Abstract
The field of regenerative medicine is increasingly in need of effective and biocompatible materials for tissue engineering. Human acellular dermal matrix (hADM)-derived collagen matrices stand out as a particularly promising candidate. Their ability to preserve structural integrity, coupled with exceptional biocompatibility, positions them as a viable choice for tissue replacement. However, their clinical application has been largely confined to serving as scaffolds. This study aims to expand the horizon of clinical uses for collagen sheets by exploring the diverse cutting-edge clinical demands. This review illustrates the clinical utilizations of collagen sheets beyond traditional roles, such as covering skin defects or acting solely as scaffolds. In particular, the potential of Epiflex®, a commercially available and immediately clinically usable allogeneic membrane, will be evaluated. Collagen sheets have demonstrated efficacy in bone reconstruction, where they can substitute the induced Masquelet membrane in a single-stage procedure, proving to be clinically effective and safe. The application of these membranes allow the reconstruction of substantial tissue defects, without requiring extensive plastic reconstructive surgery. Additionally, they are found to be apt for addressing osteochondritis dissecans lesions and for ligament reconstruction in the carpus. The compelling clinical examples showcased in this study affirm that the applications of human ADM extend significantly beyond its initial use for skin defect treatments. hADM has proven to be highly successful and well-tolerated in managing various etiologies of bone and soft tissue defects, enhancing patient care outcomes. In particular, the application from the shelf reduces the need for additional surgery or donor site defects.
Keywords: hADM, human acellular dermis, tissue engineering, techniques, tissue regeneration, scaffold, biocompatible materials
1. Introduction
In the field of regenerative medicine, the quest for effective and biocompatible materials for tissue replacement has led to the exploration of collagen matrices as promising scaffolds [1,2,3,4]. Collagen, the most abundant protein in the human body, plays a crucial role in providing structural support to various tissues [5,6,7,8]. Harnessing the unique properties of collagen for tissue engineering has gained significant attention due to its inherent biocompatibility [9], minimal immunogenicity [10], and ability to mimic the natural extracellular matrix (ECM) [11]. The hierarchical structure of collagen, characterized by triple-helical molecules forming fibrils and fibers, closely resembles the natural architecture of many tissues in the human body. This structural similarity makes collagen an attractive candidate for tissue engineering applications, where the goal is to create scaffolds that mimic the native environment of cells [8,10,11]. The ability of collagen matrices to maintain structural integrity is crucial for successful tissue replacement [12,13]. Various techniques, such as electrospinning and freeze-drying, have been employed to create collagen scaffolds with tailored architectures that closely resemble the target tissue [14,15,16]. These techniques allow for the manipulation of pore size, porosity, and mechanical properties, ensuring optimal structural support for cell growth, migration, and tissue regeneration but remain as xenogeneic transplants with the disadvantage of being less effective than allogenic grafts [17]. One of the key advantages of collagen matrices in tissue replacement is their excellent biocompatibility [18,19]. Collagen is a natural component of the ECM, reducing the risk of immune responses and rejection when used in vivo. Furthermore, collagen’s ability to interact with cell surface receptors promotes cell adhesion, proliferation, and differentiation, facilitating tissue regeneration.
The biocompatibility of collagen matrices extends beyond mere physical compatibility; it also influences biochemical signaling. Collagen provides binding sites for various growth factors and cytokines, creating a microenvironment that supports cell signaling and tissue development [20,21,22]. This inherent ability of collagen to modulate cellular behavior contributes to its effectiveness as a scaffold material for tissue engineering. Collagen matrices have found diverse applications in regenerative medicine, spanning various tissues and organs. In skin tissue engineering, collagen scaffolds have been utilized to promote wound healing and skin regeneration. In bone tissue engineering, collagen-based matrices serve as a substrate for osteoblasts, promoting bone formation [23,24] or as a barrier membrane in one step membrane reconstructions [25]. Additionally, in cardiovascular tissue engineering, collagen matrices have been explored for their potential in creating vascular grafts and promoting angiogenesis.
This work is intended to provide an overview of the clinical applicability of collagen sheets in defect situations of the bone or soft tissue. Using the example of Epiflex®, various application scenarios for the commercially available and immediately clinically applicable allogeneic membranes are shown.
The cases presented here, although small in number, represent a significant expansion of the current range of applications according to the literature. The identification of new potential fields of application is one of the aims of this study. Although currently these still isolated cases with limited significance, they nevertheless demonstrate the possible clinical potential of collagen membranes beyond the established applications.
2. Material
There are different collagen matrixes commercially available (Table 1) one collagen matrix product that can be used as an allogenic graft is Epiflex® (DIZG, Berlin, Germany). Epiflex® is manufactured from skin recovered from consenting donors in accordance with the European Directive 2004/23/EC and national legal requirements. The processing steps have been described previously [26]. Potential donors undergo strict screening for medical history, infectious diseases, and tissue quality. The skin is processed by thawing, removing debris, and excising damaged areas. Suitable skin is then split, decellularized and sterilized using a chemical sterilization process involving peracetic acid [27]. Epiflex® is provided in a freeze-dried state allowing a shelf-life of 5 years. This human acellular dermal matrix (hADM) exhibits permeability for oxygen and water vapor [26]. This property renders it suitable for primary wound dressings, allowing oxygen to diffuse into the wound bed and moisture vapor from the wound exudate to escape. A subsequent study by Rössner et al. [28] characterized the extracellular matrix (ECM) components of hADM. The matrix comprises mainly collagen I and III but also collagen IV, fibronectin, laminin, vitronectin, and hyaluronic acid. Confocal microscopy after processing revealed that the structure of hADM remains largely intact, with an intact collagen network and extracellular matrix (ECM) protein composition [28]. DNA quantification revealed values as low as 7.86 ± 2.20 ng DNA/mg dry tissue [28]. Hence, hADM can be considered completely decellularized in accordance with the criteria (e.g., <50 ng/mg) published by Capro et al. [29]. Vitacolonna et al. [30] investigated the biomechanics of hADM in an in-vitro incubation setting simulating an open abdomen. The authors incubated hADM with various human body fluids, including Ringer’s solution, blood, urine, upper gastrointestinal tract secretion, and a bacteria mixture for up to 21 days. The ultimate strength was evaluated, and the upper gastrointerstinal (GI) secretion was the only fluid that significantly reduced the mechanical strength of hADM compared to Ringer’s solution after 21 days. The average ultimate strength of all groups at day 0 accounted for 33.42 ± 4.79 N/mm2.
Table 1.
Collection of some common commercially available collagen sheets for clinical use, research was performed on 17 June 2024. Xenogenous collagen is only marginally immunogenic, the triple helical domains of bovine, equine and porcine collagens are highly homologous to human collagen. In contrast to porcine and bovine collagens, the use of equine collagens and human acellular dermis is not restricted by religious regulations.
| Brand Name | Distributor | Characteristics | Applications | Publication |
|---|---|---|---|---|
| Epiflex | DIZG, Berlin, Germany | Human acellular dermal matrix | Wound cover, hernia surgery | Epiflex® a new decellularised human skin tissue transplant: manufacture and properties. Rössner E, Smith MD, Petschke B, Schmidt K, Vitacolonna M, Syring C, von Versen R, Hohenberger P Cell and Tissue Banking, 24 June 2010, 12(3):209–217. https://doi.org/10.1007/s10561-010-9187-3. PMID: 20574693 [26] |
| Alloderm | AbbVie, North Chicago, IL, USA | Human acellular dermal matrix | Periodontal-guided bone regeneration applications | Jansen LA, De Caigny P, Guay NA, Lineaweaver WC, Shokrollahi K. The evidence base for the acellular dermal matrix AlloDerm: a systematic review. Ann Plast Surg. May 2013;70(5):587–594. https://doi.org/10.1097/SAP.0b013e31827a2d23. PMID: 23542841. [31] |
| Puros Dermis Allograft | ZimVie, Palm Beach Gardens, FL, USA | Human acellular dermal matrix | Soft tissue augmentation, peridontal and peri-implant soft tissue management | Wang H.L. et al., J Periodontol (2014) 85: 1693–1701. [32] Silc JT, Petrungaro PS. Acellular dermal matrix grafts for root coverage procedures: review of products and introduction of a new technique. Compend Contin Educ Dent. June 2013;34(6):408–414. PMID: 25162386. [33] |
| Bio-Gide | Geistlich, Wolhusen, Switzerland | Bovine type I collagen | Wound cover, periodontal-guided bone regeneration applications | Schlegel AK, Möhler H, Busch F, Mehl A. Preclinical and clinical studies of a collagen membrane (Bio-Gide). Biomaterials. April 1997;18(7):535–538. https://doi.org/10.1016/s0142-9612(96)00175-5. PMID: 9105592. [34] |
| BioMend Absorbable Collagen Membran | ZimVie, Palm Beach Gardens, FL, USA | Bovine collagen, chemically crosslinked | Periodontal-guided bone regeneration applications. Treatment of gingival lesions |
Evaluation of the efficacy of 100% Type-I collagen membrane of bovine origin in the treatment of human gingival recession: A clinical study July 2014. Indian Journal of Dentistry 5(3):132–138 https://doi.org/10.4103/0975-962X.140822 [35] |
| Collagene AT | e.g., MPE Medical, Wesseling, Germany | Equine collagen | Dental implantology and periodontology | An Overview of the Use of Equine Collagen as Emerging Material for Biomedical Applications by Nunzia Gallo, Maria Lucia Natali, Alessandro Sannino and Luca Salvator J. Funct. Biomater. 2020, 11(4), 79; https://doi.org/10.3390/jfb11040079 [36] |
| Ossix Plus | Datum Dental Ltd., Lod, Israel | Porcine collagen, sugar cross-linked | Periodontal-guided bone regeneration applications. | Radenković M, Alkildani S, Stoewe I, Bielenstein J, Sundag B, Bellmann O, Jung O, Najman S, Stojanović S, Barbeck M. Comparative In Vivo Analysis of the Integration Behavior and Immune Response of Collagen-Based Dental Barrier Membranes for Guided Bone Regeneration (GBR). Membranes (Basel). 15 September 2021;11(9):712. https://doi.org/10.3390/membranes11090712. PMID: 34564529; PMCID: PMC8467533. [37] |
The hADM has also demonstrated the capacity to be combined with other substances, such as the addition of thrombin-derived host defense peptides [38]. Electron microscopy analyses revealed that the combination of hADM and host defense peptides resulted in reduced hADM degradation, due to peptide-mediated bacterial killing. Whereas host defense peptides (HDPs) alone induce hemolytic effects in a dose dependent fashion, the combination of hADM and HDPs remains non-hemolytic.
3. Preclinical Studies
The hADM has been employed in various preclinical studies. For instance, a rat study successfully utilized the ADM for the development of axially vascularized flaps in a 3D-printed chamber, thereby retaining a higher volume compared to another collagen/elastin matrix [39]. Furthermore, Vitacolonna and colleagues seeded fibroblasts on ADM. They observed ongoing proliferation in a dorsal skinfold chamber and a repopulation of the ADM via the hair follicle pores [40].
Own scanning electron microscopic analyses revealed a structure with an approximately parallel arrangement of collagen layers. Interruptions or empty spaces could be due to e.g., sweat glands and hair follicles. Further substructures of the collagen fibrils are visible at higher magnifications. Furthermore, the human ADM could be easily loaded with regenerative cells by using a dynamic centrifugation procedure with bone marrow mononuclear cells [41].
Several animal studies have been conducted using a plate-stabilized femoral defect in rats as a bone defect model to evaluate the potential of hADM as a replacement for the membrane in the induced membrane technique [25,41]. The collagen membrane could be used here as a suitable substitute and enables a one-stage procedure [25].
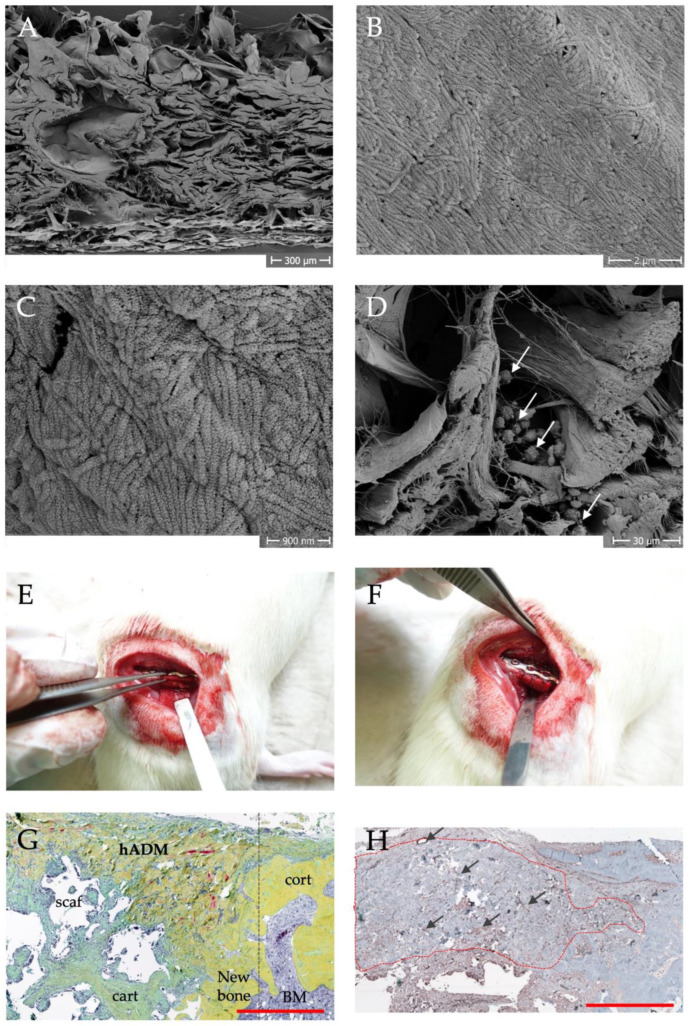
Histological analysis of the bone defects shows partial integration of the membrane into the newly formed bone tissue. Immunohistologically, a high vascular density can be observed within the hADM after 8 weeks (Figure 1) [42].
Figure 1.
Structure, vascularization and application of hADM in preclinical animal studies [42]. Scanning electron microscopy images of the structural composition at low magnification (A) and evidence of fibrillar collagen at higher magnifications (B,C). Colonization of the hADM with mononuclear cells from the bone marrow (D), arrows mark cells). In (E,F) the application of the hADM in the rat femoral defect model is shown. Movat pentachrome staining of a histological section (G) shows the integration of the membrane (hADM) into newly formed bone tissue (new bone, further abbreviations: cart = cartilage, cort = cortical bone, scaf = scaffold, dashed line shows initial defect border, scale bar = 1 mm). (H) shows the immunohistological evidence of membrane vascularization by specific staining of a-smooth muscle actin. Blood vessels appear brownish and are marked with arrows. The dashed outline indicates the hADM, scale bar = 1 mm.
4. Clinical Applications
In the clinic, the primary application for collagen hADM membranes is currently skin replacement or reconstruction in various diseases.
4.1. Skin Reconstruction
Human ADM has been clinically employed in implant-based breast reconstructions following skin- and nipple-sparing mastectomies for 84 patients [43]. The patient-reported outcomes and aesthetic results demonstrated a high level of satisfaction with high aesthetic scores. The satisfaction scores were even higher after revision surgery caused by capsular fibrosis. Paprottka et al. [44] evaluated the use of Epiflex® compared to other ADMs, including bovine and porcine, after breast surgery in 41 patients with a 3-year follow-up. The complication rate of hADM (7%) was significantly lower compared to porcine (14%) and bovine ADM (31%). Hardt et al. [45] reported their experience with laparoscopic ventral pouch pexy using hADM. In this case report, the patient remained symptom-free and showed no signs of recurrence after a 12-month follow-up.
However, other applications of collagen membranes are also clinically useful and safe, and some further cases are shown here as examples.
4.2. Collagen Membranes in Bone Reconstruction
The induced membrane concept for the reconstruction of long bone defects described by Masquelet et al. is a two-stage technique that successfully repairs bone defects of up to 25 cm [46,47]. Traditionally, in the first operation, PMMA (polymethyl methacrylate) cement spacer is inserted into the bone defect, which induces the formation of a membrane needed to avoid ingrowth of soft tissue. After 8–12 weeks, depending on the defect site [48], a second operation is conducted whereby the cement spacer is removed and the induced membrane tube is filled with iliac crest cancellous bone or RIA (Reamer-Irrigator- Aspirator) cancellous bone. Over the course of several months, total bone healing occurs. Preclinically the effect of a collagen hADM membrane was evaluated to be used in a one-step procedure. Convincing bone healing was achieved in the animal model [25]. The first clinical results of this procedure are now also available.
The aim of this paper is to illustrate and explain our experiences with hADM in various clinical applications. Application observations are currently being carried out on the individual techniques described here, but the small number of cases does not yet permit a final evaluation.
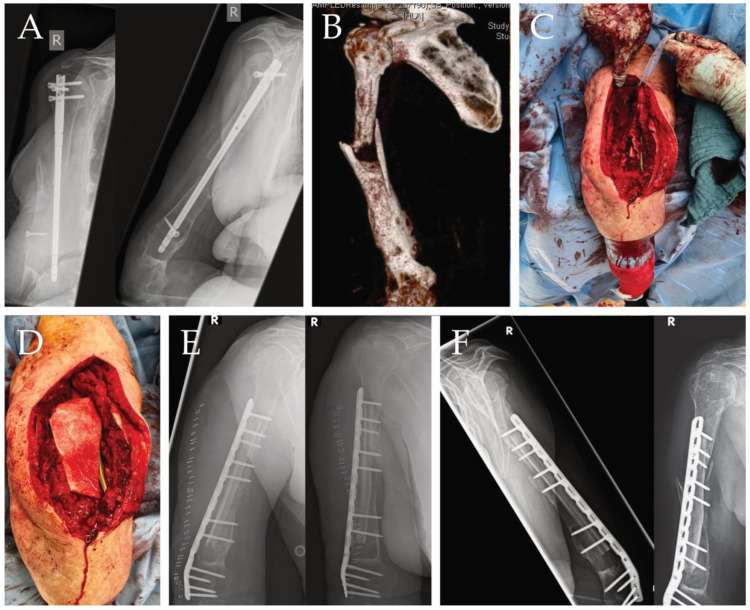
The application of hADM in bone defects as a substitute for the induction of a Masquelet membrane has been performed several times and showed good results; here we show the case of an 80-year-old woman with a humeral shaft fracture treated with intramedullary nailing at an external hospital who experienced a fall during rehabilitation, resulting in an undiagnosed periprosthetic fracture. Several weeks later, she presented with severe pain. During diagnostics, a dislocated humerus nail was detected (Figure 2A). The nail was removed, and samples were taken to rule out an infection. A CT scan was performed postoperatively (Figure 2B). No infection was detected in the samples taken; the next step was to reconstruct the humerus in a single operation. The large bone defect (Figure 2C) was filled with an allogenic fibula graft, bridged with a plate and wrapped with hADM using the single-stage membrane technique (Figure 2D), a good position was achieved, and the length of the humerus could be completely reconstructed (Figure 2E). After 6 months, the patient returned completely symptom free, achieving complete bony reconstruction using the technique shown here (Figure 2F).
Figure 2.
(A) X-ray image of dislocated humerus nail with the large defect zone clearly visible in two planes. (B) CT imaging after nail removal and sampling. (C) intraoperative situs, large defect area clearly visible. (D) operative situs after plate application and installation of a fibula graph as well as defect coverage by a collagen hADM membrane. (E) postoperative imaging, defect completely bridged. (F) healing after six months, clear bony overgrowth of the large defect zone.
However, collagen matrices are a possible treatment option not only for pure bone defects, but also for interface defects. Here, hADM is very suitable because the material can be used to achieve good coverage of defective tissue, but also a good connection between hard and soft tissue.
4.3. Collagen Membranes in Interface Defects and Cartilage Reconstruction
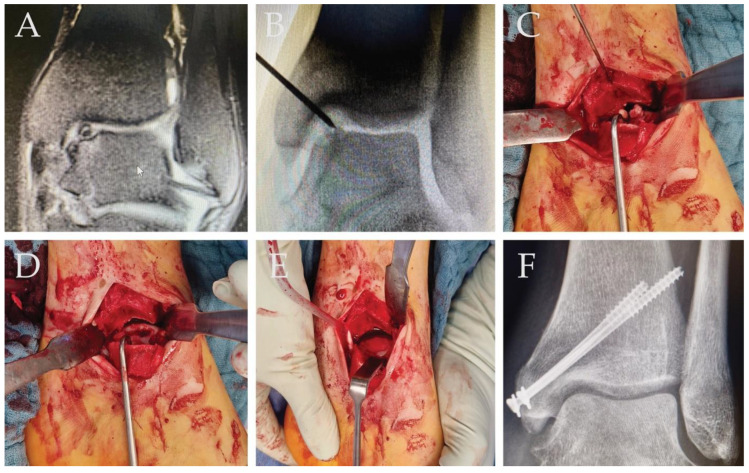
In the case of a 27-year-old patient with 4° osteochondritis dissecans (OD) of the left medial talus shoulder (Figure 3A), an arthrotomy with excision of the defect was performed. The large defect with free joint bodies is clearly visible in the intraoperative images (Figure 3B,C). After removal of the defect, the defect zone was filled with iliac crest cancellous bone and covered with a collagen hADM membrane, which was fixed with a fibrin spray and the medial malleolus was re-retained with screws (Figure 3D,E). After 6 weeks of weight-bearing, the healing process looked good (Figure 3F). The patient was pain-free during further weight bearing.
Figure 3.
(A) Preoperative MRI Image 4° osteochondritis dissecans of the left medial talus shoulder. (B) Intraoperative X-Ray with the big visible defect. (C) Intraoperative situs, visible free joint bodies. (D) Big defect after clearance of the OD. (E) Defect is filled with iliac crest cancellous bone and covered with collagen hADM membrane; the membrane is fixed with sprayed Fibrin. (F) Six weeks postoperative, good remodeling, and defect in X-ray is not visible anymore.
4.4. Collagen Membranes in Intercarpal Stabilization/Ligament Reconstruction
For ligament reconstruction, an autologous tendon transfer is typically required, posing a risk of complications at the donor site. Consequently, there is a growing interest in the use of allogenic grafts, which help avoid donor site morbidity. Additionally, allogenic grafts offer the advantage of an unlimited graft quantity, with predictable graft sizes. Several studies indicate that the outcomes of allogenic grafting are comparable to autologous grafting [49,50]. Collagen membranes have proven successful in facilitating ligament replacement in animal models [51].
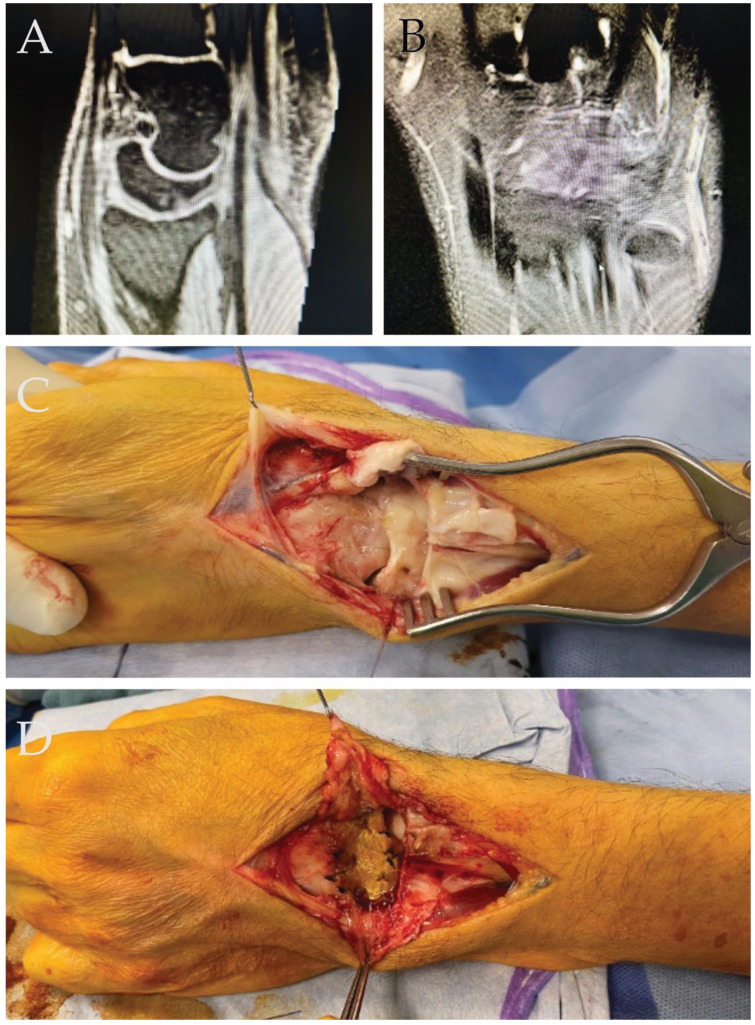
A 33-year-old male patient, experiencing severe dorsal mediocarpal instability (type II) and a positive catch-up clunk test, was enrolled for surgical wrist stabilization following failed conservative treatment (Figure 4). Prior to surgery, the patient reported load-dependent pain in the wrist and an inability to perform heavy tasks even while wearing a wrist orthosis. During surgery, multiple ganglia were typically found in the dorsal intercarpal ligaments. After removing the mucoid degeneration, a collagen hADM membrane was affixed to the scaphoid, lunate, and triquetrum using resorbable 2.4 bone anchors for reconstruction.
Figure 4.
(A) Lateral view of the wrist, indicating clear “palmar intercalated segmental instability” (PISI) observed in the MRI. (B) AP view of the mucoid-degenerated dorsal intercarpal ligaments. (C) Intraoperative perspective of the wrist revealing intercarpal instability. (D) Intraoperative image post-reconstruction of the dorsal intercarpal ligaments using a collagen hADM membrane.
Following the intervention, the wrist was immobilized for 8 weeks—initially in a forearm splint for the first 2 weeks, followed by a circular cast including the thumb for the subsequent 6 weeks. Load-bearing was prohibited for 10 weeks post-surgery. After this period, the patient underwent occupational therapy. While extension and flexion movements of the wrist were reduced to 45-0-45° as expected, the patient was able to normally use the hand pain free in everyday life with only persisting pain when lifting heavy weights for a longer period of time.
5. Discussion
The utilization of collagen sheets in clinical settings extends beyond skin replacement, yet this broader potential has not been extensively explored. Managing complex tissue defects is challenging, particularly when seeking to avoid secondary donor site morbidity. hADM has demonstrated that its biocompatibility is highly beneficial in the reconstruction of capsular defects and the augmentation of ligamentous structures. These applications leverage the inherent stability of hADM’s collagen composition, which is crucial for the repair and support of tissues that require such structural integrity. In respect to the potential support of bone healing, preclinical studies using a 5 mm rat femoral defect suggested that human ADM has clinical potential beyond the treatment of skin damage and collagenous tissue defects. In these studies, wrapping the cancellous bone-filled defect with human ADM produced a biomechanically convincing bone healing result equivalent to the classic two-stage Masquelet technique and significantly better than an uncovered bone defect (cancellous bone filling only).
The mechanism of action of the induced membrane technique is probably based on the barrier function, the sealing of the defect area from the surrounding soft tissue. This prevents the ingrowth of fibrous tissue into the defect area, which in turn reduces the formation of pseudarthrosis [52,53,54]. In addition, support of the defect via its vascularization is discussed for the induced Masquelet membrane [55]. Various regenerative growth factors (Transforming Growth Factor β (TGF-β), bone morphogenetic protein-2 (BMP-2), Vascular Endothelial Growth Factor (VEGF)) and vascularization of the membrane have been demonstrated both in animal experiments [42,56,57] and in biopsies of the human induced membrane mesenchymal stem cells (MSCs) [58,59,60].
It is very likely that the same principles also apply when using human ADM. The barrier function is immediately effective and from in vitro experiments [61] and the histological analysis of human ADM implanted around femoral defects in rats, it can be concluded that the human ADM is vascularized and colonized with cells [25], so that a possible support of bone defect healing is also conceivable when using the human ADM. However, it should be emphasized that, compared to the induced membrane, there is a delay of some days until the corresponding biological structures have formed in the membrane [62]. The contributions of the barrier function and biological activity to bone defect healing are, of course, not yet known and would have to be evaluated in appropriately designed experiments.
Looking at the clinical use of human collagen sheets, the lack of clinical studies is particularly striking. A few previously mentioned studies on the use as a dermis substitute were able to show advantages for allogeneic material, and a significantly lower complication rate was reported here [40,41]. The tolerability of allogeneic material has also been sufficiently demonstrated [43], and the same has been shown in vitro for allogeneic bone replacement material [63]. The natural occurrence of collagen in the extracellular matrix offers many potential applications for collagen sheets, but they are currently often only used as scaffolds for cell colonization.
In addition to Epiflex, other human acellular dermal matrices, such as Alloderm, are also available. Alloderm was first mentioned in the literature in 1995 [64] and, to the best of our knowledge, is one of the first human decellularized dermis products. The range of applications includes the treatment of burns, breast reconstruction or as an oral mucosa equivalent, as described in detail in the review article by Jansen et al. [31]. However, a review of the current literature also shows that the expansion of the range of applications for Alloderm described by us for Epiflex, e.g., for the treatment of large defects of the long bones, has not yet been completed. However, due to the similarity of the two products, it can be assumed that Alloderm is also likely to be effective in the cases described in this study.
Collagen matrices have been utilized in various strategies for cartilage reconstruction, including cell-based therapies, scaffold-based approaches, and combination strategies. In cell-based therapies, chondrocytes or mesenchymal stem cells (MSCs) are seeded onto collagen scaffolds, where they undergo chondrogenic differentiation and produce cartilage ECM components [65,66]. These engineered constructs can then be implanted into cartilage defects to promote tissue repair and regeneration. In scaffold-based approaches, collagen matrices serve as acellular scaffolds that facilitate endogenous cell recruitment and tissue ingrowth. These scaffolds provide a supportive environment for cell infiltration and ECM deposition, leading to the formation of neocartilage within the defect site [67]. Scaffold-based strategies are particularly advantageous for large cartilage defects or in cases where cell sourcing is challenging [68]. Combination strategies involve the incorporation of additional components, such as nanoparticles [69], or other materials, into collagen matrices to enhance their regenerative potential. For example, hybrid scaffolds composed of collagen and synthetic polymer have been developed to improve the mechanical properties and bioactivity of collagen matrices for cartilage reconstruction [70,71].
Another so far unconsidered aspect of how hADM could support the regeneration of e.g., bone or cartilage defects is the immunological shielding against tissues that are subject to different healing kinetics. Numerous studies demonstrate that fracture-related soft tissue injuries compromise bone healing [72,73], whereas muscular bed quality is essential for proper bone formation and healing [74]. Bone healing is initiated by an inflammatory cascade mediated by a number of factors, including neutrophils, macrophages, lymphocytes, and inflammatory cytokines (i.e., IL-1, IL-6, TNFα). Eliciting and maintaining an appropriate inflammatory response in early fracture healing is critical for adequate repair. Several studies have shown that interfering with the inflammatory process might either impair or improve fracture healing as reviewed by Davis et al. [75]. It is thus conceivable that the various healing phases of soft tissues (e.g., inflammation, angiogenesis, anti-inflammation, maturation and remodeling) in close proximity to the bone defect, could interfere with bone defect healing and thus affect it. The separation of the bone defect by a collagenous membrane might buffer these processes.
Another aspect that could possibly contribute to improved healing of hADM-coated bone defects are the guiding structural properties of collagen, which enable the migration of stem cells from the neighboring periosteum. MSCs have collagen receptors, such as integrin α1β1, with which they can adhere to collagen fibrils [76]. Integrins mediate cell adhesion to extracellular matrix components. In addition, MSC express the discoidin domain receptor 1 (DDR1), a collagen-activated receptor tyrosine kinase. This may help MSCs to sense the 3D conformation of collagen and respond to the 3D environment [77]. Due to the colonization of the collagen membrane with MSC and the vascularization that has been shown to occur, the hADM could function as a periosteal analogue. The histologically detectable integration of the hADM into periosteal structures (Figure 1G) could indicate that the hADM could function as a periosteal analogue due to the colonization of the collagen membrane with MSC and the vascularization that has been shown to occur. The histologically detectable integration of hADM into periosteal structures (Figure 1G) might indicate this.
This study presents various clinical applications of the technology in question and chronicles the outcomes observed throughout the treatment processes. In all documented instances, no adverse reactions or intolerance were reported, allowing us to regard the results as favorable. However, it is important to note that there is a scarcity of clinical trials focusing on these specific applications, indicating a need for more rigorous research to validate the findings. Due to the circumstances and variability of indications for this membrane, randomized clinical trials are most likely not manageable, as every patient is mostly very different to others. Thus, other means to further underline the potential for the clinical approach with ADM might be necessary.
6. Conclusions
Collagen matrices stand at the forefront of tissue replacement strategies in regenerative medicine, offering a unique combination of structural integrity and biocompatibility. The ability of collagen to closely mimic the natural ECM and its favorable interactions with cells make it a versatile scaffold material for a wide range of tissues.
The convincing clinical cases presented here prove that the clinical application spectrum of human ADM goes far beyond the original application, the treatment of skin defects. Human ADM can be used successfully and with good tolerability for the treatment of bone defects of various etiologies and for soft tissue defects for the benefit of the patient. However, it must be emphasized at this point that this paper represents an initial collection of relatively heterogeneous clinical cases that indicate a beneficial effect of human ADM in the surgical treatment of bone and tissue defects. In a next step, the collection and evaluation of further clinical cases could help to specify the range of applications and thus lay the foundation for planning and conducting a clinical study in accordance with international standards.
Acknowledgments
This work was in part supported by material donations from the German Institute for Cell and Tissue Replacement (DIZG, non-profit GmbH), Berlin, Germany.
Author Contributions
Conceptualization, J.F., I.M., D.H., C.N. and R.D.V.; resources, I.M., J.F. and D.H.; data curation, R.D.V.; writing—original draft preparation R.D.V., K.S. and D.H.; writing—review and editing R.D.V., D.H., M.J., K.S., N.S., J.N., B.W., J.F., I.M. and C.N.; visualization, D.H. and R.D.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of Goethe University Frankfurt (EV 19-491).
Informed Consent Statement
Patient consent was waived. The data have been stored in a pseudonymized form after the clinical parameters were assigned accordingly. In the context of any publication, it is not apparent who participated in this study. All data for publication are anonymized; it is not possible to draw conclusions about the patients from the study data.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that preclinical experimental studies on the effect of Epiflex have been, in part, supported by Institute for Cell and Tissue Replacement (DIZG, non-profit GmbH), Berlin, Germany. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Funding Statement
Financial support was provided in part by a Deutsche Forschungsgemeinschaft (DFG) grant NA 1712/2-1; 464606918 and by the German Institute for Cell and Tissue Replacement (DIZG, non-profit GmbH), 12555 Berlin, Germany.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Anz A.W., Jordan S.E., Ostrander R.V., 3rd, Branch E.A., Denney T.S., Cohen A., Andrews J.R. Augmentation of ACL Autograft Reconstruction with an Amnion Collagen Matrix Wrap and Bone Marrow Aspirate Concentrate: A Pilot Randomized Controlled Trial with 2-Year Follow-up. Orthop. J. Sports Med. 2023;11:23259671231210035. doi: 10.1177/23259671231210035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botella-Garcia J., Balboa M., Romera-Romero P., Stijnen T., Sanchez-Fortun A., Mercieca K., Loscos-Arenas J. Efficacy and safety of deep sclerectomy with uveoscleral implant plus collagen matrix implant overcoming the superficial scleral flap in glaucoma surgery. Int. J. Ophthalmol. 2023;16:1806–1813. doi: 10.18240/ijo.2023.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou Y., Wang X., Wang Y., Chen X., Wei B., Zhang J., Zhu L., Kou H., Li W., Wang H. Electrospun Nanofibrous Conduit Filled with a Collagen-Based Matrix (ColM) for Nerve Regeneration. Molecules. 2023;28:7675. doi: 10.3390/molecules28227675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y., Yang M., Xu W., Zhang Y., Pan L., Wang L., Wang F., Lu Y. The collagen matrix regulates the survival and function of pancreatic islets. Endocrine. 2023;83:537–547. doi: 10.1007/s12020-023-03592-4. [DOI] [PubMed] [Google Scholar]
- 5.Pitaru S., Tal H., Soldinger M., Noff M. Collagen membranes prevent apical migration of epithelium and support new connective tissue attachment during periodontal wound healing in dogs. J. Periodontal Res. 1989;24:247–253. doi: 10.1111/j.1600-0765.1989.tb01789.x. [DOI] [PubMed] [Google Scholar]
- 6.Negri S., Fila C., Farinato S., Bellomi A., Pagliaro P.P. Tissue engineering: Chondrocyte culture on type 1 collagen support. Cytohistological and immunohistochemical study. J. Tissue Eng. Regen. Med. 2007;1:158–159. doi: 10.1002/term.15. [DOI] [PubMed] [Google Scholar]
- 7.Leonida A., Caccianiga G., Rey G., Longoni S., Ceraulo S., Baldoni M. The use of a new collagen matrix to support the regeneration of peri-implant soft tissue laser-assisted: Case report. J. Biol. Regul. Homeost. Agents. 2019;33:77–88. [PubMed] [Google Scholar]
- 8.Dai N.T., Williamson M.R., Khammo N., Adams E.F., Coombes A.G. Composite cell support membranes based on collagen and polycaprolactone for tissue engineering of skin. Biomaterials. 2004;25:4263–4271. doi: 10.1016/j.biomaterials.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 9.Alshangiti D.M., El-Damhougy T.K., Zaher A., Madani M., Mohamady Ghobashy M. Revolutionizing biomedicine: Advancements, applications, and prospects of nanocomposite macromolecular carbohydrate-based hydrogel biomaterials: A review. RSC Adv. 2023;13:35251–35291. doi: 10.1039/D3RA07391B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gierek M., Labus W., Kitala D., Lorek A., Ochala-Gierek G., Zagorska K.M., Waniczek D., Szyluk K., Niemiec P. Human Acellular Dermal Matrix in Reconstructive Surgery—A Review. Biomedicines. 2022;10:2870. doi: 10.3390/biomedicines10112870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown B.N., Badylak S.F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. 2014;163:268–285. doi: 10.1016/j.trsl.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes C.A., Brison J., Michel R., Brown B.N., Castner D.G., Badylak S.F., Ratner B.D. The surface molecular functionality of decellularized extracellular matrices. Biomaterials. 2011;32:137–143. doi: 10.1016/j.biomaterials.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badylak S.F., Brown B.N., Gilbert T.W., Daly K.A., Huber A., Turner N.J. Biologic scaffolds for constructive tissue remodeling. Biomaterials. 2011;32:316–319. doi: 10.1016/j.biomaterials.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Pien N., Di Francesco D., Copes F., Bartolf-Kopp M., Chausse V., Meeremans M., Pegueroles M., Jungst T., De Schauwer C., Boccafoschi F., et al. Polymeric reinforcements for cellularized collagen-based vascular wall models: Influence of the scaffold architecture on the mechanical and biological properties. Front. Bioeng. Biotechnol. 2023;11:1285565. doi: 10.3389/fbioe.2023.1285565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo Y., Zheng S., Wang K., Luo H., Shi H., Cui Y., Li B., He H., Wu J. Drug cross-linking electrospun fiber for effective infected wound healing. Bioeng. Transl. Med. 2023;8:e10540. doi: 10.1002/btm2.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Javadi P., Derakhshan M.A., Asghari F., Kharrazi S., Faridi-Majidi R. Co-electrospun PCL-collagen nanofibers containing saffron-synthesized gold nanoparticles as an antioxidant wound dressing. Proc. Inst. Mech. Eng. H. 2023;237:1318–1329. doi: 10.1177/09544119231203773. [DOI] [PubMed] [Google Scholar]
- 17.Ashurko I., Tarasenko S., Magdalyanova M., Bokareva S., Balyasin M., Galyas A., Khamidova M., Zhornik M., Unkovskiy A. Comparative analysis of xenogeneic collagen matrix and autogenous subepithelial connective tissue graft to increase soft tissue volume around dental implants: A systematic review and meta-analysis. BMC Oral Health. 2023;23:741. doi: 10.1186/s12903-023-03475-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishihara M., Kishimoto S., Nakamura S., Fukuda K., Sato Y., Hattori H. Biomaterials as cell carriers for augmentation of adipose tissue-derived stromal cell transplantation. Biomed. Mater. Eng. 2018;29:567–585. doi: 10.3233/BME-181009. [DOI] [PubMed] [Google Scholar]
- 19.Hoganson D.M., Meppelink A.M., Hinkel C.J., Goldman S.M., Liu X.H., Nunley R.M., Gaut J.P., Vacanti J.P. Differentiation of human bone marrow mesenchymal stem cells on decellularized extracellular matrix materials. J. Biomed. Mater. Res. A. 2014;102:2875–2883. doi: 10.1002/jbm.a.34941. [DOI] [PubMed] [Google Scholar]
- 20.Manka S.W. Mapping the Binding Sites of MMPs on Types II and III Collagens Using Triple-Helical Peptide Toolkits. Methods Mol. Biol. 2024;2747:75–82. doi: 10.1007/978-1-0716-3589-6_7. [DOI] [PubMed] [Google Scholar]
- 21.Wu H., Shao C., Shi J., Hu Z., Zhou Y., Chen Z., Tang R., Xie Z., Jin W. Hyaluronic acid-mediated collagen intrafibrillar mineralization and enhancement of dentin remineralization. Carbohydr. Polym. 2023;319:121174. doi: 10.1016/j.carbpol.2023.121174. [DOI] [PubMed] [Google Scholar]
- 22.Eni-Aganga I., Lanaghan Z.M., Ismail F., Korolkova O., Goodwin J.S., Balasubramaniam M., Dash C., Pandhare J. KLF6 activates Sp1-mediated prolidase transcription during TGF-beta(1) signaling. J. Biol. Chem. 2023;300:105605. doi: 10.1016/j.jbc.2023.105605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayin E., Rashid R.H., Rodriguez-Cabello J.C., Elsheikh A., Baran E.T., Hasirci V. Human adipose derived stem cells are superior to human osteoblasts (HOB) in bone tissue engineering on a collagen-fibroin-ELR blend. Bioact. Mater. 2017;2:71–81. doi: 10.1016/j.bioactmat.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vozzi G., Corallo C., Carta S., Fortina M., Gattazzo F., Galletti M., Giordano N. Collagen-gelatin-genipin-hydroxyapatite composite scaffolds colonized by human primary osteoblasts are suitable for bone tissue engineering applications: In vitro evidences. J. Biomed. Mater. Res. A. 2014;102:1415–1421. doi: 10.1002/jbm.a.34823. [DOI] [PubMed] [Google Scholar]
- 25.Verboket R.D., Leiblein M., Janko M., Schaible A., Brune J.C., Schroder K., Heilani M., Fremdling C., Busche Y., Irrle T., et al. From two stages to one: Acceleration of the induced membrane (Masquelet) technique using human acellular dermis for the treatment of non-infectious large bone defects. Eur. J. Trauma Emerg. Surg. 2020;46:317–327. doi: 10.1007/s00068-019-01296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossner E., Smith M.D., Petschke B., Schmidt K., Vitacolonna M., Syring C., von Versen R., Hohenberger P. Epiflex((R)) a new decellularised human skin tissue transplant: Manufacture and properties. Cell Tissue Bank. 2011;12:209–217. doi: 10.1007/s10561-010-9187-3. [DOI] [PubMed] [Google Scholar]
- 27.Pruss A., Baumann B., Seibold M., Kao M., Tintelnot K., von Versen R., Radtke H., Dorner T., Pauli G., Gobel U.B. Validation of the sterilization procedure of allogeneic avital bone transplants using peracetic acid-ethanol. Biologicals. 2001;29:59–66. doi: 10.1006/biol.2001.0286. [DOI] [PubMed] [Google Scholar]
- 28.Roessner E.D., Vitacolonna M., Hohenberger P. Confocal laser scanning microscopy evaluation of an acellular dermis tissue transplant (Epiflex(R)) PLoS ONE. 2012;7:e45991. doi: 10.1371/journal.pone.0045991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crapo P.M., Gilbert T.W., Badylak S.F. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitacolonna M., Mularczyk M., Herrle F., Schulze T.J., Haupt H., Oechsner M., Pilz L.R., Hohenberger P., Rossner E.D. Effect on the tensile strength of human acellular dermis (Epiflex(R)) of in-vitro incubation simulating an open abdomen setting. BMC Surg. 2014;14:7. doi: 10.1186/1471-2482-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen L.A., De Caigny P., Guay N.A., Lineaweaver W.C., Shokrollahi K. The evidence base for the acellular dermal matrix AlloDerm: A systematic review. Ann. Plast. Surg. 2013;70:587–594. doi: 10.1097/SAP.0b013e31827a2d23. [DOI] [PubMed] [Google Scholar]
- 32.Wang H.L., Romanos G.E., Geurs N.C., Sullivan A., Suárez-López del Amo F., Eber R.M. Comparison of Two Differently Processed Acellular Dermal Matrix Products for Root Coverage Procedures: A Prospective, Randomized Multicenter Study. J. Periodontol. 2014;85:1693–1701. doi: 10.1902/jop.2014.140198. [DOI] [PubMed] [Google Scholar]
- 33.Silc J.T., Petrungaro P.S. Acellular dermal matrix grafts for root coverage procedures: Review of products and introduction of a new technique. Compend. Contin. Educ. Dent. 2013;34:408–414. [PubMed] [Google Scholar]
- 34.Schlegel A.K., Mohler H., Busch F., Mehl A. Preclinical and clinical studies of a collagen membrane (Bio-Gide) Biomaterials. 1997;18:535–538. doi: 10.1016/S0142-9612(96)00175-5. [DOI] [PubMed] [Google Scholar]
- 35.Soni N., Sikri P., Kapoor D., Soni B.W., Jain R. Evaluation of the efficacy of 100% Type-I collagen membrane of bovine origin in the treatment of human gingival recession: A clinical study. Indian J. Dent. 2014;5:132–138. doi: 10.4103/0975-962X.140822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallo N., Natali M.L., Sannino A., Salvatore L. An Overview of the Use of Equine Collagen as Emerging Material for Biomedical Applications. J. Funct. Biomater. 2020;11:79. doi: 10.3390/jfb11040079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radenkovic M., Alkildani S., Stoewe I., Bielenstein J., Sundag B., Bellmann O., Jung O., Najman S., Stojanovic S., Barbeck M. Comparative In Vivo Analysis of the Integration Behavior and Immune Response of Collagen-Based Dental Barrier Membranes for Guided Bone Regeneration (GBR) Membranes. 2021;11:712. doi: 10.3390/membranes11090712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasetty G., Kalle M., Morgelin M., Brune J.C., Schmidtchen A. Anti-endotoxic and antibacterial effects of a dermal substitute coated with host defense peptides. Biomaterials. 2015;53:415–425. doi: 10.1016/j.biomaterials.2015.02.111. [DOI] [PubMed] [Google Scholar]
- 39.Falkner F., Mayer S.A., Heuer M., Brune J., Helt H., Bigdeli A.K., Dimmler A., Heimel P., Thiele W., Sleeman J., et al. Comparison of Decellularized Human Dermal Scaffolds versus Bovine Collagen/Elastin Matrices for in vivo Engineering of Axially Vascularized Soft Tissue Flaps in Rats. Plast. Reconstr. Surg. 2023;153:130–141. doi: 10.1097/PRS.0000000000010511. [DOI] [PubMed] [Google Scholar]
- 40.Vitacolonna M., Belharazem D., Maier P., Hohenberger P., Roessner E.D. In vivo Quantification of the Effects of Radiation and Presence of Hair Follicle Pores on the Proliferation of Fibroblasts in an Acellular Human Dermis in a Dorsal Skinfold Chamber: Relevance for Tissue Reconstruction following Neoadjuvant Therapy. PLoS ONE. 2015;10:e0125689. doi: 10.1371/journal.pone.0125689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sohling N., Heilani M., Fremdling C., Schaible A., Schroder K., Brune J.C., Eras V., Nau C., Marzi I., Henrich D., et al. One Stage Masquelets Technique: Evaluation of Different Forms of Membrane Filling with and without Bone Marrow Mononuclear Cells (BMC) in Large Femoral Bone Defects in Rats. Cells. 2023;12:1289. doi: 10.3390/cells12091289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henrich D., Seebach C., Nau C., Basan S., Relja B., Wilhelm K., Schaible A., Frank J., Barker J., Marzi I. Establishment and characterization of the Masquelet induced membrane technique in a rat femur critical-sized defect model. J. Tissue Eng. Regen. Med. 2016;10:E382–E396. doi: 10.1002/term.1826. [DOI] [PubMed] [Google Scholar]
- 43.Blohmer J.-U., Beier L., Faridi A., Ankel C., Krause-Bergmann B., Paepke S., Mau C., Keller M., Strittmatter H.J., Karsten M.M. Patient-Reported Outcomes and Aesthetic Results after Immediate Breast Reconstruction Using Human Acellular Dermal Matrices: Results of a Multicenter, Prospective, Observational NOGGO-AWOGyn Study. Breast Care. 2020;16:335–342. doi: 10.1159/000509568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paprottka F.J., Krezdorn N., Sorg H., Konneker S., Bontikous S., Robertson I., Schlett C.L., Dohse N.K., Hebebrand D. Evaluation of Complication Rates after Breast Surgery Using Acellular Dermal Matrix: Median Follow-Up of Three Years. Plast. Surg. Int. 2017;2017:1283735. doi: 10.1155/2017/1283735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hardt J., Kienle P. Laparoscopic ventral pouch pexy with acellular dermal matrix (ADM)—A novel technique for the treatment of full-thickness pouch prolapse after restorative proctocolectomy and j-pouch. Int. J. Colorectal Dis. 2018;33:1643–1646. doi: 10.1007/s00384-018-3135-2. [DOI] [PubMed] [Google Scholar]
- 46.Masquelet A.C., Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop. Clin. N. Am. 2010;41:27–37. doi: 10.1016/j.ocl.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Masquelet A.C., Fitoussi F., Begue T., Muller G.P. [Reconstruction of the long bones by the induced membrane and spongy autograft] Ann. Chir. Plast. Esthet. 2000;45:346–353. [PubMed] [Google Scholar]
- 48.Morelli I., Drago L., George D.A., Gallazzi E., Scarponi S., Romano C.L. Masquelet technique: Myth or reality? A systematic review and meta-analysis. Injury. 2016;47((Suppl. S6)):S68–S76. doi: 10.1016/S0020-1383(16)30842-7. [DOI] [PubMed] [Google Scholar]
- 49.Baer G.S., Harner C.D. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin. Sports Med. 2007;26:661–681. doi: 10.1016/j.csm.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 50.Miller A.G., Raikin S.M., Ahmad J. Near-anatomic allograft tenodesis of chronic lateral ankle instability. Foot Ankle Int. 2013;34:1501–1507. doi: 10.1177/1071100713494377. [DOI] [PubMed] [Google Scholar]
- 51.Ledet E.H., Carl A.L., DiRisio D.J., Tymeson M.P., Andersen L.B., Sheehan C.E., Kallakury B., Slivka M., Serhan H. A pilot study to evaluate the effectiveness of small intestinal submucosa used to repair spinal ligaments in the goat. Spine J. 2002;2:188–196. doi: 10.1016/S1529-9430(02)00182-1. [DOI] [PubMed] [Google Scholar]
- 52.Viateau V., Guillemin G., Calando Y., Logeart D., Oudina K., Sedel L., Hannouche D., Bousson V., Petite H. Induction of a barrier membrane to facilitate reconstruction of massive segmental diaphyseal bone defects: An ovine model. Vet. Surg. 2006;35:445–452. doi: 10.1111/j.1532-950X.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 53.Kanakaris N.K., Harwood P.J., Mujica-Mota R., Mohrir G., Chloros G., Giannoudis P.V. Treatment of tibial bone defects: Pilot analysis of direct medical costs between distraction osteogenesis with an Ilizarov frame and the Masquelet technique. Eur. J. Trauma Emerg. Surg. 2023;49:951–964. doi: 10.1007/s00068-022-02162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liodakis E., Giannoudis V.P., Sehmisch S., Jha A., Giannoudis P.V. Bone defect treatment: Does the type and properties of the spacer affect the induction of Masquelet membrane? Evidence today. Eur. J. Trauma Emerg. Surg. 2022;48:4403–4424. doi: 10.1007/s00068-022-02005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akhoundzadeh D., Bloemers F.W., Verhofstad M.H.J., Schoonmade L.J., Geeraedts L.M.G., Jr. Which surgical technique may yield the best results in large, infected, segmental non-unions of the tibial shaft? A scoping review. Eur. J. Trauma Emerg. Surg. 2024. Online ahead of print . [DOI] [PMC free article] [PubMed]
- 56.Leiblein M., Winkenbach A., Koch E., Schaible A., Buchner H., Marzi I., Henrich D., Nau C. Impact of scaffold granule size use in Masquelet technique on periosteal reaction: A study in rat femur critical size bone defect model. Eur. J. Trauma Emerg. Surg. 2022;48:679–687. doi: 10.1007/s00068-020-01516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verboket R.D., Irrle T., Busche Y., Schaible A., Schroder K., Brune J.C., Marzi I., Nau C., Henrich D. Fibrous Demineralized Bone Matrix (DBM) Improves Bone Marrow Mononuclear Cell (BMC)-Supported Bone Healing in Large Femoral Bone Defects in Rats. Cells. 2021;10:1249. doi: 10.3390/cells10051249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gessmann J., Rosteius T., Baecker H., Sivalingam K., Peter E., Schildhauer T.A., Koller M. Is the bioactivity of induced membranes time dependent? Eur. J. Trauma Emerg. Surg. 2022;48:3051–3061. doi: 10.1007/s00068-021-01844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gindraux F., Loisel F., Bourgeois M., Oudina K., Melin M., de Billy B., Sergent P., Leclerc G., Petite H., Auber F., et al. Induced membrane maintains its osteogenic properties even when the second stage of Masquelet’s technique is performed later. Eur. J. Trauma Emerg. Surg. 2020;46:301–312. doi: 10.1007/s00068-019-01184-4. [DOI] [PubMed] [Google Scholar]
- 60.Janko M., Pollinger S., Schaible A., Bellen M., Schroder K., Heilani M., Fremdling C., Marzi I., Nau C., Henrich D., et al. Determination of the effective dose of bone marrow mononuclear cell therapy for bone healing in vivo. Eur. J. Trauma Emerg. Surg. 2020;46:265–276. doi: 10.1007/s00068-020-01331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vitacolonna M., Belharazem D., Hohenberger P., Roessner E.D. In-vivo quantification of the revascularization of a human acellular dermis seeded with EPCs and MSCs in co-culture with fibroblasts and pericytes in the dorsal chamber model in pre-irradiated tissue. Cell Tissue Bank. 2017;18:27–43. doi: 10.1007/s10561-016-9606-1. [DOI] [PubMed] [Google Scholar]
- 62.Hatashita S., Kawakami R., Ejiri S., Sasaki N., Toshiki N., Ito M., Konno S.I., Hakozaki M. ‘Acute Masquelet technique’ for reconstructing bone defects of an open lower limb fracture. Eur. J. Trauma Emerg. Surg. 2021;47:1153–1162. doi: 10.1007/s00068-019-01291-2. [DOI] [PubMed] [Google Scholar]
- 63.Sohling N., Leiblein M., Schaible A., Janko M., Schwable J., Seidl C., Brune J.C., Nau C., Marzi I., Henrich D., et al. First Human Leucocyte Antigen (HLA) Response and Safety Evaluation of Fibrous Demineralized Bone Matrix in a Critical Size Femoral Defect Model of the Sprague-Dawley Rat. Materials. 2020;13:3120. doi: 10.3390/ma13143120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wainwright D.J. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995;21:243–248. doi: 10.1016/0305-4179(95)93866-I. [DOI] [PubMed] [Google Scholar]
- 65.Gao L.L., Wei Y., Tan Y.S., Li R.X., Zhang C.Q., Gao H. Irrigating degradation properties of silk fibroin-collagen type II composite cartilage scaffold in vitro and in vivo. Biomater. Adv. 2023;149:213389. doi: 10.1016/j.bioadv.2023.213389. [DOI] [PubMed] [Google Scholar]
- 66.Ow Z.G.W., Ting K.J.E., Wong K.L. Single-Stage Arthroscopic Cartilage Repair with Chondrectomy and Implantation of a Templated Membrane Collagen Scaffold with Bone Marrow Aspirate Concentrate Augmentation (AMIC Plus) Arthrosc. Tech. 2023;12:e2085–e2091. doi: 10.1016/j.eats.2023.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Da H., Mu Y., Wang W. [Effect of the insulation layer of tissue-engineering scaffold on collagen type II expression in the neo-cartilage] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2013;29:702–705. [PubMed] [Google Scholar]
- 68.Migliorini F., Maffulli N., Eschweiler J., Gotze C., Hildebrand F., Betsch M. Prognostic factors for the management of chondral defects of the knee and ankle joint: A systematic review. Eur. J. Trauma Emerg. Surg. 2023;49:723–745. doi: 10.1007/s00068-022-02155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghandforoushan P., Hanaee J., Aghazadeh Z., Samiei M., Navali A.M., Khatibi A., Davaran S. Enhancing the function of PLGA-collagen scaffold by incorporating TGF-beta1-loaded PLGA-PEG-PLGA nanoparticles for cartilage tissue engineering using human dental pulp stem cells. Drug Deliv. Transl. Res. 2022;12:2960–2978. doi: 10.1007/s13346-022-01161-2. [DOI] [PubMed] [Google Scholar]
- 70.Kawazoe N., Inoue C., Tateishi T., Chen G. A cell leakproof PLGA-collagen hybrid scaffold for cartilage tissue engineering. Biotechnol. Prog. 2010;26:819–826. doi: 10.1002/btpr.375. [DOI] [PubMed] [Google Scholar]
- 71.Chen G., Sato T., Ushida T., Ochiai N., Tateishi T. Tissue engineering of cartilage using a hybrid scaffold of synthetic polymer and collagen. Tissue Eng. 2004;10:323–330. doi: 10.1089/107632704323061681. [DOI] [PubMed] [Google Scholar]
- 72.Duda G.N., Taylor W.R., Winkler T., Matziolis G., Heller M.O., Haas N.P., Perka C., Schaser K.D. Biomechanical, microvascular, and cellular factors promote muscle and bone regeneration. Exerc. Sport Sci. Rev. 2008;36:64–70. doi: 10.1097/JES.0b013e318168eb88. [DOI] [PubMed] [Google Scholar]
- 73.Landry P.S., Marino A.A., Sadasivan K.K., Albright J.A. Effect of soft-tissue trauma on the early periosteal response of bone to injury. J. Trauma. 2000;48:479–483. doi: 10.1097/00005373-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 74.Stein H., Perren S.M., Cordey J., Kenwright J., Mosheiff R., Francis M.J. The muscle bed--a crucial factor for fracture healing: A physiological concept. Orthopedics. 2002;25:1379–1383. doi: 10.3928/0147-7447-20021201-16. [DOI] [PubMed] [Google Scholar]
- 75.Davis K.M., Griffin K.S., Chu T.G., Wenke J.C., Corona B.T., McKinley T.O., Kacena M.A. Muscle-bone interactions during fracture healing. J. Musculoskelet. Neuronal Interact. 2015;15:1–9. [PMC free article] [PubMed] [Google Scholar]
- 76.Popov C., Radic T., Haasters F., Prall W.C., Aszodi A., Gullberg D., Schieker M., Docheva D. Integrins α2β1 and α11β1 regulate the survival of mesenchymal stem cells on collagen I. Cell Death Dis. 2011;2:e186. doi: 10.1038/cddis.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lund A.W., Stegemann J.P., Plopper G.E. Mesenchymal Stem Cells Sense Three Dimensional Type I Collagen through Discoidin Domain Receptor 1. Open Stem Cell J. 2009;1:40–53. doi: 10.2174/1876893800901010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.