.46 Furthermore, CRISPR/Cas9 can be utilized to develop greater effective vaccines. By editing the genomes of viruses, researchers can create attenuated or inactivated versions of the pathogen that can be used as vaccines to induce a protective immune response without causing the disease.34
However, here’s a simplified table summarizing some key methods involved in genome editing using CRISPR/Cas9 for infectious disease treatment (Table 4). This table provides a brief overview of the purposes and descriptions of various CRISPR/Cas9-based genome editing methods for infectious disease treatment.47
Table 4.
Key methods in CRISPR/Cas9 for the treatment of infectious diseases.
| Method | Purpose | Description |
|---|---|---|
| Gene Knock-Out | Disrupt or delete pathogen-related genes. | Introduce double strand breaks to inactivate target genes. |
| Gene Knock-In | Introduce modifications or new genes. | Precisely insert desired DNA sequences into the host genome. |
| Epigenome Editing | Modify the epigenetic landscape. | Use CRISPR/Cas9 to target and modify specific epigenetic marks, such as DNA methylation or histone modifications. |
| Antiviral Immune System Enhancement | Boost host immune defenses. | Edit immune-related genes to enhance immune cell activity or modify signaling pathways. |
| Base Editing | Precise nucleotide changes without breaks. | Use a modified version of CRISPR/Cas9 with a deaminase enzyme to directly convert one DNA base to another. |
| Cytosolic Editing | Modify cellular components in the cytoplasm. | Edit genes involved in cytoplasmic processes or components to impact viral replication or host-pathogen interactions. |
Cancer therapies using CRISPR/Cas9
CRISPR/Cas9 has surfaced as a versatile tool for oncological interventions because of its ability to target and modify specific genes involved in cancer progression.48 Here are some key applications:
■ Gene Editing for Tumor Suppression: CRISPR/Cas9 can be employed to target and inactivate oncogenes or activate tumor suppressor genes, effectively controlling cancer cell growth. For example, researcher have successfully edited the TP53 gene, a tumor suppressor, to enhance its function in cancer cells.49
■ Immunotherapy Enhancement: CRISPR/Cas9 can ameliorate the potency of immunotherapies like CAR-T cell therapy. By editing T cells to enhance their anti-tumor properties or to remove immune checkpoints, researchers have showed improved outcomes in preclinical studies.50
■ Drug Resistance Reversal: CRISPR/Cas9 can help overcome drug resistance in cancer cells by targeting the genes responsible for resistance, In Leukemia, researchers have used CRISPR/Cas9 to disrupt genes that mediate resistance to chemotherapy drugs, making the cancer cells more susceptible to treatment.
■ Early Detection & Diagnostics: CRISPR/Cas9 based diagnostic tools, such as SHERLOCK and DETECTR, enable highly sensitive detection of cancer-associated genetic mutations, offering potential early diagnosis and personalized treatment approaches.51
While CRISPR/Cas9 holds great promise in cancer therapies, there are ethical concerns surrounding its use in gene editing. Some argue that modifying the human genome, even for therapeutic purposes, could have unintended consequences or be misused for non-medical reasons. This debate highlights the need for stringent regulations and ethical guidelines to ensure the responsible and safe application of CRISPR/Cas9 in oncological interventions. In addition to the ethical debate, there is an ongoing scientific question regarding the long-term effects of CRISPR/Cas9 gene editing in cancer therapy. Researchers are actively investigating whether edited genes remain stable over time and whether unforeseen genetic changes could lead to complications or new health risks for patients. This research is essential for understanding the full implications of using CRISPR/Cas9 in cancer treatments.52
Pros and Cons of CRISPR/Cas9 in Human Health Therapies:
-
■
Personalized Medicine: CRISPR/Cas9 enables personalized treatment approaches by targeting specific genetic anomalies, pathogens, or cancer mutations tailored to individual patients.
-
■
Innovative Therapies: The technology offers innovative solutions for previously untreatable genetic disorders, infectious diseases, and cancer, expanding the range of therapeutic options.
-
■
Research Advancements: CRISPR/Cas9 accelerates scientific research by facilitating the creation of cellular and animal models, aiding in understanding disease mechanism and testing potential therapies.
-
■
Potential for Functional Cures: In infectious diseases and cancer, CRISPR/Cas9 holds the potential to achieve functional cures by disrupting virulence factors, reversing drug resistance, and controlling tumor growth.
-
■
Targeted Treatment: CRISPR/Cas9 technology offers highly targeted treatment options, minimizing damage to healthy tissues and reducing side effects, which can enhance the overall safety and effectiveness of therapies.
-
■
Ethical and Regulatory Complexity: The ethical considerations surrounding CRISPR/Cas9 applications, along with the need for stringent regulations, add complexity to its use, requiring careful oversight.
-
■
Unpredictable Outcomes: The long-term effects of CRISPR/Cas9 gene editing are not fully understood, leading to uncertainties about potential consequences and health risks for patients.
-
■
Cost and Accessibility: The high cost of CRISPR-based therapies and technologies may limit accessibility for patients, especially in resource-constrained healthcare systems.
CRISPR/Cas9 technology has materialized as a influential instrument in heterogeneous fields, including medicine for targeted gene editing in genetic diseases and drug development, biology for engineering animals with desired traits, and biotechnology for applications in biofuel production and plant biotechnology.53
Here, we’ve delineated a pie chart (Fig. 3) demonstrating the proportional utilization of CRISPR/Cas9 technology across various domains. In fig. 3, the pie chart illustrates the distribution of CRISPR/Cas9 technology usage across various fields, including Medicine, Biotechnology, and Biology. The largest segment of the pie chart signifies the usage of CRISPR/Cas9 technology within the domain of Medical Sciences. This indicates that 40% of the overall implementations of CRISPR/Cas9 are directed towards medicine research, therapies, and advancements. The second largest segment of the pie chart, accounting for 35% embodies the exploitation of CRISPR/Cas9 technology in the arena of Biotechnology. This usage is an ample gamut assignments, such as agricultural improvements, industrial processes, and biopharmaceutical developments. The remaining portion corresponds to Biology, signifying that 25% of CRISPR/Cas9 applications are employed in biological studies and research. This includes genetic research, gene editing, and related biological investigations.54–56
Fig. 3.
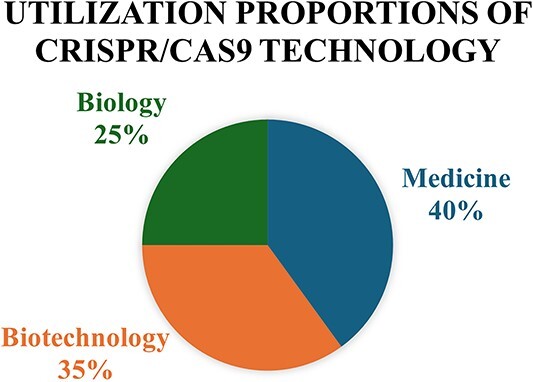
Utilization of CRISPR/Cas9 in various fields.
CRISPR/Cas9 in gene therapy: Clinical trials and future prospects
This section explainsthe ongoing clinical trials landscape, assessing successes and challenges in human trials, contemplating future directions, and elucidating potential breakthroughs. This section not only provides a comprehensive overview of the current clinical trials, but also offers insights into the dynamic trajectory of CRISPR/Cas9 in the gene therapy domain.57,58
Here’s a list providing an overview of the current clinical trials related to CRISPR/Cas9 in gene therapy.18,59 The trailing schematic overview in table 5 presents a snapshot of ongoing clinical trials utilizing the revolutionary CRISPR/Cas9 gene editing system to address specific hereditary mutations responsible for a diverse array of disorders.60
Table 5.
Overview of the clinical trials related to CRISPR/Cas9.
| Disease | Description | Clinical Trials | References |
|---|---|---|---|
| Cystic Fibrosis | Genetic Lung Disorder | CRISPR gene editing for CFTR gene in lung cells. | 79 , 80 |
| Sickle Cell Anemia | Inherited Blood Disorder | CRISPR gene editing of HBB gene in hematogenic progenitor cells. | 41 , 81 |
| Duchenne’s Dystrophy | Progressive Muscle-Wasting Disorder | CRISPR gene editing for DMD gene in muscle cells. | 82 , 83 |
| Beta-Thalassemia | Inherited Blood Disorder | CRISPR gene editing of HBB gene in blood-forming progenitor cells. | 41 , 84 |
| Leber Congenital Amaurosis | Genetic Eye Disorder | CRISPR gene editing for RPE65 gene in retinal cells. | 85 , 86 |
| Huntington’s Disease | Neurodegenerative Disorder | CRISPR gene editing for HTT gene in brain cells. | 87 |
| HIV/AIDS | Viral Infection | CRISPR gene editing of CCR5 gene in immune cells. | 88 , 89 |
| Hereditary Blindness | Genetic Vision Impairment | CRISPR gene editing for GUCY2D gene in retinal cells. | 90 |
| Alpha-1 Antitrypsin Deficiency | Genetic Lung Disorder | CRISPR gene editing for SERPINA1 gene in lung cells. | 91 , 92 |
| Hemophilia A | Genetic Blood Clotting Disorder | CRISPR gene editing for F8 gene in liver cells. | 73 |
| Phenylketonuria | Metabolic Disorder | CRISPR gene editing for PAH gene in liver cells. | 93 |
| Fanconi Anemia | Inherited Bone Marrow Failure Syndrome | CRISPR gene editing for FANCA gene in hemopoietic precursor cells. | 94 , 95 |
| Myotonic Dystrophy | Genetic Muscle Disorder | CRISPR gene editing for DMPK gene in muscle cells. | 96 |
| Beta-Amyloidosis | Neurodegenerative Disorder | CRISPR gene editing for APP gene in brain cells. | 97 |
Successes and challenges in human trials
Some early clinical trials have shown promising results, indicating the prospective of CRISPRCas9 in nurturing congenital disorders. For instance, in a study to treat beta-thalassemia and sickle cell disease, patients showed reduced symptoms and improved quality of life.41 Human trials face several challenges, including off-target effects, where CRISPR-Cas9 may unintentionally edit other genome segments, leading to unpredictable outcomes. Additionally, the conveyance of CRISPR constituents to target units efficiently persists a hurdle.61,62
Future directions and potential breakthroughs
The future of CRISPR/Cas9 revolves around refining delivery methods, enhancing specificity, and expanding disease targets, paving the way for more precise and extensive genome editing applications. These advancements promise to elevate the technology's impact and versatility.13
Advancing delivery methods
Research is ongoing to improve delivery techniques, such as using adeno-associated viruses (AAVs) for efficient and targeted transportation of CRISPR components.61 In the quest to enhance CRISPR technology’s specificity and minimize off-target effects, one scientific question emerges: What are the potential long-term consequences or unforeseen effects of using highly specific CRRISPR variants, especially when applied to the human genome. Ongoing research aims to assess the safety and stability of these advanced CRISPR technologies in the context of gene editing.63
Enhancing specificity
Developing new CRISPR variants with improved specificity may help minimize off-target effects.64 A debate surrounding the expansion of CRISPR technology to treat a broader range of diseases centers on the ethical and regulatory challenges it presents. Critics argue that as CRISPR applications expand beyond rare genetic disorders to more common conditions, there’s a risk of accessibility and affordability issues. The debate raises questions about who should have access to these treatments and how to ensure equitable distribution while maintaining rigorous safety and ethical standards.65
Expanding disease targets
CRISPR technology continues to be explored for various genetic disorders, and future breakthroughs may pave the way for treating a broader range of diseases.12
CRISPR/Cas9 and the future of genome editing
The concluding section anticipates the trajectory of this transformative technology. It has three critical dimensions: Technological advancements of CRISPR/Cas9, examining ongoing innovations; Ethical Considerations and Societal Implications, addressing ethical discourse; and Promising areas of research and development, illuminating emerging frontiers. This collective exploration not only underscores the dynamic evolution of CRISPR/Cas9 but also emphasizes the ethical considerations and promising avenues that shape the future of genome editing.66
Technological advancements in CRISPR/Cas9
Researchers have made significant strides in improving CRISPR/Cas9’s efficiency, specificity, and delivery methods. For instance, scientists have developed new CRISPR variants such as base editors42 and prime editors,67 which enable more precise changes in the DNA sequences. Delivery systems have also been enhanced to ensure safer and more efficacious conveyance of CRISPR constituents into target cells, such as using nanoparticles or viral vectors.40
On one hand, proponents argue that the technological advancements within CRISPR/Cas9 open a new window of possibilities. The advent of prime editing and base editing, for instance, represents significant strides toward precision and accuracy. These innovations address concerns about off-target effects and broaden the spectrum of diseases that could potentially be treated. It’s contended that these advancements will pave the way for therapies that were previously unimaginable, propelling medical science into a future where genetic disorders might become a thing of the past. This is cost effective and latest technique.68
Ethical considerations and societal implications
With the power to edit genes comes great responsibility. Ethical concerns surrounding CRISPR/Cas9 focus on issues like off-target effects, unintended consequences, and the potential for creating “designer babies”. The prospect of editing the germline, which would pass genetic changes to future generations, raises significant ethical debates. Balancing the potential benefits with the potential risks is a crucial aspect of using this technology responsibly.69 The widespread utilization of CRISPR/Cas9 could lead to a more personalized approach to medicine, where gene therapies are tailored to individuals’ genetic makeup. It could potentially cure genetic diseases, eliminate certain inherited conditions, and improve human health overall. However, access to this technology might be limited, leading to potential disparities in healthcare between different socio-economic groups and countries.70
However, dissenting voices caution against an overly enthusiastic embrace of these innovations. They highlight that the complexity of prime editing and base editing, while offering exciting potential, also introduces heightened complexities. The risk of introducing unintended genetic changes and the potential for long-term consequences are still not fully understood. Ethical considerations surrounding germline editing and the ability to influence future generations are formidable. Critics emphasize that technical advancement must not outpace our capacity to anticipate and address unforeseen outcomes, calling for a more cautious and reflective approach.36
In the heart of this debate lies the need to strike a harmonious balance between technological advancements and ethical contemplation. As we gaze into the future, it becomes evident that responsible progress necessitates both pushing the boundaries of science and embracing a circumspect outlook. Ongoing interdisciplinary dialogues that involve scientists, ethicists, policymakers, and the public at large are essential in navigating this valuable terrain. While the potential benefits of technological innovation are tantalizing, the ethical dimensions cannot be overlooked. Only through comprehensive discussions and collective introspection can we forge a path that steers clear of unintended consequences and safeguards the sanctity of the human genome.35
Promising areas of Research and Development
CRISPR/Cas9 has opened up exciting avenues across diverse domains of investigation and advancement by using intelligent manufacturing and latest industrial revolution technologies. Some of the promising areas includes:
■ Medicine: Gene therapies to treat genetic disorders,71 cancer, and infectious diseases (i.e. HIV, Hepatitis).
■ Agriculture: Enhancing crop resilience, disease resistance, pest resistance, and nutritional content.27,72
■ Biotechnology: Producing biofuels, industrial enzymes, and pharmaceuticals.2
■ Conservation: Restoring endangered species and ecosystems.73
■ Synthetic Biology: Designing organisms with novel functionalities.
The extensive utilization of CRISPR/Cas9 across diverse fields such as medicine, agriculture, biotechnology, conservation, and synthetic biology raises a critical scientific question: How does the application of this revolutionary genome editing tool impact the ethical and ecological landscape? The broad scope of CRISPR/Cas9’s potential for scientific advancements is undeniable, yet it introduces a host of ethical and ecological concerns.74
In the domain of medicine, where gene therapies hold great promise, the ethical imperative lies in ensuring equitable access to these transformative technologies while diligently addressing unforeseen consequences. Agricultural applications, focusing on crop modification for enhanced resilience and nutritional content, demand careful consideration of the long-term environmental effects to balance benefits against potential ecological risks. Biotechnological endeavors, such as biofuel production, bring economic advantages but necessitate robust sustainability assessments to safeguard against adverse ecological impacts.75
Conservation efforts employing CRISPR/Cas9 to revive endangered species face the challenge of weighing ethical concerns against potential ecological repercussions. In synthetic biology, the creation of novel organisms prompts questions regarding biosecurity and the complex ecological dynamics that may emerge. In essence, the overarching inquiry is how CRISPR/Cas9, while opening new frontiers in research, demands a responsible and novel approach to navigate the significant web of ethical and ecological complexities. Artificial intelligence with sustainable approach must be integrated in all types of research work. Addressing this question is paramount for realizing the full potential of genome editing technologies while upholding ethical standards and ecological integrity.76–78
Conclusion
In summary, this review artcile has explored the transformative role of CRISPR/Cas9 in genome editing with a comprehensive understanding of its natural function and its mechanism, facilitating precise DNA manipulation. The significance of CRISPR/Cas9 in affliction modeling, agriculture, biotechnology, and individuals’ health has been discussed with its wide-ranging applications and potential to revolutionize scientific research. The key findings reveal that CRISPR/Cas9 has enabled the generation of disease-specific animal models, thereby offering important knowledge about pathogenic mechanisms and novel avenues for drug discovery. In agriculture and biotechnology, CRISPR/Cas9 has manifested as a formidable tool for enhancing crop traits, increasing tillage productivity, and refining livestock breeding programs, thereby contributing to global security and sustainable resource management including sustainable development goals. The therapeutic applications of CRISPR/Cas9 in human health exhibit immense promise for targeting genetic disorders, tackling infectious diseases, and developing groundbreaking cancer therapies. Despite challenges encountered in human trials, ongoing clinical studies offer hope for realizing its full potential in clinical settings. Considering the impact of CRISPR/Cas9, it is evident that this technology has catalyzed a paradigm shift in genome editing research, accelerating progress and broadening the horizons of scientific exploration. Its versatility, precision, and efficiency have propelled it to the forefront of genetic engineering methodologies, making it an indispensable tool in diverse scientific disciplines. Looking to the future, the prospects for genome editing research are promising. Continued technological advancements, such as improved delivery methods, increased editing accuracy, and enhanced safety profiles, are likely to further strengthen the efficacy and accessibility of CRISPR/Cas9 applications. Exploration of alternative CRISPR systems and new gene editing tools may unlock additional avenues for precision genomic manipulations. The impact of CRRISPR/Cas9 transcends scientific domains and holds vast implications for medicine, chemistry, biochemistry, food, agriculture, and biotechnology. With the evolving landscape of discipline, it becomes unavoidable to deliberate upon ethical dimensions and engage in thoughtful dialogue with relevant entities to guarantee a conscientious and just utilization of this potent technology. Through sustained collaborating, rigorous research, and ethical deliberation, CRISPR/Cas9 is poised to steer humanity towards an era of groundbreaking discoveries and transformative innovations in genome editing research, including AI and all other intelligent manufacturing technologies ultimately benefiting human health, the environment, and global prosperity. Reduction of the carbon footprint through a sustainable research mentality is the ultimate goal of the next industrial revolutions (Industry 5.0; Industry 6.0). Therefore, all types of research work must adopt a sustainable approach with net zero carbon emissions. Otherwise, no research work will receive comprehensive appreciation in industry, academia, or anywhere in the world.
Contributor Information
Muskan Irfan, Department of Biotechnology, University of Management and Technology (UMT), Lahore, Sialkot Campus, Sialkot 51310, Pakistan.
Hammad Majeed, Department of Chemistry, University of Management and Technology (UMT), Lahore, Sialkot Campus, Sialkot 51310, Pakistan.
Tehreema Iftikhar, Applied Botany Lab, Department of Botany, Government College University, 54000, Lahore, Pakistan.
Pritam Kumar Ravi, Computer Applications Department, Ganesh Lal Agarwal College, Nilamber-Pitamber University, Jharkhand, 822101, India.
Author contributions
Muskan Irfan: Original Draft, Review, Writing, conceptualization. Hammad Majeed: Supervision, Conceptualization, Original Draft, Writing, Editing, Formal analysis, Methodology, Project administration. Tehreema Iftikhar: Conceptualization, Software, Validation, Visualization, Data Curation, Review, Methodology. Pritam Kumar Ravi: Visualization, Review.
Funding
No supporting funds were provided by any sponsoring bodies for this research work.
Conflict of interest statement: The authors confirm that there is no conflict of interest in this research work.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
The data related to this work has been mentioned in this manuscript.
References
- 1. Carroll D. Genome engineering with zinc-finger nucleases. Genetics. 2011:188(4):773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Doudna JA, Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science (New York, NY). 2014:346(6213):1258096. [DOI] [PubMed] [Google Scholar]
- 3. Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, et al. Multiplex genome engineering using CRISPR/Cas systems. Science (New York, NY). 2013:339(6121):819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science (New York, NY). 2013:339(6121):823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science (New York, NY). 2007:315(5819):1709–1712. [DOI] [PubMed] [Google Scholar]
- 6. Bhaya D, Davison M, Barrangou R. CRISPR-Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annu Rev Genet. 2011:45(1):273–297. [DOI] [PubMed] [Google Scholar]
- 7. Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science (New York, NY). 2012:337(6096):816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hsu PD, Scott DA, Weinstein JA, Ran FA, Konermann S, Agarwala V, Li Y, Fine EJ, Wu X, Shalem O, et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat Biotechnol. 2013:31(9):827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech L, Kempton HR, Dahlman JE, Parnas O, Eisenhaure TM, Jovanovic M, et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell. 2014:159(2):440–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaj T, Gersbach CA, Barbas CF 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013:31(7):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Komor AC, Badran AH, Liu DR. CRISPR-based Technologies for the Manipulation of eukaryotic genomes. Cell. 2017:168(1–2):20–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang JY, Doudna JA. CRISPR technology: a decade of genome editing is only the beginning. Science (New York, NY). 2023:379(6629):eadd8643. [DOI] [PubMed] [Google Scholar]
- 13. Thurtle-Schmidt DM, Lo TW. Molecular biology at the cutting edge: a review on CRISPR/CAS9 gene editing for undergraduates. Biochem Mol Biol Educ. 2018:46(2):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bashir T, Majeed H, Iftikhar T. Isolation and Screening of Thermophillic Bacteria and its Subsequent Evaluation for Lipases Production. Pak J Bot. 2024:56(2):759–64. [Google Scholar]
- 15. Bharathkumar N, Sunil A, Meera P, Aksah S, Kannan M, Saravanan KM, Anand T. CRISPR/Cas-based modifications for therapeutic applications: a review. Mol Biotechnol. 2022:64(4):355–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Makarova KS, Wolf YI, Alkhnbashi OS, Costa F, Shah SA, Saunders SJ, Barrangou R, Brouns SJ, Charpentier E, Haft DH, et al. An updated evolutionary classification of CRISPR-Cas systems. Nat Rev Microbiol. 2015:13(11):722–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naveed N, Ahmad K, Majeed H, Qureshi K, Ahmad I, Awan MF, Iftikhar T, Ahmad S, Noreen F, Amin MA, et al. The Global Impact of COVID-19: A Comprehensive Analysis of Its Effects on Various Aspects of Life. Toxicol Res. 2024:13(2):tfae045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lino CA, Harper JC, Carney JP, Timlin JA. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv. 2018:25(1):1234–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zarei A, Razban V, Hosseini SE, Tabei SMB. Creating cell and animal models of human disease by genome editing using CRISPR/Cas9. J Gene Med. 2019:21(4):e3082. [DOI] [PubMed] [Google Scholar]
- 20. Lee H, Yoon DE, Kim K. Genome editing methods in animal models. Anim Cells Syst. 2020:24(1):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu B, Saber A, Haisma HJ. CRISPR/Cas9: a powerful tool for identification of new targets for cancer treatment. Drug Discov Today. 2019:24(4):955–970. [DOI] [PubMed] [Google Scholar]
- 22.Malik S, Iftikhar T, Abbas A, Majeed H, Abdullah R. Biosynthesis of invertase by Penicillium chrysogenum using solid state fermentation technique. Int J Biosci. 2016:9(6):330–337. [Google Scholar]
- 23. Savić N, Schwank G. Advances in therapeutic CRISPR/Cas9 genome editing. Transl Res. 2016:168:15–21. [DOI] [PubMed] [Google Scholar]
- 24. Torres-Ruiz R, Rodriguez-Perales S. CRISPR-Cas9 technology: applications and human disease modelling. Brief Funct Genomics. 2017:16(1):4–12. [DOI] [PubMed] [Google Scholar]
- 25. Ahmad G, Amiji M. Use of CRISPR/Cas9 gene-editing tools for developing models in drug discovery. Drug Discov Today. 2018:23(3):519–533. [DOI] [PubMed] [Google Scholar]
- 26. Ferreira R, David F, Nielsen J. Advancing biotechnology with CRISPR/Cas9: recent applications and patent landscape. J Ind Microbiol Biotechnol. 2018:45(7):467–480. [DOI] [PubMed] [Google Scholar]
- 27. Liu Q, Yang F, Zhang J, Liu H, Rahman S, Islam S, Ma W, She M. Application of CRISPR/Cas9 in crop quality improvement. Int J Mol Sci. 2021:22(8):4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gupta D, Bhattacharjee O, Mandal D, Sen MK, Dey D, Dasgupta A, Kazi TA, Gupta R, Sinharoy S, Acharya K, et al. CRISPR-Cas9 system: a new-fangled dawn in gene editing. Life Sci. 2019:232:116636. [DOI] [PubMed] [Google Scholar]
- 29. Martinez-Lage M, Torres-Ruiz R, Rodriguez-Perales S. CRISPR/Cas9 technology: applications and human disease Modeling. Prog Mol Biol Transl Sci. 2017:152:23–48. [DOI] [PubMed] [Google Scholar]
- 30. Liu H, Zhang B. Virus-based CRISPR/Cas9 genome editing in plants. Trends Genet. 2020:36(11):810–813. [DOI] [PubMed] [Google Scholar]
- 31. Zhan C, Xia X. Research progress of CRISPR-Cas9 system for gene therapy. Sheng Wu Gong Cheng Xue Bao. 2016:32(7):861–869. [DOI] [PubMed] [Google Scholar]
- 32. Chen H, Shi M, Gilam A, Zheng Q, Zhang Y, Afrikanova I, Li J, Gluzman Z, Jiang R, Kong LJ, et al. Hemophilia a ameliorated in mice by CRISPR-based in vivo genome editing of human factor VIII. Sci Rep. 2019a:9(1):16838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gori JL, Hsu PD, Maeder ML, Shen S, Welstead GG, Bumcrot D. Delivery and specificity of CRISPR-Cas9 genome editing Technologies for Human Gene Therapy. Hum Gene Ther. 2015:26(7):443–451. [DOI] [PubMed] [Google Scholar]
- 34.Majeed H, Iftikhar T, Abbas Q. Climate resilience plastic degradation potential of Pseudomonas putida isolated from the soil of plastic waste dumping sites to reduce GHG emissions. Z Phys Chem. 2024:238(5):797–807. [Google Scholar]
- 35. Doudna JA. The promise and challenge of therapeutic genome editing. Nature. 2020:578(7794):229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li H, Yang Y, Hong W, Huang M, Wu M, Zhao X. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Target Ther. 2020b:5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iftikhar T, Majeed H, Altaf F, Khalid A. Upcycling of the industrial waste as a sustainable source of axenic fungal strain (Aspergillus oryzae) for scale up enzymatic production with kinetic analysis and Box–Behnken design application. Z Phys Chem. 2024:238(1):115–31. [Google Scholar]
- 38.Majeed H, Iftikhar T, Siddique A. Agricultural waste upcycling into improved production of triacyl glycerol acyl hydrolases. Z Phys Chem. 2024:238(5):809–827. [Google Scholar]
- 39. Sun J, Wang J, Zheng D, Hu X. Advances in therapeutic application of CRISPR-Cas9. Brief Funct Genomics. 2020:19(3):164–174. [DOI] [PubMed] [Google Scholar]
- 40.Bashir T, Iftikhar T, Majeed H. Bulk industrial textile production of bio scouring for cellulosic fabric utilizing indigenous hot springs triacylglycerol acylhydrolases from Bacillus toyonensis and Bacillus thuringiensis. Cellulose. 2024:31(2):1353–81. [Google Scholar]
- 41. Frangoul H, Altshuler D, Cappellini MD, Chen YS, Domm J, Eustace BK, Foell J, Fuente J, Grupp S, Handgretinger R, et al. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N Engl J Med. 2021:384(3):252–260. [DOI] [PubMed] [Google Scholar]
- 42. Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016:533(7603):420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang G, Yang L, Grishin D, Rios X, Ye LY, Hu Y, Li K, Zhang D, Church GM, Pu WT. Efficient, footprint-free human iPSC genome editing by consolidation of Cas9/CRISPR and piggyBac technologies. Nat Protoc. 2017:12(1):88–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yin LJ, Hu SQ, Guo F. The application of CRISPR-Cas9 gene editing technology in viral infection diseases. Yi Chuan. 2015:37(5):412–418. [DOI] [PubMed] [Google Scholar]
- 45. Bikard D, Euler CW, Jiang W, Nussenzweig PM, Goldberg GW, Duportet X, Fischetti VA, Marraffini LA. Exploiting CRISPR-Cas nucleases to produce sequence-specific antimicrobials. Nat Biotechnol. 2014:32(11):1146–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liao HK, Gu Y, Diaz A, Marlett J, Takahashi Y, Li M, Suzuki K, Xu R, Hishida T, Chang CJ, et al. Use of the CRISPR/Cas9 system as an intracellular defense against HIV-1 infection in human cells. Nat Commun. 2015:6(1):6413. [DOI] [PubMed] [Google Scholar]
- 47. Crudele JM, Chamberlain JS. Cas9 immunity creates challenges for CRISPR gene editing therapies. Nat Commun. 2018:9(1):3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Soppe JA, Lebbink RJ. Antiviral goes viral: harnessing CRISPR/Cas9 to combat viruses in humans. Trends Microbiol. 2017:25(10):833–850. [DOI] [PubMed] [Google Scholar]
- 49. Haapaniemi E, Botla S, Persson J, Schmierer B, Taipale J. CRISPR-Cas9 genome editing induces a p53-mediated DNA damage response. Nat Med. 2018:24(7):927–930. [DOI] [PubMed] [Google Scholar]
- 50. Ren J, Liu X, Fang C, Jiang S, June CH, Zhao Y. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin Cancer Res. 2017:23(9):2255–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen JS, Ma E, Harrington LB, Da Costa M, Tian X, Palefsky JM, Doudna JA. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science (New York, NY). 2018:360(6387):436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang DY, Ma N, Hui Y, Gao X. The application of CRISPR/Cas9 genome editing technology in cancer research. Yi Chuan. 2016:38(1):1–8. [DOI] [PubMed] [Google Scholar]
- 53. Chen M, Mao A, Xu M, Weng Q, Mao J, Ji J. CRISPR-Cas9 for cancer therapy: opportunities and challenges. Cancer Lett. 2019b:447:48–55. [DOI] [PubMed] [Google Scholar]
- 54.Tehreema I, Muhammad A, Sidra, Muhammad IR, Hammad M, Roheena A. Isolation, identification of an axenic fungal isolate of Aspergillus sp. (MBL-1511) and its subsequent improvement for enhanced extracellular lipolytic potential through monoculture fermentation. Pak J Bot. 2017:49(5):1981–1993. [Google Scholar]
- 55.Majeed H, Iftikhar T, Mukhtar U. Novel approach to water-efficient bulk industrial textile printing production of cotton fabric. Int J Biol Macromol. 2024:262(1):130064. [DOI] [PubMed] [Google Scholar]
- 56. Karimian A, Azizian K, Parsian H, Rafieian S, Shafiei-Irannejad V, Kheyrollah M, Yousefi M, Majidinia M, Yousefi B. CRISPR/Cas9 technology as a potent molecular tool for gene therapy. J Cell Physiol. 2019:234(8):12267–12277. [DOI] [PubMed] [Google Scholar]
- 57. Shi M, Shen Z, Zhang N, Wang L, Yu C, Yang Z. CRISPR/Cas9 technology in disease research and therapy: a review. Sheng Wu Gong Cheng Xue Bao. 2021:37(4):1205–1228. [DOI] [PubMed] [Google Scholar]
- 58. Xiao-Jie L, Hui-Ying X, Zun-Ping K, Jin-Lian C, Li-Juan J. CRISPR-Cas9: a new and promising player in gene therapy. J Med Genet. 2015:52(5):289–296. [DOI] [PubMed] [Google Scholar]
- 59. Liu W, Yang C, Liu Y, Jiang G. CRISPR/Cas9 system and its research progress in gene therapy. Anti Cancer Agents Med Chem. 2020:19(16):1912–1919. [DOI] [PubMed] [Google Scholar]
- 60. Jo YI, Suresh B, Kim H, Ramakrishna S. CRISPR/Cas9 system as an innovative genetic engineering tool: enhancements in sequence specificity and delivery methods. Biochim Biophys Acta. 2015:1856(2):234–243. [DOI] [PubMed] [Google Scholar]
- 61. Wang D, Zhang F, Gao G. CRISPR-based therapeutic genome editing: strategies and In vivo delivery by AAV vectors. Cell. 2020:181(1):136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tsai SQ, Joung JK. Defining and improving the genome-wide specificities of CRISPR-Cas9 nucleases. Nat Rev Genet. 2016:17(5):300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rajendran SR, Yau YY, Pandey D, Kumar A. CRISPR-Cas9 based genome engineering: opportunities in Agri-food-nutrition and healthcare. OMICS. 2015:19(5):261–275. [DOI] [PubMed] [Google Scholar]
- 64. Chen JS, Dagdas YS, Kleinstiver BP, Welch MM, Sousa AA, Harrington LB, Sternberg SH, Joung JK, Yildiz A, Doudna JA. Enhanced proofreading governs CRISPR-Cas9 targeting accuracy. Nature. 2017:550(7676):407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Napoletano S, Piersanti V, Rallo G. CRISPR -Cas9: a groundbreaking new technique which ushers in new prospects and just as many doubts. La Clinica Terapeutica. 2021:171(1):e52–e54. [DOI] [PubMed] [Google Scholar]
- 66. Hryhorowicz M, Lipiński D, Zeyland J, Słomski R. CRISPR/Cas9 immune system as a tool for genome engineering. Arch Immunol Ther Exp. 2017:65(3):233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, Chen PJ, Wilson C, Newby GA, Raguram A, et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019:576(7785):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sternberg SH, Doudna JA. Expanding the Biologist’s toolkit with CRISPR-Cas9. Mol Cell. 2015:58(4):568–574. [DOI] [PubMed] [Google Scholar]
- 69. National Academies of Sciences, Engineering, Medicine, National Academy of Medicine, National Academy of Sciences, Committee on Human Gene Editing: Scientific, Medical, and Ethical Considerations . Human genome editing: science, ethics, and governance. National Academies Press, Washington, DC (USA); 2017. 10.17226/24623. [DOI] [PubMed] [Google Scholar]
- 70. Brokowski C, Adli M. CRISPR ethics: moral considerations for applications of a powerful tool. J Mol Biol. 2019:431(1):88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mani I. CRISPR-Cas9 for treating hereditary diseases. Prog Mol Biol Transl Sci. 2021:181:165–183. [DOI] [PubMed] [Google Scholar]
- 72. Zhu H, Li C, Gao C. Applications of CRISPR-Cas in agriculture and plant biotechnology. Nat Rev Mol Cell Biol. 2020:21(11):661–677. [DOI] [PubMed] [Google Scholar]
- 73. Phelps MP, Seeb LW, Seeb JE. Transforming ecology and conservation biology through genome editing. Conserv Biol. 2020:34(1):54–65. [DOI] [PubMed] [Google Scholar]
- 74. Shen S, Loh TJ, Shen H, Zheng X, Shen H. CRISPR as a strong gene editing tool. BMB Rep. 2017:50(1):20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lin L, Luo Y. Tracking CRISPR's footprints. Methods Mol Biol. 2019:1961:13–28. [DOI] [PubMed] [Google Scholar]
- 76. Khan FA, Pandupuspitasari NS, Chun-Jie H, Ao Z, Jamal M, Zohaib A, Khan FA, Hakim MR, ShuJun Z. CRISPR/Cas9 therapeutics: a cure for cancer and other genetic diseases. Oncotarget. 2016:7(32):52541–52552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang C, Quan R, Wang J. Development and application of CRISPR/Cas9 technologies in genomic editing. Hum Mol Genet. 2018:27(R2):R79–R88. [DOI] [PubMed] [Google Scholar]
- 78. Cheng X, Fan S, Wen C, Du X. CRISPR/Cas9 for cancer treatment: technology, clinical applications and challenges. Brief Funct Genomics. 2020:19(3):209–214. [DOI] [PubMed] [Google Scholar]
- 79.Majeed H, Iftikhar T, Zohaib M. Extension of guava shelf life through the application of edible coating formulated with mango and lemon leaves extracts. Ind Crops Prod. 2024:216:118671. [Google Scholar]
- 80. Graham C, Hart S. CRISPR/Cas9 gene editing therapies for cystic fibrosis. Expert Opin Biol Ther. 2021:21(6):767–780. [DOI] [PubMed] [Google Scholar]
- 81. Park SH, Bao G. CRISPR/Cas9 gene editing for curing sickle cell disease. Transfus Apher Sci. 2021:60(1):103060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Erkut E, Yokota T. CRISPR therapeutics for Duchenne muscular dystrophy. Int J Mol Sci. 2022:23(3):1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Happi Mbakam C, Lamothe G, Tremblay G, Tremblay JP. CRISPR-Cas9 gene therapy for Duchenne muscular dystrophy. Neurotherapeutics. 2022:19(3):931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Khiabani A, Kohansal MH, Keshavarzi A, Shahraki H, Kooshesh M, Karimzade M, Gholizadeh Navashenaq J. CRISPR/Cas9, a promising approach for the treatment of β-thalassemia: a systematic review. Mol Genet Genomics. 2023:298(1):1–11. [DOI] [PubMed] [Google Scholar]
- 85. Jo DH, Song DW, Cho CS, Kim UG, Lee KJ, Lee K, Park SW, Kim D, Kim JH, Kim JS, et al. CRISPR-Cas9-mediated therapeutic editing of Rpe65 ameliorates the disease phenotypes in a mouse model of Leber congenital amaurosis. Sci Adv. 2019:5(10):eaax1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ruan GX, Barry E, Yu D, Lukason M, Cheng SH, Scaria A. CRISPR/Cas9- mediated genome editing as a therapeutic approach for Leber congenital Amaurosis 10. Mol Ther. 2017:25(2):331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Alkanli SS, Alkanli N, Ay A, Albeniz I. CRISPR/Cas9 mediated therapeutic approach in Huntington's disease. Mol Neurobiol. 2023:60(3):1486–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hussein M, Molina MA, Berkhout B, Herrera-Carrillo E. A CRISPR-Cas cure for HIV/AIDS. Int J Mol Sci. 2023:24(2):1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Xiao Q, Guo D, Chen S. Application of CRISPR/Cas9-based gene editing in HIV-1/AIDS therapy. Front Cell Infect Microbiol. 2019:9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ahmad I. CRISPR/Cas9-a promising therapeutic tool to cure blindness: current scenario and future prospects. Int J Mol Sci. 2022:23(19):11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shen S, Sanchez ME, Blomenkamp K, Corcoran EM, Marco E, Yudkoff CJ, Jiang H, Teckman JH, Bumcrot D, Albright CF. Amelioration of Alpha-1 antitrypsin deficiency diseases with genome editing in transgenic mice. Hum Gene Ther. 2018:29(8):861–873. [DOI] [PubMed] [Google Scholar]
- 92. Bjursell M, Porritt MJ, Ericson E, Taheri-Ghahfarokhi A, Clausen M, Magnusson L, Admyre T, Nitsch R, Mayr L, Aasehaug L, et al. Therapeutic genome editing with CRISPR/Cas9 in a humanized mouse model ameliorates α1-antitrypsin deficiency phenotype. EBioMedicine. 2018:29:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Richards DY, Winn SR, Dudley S, Nygaard S, Mighell TL, Grompe M, Harding CO. AAV-mediated CRISPR/Cas9 gene editing in murine phenylketonuria. Mol Ther Methods Clin Dev. 2019:17:234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Vrugt HJ, Harmsen T, Riepsaame J, Alexantya G, Mil SE, Vries Y, Bin Ali R, Huijbers IJ, Dorsman JC, Wolthuis RMF, et al. Effective CRISPR/Cas9-mediated correction of a Fanconi anemia defect by error-prone end joining or templated repair. Sci Rep. 2019:9(1):768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Osborn MJ, Gabriel R, Webber BR, DeFeo AP, McElroy AN, Jarjour J, Starker CG, Wagner JE, Joung JK, Voytas DF, et al. Fanconi anemia gene editing by the CRISPR/Cas9 system. Hum Gene Ther. 2015:26(2):114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Raaijmakers RHL, Ripken L, Ausems CRM, Wansink DG. CRISPR/Cas applications in myotonic dystrophy: expanding opportunities. Int J Mol Sci. 2019:20(15):3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Barman NC, Khan NM, Islam M, Nain Z, Roy RK, Haque A, Barman SK. CRISPR-Cas9: a promising genome editing therapeutic tool for Alzheimer's disease-a narrative review. Neurol Ther. 2020:9(2):419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data related to this work has been mentioned in this manuscript.


