Abstract
Viral protein R (Vpr) of human immunodeficiency virus type 1 (HIV-1) is a small accessory protein involved in the nuclear import of viral DNA and the growth arrest of host cells. Several studies have demonstrated that a significant amount of Vpr is incorporated into the virus particle via interaction with the p6 domain of Gag, and it is generally assumed that Vpr is packaged in equimolar ratio to Gag. We have quantitated the relative amount of Vpr in purified virions following [35S]cysteine labeling of infected MT-4 cells, as well as by quantitative immunoblotting and found that Vpr is present in a molar ratio of approximately 1:7 compared to capsid. Analysis of isolated core particles showed that Vpr is associated with the mature viral core, despite quantitative loss of p6 from core preparations. Metabolic labeling of infected cells with ortho[32P]phosphate revealed that a small fraction of Vpr is phosphorylated in virions and infected cells.
Viral protein R (Vpr), a polypeptide of 96 amino acids, is the major virion-associated accessory protein of human immunodeficiency virus type 1 (HIV-1). Studies in tissue culture revealed two main biological functions of Vpr. First, it prevents host cell proliferation by arresting cells in the G2 phase of the cell cycle. This effect has been related to induction of cell death as well as to increased viral gene expression, but its exact role for viral replication is unclear (15, 17, 20, 30, 33, 34, 36, 45). An independent function of Vpr is to promote the transport of the viral genome into the nucleus, thereby contributing to the ability of HIV-1 to infect nondividing cells (10, 18, 31, 32, 38). Several previous studies have demonstrated that HIV-1 Vpr as well as the related Vpx proteins of HIV-2 and simian immunodeficiency virus (SIV) are selectively packaged into virus particles (9, 46, 47), supporting a role in the early phase of virus replication. Incorporation into the virion occurs via specific interaction with the p6 domain of the Gag polyprotein (1, 3, 23, 24, 27, 29, 35), and this interaction can be exploited to target proteins in trans into the HIV particle via fusion to Vpr (43). Although the immunoprecipitation experiments which defined Vpr as a virion component did not allow exact quantitation, it is generally assumed that Vpr is present in equimolar amounts to Gag in HIV-1 particles (8).
The study presented here was aimed at a more detailed characterization of the virion-associated Vpr protein. First, we applied two different techniques to determine the amount of Vpr incorporated into virus particles. One approach involved the production of metabolically labeled virus particles from MT-4 cells. Cells were infected with HIV-1 strain NL4-3 by coculture as described previously (42). At 24 h postinfection, cells were incubated for 2 h in cysteine-free medium. Following this starvation period, steady-state labeling was performed by addition of [35S]cysteine (20 μCi/ml) to cysteine-free culture medium containing dialyzed fetal calf serum. Tissue culture supernatant was harvested following an additional 18 to 36 h of incubation. To directly visualize the major viral proteins without prior immunoprecipitation, particles were pelleted from the clarified supernatant through a 20% (wt/wt) sucrose cushion and further purified by banding on an OptiPrep velocity gradient, adapted from a recently published protocol (12). Gradient fractions containing viral particles were identified as a visible band and were collected and concentrated by centrifugation. Virion-associated proteins were then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography.
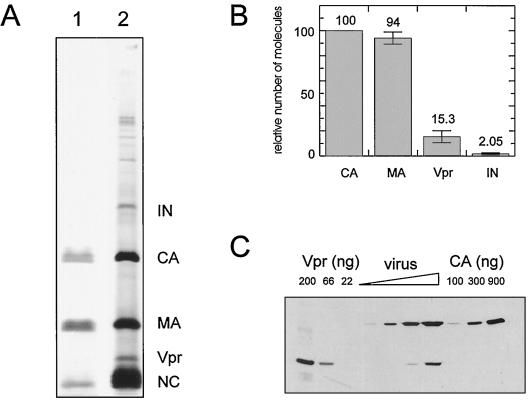
A characteristic pattern of radiolabeled protein bands was observed, and immunoprecipitation with specific polyclonal antisera was carried out to confirm the identity of particle-associated proteins (Fig. 1 and data not shown). As apparent in Fig. 1, 2A, and 4, the band corresponding to Vpr was clearly detectable in the purified virus preparations. Since virus lysates were highly pure as judged by silver staining of SDS-gels and [35S]cysteine-labeled proteins were easily identifiable without prior immunoprecipitation (Fig. 2A), the radioactivity contained in the Vpr, capsid (CA), and matrix (MA) bands could be directly quantitated by phosphorimage analysis. Virus material from three independent labeling experiments was used for evaluation. After correcting for the number of cysteines present in the proteins, amounts of MA, Vpr, and integrase (IN) in virus preparations were calculated relative to the CA protein (arbitrarily set as 100) (Fig. 2B). As expected, amounts of CA and MA calculated were nearly identical, whereas the amount of the pol-derived IN was 50-fold lower. In contrast, we found a ratio of Vpr to CA of only about 1 to 7. If one assumes an average number of 1,800 CA molecules per virion, as has been determined for retroviral particles by scanning transmission electron microscopy (39), one particle contains approximately 275 molecules of Vpr. These calculations are based on the assumption that translation efficiency and turnover of Vpr and CA are comparable, resulting in comparable relative 35S incorporation into both proteins. Since we cannot formally exclude differences in this respect, the result obtained from the labeling experiment was confirmed by an independent approach using quantitative immunoblotting. Unlabeled virus was prepared from infected MT-4 cells and purified as described above. Virion-associated Vpr and CA proteins were detected by immunoblotting with specific polyclonal antisera (Fig. 2C). For detection of Vpr, we used a polyclonal rabbit antiserum prepared against the full-length protein. Serial dilutions of recombinant HIV-1 CA and synthetic full-length Vpr peptide were analyzed in parallel. Densitometric evaluation and comparison of reactivities of the virion-derived proteins with those of the standards of known concentration yielded approximate amounts of 1.65 μg of CA and 0.11 μg of Vpr in 2 μl of virus sample, corresponding again to a molar ratio of CA to Vpr of about 7 to 1. The observation that HIV-1 particles contain Vpr in significantly lower amount than Gag is in contrast to the general assumption that Vpr and Gag are packaged in equimolar amounts in the virion; however, to our knowledge the data presented here are the first quantitative analysis of Vpr incorporation into HIV-1 particles released from infected cells not involving transcomplementation with Vpr. Since Vpr is incorporated into the particles via direct interaction with the p6 domain of Gag, packaging of a stoichiometric amount should theoretically be possible. However, the intracellular concentration of Vpr available during virus assembly may be limited due to Vpr turnover or binding to cellular factors. Although the interaction between Pr55Gag and Vpr is strong enough to be measured by in vitro assays and has been reported to be stable against high NaCl concentrations (3, 35), the affinity between the two proteins has not been quantitated. In the case of HIV-2, it has been shown that the relatively low amount of Vpr incorporated into virions is related to the short (<90-min) half-life of intracellular Vpr and can be increased by overexpression of the protein in trans, while the related Vpx protein is much more stable (half-life of >36 h) and is incorporated in comparable amounts to Gag (22). It appears less likely that a similar effect is responsible for the less than stoichiometric incorporation of HIV-1 Vpr, since its half-life in MT-4 cells has been reported to be considerably longer than 7 h (44). Nevertheless, the intracellular Vpr concentration seems to play an important role for incorporation efficiency, since in cells transfected with HIV-1 DNA the amount of virion-associated Vpr-derived protein is increased upon overexpression of Vpr fusion proteins in trans (43), suggesting that the potential interaction sites on the Gag protein are not saturated by Vpr expressed from the proviral DNA.
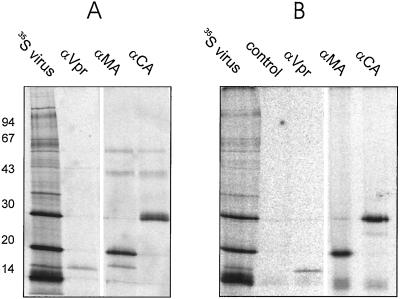
FIG. 1.
Identification of [35S]cysteine-labeled virion proteins by immunoprecipitation. A total of 3 × 105 infected cells (A) or purified virus equivalent to 2.4 ml of tissue culture supernatant (B) were lysed in 750 μl of radioimmunoprecipitation assay buffer containing 2 mM Pefabloc, 10 μM E64, and 1 μM pepstatin. Extracts were subjected to immunoprecipitation according to standard procedures (16), using polyclonal rabbit antisera directed against the indicated HIV proteins or an unrelated antibody (control) bound to protein A-agarose (Roche). Immunoprecipitates were separated by SDS-PAGE (17.5% acrylamide; acrylamide/bisacrylamide, 200:1) and visualized by phosphorimage analysis using a Fuji BAS2000 instrument. As a reference, an aliquot of purified labeled virus was also loaded directly onto the gel (lane 35S virus). Positions of marker proteins and their molecular masses in kilodaltons are indicated at the left.
FIG. 2.
Determination of the relative amounts of Vpr and CA in virus lysates. (A) Aliquot of a purified 35S-labeled virus sample used for quantitative analysis. The sample was analyzed by SDS-PAGE followed by silver staining as described by Heukeshoven and Dernick (19) (lane 1) as well as phosphorimage detection (lane 2). (B) Radioactivity in bands corresponding to Vpr, MA, CA, and IN contained in virus samples as determined by phosphorimage analysis. After normalizing for the number of cysteines present, average numbers of molecules were calculated relative to the intensity of the CA band in the same lane, which was arbitrarily set at 100%. Data shown represent the mean relative value calculated for each protein. (C) Relative amounts of virion-associated CA and Vpr as determined by immunoblotting. Various amounts of purified virus were separated by SDS-PAGE in parallel to serial dilutions of recombinant CA protein or synthetic Vpr protein of known concentration. Proteins were detected by immunoblotting using rabbit antisera directed against CA or Vpr followed by enhanced chemiluminescence staining. Band intensity was measured by densitometry using a Desaga CD50 instrument.
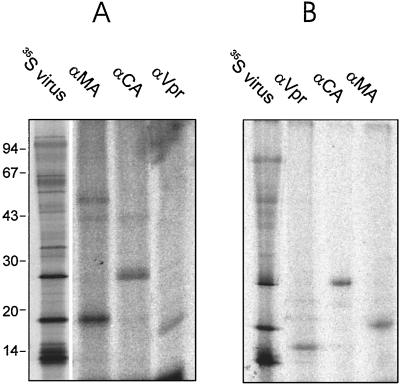
FIG. 4.
Immunoprecipitation of 32P-labeled HIV-1 proteins. Lysate from 3.5 × 106 infected cells metabolically labeled with ortho[32P]phosphate (A) or purified virus equivalent to 18 ml (Vpr) or 2.25 ml (MA and CA) of tissue culture supernatant (B) was subjected to immunoprecipitation as in Fig. 1, using the indicated antisera. Immunoprecipitates were separated by SDS-PAGE and visualized by phosphorimage analysis. As a reference, an aliquot of [35S]cysteine-labeled virus lysate was separated on the same gel (lane 35S virus). Positions of marker proteins and their molecular masses in kilodaltons are indicated at the left.
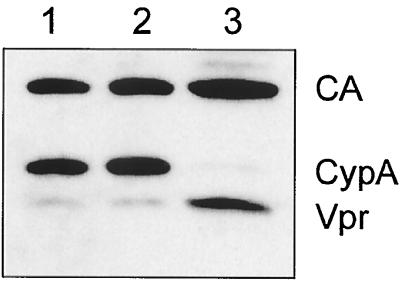
Contrasting results have been reported concerning the subviral localization of HIV-1 Vpr and the related Vpx protein of HIV-2 and SIV. Whereas immunoelectron microscopy studies suggested that HIV-1 Vpr is located mainly beneath the virion membrane (40) and Vpx from SIVmac was also detected outside the virus core (26), HIV-2 Vpx was found associated with mature cores (21). To biochemically analyze subviral Vpr localization, we adapted a method developed in our lab for preparation of intact HIV core particles by detergent stripping (42), but used gradient purified virus as starting material. Comparative immunoblot analysis of virions and isolated core particles (Fig. 3) revealed that Vpr was significantly enriched in the core preparations, whereas p6, as well as other HIV-1 structural proteins (MA) and the virion-associated cellular protein cyclophilin A, were quantitatively removed by detergent treatment (Fig. 3 and reference 42). Segregation of Vpr and p6 was surprising, because the p6 domain of Gag carries the binding site for Vpr and is presumed to recruit Vpr into the virion. Conceivably, cleaved p6 has a reduced affinity toward Vpr, resulting in dissociation of the complex upon maturation. This possibility is supported by the finding that p6, in contrast to Pr55Gag, does not display interaction with Vpr in a yeast two-hybrid analysis (35). Vpr may be retained to the core by being associated with the complex of nucleocapsid protein (NC) and the viral genomic RNA. Consistent with this hypothesis, an affinity of Vpr toward NC (11, 25, 35) as well as toward nucleic acid (48) has been reported. In any case, HIV-1 Vpr is clearly a core-associated protein, which is likely to be important for its functions in early virus replication.
FIG. 3.
Immunoblot analysis of purified HIV-1 virions and isolated core particles. Mature core particles were prepared as described by Welker et al. (42). Virus particles pelleted through a sucrose cushion (lane 1) and further purified by banding in an OptiPrep gradient (lane 2) as well as a preparation of core particles from OptiPrep gradient-purified virions (lane 3) were separated by SDS-PAGE. Similar amounts with respect to CA were loaded in each lane. Virion-associated proteins were identified by immunoblotting using a mixture of antisera against the indicated proteins and enhanced chemiluminescence staining. CypA, cyclophilin A.
Posttranslational modifications might serve to regulate the diverse functions of Vpr. Since modification of viral proteins by kinases is known as an important way to regulate viral replication, we were interested in potential phosphorylation of Vpr. Intracellular phosphorylation of several HIV-1 proteins (MA, CA, Vpu, Vif, and Nef) has been reported and, in the case of MA and Vpu, has been implicated in the regulation of differential activities of these proteins (8). To determine whether phosphorylation of Vpr occurs in infected cells, MT-4 cells were metabolically labeled with 0.5 mCi of ortho[32P]phosphate per ml at 18 to 24 h postinfection with HIV-1 strain NL4-3. Twelve hours later, virus was harvested and purified by banding in a velocity gradient as described above. Virus preparations as well as infected cells were lysed in standard radioimmunoprecipitation assay buffer (16) containing 1 mM sodium orthovanadate, 2 mM Pefabloc, 10 μM E64, and 1 μM pepstatin, and lysates were subjected to immunoprecipitation with antisera against various HIV proteins. From lysates of infected cells, antiserum against Vpr precipitated a radiolabeled protein with the expected apparent molecular weight (Fig. 4A), demonstrating that there is indeed a phosphorylated form of Vpr. In the same series of experiments we also detected phosphorylated forms of MA and CA, whose occurrence is well documented in previous reports (6, 7, 14, 28, 37). Unspecific cross-reactivity of the sera was excluded by parallel experiments using lysates of equally labeled uninfected cells, where none of the bands shown in Fig. 4 were detected (not shown). A radiolabeled Vpr band was also observed when purified virus lysate was used for immunoprecipitation (Fig. 4B), indicating that a phosphorylated form of Vpr (pVpr) is associated with virus particles.
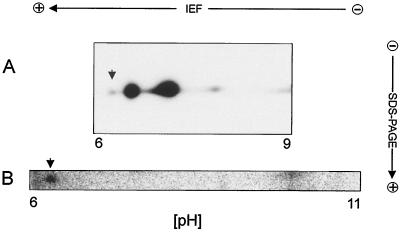
To determine the relative amount of pVpr in virus particles, we performed denaturing two-dimensional gel electrophoresis of unlabeled and 32P-labeled virus samples (Fig. 5). Several forms of Vpr with different isoelectric mobilities were detected in the unlabeled virus preparation by immunoblotting (Fig. 5A). Using several independent virus preparations, we consistently observed two major isomeric forms of Vpr with apparent isoelectric points (IEP) of approximately 7.3 and 6.8 and with minor spots focusing at pH 6.3 and 8.0, respectively. Only a single spot with the apparent molecular weight of Vpr, focusing at pH 6.3, was detected in analyses of 32P-labeled virus from two independent preparations (Fig. 5B). We conclude that pVpr corresponds to the minor form of Vpr indicated by an arrow in Fig. 5A. The difference between the nonphosphorylated Vpr forms leading to different apparent IEPs may be due to proteolytic removal of charged amino acids or other minor modifications like the change of an amide side group to a carboxy group. Analyses of [35S]cysteine-labeled virus (not shown) also revealed the Vpr isoform focusing at pH 6.3, and phosphorimage analyses allowed us to estimate that pVpr represents approximately 5% of total virion-associated Vpr. This result is supported by comparison with the relative labeling intensity of phosphorylated CA. Parallel two-dimensional analyses of labeled and unlabeled virion-associated CA (not shown) revealed a single phosphorylated form, representing approximately 5% of virion-associated CA. In the experiment shown in Fig. 4B, the labeling intensities of the immunoprecipitated Vpr, MA, and CA bands were almost identical. Since in this case eight times more virus lysate was used for immunoprecipitation with anti-Vpr serum than for precipitation with anti-MA or anti-CA serum, correcting for the different relative amounts of these proteins in the virion, we estimate that virus-associated Vpr is phosphorylated to a similar extent as CA. Virion-associated MA was also found to be phosphorylated to a similar degree.
FIG. 5.
Analysis of virion-associated Vpr by two-dimensional gel electrophoresis. (A) Unlabeled virus released from infected MT-4 cells was gradient purified as described for the labeled virus preparations. A sample corresponding to 15 μg of CA was applied to an Immobiline DryStrip 3-10L (Amersham Pharmacia) and subjected to isoelectric focusing (IEF) under denaturing conditions (8 M urea) according to the manufacturer's instructions, using an IGphor unit. Separation in the second dimension was performed by SDS-PAGE; subsequently, Vpr was detected by immunoblotting using antiserum directed against synthetic Vpr. (B) Gradient-purified 32P-labeled virus equivalent to 20 ml of tissue culture supernatant was separated by IEF (Immobiline DryStrip 6-11L) followed by SDS-PAGE. Radiolabeled protein was detected by phosphorimage analysis. Arrows indicate a single phosphorylated protein spot of the apparent molecular weight of Vpr, corresponding to an IEP of 6.3 (B) and an immunoreactive spot corresponding to the same IEP (A).
Vpr of NL4-3 contains 11 residues (four Ser, four Thr, and three Tyr) which theoretically could be phosphorylated. We have not yet mapped the modified residue(s), but one might consider Ser79 as a candidate phosphorylation site, based on sequence- and structure-dependent computer prediction according to Blom et al. (4) together with the absolute conservation of this residue in HIV-1 Vpr. It is tempting to speculate that Vpr phosphorylation plays a role in regulating the multiple functions of the protein in virus replication. Whereas in the cases of HIV-2, SIVsm, and SIVmac two independent proteins, Vpr and Vpx, are required for the nuclear import of the viral genome and the induction of host cell growth arrest, in the case of HIV-1 a single protein is responsible for both functions. Vpr also displays numerous other activities in tissue culture, like transcriptional activation, cell killing, or induction of cell differentiation. Consistent with that, the association of Vpr with a number of viral and cellular factors has been reported (2, 5, 11, 13, 25, 32, 38, 41). Vpr phosphorylation and dephosphorylation may be used to modulate these interactions throughout the viral replication cycle. Further studies are aimed at identification of the modified amino acid residue(s) as a prerequisite for testing this hypothesis.
Acknowledgments
We thank P. Henklein (Humboldt Universität, Berlin, Germany) for providing synthetic Vpr and K. Wiegers for helpful suggestions and discussions.
REFERENCES
- 1.Accola M A, Bukovsky A A, Jones M S, Göttlinger H G. A conserved dileucine-containing motif in p6gag governs the particle association of Vpx and Vpr of simian immunodeficiency viruses SIVmac and SIVagm. J Virol. 1999;73:9992–9999. doi: 10.1128/jvi.73.12.9992-9999.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agostini I, Navarro J M, Rey F, Bouhamdan M, Spire B, Vigne R, Sire J. The human immunodeficiency virus type 1 Vpr transactivator: cooperation with promoter-bound activator domains and binding to TFIIB. J Mol Biol. 1996;261:599–606. doi: 10.1006/jmbi.1996.0485. [DOI] [PubMed] [Google Scholar]
- 3.Bachand F, Yao X J, Hrimech M, Rougeau N, Cohen E A. Incorporation of Vpr into human immunodeficiency virus type 1 requires a direct interaction with the p6 domain of the p55 Gag precursor. J Biol Chem. 1999;274:9083–9091. doi: 10.1074/jbc.274.13.9083. [DOI] [PubMed] [Google Scholar]
- 4.Blom N, Gammeltoft S, Brunak S. Sequence- and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 5.Bouhamdan M, Benichou S, Rey F, Navarro J-M, Agostini I, Spire B, Camonis J, Slupphaug G, Vigne R, Benarous R, Sire J. Human immunodeficiency virus type 1 Vpr protein binds to the uracil DNA glycosylase DNA repair enzyme. J Virol. 1996;70:697–704. doi: 10.1128/jvi.70.2.697-704.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukrinskaya A G, Ghorpade A, Heinzinger N K, Smithgall T E, Lewis R E, Stevenson M. Phosphorylation-dependent human immunodeficiency virus type 1 infection and nuclear targeting of viral DNA. Proc Natl Acad Sci USA. 1996;93:367–371. doi: 10.1073/pnas.93.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cartier C, Sivard P, Tranchat C, Decim D, Desgranges C, Boyer V. Identification of three major phosphorylation sites within HIV-1 capsid. J Biol Chem. 1999;274:19434–19440. doi: 10.1074/jbc.274.27.19434. [DOI] [PubMed] [Google Scholar]
- 8.Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- 9.Cohen E A, Dehni G, Sodroski J G, Haseltine W A. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J Virol. 1990;64:3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor R I, Chen B K, Choe S, Landau N R. Vpr is required for efficient replication of human immunodeficiency virus type 1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 11.De Rocquigny H, Petitjean P, Tanchou V, Drouot L, Delaunay T, Darlix J-L, Rocques B P. The zinc fingers of HIV nucleocapsid protein NCp7 direct interactions with the viral regulatory protein Vpr. J Biol Chem. 1997;272:30753–30759. doi: 10.1074/jbc.272.49.30753. [DOI] [PubMed] [Google Scholar]
- 12.Dettenhofer M, Yu X F. Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif in virions. J Virol. 1999;73:1460–1467. doi: 10.1128/jvi.73.2.1460-1467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fouchier R A, Meyer B E, Simon J H, Fischer U, Albright A V, Gonzalez-Scarano F, Malim M H. Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex. J Virol. 1998;72:6004–6013. doi: 10.1128/jvi.72.7.6004-6013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: phosphorylation of the viral matrix protein is a key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 15.Goh W C, Rogel M E, Kinsey C M, Michael S F, Fultz P N, Nowak M A, Hahn B H, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- 16.Harlow E, Lane D. Antibodies. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 17.He J, Choe S, Walker R, DiMarzio P, Morgan D O, Landau N R. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinzinger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M, Gendelman H, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heukeshoven J, Dernick R. Improved silver staining procedure for fast staining in PhastSystem Development Unit. I. Staining of sodium dodecyl sulfate gels. Electrophoresis. 1988;9:28–32. doi: 10.1002/elps.1150090106. [DOI] [PubMed] [Google Scholar]
- 20.Jowett J B M, Planelles V, Poon B, Shah N P, Chen M-L, Chen I S Y. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2+M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kewalramani V N, Emerman M. Vpx association with mature core structures of HIV-2. Virology. 1996;218:159–168. doi: 10.1006/viro.1996.0176. [DOI] [PubMed] [Google Scholar]
- 22.Kewalramani V N, Park C S, Gallombardo P A, Emerman M. Protein stability influences human immunodeficiency virus type 2 Vpr virion incorporation and cell cycle effect. Virology. 1996;218:326–334. doi: 10.1006/viro.1996.0201. [DOI] [PubMed] [Google Scholar]
- 23.Kondo E, Mammano F, Cohen E A, Göttlinger H G. The p6gag domain of human immunodeficiency virus type 1 is sufficient for the incorporation of Vpr into heterologous viral particles. J Virol. 1995;69:2759–2764. doi: 10.1128/jvi.69.5.2759-2764.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavallee C, Yao X J, Ladha A, Göttlinger H, Haseltine W A, Cohen E A. Requirement of the Pr55Gag precursor for incorporation of the Vpr product into human immunodeficiency virus type 1 viral particles. J Virol. 1994;68:1926–1934. doi: 10.1128/jvi.68.3.1926-1934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M S, Garcia-Asua G, Bhattacharyya U, Mascagni P, Austen B M, Roberts M M. The Vpr protein of human immunodeficiency virus type 1 binds to nucleocapsid protein p7 in vitro. Biochem Biophys Res Commun. 1996;218:352–355. doi: 10.1006/bbrc.1996.0061. [DOI] [PubMed] [Google Scholar]
- 26.Liska V, Spehner D, Mehtali M, Schmitt D, Kirn A, Aubertin A M. Localization of viral protein X in simian immunodeficiency virus macaque strain and analysis of packaging requirements. J Gen Virol. 1994;75:2955–2962. doi: 10.1099/0022-1317-75-11-2955. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y L, Bennet R P, Wills J W, Gorelick R, Ratner L. A leucine triplet repeat sequence (LXX)4 in p6gag is important for Vpr incorporation into human immunodeficiency virus type 1 particles. J Virol. 1995;69:6873–6879. doi: 10.1128/jvi.69.11.6873-6879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mervis R J, Ahmad N, Lillehoj E P, Raum M G, Salazar F H, Chan H W, Venkatesan S. The gag gene products of human immunodeficiency virus type 1: alignment within the gag open reading frame, identification of posttranslational modifications, and evidence for alternative gag precursors. J Virol. 1988;62:3993–4002. doi: 10.1128/jvi.62.11.3993-4002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paxton W, Connor R I, Landau N R. Incorporation of Vpr into human immunodeficiency virus type 1 virions: requirement for the p6 region of Gag and mutational analysis. J Virol. 1993;67:7229–7237. doi: 10.1128/jvi.67.12.7229-7237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Planelles V, Bachelerie F, Jowett J B, Haislip A, Xie Y, Banooni P, Masuda T, Chen I S. Fate of the human immunodeficiency virus type 1 provirus in infected cells: a role for Vpr. J Virol. 1995;69:5883–5889. doi: 10.1128/jvi.69.9.5883-5889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popov S, Rexach M, Zybarth G, Reiling N, Lee M A, Ratner L, Lane C M, Moore M S, Blobel G, Bukrinsky M. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 1998;17:909–917. doi: 10.1093/emboj/17.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Popov S, Rexach M, Ratner L, Blobel G, Bukrinsky M. Viral protein R regulates docking of the HIV-1 preintegration complex to the nuclear pore complex. J Biol Chem. 1998;273:13347–13352. doi: 10.1074/jbc.273.21.13347. [DOI] [PubMed] [Google Scholar]
- 33.Re F, Braaten D, Franke E K, Luban J. Human immunodeficiency virus type 1 vpr arrest the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogel M E, Wu L I, Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selig L, Pages J C, Tanchou V, Preveral S, Berlioz-Torrent C, Liu L X, Erdtmann L, Darlix J-L, Benarous R, Benichou S. Interaction with the p6 domain of the Gag precursor mediates incorporation into virions of Vpr and Vpx proteins from primate lentiviruses. J Virol. 1999;73:592–600. doi: 10.1128/jvi.73.1.592-600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart S A, Poon B, Jowett J B M, Chen I S Y. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veronese F D, Copeland T D, Oroszlan S, Gallo R C, Sarngadharan M G. Biochemical and immunological analysis of human immunodeficiency virus gag gene products p17 and p24. J Virol. 1988;62:795–801. doi: 10.1128/jvi.62.3.795-801.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vodicka M A, Koepp D M, Silver P A, Emerman M. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 1998;12:175–185. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogt V M, Simon M N. Mass determination of Rous sarcoma virus virions by scanning transmission electron microscopy. J Virol. 1999;73:7050–7055. doi: 10.1128/jvi.73.8.7050-7055.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J J, Lu Y, Ratner L. Particle assembly and Vpr expression in human immunodeficiency virus type 1 infected cells demonstrated by immunoelectron microscopy. J Gen Virol. 1994;75:2607–2614. doi: 10.1099/0022-1317-75-10-2607. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Mukherjee S, Jia F, Narayan O, Zhao L J. Interaction of virion protein Vpr of human immunodeficiency virus type 1 with cellular transcription factor Sp1 and trans-activation of viral long terminal repeat. J Biol Chem. 1995;270:25564–25569. doi: 10.1074/jbc.270.43.25564. [DOI] [PubMed] [Google Scholar]
- 42.Welker R, Hohenberg H, Tessmer U, Huckhagel C, Kräusslich H-G. Biochemical and structural analysis of isolated mature cores of human immunodeficiency virus type 1. J Virol. 2000;74:1168–1177. doi: 10.1128/jvi.74.3.1168-1177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu X, Liu H, Xiao H, Kim J, Seshaiah P, Natsoulis G, Boeke J D, Hahn B H, Kappes J C. Targeting foreign proteins to human immunodeficiency virus particles via fusion with Vpr and Vpx. J Virol. 1995;69:3389–3398. doi: 10.1128/jvi.69.6.3389-3398.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao X J, Subbramanian R A, Rougeau N, Boisvert F, Bergeron D, Cohen E A. Mutagenic analysis of human immunodeficiency virus type 1 Vpr: role of a predicted N-terminal alpha-helical structure in Vpr localization and virion incorporation. J Virol. 1995;69:7032–7044. doi: 10.1128/jvi.69.11.7032-7044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao X J, Mouland A J, Subbramanian R A, Forget J, Rougeau N, Bergeron D, Cohen E A. Vpr stimulates viral expression and induces cell killing in human immunodeficiency virus type 1-infected dividing Jurkat T cells. J Virol. 1998;72:4686–4693. doi: 10.1128/jvi.72.6.4686-4693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu X F, Matsuda M, Essex M, Lee T H. Open reading frame vpr of simian immunodeficiency virus encodes a virion-associated protein. J Virol. 1990;64:5688–5693. doi: 10.1128/jvi.64.11.5688-5693.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan X, Matsuda Z, Matsuda M, Essex M, Lee T H. Human immunodeficiency virus vpr gene encodes a virion-associated protein. AIDS Res Hum Retroviruses. 1990;6:1265–1271. doi: 10.1089/aid.1990.6.1265. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S, Pointer D, Singer G, Feng Y, Park K, Zhao L-J. Direct binding to nucleic acids by Vpr of human immunodeficiency virus type 1. Gene. 1998;212:157–166. doi: 10.1016/s0378-1119(98)00178-4. [DOI] [PubMed] [Google Scholar]