Abstract
Organoids are 3D cultured tissues derived from stem cells that resemble the structure of living organs. Based on the accumulated knowledge of neural development, neural organoids that recapitulate neural tissue have been created by inducing self-organized neural differentiation of stem cells. Neural organoid techniques have been applied to human pluripotent stem cells to differentiate 3D human neural tissues in culture. Various methods have been developed to generate neural tissues of different regions. Currently, neural organoid technology has several significant limitations, which are being overcome in an attempt to create neural organoids that more faithfully recapitulate the living brain. The rapidly advancing neural organoid technology enables the use of living human neural tissue as research material and contributes to our understanding of the development, structure and function of the human nervous system, and is expected to be used to overcome neurological diseases and for regenerative medicine.
Keywords: Neural organoid, Pluripotent stem cells, 3D culture, Self-organization, Neural induction, Regional patterning, Human brain development
1. Introduction
The major goals of neuroscience are to understand the development, structure and function of the human brain, and to use this knowledge to overcome neurological and mental disorders. However, most research has been limited to non-invasive imaging analysis and methods using autopsy brains and cell lines, making it difficult to conduct experimental research on the human brain itself. Animal models have long been used as a means of analogizing the human brain, but it is unclear whether they are sufficient to elucidate the unique structure and function of the human brain, which differs from that of animals. Under these circumstances, the discovery of human pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), has expanded the possibilities for human biological research. In the 2000 s, a series of technologies were developed to induce differentiation of PSCs into neural cells and further neural tissue with three-dimensional structure. Such PSC-derived 3D neural tissues used to be variously called brain organoids, minibrains, or SFEBq cultures or spheroids, but recently uniformly referred to as “neural organoids” (Pasca et al., 2022). Organoids are miniature organ-like 3D structures that are differentiated in vitro from stem cells to recapitulate the cytoarchitecture of in vivo organs. Neural organoids are differentiated by utilizing the self-organizing properties of stem cells to recapitulate the process of 3D organogenesis. To date, various protocols have been developed for constructing neural organoids for various brain regions. Neural organoids, which serve as substitute specimens for living human brain tissue, are revolutionizing human brain research because they enable studies at the molecular, cellular, and tissue levels. In this article, we review recent development of technologies on neural organoids and discuss the current status and future direction of these technologies and their application to human brain research.
2. Emergence of neural organoids as 3D culture models for neural development
ESCs, derived from the inner cell mass of blastocysts of mouse (Evans and Kaufman, 1981) and human (Thomson et al., 1998), can differentiate into any types of embryonic tissues, representing their pluripotent nature. iPSCs, another type of cells with pluripotency, were later generated by reprogramming of somatic cells (Takahashi and Yamanaka, 2006). These PSCs have been extensively used for biological research including stem cell biology and developmental biology. In the 1990 s, significant progress was made in the field of developmental biology on the mechanism of the neural induction. Neural induction is the earliest step in the neural fate determination in the embryonic ectoderm. Neural fate is induced by neural inducers, including Noggin, Follistatin and Chordin (Smith and Harland, 1992, Lamb et al., 1993, Hemmati-Brivanlou and Melton, 1994, Sasai et al., 1994, Sasai et al., 1995), which antagonize the activity of BMP, an inducer of the mesoderm and endoderm (Sasai et al., 1995, Piccolo et al., 1996, Zimmerman et al., 1996, Fainsod et al., 1997). The findings led to the “neural default model”, in which ectodermal cells are autonomously differentiated into a neural fate in the absence of BMP signaling (Hemmati-Brivanlou and Melton, 1997, Sasai and De Robertis, 1997). In support of this model, mouse ESCs selectively differentiate into neural progenitors when cultured in serum-free media without patterning factors (Fig. 1) (Tropepe et al., 2001, Ying et al., 2003, Watanabe et al., 2005, Smukler et al., 2006). Neural differentiation was facilitated by suppression of both BMP and Nodal/Activin signaling (dual SMAD inhibition) (Chambers et al., 2009). The regional properties of neural progenitors are specified by a combination of embryonic patterning signals along the dorsal-ventral (DV) and anterior-posterior (AP) axes including Sonic hedgehog (SHH), retinoic acid, and WNTs (Nordstrom et al., 2002, Wichterle et al., 2002, Mizuseki et al., 2003, Muguruma et al., 2010, Niehrs, 2010). Serum-free media with an inhibitor of SHH enabled differentiation of cortical neurons in a sequential order as in vivo (Gaspard et al., 2008).
Fig. 1.
Emergence and innovation of neural organoid protocols. Chronology of the emergence of key methods related to neural organoid differentiation. Blue, yellow, magenta and green colors indicate differentiation protocols for human neural cells, 3D neural organoids, nonneuronal cells and advanced protocols, respectively.
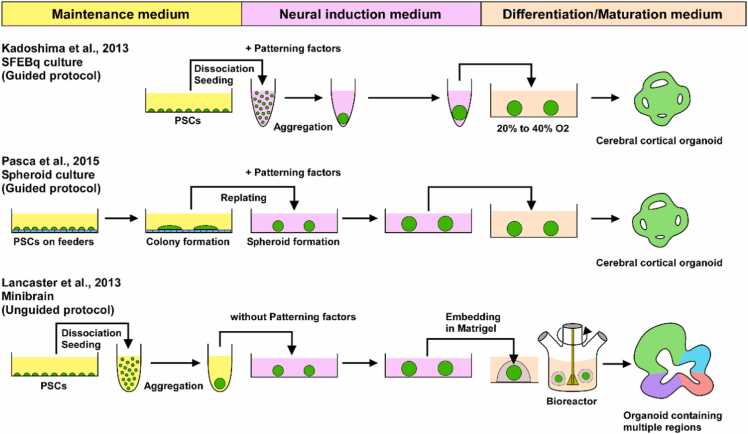
When cultured in suspension on non-adhesive substrates, PSCs form 3D aggregates called embryoid bodies (EBs) (Evans and Kaufman, 1981, Martin, 1981). EBs spontaneously differentiate into derivatives of the three germ layers, ectoderm, mesoderm and endoderm. They often exhibit tissue-like morphogenesis including neural structures. Based on the findings on EB studies and the neural induction in serum-free conditions, ESCs were efficiently differentiated into 3D neural tissues in serum-free culture of embryoid body-like aggregates (SFEB culture) (Watanabe et al., 2005). The SFEB method was later modified to facilitate 3D neural tissue differentiation by combining it with quick reaggregation of dissociated PSCs, which was called SFEBq method (SFEB with quick reaggregation) (Eiraku et al., 2008, Wataya et al., 2008). In SFEBq culture, ESCs are dissociated to minimize the effect of substrate or matrix, and subsequently reaggregated in a low-attachment 96 well plate. Neural differentiation occurs autonomously in the absence of extrinsic neural inducers. ESCs cultured in this manner efficiently generated cortical progenitors, which further form 3D tissue-like structures with layer-specific neurons in a sequential manner similar to the cortical neuroepithelium (Eiraku et al., 2008). SFEBq culture recapitulated in vivo neural development and formed 3D tissues resembling the embryonic neural tissues, later termed “neural organoids” (Pasca et al., 2022). Importantly, SFEBq-cultured PSCs have a tendency to adopt the rostral forebrain fate unless they receive any patterning signals. ESCs efficiently generated telencephalic progenitors when endogenous WNT signals were suppressed by WNT inhibitors (Eiraku et al., 2008). Cultured in complete growth factor-free medium, even without insulin, ESCs differentiated into the rostral hypothalamus (Wataya et al., 2008), consistent with the notion that the rostral hypothalamus could be assigned as the rostral-most region of the neural tube and the default status of the naive neuroectoderm. During embryonic development, the nervous system differentiates into distinct regions with different functions depending on their position along the AP and DV axes. The fate of the neural progenitors is determined by positional information provided by morphogenic patterning signals. Based on this idea, mouse ESCs were differentiated into 3D neural tissues with the characteristics of specific regions, including cerebral cortex (Eiraku et al., 2008), hypothalamus (Wataya et al., 2008), retina (Eiraku et al., 2011), adenohypophysis (Suga et al., 2011), cerebellum (Muguruma et al., 2010) and thalamus (Shiraishi et al., 2017). SFEBq culture was further applied to human ESCs and iPSCs, and succeeded in differentiation of 3D human neural tissues, including cerebral cortex (Kadoshima et al., 2013), hippocampus (Sakaguchi et al., 2015), retina (Nakano et al., 2012), cerebellum (Muguruma et al., 2015). SFEBq-based organoids that generate specific regions of neural tissues by the addition of patterning signals have been classified as patterned organoids, specified organoids or directed organoids, but have recently been uniformly referred to as guided organoids (Pasca et al., 2022).
Independently of the SFEBq method, Paşca et al. reported a technique to generate guided neural organoids using a slightly different method, called the spheroid culture (Pasca et al., 2015). In this method, pluripotent stem cells are first cultured on feeder cells while maintaining their undifferentiated nature, and then these colonies are transferred to low-attachment plates and 2D-cultured in neural induction medium with dual SMAD inhibitors to produce neural spheroids. The neural spheroids were further differentiated in neural differentiation medium, forming large 3D neural organoids. This method efficiently produces neural organoids with the property of pallium or dorsal forebrain, whereas culture in the presence of ventralizing factors directs them into the property of subpallium (Pasca et al., 2015, Sloan et al., 2018).
Both the SFEBq and spheroid methods have succeeded in efficiently producing region-specific brain tissues by directional differentiation of multicellular populations with patterning molecules. On the other hand, the group of Knoblich developed a technique to produce organoids containing tissues from multiple regions, called “mini-brains” (Lancaster et al., 2013). They first encapsulated EBs derived from pluripotent stem cells in Matrigel, a basement membrane matrix, and cultured them in a medium for neural differentiation that did not contain patterning molecules. This method was later classified as “unguided protocol”. Differentiation would be induced in a relatively free environment without specific positional information. The resulting organoids are dorsal forebrain-dominant but contain different regions such as the midbrain and hindbrain at the same time. Minibrains have the advantage of producing tissue from the entire brain at once, but the frequency of appearance of each tissue is stochastic and biased, making it difficult to control reproducibility and brain region specificity.
3. Construction of human neural organoids
Neural organoids were initially generated from mouse ESCs, but have since been applied to human ESCs and recently to iPSCs. Unlike mouse PSCs, human PSCs are susceptible to apoptotic death by anoikis in a dispersed state detached from the extracellular matrix ECM. Thus, the cell dissociation step in human organoid differentiation requires the addition of ROCK inhibitor, which suppresses the cell death and promotes cell proliferation (Watanabe et al., 2007). Currently, various protocols have been developed to construct neural organoids for different regions of the human nervous system. In Fig. 2, we summarize the three fundamental protocols for neural organoid differentiation that are the basis for the various improved methods. Human neural organoids can recapitulate human developmental processes to a certain extent in a culture system, and are being applied in many fields, including developmental research, elucidation of neural functions, pathophysiology of neurological and psychiatric disorders, and regenerative medicine of the nervous system. In this section, we describe the methods used to produce different types of neural organoids as well as the results of research using the organoids generated by these methods.
Fig. 2.
Basic protocols for neural organoids. Timeline of the three fundamental protocols for differentiation of neural organoids from human PSCs.
3.1. Unguided organoids
In an unguided approach (Lancaster et al., 2013, Lancaster and Knoblich, 2014), PSCs were aggregated in a low-attachment well plate to form EBs, which were further induced into a neuroectodermal fate in neural induction medium without the addition of extrinsic patterning factors. The neuroectodermal EBs were then embedded in droplets of Matrigel. Matrigel, prepared from extracts of Engelbreth-Holm-Swarm mouse sarcoma, contains complex and varying amounts of components, but is enriched in ECM basement membrane components, such as laminin, entactin, collagen, proteoglycans and growth factors. Matrigel promotes the protrusion of continuous neuroepithelial buds and the formation of a luminal structure with apical-basal polarity. Although Matrigel embedding is a key step in the construction of organoids, the undefined nature and lot-to-lot variations of Matrigel and batch-to-batch variations in embedding procedures may be the cause of reduced reproducibility and success rate in organoid differentiation. As another key step of the protocol, Matrigel-embedded EBs are cultured in a differentiation medium in a spinning bioreactor or orbital shaker. The continuous agitation, facilitating the diffusion of oxygen and nutrients, promotes the growth and maturation of the organoids. The unguided protocol, which largely depends on the self-organizing activity, generates diverse tissues with multiple regional identities. Immunohistochemical and RNA expression analyses revealed that the organoids generate a broad diversity of cells from the forebrain including the cerebral cortex, the midbrain, the hindbrain, the retina and the choroid plexus (Lancaster et al., 2013, Quadrato et al., 2017). Functional analysis using electrophysiological recording and calcium imaging revealed that the organoids elicit neuronal activity and can respond to light stimulation through photoreceptors in the retinal tissue (Lancaster et al., 2013, Quadrato et al., 2017). The unguided protocol was further improved for efficient growth of organoids by pulse application of CHIR99021, GSK3β inhibitor that activates non-canonical WNT pathway, and by usage of microfilaments as a scaffold of organoids (Lancaster et al., 2017). The unguided protocol tends to induce forebrain, especially the cerebral cortex. This means that the forebrain is more likely to be induced in the absence of patterning signals (Watanabe et al., 2005, Gaspard et al., 2008, Wataya et al., 2008). The activity of intrinsic signals is largely responsible for the batch-to-batch differences. Studies involving comparative analysis should take into account intrinsic signaling activities in the organoids.
As mentioned above, even in the organoids induced by the unguided protocol, the main area of analysis is the cerebral cortex. So far, the unguided protocol has been used in the modeling of microcephaly, the analysis of high-risk genes for autism spectrum disorder and the comparative studies with non-human apes in the evolutionary developmental biology (Lancaster et al., 2013, Benito-Kwiecinski et al., 2021, Li et al., 2023). Interestingly from an evolutionary perspective, human brain organoids are more expanded in size than nonhuman primate organoids (Benito-Kwiecinski et al., 2021). One of the characteristics of the primate brain, including humans, is the formation of the cerebral gyrus, but the gyrification is rarely recapitulated in human organoids. However, it has been reported that human organoids, but not mouse organoids can expand and induce gyrus-like folding by enhancing PTEN-AKT signaling (Li et al., 2017). Recently, to avoid significant batch-to-batch variability and low reproducibility of cell and regional identities due to the unguided procedure complexity, a simple culture protocol was reported that omit complex media exchange and Matrigel embedding (Eigenhuis et al., 2023). The simplified culture protocol tends to induce primarily the cortex, reproducing previous reports that the forebrain is more likely to be induced in the absence of patterning signals (Watanabe et al., 2005, Gaspard et al., 2008, Wataya et al., 2008).
3.2. Guided organoids
3.2.1. Cerebral cortex
An important feature of PSC-derived neural progenitors is that they adopt the rostral forebrain fate unless they receive caudalizing signals, in accordance with the “Nieuwkoop’s activation-transformation model” (Stern, 2001, Rallu et al., 2002, Wilson and Houart, 2004, Levine and Brivanlou, 2007). In the guided approach, PSCs are differentiated into specific neural identities in the presence of extrinsic patterning signals. During neural development of amniotes, the regional patterning occurs first along the AP axis and then along the DV axis. A number of guided protocols have been reported and an increasing number of analyses have been performed using them. However, some of these, although termed organoids, lack adequate histological analysis and some reports only analyze features at the single cell level, which does not guarantee the quality of the organoids. This review focused on reports in which certainly of organoid formation has been assessed by histological analysis, such as immunohistochemistry.
During embryogenesis, the telencephalic anlage is subdivided into the PAX6+ dorsal region (the pallium), which forms the cortex, cortical hem and choroid plexus, and the PAX6– ventral region (the subpallium), which forms the basal ganglia. The mammalian neocortex has a multilayered structure (layers I-VI), which arises from the neuroepithelium of the dorsal telencephalon. Layer I, known as the marginal zone (MZ) of the fetal primordium, consists mainly of RELN+ Cajal-Retzius cells. It is qualitatively different from other layers. The rest of the cortical layers are generated with the “inside-out” pattern: the deeper the layer, the earlier the neurons are born from the cortical progenitors and these neurons express TBR1 and CTIP2. The neurons of the upper layer, which express SATB2 and CUX1, are found later in the organoids.
Sasai group reported one of the first descriptions of regionalized brain organoids. SFEBq in combination with dual SMAD and WNT inhibitions resulted in cortical induction (Eiraku et al., 2008, Kadoshima et al., 2013). In this self-organization culture, cortical NE self-form and spontaneously develop ventricular zone (VZ) (SOX2+, PAX6+), CP, and MZ by the culture day 40–45. The cortical organoids recapitulate the features of cortical development such as temporal order of neurogenesis. Several groups have also used dual SMAD inhibition (e.g., SB431542, LDN193189, dorsomorphin, Noggin) and WNT (e.g., IWP2, IWR-1-endo, XAV939) inhibition to generate cortical organoids (Mariani et al., 2015, Pasca et al., 2015, Qian et al., 2016, Xiang et al., 2017, Qian et al., 2020).
The human cerebral cortex contains cortical stem cells known as outer radial glia (oRG), expressing HOPX and PTPRZ1. These are located in the outer subventricular zone (oSVZ) and are not observed in the rodent cortex. RG development is essential for expansion, cellular diversity and organization in the cerebral cortex. oRG cells express receptors for the leukemia inhibitory factor (LIF) during neurogenesis, which is consistent with a role for stem cells in self-renewal. The cortical organoids produced a well-developed oSVZ and a diversity of neuronal subtypes. Recent studies using cortical organoids observed that LIF was sufficient to increase the size of the oRG domain and the production of astrocytes and inhibitory interneurons (Dezonne et al., 2017, Watanabe et al., 2017, Andrews et al., 2023). In addition, large-scale experiments comparing gene expression between organoids and human fetal tissues (Amiri et al., 2018, Pollen et al., 2019, Velasco et al., 2019, Gordon et al., 2021), as well as comparing organoids using different protocols (Bhaduri et al., 2020, Tanaka et al., 2020), have been made possible by recent advances in scRNA-seq. This makes it possible to study the specific features of human cortical development and disease modeling.
3.2.2. Dorsomedial telencephalon
The dorsocaudal side of the neocortex is flanked by the cortical hem, whereas its ventrorostral side is neighbored by the lateral ganglionic eminence (LGE; striatum anlage) and septum. The cortical hem plays a key role in the formation of dorsomedial telencephalic tissues, such as the hippocampus and choroid plexus, and is a source of WNT and BMP signaling in the dorsomedial telencephalon. In line with this principle of development, the Sasai group used temporal WNT activation and BMP4 treatment of neuroectodermal organoids for the recapitulation of hippocampal primordium tissue (Sakaguchi et al., 2015). Further, prolonged activation of WNT and BMP gave rise to choroid plexus organoids (Pellegrini et al., 2020). The choroid plexus organoids formed a tight barrier and produced a fluid that is similar to the cerebrospinal fluid. This organoid allows us to study the function of the choroid plexus during brain development and provides a useful tool for assessing the permeability of new drugs.
3.2.3. Ventral telencephalon
The ventral telencephalon (subpallium) is further specified to form three ganglionic eminences, the septum, and the ventral-most telencephalic stalk regions. The ganglionic eminences consist of three anatomical subdivisions, lateral (LGE), medial (MGE), and caudal (CGE), which are distinguished by molecular markers and the cell types that they produce. The LGE and MGE generate the two large nuclei in the telencephalic basal ganglia, the striatum and the globus pallidus, respectively. A substantial portion of the MGE and CGE derivatives migrate dorsally and give rise to cortical interneurons (Hansen et al., 2010, Ma et al., 2022). During forebrain development, SHH signaling is critical for dorsal-ventral patterning. By combining the activation of SHH signaling (e.g., purmorphamine, smoothened agonist SAG) with organoids differentiated into neural ectoderm by dual SMAD inhibition, ventral forebrain organoids including the ganglionic eminence are induced (Kadoshima et al., 2013, Bagley et al., 2017, Birey et al., 2017, Watanabe et al., 2017, Xiang et al., 2017, Kim et al., 2019, Miura et al., 2020). The ventral forebrain organoids and ganglionic eminence organoids contained GABAergic interneurons with physiological properties. Interestingly, co-culturing ventral and dorsal forebrain organoids in fused explant culture (called an assembloid), as is common in embryology, recapitulate the tangential migration of GABAergic interneurons seen in vivo (Bagley et al., 2017, Birey et al., 2017, Xiang et al., 2017, Kim et al., 2019, Miura et al., 2020).
3.2.4. Diencephalon
Although most studies have focused on brain organoids that represent the forebrain of telencephalic origin, a number of other region-specific brain organoids have been described that take advantage of the ability to drive region-specific patterning as described above. The diencephalon, which gives rise to the thalamus and hypothalamus, is a part of the vertebrate neural tube. A combination of WNT, BMP4 and SHH (including SAG) induced the hypothalamic organoids containing neuropeptidergic neurons (Qian et al., 2016, Miwata et al., 2023) and hypothalamic arcuate organoids (Huang et al., 2021). The thalamic organoids were generated by prepatterning with insulin and MAPK/ERK inhibitors. Insulin is essential for cell survival but has a caudalizing effect on neural tube (Wataya et al., 2008, Muguruma et al., 2010). The MAPK/ERK inhibitors (e.g., PD325901) were used to prevent insulin-induced excessive caudalization toward the mesencephalon. BMP7 stimulation accelerates the commitment of the diencephalic fate into the thalamic identity (Xiang et al., 2019, Kiral et al., 2023). The assembly of the thalamic organoid with the cortical organoid created the reciprocal projections as seen in vivo. This system will provide a model for investigation of cortico-thalamic neural network in human.
3.2.5. Retina
Retinal organoids are one of the best characterized organoid models. The retina is derived from the rostral diencephalon. During early development, the retinal neuroepithelium, expressing RAX, expands outward (evaginates) from the diencephalic wall to form an optic vesicle. The distal part of the vesicle, which is in contact with the surface ectoderm, becomes fated to the neural retina (NR), whereas the proximal part differentiates into the retinal pigment epithelium (RPE). The optic vesicle then folds inwards (invaginates) at its distal region to form a two-layered cup-like structure, the optic cup, with the NR and RPE being the inner and outer layers, respectively. In the postnatal eye, the NR shows a stratified structure with different types of components including photoreceptors, horizontal cells, and ganglion cells. Over the years, it had generally been thought that this dynamic transformation is triggered by chemical and physical influences from other tissues, such as lens or cornea (for a more depth review, see (Sasai et al., 2012)).
Sasai group established a series of techniques based on the SFEBq culture. The RPE and NR of the outer and inner cell layers of the spontaneously formed tissues were confirmed by gene expression, indicating that the development of the optic cup had been recapitulated in vitro. Importantly, this occurred in the absence of external signaling sources, such as the lens, demonstrating the ability to self-organization (Nakano et al., 2012). Then they developed a culture method for the selective differentiation of NR by timed BMP4 treatment. They found that inhibition of GSK3 and FGFR induced a transition from NR tissue to RPE, and that removal of this inhibition caused the RPE-like tissue to revert back to NR fate (Kuwahara et al., 2015). This stepwise induction-reversion method produced retinal organoids with RPE at the margin of central-peripherally polarized NR. In the retinal organoids, rod and cone photoreceptors and ganglion cells are differentiated and organized into multilayered tissue. Recently, retinal organoid culture protocols have been developed to elucidate the molecular mechanism of retinal development and for medical application (Meyer et al., 2011, Zhong et al., 2014, Wahlin et al., 2017, Kuwahara et al., 2019, Kruczek and Swaroop, 2020).
3.2.6. Pituitary
The optic cup self-formation occurs homogeneous cells without external interactions. In contrast, the development of anterior pituitary gland (adenohypophysis) is a more complex process. The anterior pituitary gland secretes several important systemic hormones, such as adrenocorticotropic hormone (ACTH) and growth hormone. The rest of the pituitary gland is the neurohypophysis (posterior pituitary), which contains axons of hypothalamic vasopressin- and oxytocin-producing neurons. During early development, the adenohypophysis anlage originates as a placode in the non-neural head ectoderm adjacent to the anterior neural plate. The thickened placodal epithelium invaginates and subsequently detaches from the oral ectoderm, becoming a hollowed epithelial vesicle, Rathke’s pouch. The development of Rathke’s pouch depends on tissue interactions between the rostral head ectoderm and the rostral hypothalamus (Kelberman et al., 2009). Sasai group showed that the default fate of PSCs in SFEBq culture is rostral hypothalamus (Wataya et al., 2008). The anterior pituitary organoids were generated by modifying this culture system to induce oral ectoderm in addition to the hypothalamus (Ozone et al., 2016). To co-induce non-neural oral ectoderm and hypothalamic neuroepithelium in the organoids, BMP4 and SHH agonist SAG is critical for initial step in culture. Recently, to elucidate the molecular mechanism of development and for medical application, the pituitary organoid culture protocols have been developed (Kanie et al., 2019, Matsumoto et al., 2020, Taga et al., 2023).
3.2.7. Midbrain
The midbrain, or mesencephalon, is anatomically connected caudally to the hindbrain and rostrally to the diencephalon. The initial phase of midbrain development depends on the formation and function of the isthmic organizer, which lies at the midbrain-hindbrain boundary (MHB). This organizer is formed by the intricate regulatory functions of region-specific transcription factors (Nakamura et al., 2005). The transcription factors are under the control of FGF8, which is secreted by the isthmic organizer itself. Following the onset of FGF8 expression, the expression of Wnt1 starts in the MHB. These two factors form a positive feedback loop to drive each other’s expression and act as organizer factors that pattern the tissues around the MHB.
The stratified midbrain organoids were generated to contain functional neurons such as midbrain dopaminergic neurons and neuromelanin-containing subtypes (Tieng et al., 2014, Jo et al., 2016, Qian et al., 2016, Monzel et al., 2017, Smits et al., 2019, Kwak et al., 2020). The neural ectoderm generated by dual SMAD inhibition was treated with WNT agonist CHIR99021 and further exposed SHH (including SAG, purmorphamine) along with FGF8 for floor plate induction. Song lab used a very similar approach as mentioned above, but after the derivation of floor plate they were transferred to miniaturized spinning bioreactor, produced midbrain organoids that contained TH-positive dopaminergic neurons (Qian et al., 2016).
3.2.8. Hindbrain
In the cerebellar organoid differentiation, neural induction is achieved by dual SMAD inhibition. Subsequent addition of FGF2 and insulin promotes the formation of isthmic organizer-like structures. The stepwise progression of cerebellar patterning is realized by the initial induction of isthmic organizer, closely mimicking the in vivo situation (Muguruma et al., 2015). FGF2 promotes the generation of cerebellar neurons, including Purkinje cells and other GABAergic cell types. The addition of FGF19 and SDF1 results in the production of apically-basally polarized cerebellar plate NEs that contain rhombic lip-like structure from which cerebellar granule cells are generated. The cerebellar plate NE in the organoids consisted of a multilayered structure, VZ, intermediate layer containing progenitors of Purkinje cells, and an outermost layer occupied by derivatives of the rhombic lip (Muguruma et al., 2015). scRNA-seq experiments suggested that the major cell types of the cerebellum are produced in human cerebellar organoids (Ballabio et al., 2020, Nayler et al., 2021). The developmental steps of cerebellar organoids recapitulate in vivo (van Essen et al., 2020, Nayler et al., 2021). The cerebellar organoids have been used to elucidate the molecular mechanism of cerebellar development and for medical application (van Essen et al., 2020, Nato et al., 2021, Nayler et al., 2021, Kamei et al., 2023).
3.2.9. Spinal cord
The spinal cord is formed via neurulation during early embryonic development. The posterior part of the neural tube develops into the spinal cord, which contains more than 20 classes of neurons. Spinal cord identity can be induced by the combinational addition of caudalizing signals such as retinoic acid, WNT and FGF. The distinct neuronal subtypes are determined by WNT emanating from the roof plate and SHH secreted from the floor plate. Recently, several groups have reported the generation of spinal cord organoids (Hor et al., 2018, Ogura et al., 2018, Andersen et al., 2020, Faustino Martins et al., 2020, Lee et al., 2022). Andersen et al. generated human cortico-spinal-muscle assembloids with synaptically connected projections leading to the control of muscle cell contraction (Andersen et al., 2020). Interestingly, Faustino Martins et al. generated neuro-muscular organoids through neuromesodermal progenitors derived from human PSCs (Faustino Martins et al., 2020). These spinal cord organoids will provide useful model for investigation of development and medical applications.
4. Advance in organoid technologies
Various methods have been developed to generate different types of neural organoids, and have succeeded to some extent in recapitulating the development of nervous system in culture, but these methods still have many limitations. In this section, we will discuss the problems faced by current methods and attempts to improve them in order to faithfully recapitulate the neural development.
One of the major problems in organoid production is the issue of reproducibility, as the quality of the organoids produced varies from cell line to cell line and from batch to batch, even when using the same protocol. In unguided protocols with a high degree of freedom in differentiation, stochastic generation of diverse tissues is inevitable (Lancaster et al., 2013, Lancaster and Knoblich, 2014), but even in guided protocols where patterning signals provide directional differentiation, considerable variation in differentiation can occur. The low success rate and inconsistency of tissue formation has been reported to be improved by using artificial microfilaments as scaffolds for organoids and by adding extracellular matrix to the maturation process (Lancaster et al., 2017). Organoid differentiation starts with culturing PSCs in an undifferentiated state and the condition of PSCs at this stage affects their ability to form organoids. PSCs used to be cultured on fibroblast feeders to keep them in an undifferentiated state, but recently they are often cultured in a feeder-free state due to the ease of handling and to avoidance of animal-derived components (xeno-free condition). Recent studies suggested that PSCs cultured without feeders may exhibit a reduced capacity to form organoids compared to PSCs cultured on feeders (Kuwahara et al., 2019, Ideno et al., 2022, Watanabe et al., 2022), possibly due to differences in epigenetic status of PSCs. The low competence in organoid differentiation can be improved by preconditioning with patterning signals (Kuwahara et al., 2019, Taga et al., 2023), adding TGFβ family growth factors (Watanabe et al., 2022) or suppression of FGF signaling (Ideno et al., 2022) during maintenance culture of PSCs prior to neural differentiation.
Organoids differentiate into neural tissues through a neural induction step. Then they grow larger by being cultured in the medium for differentiation and maturation. Unlike when started from mouse PSCs (Eiraku et al., 2008, Eiraku et al., 2011), organoids derived from human PSCs are larger in size and form more complex structures (Nakano et al., 2012, Kadoshima et al., 2013), reflecting differences in in vivo structures between species. Even among primate species, neural organoids from human PSCs have been reported to be more expanded in size than organoids from nonhuman primate PSCs (Benito-Kwiecinski et al., 2021). It appears that the species of the source PSCs may influence the size and complexity of the resulting organoids. They can grow up to several millimeters in diameter over several months, but their growth rate slows down once they reach a certain size. The surface of the organoid expands the neural tissue structures, but its center becomes filled with necrotic tissue (Kadoshima et al., 2013). In fact, it has been reported that in organoids cultured for long periods of time, a group of genes related to cellular stress are upregulated (Pollen et al., 2019, Bhaduri et al., 2020, Gordon et al., 2021). Organoids get nutrients and oxygen from the medium and release waste products to the outside, but they rely on passive diffusion of liquid and gas for these exchanges. As the organoids grow larger, it's not enough for liquid or gas to move by itself, which could suppress cell growth and cause cell death. This problem of diffusion limit can be ameliorated to some extent by continuous agitation of the medium in the bioreactor (Lancaster and Knoblich, 2014, Lancaster et al., 2017), culturing under high oxygen concentrations (Kadoshima et al., 2013) or regular cutting of the organoids in half every two weeks (Kadoshima et al., 2013). This problem is more effectively solved by slicing the organoids and culturing them at the air-liquid interface (Giandomenico et al., 2019, Qian et al., 2020), which increases the diffusion and cell survival at the expense of a partial loss of 3D tissue structure.
An alternative approach to enhance organoid maturation would be to vascularize the organoids. The living brain becomes vascularized as development proceeds, but vascular cells of mesodermal origin rarely arise in neural organoids of neuroectodermal origin. Attempts are being made to integrate vascular endothelial cells into neural organoids. Incorporation of human umbilical vein endothelial cells (Shi et al., 2020), directly converted endothelial cells by forced expression of transcription factors (Cakir et al., 2019) or mesodermal progenitors (Worsdorfer et al., 2019) facilitates the formation of endothelial network surrounding the neural organoids, though the functional improvement remains unclear.
Although neural organoids recapitulate the differentiation of various cell types that constitute human neural tissues, they rarely generate certain cell types such as glial cells. Similar to in vivo neural development, neural stem cells in the organoids first generate neurons and subsequently produces astrocytes. Astrocytes are efficiently generated and maturated in long-term culture of cortical organoids (Pasca et al., 2015, Sloan et al., 2017). Oligodendrocytes were rarely generated in cortical organoids, but addition of supplemental growth factors such as PDGF and T3 (triiodo-L-thyronine) can induce oligodendrocyte precursor cells and differentiation of oligodendrocytes for myelination (Hubler et al., 2018, Madhavan et al., 2018, Marton et al., 2019). Microglia, the mesoderm-derived immune cells in the CNS, are not usually generated in the guided neural organoids. However, the unguided protocol without using dual SMAD inhibitors can spontaneously differentiate mesodermal cells with similar characteristics to microglia (Ormel et al., 2018). In vitro protocols have been developed to differentiate PSCs into microglial cells (Muffat et al., 2016), which can be integrated into neural organoids to study neuro-immune interactions (Abud et al., 2017, Brownjohn et al., 2018, Lin et al., 2018, Song et al., 2019, Xu et al., 2021).
Because guided protocols are optimized to induce neural tissue in specific regions, they are suitable for studying individual regions, but not for studying large regions or interactions between regions such as long-range neural circuits. On the other hand, unguided protocols induce neural tissue over a wide area of the brain in the same organoid, but the regions generated are stochastic and unpredictable, making them difficult to control. This problem can be solved by the “assembloid” approach, in which multiple organoids that are separately differentiated into specific brain regions using guided protocols are fused and co-cultured together (Bagley et al., 2017, Birey et al., 2017, Xiang et al., 2017). In the first series of studies on the assembloid, two types of organoids, dorsal forebrain and ventral forebrain, were combined in co-culture to recapitulate cell migration of GABAergic interneurons from the ventral ganglionic eminence to the dorsal cerebral cortex (Bagley et al., 2017, Birey et al., 2017, Xiang et al., 2017). The assembloid protocols were further applied to coculture of different combination of organoids for investigation of long-range axonal connections that include thalamo-cortical and cortico-thalamic (Xiang et al., 2019), cortico-striatal (Miura et al., 2020), hypothalamo-pituitary (Kasai et al., 2020), cortico-spinal-muscle (Andersen et al., 2020) and retino-thalamic (Fligor et al., 2021) projections.
Another limitation of neural organoid protocols is the lack of topographic organization within the organoids. Although AP or DV polarity is seen in some cases (Kadoshima et al., 2013, Muguruma et al., 2015), clear and consistent topographic patterning was not created within the organoids. One approach to facilitate topographic patterning is to provide a signaling center by applying a point source of morphogen. For example, cell aggregates expressing SHH, a ventralizing signaling protein, were placed at one pole of a telencephalic organoid to generate dorsal-to-ventral telencephalic tissue in a spatially graded manner (Cederquist et al., 2019). Regional patterning can also be achieved in a more sophisticated way by use of microfluidic devices. The spatial gradient of WNT signals provided by microfluidics has been used to achieve AP patterning in the neural organoids (Rifes et al., 2020).
Neural organoids have succeeded in recapitulating human neural development to some extent, but they do not have exactly the same 3D cytoarchitecture as the living brain. In human ontogeny, the neural ectoderm-derived neural plate invaginates and folds at the midline to form the neural tube, which give rise to the CNS. The neural tube is a single continuous neuroepithelial structure surrounding a single lumen with an apical-in and basal-out orientation. In addition to the apical-basal polarity, the neural tube is also polarized along the AP and DV axes. On the other hand, neuroepithelial tissues in organoids, regardless to the differentiation protocol used, tend to be divided into a number of small pieces, each of which takes the form of a sphere called a neural rosette (e.g., (Kadoshima et al., 2013, Lancaster et al., 2013, Pasca et al., 2015). The neural rosette locally recapitulates the neuroepithelial structure with a proper apical-basal orientation and concentric laminar organization. However, such formation of neural rosettes seen in differentiating organoids is a phenomenon that is not seen during the formation of the neural tube in ontogeny. The assembly of fragmented neuronal rosettes has a geometrical structure that is quite different from that of living brain tissues without obvious AP and DV polarity. Such fragmentation may be the limiting factor for the long-term growth of organoids and the formation of large, polarized tissues that faithfully recapitulate the 3D cytoarchitecture of the brain. Kadoshima et al. reported that neuroepithelial tissue, which initially surrounds the organoid surface during the neural induction stage, invaginates to form spherical neural rosettes with apical-in polarity during the stage of differentiation (Kadoshima et al., 2013). This invagination process can occur stochastically at multiple locations on the surface, which could lead to the breakdown of the neuroepithelium and the formation of fragmented rosettes.
Attempts have been made to generate continuous neuroepithelial structures with the correct polarity. In mouse ESCs, the addition of the ECM components laminin and entactin during the neuronal induction stage was successful in producing continuous, polarized neuroepithelial structures (Nasu et al., 2012), although the effectivity to differentiation of human PSCs remains unknown. ECM, which is abundant at the basal surface of the epithelium, functions in the establishment of apical-basal polarity. An abundance of ECM in the environment leads to an apical-in polarity, whereas its deficiency leads to an apical-out polarity in the epithelium formed by 3D cell aggregates (Yonemura, 2014). ECM components facilitate the spontaneous formation of epithelial structure with a lumen in 3D culture of PSCs (Taniguchi et al., 2015), which further recapitulates the development of epiblast and amniotic sac (Shao et al., 2017). It is expected that ECM may be involved in the formation of large contiguous neuroepithelial tissues in neural organoids, but the effect of ECM is a subject for further study.
We have discussed the generation of organoids with a focus on nervous system differentiation. Recently, using an approach different from neural organoids, attempts have been made to recapitulate human embryogenesis by differentiation of PSCs (Shao and Fu, 2022). When cultured on 2D micropatterned ECM substrates under dual SMAD inhibition, PSCs differentiate into neuroectoderm. Subsequent BMP4 stimulation induces non-neural ectoderm differentiation in the periphery and medial-lateral patterning, forming concentric domains of neural plate, neural crest, sensory placode and epidermis (Xue et al., 2018, Britton et al., 2019, Tewary et al., 2019). Extended culture results in the formation of 2.5D flattened luminal neural tube-like structure called neuruloid, recapitulating the neurulation process (Haremaki et al., 2019). Further, Karzbrun et al. succeeded in forming of 3D neural tube-like structures (Karzbrun et al., 2021). Matrigel was added to PSCs seeded on 2D micropatterned substrates to create 3D epithelial structures with a single lumen. Then, the neuroectoderm was induced and stimulated with BMP4, leading to the folding of the central neuroectoderm by the peripheral non-neural ectoderm, resulting in the formation of a continuous neuroepithelium with a single lumen similar to the neural tube found in vivo. The neuruloid model has the potential to complement conventional neural organoid models and can be used to differentiate neural tissues that more faithfully recapitulate the human brain development.
Neural organoids can form tissues that resemble human brains in culture, but likely due to limitations of the culture environment, they are not able to fully recapitulate a living brain. One approach to evaluate the ability of neural organoids to differentiate and maturate is to transplant the organoids into experimental animals. Human neural organoids generated using guided or unguided protocols have been transplanted and integrated into the cerebral cortex (Daviaud et al., 2018, Mansour et al., 2018, Kitahara et al., 2020, Shi et al., 2020, Wang et al., 2020a, Wang et al., 2020b, Dong et al., 2021, Revah et al., 2022, Wilson et al., 2022, Jgamadze et al., 2023) and the cerebellum (Kamei et al., 2023) of rodents, and the cerebral cortex of primates (Kitahara et al., 2020). Long-range projections of efferents (Kitahara et al., 2020, Dong et al., 2021, Revah et al., 2022, Jgamadze et al., 2023) from and to the organoids were demonstrated. Furthermore, it has been shown that organoids implanted in the somatosensory cortex respond to sensory stimuli, and that stimulation of the organoids elicits reward-seeking behavior in the animals (Revah et al., 2022). In the visual system, implanted organoids have been shown to respond to visual stimuli (Wilson et al., 2022, Jgamadze et al., 2023) and even to acquire orientation selectivity as seen in vivo (Jgamadze et al., 2023). Thus, transplantation studies are expected to be useful in assessing the functional aspects of neural organoids.
5. Conclusion
Taking advantage of advances in stem cell biology and developmental biology, the neural organoid technology emerged to generate neural tissues from pluripotent stem cells by recapitulating in vivo neural development. Since then, various protocols have been developed to generate a variety of neural tissues. These neural organoid technologies provide excellent experimental models for studying human neural development. Organoid technology is currently advancing rapidly, but at the same time faces significant problems that need to be solved. Current limitations include insufficient reproducibility of organoid formation, cessation of growth at certain stages, and inability to fully reproduce mature tissue structures. In the future, it is expected that these problems will be overcome and that technologies will be developed to more faithfully construct human neural tissues, leading to a better understanding of the human nervous system. In this article, we have reviewed various studies related to the induction of neural organoid differentiation, with a focus on recapitulating human neural ontogenesis and elucidating the developmental mechanisms. From an application perspective, neural organoid technology is expected to be used in regenerative medicine, modeling of neurological diseases, and screening of therapeutic agents for diseases.
Research Ethics
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript. IRB approval was obtained (required for studies and series of 3 or more cases). Written consent to publish potentially identifying information, such as details or the case and photographs, was obtained from the patient(s) or their legal guardian(s).
Funding
Funding was received for this work. All of the sources of funding for the work described in this publication are acknowledged below: JSPS KAKENHI (21H03812 to K.M., 22H02961 to A.T.).
CRediT authorship contribution statement
Atsushi Tamada wrote the manuscript with Keiko Muguruma. All authors approved the final manuscript.
Declaration of Competing Interest
No conflict of interest exists.
Acknowledgments
We would like to thank Dr. Toshiya Kimura for valuable comments. K.M. express special thanks to Yoshiki Sasai with respect to his legacy in science. This research was supported by JSPS KAKENHI (21H03812 to K.M., 22H02961 to A.T.).
Authorship
The International Committee of Medical Journal Editors (ICMJE) recommends that authorship be based on the following four criteria:
-
1.
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
-
2.
Drafting the work or revising it critically for important intellectual content; AND
-
3.
Final approval of the version to be published; AND
-
4.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author Information
The authors declare no competing interests. Correspondence should be addressed to K.M.
References
- Abud E.M., Ramirez R.N., Martinez E.S., Healy L.M., Nguyen C.H.H., Newman S.A., Yeromin A.V., Scarfone V.M., Marsh S.E., Fimbres C., Caraway C.A., Fote G.M., Madany A.M., Agrawal A., Kayed R., Gylys K.H., Cahalan M.D., Cummings B.J., Antel J.P., Mortazavi A., Carson M.J., Poon W.W., Blurton-Jones M. iPSC-derived human microglia-like cells to study neurological diseases. Neuron. 2017;94:278–293. doi: 10.1016/j.neuron.2017.03.042. e279. https://doi.org/S0896-6273(17)30286-6 [pii] 10.1016/j.neuron.2017.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri A., Coppola G., Scuderi S., Wu F., Roychowdhury T., Liu F., Pochareddy S., Shin Y., Safi A., Song L., Zhu Y., Sousa A.M.M., Gerstein M., Crawford G.E., Sestan N., Abyzov A., Vaccarino F.M. Transcriptome and epigenome landscape of human cortical development modeled in organoids. Science. 2018:362. doi: 10.1126/science.aat6720. https://doi.org/10.1126/science.aat6720 eaat6720 362/6420/eaat6720 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J., Revah O., Miura Y., Thom N., Amin N.D., Kelley K.W., Singh M., Chen X., Thete M.V., Walczak E.M., Vogel H., Fan H.C., Pasca S.P. Generation of Functional Human 3D Cortico-Motor Assembloids. Cell. 2020;183:1913–1929. doi: 10.1016/j.cell.2020.11.017. e1926. https://doi.org/S0092-8674(20)31534-8 [pii] 10.1016/j.cell.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews M.G., Siebert C., Wang L., White M.L., Ross J., Morales R., Donnay M., Bamfonga G., Mukhtar T., McKinney A.A., Gemenes K., Wang S., Bi Q., Crouch E.E., Parikshak N., Panagiotakos G., Huang E., Bhaduri A., Kriegstein A.R. LIF signaling regulates outer radial glial to interneuron fate during human cortical development. Cell Stem Cell. 2023 doi: 10.1016/j.stem.2023.08.009. https://doi.org/S1934-5909(23)00292-8 [pii] 10.1016/j.stem.2023.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley J.A., Reumann D., Bian S., Levi-Strauss J., Knoblich J.A. Fused cerebral organoids model interactions between brain regions. Nat. Methods. 2017;14:743–751. doi: 10.1038/nmeth.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabio C., Anderle M., Gianesello M., Lago C., Miele E., Cardano M., Aiello G., Piazza S., Caron D., Gianno F., Ciolfi A., Pedace L., Mastronuzzi A., Tartaglia M., Locatelli F., Ferretti E., Giangaspero F., Tiberi L. Modeling medulloblastoma in vivo and with human cerebellar organoids. Nat. Commun. 2020;11:583. doi: 10.1038/s41467-019-13989-3. https://doi.org/10.1038/s41467-019-13989-3 583 10.1038/s41467-019-13989-3 [pii] 13989 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-Kwiecinski S., Giandomenico S.L., Sutcliffe M., Riis E.S., Freire-Pritchett P., Kelava I., Wunderlich S., Martin U., Wray G.A., McDole K., Lancaster M.A. An early cell shape transition drives evolutionary expansion of the human forebrain. Cell. 2021;184:2084–2102. doi: 10.1016/j.cell.2021.02.050. e2019. https://doi.org/S0092-8674(21)00239-7 [pii] 10.1016/j.cell.2021.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaduri A., Andrews M.G., Mancia Leon W., Jung D., Shin D., Allen D., Schmunk G., Haeussler M., Salma J., Pollen A.A., Nowakowski T.J., Kriegstein A.R. Cell stress in cortical organoids impairs molecular subtype specification. Nature. 2020;578:142–148. doi: 10.1038/s41586-020-1962-0. https://doi.org/10.1038/s41586-020-1962-0 10.1038/s41586-020-1962-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birey F., Andersen J., Makinson C.D., Islam S., Wei W., Huber N., Fan H.C., Metzler K.R.C., Panagiotakos G., Thom N., O'Rourke N.A., Steinmetz L.M., Bernstein J.A., Hallmayer J., Huguenard J.R., Pasca S.P. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545:54–59. doi: 10.1038/nature22330. https://doi.org/10.1038/nature22330 nature22330 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton G., Heemskerk I., Hodge R., Qutub A.A., Warmflash A. A novel self-organizing embryonic stem cell system reveals signaling logic underlying the patterning of human ectoderm. Development. 2019;146 doi: 10.1242/dev.179093. https://doi.org/10.1242/dev.179093 dev179093 dev.179093 [pii] DEV179093 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownjohn P.W., Smith J., Solanki R., Lohmann E., Houlden H., Hardy J., Dietmann S., Livesey F.J. Functional Studies of Missense TREM2 Mutations in Human Stem Cell-Derived Microglia. Stem Cell Rep. 2018;10:1294–1307. doi: 10.1016/j.stemcr.2018.03.003. https://doi.org/S2213-6711(18)30109-7 [pii] 10.1016/j.stemcr.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir B., Xiang Y., Tanaka Y., Kural M.H., Parent M., Kang Y.J., Chapeton K., Patterson B., Yuan Y., He C.S., Raredon M.S.B., Dengelegi J., Kim K.Y., Sun P., Zhong M., Lee S., Patra P., Hyder F., Niklason L.E., Lee S.H., Yoon Y.S., Park I.H. Engineering of human brain organoids with a functional vascular-like system. Nat. Methods. 2019;16:1169–1175. doi: 10.1038/s41592-019-0586-5. https://doi.org/10.1038/s41592-019-0586-5 10.1038/s41592-019-0586-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederquist G.Y., Asciolla J.J., Tchieu J., Walsh R.M., Cornacchia D., Resh M.D., Studer L. Specification of positional identity in forebrain organoids. Nat. Biotechnol. 2019;37:436–444. doi: 10.1038/s41587-019-0085-3. https://doi.org/10.1038/s41587-019-0085-3 10.1038/s41587-019-0085-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. https://doi.org/10.1038/nbt.1529 nbt.1529 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviaud N., Friedel R.H., Zou H. Vascularization and Engraftment of Transplanted Human Cerebral Organoids in Mouse Cortex. eNeuro. 2018;5 doi: 10.1523/ENEURO.0219-18.2018. https://doi.org/10.1523/ENEURO.0219-18.2018 ENEURO.0219-18.2018 eN-NWR-0219-18 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezonne R.S., Sartore R.C., Nascimento J.M., Saia-Cereda V.M., Romao L.F., Alves-Leon S.V., de Souza J.M., Martins-de-Souza D., Rehen S.K., Gomes F.C. Derivation of Functional Human Astrocytes from Cerebral Organoids. Sci. Rep. 2017;7:45091. doi: 10.1038/srep45091. https://doi.org/10.1038/srep45091 45091 srep45091 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Xu S.B., Chen X., Tao M., Tang X.Y., Fang K.H., Xu M., Pan Y., Chen Y., He S., Liu Y. Human cerebral organoids establish subcortical projections in the mouse brain after transplantation. Mol. Psychiatry. 2021;26:2964–2976. doi: 10.1038/s41380-020-00910-4. https://doi.org/10.1038/s41380-020-00910-4 10.1038/s41380-020-00910-4 [pii] 910 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenhuis K.N., Somsen H.B., van der Kroeg M., Smeenk H., Korporaal A.L., Kushner S.A., de Vrij F.M.S., van den Berg D.L.C. A simplified protocol for the generation of cortical brain organoids. Front Cell Neurosci. 2023;17:1114420. doi: 10.3389/fncel.2023.1114420. https://doi.org/10.3389/fncel.2023.1114420 1114420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M., Takata N., Ishibashi H., Kawada M., Sakakura E., Okuda S., Sekiguchi K., Adachi T., Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. doi: 10.1038/nature09941. https://doi.org/10.1038/nature09941 nature09941 [pii] [DOI] [PubMed] [Google Scholar]
- Eiraku M., Watanabe K., Matsuo-Takasaki M., Kawada M., Yonemura S., Matsumura M., Wataya T., Nishiyama A., Muguruma K., Sasai Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. https://doi.org/10.1016/j.stem.2008.09.002 S1934-5909(08)00455-4 [pii] [DOI] [PubMed] [Google Scholar]
- Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Fainsod A., Deissler K., Yelin R., Marom K., Epstein M., Pillemer G., Steinbeisser H., Blum M. The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech. Dev. 1997;63:39–50. doi: 10.1016/s0925-4773(97)00673-4. https://doi.org/S0925-4773(97)00673-4 [pii] 10.1016/s0925-4773(97)00673-4. [DOI] [PubMed] [Google Scholar]
- Faustino Martins J.M., Fischer C., Urzi A., Vidal R., Kunz S., Ruffault P.L., Kabuss L., Hube I., Gazzerro E., Birchmeier C., Spuler S., Sauer S., Gouti M. Self-Organizing 3D Human Trunk Neuromuscular Organoids. Cell Stem Cell. 2020;27:498. doi: 10.1016/j.stem.2020.08.011. https://doi.org/S1934-5909(20)30407-0 [pii] 10.1016/j.stem.2020.08.011. [DOI] [PubMed] [Google Scholar]
- Fligor C.M., Lavekar S.S., Harkin J., Shields P.K., VanderWall K.B., Huang K.C., Gomes C., Meyer J.S. Extension of retinofugal projections in an assembled model of human pluripotent stem cell-derived organoids. Stem Cell Rep. 2021;16:2228–2241. doi: 10.1016/j.stemcr.2021.05.009. https://doi.org/S2213-6711(21)00260-5 [pii] 10.1016/j.stemcr.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspard N., Bouschet T., Hourez R., Dimidschstein J., Naeije G., van den Ameele J., Espuny-Camacho I., Herpoel A., Passante L., Schiffmann S.N., Gaillard A., Vanderhaeghen P. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. https://doi.org/10.1038/nature07287 nature07287 [pii] [DOI] [PubMed] [Google Scholar]
- Giandomenico S.L., Mierau S.B., Gibbons G.M., Wenger L.M.D., Masullo L., Sit T., Sutcliffe M., Boulanger J., Tripodi M., Derivery E., Paulsen O., Lakatos A., Lancaster M.A. Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat. Neurosci. 2019;22:669–679. doi: 10.1038/s41593-019-0350-2. https://doi.org/10.1038/s41593-019-0350-2 10.1038/s41593-019-0350-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A., Yoon S.J., Tran S.S., Makinson C.D., Park J.Y., Andersen J., Valencia A.M., Horvath S., Xiao X., Huguenard J.R., Pasca S.P., Geschwind D.H. Long-term maturation of human cortical organoids matches key early postnatal transitions. Nat. Neurosci. 2021;24:331–342. doi: 10.1038/s41593-021-00802-y. https://doi.org/10.1038/s41593-021-00802-y 10.1038/s41593-021-00802-y [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D.V., Lui J.H., Parker P.R., Kriegstein A.R. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. https://doi.org/10.1038/nature08845 nature08845 [pii] [DOI] [PubMed] [Google Scholar]
- Haremaki T., Metzger J.J., Rito T., Ozair M.Z., Etoc F., Brivanlou A.H. Self-organizing neuruloids model developmental aspects of Huntington's disease in the ectodermal compartment. Nat. Biotechnol. 2019;37:1198–1208. doi: 10.1038/s41587-019-0237-5. https://doi.org/10.1038/s41587-019-0237-5 10.1038/s41587-019-0237-5 [pii] [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Melton D. Vertebrate neural induction. Annu Rev. Neurosci. 1997;20:43–60. doi: 10.1146/annurev.neuro.20.1.43. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A., Melton D.A. Inhibition of activin receptor signaling promotes neuralization in Xenopus. Cell. 1994;77:273–281. doi: 10.1016/0092-8674(94)90319-0. https://doi.org/0092-8674(94)90319-0 [pii] 10.1016/0092-8674(94)90319-0. [DOI] [PubMed] [Google Scholar]
- Hor J.H., Soh E.S., Tan L.Y., Lim V.J.W., Santosa M.M., Winanto Ho.B.X., Fan Y., Soh B.S., Ng S.Y. Cell cycle inhibitors protect motor neurons in an organoid model of Spinal Muscular Atrophy. Cell Death Dis. 2018;9:1100. doi: 10.1038/s41419-018-1081-0. https://doi.org/10.1038/s41419-018-1081-0 1100 10.1038/s41419-018-1081-0 [pii] 1081 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.K., Wong S.Z.H., Pather S.R., Nguyen P.T.T., Zhang F., Zhang D.Y., Zhang Z., Lu L., Fang W., Chen L., Fernandes A., Su Y., Song H., Ming G.L. Generation of hypothalamic arcuate organoids from human induced pluripotent stem cells. Cell Stem Cell. 2021;28:1657–1670. doi: 10.1016/j.stem.2021.04.006. e1610. https://doi.org/S1934-5909(21)00163-6 [pii] 10.1016/j.stem.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubler Z., Allimuthu D., Bederman I., Elitt M.S., Madhavan M., Allan K.C., Shick H.E., Garrison E., M T.K., Factor D.C., Nevin Z.S., Sax J.L., Thompson M.A., Fedorov Y., Jin J., Wilson W.K., Giera M., Bracher F., Miller R.H., Tesar P.J., Adams D.J. Accumulation of 8,9-unsaturated sterols drives oligodendrocyte formation and remyelination. Nature. 2018;560:372–376. doi: 10.1038/s41586-018-0360-3. https://doi.org/10.1038/s41586-018-0360-3 10.1038/s41586-018-0360-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideno H., Imaizumi K., Shimada H., Sanosaka T., Nemoto A., Kohyama J., Okano H. Human PSCs determine the competency of cerebral organoid differentiation via FGF signaling and epigenetic mechanisms. iScience. 2022;25 doi: 10.1016/j.isci.2022.105140. https://doi.org/10.1016/j.isci.2022.105140 105140 S2589-0042(22)01412-2 [pii] 105140 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jgamadze D., Lim J.T., Zhang Z., Harary P.M., Germi J., Mensah-Brown K., Adam C.D., Mirzakhalili E., Singh S., Gu J.B., Blue R., Dedhia M., Fu M., Jacob F., Qian X., Gagnon K., Sergison M., Fruchet O., Rahaman I., Wang H., Xu F., Xiao R., Contreras D., Wolf J.A., Song H., Ming G.L., Chen H.I. Structural and functional integration of human forebrain organoids with the injured adult rat visual system. Cell Stem Cell. 2023;30:137–152. doi: 10.1016/j.stem.2023.01.004. e137. https://doi.org/S1934-5909(23)00004-8 [pii] 10.1016/j.stem.2023.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J., Xiao Y., Sun A.X., Cukuroglu E., Tran H.D., Goke J., Tan Z.Y., Saw T.Y., Tan C.P., Lokman H., Lee Y., Kim D., Ko H.S., Kim S.O., Park J.H., Cho N.J., Hyde T.M., Kleinman J.E., Shin J.H., Weinberger D.R., Tan E.K., Je H.S., Ng H.H. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell. 2016;19:248–257. doi: 10.1016/j.stem.2016.07.005. https://doi.org/S1934-5909(16)30200-4 [pii] 10.1016/j.stem.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoshima T., Sakaguchi H., Nakano T., Soen M., Ando S., Eiraku M., Sasai Y. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl. Acad. Sci. USA. 2013;110:20284–20289. doi: 10.1073/pnas.1315710110. https://doi.org/10.1073/pnas.1315710110 1315710110 [pii] 201315710 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei T., Tamada A., Kimura T., Kakizuka A., Asai A., Muguruma K. Survival and process outgrowth of human iPSC-derived cells expressing Purkinje cell markers in a mouse model for spinocerebellar degenerative disease. Exp. Neurol. 2023;369 doi: 10.1016/j.expneurol.2023.114511. https://doi.org/S0014-4886(23)00196-6 [pii] 10.1016/j.expneurol.2023.114511. [DOI] [PubMed] [Google Scholar]
- Kanie K., Bando H., Iguchi G., Muguruma K., Matsumoto R., Hidaka-Takeno R., Okimura Y., Yamamoto M., Fujita Y., Fukuoka H., Yoshida K., Suda K., Nishizawa H., Ogawa W., Takahashi Y. Pathogenesis of Anti-PIT-1 Antibody Syndrome: PIT-1 Presentation by HLA Class I on Anterior Pituitary Cells. J. Endocr. Soc. 2019;3:1969–1978. doi: 10.1210/js.2019-00243. https://doi.org/10.1210/js.2019-00243 201900243 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karzbrun E., Khankhel A.H., Megale H.C., Glasauer S.M.K., Wyle Y., Britton G., Warmflash A., Kosik K.S., Siggia E.D., Shraiman B.I., Streichan S.J. Human neural tube morphogenesis in vitro by geometric constraints. Nature. 2021;599:268–272. doi: 10.1038/s41586-021-04026-9. https://doi.org/10.1038/s41586-021-04026-9 10.1038/s41586-021-04026-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai T., Suga H., Sakakibara M., Ozone C., Matsumoto R., Kano M., Mitsumoto K., Ogawa K., Kodani Y., Nagasaki H., Inoshita N., Sugiyama M., Onoue T., Tsunekawa T., Ito Y., Takagi H., Hagiwara D., Iwama S., Goto M., Banno R., Takahashi J., Arima H. Hypothalamic Contribution to Pituitary Functions Is Recapitulated In Vitro Using 3D-Cultured Human iPS Cells. Cell Rep. 2020;30:18–24. doi: 10.1016/j.celrep.2019.12.009. e15. https://doi.org/S2211-1247(19)31648-1 [pii] 10.1016/j.celrep.2019.12.009. [DOI] [PubMed] [Google Scholar]
- Kelberman D., Rizzoti K., Lovell-Badge R., Robinson I.C., Dattani M.T. Genetic regulation of pituitary gland development in human and mouse. Endocr. Rev. 2009;30:790–829. doi: 10.1210/er.2009-0008. https://doi.org/10.1210/er.2009-0008 er.2009-0008 [pii] 2733 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Xu R., Padmashri R., Dunaevsky A., Liu Y., Dreyfus C.F., Jiang P. Pluripotent Stem Cell-Derived Cerebral Organoids Reveal Human Oligodendrogenesis with Dorsal and Ventral Origins. Stem Cell Rep. 2019;12:890–905. doi: 10.1016/j.stemcr.2019.04.011. https://doi.org/S2213-6711(19)30133-X [pii] 10.1016/j.stemcr.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiral F.R., Cakir B., Tanaka Y., Kim J., Yang W.S., Wehbe F., Kang Y.J., Zhong M., Sancer G., Lee S.H., Xiang Y., Park I.H. Generation of ventralized human thalamic organoids with thalamic reticular nucleus. Cell Stem Cell. 2023;30:677–688. doi: 10.1016/j.stem.2023.03.007. e675. https://doi.org/S1934-5909(23)00078-4 [pii] 10.1016/j.stem.2023.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara T., Sakaguchi H., Morizane A., Kikuchi T., Miyamoto S., Takahashi J. Axonal Extensions along Corticospinal Tracts from Transplanted Human Cerebral Organoids. Stem Cell Rep. 2020;15:467–481. doi: 10.1016/j.stemcr.2020.06.016. https://doi.org/S2213-6711(20)30237-X [pii] 10.1016/j.stemcr.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruczek K., Swaroop A. Pluripotent stem cell-derived retinal organoids for disease modeling and development of therapies. Stem Cells. 2020;38:1206–1215. doi: 10.1002/stem.3239. https://doi.org/10.1002/stem.3239 STEM3239 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara A., Ozone C., Nakano T., Saito K., Eiraku M., Sasai Y. (2015) Generation of a ciliary margin-like stem cell niche from self-organizing human retinal tissue. Nat Commun 6:6286. https://doi.org/10.1038/ncomms7286 ncomms7286 [pii]. [DOI] [PubMed]
- Kuwahara A., Yamasaki S., Mandai M., Watari K., Matsushita K., Fujiwara M., Hori Y., Hiramine Y., Nukaya D., Iwata M., Kishino A., Takahashi M., Sasai Y., Kimura T. Preconditioning the Initial State of Feeder-free Human Pluripotent Stem Cells Promotes Self-formation of Three-dimensional Retinal Tissue. Sci. Rep. 2019;9:18936. doi: 10.1038/s41598-019-55130-w. https://doi.org/10.1038/s41598-019-55130-w 18936 10.1038/s41598-019-55130-w [pii] 55130 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak T.H., Kang J.H., Hali S., Kim J., Kim K.P., Park C., Lee J.H., Ryu H.K., Na J.E., Jo J., Je H.S., Ng H.H., Kwon J., Kim N.H., Hong K.H., Sun W., Chung C.H., Rhyu I.J., Han D.W. Generation of homogeneous midbrain organoids with in vivo-like cellular composition facilitates neurotoxin-based Parkinson's disease modeling. Stem Cells. 2020;38:727–740. doi: 10.1002/stem.3163. [DOI] [PubMed] [Google Scholar]
- Lamb T.M., Knecht A.K., Smith W.C., Stachel S.E., Economides A.N., Stahl N., Yancopolous G.D., Harland R.M. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Lancaster M.A., Corsini N.S., Wolfinger S., Gustafson E.H., Phillips A.W., Burkard T.R., Otani T., Livesey F.J., Knoblich J.A. Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechnol. 2017;35:659–666. doi: 10.1038/nbt.3906. https://doi.org/10.1038/nbt.3906 nbt.3906 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., Knoblich J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc. 2014;9:2329–2340. doi: 10.1038/nprot.2014.158. https://doi.org/10.1038/nprot.2014.158 nprot.2014.158 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., Renner M., Martin C.A., Wenzel D., Bicknell L.S., Hurles M.E., Homfray T., Penninger J.M., Jackson A.P., Knoblich J.A. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. https://doi.org/10.1038/nature12517 nature12517 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Shin H., Shaker M.R., Kim H.J., Park S.H., Kim J.H., Lee N., Kang M., Cho S., Kwak T.H., Kim J.W., Song M.R., Kwon S.H., Han D.W., Lee S., Choi S.Y., Rhyu I.J., Kim H., Geum D., Cho I.J., Sun W. Production of human spinal-cord organoids recapitulating neural-tube morphogenesis. Nat. Biomed. Eng. 2022;6:435–448. doi: 10.1038/s41551-022-00868-4. https://doi.org/10.1038/s41551-022-00868-4 10.1038/s41551-022-00868-4 [pii] [DOI] [PubMed] [Google Scholar]
- Levine A.J., Brivanlou A.H. Proposal of a model of mammalian neural induction. Dev. Biol. 2007;308:247–256. doi: 10.1016/j.ydbio.2007.05.036. https://doi.org/S0012-1606(07)01092-5 [pii] 10.1016/j.ydbio.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Fleck J.S., Martins-Costa C., Burkard T.R., Themann J., Stuempflen M., Peer A.M., Vertesy A., Littleboy J.B., Esk C., Elling U., Kasprian G., Corsini N.S., Treutlein B., Knoblich J.A. Single-cell brain organoid screening identifies developmental defects in autism. Nature. 2023;621:373–380. doi: 10.1038/s41586-023-06473-y. https://doi.org/10.1038/s41586-023-06473-y 10.1038/s41586-023-06473-y [pii] 6473 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Muffat J., Omer A., Bosch I., Lancaster M.A., Sur M., Gehrke L., Knoblich J.A., Jaenisch R. (2017) Induction of Expansion and Folding in Human Cerebral Organoids. Cell Stem Cell 20:385–396 e383. https://doi.org/10.1016/j.stem.2016.11.017. [DOI] [PMC free article] [PubMed]
- Lin Y.T., Seo J., Gao F., Feldman H.M., Wen H.L., Penney J., Cam H.P., Gjoneska E., Raja W.K., Cheng J., Rueda R., Kritskiy O., Abdurrob F., Peng Z., Milo B., Yu C.J., Elmsaouri S., Dey D., Ko T., Yankner B.A., Tsai L.H. (2018) APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer's Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron 98:1294. https://doi.org/S0896–6273(18)30479–3 [pii] 10.1016/j.neuron.2018.06.011. [DOI] [PMC free article] [PubMed]
- Ma H., Chen J., Deng Z., Sun T., Luo Q., Gong H., Li X., Long B. Multiscale Analysis of Cellular Composition and Morphology in Intact Cerebral Organoids. Biol. (Basel) 2022;11 doi: 10.3390/biology11091270. https://doi.org/10.3390/biology11091270 1270 biology11091270 [pii] biology-11-01270 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan M., Nevin Z.S., Shick H.E., Garrison E., Clarkson-Paredes C., Karl M., Clayton B.L.L., Factor D.C., Allan K.C., Barbar L., Jain T., Douvaras P., Fossati V., Miller R.H., Tesar P.J. Induction of myelinating oligodendrocytes in human cortical spheroids. Nat. Methods. 2018;15:700–706. doi: 10.1038/s41592-018-0081-4. https://doi.org/10.1038/s41592-018-0081-4 10.1038/s41592-018-0081-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A.A., Goncalves J.T., Bloyd C.W., Li H., Fernandes S., Quang D., Johnston S., Parylak S.L., Jin X., Gage F.H. An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol. 2018;36:432–441. doi: 10.1038/nbt.4127. https://doi.org/10.1038/nbt.4127 nbt.4127 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J., Coppola G., Zhang P., Abyzov A., Provini L., Tomasini L., Amenduni M., Szekely A., Palejev D., Wilson M., Gerstein M., Grigorenko E.L., Chawarska K., Pelphrey K.A., Howe J.R., Vaccarino F.M. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell. 2015;162:375–390. doi: 10.1016/j.cell.2015.06.034. https://doi.org/S0092-8674(15)00759-X [pii] 10.1016/j.cell.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton R.M., Miura Y., Sloan S.A., Li Q., Revah O., Levy R.J., Huguenard J.R., Pasca S.P. Differentiation and maturation of oligodendrocytes in human three-dimensional neural cultures. Nat. Neurosci. 2019;22:484–491. doi: 10.1038/s41593-018-0316-9. https://doi.org/10.1038/s41593-018-0316-9 10.1038/s41593-018-0316-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto R., Suga H., Aoi T., Bando H., Fukuoka H., Iguchi G., Narumi S., Hasegawa T., Muguruma K., Ogawa W., Takahashi Y. Congenital pituitary hypoplasia model demonstrates hypothalamic OTX2 regulation of pituitary progenitor cells. J. Clin. Invest. 2020;130:641–654. doi: 10.1172/JCI127378. https://doi.org/127378 [pii] 10.1172/JCI127378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J.S., Howden S.E., Wallace K.A., Verhoeven A.D., Wright L.S., Capowski E.E., Pinilla I., Martin J.M., Tian S., Stewart R., Pattnaik B., Thomson J.A., Gamm D.M. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011;29:1206–1218. doi: 10.1002/stem.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y., Li M.Y., Birey F., Ikeda K., Revah O., Thete M.V., Park J.Y., Puno A., Lee S.H., Porteus M.H., Pasca S.P. Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nat. Biotechnol. 2020;38:1421–1430. doi: 10.1038/s41587-020-00763-w. https://doi.org/10.1038/s41587-020-00763-w 10.1038/s41587-020-00763-w [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwata T., Suga H., Kawaguchi Y., Sakakibara M., Kano M., Taga S., Soen M., Ozaki H., Asano T., Sasaki H., Miyata T., Yasuda Y., Kobayashi T., Sugiyama M., Onoue T., Takagi H., Hagiwara D., Iwama S., Arima H. Generation of hypothalamic neural stem cell-like cells in vitro from human pluripotent stem cells. Stem Cell Rep. 2023;18:869–883. doi: 10.1016/j.stemcr.2023.02.006. https://doi.org/S2213-6711(23)00053-X [pii] 10.1016/j.stemcr.2023.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuseki K., Sakamoto T., Watanabe K., Muguruma K., Ikeya M., Nishiyama A., Arakawa A., Suemori H., Nakatsuji N., Kawasaki H., Murakami F., Sasai Y. Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2003;100:5828–5833. doi: 10.1073/pnas.1037282100. https://doi.org/1037282100 [pii] 7282 [pii] 10.1073/pnas.1037282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzel A.S., Smits L.M., Hemmer K., Hachi S., Moreno E.L., van Wuellen T., Jarazo J., Walter J., Bruggemann I., Boussaad I., Berger E., Fleming R.M.T., Bolognin S., Schwamborn J.C. Derivation of Human Midbrain-Specific Organoids from Neuroepithelial Stem Cells. Stem Cell Rep. 2017;8:1144–1154. doi: 10.1016/j.stemcr.2017.03.010. https://doi.org/S2213-6711(17)30116-9 [pii] 10.1016/j.stemcr.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muffat J., Li Y., Yuan B., Mitalipova M., Omer A., Corcoran S., Bakiasi G., Tsai L.H., Aubourg P., Ransohoff R.M., Jaenisch R. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat. Med. 2016;22:1358–1367. doi: 10.1038/nm.4189. https://doi.org/10.1038/nm.4189 nm.4189 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muguruma K., Nishiyama A., Kawakami H., Hashimoto K., Sasai Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 2015;10:537–550. doi: 10.1016/j.celrep.2014.12.051. https://doi.org/S2211-1247(14)01104-8 [pii] 10.1016/j.celrep.2014.12.051. [DOI] [PubMed] [Google Scholar]
- Muguruma K., Nishiyama A., Ono Y., Miyawaki H., Mizuhara E., Hori S., Kakizuka A., Obata K., Yanagawa Y., Hirano T., Sasai Y. Ontogeny-recapitulating generation and tissue integration of ES cell-derived Purkinje cells. Nat. Neurosci. 2010;13:1171–1180. doi: 10.1038/nn.2638. https://doi.org/10.1038/nn.2638 nn.2638 [pii] [DOI] [PubMed] [Google Scholar]
- Nakamura H., Katahira T., Matsunaga E., Sato T. Isthmus organizer for midbrain and hindbrain development. Brain Res Brain Res Rev. 2005;49:120–126. doi: 10.1016/j.brainresrev.2004.10.005. https://doi.org/S0165-0173(04)00170-5 [pii] 10.1016/j.brainresrev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Nakano T., Ando S., Takata N., Kawada M., Muguruma K., Sekiguchi K., Saito K., Yonemura S., Eiraku M., Sasai Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–785. doi: 10.1016/j.stem.2012.05.009. https://doi.org/S1934-5909(12)00242-1 [pii] 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Nasu M., Takata N., Danjo T., Sakaguchi H., Kadoshima T., Futaki S., Sekiguchi K., Eiraku M., Sasai Y. Robust formation and maintenance of continuous stratified cortical neuroepithelium by laminin-containing matrix in mouse ES cell culture. PLoS One 7. 2012 doi: 10.1371/journal.pone.0053024. https://doi.org/10.1371/journal.pone.0053024 e53024 PONE-D-12-23290 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nato G., Corti A., Parmigiani E., Jachetti E., Lecis D., Colombo M.P., Delia D., Buffo A., Magrassi L. Immune-tolerance to human iPS-derived neural progenitors xenografted into the immature cerebellum is overridden by species-specific differences in differentiation timing. Sci. Rep. 2021;11:651. doi: 10.1038/s41598-020-79502-9. https://doi.org/10.1038/s41598-020-79502-9 651 10.1038/s41598-020-79502-9 [pii] 79502 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayler S., Agarwal D., Curion F., Bowden R., Becker E.B.E. High-resolution transcriptional landscape of xeno-free human induced pluripotent stem cell-derived cerebellar organoids. Sci. Rep. 2021;11:12959. doi: 10.1038/s41598-021-91846-4. https://doi.org/10.1038/s41598-021-91846-4 12959 10.1038/s41598-021-91846-4 [pii] 91846 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C. On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development. 2010;137:845–857. doi: 10.1242/dev.039651. https://doi.org/10.1242/dev.039651 137/6/845 [pii] [DOI] [PubMed] [Google Scholar]
- Nordstrom U., Jessell T.M., Edlund T. Progressive induction of caudal neural character by graded Wnt signaling. Nat. Neurosci. 2002;5:525–532. doi: 10.1038/nn0602-854. https://doi.org/nn854 [pii] 10.1038/nn0602-854. [DOI] [PubMed] [Google Scholar]
- Ogura T., Sakaguchi H., Miyamoto S., Takahashi J. Three-dimensional induction of dorsal, intermediate and ventral spinal cord tissues from human pluripotent stem cells. Development. 2018;145 doi: 10.1242/dev.162214. https://doi.org/10.1242/dev.162214 dev162214 145/16/dev162214 [pii] DEV162214 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel P.R., Vieira de Sa R., van Bodegraven E.J., Karst H., Harschnitz O., Sneeboer M.A.M., Johansen L.E., van Dijk R.E., Scheefhals N., Berdenis van Berlekom A., Ribes Martinez E., Kling S., MacGillavry H.D., van den Berg L.H., Kahn R.S., Hol E.M., de Witte L.D., Pasterkamp R.J. Microglia innately develop within cerebral organoids. Nat. Commun. 2018;9:4167. doi: 10.1038/s41467-018-06684-2. https://doi.org/10.1038/s41467-018-06684-2 4167 10.1038/s41467-018-06684-2 [pii] 6684 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozone C., Suga H., Eiraku M., Kadoshima T., Yonemura S., Takata N., Oiso Y., Tsuji T., Sasai Y. Functional anterior pituitary generated in self-organizing culture of human embryonic stem cells. Nat. Commun. 2016;7:10351. doi: 10.1038/ncomms10351. https://doi.org/10.1038/ncomms10351 10351 ncomms10351 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca A.M., Sloan S.A., Clarke L.E., Tian Y., Makinson C.D., Huber N., Kim C.H., Park J.Y., O'Rourke N.A., Nguyen K.D., Smith S.J., Huguenard J.R., Geschwind D.H., Barres B.A., Pasca S.P. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods. 2015;12:671–678. doi: 10.1038/nmeth.3415. https://doi.org/10.1038/nmeth.3415 nmeth.3415 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca S.P., Arlotta P., Bateup H.S., Camp J.G., Cappello S., Gage F.H., Knoblich J.A., Kriegstein A.R., Lancaster M.A., Ming G.L., Muotri A.R., Park I.H., Reiner O., Song H., Studer L., Temple S., Testa G., Treutlein B., Vaccarino F.M. A nomenclature consensus for nervous system organoids and assembloids. Nature. 2022;609:907–910. doi: 10.1038/s41586-022-05219-6. https://doi.org/10.1038/s41586-022-05219-6 10.1038/s41586-022-05219-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini L., Albecka A., Mallery D.L., Kellner M.J., Paul D., Carter A.P., James L.C., Lancaster M.A. SARS-CoV-2 Infects the Brain Choroid Plexus and Disrupts the Blood-CSF Barrier in Human Brain Organoids. Cell Stem Cell. 2020;27:951–961. doi: 10.1016/j.stem.2020.10.001. e955. https://doi.org/S1934-5909(20)30495-1 [pii] 10.1016/j.stem.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S., Sasai Y., Lu B., De Robertis E.M. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. https://doi.org/S0092-8674(00)80132-4 [pii] 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollen A.A., Bhaduri A., Andrews M.G., Nowakowski T.J., Meyerson O.S., Mostajo-Radji M.A., Di Lullo E., Alvarado B., Bedolli M., Dougherty M.L., Fiddes I.T., Kronenberg Z.N., Shuga J., Leyrat A.A., West J.A., Bershteyn M., Lowe C.B., Pavlovic B.J., Salama S.R., Haussler D., Eichler E.E., Kriegstein A.R. Establishing Cerebral Organoids as Models of Human-Specific Brain Evolution. Cell. 2019;176:743–756. doi: 10.1016/j.cell.2019.01.017. e717. https://doi.org/S0092-8674(19)30050-9 [pii] 10.1016/j.cell.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Nguyen H.N., Song M.M., Hadiono C., Ogden S.C., Hammack C., Yao B., Hamersky G.R., Jacob F., Zhong C., Yoon K.J., Jeang W., Lin L., Li Y., Thakor J., Berg D.A., Zhang C., Kang E., Chickering M., Nauen D., Ho C.Y., Wen Z., Christian K.M., Shi P.Y., Maher B.J., Wu H., Jin P., Tang H., Song H., Ming G.L. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. https://doi.org/S0092-8674(16)30467-6 [pii] 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Su Y., Adam C.D., Deutschmann A.U., Pather S.R., Goldberg E.M., Su K., Li S., Lu L., Jacob F., Nguyen P.T.T., Huh S., Hoke A., Swinford-Jackson S.E., Wen Z., Gu X., Pierce R.C., Wu H., Briand L.A., Chen H.I., Wolf J.A., Song H., Ming G.L. Sliced Human Cortical Organoids for Modeling Distinct Cortical Layer Formation. Cell Stem Cell. 2020;26:766–781. doi: 10.1016/j.stem.2020.02.002. e769. https://doi.org/S1934-5909(20)30055-2 [pii] 10.1016/j.stem.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadrato G., Nguyen T., Macosko E.Z., Sherwood J.L., Min Yang S., Berger D.R., Maria N., Scholvin J., Goldman M., Kinney J.P., Boyden E.S., Lichtman J.W., Williams Z.M., McCarroll S.A., Arlotta P. Cell diversity and network dynamics in photosensitive human brain organoids. Nature. 2017;545:48–53. doi: 10.1038/nature22047. https://doi.org/10.1038/nature22047 nature22047 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rallu M., Corbin J.G., Fishell G. (2002) Parsing the prosencephalon. Nat Rev Neurosci 3:943–951. https://doi.org/nrn989 [pii] 10.1038/nrn989. [DOI] [PubMed]
- Revah O., Gore F., Kelley K.W., Andersen J., Sakai N., Chen X., Li M.Y., Birey F., Yang X., Saw N.L., Baker S.W., Amin N.D., Kulkarni S., Mudipalli R., Cui B., Nishino S., Grant G.A., Knowles J.K., Shamloo M., Huguenard J.R., Deisseroth K., Pasca S.P. Maturation and circuit integration of transplanted human cortical organoids. Nature 610. 2022:319–326. doi: 10.1038/s41586-022-05277-w. https://doi.org/10.1038/s41586-022-05277-w 10.1038/s41586-022-05277-w [pii] 5277 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifes P., Isaksson M., Rathore G.S., Aldrin-Kirk P., Moller O.K., Barzaghi G., Lee J., Egerod K.L., Rausch D.M., Parmar M., Pers T.H., Laurell T., Kirkeby A. Modeling neural tube development by differentiation of human embryonic stem cells in a microfluidic WNT gradient. Nat. Biotechnol. 2020;38:1265–1273. doi: 10.1038/s41587-020-0525-0. https://doi.org/10.1038/s41587-020-0525-0 10.1038/s41587-020-0525-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi H., Kadoshima T., Soen M., Narii N., Ishida Y., Ohgushi M., Takahashi J., Eiraku M., Sasai Y. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat. Commun. 6:8896. 2015 doi: 10.1038/ncomms9896. https://doi.org/10.1038/ncomms9896 8896 ncomms9896 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y., De Robertis E.M. Ectodermal patterning in vertebrate embryos. Dev. Biol. 1997;182:5–20. doi: 10.1006/dbio.1996.8445. https://doi.org/S0012-1606(96)98445-6 [pii] 10.1006/dbio.1996.8445. [DOI] [PubMed] [Google Scholar]
- Sasai Y., Eiraku M., Suga H. In vitro organogenesis in three dimensions: self-organising stem cells. Development. 2012;139(4111-4121) doi: 10.1242/dev.079590. https://doi.org/10.1242/dev.079590 139/22/4111 [pii] [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., De Robertis E.M. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995;377:757. doi: 10.1038/377757a0. [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., Geissert D., Gont L.K., De Robertis E.M. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. https://doi.org/0092-8674(94)90068-X [pii] 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Fu J. Engineering multiscale structural orders for high-fidelity embryoids and organoids. Cell Stem Cell. 2022;29:722–743. doi: 10.1016/j.stem.2022.04.003. https://doi.org/S1934-5909(22)00156-4 [pii] 10.1016/j.stem.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Taniguchi K., Townshend R.F., Miki T., Gumucio D.L., Fu J. A pluripotent stem cell-based model for post-implantation human amniotic sac development. Nat. Commun. 2017;8:208. doi: 10.1038/s41467-017-00236-w. https://doi.org/10.1038/s41467-017-00236-w 208 10.1038/s41467-017-00236-w [pii] 236 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Sun L., Wang M., Liu J., Zhong S., Li R., Li P., Guo L., Fang A., Chen R., Ge W.P., Wu Q., Wang X. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000705. https://doi.org/10.1371/journal.pbio.3000705 e3000705 PBIOLOGY-D-19-01969 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi A., Muguruma K., Sasai Y. Generation of thalamic neurons from mouse embryonic stem cells. Development. 2017;144:1211–1220. doi: 10.1242/dev.144071. https://doi.org/10.1242/dev.144071 dev.144071 [pii] [DOI] [PubMed] [Google Scholar]
- Sloan S.A., Andersen J., Pasca A.M., Birey F., Pasca S.P. Generation and assembly of human brain region-specific three-dimensional cultures. Nat. Protoc. 2018;13:2062–2085. doi: 10.1038/s41596-018-0032-7. https://doi.org/10.1038/s41596-018-0032-7 10.1038/s41596-018-0032-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan S.A., Darmanis S., Huber N., Khan T.A., Birey F., Caneda C., Reimer R., Quake S.R., Barres B.A., Pasca S.P. Human Astrocyte Maturation Captured in 3D Cerebral Cortical Spheroids Derived from Pluripotent Stem Cells. Neuron. 2017;95:779–790. doi: 10.1016/j.neuron.2017.07.035. e776. https://doi.org/S0896-6273(17)30683-9 [pii] 10.1016/j.neuron.2017.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W.C., Harland R.M. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. https://doi.org/0092-8674(92)90316-5 [pii] 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smits L.M., Reinhardt L., Reinhardt P., Glatza M., Monzel A.S., Stanslowsky N., Rosato-Siri M.D., Zanon A., Antony P.M., Bellmann J., Nicklas S.M., Hemmer K., Qing X., Berger E., Kalmbach N., Ehrlich M., Bolognin S., Hicks A.A., Wegner F., Sterneckert J.L., Schwamborn J.C. Modeling Parkinson's disease in midbrain-like organoids. NPJ Park. Dis. 2019;5:5. doi: 10.1038/s41531-019-0078-4. https://doi.org/10.1038/s41531-019-0078-4 5 78 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smukler S.R., Runciman S.B., Xu S., van der Kooy D. Embryonic stem cells assume a primitive neural stem cell fate in the absence of extrinsic influences. J. Cell Biol. 2006;172:79–90. doi: 10.1083/jcb.200508085. https://doi.org/jcb.200508085 [pii] 200508085 [pii] 10.1083/jcb.200508085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L., Yuan X., Jones Z., Vied C., Miao Y., Marzano M., Hua T., Sang Q.A., Guan J., Ma T., Zhou Y., Li Y. Functionalization of Brain Region-specific Spheroids with Isogenic Microglia-like Cells. Sci. Rep. 9. 2019:11055. doi: 10.1038/s41598-019-47444-6. https://doi.org/10.1038/s41598-019-47444-6 11055 10.1038/s41598-019-47444-6 [pii] 47444 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern C.D. Initial patterning of the central nervous system: how many organizers. Nat. Rev. Neurosci. 2001;2:92–98. doi: 10.1038/35053563. [DOI] [PubMed] [Google Scholar]
- Suga H., Kadoshima T., Minaguchi M., Ohgushi M., Soen M., Nakano T., Takata N., Wataya T., Muguruma K., Miyoshi H., Yonemura S., Oiso Y., Sasai Y. Self-formation of functional adenohypophysis in three-dimensional culture. Nature. 2011;480:57–62. doi: 10.1038/nature10637. https://doi.org/10.1038/nature10637 nature10637 [pii] [DOI] [PubMed] [Google Scholar]
- Taga S., Suga H., Nakano T., Kuwahara A., Inoshita N., Kodani Y., Nagasaki H., Sato Y., Tsumura Y., Sakakibara M., Soen M., Miwata T., Ozaki H., Kano M., Watari K., Ikeda A., Yamanaka M., Takahashi Y., Kitamoto S., Kawaguchi Y., Miyata T., Kobayashi T., Sugiyama M., Onoue T., Yasuda Y., Hagiwara D., Iwama S., Tomigahara Y., Kimura T., Arima H. Generation and purification of ACTH-secreting hPSC-derived pituitary cells for effective transplantation. Stem Cell Rep. 2023;18:1657–1671. doi: 10.1016/j.stemcr.2023.05.002. https://doi.org/S2213-6711(23)00180-7 [pii] 10.1016/j.stemcr.2023.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. https://doi.org/S0092-8674(06)00976-7 [pii] 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Cakir B., Xiang Y., Sullivan G.J., Park I.H. Synthetic Analyses of Single-Cell Transcriptomes from Multiple Brain Organoids and Fetal Brain. Cell Rep. 2020;30:1682–1689. doi: 10.1016/j.celrep.2020.01.038. e1683. https://doi.org/S2211-1247(20)30053-X [pii] 10.1016/j.celrep.2020.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Shao Y., Townshend R.F., Tsai Y.H., DeLong C.J., Lopez S.A., Gayen S., Freddo A.M., Chue D.J., Thomas D.J., Spence J.R., Margolis B., Kalantry S., Fu J., O'Shea K.S., Gumucio D.L. Lumen Formation Is an Intrinsic Property of Isolated Human Pluripotent Stem Cells. Stem Cell Rep. 2015;5:954–962. doi: 10.1016/j.stemcr.2015.10.015. https://doi.org/S2213-6711(15)00312-4 [pii] 10.1016/j.stemcr.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewary M., Dziedzicka D., Ostblom J., Prochazka L., Shakiba N., Heydari T., Aguilar-Hidalgo D., Woodford C., Piccinini E., Becerra-Alonso D., Vickers A., Louis B., Rahman N., Danovi D., Geens M., Watt F.M., Zandstra P.W. High-throughput micropatterning platform reveals Nodal-dependent bisection of peri-gastrulation-associated versus preneurulation-associated fate patterning. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000081. https://doi.org/10.1371/journal.pbio.3000081 e3000081 PBIOLOGY-D-18-00978 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tieng V., Stoppini L., Villy S., Fathi M., Dubois-Dauphin M., Krause K.H. Engineering of midbrain organoids containing long-lived dopaminergic neurons. Stem Cells Dev. 2014;23:1535–1547. doi: 10.1089/scd.2013.0442. [DOI] [PubMed] [Google Scholar]
- Tropepe V., Hitoshi S., Sirard C., Mak T.W., Rossant J., van der Kooy D. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30:65–78. doi: 10.1016/s0896-6273(01)00263-x. https://doi.org/S0896-6273(01)00263-X [pii] 10.1016/s0896-6273(01)00263-x. [DOI] [PubMed] [Google Scholar]
- van Essen M.J., Nayler S., Becker E.B.E., Jacob J. Deconstructing cerebellar development cell by cell. PLoS Genet. 2020;16 doi: 10.1371/journal.pgen.1008630. e1008630 PGENETICS-D-19-01838 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco S., Kedaigle A.J., Simmons S.K., Nash A., Rocha M., Quadrato G., Paulsen B., Nguyen L., Adiconis X., Regev A., Levin J.Z., Arlotta P. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature. 2019;570:523–527. doi: 10.1038/s41586-019-1289-x. https://doi.org/10.1038/s41586-019-1289-x 10.1038/s41586-019-1289-x [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlin K.J., Maruotti J.A., Sripathi S.R., Ball J., Angueyra J.M., Kim C., Grebe R., Li W., Jones B.W., Zack D.J. Photoreceptor Outer Segment-like Structures in Long-Term 3D Retinas from Human Pluripotent Stem Cells. Sci. Rep. 2017;7:766. doi: 10.1038/s41598-017-00774-9. https://doi.org/10.1038/s41598-017-00774-9 766 10.1038/s41598-017-00774-9 [pii] 774 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.N., Wang Z., Xu T.Y., Cheng M.H., Li W.L., Miao C.Y. Cerebral Organoids Repair Ischemic Stroke Brain Injury. Transl. Stroke Res 11:983-1000. 2020 doi: 10.1007/s12975-019-00773-0. https://doi.org/10.1007/s12975-019-00773-0 10.1007/s12975-019-00773-0 [pii] 773 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wang S.N., Xu T.Y., Hong C., Cheng M.H., Zhu P.X., Lin J.S., Su D.F., Miao C.Y. Cerebral organoids transplantation improves neurological motor function in rat brain injury. CNS Neurosci. Ther. 2020;26:682–697. doi: 10.1111/cns.13286. CNS13286 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Kamiya D., Nishiyama A., Katayama T., Nozaki S., Kawasaki H., Watanabe Y., Mizuseki K., Sasai Y. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat. Neurosci. 2005;8:288–296. doi: 10.1038/nn1402. https://doi.org/nn1402 [pii] 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Ueno M., Kamiya D., Nishiyama A., Matsumura M., Wataya T., Takahashi J.B., Nishikawa S., Muguruma K., Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. https://doi.org/nbt1310 [pii] 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Buth J.E., Haney J.R., Vishlaghi N., Turcios F., Elahi L.S., Gu W., Pearson C.A., Kurdian A., Baliaouri N.V., Collier A.J., Miranda O.A., Dunn N., Chen D., Sabri S., Torre-Ubieta L., Clark A.T., Plath K., Christofk H.R., Kornblum H.I., Gandal M.J., Novitch B.G. TGFbeta superfamily signaling regulates the state of human stem cell pluripotency and capacity to create well-structured telencephalic organoids. Stem Cell Rep. 2022;17:2220–2238. doi: 10.1016/j.stemcr.2022.08.013. https://doi.org/S2213-6711(22)00422-2 [pii] 10.1016/j.stemcr.2022.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Buth J.E., Vishlaghi N., de la Torre-Ubieta L., Taxidis J., Khakh B.S., Coppola G., Pearson C.A., Yamauchi K., Gong D., Dai X., Damoiseaux R., Aliyari R., Liebscher S., Schenke-Layland K., Caneda C., Huang E.J., Zhang Y., Cheng G., Geschwind D.H., Golshani P., Sun R., Novitch B.G. Self-Organized Cerebral Organoids with Human-Specific Features Predict Effective Drugs to Combat Zika Virus Infection. Cell Rep. 2017;21:517–532. doi: 10.1016/j.celrep.2017.09.047. https://doi.org/S2211-1247(17)31337-2 [pii] 10.1016/j.celrep.2017.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wataya T., Ando S., Muguruma K., Ikeda H., Watanabe K., Eiraku M., Kawada M., Takahashi J., Hashimoto N., Sasai Y. Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. Proc. Natl. Acad. Sci. USA. 2008;105(11796-11801) doi: 10.1073/pnas.0803078105. https://doi.org/10.1073/pnas.0803078105 0803078105 [pii]4238 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H., Lieberam I., Porter J.A., Jessell T.M. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. https://doi.org/S0092867402008358 [pii] 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Wilson M.N., Thunemann M., Liu X., Lu Y., Puppo F., Adams J.W., Kim J.H., Ramezani M., Pizzo D.P., Djurovic S., Andreassen O.A., Mansour A.A., Gage F.H., Muotri A.R., Devor A., Kuzum D. Multimodal monitoring of human cortical organoids implanted in mice reveal functional connection with visual cortex. Nat. Commun. 2022;13:7945. doi: 10.1038/s41467-022-35536-3. https://doi.org/10.1038/s41467-022-35536-3 7945 10.1038/s41467-022-35536-3 [pii] 35536 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S.W., Houart C. Early steps in the development of the forebrain. Dev. Cell. 2004;6:167–181. doi: 10.1016/s1534-5807(04)00027-9. https://doi.org/S1534-5807(04)00027-9 [pii] 10.1016/s1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsdorfer P., Dalda N., Kern A., Kruger S., Wagner N., Kwok C.K., Henke E., Ergun S. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci. Rep. 2019;9:15663. doi: 10.1038/s41598-019-52204-7. https://doi.org/10.1038/s41598-019-52204-7 15663 10.1038/s41598-019-52204-7 [pii] 52204 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Tanaka Y., Cakir B., Patterson B., Kim K.Y., Sun P., Kang Y.J., Zhong M., Liu X., Patra P., Lee S.H., Weissman S.M., Park I.H. hESC-Derived Thalamic Organoids Form Reciprocal Projections When Fused with Cortical Organoids. Cell Stem Cell. 2019;24:487–497. doi: 10.1016/j.stem.2018.12.015. e487. https://doi.org/S1934-5909(18)30605-2 [pii] 10.1016/j.stem.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Tanaka Y., Patterson B., Kang Y.J., Govindaiah G., Roselaar N., Cakir B., Kim K.Y., Lombroso A.P., Hwang S.M., Zhong M., Stanley E.G., Elefanty A.G., Naegele J.R., Lee S.H., Weissman S.M., Park I.H. Fusion of Regionally Specified hPSC-Derived Organoids Models Human Brain Development and Interneuron Migration. Cell Stem Cell. 2017;21:383–398. doi: 10.1016/j.stem.2017.07.007. e387. https://doi.org/S1934-5909(17)30286-2 [pii] 10.1016/j.stem.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Boreland A.J., Li X., Erickson C., Jin M., Atkins C., Pang Z.P., Daniels B.P., Jiang P. Developing human pluripotent stem cell-based cerebral organoids with a controllable microglia ratio for modeling brain development and pathology. Stem Cell Rep. 2021;16:1923–1937. doi: 10.1016/j.stemcr.2021.06.011. https://doi.org/S2213-6711(21)00317-9 [pii] 10.1016/j.stemcr.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X., Sun Y., Resto-Irizarry A.M., Yuan Y., Aw Yong K.M., Zheng Y., Weng S., Shao Y., Chai Y., Studer L., Fu J. Mechanics-guided embryonic patterning of neuroectoderm tissue from human pluripotent stem cells. Nat. Mater. 2018;17:633–641. doi: 10.1038/s41563-018-0082-9. https://doi.org/10.1038/s41563-018-0082-9 10.1038/s41563-018-0082-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Q.L., Stavridis M., Griffiths D., Li M., Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. https://doi.org/nbt780 [pii] 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- Yonemura S. Differential sensitivity of epithelial cells to extracellular matrix in polarity establishment. PLoS One 9. 2014 doi: 10.1371/journal.pone.0112922. https://doi.org/10.1371/journal.pone.0112922 e112922 PONE-D-14-36395 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Gutierrez C., Xue T., Hampton C., Vergara M.N., Cao L.H., Peters A., Park T.S., Zambidis E.T., Meyer J.S., Gamm D.M., Yau K.W., Canto-Soler M.V. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat. Commun. 2014;5:4047. doi: 10.1038/ncomms5047. https://doi.org/10.1038/ncomms5047 ncomms5047 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman L.B., De Jesus-Escobar J.M., Harland R.M. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. https://doi.org/S0092-8674(00)80133-6 [pii] 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]