Abstract
Background
The increasing incidence of brain metastases (BMs) and improved survival rates underscore the necessity to investigate the effects of treatments on individuals. The aim of this study was to evaluate the individual trajectories of subjective and objective cognitive performance after radiotherapy in patients with BMs.
Methods
The study population consisted of adult patients with BMs referred for radiotherapy. A semi-structured interview and comprehensive neurocognitive assessment (NCA) were used to assess both subjective and objective cognitive performance before, 3 months and ≥ 11 months after radiotherapy. Reliable change indices were used to identify individual, clinically meaningful changes.
Results
Thirty-six patients completed the 3-month follow-up, and 14 patients completed the ≥ 11-months follow-up. Depending on the domain, subjective cognitive decline was reported by 11–22% of patients. In total, 50% of patients reported subjective decline in at least one cognitive domain. Intracranial progression 3 months postradiotherapy was a risk-factor for self-reported deterioration (P = .031). Objective changes were observed across all domains, with a particular vulnerability for decline in memory at 3 months postradiotherapy. The majority of patients (81%) experienced both a deterioration as well as improvement (eg, mixed response) in objective cognitive functioning. Results were similar for the long-term follow-up (3 to ≥11 months). No risk factors for objective cognitive change 3 months postradiotherapy were identified.
Conclusions
Our study revealed that the majority of patients with BMs will show a mixed cognitive response following radiotherapy, reflecting the complex impact. This underscores the importance of patient-tailored NCAs 3 months postradiotherapy to guide optimal rehabilitation strategies.
Keywords: brain metastases, neurocognitive functioning, radiotherapy
Brain metastases (BMs) represent a rapidly growing population currently encompassing 10–30% of the adult cancer population.1,2 This number is expected to increase due to earlier detection through enhanced imaging techniques and advancements in medical treatment improving survival rates. Overall survival rates currently range from months to several years.2–9 Treatment consists of different options, including radiotherapy, surgery, chemotherapy, immunotherapy, or a combination.10 However, with prolonged survival comes the increased likelihood of experiencing cognitive side-effects from these treatments, underscoring the urgency of research into the impact of treatment on patients’ cognitive function. The ultimate goal of research is to enhance patient-centered care by providing well-informed psycho-education.
Prior to the start of treatment for BMs, a significant proportion of patients already experience cognitive difficulties; at least one out of every 2 patients demonstrates cognitive impairment on minimally one cognitive domain.11–14 Cancer treatment can lead to further deterioration of neurocognitive functioning with declines observed both after systemic therapy (ie, chemo- and immunotherapy)15–18 and local therapies (ie, brain radiotherapy). Multiple cognitive domains can be affected after brain radiotherapy with impairments reported in memory, executive function, and processing speed.11 On a group level, most patients exhibit a decline in neurocognitive performance during 8 months after whole-brain radiotherapy (WBRT), whereas, after stereotactic radiosurgery (SRS), the majority of patients maintain a stable cognitive performance.11 However, substantial variety exists both within and between subjects in terms of which cognitive domains are affected and to what extent. Previous research indicated stable cognitive performance up to 9 months after SRS at group-level, while almost 40% showed declined performance on the individual level.19,20
Despite significant progress, many studies had limited follow-up durations and small sample sizes. Hence, it is crucial to confirm and continue to build upon previous findings. Therefore, the current study evaluated the individual trajectories of both subjective and objective cognitive performance in patients with BMs in the short-term (ie, 3 months) and in the long-term (ie, ≥11 months) after radiotherapy. By using a reliable change index (RCI)21,22 we consider the test–retest reliability of neurocognitive tasks, enabling us to identify individual, clinically meaningful changes in cognitive functioning. This study aims to gain insights into the impact of treatment for BMs on patients’ lives by investigating individual cognitive functioning within this heterogeneous group, considering subjective experiences, and focusing on long-term effects.
Methods
Study Set-Up and Population
Study procedures have been described previously.23 Data was prospectively collected from the Cohort for patient-reported outcomes, imaging, and trial inclusion in Metastatic BRAin disease (COIMBRA, NCT05267158) and the Assessing and Predicting Radiation Influence on Cognitive Outcome using the cerebrovascular stress Test (APRICOT) study. The study population consisted of adult patients (≥18 years) with either radiographic and/or histologic proof of metastatic brain disease referred to the University Medical Center Utrecht (UMCU) for brain radiotherapy. For both studies, neurocognitive assessments (NCAs), including semi-structured interviews, were performed before, 3 months and ≥ 11 months after radiotherapy. The studies were performed in accordance with the Declaration of Helsinki24 and the UMCU institutional ethical review approved both the COIMBRA and APRICOT study (#18-642 and #18-747, respectively). Written informed consent was obtained from all participants prior to participation.
Data Collection
Semi-structured interview
Prior to each neurocognitive assessment (NCA), subjective cognitive experience was assessed using a semi-structured interview. For the current analyses, the subjective cognitive ratings using the visual analog scales (VAS) were used. In brief, patients were asked to assess their performance regarding thinking, memory, attention, perception, language, and processing speed using VAS, similar to Schoo et al.25 The VAS consisted of a 100 mm vertical line on A3-sized paper, where the top (+) represents perfect performance and the bottom (−) represents worst performance. Patients marked the line at their experienced premorbid performance level (ie, prior to the primary cancer diagnosis and antitumor treatment) as well as their current experience. This resulted in an intra-individual estimation ranging from 0 (−) to 100 (+). A difference score was calculated for each cognitive concept to assess change in performance. This was categorized into stable performance (±5), subtle improvement or decline (±6 to 25), substantial improvement or decline (±26 to 50), and extreme improvement or decline (±>50).
Neurocognitive assessment
A comprehensive NCA was used to assess objective cognitive performance. All tests are internationally widely used standardized psychometric instruments designed to assess neurocognitive deficits in the major neurocognitive domains. This battery encompasses all tests advised by the International Cancer and Cognition Task Force (ICCTF)26 and supplemented with additional neuropsychological tests (Supplementary Table 1). At repeated testing, alternate forms were used to minimize practice effects. While neuropsychological tests often evaluate more than one neurocognitive domain, tests were classified into different neurocognitive domains based on available literature and clinical experience. All NCAs were performed in-person by trained psychologists and were planned to be completed within approximately 90 min.
To assess neurocognitive impairment, each neuropsychological test was scored according to standardized scoring criteria. The uncorrected scores were transformed into z-scores based on the mean and SD of control populations derived from published norm data and corrected for age and education where appropriate. Neurocognitive impairment in each domain was defined as a Z-score ≤ −1.5 on any of the administered tests within the domain.
Individual change in neurocognitive performance was assessed using the RCI as formulated by Jacobson and Truax.21,22 Using the uncorrected score, this RCI accounts for the test–retest reliability of the task based on published normative data (Supplementary Table 2). RCI values of ≥1.645 indicate improvement, ≤−1.645 decline, and values within ±1.645 indicate stable cognitive performance.27 Change in neurocognitive performance per domain was defined as improved or declined if at least one task within that domain showed improvement or decline, respectively, as mixed if at least one task indicated improvement and one task indicated decline, and as stable when all tasks within that domain demonstrated stable performance.
Patient characteristics
Patient characteristics were obtained from the semi-structured interview and from the hospital’s electronic healthrecord (HiX, Chipsoft, The Netherlands). This data included sex, age at inclusion, level of education according to the Verhage criteria,28 handedness, Karnofsky Performance Status (KPS),6 primary tumor origins, presence of extracranial metastases, time since BMs diagnosis, previous anti-tumor therapy, dexamethasone dose 1–5 days prior to radiotherapy, and symptoms at BMs diagnosis. As part of standard medical care, the preradiotherapy MRI scans of each patient were evaluated to determine the number of BMs, hemisphere, and lobe involvement. Additionally, for those patients who completed follow-up NCAs, the clinical MRI follow-up scans were evaluated to determine intracranial progression during follow-up, and new radiotherapy treatments were registered.
Statistical Analyses
For this study, the subjective and objective cognitive data acquired from October 2020 to May 2023 was used. Analyses were performed using SPSS (IBM SPSS Statistics, 25.0.0). Statistical significance was set at P < .05, adjusted for multiple comparisons when necessary. We anticipated that the assumption of normally distributed data would be violated due to the sample size and selected nonparametric alternatives for all statistical tests. Differences between the patient completing and not completing the follow-up NCAs were assessed using chi-square test for categorical data and Mann–Whitney U-tests for continuous data.
At each time point, the percentage of patients with a cognitive performance below the impairment threshold (Z ≤ −1.5) was calculated for each task as well as for each domain. Additionally, individual changes in both subjective and objective cognitive performance were calculated for (1) each domain (“domain-level”) and (2) across all domains (“overall-level”). Above-described cut-off scores were used to determine an improvement, deterioration, mixed, or stable score. Changes in scores were calculated for baseline versus 3 months, and 3 versus ≥ 11 months.
For the overall-level, patients were categorized into 4 categories for subjective and objective cognitive performance separately: (1) decline, (2) improvement, (3) mixed, and (4) stable performance. Decline and improvement were defined as either a decrease or increase in at least one cognitive domain, respectively. The category “mixed” included patients who showed both declined and improved performance across the domain and/or, for objective cognitive performance only, within one domain. Patients were categorized into “stable” if performance across all domains remained unchanged. Subsequently, age, baseline KPS, primary tumor, presence of extracranial metastases, number of BMs, symptomatic BMs, synchronous diagnosis of BMs, intracranial progression at 3 months as determined by clinical follow-up scans, and baseline cognitive impairment were assessed for the 4 categories, separately for the 2 time periods and for subjective and objective cognitive performance. As this analysis aimed to explore possible risk factors for cognitive decline, no corrections for multiple comparisons were performed.
Results
Compliance
Thirty-six out of the original 60 (60%) patients were eligible for analysis, having completed the 3-months follow-up NCA (Figure 1). Of the 24 patients eligible for ≥11-months follow-up, 14 (58%) patients completed this assessment. Reasons for noncompliance were poor medical condition, death, refusal because testing was considered too burdensome, and time constraints of the patient.
Figure 1.
Flow-chart of the patients completing the pre-radiotherapy, 3-months and ≥11-months NCA including reasons for patients lost in follow-up.
Patients who did not complete the 3-months follow-up had a lower KPS than patients who did complete the 3 months follow-up (P = .001). More patients who completed the ≥11-months follow-up had BMs as their first symptom of cancer (ie, synchronous diagnosis, 57%) than patients who did not complete the ≥11-months follow-up (10%; P = .019). None of the other characteristics as shown in Table 1 significantly differed between patient groups. Moreover, there were no differences regarding preradiotherapy cognitive performance (domain-level) or number of patients with a cognitive impairment between patients who completed or not-completed the 3-months NCA nor between patients who completed or not-completed the ≥11-months NCA.
Table 1.
Preradiotherapy Sociodemographic and Clinical Characteristics of the Patient Population
| Patients without follow-up | Patients with 3 months follow-up | Patients with ≥11 months follow-up | |
|---|---|---|---|
| N | 24 | 36 | 14 |
| Age, years, median (IQR) | 68 (63–73) | 63 (56–71) | 67 (56–73) |
| Sex (male), n (%) | 13 (54) | 19 (53) | 8 (57) |
| Educational levela, n (%) | |||
| 3 | 0 (0) | 3 (8) | 2 (14) |
| 4 | 4 (17) | 7 (19) | 3 (21) |
| 5 | 12 (50) | 10 (28) | 5 (36) |
| 6 | 5 (21) | 10 (28) | 3 (21) |
| 7 | 3 (13) | 6 (17) | 1 (7) |
| Ravens matrices,29 median percentile (IQR) | 62.5 (28-75) | 69 (38-90) | 69 (47-84) |
| Handednessb, n (%) | |||
| Left | 5 (21) | 3 (8) | 2 (14) |
| Right | 18 (75) | 32 (89) | 11 (79) |
| Ambidextrous | 1 (4) | 1 (3) | 1 (7) |
| KPS, median (IQR) | 75 (63-80) | 80 (80-90) | 80 (78-90) |
| KPS ≥ 90, n (%) | 2 (8) | 14 (39) | 6 (43) |
| Missing | 5 (21) | 0 (0) | 0 (0) |
| No. of BMs, n (%) | |||
| 1 | 7 (33) | 11 (31) | 5 (36) |
| 2–4 | 12 (50) | 14 (39) | 5 (36) |
| 5–10 | 2 (8) | 7 (19) | 3 (21) |
| >10 | 3 (13) | 4 (11) | 1 (7) |
| Hemisphere involvement BMs, n (%) | |||
| Left | 7 (29) | 9 (25) | 2 (14) |
| Right | 5 (21) | 7 (19) | 4 (29) |
| Bilateral | 12 (50) | 20 (56) | 8 (57) |
| Lobe involvement, n (%) | |||
| Frontal | 11 (46) | 23 (64) | 9 (64) |
| Temporal | 5 (21) | 8 (22) | 4 (29) |
| Occipital | 8 (33) | 13 (36) | 4 (29) |
| Parietal | 8 (33) | 17 (47) | 7 (50) |
| Cerebellum | 12 (50) | 15 (42) | 7 (50) |
| Brainstem | 1 (4) | 2 (6) | 0 (0) |
| Primary tumor origin, n (%) | |||
| Lung cancer | 13 (54) | 17 (47) | 9 (64) |
| Melanoma | 4 (17) | 9 (25) | 3 (21) |
| Breast Cancer | 1 (4) | 2 (5) | 0 (0) |
| Renal cell carcinoma | 1 (4) | 3 (8) | 2 (14) |
| Other | 5 (21) | 5 (14) | 0 (0) |
| Extracranial metastases, n (%) | 15 (63) | 21 (58) | 9 (64) |
| BMs as first symptom of cancer diagnosis, n (%) | 7 (29) | 12 (33) | 8 (57) |
| Previous brain RT, n (%) | 2 (8) | 7 (19) | 2 (14) |
| Previous BMs resection, n (%) | 7 (29) | 10 (28) | 4 (29) |
| Previous immuno-/chemotherapy, n (%) | 18 (75) | 10 (28) | 9 (64) |
| Type of RT | |||
| SRS | 19 (79) | 34 (94) | 14 (100) |
| WBRT | 4 (17) | 2 (6) | 0 (0) |
| WBRT + SRS | 1 (4) | 0 (0) | 0 (0) |
| Dexamethasone use prior to RT, mg/day, median (IQR) | 4 (0-4) | 0 (0-4) | 1 (0-4) |
| Symptomatic BMs at diagnosis, n (%) | 16 (67) | 21 (58) | 8 (57) |
| Epilepsy c | 3 (19) | 8 (38) | 4 (50) |
| Motor c | 5 (31) | 7 (33) | 3 (38) |
| Sensory c | 0 (0) | 3 (14) | 1 (13) |
| Balance c | 5 (31) | 4 (19) | 2 (25) |
| Language c | 0 (0) | 2 (10) | 1 (13) |
| Visual c | 4 (25) | 4 (19) | 2 (25) |
| Cognitive c | 6 (38) | 3 (14) | 1 (13) |
| Headache c | 5 (31) | 7 (33) | 2 (25) |
| Other c | 5 (31) | 4 (19) | 1 (13) |
aAccording to Verhage classification30, bself-reported, cPercentage of patients with symptomatic BMS at diagnosis.
Due to rounding, not all percentages add up to 100%.
Abbreviations: BMs, brain metastases; IQR, interquartile range; RT, radiotherapy; SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
Clinical Characteristics
Baseline sociodemographic and clinical characteristics are shown in Table 1. In total 36 patients (19 male) finished the 3-months follow-up NCA at a median of 16 weeks from baseline (IQR 14-17). Median long-term follow-up time was 61 weeks from baseline (IQR 52–76). The median age was 63 years, and the primary tumor was most frequently lung cancer (47%). Most patients received SRS (94%) for 2–4 BMs (39%).
During the 3-months follow-up, intracranial progression was observed in 13/36 patients (36%) of which 3 patients had received additional radiotherapy for these new BMs before the follow-up NCA. From the 3-months to the ≥11-months follow-up NCA, intracranial progression was observed in 6/14 patients of which 5 patients had received additional radiotherapy for these new BMs before the ≥11-months follow-up NCA.
Subjective Cognitive Functioning
Preradiotherapy
Preradiotherapy subjective performance of a larger sample has been reported previously.23 Of the currently included sample, 11/36 (31%) reported stable subjective cognitive performance across all domains, while the majority of patients (24/36, 67%), report lower subjective cognitive performance than their premorbid levels on at least one cognitive domain (Supplementary Figure 1). Declines were reported across all domains, but most common for attention, thinking, memory, and language (all 4 domains: 14/36, 39%).
Postradiotherapy changes
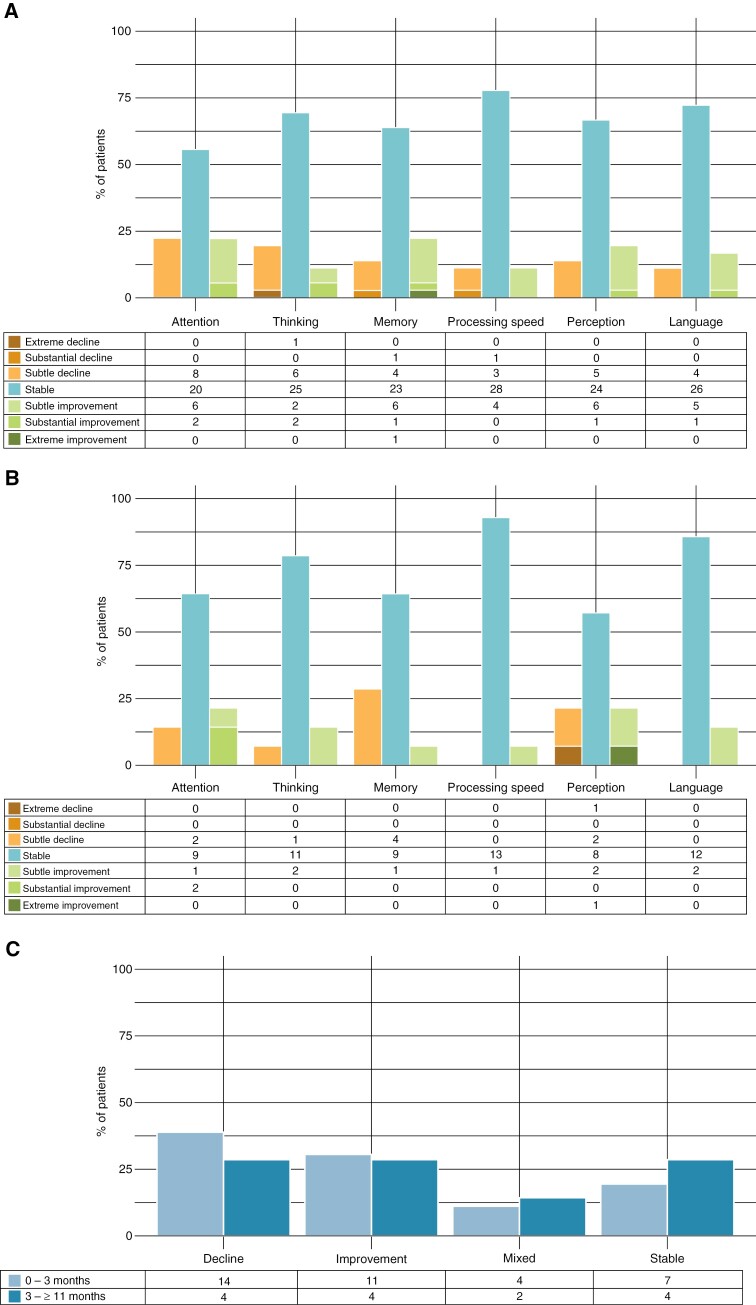
On the domain-level, subjective declines in cognitive performance were reported across all domains 3 months postradiotherapy (Figure 2a). Declines were most frequently reported for attention (22%) and thinking (19%). For most domains, the percentage of patients that reported a decline was balanced out by the percentage of patients that reported an improvement (11–22%). Improvements were most often reported for attention (22%) and memory (22%). A stable score was reported across domains by 56–78% of patients. Stable scores were most often reported for processing speed (78%), language (72%), and thinking (69%).
Figure 2.
Change in subjective cognitive performance calculated from (a) baseline-3 months and (b) 3 to ≥11 months at the domain-level and (c) at overall-level. Tables below the graph indicate the number of patients. Note: stable performance (±5), subtle improvement or decline (±6 to 25), substantial improvement or decline (±26 to 50), and extreme improvement or decline (±>50).
From 3 to ≥11 months after radiotherapy, (further) subjective declines in cognitive performance were reported for memory (29%), perception (21%), attention (14%), and thinking (7%; Figure 2b). No declines were reported for processing speed or language. Improvements were also reported across all domains, but most frequently for attention (21%) and perception (21%). Stable scores were again most often observed for processing speed (93%) and language (86%).
Overall, 3 months postradiotherapy 39% of patients reported a decline, 31% an improvement, 11% mixed performance, and 19% stable subjective cognitive performance (Figure 2c). In the time period from 3 to ≥11 months after radiotherapy similar results were observed, with decline, improvement, and stable performance in 29% of patients and mixed in 14%. More patients with intracranial progression showed a decline across all cognitive domains 3 months postradiotherapy compared to patients without intracranial progression (X2(3) = 8.896, P = 0.031). Age, KPS at baseline, number of BMs, synchronous BMs diagnosis, primary tumor, extracranial metastases, symptomatic BMs, and number of cognitive domain impairments at baseline did not significantly differ between the 4 categories from baseline to 3 months after radiotherapy (Supplementary Table 3).
Objective Neurocognitive Functioning
Preradiotherapy
An elaborate evaluation of preradiotherapy cognitive performance in a larger sample of this subset has been reported previously.23 For the currently included sample, 29/36 (81%) showed impairments (Z ≤ −1.5) in at least one cognitive domain, and 18/36 (50%) with impairments in at least 2 domains. Memory was most frequently affected (47%), followed by psychomotor speed (36%), and processing speed (31%; Supplementary Results).
Short-term postradiotherapy
Three months after radiotherapy, a cognitive impairment (Z ≤ −1.5) in ≥1 domain was observed for nearly all patients 35/36 (97%), whereby more than half of all patients (68%) showed impaired performance in at least 2 domains. Memory was most often impaired (78%), followed by psychomotor speed (39%), and attention (31%; Supplementary Results).
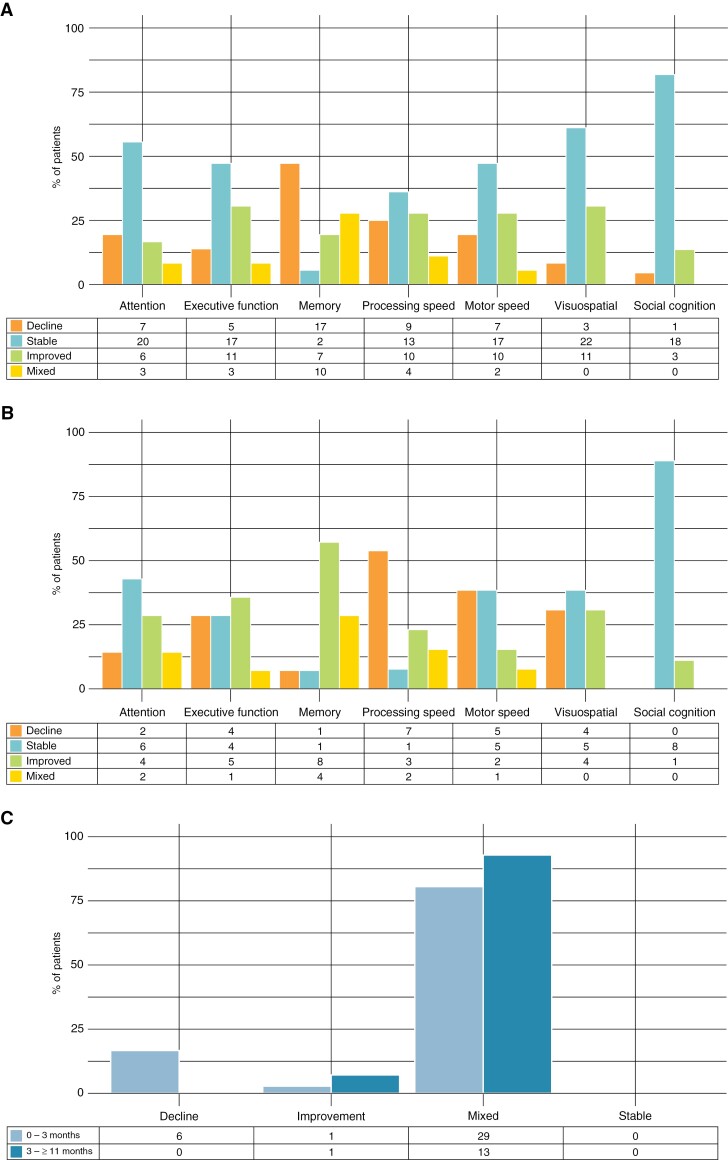
Using the RCI, a decline in cognitive performance was observed across all domains, but most frequently for memory (47%) and processing speed (35%; Figure 3a). Improvements were most often observed for executive function and visuospatial functioning (both 31%) and processing and psychomotor speed (both 28%). Mixed cognitive changes across the tests within a cognitive domain were seen for all domains, except visuospatial functioning and social cognition. Memory showed the highest frequency of mixed responses (28%). Stable cognitive performance was most often observed for social cognition (82%) and for visuospatial functioning (61%).
Figure 3.
Change in objective cognitive performance calculated from (a) baseline-3 months and (b) 3 to ≥11 months at the domain-level, and (c) at overall-level. Tables below the graph indicate the number of patients.
Overall, none of the patients showed stable performance across all domains, with most 29/36 (81%) showing both improvement and decline in different cognitive domains (Figure 3c). Solely declined performance was observed in 6/36 (17%). Age, KPS at baseline, number of BMs, synchronous BMs diagnosis, primary tumor, extracranial metastases, symptomatic BMs, intracranial progression, and number of cognitive domain impairments at baseline did not differ between the 4 categories from baseline to 3 months after radiotherapy (Supplementary Table 5).
Long-term postradiotherapy
At least 11 months after radiotherapy, a cognitive impairment (Z ≤ −1.5) in at least 1 domain was found for 11/14 (79%) of patients, whereby more than half of the patients (71%) showed impaired performance in ≥2 domains. Memory was most often impaired (71%), followed by social cognition (43%), and psychomotor speed (39%; Supplementary Results).
The RCI indicated a decline in cognitive performance across all domains except social cognition (Figure 3b). Declines were most frequently observed for processing speed (54%) and psychomotor speed (39%). Improvements were most often observed regarding memory (57%) and executive function (36%). Mixed responses across the tests within a cognitive domain were seen for all domains, except visuospatial functioning and social cognition, and were most common for memory (29%). Stable cognitive performance was most often observed for social cognition (89%) and attention (43%). Overall, none of the patients showed declined or stable performance across all domains, with most 13/14 (93%) showing mixed performance (Figure 3c). Solely improved performance was observed in 1/14 (7%).
Discussion
To be able to provide patient-tailored cancer care, including personalized psycho-education, we first need individualized research to inform on the effects of cancer treatment on individuals. Therefore, the aim of this study was to evaluate the individual trajectories of subjective and objective cognitive performance after radiotherapy in patients with BMs both in the short-term (ie, 3 months) and in the long-term (ie, ≥11 months) to provide insight into the cognitive impact of treatment. Our findings demonstrate that on a group-level, the incidence of patients displaying cognitive decline is counterbalanced by those demonstrating improvements within the same cognitive domain. The individualized results reveal a nuanced picture, where the majority of patients who exhibit improvements in one domain also experience a decline in another. Thus, impact of radiotherapy on cognitive performance is complex and multidimensional.
The cognitive impact of radiotherapy was also subjectively reported by the majority of patients. Using VAS, patients were able to provide a differentiated profile of subjective cognitive decline over time. Three months postradiotherapy, half of the patients experienced a decline in subjective cognitive performance, most frequently involving attention and thinking. In the long term, memory complaints were more prominent. Considering that intracranial progression emerged as a risk factor for self-reported cognitive decline 3 months postradiotherapy, these cognitive complaints may primarily reflect new or aggravated symptoms resulting from the presence of new BMs. Based on previous research, it was anticipated that the specific cognitive domains would not fully align with those observed in the objective cognitive assessment.31 However, there was a consistency between the number of patients reporting subjective cognitive decline and those demonstrating objective cognitive decline. To illustrate, patients may have reported complaints in attention, while these were objectively reflected by worse memory performance. This underlines that while patients may label cognitive complaints differently, it is essential to incorporate subjective assessments to also capture patients’ experiences of cognitive difficulties.
Regarding objective cognitive performance, multiple cognitive domains were affected postradiotherapy, rather than a single domain universally affected in all patients. Nevertheless, memory appeared particularly susceptible to the negative effects of treatment, reflected by the 80% of patients with a memory impairment 3 months postradiotherapy and 75% of patients exhibiting declined memory performance compared to preradiotherapy. After excluding the 2 patients who received WBRT, these percentages remained unchanged, highlighting the notable prevalence of memory decline within our SRS patient sample. The negative impact of radiotherapy on learning and memory performance has received widespread recognition. As the hippocampus is the primary brain region responsible for learning and memory, a great deal of interest has now been put into hippocampal avoidance WBRT (HA-WBRT)30,32,33 and now even HA-SRS29 for the possible preservation of neurocognition. While damage to the hippocampus has indeed been implicated in cognitive decline following brain radiotherapy, recent findings underscore concomitant shrinkage in other subcortical brain structures, as well as damage to both white matter and other cortical territories.34–40 Moreover, contemporary perspectives on cognitive functioning emphasize its network-based nature, moving beyond mere localization.41,42 This recognition complicates the straightforward attribution of cognitive side-effects exclusively to specific radiotherapy-vulnerable brain regions. Further research is needed to better understand the complex interplay between radiotherapy, brain structures, and cognitive functioning in order to optimize treatment outcomes in clinical settings.
In the long-term, slowing of both processing and psychomotor speed was most prominent, while this was not reflected accordingly in self-reported cognitive changes. Multiple factors could have contributed to the observed slowing as processing and psychomotor speed both rely on a widespread neural network.43,44 As most patients with BMs receive systemic treatment after brain radiotherapy, the long-term psychomotor slowing could also be a consequence of chemotherapy-induced neuropathy rather than long-term radiotherapy effects.45,46
In our sample, a decline in objective cognitive performance was observed in nearly all patients. Interestingly, for the majority, this decline was accompanied by improvement in another cognitive domain. This pattern of mixed responses persisted in the long term and was irrespective of patients’ preradiotherapy cognitive impairment. While not statistically significant, a higher number of BMs and metasynchronous BMs diagnoses appeared more common among patients with cognitive decline 3 months postradiotherapy, suggesting potential areas for future investigation. As mixed responses were predominant, no other clear risk factors for postradiotherapy cognitive changes were found. It is important to acknowledge that cognitive improvement does not necessarily indicate the absence of cognitive impairment, as demonstrated by the small percentage of patients without any cognitive impairment at both follow-up times (3–21%). Nonetheless, the RCI enabled us to identify meaningful changes, indicating that both the observed positive and negative changes are likely to have a significant impact on the daily lives of these patients. The heterogeneity in cognitive outcomes can likely be attributed to a range of factors, including the extent and location of metastases, individual variabilities in treatment response, and preexisting cognitive or neural vulnerabilities. Future studies incorporating these different variables in large cohorts could advance our understanding of the complex interplay between treatment-related factors and patient-specific factors in shaping cognitive outcomes after radiotherapy. The current findings provide a snapshot of the currently available evidence on cognitive trajectories of individual patients with BMs after receiving radiotherapy. Yet future research still faces numerous challenges and opportunities. For example, while the clinical observation of the unfragmented Hooper Visual Organization test indicated no severe naming difficulties in our sample, the absence of normative data hinders evaluating the relative impact of radiotherapy on language function compared to other cognitive domains. Future studies should include formal language assessments to fill this gap. Moreover, it is challenging to discern the effects of radiotherapy from those of other treatments and disease progression, as adjuvant systemic treatments during the study period may have contributed to declines in cognitive performance. Due to the inevitable deterioration in the medical condition of this population, results are mainly representative of the group of patients fit enough 3 months postradiotherapy. Nevertheless, both compliance rates and available patients for follow-up, especially in the long-term, were comparable to or higher than previous studies.13,14,20,47–49 Most importantly, these results can be used to design future studies to best capture the complexity of individual cognitive changes in patients with BMs. For example, as memory seems particularly vulnerable, multiple tests each capturing different aspects of this multifaceted cognitive function should be incorporated.
Conclusions
The increasing incidence of BMs and improved survival rates underscore the urgent need to investigate the effects of treatments on individuals because group-level information alone is insufficient when conveying the potential treatment effects to patients and caregivers. Our study revealed a complex impact of radiotherapy on subjective and objective cognitive performance, involving both positive and negative changes across various cognitive domains. Particularly, memory showed vulnerability in the early postradiotherapy period. The observed within-individual variation emphasizes the involvement of intricate underlying mechanisms, highlighting the need for further investigation. Despite current advancements in treatment patients remain at risk for intracranial progression, which was a clear risk factor for subjective cognitive decline in this study. These findings are specific to patients with BMs who have undergone radiotherapy and should be considered in light of the potential trade-off between cognitive difficulties and survival benefits. Our results show the heterogeneity of cognitive profiles postradiotherapy, thereby underscoring the importance of patient-tailored NCAs three months postradiotherapy to guide optimal rehabilitation strategies.
Supplementary Material
Acknowledgments
We would like to thank all patients who participated in the APRICOT and COIMBRA study for contributing their time to these research projects. Additionally, we would like to thank the master students who assisted in the data collection for the COIMBRA and APRICOT study as part of their research internship: Celeste Hinkert, Charlotte Doll, Eline van Daele, Gelena Mahmoud, Janneke Verhoeven, Manon Kraaij, Thirza Vrolijk.
Contributor Information
Eva E van Grinsven, Department of Neurology and Neurosurgery, University Medical Center Utrecht Brain Center, Utrecht University, Utrecht, The Netherlands.
Fia Cialdella, Department of Radiation Oncology, University Medical Center Utrecht, Utrecht, The Netherlands; Department of Medical Oncology, University Medical Center Utrecht, Utrecht, The Netherlands.
Yoniet Gmelich Meijling, Department of Neurology and Neurosurgery, University Medical Center Utrecht Brain Center, Utrecht University, Utrecht, The Netherlands.
Joost J C Verhoeff, Department of Radiation Oncology, University Medical Center Utrecht, Utrecht, The Netherlands.
Marielle E P Philippens, Department of Radiation Oncology, University Medical Center Utrecht, Utrecht, The Netherlands.
Martine J E van Zandvoort, Department of Neurology and Neurosurgery, University Medical Center Utrecht Brain Center, Utrecht University, Utrecht, The Netherlands; Department of Experimental Psychology and Helmholtz Institute, Utrecht University, The Netherlands.
Funding
The APRICOT study described in the current manuscript as well as the first author (E.G), were supported by research funding from the Dutch Cancer Society “Koningin Wilhelmina Fonds (KWF)” (#11110). The funding sources had no role in conducting this research or preparation of this manuscript.
Conflict of interest statement
The authors declare no conflict of interest.
Authorship statement
E.v.G.: conceptualization, data curation, methodology, formal analysis, investigation, visualization, writing—original draft, writing—review and editing. F.C.: conceptualization, investigation, writing—review and editing. J.V.: conceptualization, supervision, writing—review and editing. M.P.: conceptualization, supervision, funding acquisition, writing—review and editing. M.v.Z.: conceptualization, methodology, supervision, writing—review and editing.
Data availability
Upon completion of the APRICOT and COIMBRA trial data can be made available pending a formal research proposal.
References
- 1. Gerstenecker A, Nabors LB, Meneses K, et al. Cognition in patients with newly diagnosed brain metastasis: profiles and implications. J Neurooncol. 2014;120(1):179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Achrol AS, Rennert RC, Anders C, et al. Brain metastases. Nat Rev Dis Primers. 2019;5(1):5. [DOI] [PubMed] [Google Scholar]
- 3. Nieder C, Spanne O, Mehta MP, Grosu AL, Geinitz H.. Presentation, patterns of care, and survival in patients with brain metastases: What has changed in the last 20 years? Cancer. 2011;117(11):2505–2512. [DOI] [PubMed] [Google Scholar]
- 4. Lanier CM, Hughes R, Ahmed T, et al. Immunotherapy is associated with improved survival and decreased neurologic death after SRS for brain metastases from lung and melanoma primaries. Neurooncol Pract. 2019;6(5):402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nayak L, Lee EQ, Wen PY.. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48–54. [DOI] [PubMed] [Google Scholar]
- 6. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–656. [PubMed] [Google Scholar]
- 7. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37(4):745–751. [DOI] [PubMed] [Google Scholar]
- 8. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655–661. [DOI] [PubMed] [Google Scholar]
- 10. Eichler AF, Loeffler JS.. Multidisciplinary management of brain metastases. Oncologist. 2007;12(7):884–898. [DOI] [PubMed] [Google Scholar]
- 11. Van Grinsven EE, Nagtegaal SHJ, Verhoeff JJC, Van Zandvoort MJE.. The impact of stereotactic or whole brain radiotherapy on neurocognitive functioning in adult patients with brain metastases: a systematic review and meta-analysis. Oncol Res Treat. 2021;44(11):622–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mehta MP, Rodrigus P, Terhaard CHJ, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21(13):2529–2536. [DOI] [PubMed] [Google Scholar]
- 13. Chang EL, Wefel JS, Maor MH, et al. A pilot study of neurocognitive function in patients with one to three new brain metastases initially treated with stereotactic radiosurgery alone. Neurosurgery. 2007;60(2):277–83; discussion 283. [DOI] [PubMed] [Google Scholar]
- 14. Habets EJJ, Dirven L, Wiggenraad RG, et al. Neurocognitive functioning and health-related quality of life in patients treated with stereotactic radiotherapy for brain metastases: a prospective study. Neuro Oncol. 2016;18(3):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hodgson KD, Hutchinson AD, Wilson CJ, Nettelbeck T.. A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev. 2013;39(3):297–304. [DOI] [PubMed] [Google Scholar]
- 16. Joly F, Castel H, Tron L, Lange M, Vardy J.. Potential effect of immunotherapy agents on cognitive function in cancer patients. J Natl Cancer Inst. 2020;112(2):123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wefel JS, Schagen SB.. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12(3):267–275. [DOI] [PubMed] [Google Scholar]
- 18. Schagen SB, Tsvetkov AS, Compter A, Wefel JS.. Cognitive adverse effects of chemotherapy and immunotherapy: are interventions within reach? Nat Rev Neurol. 2022;18(3):173–185. [DOI] [PubMed] [Google Scholar]
- 19. Schimmel WCM, Verhaak E, Bakker M, et al. Group and individual change in cognitive functioning in patients with 1 to 10 brain metastases following gamma knife radiosurgery. Clin Oncol. 2021;33(5):314–321. [DOI] [PubMed] [Google Scholar]
- 20. van der Meer PB, Habets EJJ, Wiggenraad RG, et al. Individual changes in neurocognitive functioning and health-related quality of life in patients with brain oligometastases treated with stereotactic radiotherapy. J Neurooncol. 2018;139(2):359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jacobson NS, Roberts LJ, Berns SB, McGlinchey JB.. Methods for defining and determining the clinical significance of treatment effects: description, application, and alternatives. J Consult Clin Psychol. 1999;67(3):300–307. [DOI] [PubMed] [Google Scholar]
- 22. Jacobson NS, Truax P.. Clinical significance: a statistical approach to denning meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12–19. [DOI] [PubMed] [Google Scholar]
- 23. van Grinsven E, Cialdella F, Verhoeff J, Philippens M, Zandvoort M.. Different profiles of neurocognitive functioning in patients with brain metastases prior to brain radiotherapy. J Psycho-Oncol. 2023;32(11):1752–1761. [DOI] [PubMed] [Google Scholar]
- 24. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. [DOI] [PubMed] [Google Scholar]
- 25. Schoo LA, Van Zandvoort MJE, Biessels GJ, Kappelle LJ, Postma A.. Insight in cognition: self-awareness of performance across cognitive domains. Appl Neuropsychol Adult. 2013;20(2):95–102. [DOI] [PubMed] [Google Scholar]
- 26. Wefel JS, Vardy J, Ahles T, Schagen SB.. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. [DOI] [PubMed] [Google Scholar]
- 27. Duff K. Current topics in science and practice evidence-based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Arch Clin Neuropsychol. 2012;27(3):248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verhage F. Intelligentie en leeftijd bij volwassenen en bejaarden. Koninklijke van Gorcum. 1964:98. [Google Scholar]
- 29. Burgess L, Nair V, Gratton J, et al. Stereotactic radiosurgery optimization with hippocampal-sparing in patients treated for brain metastases. Phys Imaging Radiat Oncol. 2021;17(11):106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wefel JS, Armstrong TS, Tome WA, et al. Sustained preservation of cognition and prevention of patient-reported symptoms with hippocampal avoidance during whole-brain radiotherapy for brain metastases: final results of NRG Oncology CC001. Int J Radiat Oncol Biol Phys. 2023;117(3):571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Green HJ, Pakenham KI, Gardiner RA.. Cognitive deficits associated with cancer: a model of subjective and objective outcomes. Psychol Health Med. 2005;10(2):145–160. [Google Scholar]
- 32. Popp I, Rau A, Kellner E, et al. Hippocampus-avoidance whole-brain radiation therapy is efficient in the long-term preservation of hippocampal volume. Front Oncol. 2021;11(August):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang WC, Chen YF, Yang CC, et al. Hippocampal avoidance whole-brain radiotherapy without memantine in preserving neurocognitive function for brain metastases: a phase II blinded randomized trial. Neuro Oncol. 2020;23(August):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Makale MT, McDonald CR, Hattangadi-Gluth JA, Kesari S.. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol. 2017;13(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nagtegaal SHJ, David S, Philippens MEP, et al. Dose-dependent volume loss in subcortical deep grey matter structures after cranial radiotherapy. Clin Transl Radiat Oncol. 2021;26(15):35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagtegaal SHJ, David S, Snijders TJ, et al. Effect of radiation therapy on cerebral cortical thickness in glioma patients: treatment-induced thinning of the healthy cortex. Neurooncol Adv. 2020;2(1):vdaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nagtegaal SHJ, David S, van Grinsven EE, et al. Morphological changes after cranial fractionated photon radiotherapy: localized loss of white matter and grey matter volume with increasing dose. Clin Transl Radiat Oncol. 2021;31(January):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Connor M, Karunamuni R, McDonald C, et al. Regional susceptibility to dose-dependent white matter damage after brain radiotherapy. Radiother Oncol. 2017;123(2):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu T, Chapman CH, Tsien C, et al. Effect of the maximum dose on white matter fiber bundles using longitudinal diffusion tensor imaging. Int J Radiat Oncol Biol Phys. 2016;96(3):696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chapman CH, Zhu T, Nazem-Zadeh M, et al. Diffusion tensor imaging predicts cognitive function change following partial brain radiotherapy for low-grade and benign tumors. Radiother Oncol. 2016;120(2): 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pessoa L. Understanding brain networks and brain organization. Phys Life Rev. 2014;11(3):400–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sutterer MJ, Tranel D.. Neuropsychology and cognitive neuroscience in the fMRI era: a recapitulation of localizationist and connectionist views. Neuropsychology. 2017;31(8):972–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hua B, Ding X, Xiong M, et al. Alterations of functional and structural connectivity in patients with brain metastases. PLoS One. 2020;15(5):e0233833–e0233816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maesawa S, Bagarinao E, Fujii M, et al. Evaluation of resting state networks in patients with gliomas: connectivity changes in the unaffected side and its relation to cognitive function. PLoS One. 2015;10(2):e0118072–e0118013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miaskowski C, Mastick J, Paul SM, et al. Chemotherapy-induced neuropathy in cancer survivors. J Pain Symptom Manage. 2017;54(2):204–218.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wefel JS, Vidrine DJ, Marani SK, et al. A prospective study of cognitive function in men with non‐seminomatous germ cell tumors. Psychooncology. 2014;23(6):626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 48. Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases a randomized clinical trial. JAMA. 2016;316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Minniti G, Capone L, Nardiello B, et al. Neurological outcome and memory performance in patients with 10 or more brain metastases treated with frameless linear accelerator (LINAC)-based stereotactic radiosurgery. J Neurooncol. 2020;148(1):47–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon completion of the APRICOT and COIMBRA trial data can be made available pending a formal research proposal.