Abstract
Malignant progression is a life-threatening consequence of human papillomavirus-associated lesions. In this study, we tested the efficacy of papillomavirus early-gene-based vaccines for prevention of carcinoma development of papillomavirus-induced skin papillomas on rabbits. Rabbit skin papillomas were initiated by infection with cottontail rabbit papillomavirus (CRPV). The papillomas were allowed to grow for 3 months without any treatment intervention. Rabbits were then immunized by gene gun-mediated intracutaneous administration of four DNA plasmids encoding CRPV E1, E2, E6, and E7 genes, respectively. All eight control rabbits receiving vector alone developed invasive carcinoma within 8 to 13 months. In contrast, only two of eight vaccinated rabbits developed carcinoma at 12 and 15 months, respectively. Papilloma growth was suppressed in the majority of vaccinated rabbits but not completely eradicated. These results indicate that gene gun-mediated immunization with papillomavirus early genes may be a promising strategy for prevention of malignant progression of human papillomavirus-associated lesions in humans.
High-risk human papillomaviruses (HPVs), such as HPV type 16 (HPV16) and HPV18, first induce benign mucosal and/or cutaneous hyperproliferative lesions that may spontaneously regress or persist and in some patients progress to invasive cancer (32). Epidemiological observations (3) together with those of a functional in vitro study of papillomavirus-encoded oncogenes (20) provide strong evidence that high-risk HPV infection plays a crucial role in the development of anogenital cancer, particularly cervical carcinoma, the second most common cancer in women worldwide. HPV infection is also linked to carcinoma development at other anatomical sites (29). Animal models of papillomavirus infection which mimic features of HPV-induced carcinoma are essential for studying immune responses to these virally induced cancers. One well-characterized model is the cottontail rabbit papillomavirus (CRPV) model (this virus infects cottontail rabbits, its natural host, and domestic rabbits). CRPV infection of New Zealand White (NZW) rabbits initially induces benign skin tumors (papillomas or warts) which may spontaneously regress or progress to invasive carcinoma (16). The natural history of CRPV-induced warts resembles that of human genital HPV infection (16). Recently, the CRPV-rabbit model has been used extensively for developing and testing papillomavirus early-gene-based (13, 14, 26, 31) and late-gene-based (4) vaccines. All these early studies tested the efficacy of vaccination for protection of animals against viral challenge. In our laboratory, rabbit skin warts induced by one CRPV isolate (CRPV-Hershey) showed a very low incidence of spontaneous regression (<2%) and developed invasive carcinoma on all rabbits with persistent virus infection within about 1 year (7). The model thus provides opportunities to test therapeutic vaccination against both benign (papilloma) and malignant (carcinoma) tumor cells. Our previous study with the CRPV-rabbit model demonstrated that gene gun-mediated intracutaneous vaccination with a combination of CRPV E1, E2, E6, and E7 genes provided protection of rabbits from viral challenge (13). In this study, we tested whether the same strategy could eradicate established viral warts and/or prevent malignant progression of established but initially benign viral warts.
CRPV E1, E2, E6, and E7 genes were cloned into V1Jns plasmid (13). Recombinant plasmid DNA was precipitated onto 1.6-μm-diameter gold particles at a ratio of 1 μg of DNA/0.5 mg of gold particles. The inner surface of Tefzel tubing was then coated with gold particles following the manufacturer's protocol (Bio-Rad, Hercules, Calif.).
NZW rabbits were purchased from Covance Research Products Inc. (Denver, Pa.). CRPV stock was previously prepared and stored at −70°C (1). Prior to use, viral stocks were quickly thawed, sonicated for 1 min, and diluted 100-fold with phosphate-buffered saline. Sixteen rabbits were divided into two groups, and each rabbit was infected with CRPV at four dorsal sites. Rabbit backs were shaved with electric clippers, and 100 μl of virus suspension was administered onto each scarified site (1.5 by 1.5 cm). Scarification was achieved using a scalpel blade held perpendicular to the skin surface. Visible viral warts appeared on all infected sites within 3 to 4 weeks. Skin warts induced with this dose of CRPV had a very low incidence of spontaneous regression and high incidence of malignant progression (7). The warts were allowed to grow without any intervention for about 3 months. Spontaneous regression of the warts was not observed, indicating that antiviral immunity, if any, was weak in these rabbits. Four months following virus infection (about 3 months after wart outgrowth), one group of rabbits was immunized with four recombinant DNA plasmids encoding CRPV E1, E2, E6, and E7 genes, and rabbits in the second group received vector plasmid only as controls. Rabbits were immunized by gene gun-mediated intracutaneous delivery of plasmid DNA, in which DNA-gold particles were bombarded at 400 lb/in2 onto rabbit dorsal skin sites which were shaved and then treated with depilatory lotion (Nair roll-on hair remover; Carter-Wallace, Inc., New York, N.Y.) for the first three treatments at 3-week intervals. For the last three immunizations, DNA-gold particles were delivered onto the inner surface of the ear at 2-month intervals. For the vaccination group, individual animals received 20 μg of plasmid DNA for each construct (V1JnsE1, V1JnsE2, V1JnsE6, and V1JnsE7) for each immunization. Vaccinations were delivered to separate but adjacent sites for individual constructs. For the control group, rabbits received 20 μg of plasmid vector DNA for each immunization.
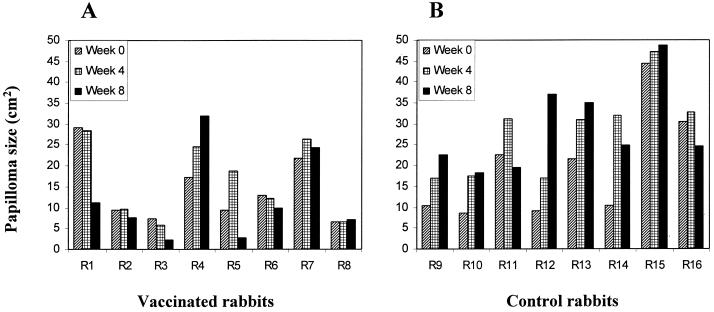
Papilloma size (width × length) was measured weekly for 8 consecutive weeks following the first vaccination. Papillomas on the rabbits showed considerable variation in size. However, before vaccination, there was no statistical difference between total papilloma size for the two groups of rabbits (P = 0.328 by t test). Papilloma growth on vaccinated rabbits, however, was suppressed (Fig. 1). At 8 weeks after the first vaccination, only one rabbit in the vaccinated group showed an increased papilloma size of more than 50%. Papilloma size showed a slight increase on two other vaccinated rabbits (+11 and +8%), and a decrease on the remaining five vaccinated rabbits (−61, −17, −67, −70, and −24%). In contrast, papilloma size on five control rabbits was markedly increased (+118, +114, +285, +61, and +140%). Papilloma sizes on one control rabbit was slightly increased, and on the remaining two rabbits, there was a decrease in papilloma sizes (−17 and −19%) (Fig. 1). Total papilloma sizes on control rabbits doubled compared to the prevaccination sizes. At the end of 8 weeks following the first vaccination, the total papilloma size on vaccinated rabbits was significantly less than that on control rabbits (P = 0.006 by t test). It is noteworthy that although papilloma growth was suppressed by early-gene vaccination in the majority of rabbits, none of the established papillomas were completely eradicated, demonstrating that the immunity so induced was insufficient to eradicate established, large viral papillomas.
FIG. 1.
Papilloma sizes on vaccinated (A) and nonvaccinated control (B) rabbits at the time point before vaccination (week 0) and at four and eight weeks following the first vaccination. Papilloma size of individual rabbits (R1 to R16) represents the sum of papilloma sizes at four infection sites.
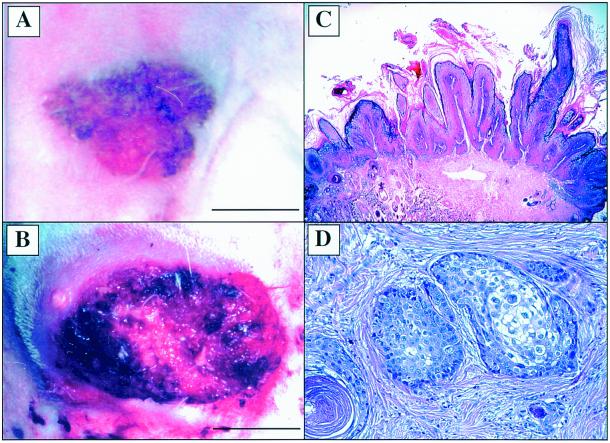
To evaluate whether the immunity so induced could prevent or delay carcinoma development of CRPV-induced papillomas, rabbits were monitored for 16 months after viral infection. Typical gross and histological characteristics of viral papillomas are shown in Fig. 2A and C, respectively. Invasive carcinoma was observed as early as 8 months after virus infection (Table 1). An early sign of malignant progression is usually light exudation on the top of the papillomas, followed by rapid growth and an ulcerating surface. Central necrosis of the papillomas and invasive growth to the adjacent tissue are typical characteristics of malignant conversion of late-stage papillomas (Fig. 2B). In this study, all carcinoma development was confirmed by histological examination. Biopsies of carcinoma showed moderately well-differentiated tumor cells in subepithelial tissue (Fig. 2D). At the end of 10 months after virus infection, three of eight control rabbits (37.5%) developed invasive carcinoma, whereas none of the eight vaccinated rabbits (0%) developed carcinoma. In the vaccinated group at 12 months postinfection, one rabbit died without cancer and one rabbit developed invasive cancer (12.5%). In contrast, five of eight (62.5%) control rabbits developed invasive cancer. By 14 months after viral infection, all eight control rabbits developed invasive carcinoma, whereas in the vaccinated group, no additional rabbits developed cancer, and one rabbit died due to other causes. At this time point, the incidences of malignant progression of papillomas were 100% (eight of eight) for control rabbits and only 16.6% (one of six) for the vaccinated rabbits. Five vaccinated rabbits without cancer were monitored for another 3 months. One rabbit developed invasive cancer at 15 months following virus infection. The remaining rabbits showed no sign of carcinoma development (Table 1). These data indicated that papillomavirus early-gene-based intracutaneous vaccination prevented and/or delayed malignant progression of papillomavirus-induced benign lesions on rabbits (eight of eight versus two of six; P = 0.015 by Fisher's exact test).
FIG. 2.
Gross and histological characteristics of papillomas and malignant tumors on vaccinated and control rabbits. (A) Papillomas on a vaccinated rabbit. The lesion shows limited amounts of horny tissue on the top of the papillomas. The photograph was taken 9 weeks following the first vaccination. Bar, 1.5 cm. (B) Carcinoma on a nonvaccinated rabbit. Cancer shows invasive growth into adjacent tissue with ulcerating surfaces and necrosis in the middle of the carcinoma. Bar, 2.0 cm. (C) Papilloma on a vaccinated rabbit showing histological characteristics of papillomavirus-infected benign epithelial lesion. Magnification, ×15. (D) Carcinomas showing tumor cells in dermis. Magnification, ×100. Hematoxylin and eosin stain was used in panels C and D.
TABLE 1.
MHC class II DRa and DQa RFLP genotypes and carcinoma development in vaccinated and control rabbits
| Rabbita | RFLP genotypeb
|
Carcinoma development (mo)c | |
|---|---|---|---|
| DRa | DQa | ||
| Vaccinated | |||
| 1 | DD | BCD | − |
| 2 | AD | CDE | − |
| 3 | BD | CD | − |
| 4d | AD | BDE | − |
| 5 | AB | BD | − |
| 6e | BD | BD | − |
| 7 | DD | BD | + (15) |
| 8 | BD | BD | + (12) |
| Control | |||
| 9 | DD | BCD | + (13) |
| 10 | AD | CDE | + (12) |
| 11 | AA | DE | + (13) |
| 12 | AA | DE | + (9) |
| 13 | DD | BCD | + (8) |
| 14 | DD | BCD | + (12) |
| 15 | BB | DE | + (10) |
| 16 | BD | CDE | + (13) |
Rabbits were vaccinated with E1, E2, E6, and E7 genes; control rabbits received vector only.
DRa and DQa RFLP alleles were named as defined previously (14). DRa alleles were EcoRI fragments of 6.0 (A), 5.6 (B), and 4.8 (D) kb. DQa alleles were PvuII fragments of 6.3 (B), 5.9 (C), 4.8 (D), and 3.0 (E) kb.
Carcinoma development (number of months following virus infection).
Died 12 months after viral infection without carcinoma development.
Died 13 months after viral infection without carcinoma development.
An early study found that the incidence of wart regression or progression was related to particular viral isolates and to different rabbit species (22). More-recent studies discovered that both regression and malignant progression of viral warts are linked to rabbit major histocompatibility complex (MHC) class II genes (10) and also to a given viral variant (5) (R. Han, unpublished observations). In order to investigate whether the increased incidence of malignant progression was linked to the MHC class II gene alleles in these rabbits, we typed rabbit MHC class II DQa and DRa genotypes by restriction fragment length polymorphism (RFLP) analysis (17). Three DRa EcoRI bands (Table 1) were found, are referred to as DRaA (6.0 kb), DRaB (5.6 kb), DRaD (4.8 kb), and are in agreement with sizes previously reported (10). Homozygous rabbits showed single-band patterns, and heterozygotes showed two-band patterns (Table 1). Four DQa PvuII bands were found in these rabbits and are referred to as DQaB (6.3 kb), DQaC (5.9 kb), DQaD (4.8 kb), and DQaE (3.0 kb) (Table 1) as defined previously (10, 17). However, we observed that some rabbits showed a three-band pattern (Table 1), suggesting that new DQa haplotypes are represented in this population of NZW rabbits. Table 1 showed DRa and DQa RFLP genotypes for each rabbit. None of the DRa and DQa RFLP alleles was found to be linked to an increased incidence of malignant progression.
We evaluated T-cell-mediated immunity by in vitro proliferation assays 1 week after the third immunization (13). Peripheral blood mononuclear cells (PBMCs) were prepared from ear arterial blood and stimulated in vitro with CRPV E1, E2, E6, or E7 proteins that were prepared using the baculovirus expression system as previously described (13). Stimulation indices (SI) are shown in Table 2. The observation that control rabbits also had weak proliferative responses indicated that the viral papillomas alone could prime cell-mediated immune responses, and these data agree with those of a prior study (25). Negative responses (SI of <2) were seen in both vaccinated and control rabbits. In general, there were stronger positive responses in the vaccinated group than in the control group (Table 2). Unexpectedly, all tested rabbits showed negative responses to E6 protein. We do not believe the negative responses resulted from general technical failure of this assay, because all four proliferation assays were conducted concurrently. In addition, the E6 fusion protein was quantified and checked by Western blotting. However, we cannot conclude that E6 vaccination played no role in the prevention of malignant progression. In our earlier study, we found that the magnitude of in vitro proliferative responses did not correlate with in vivo protective immunity (13).
TABLE 2.
PBMC proliferation in response to E1, E2, E6, and E7 stimulation in vitro in vaccinated and control rabbits
| Rabbita | PBMC proliferation (103 cpm; mean ± SEM)b
|
SIc
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| E1 | E2 | E6 | E7 | No antigen | E1 | E2 | E6 | E7 | |
| Vaccinated | |||||||||
| 1 | 4.442 ± 0.382 | 1.083 ± 0.162 | 0.382 ± 0.025 | 0.699 ± 0.046 | 0.346 ± 0.039 | 12.8 | 3.0 | 1.1 | 2.0 |
| 2 | 0.613 ± 0.064 | 0.584 ± 0.068 | 0.489 ± 0.106 | 0.778 ± 0.100 | 0.328 ± 0.058 | 1.9 | 1.8 | 1.5 | 2.4 |
| 3 | 3.223 ± 0.437 | 2.179 ± 0.232 | 0.681 ± 0.081 | 1.809 ± 0.236 | 0.457 ± 0.050 | 7.1 | 4.8 | 1.5 | 4.0 |
| 4d | NDe | ND | ND | ND | ND | ND | ND | ND | ND |
| 5 | 16.50 ± 2.923 | 4.124 ± 1.658 | 0.327 ± 0.061 | 0.523 ± 0.164 | 0.200 ± 0.067 | 82.5 | 20.6 | 1.6 | 2.6 |
| 6 | 0.814 ± 0.049 | 0.635 ± 0.045 | 0.530 ± 0.068 | 1.540 ± 0.367 | 0.421 ± 0.016 | 1.9 | 1.5 | 1.3 | 3.7 |
| 7 | 0.368 ± 0.016 | 0.430 ± 0.054 | 0.146 ± 0.016 | 0.253 ± 0.031 | 0.142 ± 0.007 | 2.6 | 3.0 | 1.0 | 1.8 |
| 8 | 0.696 ± 0.120 | 0.264 ± 0.013 | 0.222 ± 0.016 | 0.312 ± 0.060 | 0.204 ± 0.020 | 3.4 | 1.3 | 1.1 | 1.5 |
| Control | |||||||||
| 9 | 1.554 ± 0.222 | 0.675 ± 0.142 | 0.322 ± 0.015 | 0.417 ± 0.014 | 0.246 ± 0.019 | 6.3 | 2.7 | 1.3 | 1.7 |
| 10 | 0.628 ± 0.161 | 0.382 ± 0.094 | 0.265 ± 0.080 | 0.393 ± 0.019 | 0.199 ± 0.008 | 3.1 | 1.9 | 1.3 | 2.0 |
| 11 | 0.324 ± 0.065 | 0.265 ± 0.022 | 0.324 ± 0.072 | 0.836 ± 0.219 | 0.310 ± 0.054 | 1.0 | 0.9 | 1.0 | 2.6 |
| 12d | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 13 | 1.616 ± 0.301 | 0.657 ± 0.047 | 0.406 ± 0.010 | 0.504 ± 0.080 | 0.428 ± 0.027 | 3.8 | 1.5 | 0.9 | 1.2 |
| 14 | 0.624 ± 0.068 | 0.665 ± 0.052 | 0.359 ± 0.068 | 0.408 ± 0.036 | 0.312 ± 0.023 | 2.0 | 2.1 | 1.6 | 1.3 |
| 15 | 0.478 ± 0.065 | 0.730 ± 0.094 | 0.381 ± 0.068 | 0.255 ± 0.039 | 0.252 ± 0.051 | 1.9 | 2.9 | 1.5 | 1.0 |
| 16 | 0.261 ± 0.022 | 0.309 ± 0.043 | 0.208 ± 0.012 | 0.228 ± 0.017 | 0.165 ± 0.016 | 1.6 | 1.9 | 1.3 | 1.4 |
Rabbits were vaccinated with E1, E2, E6, and E7 genes; control rabbits received vector alone.
Values for three wells. All values were determined from assays done on day 4.
Ratio of counts per minute in response to specific antigen to counts per minute in response to no antigen. A SI greater than 2 was considered positive and is shown in bold type.
Insufficient PBMC for assays.
ND, not done.
We previously demonstrated that gene gun-mediated intracutaneous vaccination with the combination of CRPV E1, E2, E6, and E7 genes protected rabbits against virus challenge (13). In this study, we showed that the immunity induced after papillomas had developed suppressed papilloma growth and prevented and/or delayed the development of invasive carcinoma. However, established papillomas could not be eradicated, suggesting that the vaccination-induced immunity was insufficient to accomplish a complete therapeutic response. This may be due to the mass of established papillomas or to a locally unfavorable environment that may reduce the efficacy of effector cells that can kill tumor cells. For example, tumor mass may be determined by a balance between tumor cell proliferation and tumor cell destruction. If the number of tumor cells destroyed by immune effector cells is less than the tumor cell proliferation rate, then tumor will continue to grow. It was observed that infiltration of lymphocytes are mainly located in the dermis beneath viral lesions (27). We propose that the basal lamina is likely a barrier for lymphocyte penetration into the epidermis to kill virus-infected cells. However, malignant transformed cells in the epidermis first invade through the basal lamina and form microinvasive lesions in the dermis that are exposed to infiltrating lymphocytes which may effectively destroy these malignant tumor cells. This may explain why the vaccination-induced immunity was not effective in the eradication of established lesions but effective in the prevention of malignant progression. In this study, two of eight vaccinated rabbits developed invasive carcinoma which occurred later than carcinoma onset in control rabbits. These observations suggest that stronger immunity in these two rabbits is needed to accomplish complete prevention of invasive carcinoma development.
Malignant progression of papillomavirus-induced lesions is proposed to proceed through a multistage process. In vitro studies showed that CRPV E6, E7, E8, and E5 have transforming potential (11, 19). Cell-mediated immunity is likely to control this process by elimination of malignantly transformed cells and/or by a reduction of the number of virus-infected cells which have the potential to become transformed. In high-risk HPV-infected lesions (30) and CRPV-induced skin papillomas (23), expression of E1, E2, E6, and E7 genes have been detected in the epithelial basal and suprabasal layers by in situ RNA-RNA hybridization. Theoretically, cytotoxic T lymphocytes (CTLs) specific for E1 and E2 antigens can kill virus-infected cells in the basal and suprabasal layers of epithelium, thus suppressing viral tumor growth and reducing the rate of malignant transformation. E6 and E7 but not E1 and E2 are selectively retained and expressed in malignantly transformed cells (15, 24). Therefore, CTLs specific for E6 and E7 antigen may be the only effector cells with the capacity to eradicate malignantly transformed cells, thus directly preventing carcinoma development. In murine models, immunization of mice with HPV16 E6 and E7 proteins impeded the growth of syngeneic tumor cells expressing E6 or E7 proteins (6, 18). Vaccination with the combination of E1, E2, E6, and E7 genes thus increases the efficacy in the prevention of carcinoma development.
T-cell-mediated immunity plays a critical role in immune surveillance of HPV infection and the development of papillomavirus-associated carcinoma (28). Gene gun-mediated intracutaneous genetic immunization directly transfects dermal dendrocytes, potent antigen-presenting cells, which subsequently migrate into local lymph nodes and prime immune responses (8, 21). It has been demonstrated that a predominant role for directly transfected dendritic cells in antigen presentation is to prime CD8+ cells after gene gun immunization (21). Gene gun immunization thus represents an effective strategy for papillomavirus vaccines. Another important advantage for gene gun-based immunization is that it can be used repeatedly to boost immune responses without provoking an immune attack against the vectors themselves. This contrasts with viral vector-based booster vaccinations which can induce antibody-mediated neutralization of viral vector. One caveat of gene gun-based delivery of E6 and E7 oncogenes into live cells is the potential to induce malignant transformation of transfected cells in vivo. Genetic engineering of these oncogenes to abolish their transforming activity while preserving their antigenicity may provide a solution for these safety issues.
There was a poor correlation between in vitro PBMC proliferative responses to antigen stimulation and protective immunity against viral infection in vivo in this and our previous studies (12, 13). In vitro PBMC proliferative responses to soluble antigens induce both CD4+ and CD8+ T-cell proliferation. However, these proliferative responses mainly reflect CD4+ T-cell-mediated immunity. Theoretically, CD4+ T lymphocytes are not the main effector cells for the eradication of virus-infected cells. Unfortunately, evaluation of CTL-mediated immunity is not possible in this group of outbred rabbits due to the lack of appropriate reagents, including target cells, and rabbit cytokine assays. In a number of clinical trials, immunization of cancer patients with synthetic and natural peptides also showed a poor correlation between specific T-cell-mediated immune responses evaluated in vitro and clinical responses in vivo (2). This poor correlation may be due to the magnitude of the in vitro immune responses in immune assays not correlating with the avidity of CTLs to their targets (9). Several studies (reviewed in reference 9) have shown that whereas low-avidity CTLs can be readily detected by standard immunological assays, only high-avidity CTLs exert biological function in vivo in viral or tumor models.
The development of cancer vaccines is a major goal for the therapeutic treatment of cancers. Immunotherapeutic approaches, however, have proven difficult to develop because of the lack of well-defined tumor-specific antigens and effective immunization protocols that elicit long-lasting cell-mediated immunity. Papillomavirus-associated cancer selectively express papillomavirus early gene products which may serve as tumor-specific antigens. Gene gun-mediated immunization can induce potent cell-mediated immunity responses (8, 21). These observations together with our studies with the CRPV-rabbit model suggest that gene gun immunization with papillomavirus early genes may be a promising strategy for prevention of HPV-associated carcinoma.
Acknowledgments
We thank Satvir Tevethia for critical review of the manuscript and for fruitful discussions.
This study was supported by grant RO1 CA47622 and the Jake Gittlen Memorial Golf Tournament. R. Han is the recipient of the 1996–1998 American Social Health Association/Merck Foundation Research Fellowship in sexually transmitted diseases.
REFERENCES
- 1.Angell M G, Christensen N D, Kreider J W. An in vitro system for studying the initial stages of cottontail rabbit papillomavirus infection. J Virol Methods. 1992;39:207–216. doi: 10.1016/0166-0934(92)90139-5. [DOI] [PubMed] [Google Scholar]
- 2.Bellone M, Lezzi G, Imro M A, Protti M P. Cancer immunotherapy: synthetic and natural peptides in the balance. Immunol Today. 1999;20:457–462. doi: 10.1016/s0167-5699(99)01503-0. [DOI] [PubMed] [Google Scholar]
- 3.Bosch F X, Manos M M, Munoz N, Sherman M, Jansen A M, Peto J, Schiffman M H, Moreno V, Kurman R, Shah K V. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 4.Breitburd F, Kirnbauer R, Hubbert N L, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller J T, Lowy D R. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breitburd F, Salmon J, Orth G. The rabbit viral skin papillomas and carcinomas: a model for the immunogenetics of HPV-associated carcinogenesis. Clin Dermatol. 1997;15:237–247. doi: 10.1016/s0738-081x(97)00009-6. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Thomas E K, Hu S-L, Hellström I, Hellström K E. Human papillomavirus type 16 nucleoprotein E7 is a tumor rejection antigen. Proc Natl Acad Sci USA. 1991;88:110–114. doi: 10.1073/pnas.88.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen N D, Han R, Kreider J W. Cottontail rabbit papillomavirus. In: Ahmed R, Chen I S Y, editors. Persistent viral infections. Chichester, United Kingdom: John Wiley & Sons; 1999. pp. 485–502. [Google Scholar]
- 8.Condon C, Watkins S C, Celluzzi C M, Thompson K, Falo L D. DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 9.Gilboa E. The makings of a tumor rejection antigen. Immunity. 1999;11:263–270. doi: 10.1016/s1074-7613(00)80101-6. [DOI] [PubMed] [Google Scholar]
- 10.Han R, Breitburd F, Marche P N, Orth G. Linkage of regression and malignant conversion of rabbit viral papillomas to MHC class II genes. Nature. 1992;356:66–68. doi: 10.1038/356066a0. [DOI] [PubMed] [Google Scholar]
- 11.Han R, Cladel N M, Reed C, Christensen N D. Characterization of transformation function of cottontail rabbit papillomavirus E5 and E8 genes. Virology. 1998;251:253–263. doi: 10.1006/viro.1998.9416. [DOI] [PubMed] [Google Scholar]
- 12.Han R, Cladel N M, Reed C, Christensen N D. Intramuscular injection of plasmid DNA encoding cottontail rabbit papillomavirus E1, E2, E6 and E7 induces cell-mediated but not humoral immune responses in rabbits. Vaccine. 1999;17:1558–1566. doi: 10.1016/s0264-410x(98)00356-9. [DOI] [PubMed] [Google Scholar]
- 13.Han R, Cladel N M, Reed C A, Peng X, Christensen N D. Protection of rabbits from viral challenge by gene gun-based intracutaneous vaccination with a combination of cottontail rabbit papillomavirus E1, E2, E6, and E7 genes. J Virol. 1999;73:7039–7043. doi: 10.1128/jvi.73.8.7039-7043.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen E R, Selvakumar R, Shen H, Ahmed R, Wettstein F O, Miller J F. Recombinant Listeria monocytogenes vaccination eliminates papillomavirus-induced tumors and prevents papilloma formation from viral DNA. J Virol. 1997;71:8467–8474. doi: 10.1128/jvi.71.11.8467-8474.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeon S, Lambert P F. Integration of HPV-16 DNA into the human genome leads to increased stability of E6/E7 mRNAs: implications for cervical carcinogenesis. Proc Natl Acad Sci USA. 1993;92:1654–1658. doi: 10.1073/pnas.92.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreider J W, Bartlett G L. The Shope papilloma-carcinoma complex of rabbits: a model system of neoplastic progression and spontaneous regression. Adv Cancer Res. 1981;35:81–110. doi: 10.1016/s0065-230x(08)60909-4. [DOI] [PubMed] [Google Scholar]
- 17.Marche P N, Rebiére M C, Laverrière A, English D W, LeGuern C, Kindt T J. Definition of rabbit class I and class II MHC gene haplotypes using molecular typing procedures. Immunogenetics. 1989;29:273–276. doi: 10.1007/BF00717913. [DOI] [PubMed] [Google Scholar]
- 18.Meneguzzi G, Cerni C, Kieny M P, Lathe R. Immunization against human papillomavirus type 16 tumor cells with recombinant vaccinia viruses expressing E6 and E7. Virology. 1991;181:62–69. doi: 10.1016/0042-6822(91)90470-v. [DOI] [PubMed] [Google Scholar]
- 19.Meyers C, Harry J, Lin Y-L, Wettstein F O. Identification of three transforming proteins encoded by cottontail rabbit papillomavirus. J Virol. 1992;66:1655–1664. doi: 10.1128/jvi.66.3.1655-1664.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Münger K, Phelps W C, Bubb V, Howley P M, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63:4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porgador A, Irvine K R, Iwasaki A, Barber B H, Restifo N P, Germain R N. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ cells after gene gun immunization. J Exp Med. 1998;188:1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rous P, Kidd J G, Beard J W. Observations on the relation of virus causing rabbit papillomas to cancers deriving therefrom. I. The influence of host species and of the pathogenic activity and concentration of virus. J Exp Med. 1936;64:385–400. doi: 10.1084/jem.64.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitt A, Rochat A, Zeltner R, Borenstein L, Barrandon Y, Wettstein F O, Iftner T. The primary target cells of the high-risk cottontail rabbit papillomavirus colocalize with hair follicle stem cells. J Virol. 1996;70:1912–1922. doi: 10.1128/jvi.70.3.1912-1922.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz E, Freese U K, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314:111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- 25.Selvakumar R, Ahmed R, Wettstein F O. Tumor regression is associated with a specific immune response to the E2 protein of cottontail rabbit papillomavirus. Virology. 1995;208:298–302. doi: 10.1006/viro.1995.1152. [DOI] [PubMed] [Google Scholar]
- 26.Selvakumar R, Borenstein L A, Lin Y-L, Ahmed R, Wettstein F O. Immunization with nonstructural proteins E1 and E2 of cottontail rabbit papillomavirus stimulates regression of virus-induced papillomas. J Virol. 1995;69:602–605. doi: 10.1128/jvi.69.1.602-605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selvakumar R, Schmitt A, Iftner T, Ahmed R, Wettstein F O. Regression of papillomas induced by cottontail rabbit papillomavirus is associated with infiltration of CD8+ cells and persistence of viral DNA after regression. J Virol. 1997;71:5540–5548. doi: 10.1128/jvi.71.7.5540-5548.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shamanin V, zur Hausen H, Lavergne D, Proby C M, Leigh I M, Neumann C, Hamm H, Goos M, Haustein U-F, Jung E G, Plewig G, Wolff H, de Villes E. Human papillomavirus infections in nonmelanoma skin cancers from renal transplant recipients and nonimmunosuppressed patients. J Nat Cancer Inst. 1996;88:802–811. doi: 10.1093/jnci/88.12.802. [DOI] [PubMed] [Google Scholar]
- 29.Snijders P J, Scholes A G, Hart C A, Jones A S, Vaughan E D, Woolgar J A, Meijer C J, Walboomners J M, Field J K. Prevalence of mucosotropic human papillomaviruses in squamous-cell carcinoma of the head and neck. Int J Cancer. 1996;66:464–469. doi: 10.1002/(SICI)1097-0215(19960516)66:4<464::AID-IJC9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 30.Stoler M H, Rhodes C R, Whitbeck A, Wolinsky S M, Chow L T, Broker T R. Human papillomavirus 16 and 18 gene expression in cervical neoplasia. Hum Pathol. 1992;23:117–128. doi: 10.1016/0046-8177(92)90232-r. [DOI] [PubMed] [Google Scholar]
- 31.Sundaram P, Tigelaar R E, Xiao W, Brandsma J L. Intracutaneous vaccination of rabbits with the E6 gene of cottontail rabbit papillomavirus provides partial protection against virus challenge. Vaccine. 1998;16:613–623. doi: 10.1016/s0264-410x(97)84510-0. [DOI] [PubMed] [Google Scholar]
- 32.zur Hausen H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr Top Microbiol Immunol. 1994;186:131–156. doi: 10.1007/978-3-642-78487-3_8. [DOI] [PubMed] [Google Scholar]