Abstract
We show the direct production and detection of 13C-hyperpolarized fumarate by parahydrogen-induced polarization (PHIP) in a microfluidic lab-on-a-chip (LoC) device and achieve 8.5% 13C polarization. This is the first demonstration of 13C-hyperpolarization of a metabolite by PHIP in a microfluidic device. LoC technology allows the culture of mammalian cells in a highly controlled environment, providing an important tool for the life sciences. In-situ preparation of hyperpolarized metabolites greatly enhances the ability to quantify metabolic processes in such systems by microfluidic NMR. PHIP of 1H nuclei has been successfully implemented in microfluidic systems, with mass sensitivities in the range of pmol/s. However, metabolic NMR requires high-yield production of hyperpolarized metabolites with longer spin life times than is possible with 1H. This can be achieved by transfer of the polarization onto 13C nuclei, which exhibit much longer T1 relaxation times. We report an improved microfluidic PHIP device, optimized using a finite element model, that enables the direct and efficient production of 13C-hyperpolarized fumarate.
Introduction
Lab-on-a-chip (LoC) systems that can culture cells, cell aggregates, or tissues, are increasingly adopted as a research tool in the life sciences, especially in drug development.1−3 While this is partly driven by the widely recognized need to reduce animal testing, LoC cultures allow the use of human cells and can therefore provide more relevant models of human disease. Microfluidic technology enables precise control over the cellular growth environment, and offers high throughput and a high degree of reproducibility. In this way, cellular processes and functions as well as their response to external stimuli such as drugs,4 therapeutic targets,5−7 toxins,8,9 and oxygen or nutrient supply10,11 can be studied systematically. Microfluidic NMR12−14 allows noninvasive and real-time operando quantitative characterization of metabolic15−17 and chemical18 processes in LoC devices. However, sensitivity is limited in these systems due to their small size. Hyperpolarization of the nuclear spins19 could address this, but requires preparation of hyperpolarized species that can be metabolized by the cultured cells, with a lifetime of the spin order long enough to detect downstream metabolic products.
Hyperpolarized metabolites have great potential as contrast agents for magnetic resonance imaging (MRI) and magnetic resonance spectroscopic imaging (MRSI), providing real-time and quantitative information on active metabolic pathways in healthy and diseased tissues.20,21 This approach has been used in vivo for metabolic profiling of tumors such glioma,22,23 hepatocellular carcinoma, lymphoma,24,25 pancreatic26 and breast cancers.27,28 In this modality, relatively large amounts (several g) of hyperpolarized material (most commonly pyruvate) are prepared and injected intravenously into the patient. Preparation relies on either dissolution dynamic nuclear polarization29,30 or on low-field polarization transfer based on parahydrogen-induced polarization (PHIP).31,32 The batch mode of operation of these methods does not lend itself to LoC culture devices, where a steady supply of much smaller amounts of hyperpolarized metabolites is needed. In this case, preparation methods that operate continuously at flow rates compatible with microfluidic systems (up to a few μL/min) are required.33 Additionally, as the lifetime of hyperpolarized species is limited by nuclear relaxation, it is crucial to produce them directly on the microfluidic device, in immediate proximity of their usage.
PHIP makes it possible to enhance NMR signals by up to 5 orders of magnitude.34,35 It utilizes para-hydrogen (p-H2), the singlet nuclear spin isomer of molecular hydrogen, as a source of spin order. The nuclear spin order is transferred to a target molecule via a chemical reaction of p-H2 with an unsaturated molecule in the presence of an organometallic catalyst. The chemical reaction is followed by spin manipulations to transfer the parahydrogen-derived spin order to a desired nucleus, and may include purification steps to remove unwanted compounds.36
LoC devices can be used to implement some or all of these processes. Eills et al. have reported mass sensitives of the order of pmol/s for 1H in a microfluidic PHIP device37 based on diffusion of p-H2 through a silicone membrane, using propargyl acetate in methanol as a substrate. Barker et al. have subsequently shown that the same design can be used to directly hydrogenate acetylene dicarboxylic acid to produce 1H-hyperpolarized fumarate.38 However, the yield obtained in both cases falls short of the requirements for biological applications, particularly since further transformations such as purification and cleavage are required to effectively utilize the hyperpolarized material. To understand the interplay of the chemical, spatial and spin dynamics occurring on the microfluidic device proposed by Eills et al. Ostrowska et al.39 developed a finite element model of reaction and found that insufficient uptake of hydrogen was the limiting factor of the reaction.
In the present contribution, we report an improved device design, optimized using this finite element model to maximize hydrogen uptake. Additionally, we introduce a variable temperature control to regulate the temperature at the sample detection chamber. It is shown that these improvements, taken together, increase the yield to such a point that the production and detection of 13C-hyperpolarized fumarate becomes possible. To the best of our knowledge, this is the first report of PHIP-based 13C hyperpolarization in a microfluidic system.
Materials and Methods
Microfluidic Setup
The microfluidic device was manufactured from polycarbonate (PC) (Self Adhesive Supplies, UK) following the protocol given in ref (38). Briefly, devices were cut out with a LS3040 CO2 laser cutter (HPC Laser, United Kingdom) from three layers of polycarbonate sheet material with 0.25, 0.5, and 0.25 mm thickness for the top, middle, and bottom layers, respectively. The sample detection chamber in the middle layer and channels in the top layer were cut through, while the channels in the bottom and middle layers were engraved. After plasma activating using Corona Treater (Electro-Technic Products, USA), each layer was coated with 18 μL of plasticizer (5 v/v% dibutyl phtalate in isopropyl alcohol). Then the layers were dried for 15 min at 65 °C, assembled and bonded together under pressure and heat (5 tonnes, 85 °C).
The microfluidic assembly consisted of the chip interposed between two 1 mm PDMS membranes (Shielding Solutions, UK) held together by a fluidic interface (ProtoLabs, UK). Connectors for 1/16” fluid and gas lines (Cole Parmer, UK) facilitated the delivery of substrates onto the chip shown in Figure 1. PDMS membranes that covered the upper part of the chip served a dual purpose. First they promoted diffusion of hydrogen into the liquid channel and second, they enabled sealing of the assembly.
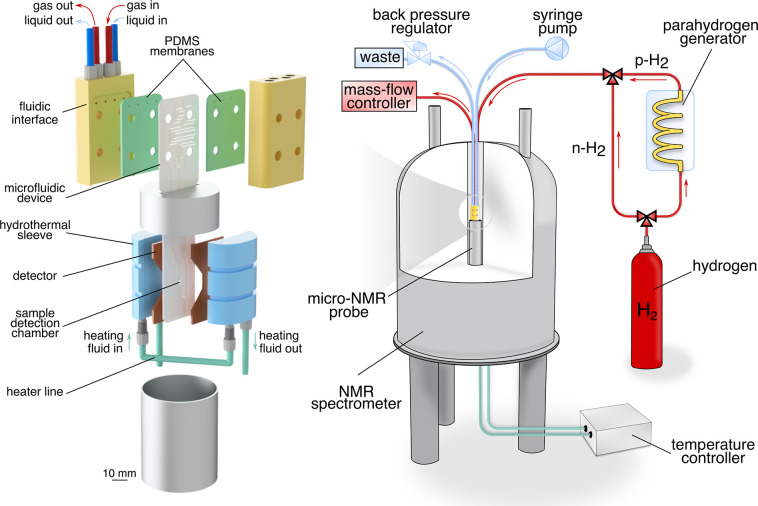
Figure 1.
Experimental setup. The microfluidic chip assembly consists of a microfluidic device interposed between two PDMS membranes. These are held together by the fluidic interface that enables delivery of substrates into the chip. All experiments were performed inside of a high-field NMR spectrometer. Hydrogen/parahydrogen gas was supplied from a gas cylinder, while the precursor solution was introduced into the device using a syringe pump located outside of the spectrometer. The device was placed into the micro-NMR probe for detection. The probe was also equipped with hydrothermal sleeves regulated by a temperature controller that enabled efficient heating of the sample detection chamber.
All experiments were conducted on a Bruker AVANCE III spectrometer operating at 11.7 T magnetic field. The microfluidic assembly was placed inside of a stripline-based micro-NMR probe for detection14 as shown in Figure 1. The probe was equipped with hydrothermal sleeves that housed a thermistor regulated by temperature controller, allowing efficient heating of the sample detection chamber only. The calibration of the heater was recorded by Rogers et al. and shows temperature fluctuations of less than 0.1 °C.16
The precursor solution was delivered into the chip using a syringe pump (Cole-Parmer, United Kingdom) located outside of the NMR spectrometer as illustrated in Figure 1. Hydrogen gas (gas purity 99.995%) was delivered from a cyliner located outside of the spectrometer at a flow rate set to 20 mL min–1 controlled using a mass-flow controller at the end of the gas line. The gas line was equipped with a valve selecting a flow of either hydrogen in thermal equilibrium or parahydrogen. Parahydrogen gas was obtained with 50% enrichment using a home-built parahydrogen generator filled with iron(III) oxide and cooled to 77 K.
All chemicals were purchased from Merck KGaA (Germany) and were used as received.
Quantification of Hydrogen Uptake into the β-Chip
The uptake of hydrogen into the β-chip was quantified by flowing
a solution of 20 mM sodium acetate dissolved in methanol-d4. In the gas channel, hydrogen in thermal equilibrium was supplied
at 5 bar. The flow rate of hydrogen was controlled using a mass-flow
controller positioned at the end of the gas line, set to a constant
rate of 20 mL min–1. The flow rate of the liquid
was varied from 2 to 20 μL min–1 in steps
of 2 μL min–1 and the solution was
left to equilibrate for 10 min at each flow rate. Then, 64 scans were
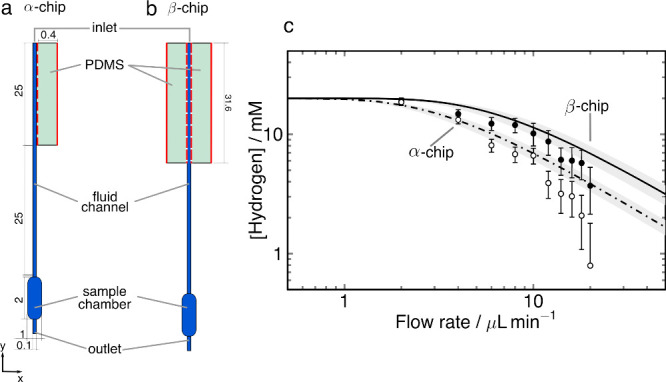
acquired after the application of a 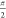 pulse with a recycle delay of 20 s. The
NMR signal at 4.55 ppm was integrated to determine the H2 concentration.
pulse with a recycle delay of 20 s. The
NMR signal at 4.55 ppm was integrated to determine the H2 concentration.
Finite Element Modeling
Finite element simulations were performed using COMSOL Multiphysics version 5.4. Figure 3a and Figure 3b show simulation domains for the α- and β-chips, respectively. The key functional components are the fluid channel, the sample chamber and PDMS membranes. The total volume of the β-chip was calculated as 7 μL. The simulation protocol and detailed results are given in the SI.
Figure 3.
(a) α-Chip simulation domain. Adapted from ref (39). Available under CC BY 4.0. Copyright Ostrowska et al. (b) β-Chip simulation domain. (c) Hydrogen uptake into the chip as a function of flow rate of the liquid. The solid empty and black circles represent the NMR data for α- and β-chips, respectively. The solid and dash–dotted lines are the results of FEM simulations. The gray shadows represent ±1.5 μL error in the volume of the chip. Data for the α-chip was obtained from Eills et al.37
Formation of 13C-Hyperpolarized Fumarate
The precursor solution contained 100 mM acetylene dicarboxylic acid
[1-13C] disodium salt, 6 mM 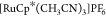 catalyst and 200 mM sodium sulfite dissolved
in D2O at 50 °C. The heater temperature was set to
58 °C. Flow rates from 2 to 16 μL min–1 in steps of 2 μL min–1 were studied. Parahydrogen
pressure was set to 6 bar. The probe delivered nutation frequencies
for 13C RF pulses of 12.5 kHz. Spectra were collected with
a 200 ppm spectral width, and 8 k data points were acquired. Proton
singlet order in [1-13C]fumarate was converted into the
observable carbon magnetization using the singlet-to-heteronuclear-magnetization
(S2hM) pulse sequence.41 The maximum efficiency
was achieved using the following parameters: τ = 15.7 ms, n2 = 7, n1 = 7. The
repetition delay was set to 60 s. The yield of fumarate was determined
by comparing the integral of the fumarate peak at 6.8 ppm to the catalyst
Cp* peak at 2.35 ppm (spectrum shown in the SI) and accounting for the difference in the number of protons. To
calculate the enhancement factor for carbon polarization, the SNR
of in the hyperpolarized spectrum was compared with the SNR obtained
form a spectrum of 1 M d-glucose-1-13C averaged
over 32 scans.
catalyst and 200 mM sodium sulfite dissolved
in D2O at 50 °C. The heater temperature was set to
58 °C. Flow rates from 2 to 16 μL min–1 in steps of 2 μL min–1 were studied. Parahydrogen
pressure was set to 6 bar. The probe delivered nutation frequencies
for 13C RF pulses of 12.5 kHz. Spectra were collected with
a 200 ppm spectral width, and 8 k data points were acquired. Proton
singlet order in [1-13C]fumarate was converted into the
observable carbon magnetization using the singlet-to-heteronuclear-magnetization
(S2hM) pulse sequence.41 The maximum efficiency
was achieved using the following parameters: τ = 15.7 ms, n2 = 7, n1 = 7. The
repetition delay was set to 60 s. The yield of fumarate was determined
by comparing the integral of the fumarate peak at 6.8 ppm to the catalyst
Cp* peak at 2.35 ppm (spectrum shown in the SI) and accounting for the difference in the number of protons. To
calculate the enhancement factor for carbon polarization, the SNR
of in the hyperpolarized spectrum was compared with the SNR obtained
form a spectrum of 1 M d-glucose-1-13C averaged
over 32 scans.
Results and Discussion
The basic principle of operation of our PHIP device is shown schematically in Figure 2. The solution containing an unsaturated precursor flows through the channel indicated in blue, next to a channel containing parahydrogen gas under pressure, shown in red. Both channels are covered by a PDMS membrane, through which the molecular hydrogen diffuses efficiently (Figure 2c). Figure 2 compares the original chip design used by Eills et al.37 (a) with an improved design used here (b). The length of the fluid path has been increased, and the fluid path is now flanked by the hydrogen gas channel on either side.
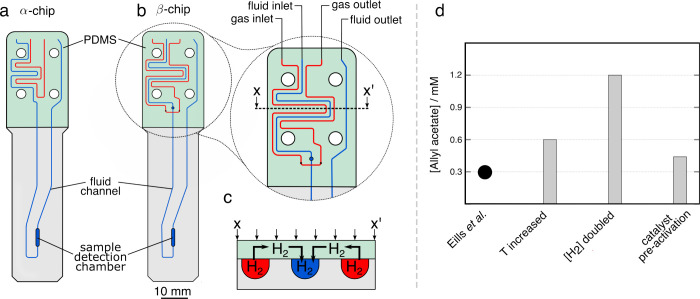
Figure 2.
Top view of the microfluidic devices. (a) The α-chip used by Eills et al.37 Adapted from ref (39). Available under CC BY 4.0. Copyright Ostrowska et al. (b) The β-chip. The key functional area of the β-chip was enlarged. (c) Cross section of the β-chip. The PDMS membrane (green) acts as a bridge between the fluid (blue) and two gas (red) channels, enabling hydrogen to diffuse into the solution. (d) Concentration of allyl acetate reported by Eills et al.37 and three independent scenarios predicted by the model developed in ref (39).
An FEM model of the transport and chemical kinetics of the para-hydrogenation of propargyl acetate to allyl acetate has been previously reported.39Figure 2d shows the experimental yield of hyperpolarized allyl acetate reported by Eills et al.37 along with the prediction of the FEM model, whose kinetic parameters have been obtained from independent experiments at large scale.39 The model was used to explore three different hypothetical scenarios. In the first scenario all reaction rate constants were increased by a factor of 2, approximating a temperature increase by about 10 °C. As shown in Figure 2d, this leads to an increase in the yield by about a factor of 2 as expected. In the second case the partial pressure of hydrogen in the gas supply was doubled. The model predicts a massive increase in yield by a factor of 4. Finally, the catalyst activation rate was increased 10 times, simulating a situation where the protection group of the catalyst was replaced with one that is easier to remove. This led only to a modest increase in the yield. From these findings, we concluded that improvement of the hydrogen uptake was the most efficient way of increasing the yield of hyperpolarized product.
Enhancing Hydrogen Uptake
Experiments by Eills et al. had been carried out with hydrogen gas at 5 bar. Simply elevating hydrogen pressure in the chip is not viable as it tends to cause delamination and leakages, and high hydrogen pressures pose a safety hazard. Instead, the channel network can be modified to maximize the gas uptake. The fluidic design in the α-chip used by Ellis et al. consisted of one gas and one fluid channel in a side-by-side arrangement, with a PDMS membrane covering both channels and serving as a diffusion conduit for H2. The fluid channel in the β-chip design was positioned between two gas pathways, as shown in Figure 3a and 3b. Additionally, the fluid pathway in contact with the PDMS membrane was extended by nearly 30% in length. The finite element simulation domains for the α – and β-chips are shown in Figure 3.
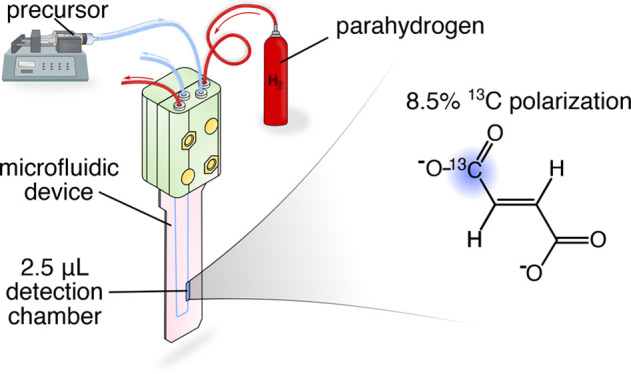
To experimentally measure the uptake of hydrogen gas into the β-chip, methanol was flowed into the fluid channel by means of a syringe pump located outside of the NMR spectrometer as shown in Figure 1. The chip was pressurized to 5 bar with hydrogen gas and its flow was controlled using a mass-flow controller set to 20 mL min–1. Dissolved hydrogen was detected by NMR in the 2.5 μL sample chamber on the chip.
Figure 3c shows the concentration of hydrogen in the sample chamber as a function of flow rate; 20 mM of sodium acetate was used as the concentration standard. The solid empty and black circles represent the NMR data for α- and β-chips, respectively. Error bars represent integrated rms noise in the spectra. Experimental NMR data for the α-chip was taken from ref (37). At 2 μL min–1 flow rate, the flowing liquid in both devices is fully saturated with hydrogen. However, as the flow rate increases to 10 μL min–1, the concentration of hydrogen in the β-chip is 11.3 mM versus only ∼6 mM in the α-chip. At a higher flow rate of 18 μL min–1 there is 3 times more hydrogen dissolved in the β-chip compared to the α-chip. The solid and dash-dotted lines are the FEM simulations and the gray shadows represent uncertainty due to fabrication tolerances of the chips. Simulations for both the α- and the β-chip are in good agreement with the experimental data for flow rates up to 10 μL min–1. Above this flow rate, the model consistently overestimates the hydrogen uptake. This discrepancy is not well understood yet, it was proposed that this could be due to the deformation of the PDMS membrane.37 However, simulations and experiments both suggest that the hydrogen uptake of the β-chip is higher by a factor of 2 for flow rates above 2 μL/min.
The PHIP performance of the microfluidic chip was compared to the results obtained by Eills et al. To this effect, the precursor solution containing 20 mM of propargyl acetate and 5 mM of rhodium catalyst flowed in the solution channel, while 5 bar of para-enriched hydrogen gas was supplied into the gas channel. The experimental setup is shown schematically in Figure 1. Hydrothermal sleeves were incorporated between the stripline detector and the microfluidic device housing, which facilitated efficient heating of the sample chamber up to 58 °C. The experiments and results are described in detail in the SI. Briefly, at the optimal flow rate of 5 μL min–1, the concentration of allyl acetate at 25 °C was determined to be 4.9 ± 0.2 mM, corresponding to a yield of 24.5 ± 1%. Compared to the results reported by Eills et al, this represents an increase in yield by a factor of 15. Increasing the temperature to 37 °C led to the concentration of allyl acetate of 7.0 ± 0.2 mM, corresponding to a yield of 35 ± 1%. This represents a further 10% increase in yield compared to the initial conditions. Elevation of the temperature to 47 °C led to a decrease in the concentration of allyl acetate to 5.4 ± 0.2 mM.
Formation of 13C-Hyperpolarized Fumarate
The short lifetime of 1H polarization, of the order of seconds, limits application of 1H-hyperpolarization to track metabolic processes. This can be overcome by transferring the polarization to a longer-lived nucleus such as 13C or 15N. Hyperpolarized fumarate is a promising target for in vivo detection of necrosis and therefore has been extensively used as a hyperpolarization target.43−46 However, the trans-hydrogenation reaction to synthesize hyperpolarized fumarate is challenging as it is slow compared to the time frame in which the hyperpolarization returns to thermal equilibrium.47 As will be shown in the following, the enhanced hydrogen uptake of the β-chip together with the ability to run the reaction at slightly elevated temperature make it possible to hyperpolarize fumarate more efficiently.
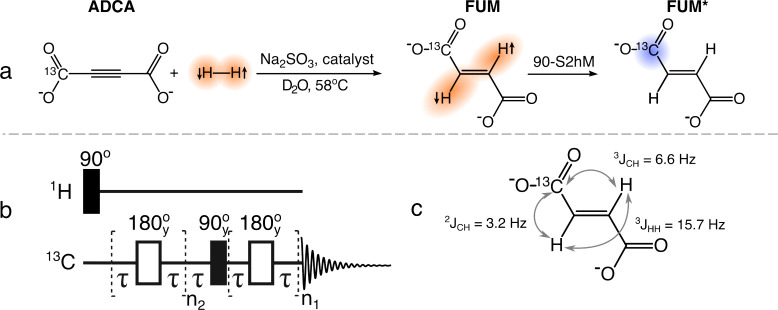
As shown in Figure 4a hyperpolarized fumarate was generated in aqueous solution via a reaction of [1 – 13C]-acetylenedicarboxylic acid disodium salt (ADCA) with para – hydrogen in the presence of a ruthenium catalyst, resulting in [1 – 13C]fumarate (FUM). Since the added protons are chemically and magnetically equivalent, a 13C label is required to to break the symmetry and enable observation of the spin order by NMR. The pulse sequence to convert the resulting singlet spin order into 13C magnetization is shown in Figure 4b. It consists of an initial purge pulse on the 1H channel, followed by an S2hM sequence41 on the 13C channel. This hydrogenation reaction is known to be affected by singlet–triplet (S-T) mixing, which can lead to a reduction of observable PHIP signal.48 S-T mixing occurs when molecules of hydrogen form intermediate hydride species with the catalyst metal center. At high magnetic fields the two protons experience a chemical shift difference in the hydride, which can lead to significant leakage from the proton singlet state (|S0⟩) to the central triplet state (|T0⟩).49 Partial signal cancellation occurs after S2M or S2hM sequences are applied which convert these states to either 1H or 13C magnetization but with opposite phases. There are methods for mitigating so-called S-T mixing.48,50,51 A π/2 ”purge” pulse prior to the S2M sequence was found to improve the efficiency of the sequence in microfluidic chips.38 The purge pulse removes the detrimental population of the |T0⟩ state by transferring it to the two outer |T±⟩ states where it has no effect on the polarization transfer. Here, the purge pulse was applied on the 1H channel prior to application of the S2hM sequence on the 13C channel as shown in Figure 4b.
Figure 4.
(a) Formation of 13C-hyperpolarized fumarate. Acetylene
dicarboxylic acid [1-13C] disodium salt labeled as molecule ADCA reacts with parahydrogen in the presence of sodium sulfite
and the catalyst 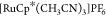 in D2O. The reaction results
in a production of disodium [1-13C]fumarate, molecule FUM, with the two protons in a singlet state. Application
of the S2hM pulse sequence converts the singlet state into observable 13C magnetization FUM*. (b) 90-S2hM pulse sequence
used to transfer the polarization from the proton singlet state to
carbon. (c) The J-coupling network of [1-13C]fumarate. The J-coupling values were taken from
ref (43).
in D2O. The reaction results
in a production of disodium [1-13C]fumarate, molecule FUM, with the two protons in a singlet state. Application
of the S2hM pulse sequence converts the singlet state into observable 13C magnetization FUM*. (b) 90-S2hM pulse sequence
used to transfer the polarization from the proton singlet state to
carbon. (c) The J-coupling network of [1-13C]fumarate. The J-coupling values were taken from
ref (43).
Figure 5a shows single scan 13C NMR spectra of 13C-hyperpolarized fumarate obtained at different flow rates using the setup depicted in Figure 1 and the β-chip at a temperature of 58 °C. At 2 μL min–1, the carbon signal is barely distinguishable from the noise but as the flow rate increases, the signal intensity increases. The change in signal intensity as a function of flow rate is displayed in Figure 5b. There is a gradual increase in signal intensity up to 8 μL min–1, followed by a plateau. This behavior is markedly different to what has been reported by Eills et al. for 1H hyperpolarization,37 which exhibited a sharp maximum at the optimum flow rate. At very low flow rates the time it takes for the product to be delivered into the sample chamber is greater that the spin relaxation time. This seems to be the case at 2 μL min–1 and below. It should be noted that since the polarization transfer only takes place in the sample detection region, it is the 1H singlet lifetime that is relevant here, not the 13C T1. Between 2 and 8 μL min–1 a gradually increasing amount of hyperpolarized material reaches the sample chamber. As shown in Figure 3, the hydrogen uptake decreases rapidly with increasing flow rate. It appears that this effect, which must lead to a decreasing yield of hydrogenation product with increasing flow rate, is almost perfectly compensated by the shorter amount of time needed for the product to reach the detection chamber at flow rates between 8 and 16 μL min–1. This gives rise to the hope that the 13C polarization could be substantially improved if the polarization transfer step could be carried out further upstream in the chip. Further experiments and detailed simulations are needed to clarify this point in support of a corresponding redesign of the microfluidic setup.
Figure 5.
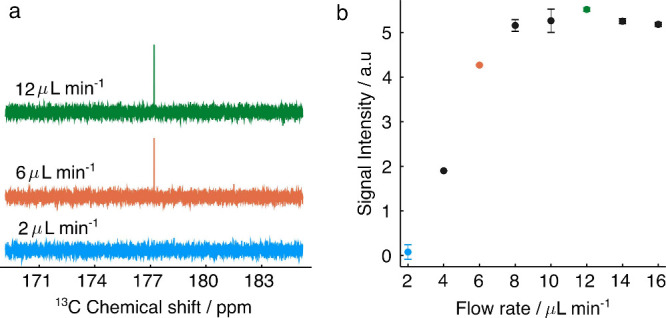
(a) 13C spectra of [1-13C]fumarate at different flow rates. (b) Hyperpolarized 13C signal intensity of [1-13C]fumarate as a function of fluid flow rate.
A straightforward way to quantify the enhancement
factor is to
run the same experiment with hydrogen in thermal equilibrium. Unfortunately,
the concentration of fumarate was too low for the thermal 13C signal to be directly observed using our home-built transmission
line probe, which is not optimized for sensitivity on the low frequency
channel. To estimate the signal enhancement, the hyperpolarized spectrum
was compared with a spectrum of 1 M d-Glucose-1-13C obtained after the application of 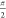 pulse (see SI). The SNR in the glucose spectrum is 2:1, while in the hyperpolarized
spectrum of fumarate the SNR is 9:1. Since the glucose spectrum was
obtained with 32 scans, the SNR from a single scan is
pulse (see SI). The SNR in the glucose spectrum is 2:1, while in the hyperpolarized
spectrum of fumarate the SNR is 9:1. Since the glucose spectrum was
obtained with 32 scans, the SNR from a single scan is 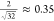 . Accounting for the fact that glucose spectrum
was obtained from a 1 M sample and the spectrum of fumarate was obtained
from a 3 mM sample. This leads to the signal enhancement factor of
. Accounting for the fact that glucose spectrum
was obtained from a 1 M sample and the spectrum of fumarate was obtained
from a 3 mM sample. This leads to the signal enhancement factor of 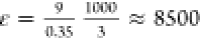 , corresponding to 8.5% 13C polarization.
, corresponding to 8.5% 13C polarization.
Conclusions
In this work we have used finite element simulation results to inform the design of an optimized microfluidic device for performing PHIP reactions. FEM of the chip reported by Ostrowska et al.39 identified that inadequate uptake of hydrogen into the device is the limiting factor for the reaction, which resulted in submilimolar reaction yield. Introduction of an additional hydrogenation channel resulted in a 15-fold increase in the yield of hyperpolarized product compared with previously reported α-chip.37 Heating the sample chamber of the chip led to a further improvement of the yield. With these improvements, it has become possible for the first time to demonstrate the production and observation of the 13C hyperpolarized metabolite fumarate in a microfluidic device, with a 13C polarization of 8.5%. Further improvements are possible by optimization of the fluidic design, as well as by improvement of the 13C sensitivity of the microfluidic NMR probe. The present results represent an important step toward the integrated production of hyperpolarized materials and microfluidic cell culture.15,16 However, this requires integration of cleanup steps into the microfluidic system to remove the potentially toxic catalyst and reaction products. Research in this direction is underway in our laboratory, and will be reported at a later occasion.
Acknowledgments
The authors thank Dr. Manvendra Sharma for help with the design and setup of the microfluidic NMR probe and fluidic support systems. This work was supported by the UK Engineering and Physical Sciences Research Council (EPSRC) through grants EP/W020343/1 paraQchip and EP/V055593/1 and the iCASE studentship EP/R513325/1 to S.J.B., cofunded by Bruker UK Ltd., as well as by the European Research Council grant 786707-FunMagResBeacons.
Data Availability Statement
All raw experimental and simulation data have been deposited on zenodo.org, organized by figure.52
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.4c03271.
Data on allyl acetate pH2 experiments, details on the FEM simulation model, the 1H NMR spectrum of fumarate, as well as technical drawings of the microfluidic device (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Leung C. M.; de Haan P.; Ronaldson-Bouchard K.; Kim G.-A.; Ko J.; Rho H. S.; Chen Z.; Habibovic P.; Jeon N. L.; Takayama S.; Shuler M. L.; Vunjak-Novakovic G.; Frey O.; Verpoorte E.; Toh Y.-C. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2022, 2, 1–29. 10.1038/s43586-022-00118-6. [DOI] [Google Scholar]
- Ma C.; Peng Y.; Li H.; Chen W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol. Sci. 2021, 42, 119. 10.1016/j.tips.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E. Human Organs-on-Chips for Disease Modelling, Drug Development and Personalized Medicine. Nat. Rev. Genet. 2022, 23, 467–491. 10.1038/s41576-022-00466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si L.; Bai H.; Rodas M.; Cao W.; Oh C. Y.; Jiang A.; Moller R.; Hoagland D.; Oishi K.; Horiuchi S.; Uhl S.; Blanco-Melo D.; Albrecht R. A.; Liu W.-C.; Jordan T.; Nilsson-Payant B. E.; Golynker I.; Frere J.; Logue J.; Haupt R.; McGrath M.; Weston S.; Zhang T.; Plebani R.; Soong M.; Nurani A.; Kim S. M.; Zhu D. Y.; Benam K. H.; Goyal G.; Gilpin S. E.; Prantil-Baun R.; Gygi S. P.; Powers R. K.; Carlson K. E.; Frieman M.; TenOever B. R.; Ingber D. E. A Human-Airway-on-a-Chip for the Rapid Identification of Candidate Antiviral Therapeutics and Prophylactics. Nat. Biomed. Eng. 2021, 5, 815–829. 10.1038/s41551-021-00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q.; Liu J.; Wang X.; Feng L.; Wu J.; Zhu X.; Wen W.; Gong X. Organ-on-a-chip: Recent Breakthroughs and Future Prospects. Biomed. Eng. Online 2020, 19, 1–19. 10.1186/s12938-020-0752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri N.; Klapperich C. M.; Cabodi M. Towards Lab-on-a-Chip Diagnostics for Malaria Elimination. Lab Chip 2018, 18, 75–94. 10.1039/C7LC00758B. [DOI] [PubMed] [Google Scholar]
- Wu J.; Dong M.; Rigatto C.; Liu Y.; Lin F. Lab-on-Chip Technology for Chronic Disease Diagnosis. NPJ. Digit. Med. 2018, 1, 1–11. 10.1038/s41746-017-0014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodat Y. A.; Kang M. G.; Kiaee K.; Kim G. J.; Martinez A. F. H.; Rosenkranz A.; Bae H.; Shin S. R. Human-Derived Organ-on-a-Chip for Personalized Drug Development. Curr. Pharm. Des. 2019, 24, 5471–5486. 10.2174/1381612825666190308150055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y.; Han X.; Wang Y.; Chen Z.; Lu Y.; Liu T.; Wu Z.; Jin Y.; Luo Y.; Zhang X. Drug Toxicity Evaluation Based on Organ-on-a-chip Technology: A Review. Micromachines 2020, 11, 381. 10.3390/mi11040381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komen J.; Westerbeek E. Y.; Kolkman R. W.; Roesthuis J.; Lievens C.; van den Berg A.; van der Meer A. D. Controlled Pharmacokinetic Anti-Cancer Drug Concentration Profiles Lead to Growth Inhibition of Colorectal Cancer Cells in a Microfluidic Device. Lab Chip 2020, 20, 3167–3178. 10.1039/D0LC00419G. [DOI] [PubMed] [Google Scholar]
- Emami Nejad A.; Najafgholian S.; Rostami A.; Sistani A.; Shojaeifar S.; Esparvarinha M.; Nedaeinia R.; Haghjooy Javanmard S.; Taherian M.; Ahmadlou M.; Salehi R.; Sadeghi B.; Manian M. The Role of Hypoxia in the Tumor Microenvironment and Development of Cancer Stem Cell: A Novel Approach to Developing Treatment. Cancer Cell Int. 2021, 21, 1–26. 10.1186/s12935-020-01719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badilita V.; Meier R. Ch.; Spengler N.; Wallrabe U.; Utz M.; Korvink J. G. Microscale Nuclear Magnetic Resonance: A Tool for Soft Matter Research. Soft Matter 2012, 8, 10583–10597. 10.1039/c2sm26065d. [DOI] [Google Scholar]
- Finch G.; Yilmaz A.; Utz M. An Optimised Detector for In-Situ High-Resolution NMR in Microfluidic Devices. Journal of Magnetic Resonance 2016, 262, 73–80. 10.1016/j.jmr.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Sharma M.; Utz M. Modular Transmission Line Probes for Microfluidic Nuclear Magnetic Resonance Spectroscopy and Imaging. Journal of Magnetic Resonance 2019, 303, 75–81. 10.1016/j.jmr.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Patra B.; Sharma M.; Hale W.; Utz M. Time-Resolved Non-Invasive Metabolomic Monitoring of a Single Cancer Spheroid by Microfluidic NMR. Sci. Rep. 2021, 11, 53. 10.1038/s41598-020-79693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers G.; Barker S.; Sharma M.; Khakoo S.; Utz M. Operando NMR Metabolomics of a Microfluidic Cell Culture. J. Magn. Reson. 2023, 349, 107405. 10.1016/j.jmr.2023.107405. [DOI] [PubMed] [Google Scholar]
- Jenne A.; von der Ecken S.; Moxley-Paquette V.; Soong R.; Swyer I.; Bastawrous M.; Busse F.; Bermel W.; Schmidig D.; Kuehn T.; Kuemmerle R.; Al Adwan-Stojilkovic D.; Graf S.; Frei T.; Monette M.; Wheeler A. R.; Simpson A. J. Integrated Digital Microfluidics NMR Spectroscopy: A Key Step toward Automated In Vivo Metabolomics. Anal. Chem. 2023, 95, 5858–5866. 10.1021/acs.analchem.2c04201. [DOI] [PubMed] [Google Scholar]
- Chen J.; Tian J.; Chen Y.; Wu T.; Sun H.; Xie J.; You X.; Chen Z. Probing the Kinetics of Chemical Reactions in Ultra-Small Droplet Samples Using Digital Microfluidic Nuclear Magnetic Resonance Spectroscopy. Microchem. J. 2023, 193, 108984. 10.1016/j.microc.2023.108984. [DOI] [Google Scholar]
- Eills J.; Budker D.; Cavagnero S.; Chekmenev E. Y.; Elliott S. J.; Jannin S.; Lesage A.; Matysik J.; Meersmann T.; Prisner T.; Reimer J. A.; Yang H.; Koptyug I. V. Spin Hyperpolarization in Modern Magnetic Resonance.. Chem. Rev. 2023, 123, 1417–1551. 10.1021/acs.chemrev.2c00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaeggemose M.; Schulte F. R.; Laustsen C. Comprehensive Literature Review of Hyperpolarized Carbon-13 MRI: The Road to Clinical Application. Metabolites 2021, 11, 219. 10.3390/metabo11040219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woitek R.; Gallagher F. A. The Use of Hyperpolarised 13C-MRI in Clinical Body Imaging to Probe Cancer Metabolism. Br. J. Cancer 2021, 124, 1187–1198. 10.1038/s41416-020-01224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.; Rintaro H.; Kim S. K.; Park I. Characterization of Distinctive In Vivo Metabolism between Enhancing and Non-Enhancing Gliomas Using Hyperpolarized Carbon-13 MRI. Metabolites 2021, 11, 504. 10.3390/metabo11080504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I.; Kim S.; Pucciarelli D.; Song J.; Choi J. M.; Lee K.-H.; Kim Y. H.; Jung S.; Yoon W.; Nakamura J. L. Differentiating Radiation Necrosis from Brain Tumor Using Hyperpolarized Carbon-13 MR Metabolic Imaging. Mol. Imaging Biol. 2021, 23, 417–426. 10.1007/s11307-020-01574-w. [DOI] [PubMed] [Google Scholar]
- Perkons N. R.; Johnson O.; Pilla G.; Profka E.; Mercadante M.; Ackerman D.; Gade T. P. F. Functional Genetic Screening Enables Theranostic Molecular Imaging in Cancer. Clin. Cancer Res. 2020, 26, 4581–4589. 10.1158/1078-0432.CCR-20-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkons N. R.; Johnson O.; Pilla G.; Gade T. P. F. Pharmacodynamics and Pharmacokinetics of Hyperpolarized [1 – 13C]-Pyruvate in a Translational Oncologic Model. NMR Biomed 2021, 34, e4502 10.1002/nbm.4502. [DOI] [PubMed] [Google Scholar]
- Martinho R. P.; Bao Q.; Markovic S.; Preise D.; Sasson K.; Agemy L.; Scherz A.; Frydman L. Identification of Variable stages in Murine Pancreatic Tumors by a Multiparametric Approach Employing Hyperpolarized 13C MRSI, 1H Diffusivity and 1H T1 MRI. NMR Biomed 2021, 34, e4446 10.1002/nbm.4446. [DOI] [PubMed] [Google Scholar]
- Woitek R.; McLean M. A.; Gill A. B.; Grist J. T.; Provenzano E.; Patterson A. J.; Ursprung S.; Torheim T.; Zaccagna F.; Locke M.; Laurent M.-C.; Hilborne S.; Frary A.; Beer L.; Rundo L.; Patterson I.; Slough R.; Kane J.; Biggs H.; Harrison E.; Lanz T.; Basu B.; Baird R.; Sala E.; Graves M. J.; Gilbert F. J.; Abraham J. E.; Caldas C.; Brindle K. M.; Gallagher F. A. Hyperpolarized 13C MRI of Tumor Metabolism Demonstrates Early Metabolic Response to Neoadjuvant Chemotherapy in Breast Cancer. Radiol. Imaging Cancer 2020, 2, e200017 10.1148/rycan.2020200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woitek R.; McLean M. A.; Ursprung S.; Rueda O. M.; Garcia R. M.; Locke M. J.; Beer L.; Baxter G.; Rundo L.; Provenzano E.; Kaggie J.; Patterson A.; Frary A.; Field-Rayner J.; Papalouka V.; Kane J.; Benjamin A. J. V.; Gill A. B.; Priest A. N.; Lewis D. Y.; Russell R.; Grimmer A.; White B.; Latimer-Bowman B.; Patterson I.; Schiller A.; Carmo B.; Slough R.; Lanz T.; Wason J.; Schulte R. F.; Chin S.-F.; Graves M. J.; Gilbert F. J.; Abraham J. E.; Caldas C.; Brindle K. M.; Sala E.; Gallagher F. A. Hyperpolarized Carbon-13 MRI for Early Response Assessment of Neoadjuvant Chemotherapy in Breast Cancer Patients. Cancer Res. 2021, 81, 6004–6017. 10.1158/0008-5472.CAN-21-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardenkjær-Larsen J. H.; Fridlund B.; Gram A.; Hansson G.; Hansson L.; Lerche M. H.; Servin R.; Thaning M.; Golman K. Increase in Signal-to-Noise Ratio of > 10,000 Times in Liquid-State NMR. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 10158–10163. 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurhanewicz J.; Vigneron D. B.; Ardenkjaer-Larsen J. H.; Bankson J. A.; Brindle K.; Cunningham C. H.; Gallagher F. A.; Keshari K. R.; Kjaer A.; Laustsen C.; Mankoff D. A.; Merritt M. E.; Nelson S. J.; Pauly J. M.; Lee P.; Ronen S.; Tyler D. J.; Rajan S. S.; Spielman D. M.; Wald L.; Zhang X.; Malloy C. R.; Rizi R. Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019, 21, 1–16. 10.1016/j.neo.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hövener J.-B.; Pravdivtsev A. N.; Kidd B.; Bowers C. R.; Glöggler S.; Kovtunov K. V.; Plaumann M.; Katz-Brull R.; Buckenmaier K.; Jerschow A.; Reineri F.; Theis T.; Shchepin R. V.; Wagner S.; Bhattacharya P.; Zacharias N. M.; Chekmenev E. Y. Parahydrogen-Based Hyperpolarization for Biomedicine. Angew Chem Int Ed 2018, 57, 11140–11162. 10.1002/anie.201711842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel L.; Gierse M.; Gottwald W.; Ahmadova Z.; Grashei M.; Wolff P.; Josten F.; Karaali S.; Müller C. A.; Lucas S.; Scheuer J.; Müller C.; Blanchard J.; Topping G. J.; Wendlinger A.; Setzer N.; Sühnel S.; Handwerker J.; Vassiliou C.; van Heijster Frits H. A.; Knecht S.; Keim M.; Schilling F.; Schwartz I. Parahydrogen-Polarized [1–13C]Pyruvate for Reliable and Fast Preclinical Metabolic Magnetic Resonance Imaging. Advanced Science 2023, 10, 2303441. 10.1002/advs.202303441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eills J.; Hale W.; Utz M. Synergies Between Hyperpolarized NMR and Microfluidics: A Review. Prog. Nucl. Magn. Reson. Spectrosc. 2022, 128, 44–69. 10.1016/j.pnmrs.2021.09.001. [DOI] [PubMed] [Google Scholar]
- Bowers C. R.; Weitekamp D. P. Parahydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alignment. J. Am. Chem. Soc. 1987, 109, 5541–5542. 10.1021/ja00252a049. [DOI] [Google Scholar]
- Duckett S. B.; Sleigh C. J. Applications of the Parahydrogen Phenomenon: A Chemical Perspective. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 34, 71–92. 10.1016/S0079-6565(98)00027-2. [DOI] [Google Scholar]
- Reineri F.; Cavallari E.; Carrera C.; Aime S. Hydrogenative-PHIP Polarized Metabolites for Biological Studies. MAGMA 2021, 34, 25–47. 10.1007/s10334-020-00904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eills J.; Hale W.; Sharma M.; Rossetto M.; Levitt M. H.; Utz M. High-Resolution Nuclear Magnetic Resonance Spectroscopy with Picomole Sensitivity by Hyperpolarization on a Chip. J. Am. Chem. Soc. 2019, 141, 9955–9963. 10.1021/jacs.9b03507. [DOI] [PubMed] [Google Scholar]
- Barker S. J.; Dagys L.; Hale W.; Ripka B.; Eills J.; Sharma M.; Levitt M. H.; Utz M. Direct Production of a Hyperpolarized Metabolite on a Microfluidic Chip. Anal. Chem. 2022, 94, 3260–3267. 10.1021/acs.analchem.1c05030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowska S. J.; Rana A.; Utz M. Spatially Resolved Kinetic Model of Parahydrogen Induced Polarisation (PHIP) in a Microfluidic Chip. ChemPhysChem 2021, 22, 2004–2013. 10.1002/cphc.202100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevanato G.; Eills J.; Bengs C.; Pileio G. A Pulse Sequence for Singlet to Heteronuclear Magnetization Transfer: S2hM. J. Magn. Reson. 2017, 277, 169–178. 10.1016/j.jmr.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Ripka B.; Eills J.; Kouřilová H.; Leutzsch M.; Levitt M. H.; Münnemann K. Hyperpolarized Fumarate via Parahydrogen. Chem. Commun. 2018, 54, 12246–12249. 10.1039/C8CC06636A. [DOI] [PubMed] [Google Scholar]
- Stewart N. J.; Nakano H.; Sugai S.; Tomohiro M.; Kase Y.; Uchio Y.; Yamaguchi T.; Matsuo Y.; Naganuma T.; Takeda N.; Nishimura I.; Hirata H.; Hashimoto T.; Matsumoto S. Hyperpolarized 13C Magnetic Resonance Imaging of Fumarate Metabolism by Parahydrogen-induced Polarization: A Proof-of-Concept in vivo Study. ChemPhysChem 2021, 22, 915–923. 10.1002/cphc.202001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S.; Blanchard J. W.; Barskiy D.; Cavallari E.; Dagys L.; Van Dyke E.; Tsukanov M.; Bliemel B.; Münnemann K.; Aime S.; Reineri F.; Levitt M. H.; Buntkowsky G.; Pines A.; Blümler P.; Budker D.; Eills J. Rapid Hyperpolarization and Purification of the Metabolite fumarate in Aqueous Solution. Proc. Natl. Acad. Sci. U.S.A. 2021, 118, e2025383118 10.1073/pnas.2025383118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eills J.; Cavallari E.; Carrera C.; Budker D.; Aime S.; Reineri F. Real-Time Nuclear Magnetic Resonance Detection of Fumarase Activity Using Parahydrogen-Hyperpolarized [1-13C]Fumarate. J. Am. Chem. Soc. 2019, 141, 20209–20214. 10.1021/jacs.9b10094. [DOI] [PubMed] [Google Scholar]
- Wienands L.; Theiß F.; Eills J.; Rösler L.; Knecht S.; Buntkowsky G. Optimizing the Reaction Conditions for the Formation of Fumarate via Trans-Hydrogenation. Appl. Magn. Reson. 2022, 53, 615–634. 10.1007/s00723-021-01371-w. [DOI] [Google Scholar]
- Kating P.; Wandelt A.; Selke R.; Bargon J. Nuclear Singlet/Triplet Mixing During Hydrogenations with Parahydrogen: an in situ NMR Method to Investigate Catalytic Reaction Mechanisms and Their Kinetics. 2. Homogeneous Hydrogenation of 1,4-dihydro-1,4-epoxynaphthalene Using Different Rhodium Catalysts. J. Phys. Chem. 1993, 97, 13313–13317. 10.1021/j100152a040. [DOI] [Google Scholar]
- Markelov D. A.; Kozinenko V. P.; Knecht S.; Kiryutin A. S.; Yurkovskaya A. V.; Ivanov K. L. Singlet to Triplet Conversion in Molecular Hydrogen and its Role in Parahydrogen Induced Polarization. Phys. Chem. Chem. Phys. 2021, 23, 20936–20944. 10.1039/D1CP03164C. [DOI] [PubMed] [Google Scholar]
- Bargon J.; Kandels J.; Kating P. Nuclear Magnetic Resonance Studies of Homogeneous Catalysis Using Parahydrogen: Analysis of Nuclear Singlet–Triplet Mixing as a Diagnostic Tool to Characterize Intermediates. J. Chem. Phys. 1993, 98, 6150–6153. 10.1063/1.464853. [DOI] [Google Scholar]
- Dagys L.; Bengs C.; Levitt M. H. Low-frequency Excitation of Singlet–Triplet Transitions. Application to Nuclear Hyperpolarization. J. Chem. Phys. 2021, 155, 154201. 10.1063/5.0065863. [DOI] [PubMed] [Google Scholar]
- Ostrowska S.; Dagys L.; Utz M.; Levitt M.. Raw Data for: Efficient Parahydrogen Induced 13C Hyperpolarization on a Microfluidic Device. https://zenodo.org/records/11108345. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw experimental and simulation data have been deposited on zenodo.org, organized by figure.52