Abstract
Disruption of the blood-central nervous system barrier (BCB) is increasingly recognized as a pathological factor in diseases and trauma of the central nervous system. Despite the neuropathological impact, current treatment modalities do not target the BCB; strategies to reconstitute the impaired BCB have been restricted to nutritional and dietary remedies. As an integral cell type in the neurovascular unit, pericytes are crucial to the development, maintenance, and repair of the BCB. As such, pericytes are well poised as cellular agents for reconstitution of the impaired BCB. Here, we summarize recent revelations regarding the role of BCB disruption in diseases and trauma of the central nervous system and highlight how pericytes are harnessed to provide targeted therapeutic effect in each case. This review will also address how recent advances in pericyte derivation strategies can serve to overcome practical hurdles in the clinical use of pericytes.
Keywords: Pericytes, Cell therapy, Blood-CNS barrier, Adult stem cells
Highlights
-
•
Disruption of the blood-central nervous system barrier (BCB) underlies diverse CNS disorders.
-
•
Pericytes are integral to the development, maintenance, and repair of the BCB.
-
•
The efficacy of pericyte transplantation is well supported by in vitro and rodent models.
-
•
Advances in pericyte derivation strategies overcome hurdles in the clinical use of pericytes.
1. Introduction
Disruption of the blood-central nervous system barrier (BCB) has been overlooked as an etiological factor in many central nervous system (CNS) disease (Sweeney et al., 2018). Given the neuropathological implications, re-examination of strategies that restore the BCB may open the way for new and effective treatments. In neurodegenerative diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS), loss of BCB integrity precedes the emergence of behavioral symptoms linked to events that culminate in neuron death (Bartels et al., 2008, Desai et al., 2007, Erickson and Banks, 2013, Kim et al., 2020, Winkler et al., 2014b, Zenaro et al., 2017). In acute CNS disorders such as stroke or spinal cord injury, BCB breakdown in the early phase of injury plays key roles in delaying, and even preventing, neurological recovery in patients (Blume et al., 2020, Cohen et al., 2009, Lee et al., 2012, Nadareishvili et al., 2019, Okada et al., 2020).
Recently, transplantation of pericytes for the treatment of neurovascular disruptions has gained interest due to the ability of pericytes in promoting regeneration and restoration of the BCB (Zhu et al., 2022). With pericyte transplantation in animal models of ischemic stroke and Alzheimer’s disease, reports indicated restoration of BCB and associated functions (Hou et al., 2020, Sun et al., 2020, Tachibana et al., 2018). However, the clinical effectiveness of pericyte transplantation in human patients remains to be established. In this review, we will explore pericyte transplantation as a novel therapeutic intervention to reconstitute the disrupted BCB and discuss practical considerations for clinical application of the strategy in CNS diseases.
2. What are pericytes?
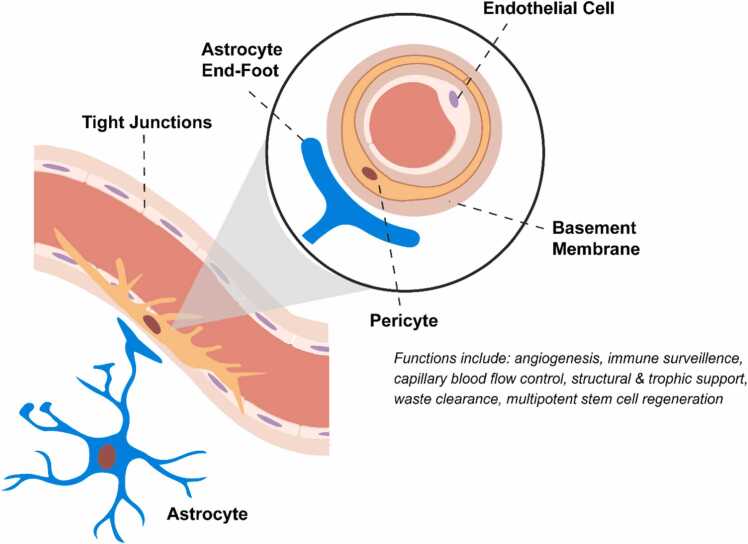
Located at the interface between the CNS parenchyma and vasculature, CNS pericytes are mobile, multipotent, and specialized CNS vascular cells embedded in the basement membrane of capillaries, pre-capillary arterioles, and post-capillary venules throughout the body (Armulik et al., 2010, Attwell et al., 2016, Daneman et al., 2010, Laredo et al., 2019, Sweeney et al., 2016). In general, vascular pericytes regulate angiogenesis (Ribatti et al., 2011), capillary blood flow (Hartmann et al., 2021), inflammation (Thomas et al., 2017), waste clearance (Gouveia-Freitas and Bastos-Leite, 2021), and stem cell regeneration (Ahmed and El-Badri, 2018, Song et al., 2022) in an organ- and tissue-specific manner. In the CNS, specialized CNS pericytes form the BCB and provide structural & trophic support to maintain the BCB (Fig. 1). Non-fenestrated endothelial cells that ensheathe astrocyte end-feet together with CNS pericytes embedded in the basement membrane form the BCB which not only prevents non-selective transport but also facilitates selective transport of solutes across the BCB. Specialized CNS pericytes will be referred simply as pericytes henceforth.
Fig. 1.
Components of the blood-CNS barrier and CNS pericyte functions. Situated at the interface between the vascular system and the CNS, CNS pericytes embedded in the basement membrane are a crucial part of the neurovascular unit.
2.1. Pericytes at the BCB
Pericytes play a significant role in forming the BCB during development and maintaining barrier properties of the mature BCB. By embryonic day 12 in rats, a functional BCB across nascent blood vessels in the neural tissue was observed with specialized endothelial cells that express BCB-specific influx and efflux transporters (Daneman et al., 2010). The onset of barrier function was coincident with the recruitment of pericytes to the vasculature. In mice, BCB permeability was further found to correlate with vessel coverage by pericytes (Daneman et al., 2010, Lindahl et al., 1997).
The role that pericytes play in maintaining BCB integrity is nuanced given that deficiency in pericytes did not directly lead to downregulation of BBB-specific genes in endothelial cells, but instead resulted in upregulation of genes associated with leukocyte infiltration and transcytosis (Daneman et al., 2010). Endothelial cells in pericyte-deficient mice showed increase in cytoplasmic vesicles. Although tight junction proteins remained observable in such vessels, they were misaligned with the vascular lumen (Daneman et al., 2010). In such mice, defective maturation of astrocyte end-feet was found to correlate with mis-localization of aquaporin 4 (AQP4) to pericytes that had detached from blood vessels (Armulik et al., 2010). The findings indicate that pericytes direct astrocyte end-feet polarization to the blood vessels (Armulik et al., 2010). A mechanistic link between pericyte loss and accumulation of such pathological protein aggregates as hyperphosphorylated tau (p-Tau) and Aβ was thus revealed. Correct localization of AQP4 is thus essential for maintaining interstitial flow which in turn mediates lymphatic clearance of metabolic waste and pathological proteins (Mestre et al., 2018, Sagare et al., 2013, Silva et al., 2021, Winkler et al., 2014a).
Apart from maintenance of BCB integrity, pericytes in the CNS carry out other important functions such as facilitating vascular remodeling, controlling cerebral blood flow, and modulating inflammation. Endothelial cells secrete platelet-derived growth factor BB (PDGF-BB), which binds to platelet-derived growth factor receptor beta (PDGFRβ) on pericytes, activating the Ras/Raf/MEK/ERK cascade leading to pericyte detachment and chemotactic migration towards PDGF-BB (Laredo et al., 2019). Migrating pericytes in turn secrete vascular endothelial growth factor (VEGF), which induces migration and proliferation of endothelial cells, resulting in angiogenesis (Darland et al., 2003). Upon stimulation by glutamate associated with neuronal activity, contractile pericytes induce capillary dilation; it is estimated that up to 84% of the increase in cerebral blood flow in response to neuronal activity is due to brain capillary dilation mediated by CNS pericytes (Hall et al., 2014). CNS pericytes also facilitate inflammatory responses in the brain, chiefly by facilitating transmigration of leukocytes through the BCB. It was shown in pericyte-deficient mouse models that leukocyte infiltration was inversely proportional to pericyte coverage, and upon experimental autoimmune encephalomyelitis (EAE) these mice were more susceptible to death arising from aggravated neuroinflammation caused by influx of leukocytes (Torok et al., 2021).
2.2. Pericyte markers & subtypes
No definitive set of pericyte/ pericyte subtype markers has yet to be agreed upon by the scientific community. This is largely due to the heterogeneity of cells that make up the vasculature. In general, pericytes express PDGFRβ, NG2, and CD146, and are distinguished from perivascular fibroblasts by their absence in the interstitial space (Vanlandewijck et al., 2018). αSMA expression on pericytes indicate a smooth muscle cell-like pericyte subtype that is located in pre-capillary arteriole and post-capillary venules, controlling cerebral blood flow (Vanlandewijck et al., 2018). On the other hand, lack of αSMA expression indicates a mid-capillary pericyte subtype that is more associated with BCB function (Chasseigneaux et al., 2018). That being said, both αSMA+ and αSMA– pericyte subtypes are required to form and maintain the BCB (Wilhelm et al., 2016, Zhao et al., 2015). Furthermore, GLAST Type 1+ (GLAST1+) pericytes, which supposedly make up 10% of the pericyte population in the CNS, are known to proliferate during CNS injury and form the core of the glial scar (Dias et al., 2021, Dias et al., 2018). However, single cell transcriptomics (scRNAseq) studies using human and mouse brains did not reveal such pericyte subtype, instead GLAST expression was found to be highly upregulated in astrocytes and perivascular fibroblasts (Gastfriend et al., 2021, Vanlandewijck et al., 2018).
3. Pericyte derivation & transplantation
3.1. Pericyte origin in development
Lineage tracing and quail-chick chimera studies have shown that brain pericytes arise from the neural crest, while those in the brainstem and spinal cord are of a mutually exclusive mesoderm origin (Etchevers et al., 2001). This may partly explain the lower permeability of the blood-brain barrier compared to that of the blood-spinal cord barrier (Bartanusz et al., 2011). There is also evidence that neural crest-derived mesenchymal stem cells (MSCs) in the bone marrow serves as a reservoir of pericyte progenitor cells, which may migrate to sites of vascular remodeling and give rise to pericytes and smooth muscle cells (Coste et al., 2017, Isern et al., 2014, Lamagna and Bergers, 2006). However, the neural crest origin of MSCs and CNS pericyte progenitors in the bone marrow has yet to be validated by definitive lineage tracing studies. Yet another subset of CNS pericytes arises from mature macrophages during development, highlighting the multi-lineage nature of CNS pericytes (Yamamoto et al., 2017).
3.2. Pericyte derivation & cell sources
At present, pericyte differentiation is induced by commercial MSC-supporting medium containing fetal bovine serum (FBS), supplemented with a combination of PDGF-BB, basic fibroblast growth factor (bFGF), or transforming growth factor β (TGF-β) (Faal et al., 2019, Stebbins et al., 2019, Sun et al., 2020, Vidal et al., 2015). Binding of TGF-β to TGFβR2 in pericytes results in the phosphorylation of Alk5 and activation of the Smad signaling cascade. This in turn promotes differentiation of progenitors into pericytes, as well as maturation, proliferation, and migration of pericyte cells (Sweeney et al., 2016). After establishment of pericyte cell-fate, binding of PDGF-BB to PDGFRs on pericytes activates several downstream signaling pathways involving Stat5, Ras/MAPK, PI3K/Akt, and PLCγ, resulting in pericyte proliferation, survival, and migration (Smyth et al., 2022, Sweeney et al., 2016). Similarly, bFGF is known to activate RAS/MAPK, PI3k/AKT, and PLCγ pathways (Goldfarb, 2001). Though the precise mechanism is not well understood, bFGF works in complement with PDGF-BB by upregulating PDGFRβ expression in pericytes (Nakamura et al., 2016, Nissen et al., 2007).
Such a strategy was used to obtain pericytes from embryonic mouse MSCs (Tachibana et al., 2018). Pericytes were also obtained from human pluripotent stem cells (hPSC) or human induced pluripotent stem cells (iPSCs)-derived from human embryonic fibroblasts after initial neural crest fate induction (Faal et al., 2019, Stebbins et al., 2019, Sun et al., 2020). Pericyte induction from these neural crest-like cells can then proceed with addition of bFGF and PDGF-BB. Theoretically, any accessible adult tissue harboring mesoderm- or neural crest-derived stem cells can be harnessed to produce pericytes, though cranial neural crest-derived stem cells may be advantageous for subsequent transplantation into the brain (Etchevers et al., 2001). With that said, autologous pericytes derived from mature adipose tissue have been successfully engrafted into the brain vasculature in canines, raising the possibility that non-cranial neural crest-derived pericytes may also home to the cerebral vasculature to confer therapeutic benefits (Youn et al., 2015).
Outside the context of CNS diseases, pericytes derived or culture expanded from bone marrow, adipose tissue, skeletal muscles, cochlear ducts, saphenous vein, and kidneys have demonstrated therapeutic effects upon transplantation in various diseases models by facilitating tissue repair and modulating blood flow (Amos et al., 2011, Hou et al., 2020, Katare et al., 2011, Konig et al., 2016, Munroe et al., 2019, Song et al., 2022, Youn et al., 2015). Successful induction of brain pericytes from these clinically accessible sources of MSCs will be a significant step towards pericyte transplantation as therapy.
Addition of PDGF-BB, bFGF, and TGF-β is insufficient for deriving pericytes as pericyte induction fails without FBS, meaning there are other growth factors & compounds that support pericyte growth that remain unknown (Stebbins et al., 2019). This highlights the possibility to improve derivation protocols for pericytes. Collection of scRNAseq data from pericyte derivation studies can inform more rapid and higher purity differentiation protocols by identifying druggable targets and growth factors that upregulate enriched pathways associated with pericyte differentiation (Cai et al., 2022, Wu et al., 2021). Conducting pathway enrichment analysis with available scRNAseq data on in vivo pericytes and cross-referencing the results with the proteomic profile of FBS may help researchers identify key factors involved in pericyte-fate determination to construct an efficient and standardized serum-free pericyte induction medium, which will expedite quality control and accelerate clinical translation (Reimand et al., 2019, Zheng et al., 2006). These recent advances in omics technology and data processing will be instrumental for optimizing and standardizing pericyte derivation protocols that are necessary for clinical translation (Roddie et al., 2019).
3.3. Transplantation routes
Both intravenous and intracranial delivery of pericytes have led to efficient cell engraftment and therapeutic benefits in rodent models of ischemic stroke and Alzheimer’s disease, respectively (Sun et al., 2020, Tachibana et al., 2018). However, it is difficult to conclusively determine the optimal method of pericyte delivery in various CNS disease contexts due to the lack of comparative studies. There is a plethora of studies on differential MSC engraftment rates and efficacy depending on transplantation route, which can be used as reference. Minimally invasive delivery routes, such as intra-venous or intra-arterial injection through the carotid artery are often favored as they have less risk than direct injection to neural tissue. Furthermore, cell delivery via the circulatory system allows repeated transplantations (Fricova et al., 2020, Hernandez and Garcia, 2021, Turnbull et al., 2019). However, studies reported that MSCs delivered via the intravenous route are mostly retained in the lungs, resulting in reduced engraftment in CNS tissue (Cerri et al., 2015, Fischer et al., 2009, Li et al., 2021). Therefore these methods generally had lower efficacy and reduced homing to target tissues compared to more invasive, direct routes of delivery, such as intralesional, intracerebral, or intraspinal injections (Jin et al., 2005). On the other hand, surgical transplantation of cells into the neural tissue suffer from inherent surgery-related risks and possible post-surgical complications, which make multiple transplantation undesirable and costly (Boltze et al., 2015).
As such, invasive yet more effective routes of delivery are favored for traumatic or acute CNS diseases where operative care is necessary regardless of whether cell therapy is undertaken, while minimally invasive methods of cell transplantation are favored for chronic degenerative CNS diseases in which multiple injections may be required and surgery may exacerbate neurological damage (Boltze et al., 2015, Hernandez and Garcia, 2021). Recent studies have also shown that delivery routes such as intranasal delivery or intrathecal injection can still attain low peripheral retainment, while offering similar efficacy and homing ability as direct transplantation into the brain and spinal cord (Danielyan et al., 2014, Danielyan et al., 2009, de Araujo et al., 2022, Liau et al., 2020).
Given that MSCs and pericyte cells are both cellular agents that share similar marker expression and functional profile (Crisan et al., 2008, Dore-Duffy et al., 2006), and that pericytes have superior migratory and chemotactic properties compared to MSCs, it is expected that pericytes will show better homing compared to MSCs. Indeed, a recent study showed that a subset of placental MSCs with significantly enhanced migratory and angiogenic properties is marked by PDGFRβ expression, the canonical marker for pericytes (Wang et al., 2018). Though this subject remains controversial, tissues sources of what were regarded as MSCs may in fact harbor pericytes or pericyte progenitor cells that are highly migratory and can robustly home to vascular beds throughout the body and in the CNS (Caplan, 2008, de Souza et al., 2016).
4. Value of pericyte transplantation in various CNS diseases and trauma
The BCB guards against entry of blood components into the CNS - exposure to serum can initiate neuronal death, whereas influx of blood cells or clotting factors causes oxidative stress and instigates inflammatory cascades that ultimately lead to apoptosis (Garcia et al., 1992, Stokum et al., 2021). Despite the catastrophic consequences of BCB breakdown, there are limited treatment options to protect or restore the BCB in various CNS disorders that are characterized by chronic or acute BCB disruption. In this final section, we explore how pericyte transplantation may be beneficial towards such CNS disorders.
4.1. Neurodegenerative diseases
Signs of BCB dysfunction in the brain can be detected early on in AD patients, preceding cognitive decline (Montagne et al., 2017). In a post-mortem study, AD brain samples had significantly lower pericyte vascular coverage in the cortex and hippocampus compared to healthy adults and the lack of pericyte coverage correlated with the severity of serum protein extravasation, an indicator of BCB breakdown (Sengillo et al., 2013). AD progression in patients with the major risk allele apolipoprotein E4 (APOE4) is also characterized by accelerated CNS pericyte degradation (Halliday et al., 2016). On the other hand, while a causative link between pericyte deficiency or dysfunction and PD has yet to be established, significant BCB disruption characterized by serum extravasation and disruption of BCB efflux is observed clinically (Bartels et al., 2008, Gray and Woulfe, 2015).
Nonetheless, pericyte transplantation may help increase pericyte coverage in AD and PD patients to restore BCB integrity, reduce serum extravasation, and normalize efflux (Sun et al., 2020). Moreover, pericyte transplantation have been shown to increase low density lipoprotein receptor-related protein 1 (LRP1)-mediated Aβ clearance in mouse models of AD (Tachibana et al., 2018).
Pervasive BCB breakdown is also observed in post-mortem spinal cord samples obtained from ALS and multiple sclerosis (MS) patients (Garbuzova-Davis et al., 2012, Kirk et al., 2003, Ortiz et al., 2014, Winkler et al., 2013). This was characterized by pericyte degeneration, edema, extravasation of IgG, and reduced number of tight junctions in sporadic ALS patients, and tissue accumulation of serum proteins and hemoglobin deposits in MS patients. The extent of extravasation was correlated with reduced pericyte coverage (Garbuzova-Davis et al., 2012, Winkler et al., 2013). In a familial ALS mouse model harbouring superoxide dismutase 1 (SOD1) mutation, BCB damage is observed early on, with motor-neuron dysfunction proportional to the extent of BCB damage (Winkler et al., 2014b). Early dysfunction of BCB in MS patients has been attributed to pathological activation of lymphocytes and release of proinflammatory cytokines (Kirk et al., 2003, Ortiz et al., 2014). In a EAE mouse model of MS, regions with fibrinogen leakage due to compromised BCB induced rapid microglial activation that resulted in the release of reactive oxygen species and axonal damage (Davalos et al., 2012). Notably, recent studies show that pericyte presence is crucial for differentiation of oligodendrocyte precursor cells (OPC) and remyelination (Azevedo et al., 2018, de La Fuente et al., 2017). Thus pericyte transplantation in ALS and MS not only has the potential to reverse BCB damage thereby reducing cell death resulting from serum and leukocyte extravasation, the action of pericytes in supporting OPC differentiation and remyelination has potential to counteract autoimmune destruction of myelin in MS.
4.2. Stroke
Apart from preserving brain function in neurodegenerative diseases, pericyte transplant can also alleviate progression of acute neural trauma. At the onset of ischemic stroke, tissue ischemia causes cytotoxic edema and rapid loss of pericytes, leading to gradual BBB breakdown (Fernandez-Klett et al., 2013, Krueger et al., 2019, Schellinger and Warach, 2004). During the acute phase (6 h – 6 days), reperfusion therapy is conducted and BBB permeability peaks as a result of neuroinflammation and reperfusion injury. This is followed by decreased BBB permeability during the sub-acute phase (1 – 3 weeks) and gradual stabilization in the chronic phase (> 6 weeks) (Bernardo-Castro et al., 2020). In contrast, intracerebral hemorrhage (ICH) results in immediate BCB breakdown, leading to hematoma expansion in the first 24 h (Brott et al., 1997). This is followed by secondary brain injury that is characterized by delayed BCB permeability as caused by oxidative stress, release of proinflammatory cytokines, and edema (Aiyagari, 2015, Keep et al., 2014). Interestingly, elevated BBB permeability during the sub-acute phase is beneficial towards angiogenesis for revascularization, and thus reduce cerebral infarction and improve prospects of neurological recovery in patients (Rust et al., 2019, Tang et al., 2007, Yin et al., 2015).
Although this remains contested, endogenous pericytes can undergo pathological activation and act to restrict cerebral blood flow during ischemic stroke onset, impeding reperfusion after recanalization therapy (Vates et al., 2010, Yemisci et al., 2009). As such, while pericyte delivery during the acute phase can potentially attenuate inflammation and jump-start BCB reconstitution, exposure of transplanted pericytes to the acute post-stroke microenvironment may result in their undesirable pathological activation. Perhaps the optimal timepoint for pericyte transplant lies between the acute and sub-acute phases when the transplanted cells can still stimulate recovery but are less likely to be pathologically activated. In mice, pericytes injected 3-day post-ischemia were still able to bolster pericyte vessel coverage, enhance BCB integrity, and improve behavior (Sun et al., 2020). This supports the notion that immediate pericyte transplant might not always be the optimal solution.
Interestingly, pericyte loss has been indicated as a key risk factor for intracerebral hemorrhage, with loss of pericytes shown to induce microhemorrhages in humans and rodents (Tang et al., 2007, Winkler et al., 2018). Pericyte degeneration is also involved in other risk factors of intracerebral hemorrhage such as hypertension and cerebral amyloid angiopathy (Gatti et al., 2020, Shi et al., 2020, Tagami et al., 1990).
4.3. Neurotrauma
After an initial peak in BCB permeability after acute spinal cord injury, there is a second peak in BSCB permeability between 3 and 7 days post-injury, corresponding to a period of vascular remodeling and inflammation (Whetstone et al., 2003). After 2 weeks, spinal cord edema is generally resolved and BCB integrity begins to re-stabilize (Cho and Fehlings, 2017, Cohen et al., 2009, Kisler et al., 2017). Much like acute spinal cord injury, the acute phase of traumatic brain injury is characterized by diffuse pericyte loss, impairment of pericyte-endothelial interactions, loss of basement membrane protein and tight junctions, and vasogenic edema caused by BBB breakdown. During the subacute phase, PDGFRβ+ pericytes proliferate in the trauma zone and contribute to glial scarring (Bhowmick et al., 2019, Sakai et al., 2021, Zehendner et al., 2015).
In traumatic encephalopathy (CTE), chronic BBB damage by mild repetitive brain injury is often the root cause of neurodegeneration and pursuant dementia and schizophrenia over time (Doherty et al., 2016). In CTE patients, regions of BCB damage evidenced by decreased number of tight junctions and extravasation of serum coincided with regions with p-Tau deposition (Farrell et al., 2019). Though pericyte loss has not yet been explored in the context of CTE, pericyte degeneration can be expected considering the BCB damage and accumulation of p-Tau. In chronic spinal cord myelopathy, signs of BCB damage can be observed in patients with moderate to severe compression and after decompression surgery (Blume et al., 2020, Hasegawa et al., 2007, Tachibana et al., 2019). As in the case for ischemic stroke, increased BCB permeability after decompression results from reperfusion injury and vascular remodeling (Figley et al., 2014, Nishinaka et al., 2018, Vidal et al., 2017, Yang and Torbey, 2020).
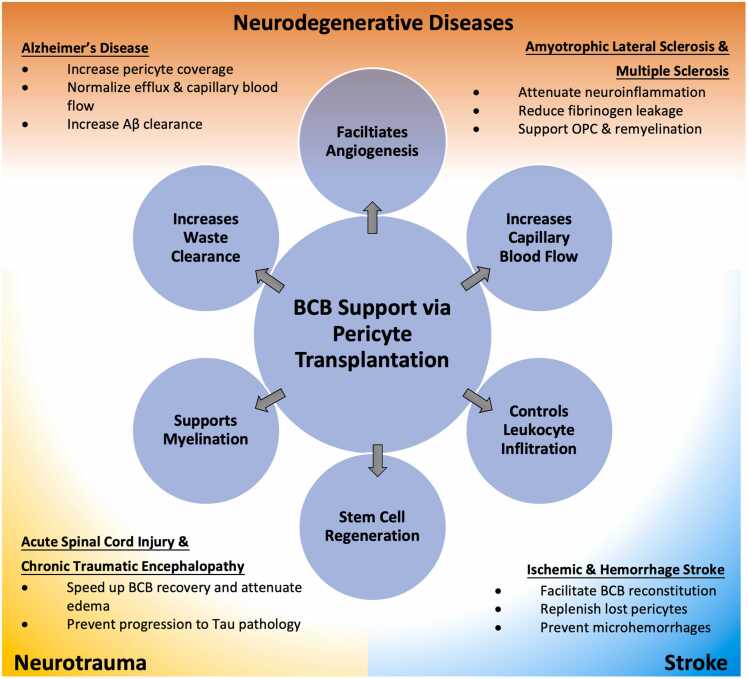
The role of pericytes in disease pathophysiology of neurotrauma is complex. There is evidence that pericytes may induce spinal cord ischemia through contraction during spinal cord injury and may impede neurological recovery by promoting fibrotic scarring (Almeida et al., 2018, Picoli et al., 2019). Transplantation of pericytes may also introduce a subset of GLAST+ pericytes that can hyperproliferate and contribute to astrogliosis (Dias et al., 2021). To mitigate this, pre-purification of pericytes with FACS or selective culture expansion of GLAST– and αSMA– pericyte subtypes may be necessary before transplantation. Given that survival of transplanted pericytes is likely low during the acute phase of spinal cord injury or traumatic brain injury, transplantation of GLAST– and αSMA– pericytes in the subacute phase is a testable alternative for improving prospects of BCB reconstitution and vascular repair (Fig. 2).
Fig. 2.
Therapeutic effects of pericyte transplantation and its clinical applications. Pericyte loss or dysfunction is observed in neurodegenerative diseases, neurotrauma, and stroke. Due to its multifaceted functional profile and high relevance in various CNS pathology, pericyte transplantation is an attractive therapeutic agent that is also broadly applicable.
5. Conclusion
Diverse CNS disorders are characterized by BCB disruption, with early loss of pericytes often preceding appearance of neurological symptoms. While pericytes modulate inflammation and clearance of CNS waste, they themselves are highly sensitive to proinflammatory cytokines and susceptible to toxic protein aggregates (Berthiaume et al., 2018, Yamanaka et al., 2021). This suggests the possibility of a vicious cycle whereby pericyte degeneration caused by chronic inflammation and accumulation of pathological protein aggregates in early neurodegeneration starts a “snowballing” effect that further exacerbates pericyte loss, vascular pathology and BCB breakdown (Berthiaume et al., 2018). Transplantation of functional CNS pericytes represents a possibility to break the cycle and thus to allow for recovery from CNS disorders. With recent advances in in vitro pericyte induction from clinically accessible sources, further preclinical & clinical exploration in various CNS disease contexts is warranted.
Contributor Information
Kenneth Lap Kei Wu, Email: kennethlkwu@gmail.com.
Daisy Kwok-Yan Shum, Email: shumdkhk@hku.hk.
References
- Ahmed T.A., El-Badri N. Pericytes: The role of multipotent stem cells in vascular maintenance and regenerative medicine. Adv. Exp. Med Biol. 2018;1079:69–86. doi: 10.1007/5584_2017_138. [DOI] [PubMed] [Google Scholar]
- Aiyagari V. The clinical management of acute intracerebral hemorrhage. Expert Rev. Neurother. 2015;15:1421–1432. doi: 10.1586/14737175.2015.1113876. [DOI] [PubMed] [Google Scholar]
- Almeida V.M., Paiva A.E., Sena I.F.G., Mintz A., Magno L.A. v, Birbrair A. Pericytes Make Spinal Cord Breathless after Injury. Neuroscientist. 2018;24:440–447. doi: 10.1177/1073858417731522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos P.J., Mulvey C.L., Seaman S.A., Walpole J., Degen K.E., Shang H., Katz A.J., Peirce S.M. Hypoxic culture and in vivo inflammatory environments affect the assumption of pericyte characteristics by human adipose and bone marrow progenitor cells. Am. J. Physiol. Cell Physiol. 2011;301 doi: 10.1152/ajpcell.00460.2010. C1378-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A., Genove G., Mae M., Nisancioglu M.H., Wallgard E., Niaudet C., He L., Norlin J., Lindblom P., Strittmatter K., Johansson B.R., Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Attwell D., Mishra A., Hall C.N., O’Farrell F.M., Dalkara T. What is a pericyte? J. Cereb. Blood Flow. Metab. 2016;36:451–455. doi: 10.1177/0271678X15610340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo P.O., Sena I.F.G., Andreotti J.P., Carvalho-Tavares J., Alves-Filho J.C., Cunha T.M., Cunha F.Q., Mintz A., Birbrair A. Pericytes modulate myelination in the central nervous system. J. Cell Physiol. 2018;233:5523–5529. doi: 10.1002/jcp.26348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartanusz V., Jezova D., Alajajian B., Digicaylioglu M. The blood-spinal cord barrier: morphology and clinical implications. Ann. Neurol. 2011;70:194–206. doi: 10.1002/ana.22421. [DOI] [PubMed] [Google Scholar]
- Bartels A.L., Willemsen A.T., Kortekaas R., de Jong B.M., de Vries R., de Klerk O., van Oostrom J.C., Portman A., Leenders K.L. Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson’s disease, PSP and MSA. J. Neural Transm. (Vienna) 2008;115:1001–1009. doi: 10.1007/s00702-008-0030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo-Castro S., Sousa J.A., Bras A., Cecilia C., Rodrigues B., Almendra L., Machado C., Santo G., Silva F., Ferreira L., Santana I., Sargento-Freitas J. Pathophysiology of blood-brain barrier prmeability throughout the different stages of ischemic stroke and its implication on hemorrhagic transformation and recovery. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.594672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiaume A.A., Hartmann D.A., Majesky M.W., Bhat N.R., Shih A.Y. Pericyte structural remodeling in cerebrovascular health and homeostasis. Front Aging Neurosci. 2018;10 doi: 10.3389/fnagi.2018.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick S., D’Mello V., Caruso D., Wallerstein A., Abdul-Muneer P.M. Impairment of pericyte-endothelium crosstalk leads to blood-brain barrier dysfunction following traumatic brain injury. Exp. Neurol. 2019;317:260–270. doi: 10.1016/j.expneurol.2019.03.014. [DOI] [PubMed] [Google Scholar]
- Blume C., Geiger M.F., Brandenburg L.O., Muller M., Mainz V., Kalder J., Albanna W., Clusmann H., Mueller C.A. Patients with degenerative cervical myelopathy have signs of blood spinal cord barrier disruption, and its magnitude correlates with myelopathy severity: a prospective comparative cohort study. Eur. Spine J. 2020;29:986–993. doi: 10.1007/s00586-020-06298-7. [DOI] [PubMed] [Google Scholar]
- Boltze J., Arnold A., Walczak P., Jolkkonen J., Cui L., Wagner D.C. The dark side of the force - constraints and complications of cell therapies for stroke. Front Neurol. 2015;6:155. doi: 10.3389/fneur.2015.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brott T., Broderick J., Kothari R., Barsan W., Tomsick T., Sauerbeck L., Spilker J., Duldner J., Khoury J. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- Cai S., Fan C., Xie L., Zhong H., Li A., Lv S., Liao M., Yang X., Su X., Wang Y., Wang H., Wang M., Huang P., Liu Y., Wang Y., Liu Y., Wang T., Zhong Y., Ma L. Single-cell RNA sequencing reveals the potential mechanism of heterogeneity of immunomodulatory properties of foreskin and umbilical cord mesenchymal stromal cells. Cell Biosci. 2022;12 doi: 10.1186/s13578-022-00848-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan A.I. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Cerri S., Greco R., Levandis G., Ghezzi C., Mangione A.S., Fuzzati-Armentero M.T., Bonizzi A., Avanzini M.A., Maccario R., Blandini F. Intracarotid infusion of mesenchymal stem cells in an animal model of Parkinson’s Disease, focusing on cell distribution and neuroprotective and behavioral effects. Stem Cells Transl. Med. 2015;4:1073–1085. doi: 10.5966/sctm.2015-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasseigneaux S., Moraca Y., Cochois-Guegan V., Boulay A.C., Gilbert A., le Crom S., Blugeon C., Firmo C., Cisternino S., Laplanche J.L., Curis E., Decleves X., Saubamea B. Isolation and differential transcriptome of vascular smooth muscle cells and mid-capillary pericytes from the rat brain. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-30739-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho N., Fehlings M.G. Chapter 14 - Spinal cord edema after spinal cord injury: from pathogenesis to management, in. brain edema Mol. Mech. Clin. Pract. 2017:261–275. doi: 10.1016/B978-0-12-803196-4.00014-X. [DOI] [Google Scholar]
- Cohen D.M., Patel C.B., Ahobila-Vajjula P., Sundberg L.M., Chacko T., Liu S.J., Narayana P.A. Blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced MRI. NMR Biomed. 2009;22:332–341. doi: 10.1002/nbm.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste C., Neirinckx V., Sharma A., Agirman G., Rogister B., Foguenne J., Lallemend F., Gothot A., Wislet S. Human bone marrow harbors cells with neural crest-associated characteristics like human adipose and dermis tissues. PLoS One. 2017;12 doi: 10.1371/journal.pone.0177962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M., Yap S., Casteilla L., Chen C.W., Corselli M., Park T.S., Andriolo G., Sun B., Zheng B., Zhang L., Norotte C., Teng P.N., Traas J., Schugar R., Deasy B.M., Badylak S., Buhring H.J., Giacobino J.P., Lazzari L., Huard J., Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Daneman R., Zhou L., Kebede A.A., Barres B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielyan L., Beer-Hammer S., Stolzing A., Schafer R., Siegel G., Fabian C., Kahle P., Biedermann T., Lourhmati A., Buadze M., Novakovic A., Proksch B., Gleiter C.H., Frey W.H., Schwab M. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer’s and Parkinson’s disease. Cell Transpl. 2014;23(Suppl 1) doi: 10.3727/096368914X684970. S123-39. [DOI] [PubMed] [Google Scholar]
- Danielyan L., Schafer R., von Ameln-Mayerhofer A., Buadze M., Geisler J., Klopfer T., Burkhardt U., Proksch B., Verleysdonk S., Ayturan M., Buniatian G.H., Gleiter C.H., Frey 2nd W.H. Intranasal delivery of cells to the brain. Eur. J. Cell Biol. 2009;88:315–324. doi: 10.1016/j.ejcb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Darland D.C., Massingham L.J., Smith S.R., Piek E., Saint-Geniez M., D’Amore P.A. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev. Biol. 2003;264:275–288. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Davalos D., Ryu J.K., Merlini M., Baeten K.M., le Moan N., Petersen M.A., Deerinck T.J., Smirnoff D.S., Bedard C., Hakozaki H., Gonias Murray S., Ling J.B., Lassmann H., Degen J.L., Ellisman M.H., Akassoglou K. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat. Commun. 2012;3 doi: 10.1038/ncomms2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araujo L.T., Macedo C.T., Damasceno P.K.F., das Neves I.G.C., de Lima C.S., Santos G.C., de Santana T.A., Sampaio G.L.A., Silva D.N., Villarreal C.F., Chaguri A.C.C., da Silva C.G., Mota A.C.A., Badaro R., Ribeiro Dos Santos R., Soares M.B.P. Clinical trials using mesenchymal stem cells for spinal cord injury: Challenges in Generating Evidence. Cells. 2022;11 doi: 10.3390/cells11061019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de La Fuente A.G., Lange S., Silva M.E., Gonzalez G.A., Tempfer H., van Wijngaarden P., Zhao C., di Canio L., Trost A., Bieler L., Zaunmair P., Rotheneichner P., O’Sullivan A., Couillard-Despres S., Errea O., Mae M.A., Andrae J., He L., Keller A., Batiz L.F., Betsholtz C., Aigner L., Franklin R.J.M., Rivera F.J. Pericytes stimulate oligodendrocyte progenitor cell differentiation during CNS remyelination. Cell Rep. 2017;20:1755–1764. doi: 10.1016/j.celrep.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza L.E., Malta T.M., Kashima Haddad S., Covas D.T. Mesenchymal stem cells and pericytes: To what extent are they related? Stem Cells Dev. 2016;25:1843–1852. doi: 10.1089/scd.2016.0109. [DOI] [PubMed] [Google Scholar]
- Desai B.S., Monahan A.J., Carvey P.M., Hendey B. Blood-brain barrier pathology in Alzheimer’s and Parkinson’s disease: implications for drug therapy. Cell Transpl. 2007;16:285–299. doi: 10.3727/000000007783464731. [DOI] [PubMed] [Google Scholar]
- Dias D.O., Kalkitsas J., Kelahmetoglu Y., Estrada C.P., Tatarishvili J., Holl D., Jansson L., Banitalebi S., Amiry-Moghaddam M., Ernst A., Huttner H.B., Kokaia Z., Lindvall O., Brundin L., Frisen J., Goritz C. Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-25585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias D.O., Kim H., Holl D., Werne Solnestam B., Lundeberg J., Carlen M., Goritz C., Frisen J. Reducing pericyte-derived scarring promotes recovery after spinal cord injury. Cell. 2018;173:153–165. doi: 10.1016/j.cell.2018.02.004. e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty C.P., O’Keefe E., Wallace E., Loftus T., Keaney J., Kealy J., Humphries M.M., Molloy M.G., Meaney J.F., Farrell M., Campbell M. Blood-brain barrier dysfunction as a hallmark pathology in chronic traumatic encephalopathy. J. Neuropathol. Exp. Neurol. 2016;75:656–662. doi: 10.1093/jnen/nlw036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore-Duffy P., Katychev A., Wang X., van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J. Cereb. Blood Flow. Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Erickson M.A., Banks W.A. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J. Cereb. Blood Flow. Metab. 2013;33:1500–1513. doi: 10.1038/jcbfm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchevers H.C., Vincent C., le Douarin N.M., Couly G.F. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development. 2001;128:1059–1068. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
- Faal T., Phan D.T.T., Davtyan H., Scarfone V.M., Varady E., Blurton-Jones M., Hughes C.C.W., Inlay M.A. Induction of mesoderm and neural crest-derived pericytes from human pluripotent stem cells to study blood-brain barrier interactions. Stem Cell Rep. 2019;12:451–460. doi: 10.1016/j.stemcr.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M., Aherne S., O’Riordan S., O’Keeffe E., Greene C., Campbell M. Blood-brain barrier dysfunction in a boxer with chronic traumatic encephalopathy and schizophrenia. Clin. Neuropathol. 2019;38:51–58. doi: 10.5414/NP301130. [DOI] [PubMed] [Google Scholar]
- Fernandez-Klett F., Potas J.R., Hilpert D., Blazej K., Radke J., Huck J., Engel O., Stenzel W., Genove G., Priller J. Early loss of pericytes and perivascular stromal cell-induced scar formation after stroke. J. Cereb. Blood Flow. Metab. 2013;33:428–439. doi: 10.1038/jcbfm.2012.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figley S.A., Khosravi R., Legasto J.M., Tseng Y.F., Fehlings M.G. Characterization of vascular disruption and blood-spinal cord barrier permeability following traumatic spinal cord injury. J. Neurotrauma. 2014;31:541–552. doi: 10.1089/neu.2013.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U.M., Harting M.T., Jimenez F., Monzon-Posadas W.O., Xue H., Savitz S.I., Laine G.A., Cox C.S., Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–692. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricova D., Korchak J.A., Zubair A.C. Challenges and translational considerations of mesenchymal stem/stromal cell therapy for Parkinson’s disease. NPJ Regen. Med. 2020;5 doi: 10.1038/s41536-020-00106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzova-Davis S., Hernandez-Ontiveros D.G., Rodrigues M.C., Haller E., Frisina-Deyo A., Mirtyl S., Sallot S., Saporta S., Borlongan C. v, Sanberg P.R. Impaired blood-brain/spinal cord barrier in ALS patients. Brain Res. 2012;1469:114–128. doi: 10.1016/j.brainres.2012.05.056. [DOI] [PubMed] [Google Scholar]
- Garcia J.E., Jr., Nonner D., Ross D., Barrett J.N. Neurotoxic components in normal serum. Exp. Neurol. 1992;118:309–316. doi: 10.1016/0014-4886(92)90188-v. [DOI] [PubMed] [Google Scholar]
- Gastfriend B.D., Foreman K.L., Katt M.E., Palecek S.P., Shusta E. v. Integrative analysis of the human brain mural cell transcriptome. J. Cereb. Blood Flow. Metab. 2021;41:3052–3068. doi: 10.1177/0271678X211013700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti L., Tinelli F., Scelzo E., Arioli F., di Fede G., Obici L., Pantoni L., Giaccone G., Caroppo P., Parati E.A., Bersano A. Understanding the pathophysiology of cerebral amyloid angiopathy. Int J. Mol. Sci. 2020;21 doi: 10.3390/ijms21103435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M. Signaling by fibroblast growth factors: the inside story. Sci. STKE. 2001;2001:pe37. doi: 10.1126/stke.2001.106.pe37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia-Freitas K., Bastos-Leite A.J. Perivascular spaces and brain waste clearance systems: relevance for neurodegenerative and cerebrovascular pathology. Neuroradiology. 2021;63:1581–1597. doi: 10.1007/s00234-021-02718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M.T., Woulfe J.M. Striatal blood-brain barrier permeability in Parkinson’s disease. J. Cereb. Blood Flow. Metab. 2015;35:747–750. doi: 10.1038/jcbfm.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C.N., Reynell C., Gesslein B., Hamilton N.B., Mishra A., Sutherland B.A., O’Farrell F.M., Buchan A.M., Lauritzen M., Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday M.R., Rege S. v, Ma Q., Zhao Z., Miller C.A., Winkler E.A., Zlokovic B. v. Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow. Metab. 2016;36:216–227. doi: 10.1038/jcbfm.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann D.A., Berthiaume A.A., Grant R.I., Harrill S.A., Koski T., Tieu T., McDowell K.P., Faino A. v, Kelly A.L., Shih A.Y. Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat. Neurosci. 2021;24:633–645. doi: 10.1038/s41593-020-00793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K., Homma T., Chiba Y. Upper extremity palsy following cervical decompression surgery results from a transient spinal cord lesion. Spine (Philos. Pa 1976) 2007;32:E197–E202. doi: 10.1097/01.brs.0000257576.84646.49. [DOI] [PubMed] [Google Scholar]
- Hernandez A.E., Garcia E. Mesenchymal stem cell therapy for Alzheimer’s Disease. Stem Cells Int. 2021;2021 doi: 10.1155/2021/7834421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z., Neng L., Zhang J., Cai J., Wang X., Zhang Y., Lopez I.A., Shi X. Acoustic trauma causes cochlear pericyte-to-myofibroblast-like cell transformation and vascular degeneration, and transplantation of new pericytes prevents vascular atrophy. Am. J. Pathol. 2020;190:1943–1959. doi: 10.1016/j.ajpath.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isern J., Garcia-Garcia A., Martin A.M., Arranz L., Martin-Perez D., Torroja C., Sanchez-Cabo F., Mendez-Ferrer S. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife. 2014;3 doi: 10.7554/eLife.03696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K., Sun Y., Xie L., Mao X.O., Childs J., Peel A., Logvinova A., Banwait S., Greenberg D.A. Comparison of ischemia-directed migration of neural precursor cells after intrastriatal, intraventricular, or intravenous transplantation in the rat. Neurobiol. Dis. 2005;18:366–374. doi: 10.1016/j.nbd.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Katare R., Riu F., Mitchell K., Gubernator M., Campagnolo P., Cui Y., Fortunato O., Avolio E., Cesselli D., Beltrami A.P., Angelini G., Emanueli C., Madeddu P. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ. Res. 2011;109:894–906. doi: 10.1161/CIRCRESAHA.111.251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keep R.F., Zhou N., Xiang J., Andjelkovic A. v, Hua Y., Xi G. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS. 2014;11:18. doi: 10.1186/2045-8118-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Shin J.Y., Lee Y.S., Yun S.P., Maeng H.J., Lee Y. Brain endothelial P-glycoprotein level Is reduced in Parkinson’s Disease via a Vitamin D receptor-dependent pathway. Int J. Mol. Sci. 2020;21 doi: 10.3390/ijms21228538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk J., Plumb J., Mirakhur M., McQuaid S. Tight junctional abnormality in multiple sclerosis white matter affects all calibres of vessel and is associated with blood-brain barrier leakage and active demyelination. J. Pathol. 2003;201:319–327. doi: 10.1002/path.1434. [DOI] [PubMed] [Google Scholar]
- Kisler K., Nelson A.R., Rege S. v, Ramanathan A., Wang Y., Ahuja A., Lazic D., Tsai P.S., Zhao Z., Zhou Y., Boas D.A., Sakadzic S., Zlokovic B. v. Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat. Neurosci. 2017;20:406–416. doi: 10.1038/nn.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig M.A., Canepa D.D., Cadosch D., Casanova E., Heinzelmann M., Rittirsch D., Plecko M., Hemmi S., Simmen H.P., Cinelli P., Wanner G.A. Direct transplantation of native pericytes from adipose tissue: A new perspective to stimulate healing in critical size bone defects. Cytotherapy. 2016;18:41–52. doi: 10.1016/j.jcyt.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Krueger M., Mages B., Hobusch C., Michalski D. Endothelial edema precedes blood-brain barrier breakdown in early time points after experimental focal cerebral ischemia. Acta Neuropathol. Commun. 2019;7 doi: 10.1186/s40478-019-0671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamagna C., Bergers G. The bone marrow constitutes a reservoir of pericyte progenitors. J. Leukoc. Biol. 2006;80:677–681. doi: 10.1189/jlb.0506309. [DOI] [PubMed] [Google Scholar]
- Laredo F., Plebanski J., Tedeschi A. Pericytes: problems and promises for CNS repair. Front Cell Neurosci. 2019;13 doi: 10.3389/fncel.2019.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.Y., Kim H.S., Choi H.Y., Oh T.H., Yune T.Y. Fluoxetine inhibits matrix metalloprotease activation and prevents disruption of blood-spinal cord barrier after spinal cord injury. Brain. 2012;135:2375–2389. doi: 10.1093/brain/aws171. [DOI] [PubMed] [Google Scholar]
- Li W., Shi L., Hu B., Hong Y., Zhang H., Li X., Zhang Y. Mesenchymal stem cell-based therapy for stroke: current understanding and challenges. Front Cell Neurosci. 2021;15 doi: 10.3389/fncel.2021.628940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau L.L., Looi Q.H., Chia W.C., Subramaniam T., Ng M.H., Law J.X. Treatment of spinal cord injury with mesenchymal stem cells. Cell Biosci. 2020;10 doi: 10.1186/s13578-020-00475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P., Johansson B.R., Leveen P., Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277(1979):242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Mestre H., Hablitz L.M., Xavier A.L., Feng W., Zou W., Pu T., Monai H., Murlidharan G., Castellanos Rivera R.M., Simon M.J., Pike M.M., Pla V., Du T., Kress B.T., Wang X., Plog B.A., Thrane A.S., Lundgaard I., Abe Y., Yasui M., Thomas J.H., Xiao M., Hirase H., Asokan A., Iliff J.J., Nedergaard M. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife. 2018;7 doi: 10.7554/eLife.40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A., Zhao Z., Zlokovic B. v. Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J. Exp. Med. 2017;214:3151–3169. doi: 10.1084/jem.20171406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munroe M., Dvoretskiy S., Lopez A., Leong J., Dyle M.C., Kong H., Adams C.M., Boppart M.D. Pericyte transplantation improves skeletal muscle recovery following hindlimb immobilization. FASEB J. 2019;33:7694–7706. doi: 10.1096/fj.201802580R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadareishvili Z., Simpkins A.N., Hitomi E., Reyes D., Leigh R. Post-stroke blood-brain barrier disruption and poor functional outcome in patients receiving thrombolytic therapy. Cereb. Dis. 2019;47:135–142. doi: 10.1159/000499666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Arimura K., Nishimura A., Tachibana M., Yoshikawa Y., Makihara N., Wakisaka Y., Kuroda J., Kamouchi M., Ooboshi H., Kitazono T., Ago T. Possible involvement of basic FGF in the upregulation of PDGFRbeta in pericytes after ischemic stroke. Brain Res. 2016;1630:98–108. doi: 10.1016/j.brainres.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Nishinaka A., Inoue Y., Fuma S., Hida Y., Nakamura S., Shimazawa M., Hara H. Pathophysiological role of VEGF on retinal edema and nonperfused areas in mouse eyes with retinal vein occlusion. Invest Ophthalmol. Vis. Sci. 2018;59:4701–4713. doi: 10.1167/iovs.18-23994. [DOI] [PubMed] [Google Scholar]
- Nissen L.J., Cao R., Hedlund E.M., Wang Z., Zhao X., Wetterskog D., Funa K., Brakenhielm E., Cao Y. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J. Clin. Invest. 2007;117:2766–2777. doi: 10.1172/JCI32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T., Suzuki H., Travis Z.D., Zhang J.H. The Stroke-Induced Blood-Brain Barrier Disruption: Current progress of inspection technique, mechanism, and therapeutic target. Curr. Neuropharmacol. 2020;18:1187–1212. doi: 10.2174/1570159X18666200528143301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz G.G., Pacheco-Moises F.P., Macias-Islas M.A., Flores-Alvarado L.J., Mireles-Ramirez M.A., Gonzalez-Renovato E.D., Hernandez-Navarro V.E., Sanchez-Lopez A.L., Alatorre-Jimenez M.A. Role of the blood-brain barrier in multiple sclerosis. Arch. Med Res. 2014;45:687–697. doi: 10.1016/j.arcmed.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Picoli C.C., Coimbra-Campos L.M.C., Guerra D.A.P., Silva W.N., Prazeres P., Costa A.C., Magno L.A. v, Romano-Silva M.A., Mintz A., Birbrair A. Pericytes act as key players in spinal cord injury. Am. J. Pathol. 2019;189:1327–1337. doi: 10.1016/j.ajpath.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimand J., Isserlin R., Voisin V., Kucera M., Tannus-Lopes C., Rostamianfar A., Wadi L., Meyer M., Wong J., Xu C., Merico D., Bader G.D. Pathway enrichment analysis and visualization of omics data using g:Profiler, GSEA, Cytoscape and EnrichmentMap. Nat. Protoc. 2019;14:482–517. doi: 10.1038/s41596-018-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roddie C., O’Reilly M., Dias Alves Pinto J., Vispute K., Lowdell M. Manufacturing chimeric antigen receptor T cells: issues and challenges. Cytotherapy. 2019;21:327–340. doi: 10.1016/j.jcyt.2018.11.009. [DOI] [PubMed] [Google Scholar]
- Rust R., Gronnert L., Gantner C., Enzler A., Mulders G., Weber R.Z., Siewert A., Limasale Y.D.P., Meinhardt A., Maurer M.A., Sartori A.M., Hofer A.S., Werner C., Schwab M.E. Nogo-A targeted therapy promotes vascular repair and functional recovery following stroke. Proc. Natl. Acad. Sci. USA. 2019;116:14270–14279. doi: 10.1073/pnas.1905309116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagare A.P., Bell R.D., Zhao Z., Ma Q., Winkler E.A., Ramanathan A., Zlokovic B. v. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat. Commun. 2013;4 doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sakai K., Takata F., Yamanaka G., Yasunaga M., Hashiguchi K., Tominaga K., Itoh K., Kataoka Y., Yamauchi A., Dohgu S. Reactive pericytes in early phase are involved in glial activation and late-onset hypersusceptibility to pilocarpine-induced seizures in traumatic brain injury model mice. J. Pharm. Sci. 2021;145:155–165. doi: 10.1016/j.jphs.2020.11.008. [DOI] [PubMed] [Google Scholar]
- Schellinger P.D., Warach S. Therapeutic time window of thrombolytic therapy following stroke. Curr. Atheroscler. Rep. 2004;6:288–294. doi: 10.1007/s11883-004-0060-3. [DOI] [PubMed] [Google Scholar]
- Sengillo J.D., Winkler E.A., Walker C.T., Sullivan J.S., Johnson M., Zlokovic B. v. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol. 2013;23:303–310. doi: 10.1111/bpa.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Koronyo Y., Rentsendorj A., Regis G.C., Sheyn J., Fuchs D.T., Kramerov A.A., Ljubimov A. v, Dumitrascu O.M., Rodriguez A.R., Barron E., Hinton D.R., Black K.L., Miller C.A., Mirzaei N., Koronyo-Hamaoui M. Identification of early pericyte loss and vascular amyloidosis in Alzheimer’s disease retina. Acta Neuropathol. 2020;139:813–836. doi: 10.1007/s00401-020-02134-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva I., Silva J., Ferreira R., Trigo D. Glymphatic system, AQP4, and their implications in Alzheimer’s disease. Neurol. Res Pr. 2021;3 doi: 10.1186/s42466-021-00102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth L.C.D., Highet B., Jansson D., Wu J., Rustenhoven J., Aalderink M., Tan A., Li S., Johnson R., Coppieters N., Handley R., Narayan P., Singh-Bains M.K., Schweder P., Turner C., Mee E.W., Heppner P., Correia J., Park T.I., Curtis M.A., Faull R.L.M., Dragunow M. Characterisation of PDGF-BB:PDGFRbeta signalling pathways in human brain pericytes: evidence of disruption in Alzheimer’s disease. Commun. Biol. 2022;5 doi: 10.1038/s42003-022-03180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song T., Zhu X.Y., Eirin A., Jiang Y., Krier J.D., Tang H., Jordan K.L., Lerman A., Lerman L.O. Exogenous pericyte delivery protects the mouse kidney from chronic ischemic injury. Am. J. Physiol. Ren. Physiol. 2022 doi: 10.1152/ajprenal.00064.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins M.J., Gastfriend B.D., Canfield S.G., Lee M.S., Richards D., Faubion M.G., Li W.J., Daneman R., Palecek S.P., Shusta E. v. Human pluripotent stem cell-derived brain pericyte-like cells induce blood-brain barrier properties. Sci. Adv. 2019;5 doi: 10.1126/sciadv.aau7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokum J.A., Cannarsa G.J., Wessell A.P., Shea P., Wenger N., Simard J.M. When the Blood Hits Your Brain: The Neurotoxicity of Extravasated Blood. Int J. Mol. Sci. 2021;22 doi: 10.3390/ijms22105132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Huang Y., Gong J., Wang J., Fan Y., Cai J., Wang Y., Qiu Y., Wei Y., Xiong C., Chen J., Wang B., Ma Y., Huang L., Chen X., Zheng S., Huang W., Ke Q., Wang T., Li X., Zhang W., Xiang A.P., Li W. Transplantation of hPSC-derived pericyte-like cells promotes functional recovery in ischemic stroke mice. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-19042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M.D., Ayyadurai S., Zlokovic B. v. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 2016;19:771–783. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M.D., Sagare A.P., Zlokovic B. v. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M., Yamazaki Y., Liu C.C., Bu G., Kanekiyo T. Pericyte implantation in the brain enhances cerebral blood flow and reduces amyloid-beta pathology in amyloid model mice. Exp. Neurol. 2018;300:13–21. doi: 10.1016/j.expneurol.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana N., Oichi T., Kato S., Sato Y., Hasebe H., Hirai S., Taniguchi Y., Matsubayashi Y., Mori H., Tanaka S., Oshima Y. Spinal cord swelling in patients with cervical compression myelopathy. BMC Musculoskelet. Disord. 2019;20 doi: 10.1186/s12891-019-2673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami M., Nara Y., Kubota A., Fujino H., Yamori Y. Ultrastructural changes in cerebral pericytes and astrocytes of stroke-prone spontaneously hypertensive rats. Stroke. 1990;21:1064–1071. doi: 10.1161/01.str.21.7.1064. [DOI] [PubMed] [Google Scholar]
- Tang T., Liu X.J., Zhang Z.Q., Zhou H.J., Luo J.K., Huang J.F., Yang Q.D., Li X.Q. Cerebral angiogenesis after collagenase-induced intracerebral hemorrhage in rats. Brain Res. 2007;1175:134–142. doi: 10.1016/j.brainres.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Thomas H., Cowin A.J., Mills S.J. The importance of pericytes in healing: wounds and other pathologies. Int J. Mol. Sci. 2017;18 doi: 10.3390/ijms18061129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torok O., Schreiner B., Schaffenrath J., Tsai H.C., Maheshwari U., Stifter S.A., Welsh C., Amorim A., Sridhar S., Utz S.G., Mildenberger W., Nassiri S., Delorenzi M., Aguzzi A., Han M.H., Greter M., Becher B., Keller A. Pericytes regulate vascular immune homeostasis in the CNS. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2016587118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull M.T., Zubair A.C., Meschia J.F., Freeman W.D. Mesenchymal stem cells for hemorrhagic stroke: status of preclinical and clinical research. NPJ Regen. Med. 2019;4 doi: 10.1038/s41536-019-0073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlandewijck M., He L., Mae M.A., Andrae J., Ando K., del Gaudio F., Nahar K., Lebouvier T., Lavina B., Gouveia L., Sun Y., Raschperger E., Rasanen M., Zarb Y., Mochizuki N., Keller A., Lendahl U., Betsholtz C. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480. doi: 10.1038/nature25739. [DOI] [PubMed] [Google Scholar]
- Vates G.E., Takano T., Zlokovic B., Nedergaard M. Pericyte constriction after stroke: the jury is still out. Nat. Med. 2010;16:959. doi: 10.1038/nm0910-959. author reply 960. [DOI] [PubMed] [Google Scholar]
- Vidal M., Maniglier M., Deboux C., Bachelin C., Zujovic V., Baron-Van Evercooren A. Adult DRG stem/progenitor cells generate pericytes in the presence of central nervous system (CNS) developmental cues, and schwann cells in response to CNS demyelination. Stem Cells. 2015;33:2011–2024. doi: 10.1002/stem.1997. [DOI] [PubMed] [Google Scholar]
- Vidal P.M., Karadimas S.K., Ulndreaj A., Laliberte A.M., Tetreault L., Forner S., Wang J., Foltz W.D., Fehlings M.G. Delayed decompression exacerbates ischemia-reperfusion injury in cervical compressive myelopathy. JCI Insight. 2017;2 doi: 10.1172/jci.insight.92512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Mo M., Wang J., Sadia S., Shi B., Fu X., Yu L., Tredget E.E., Wu Y. Platelet-derived growth factor receptor beta identifies mesenchymal stem cells with enhanced engraftment to tissue injury and pro-angiogenic property. Cell Mol. Life Sci. 2018;75:547–561. doi: 10.1007/s00018-017-2641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetstone W.D., Hsu J.Y., Eisenberg M., Werb Z., Noble-Haeusslein L.J. Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J. Neurosci. Res. 2003;74:227–239. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I., Nyul-Toth A., Suciu M., Hermenean A., Krizbai I.A. Heterogeneity of the blood-brain barrier. Tissue Barriers. 2016;4 doi: 10.1080/21688370.2016.1143544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler E.A., Birk H., Burkhardt J.K., Chen X., Yue J.K., Guo D., Rutledge W.C., Lasker G.F., Partow C., Tihan T., Chang E.F., Su H., Kim H., Walcott B.P., Lawton M.T. Reductions in brain pericytes are associated with arteriovenous malformation vascular instability. J. Neurosurg. 2018;129:1464–1474. doi: 10.3171/2017.6.JNS17860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler E.A., Sagare A.P., Zlokovic B. v. The pericyte: a forgotten cell type with important implications for Alzheimer’s disease? Brain Pathol. 2014;24:371–386. doi: 10.1111/bpa.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler E.A., Sengillo J.D., Sagare A.P., Zhao Z., Ma Q., Zuniga E., Wang Y., Zhong Z., Sullivan J.S., Griffin J.H., Cleveland D.W., Zlokovic B. v. Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc. Natl. Acad. Sci. USA. 2014;111:E1035–E1042. doi: 10.1073/pnas.1401595111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler E.A., Sengillo J.D., Sullivan J.S., Henkel J.S., Appel S.H., Zlokovic B. v. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125:111–120. doi: 10.1007/s00401-012-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.L., Dicks A., Steward N., Tang R., Katz D.B., Choi Y.R., Guilak F. Single cell transcriptomic analysis of human pluripotent stem cell chondrogenesis. Nat. Commun. 2021;12 doi: 10.1038/s41467-020-20598-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Muramatsu M., Azuma E., Ikutani M., Nagai Y., Sagara H., Koo B.N., Kita S., O’Donnell E., Osawa T., Takahashi H., Takano K.I., Dohmoto M., Sugimori M., Usui I., Watanabe Y., Hatakeyama N., Iwamoto T., Komuro I., Takatsu K., Tobe K., Niida S., Matsuda N., Shibuya M., Sasahara M. A subset of cerebrovascular pericytes originates from mature macrophages in the very early phase of vascular development in CNS. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-03994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka G., Takata F., Kataoka Y., Kanou K., Morichi S., Dohgu S., Kawashima H. The neuroinflammatory role of pericytes in epilepsy. Biomedicines. 2021;9 doi: 10.3390/biomedicines9070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Torbey M.T. Angiogenesis and blood-brain barrier permeability in vascular remodeling after stroke. Curr. Neuropharmacol. 2020;18:1250–1265. doi: 10.2174/1570159X18666200720173316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemisci M., Gursoy-Ozdemir Y., Vural A., Can A., Topalkara K., Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat. Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- Yin K.J., Hamblin M., Chen Y.E. Angiogenesis-regulating microRNAs and Ischemic Stroke. Curr. Vasc. Pharm. 2015;13:352–365. doi: 10.2174/15701611113119990016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn S.W., Jung K.H., Chu K., Lee J.Y., Lee S.T., Bahn J.J., Park D.K., Yu J.S., Kim S.Y., Kim M., Lee S.K., Han M.H., Roh J.K. Feasibility and safety of intra-arterial pericyte progenitor cell delivery following mannitol-induced transient blood-brain barrier opening in a canine model. Cell Transpl. 2015;24:1469–1479. doi: 10.3727/096368914X682413. [DOI] [PubMed] [Google Scholar]
- Zehendner C.M., Sebastiani A., Hugonnet A., Bischoff F., Luhmann H.J., Thal S.C. Traumatic brain injury results in rapid pericyte loss followed by reactive pericytosis in the cerebral cortex. Sci. Rep. 2015;5 doi: 10.1038/srep13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenaro E., Piacentino G., Constantin G. The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 2017;107:41–56. doi: 10.1016/j.nbd.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Nelson A.R., Betsholtz C., Zlokovic B. v. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163:1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X., Baker, H., Hancock, W.S., Fawaz, F., McCaman, M., Pungor Jr., E., 2006, Proteomic analysis for the assessment of different lots of fetal bovine serum as a raw material for cell culture. Part IV. Application of proteomics to the manufacture of biological drugs. Biotechnol Prog 22, 1294–1300. 10.1021/bp060121o. [DOI] [PubMed]
- Zhu S., Chen M., Ying Y., Wu Q., Huang Z., Ni W., Wang X., Xu H., Bennett S., Xiao J., Xu J. Versatile subtypes of pericytes and their roles in spinal cord injury repair, bone development and repair. Bone Res. 2022;10 doi: 10.1038/s41413-022-00203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]