Abstract
Background
What kinds of fetal adverse outcomes beyond stillbirth directly correlate to the severity of intrahepatic cholestasis during pregnancy (ICP) remained tangled. Herein, we conducted a retrospective cohort study and a dose-response meta-analysis to speculate the association between the severity of ICP and its adverse outcomes.
Methods
We retrospectively collected a cohort of ICP patients from electronic records from Guangzhou Women and Children’s Medical Center between Jan 1st, 2018, and Dec 31st, 2022. Also, we searched PubMed, Cochrane, Embase, Scopus, and Web of Science to extract prior studies for meta-analysis. The Kruskal-Wallis test, a one-way or two-way variants analysis (ANOVA), and multi-variant regression are utilized for cohort study. One stage model, restricted cubic spline analysis, and fixed-effect model are applied for dose-response meta-analysis. The data analysis was performed using the R programme.
Results
Our cohort included 1,289 pregnant individuals, including 385 mild ICP cases, 601 low moderate ICP cases, 282 high moderate ICP cases, and 21 severe ICP cases. The high moderate bile acid levels were correlated to preterm birth [RR = 2.14, 95%CI 1.27 to 3.62), P < 0.01], and preterm premature rupture of membranes [RR = 0.34, 95%CI 0.19 to 0.62), P < 0.01]. We added our cases to cases reported by other studies included in the meta-analysis. There were 15,826 patients included in dose-response meta-analysis. The severity of ICP was associated with increased risks of stillbirth, spontaneous preterm birth, iatrogenic preterm birth, preterm birth, admission to neonatal intensive care unit, and meconium-stained fluid (P < 0.05).
Conclusions
Our study shows the correlation between the severity of ICP and the ascending risks of stillbirth, preterm birth, and meconium-stained fluid, providing new threshold TBA levels.
Prospero registration number
CRD42023472634.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-024-06645-2.
Keywords: Intrahepatic cholestasis during pregnancy, Adverse pregnancy outcomes, Dose-response meta-analysis
Introduction
Intrahepatic cholestasis of pregnancy (ICP), is the most common liver disease specific to pregnancy. The estimated prevalence of ICP ranges from 0.2% to 25.0%, with wide variations by race, region, and seasonal variation [1]. Its prevalence is elevated in South America and Northern Europe, with the highest occurrence typically observed during the winter season [2]. In China, the incidence rate is around 1.2%, with the highest incidence rate in the Yangtze River basins [3]. ICP is a reversible disease potentially co-existing with pruritus, elevated serum aminotransferase, or abnormal total bile acid level (TBA ≥ 10µmol/L), and TBA level (10–19µmol/L as mild, 20–39µmol/L as low moderate, 40–99µmol/L as high moderate ICP, and ≥ 100µmol/L as severe) serves as the primary indicator for diagnosis and categorization of its severity.
Previous studies documented relationships with ICP and the occurrence of adverse pregnancy outcomes like spontaneous and iatrogenic preterm delivery, amniotic fluid meconium staining, and even stillbirth [4–6]. The pathophysiological mechanisms underlying the aforementioned complications may be multifactorial. First, abnormally elevated bile acid served as a vasoconstrictive agonist in the maternal-fetal interface, causing insufficient placental blood supply and chronic hypoxia, thus possibly triggering malnutrition, neonatal asphyxia, and even stillbirth [7–9]. Second, bile acid is capable of activating oxytocin receptors, and forth reinforcing the contraction of myometrium, which may be the culprit of spontaneous preterm birth [10].
While previous research has established an association between elevated TBA levels or the severity of ICP and an elevated risk of stillbirth, the impact of its severity on other adverse outcomes remains inconclusive. Herein, we collected a large population to delve into the dose-response association between the TBA levels and risks of adverse obstetric outcomes beyond stillbirth.
Materials and methods
Patients
The diagnosis of ICP is based on the measurement of TBA level, with a threshold of 10µmol/L. The retrospective cohort of pregnant individuals with TBA ≥ 10µmol/L was derived from Guangzhou Women and Children’s Center from January 1st, 2018 to December 31st, 2022. In our study, we excluded patients with twin or multiple pregnancies, hepatitis B, or other severe infectious diseases. Characteristics of pregnant individuals, baseline information of neonates, and adverse pregnancy outcomes were extracted from an electronic medical system. The process of data collection has been approved by the institutional review board approval of Guangzhou Women and Children’s Medical Center.
Search strategy
In the meta-analysis section of our study, we conducted a comprehensive analysis of existing research literature. Two researchers independently searched PubMed, Scopus, Cochrane Library, and Embase databases, using MeSH terms and Entry terms, and screened eligible articles based on inclusion and exclusion criteria by October 3rd, 2023. The MeSH words include "Intrahepatic Cholestasis of Pregnancy". However, a part of the free term which had not to correspond with Mesh terms, including"Familial intrahepatic cholestasis of pregnancy", "Recurrent intrahepatic cholestasis of pregnancy", "Pregnancy-related cholestasis" and "Pregnancy-Related Cholestasis". We also checked the list of references and performed citation searching (Web of Science, v.5.23.2, and ClinicalTrials.gov up to October 3rd, 2023) of included studies to identify other potentially relevant studies.
Included criteria and excluded criteria for meta-analysis
The inclusion criteria are as follows: (1) patients with ICP; (2) Singleton pregnancies; (3) Studies with minimal number of multiple pregnancies [11–15]; (4) Patients were grouped by TBA levels. The exclusion criteria are defined as follows: (1) patients suffering from hepatic diseases; (2) patients with other complications of pregnancy including hematological disease, acute abdomen, hyperthyroidism, and other conditions; (3) patients with fetal anomalies.
Data extraction and quality assessment for meta-analysis
Two investigators independently conducted the data extraction procedure. When dissents occurred between the two investigators, a third-party investigator assessed the study findings.
Two researchers used the Newcastle-Ottawa Scale (NOS) to evaluate the quality of the articles. During the evaluation process, each aspect is assigned a certain number of points, with a total of 9 points. A score of 7–9 is considered high-quality research, 4–6 is moderate-quality research, and less than 4 is considered low-quality research.
Data analysis
In our cohort, we identified the severity of ICP by pregnancy peak TBA levels (µmol/L). Further, we classified the total individuals into the following categories: mild ICP (10–19), low moderate ICP (20–39), high moderate ICP (40–99), and severe ICP (≥ 100). The cohort study employed a range of statistical methods: The Shapiro-Wilk test was utilized for assessing the normality of the data, while ANOVA and the Kruskal-Wallis test were employed for comparing differences among multiple groups. Additionally, linear regression and logistic regression were used to examine the association between TBA levels and adverse outcomes.
We calculated or extracted the 95% confidence interval (CI), the log-transformed RR, the mean number, and SDs for the “1stage” dose-response meta-analysis. Knots of restricted cubic spline (RCS) analysis were set at percentiles 5.0%, 35.0%, 65.0%, and 95.0%. Underpinned by a fixed effect model meta-analysis, we visualized the pooled relationship between different doses of TBA and adverse pregnancy outcomes via RCS. The wald test was conducted to estimate the non-linear relation of the RCS model. To assess the heterogeneity, we performed the Cochrane Q test and computed the I2 value. The publication bias was tested via Egger’s test. In this study, data analysis was performed by R program 4.3.0. completely. P value < 0.05 is the token of statistical significance.
Results
Patients
During the period from January 1, 2018, to December 31, 2022, there were 3,252 cases of ICP diagnosed in our hospital and 2,047 mothers had a record of delivery in the hospital, of which 1,943 were singleton pregnancies and 104 were twin pregnancies. After excluding missing data and twin pregnancies, the cohort study comprised 1,425 individuals. Subsequently, 136 patients with hepatitis B infection were excluded, resulting in a final inclusion of 1,289 cases.
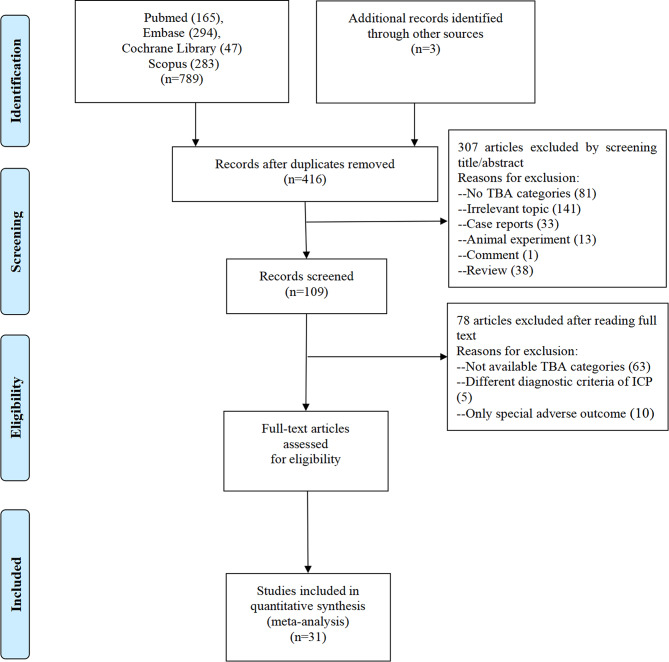
Figure 1 shows the results of the article research filtering. A total of 789 studies were collected from PubMed (n = 165), Embase (n = 294), Cochrane Library (n = 47), Scopus (n = 283), and additional records were identified through other sources (n = 3). The remaining 416 articles were found to be relevant for this meta-analysis after excluding duplications and studies that did not align with inclusion criteria. 78 articles were excluded after evaluating the full text. Eventually, 31 articles were enrolled [7, 11–40]. Additionally, we obtained the ICP cohort from the Guangzhou Women and Children’s Center and included it in the dose-response meta-analysis.
Fig. 1.
Flow graph of study selection
Baseline information
In the cohort study, among these 1,289 pregnant individuals, 385 had mild ICP, 601 cases in the low moderate ICP group, 282 cases in the high moderate ICP group, and 21 cases in the severe ICP group. In these groups, there was no statistical difference between the severity of ICP and the number of parities (P = 0.16), gravidity (P = 0.93), gestational diabetes mellitus (P = 0.29), and the mode of delivery (P = 0.07) Among patients with varying degrees of ICP, differences were observed in maternal weight (P < 0.01), body mass index (P < 0.01), systolic blood pressure (P = 0.01), gestational week (P < 0.01), and report week (P < 0.01)(Table 1).
Table 1.
Baseline information of the cohort with 1,289 ICP patients
| Mild (N = 385) |
Low Moderate (N = 601) |
High Moderate (N = 282) |
Severe (N = 21) |
Overall (N = 1289) |
P-value | |
|---|---|---|---|---|---|---|
| Age, mean ± SD | 30.5 (4.56) | 30.7 (4.57) | 30.8 (4.62) | 31.3 (4.95) | 30.7 (4.58) | 0.72 |
| Height, mean ± SD | 159 (5.14) | 159 (5.28) | 159 (5.01) | 158 (5.03) | 159 (5.18) | 0.15 |
| Weight, mean ± SD | 66.3 (10.2) | 65.5 (8.76) | 63.2 (9.54) | 60.0 (6.75) | 65.2 (9.46) | < 0.01 |
|
BMI, median[min, max] |
25.7 [14.2, 57.8] | 25.4 [17.6, 36.1] | 24.9 [19.4, 39.1] | 24.1 [18.0, 28.4] | 25.4 [14.2, 57.8] | < 0.01 |
| SBP, mean ± SD | 119 (12.9) | 117 (10.9) | 116 (11.1) | 113 (11.4) | 117 (11.6) | 0.01 |
| DBP, mean ± SD | 76.2 (8.85) | 75.5 (8.69) | 74.6 (8.20) | 72.7 (10.0) | 75.4 (8.67) | 0.06 |
|
Parity, median[min, max] |
0 [0, 3] | 1 [0, 4] | 0 [0, 3] | 0 [0, 2] | 0 [0, 4] | 0.16 |
| Gravidity, median[min, max] | 2 [1, 7] | 2 [1, 8] | 2 [1, 7] | 2 [1, 7] | 2 [1, 8] | 0.93 |
| Gestational week, median[min, max] | 38.7 [27.0, 41.1] | 38.0 [31.0, 41.3] | 37.7 [30.0, 41.0] | 36.0 [33.0, 38.4] | 38.0 [27.0, 41.3] | < 0.01 |
| Report week, median[min, max] | 37.0 [0, 41.0] | 35.0 [0, 41.0] | 36.0 [0, 41.0] | 34.0 [8.00, 38.0] | 36.0 [0, 41.0] | < 0.01 |
| GDM, n (%) | 43 (15.2%) | 121 (20.1%) | 60 (15.6%) | 3 (14.3%) | 227 (17.6%) | 0.29 |
| Birth sex, n (%) | 0.96 | |||||
| boy | 206 (53.5%) | 336 (55.9%) | 154 (54.6%) | 11 (52.4%) | 707 (54.8%) | |
| girl | 179 (46.5%) | 265 (44.1%) | 128 (45.4%) | 10 (47.6%) | 582 (45.2%) | |
| Delivery mode, n (%) | 0.07 | |||||
| Viginal | 230 (59.7%) | 352 (58.6%) | 157 (55.7%) | 6 (28.6%) | 745 (57.8%) | |
| Cesarean | 155 (40.3%) | 249 (41.4%) | 125 (44.3%) | 15 (71.4%) | 544 (42.2%) |
*BMI: body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; GDM: Gestational diabetes melitus
Quality assessment and study characteristics
The characteristics of the eligible articles for meta-analysis (comprising a total of 32 articles, including 19 retrospective cohorts, 9 case-control cohorts, and 4 prospective cohorts) were summarized in Supplementary Table 1. Out of the thirty-two studies included in the analysis, thirty were classified as high quality based on the NOS assessment, while the remaining two studies were determined to be of moderate quality. All studies were published between 2004 and 2023. Our study included a total of 15,862 cases of ICP, with the number of cases in each study ranging from 47 to 4,329. The follow-up duration across the studies ranged from 2 years to 15 years.
Primary outcomes
Results of cohort study
Table 2 details the major outcome indicators, including mothers and infants. The difference in preterm birth (P < 0.01) and PROM (P = 0.01) between different severity groups of ICP was statistically significant. Through univariate analysis, we observed a significant association between the severity of ICP and an elevated incidence of preterm birth (P < 0.01), except for the mild ICP group. Before childbirth, our data revealed that, except for the severe ICP group, increasing TBA concentrations were associated with a decreased risk of preterm premature rupture of membranes in the moderate ICP group (RR < 1, P ≤ 0.05). With regards to the relationship between ICP severity and fetal outcomes, ICP severity was significantly correlated with newborns’ birth height and birth weight (P < 0.01). However, our analysis revealed no significant correlation between ICP severity and Apgar scores.
Table 2.
Adverse pregnancy outcomes of the cohort with 1,289 ICP patients
| Mild (N = 385) |
Low Moderate (N = 601) |
High Moderate (N = 282) |
Severe (N = 21) |
Overall (N = 1289) |
P-value | |
|---|---|---|---|---|---|---|
| Preterm birth, n (%) | 27 (7.0%) | 40 (6.7%) | 40 (14.2%) | 10 (47.6%) | 117 (9.1%) | < 0.01 |
| Hypertension disorder of pregnancy, n (%) | 32 (8.3%) | 38 (6.3%) | 13 (4.6%) | 1 (4.8%) | 84 (6.5%) | 0.43 |
| PROM, n (%) | 55 (14.3%) | 62 (10.3%) | 16 (5.7%) | 3 (14.3%) | 136 (10.6%) | 0.01 |
| Meconium-stained fluid, n (%) | 18 (4.7%) | 18 (3.0%) | 6 (2.1%) | 1 (4.8%) | 43 (3.3%) | 0.44 |
| Polyhydramnios, n (%) | 14 (3.6%) | 12 (2.0%) | 8 (2.8%) | 0 (0%) | 34 (2.6%) | 0.55 |
| Oligoamnios, n (%) | 23 (6.0%) | 19 (3.2%) | 6 (2.1%) | 0 (0%) | 48 (3.7%) | 0.07 |
| Birth height, mean ± SD | 49.2 (2.25) | 49.0 (1.90) | 48.4 (2.32) | 46.3 (2.72) | 48.9 (2.16) | < 0.01 |
| Birth weight, mean ± SD | 3110 (474) | 3040 (431) | 2920 (425) | 2570 (395) | 3030 (451) | < 0.01 |
| Apgar1 | 9.00 [3.00, 10.0] | 9.00 [5.00, 10.0] | 9.00 [1.00, 10.0] | 9.00 [8.00, 10.0] | 9.00 [1.00, 10.0] | 0.12 |
| Apgar5 | 10.0 [6.00, 10.0] | 10.0 [8.00, 10.0] | 10.0 [4.00, 10.0] | 10.0 [8.00, 10.0] | 10.0 [4.00, 10.0] | 0.09 |
| Apgar10 | 10.0 [3.00, 10.0] | 10.0 [8.00, 10.0] | 10.0 [4.00, 10.0] | 10.0 [8.00, 10.0] | 10.0 [3.00, 10.0] | 0.49 |
* PROM, preterm prelabor rupture of membranes
Moreover, to account for potential confounding factors, we incorporated covariates into our analysis and employed regression model to examine the specific impact of ICP severity on outcomes within each group. Table 3 displays indicators related to motherhood after adjustment for relevant variables. After adjusting for potential confounders including report week, BMI, and SBP, it was revealed that high moderate intrahepatic cholestasis in pregnancy remained significantly associated with preterm birth [RR = 2.14, 95%CI (1.27, 3.62), P < 0.01], and preterm premature rupture of membranes [RR = 0.34, 95%CI (0.19, 0.62), P < 0.01]. When controlling for the same factors, severe ICP was associated with preterm birth [RR = 12.45, 95%CI (4.74, 32.74), P < 0.01]. In addition, high moderate ICP was also associated with hypertension disorder of pregnancy [RR = 0.41, 95%CI (0.20, 0.83), P = 0.01] after adjusted for the potential confounders of report week, gestational week and BMI. In the indicators of fetal birth weight, birth height, Apgar score in 1 min, Apgar score in 5 min, and Apgar score in 10 min, there was no statistical significance when covariates were included to adjust the regression model except for the high moderate group was adversely associated with the score of Apgar1 [RR = 0.12, 95%CI (0.02, 0.22), P = 0.02]. (Table 4)
Table 3.
Association of ICP severity and maternal outcome
| RR | 95%CI | P-value | |
|---|---|---|---|
| Preterm birth | |||
| Low Moderate | 0.92 | (0.55, 1.54) | 0.75 |
| High Moderate | 2.14 | (1.27, 3.62) | < 0.01 |
| Severe | 12.45 | (4.74, 32.74) | < 0.01 |
| Preterm premature rupture of membranes | |||
| Low Moderate | 0.67 | (0.45, 1.00) | 0.05 |
| High Moderate | 0.34 | (0.19, 0.62) | < 0.01 |
| Severe | 0.95 | (0.27, 3.39) | 0.94 |
| Hypertension disorder of pregnancy | |||
| Low Moderate | 0.72 | (0.43, 1.22) | 0.22 |
| High Moderate | 0.41 | (0.20, 0.83) | 0.01 |
| Severe | 0.16 | (0.02, 1.38) | 0.10 |
*Group, report week, BMI, and SBP were adjusted for preterm birth and Preterm premature rupture of membranes; group, report week, gestational week, and BMI were adjusted for Hypertension disorder of pregnancy
Table 4.
Association of ICP severity and fetal outcomes
| Beta | Se | 95% CI | P-value | |
|---|---|---|---|---|
| Birth weight | ||||
| Low Moderate | 10.84 | 22.86 | (-33.97, 55.64) | 0.64 |
| High Moderate | -3.93 | 27.97 | (-58.76, 50.90) | 0.89 |
| Severe | 40.37 | 80.52 | (-117.456, 198.20) | 0.62 |
| Birth height | ||||
| Low Moderate | 0.08 | 0.11 | (-0.14, 0.30) | 0.50 |
| High Moderate | -0.03 | 0.14 | (-0.30, 0.24) | 0.83 |
| Severe | -0.36 | 0.40 | (-1.15, 0.42) | 0.36 |
| Apgar1 | ||||
| Low Moderate | 0.002 | 0.04 | (-0.08, 0.08) | 0.95 |
| High Moderate | 0.12 | 0.05 | (0.02, 0.22) | 0.02 |
| Severe | 0.25 | 0.15 | (-0.04, 0.54) | 0.09 |
| Apgar5, | ||||
| Low Moderate | 0.02 | 0.03 | (-0.03, 0.07) | 0.41 |
| High Moderate | 0.03 | 0.03 | (-0.04, 0.09) | 0.43 |
| Severe | 0.03 | 0.09 | (-0.15, 0.22) | 0.73 |
| Apgar10 | ||||
| Low Moderate | 0.03 | 0.02 | (-0.01, 0.08) | 0.17 |
| High Moderate | 0.006 | 0.03 | (-0.05, 0.06) | 0.82 |
| Severe | 0.06 | 0.09 | (-0.11, 0.22) | 0.51 |
*All outcomes were adjusted with the group, report week, BMI, SBP, and gestational week
Results of meta-analysis
31 published studies and our retrospective cohort fulfilled the inclusion and exclusion criteria, and data of 15,826 patients comprising their peak TBA levels and perinatal complications were aggregated for dose-response meta-analysis.
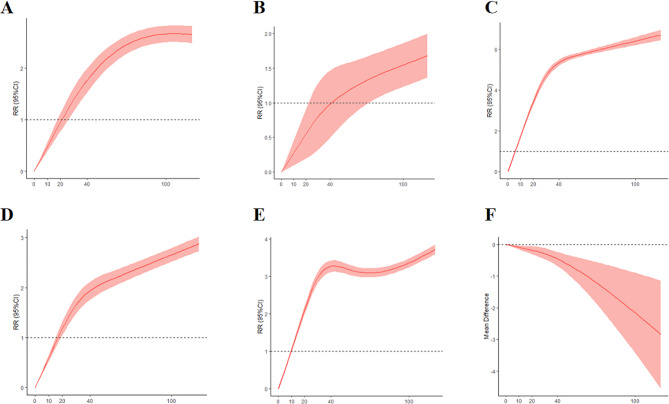
Consisting of 4,051 patients, Fig. 2A revealed TBA ≥ 20µmol/L is correlated with ascended risks of spontaneous preterm (P < 0.001). Figure 2B, which included 1,781 ICP patients, demonstrated a statistical relationship between TBA levels and iatrogenic preterm labor when TBA ≥ 40µmol/L (P < 0.001). Additionally, evidence from 8,278 patients indicated that the severity of ICP was associated with an increased incidence of stillbirth (P < 0.001), with a notable RR ≥ 1 observed when TBA ≥ 10µmol/L(Fig. 2C). Figure 2D disclosed a concomitant of the rising risk of meconium-stained fluid and ascending TBA levels when TBA ≥ 20µmol/L. In Fig. 2E, data from 5,610 patients revealed that elevated TBA levels were linked to the admission of neonatal care unit (P < 0.05). Furthermore, synthesized data from 7,267 patients in Fig. 2F demonstrated a significant decrease in mean birth weight as TBA levels increased (P < 0.05).
Fig. 2.
(A) Non-linear regression model of association between TBA levels and spontaneous preterm birth. (B) Non-linear regression model of association between TBA levels and iatrogenic preterm birth. (C) Non-linear regression model of association between TBA levels and stillbirth. (D) Non-linear regression model of association between TBA levels and meconium-stained fluid. (E) Non-linear regression model of association between TBA levels and the admission of neonatal intensive care unit. (F) Non-linear regression model of the association between TBA levels and birthweight
Notably, no statistical differences were found between TBA levels and the occurrence of fetal distress, neonatal respiratory distress syndrome, Apgar scores, postpartum hemorrhage, hyperbilirubinemia, cesarean delivery, and PROM. Supplementary Table 2 presented qualitative analysis results, showing a pooled RR of 1.00 with 0.0% heterogeneity for cesarean delivery in non-Asian countries, while Asian countries tended to perform a higher rate of cesarean delivery in the highest dose group (P < 0.01).
Discussion
Our study first revealed the worsening of ICP is synchronized with increased risks of spontaneous preterm birth, iatrogenic preterm birth, meconium-stained fluid, NICU admission, and abnormal birth weight based on a large population including 15,826 patients. This is a large single-center cohort study and a dose-response meta-analysis aiming to elucidate the severity of ICP in relation to the adverse outcomes beyond stillbirth.
Our meta-analysis confirmed a significant association between elevated TBA levels and an increased risk of spontaneous preterm birth, with a dose-response relationship observed above a threshold of 20µmol/L. The relative risk of iatrogenic preterm surpassed 1.0 as the TBA concentration increased beyond 40µmol/L. To some extent, this finding is consistent with the ACOG committee’s recommendation for ICP patients [41]. Additionally, our dose-response analysis found when TBA levels reached ≥ 10µmol/L, the RR for stillbirth reached 1.0. This finding contrasts with a previous report that indicated no significant effects on stillbirth for the TBA equal to or less than 19µmol/L [42]. In 2019, a meta-analysis of 23 studies revealed that the risk of stillbirth only increased when TBA concentrations exceeded 100µmol/L, while the risk of stillbirth remained similar to the background risk at lower concentrations [43]. Conversely, our study found that the risk of stillbirth increased when TBA concentrations exceeded 10µmol/L through dose-response meta-analysis. Our aggregated data revealed a stillbirth rate of 1.69%(supplementary Table 3) between TBA concentrations of 10µmol/L and 40µmol/L, significantly higher than the background rate. Collectively, our findings suggest that TBA concentrations peaking over 10µmol/L may serve as a warning sign for stillbirth, and women who experience such peaks warrant necessary surveillance. There requires a larger population study to estimate the threshold TBA levels showing risks of stillbirth. Also, our findings challenge previous research by showing that the risk of meconium-stained fluid is significant when TBA levels reach 20µmol/L, rather than the previously reported threshold of 40µmol/L [44].
The clinical and research implications stem from our findings, and we reckoned that regular monitoring of TBA for its volatile nature in advanced pregnancy is essential due to the various developmental changes the fetus undergoes in utero. While our results do not directly associate the optimal timing of TBA level monitoring with specific risks, expert opinions from Puljic and Lo advised 36 weeks could be the optimum time to prevent neonatal complications [45, 46]. Prospective studies with larger populations and pilot timing will be solutions to the current challenges of ICP. Our results also identified a clear relationship between TBA concentrations and the increasing risk of meconium-stained fluid and spontaneous preterm, and importantly the TBA concentration over 20µmol/L is the threshold. More frequent monitoring of the fetus and possible neonatal intensive care should be advised to patients whose TBA is beyond the threshold.
Our study has several limitations. Firstly, despite the inclusion of 1,289 individuals in the cohort study, there is a paucity of patients suffering from severe ICP (n = 22), which may impact the reliability of the results for severe ICP. Secondly, the retrospective nature of the cohort study means that clinicians were not able to manage all the ICP patients under an identical therapeutic regimen. Additionally, in the meta-analysis, variations in TBA sample collection, like the sample collected at fasting and postprandial phases, could lead to the over-diagnosis or analysis inaccuracy of ICP.
Conclusion
In conclusion, a large cohort study and meta-analysis involving 15,826 patients demonstrates a significant correlation between ICP and adverse outcomes, including preterm birth, meconium-stained amniotic fluid, and stillbirth. The study also establishes new threshold levels for TBA, providing critical insights for clinical management.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We express gratitude to Dr. Qin from the department of epidemiology in Guangzhou Medical University, for her assistance with the necessary knowledge of study design and statistical analysis.
Abbreviations
- ICP
intrahepatic cholestasis during pregnancy
- ANOVA
a one-way or two-way variants analysis
- TBA
total bile acid
- PE
pre-eclampsia
- HBsAg
B surface antigen
- PROM
pretermprelabor rupture of membranes
- NICU
neonatal intensive care unit
- NOS
Newcastle-Ottawa Scale
- RR
relative risk
- SD
standard deviation
- OR
odds ratio
- CI
confidence interval
- RCS
restrict cubic spline
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- GDM
gestational diabetes Mellitus
- ELBW
extremely low birth weight infants
- VLBW
very low birth weight infants
- LBW
low birth weight infants
- NBW
normal birth weight
Author contributions
Yi Yuan, Zi Lv and Huishu Liu: Conception and design; Qiulun Zhou, Yuying Wang, Zhuoqi He, Yannei Liang, Suyi Qiu, Yiting Chen and Yiru He: Data collection; Qiulun Zhou, Yi Yuan, Yuying Wang and Zhuoqi He: Data analysis; Qiulun Zhou, Yi Yuan, Yuying Wang, Zhuoqi He, Yannei Liang and Suyi Qiu: Figures presentation; Yi Yuan, Qiulun Zhou, Yuying Wang, Zhuoqi He, Yannei Liang, Suyi Qiu, Yiting Chen and Yiru He: Manuscript writing; Zi Lv and Huishu Liu: substantial revision of the manuscript.
Funding
This work was supported by the Guangzhou Municipal Science and Technology Bureau(202102010016), the Funding by Guangzhou Science and Technology Project(2023A04J1220), the High-tech Major Featured Technology Program of Guangzhou Municipal Health Commission(2019GX07), the Research Foundation of Guangzhou Women and Children’s Medical Center(2021BS050), and the Plan on Enhancing Scientifc Research in Guangzhou Medical University(02-408-240603131102).
Data availability
The datasets analyzed during the current study are not publicly available due to privacy but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This cohort study was approved by the institutional ethic board of Guangzhou Women and Children’s Medical Center, and all procedures were performed in accordance with the relevant ethical standards. All participants were provided with verbal and written information and written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qiulun Zhou and Yi Yuan made equal contributions to this study.
Contributor Information
Zi Lv, Email: lvzi0121@foxmail.com.
Huishu Liu, Email: huishuliu@hotmail.com.
References
- 1.Koivurova S, Hartikainen A-L, Karinen L, Gissler M, Hemminki E, Martikainen H, et al. The course of pregnancy and delivery and the use of maternal healthcare services after standard IVF in Northern Finland 1990–1995. Hum Reprod. 2002;17:2897–903. doi: 10.1093/humrep/17.11.2897. [DOI] [PubMed] [Google Scholar]
- 2.Abedin P, Weaver JB, Egginton E. Intrahepatic cholestasis of pregnancy: prevalence and ethnic distribution. Ethn Health. 1999;4:35–7. doi: 10.1080/13557859998173. [DOI] [PubMed] [Google Scholar]
- 3.Gao X-X, Ye M-Y, Liu Y, Li J-Y, Li L, Chen W, et al. Prevalence and risk factors of intrahepatic cholestasis of pregnancy in a Chinese population. Sci Rep. 2020;10:16307. doi: 10.1038/s41598-020-73378-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geenes V, Lövgren-Sandblom A, Benthin L, Lawrance D, Chambers J, Gurung V, et al. The reversed feto-maternal bile acid gradient in intrahepatic cholestasis of pregnancy is corrected by ursodeoxycholic acid. PLoS ONE. 2014;9:e83828. doi: 10.1371/journal.pone.0083828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oztekin D, Aydal I, Oztekin O, Okcu S, Borekci R, Tinar S. Predicting fetal asphyxia in intrahepatic cholestasis of pregnancy. Arch Gynecol Obstet. 2009;280:975–9. doi: 10.1007/s00404-009-1052-x. [DOI] [PubMed] [Google Scholar]
- 6.Williamson C, Hems LM, Goulis DG, Walker I, Chambers J, Donaldson O, et al. Clinical outcome in a series of cases of obstetric cholestasis identified via a patient support group. BJOG. 2004;111:676–81. doi: 10.1111/j.1471-0528.2004.00167.x. [DOI] [PubMed] [Google Scholar]
- 7.Brouwers L, Koster, Page-Christiaens, Kemperman H, Boon J, Evers, et al. Intrahepatic cholestasis of pregnancy: maternal and fetal outcomes associated with elevated bile acid levels. Am J Obstet Gynecol. 2015;212:e1001–7. doi: 10.1016/j.ajog.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 8.Sepúlveda WH, González C, Cruz MA, Rudolph MI. Vasoconstrictive effect of bile acids on isolated human placental chorionic veins. Eur J Obstet Gynecol Reprod Biol. 1991;42:211–5. doi: 10.1016/0028-2243(91)90222-7. [DOI] [PubMed] [Google Scholar]
- 9.Song F, Wu W, Qian Z, Zhang G, Cheng Y. Assessment of the Placenta in Intrauterine Growth Restriction by Diffusion-Weighted Imaging and Proton Magnetic Resonance Spectroscopy. Reprod Sci. 2017;24:575–81. doi: 10.1177/1933719116667219. [DOI] [PubMed] [Google Scholar]
- 10.Germain AM, Kato S, Carvajal JA, Valenzuela GJ, Valdes GL, Glasinovic JC. Bile acids increase response and expression of human myometrial oxytocin receptor. Am J Obstet Gynecol. 2003;189:577–82. doi: 10.1067/S0002-9378(03)00545-3. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Flores J, Cañamares M, Cruceyra M, Garicano A, Espada M, Lopez A, et al. Clinical value of maternal bile acid quantification in intrahepatic cholestasis of pregnancy as an adverse Perinatal Outcome Predictor. Gynecol Obstet Invest. 2015;79:222–8. doi: 10.1159/000370003. [DOI] [PubMed] [Google Scholar]
- 12.Huang L, Li X, Liu T, Wei L, Fan C, Tang D, et al. Effect of intrahepatic cholestasis of pregnancy on infantile food allergy: a retrospective longitudinal study cohort in Southwest China. Eur J Obstet Gynecol Reproductive Biology. 2022;272:110–5. doi: 10.1016/j.ejogrb.2022.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Gupta S, Bhattarai S, Gupta T, Arora S. Maternal and foetal outcomes in early and late intrahepatic cholestasis of pregnancy and their Association with maternal serum bile acid levels: a prospective cohort study. JCDR. 2022 doi: 10.7860/JCDR/2022/52069.16272. [DOI] [Google Scholar]
- 14.Marathe JA, Lim WH, Metz MP, Scheil W, Dekker GA, Hague WM. A retrospective cohort review of intrahepatic cholestasis of pregnancy in a South Australian population. Eur J Obstet Gynecol Reproductive Biology. 2017;218:33–8. doi: 10.1016/j.ejogrb.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Jin J, Pan S, Huang L, Yu Y, Zhong M, Zhang G. Risk factors for adverse fetal outcomes among women with early- versus late‐onset intrahepatic cholestasis of pregnancy. Intl J Gynecol Obste. 2015;128:236–40. doi: 10.1016/j.ijgo.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Proehl S, Piacquadio K, Getahun D, Fassett M, Ramos GS, Trivedi N. 773: change to bile acid level and risk of stillbirth in intrahepatic cholestasis of pregnancy. Am J Obstet Gynecol. 2017;216:S447. doi: 10.1016/j.ajog.2016.11.506. [DOI] [Google Scholar]
- 17.Golbasi C, Golbasi H, Bayraktar B, Omeroglu I, Ekin A. Asociación De Los Niveles de ácidos biliares y hormonas tiroideas en la colestasis intrahepática del embarazo. Rev Peru Ginecol Obstet. 2022;68.
- 18.Sarker M, Zamudio AR, DeBolt C, Ferrara L. Beyond stillbirth: association of intrahepatic cholestasis of pregnancy severity and adverse outcomes. American Journal of Obstetrics and Gynecology. 2022;227:517.e1-517.e7. [DOI] [PubMed]
- 19.Silver RM, Parker CB, Goldenberg R, Reddy UM, Dudley DJ, Saade GR et al. Bile acids in a multicenter, population-based case-control study of stillbirth. American Journal of Obstetrics and Gynecology. 2014;210:460.e1-460.e9. [DOI] [PMC free article] [PubMed]
- 20.Li L, Chen W, Ma L, Liu ZB, Lu X, Gao XX, et al. Continuous association of total bile acid levels with the risk of small for gestational age infants. Sci Rep. 2020;10:9257. doi: 10.1038/s41598-020-66138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juusela AL, Cordero L, Gimovsky M, Nazir M. Correlation of bile acids and aspartate-aminotransferase with outcomes in cholestasis of pregnancy. NPM. 2020;13:513–9. doi: 10.3233/NPM-190276. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Lun W, Shi W. Effects of elevated bile acid levels on fetal myocardium in intrahepatic cholestasis of pregnancy, a retrospective study from a neonatal perspective. Clin Res Hepatol Gastroenterol. 2022;46:102013. doi: 10.1016/j.clinre.2022.102013. [DOI] [PubMed] [Google Scholar]
- 23.Guszczynska-Losy M, Wirstlein PK, Wender-Ozegowska E, Kedzia M. Evaluation of predictive value of biochemical markers for adverse obstetrics outcomes in pregnancies complicated by cholestasis. Ginekol Pol. 2020;91:269–76. doi: 10.5603/GP.2020.0051. [DOI] [PubMed] [Google Scholar]
- 24.Li P, Jiang Y, Xie M, You Y. Factors associated with intrahepatic cholestasis of pregnancy and its influence on maternal and infant outcomes. Medicine. 2023;102:e32586. doi: 10.1097/MD.0000000000032586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glantz A, Marschall H-U, Mattsson L-ke. Intrahepatic cholestasis of pregnancy: relationships between bile acid levels and fetal complication rates. Hepatology. 2004;40:467–74. doi: 10.1002/hep.20336. [DOI] [PubMed] [Google Scholar]
- 26.Jhirwal M, Sharma C, Shekhar S, Singh P, Meena SP, Kathuria P, et al. Maternal and perinatal outcome in pregnancy complicated by Intrahepatic Cholestasis. Cureus. 2022 doi: 10.7759/cureus.28512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrera CA, Manuck TA, Stoddard GJ, Varner MW, Esplin S, Clark EAS, et al. Perinatal outcomes associated with intrahepatic cholestasis of pregnancy. J Maternal-Fetal Neonatal Med. 2018;31:1913–20. doi: 10.1080/14767058.2017.1332036. [DOI] [PubMed] [Google Scholar]
- 28.Furrer R, Winter K, Schäffer L, Zimmermann R, Burkhardt T, Haslinger C. Postpartum Blood loss in women treated for intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2016;128:1048–52. doi: 10.1097/AOG.0000000000001693. [DOI] [PubMed] [Google Scholar]
- 29.Kawakita T, Parikh LI, Ramsey PS, Huang C-C, Zeymo A, Fernandez M et al. Predictors of adverse neonatal outcomes in intrahepatic cholestasis of pregnancy. American Journal of Obstetrics and Gynecology. 2015;213:570.e1-570.e8. [DOI] [PMC free article] [PubMed]
- 30.Çelik S, Çalışkan CS, Çelik H, Güçlü M, Başbuğ A. Predictors of adverse perinatal outcomes in intrahepatic cholestasis of pregnancy. Ginekol Pol. 2019;90:217–22. doi: 10.5603/GP.2019.0039. [DOI] [PubMed] [Google Scholar]
- 31.Mei Y, Gao L, Lin Y, Luo D, Zhou X, He L. Predictors of adverse perinatal outcomes in intrahepatic cholestasis of pregnancy with dichorionic diamniotic twin pregnancies. J Maternal-Fetal Neonatal Med. 2019;32:472–6. doi: 10.1080/14767058.2017.1384461. [DOI] [PubMed] [Google Scholar]
- 32.Madazli R, Yuksel MA, Oncul M, Tuten A, Guralp O, Aydin B. Pregnancy outcomes and prognostic factors in patients with intrahepatic cholestasis of pregnancy. J Obstet Gynaecol. 2015;35:358–61. doi: 10.3109/01443615.2014.968102. [DOI] [PubMed] [Google Scholar]
- 33.Estiú MC, Frailuna MA, Otero C, Dericco M, Williamson C, Marin JJG, et al. Relationship between early onset severe intrahepatic cholestasis of pregnancy and higher risk of meconium-stained fluid. PLoS ONE. 2017;12:e0176504. doi: 10.1371/journal.pone.0176504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong C, Zhu Z, Mei F. Risk factors associated with cesarean section and adverse fetal outcomes in intrahepatic cholestasis of pregnancy. Front Pediatr. 2023;11:1136244. doi: 10.3389/fped.2023.1136244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu T, Zhan Y, Chen D, Deng X, Mao C, Xu J, et al. Risk-stratified management strategies for intrahepatic cholestasis of pregnancy: a tertiary center population review over nearly 5 years. Intl J Gynecol Obste. 2024;164:219–26. doi: 10.1002/ijgo.14987. [DOI] [PubMed] [Google Scholar]
- 36.Sargın Oruç A, Seçkin B, Özcan N, Özyer S, Uzunlar Ö, Danışman N. Role of postprandial bile acids in prediction of perinatal outcome in intrahepatic cholestasis of pregnancy. J Obstet Gynaecol. 2014;40:1883–9. doi: 10.1111/jog.12444. [DOI] [PubMed] [Google Scholar]
- 37.Kırlangıç MM, Sahin E, Eraslan Sahin M, Madendag Y, Col Madendag I, Ak M, et al. Severe intrahepatic cholestasis pregnancy is Associated with maternal endothelial dysfunction: a case-control study. Cureus. 2022 doi: 10.7759/cureus.32276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ataalla WM, Ziada DH, Gaber R, Ossman A, Bayomy S, Elemary BR. The impact of total bile acid levels on fetal cardiac function in intrahepatic cholestasis of pregnancy using fetal echocardiography: a tissue doppler imaging study. J Maternal-Fetal Neonatal Med. 2016;29:1445–50. doi: 10.3109/14767058.2015.1051020. [DOI] [PubMed] [Google Scholar]
- 39.Nezer M, Bas-Lando M, Farkash R, Algor N, Samueloff A, Sela H. 360: Intrahepatic cholestasis of pregnancy, outcomes related to bile acid levels. Am J Obstet Gynecol. 2015;212:S189. doi: 10.1016/j.ajog.2014.10.406. [DOI] [Google Scholar]
- 40.Roy A, Premkumar M, Mishra S, Mehtani R, Suri V, Aggarwal N, et al. Role of ursodeoxycholic acid on maternal serum bile acids and perinatal outcomes in intrahepatic cholestasis of pregnancy. Eur J Gastroenterol Hepatol. 2021;33:571–6. doi: 10.1097/MEG.0000000000001954. [DOI] [PubMed] [Google Scholar]
- 41.Medically Indicated Late-Preterm and Early-Term Deliveries ACOG Committee Opinion, Number 831. Obstet Gynecol. 2021;138:e35–9. doi: 10.1097/AOG.0000000000004447. [DOI] [PubMed] [Google Scholar]
- 42.Girling J, Knight CL, Chappell L. Intrahepatic cholestasis of pregnancy: Green-top Guideline 43 June 2022. BJOG. 2022;129:e95–114. doi: 10.1111/1471-0528.17206. [DOI] [PubMed] [Google Scholar]
- 43.Ovadia C, Seed PT, Sklavounos A, Geenes V, Di Ilio C, Chambers J, et al. Association of adverse perinatal outcomes of intrahepatic cholestasis of pregnancy with biochemical markers: results of aggregate and individual patient data meta-analyses. Lancet. 2019;393:899–909. doi: 10.1016/S0140-6736(18)31877-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vain NE, Szyld EG, Prudent LM, Wiswell TE, Aguilar AM, Vivas NI. Oropharyngeal and nasopharyngeal suctioning of meconium-stained neonates before delivery of their shoulders: multicentre, randomised controlled trial. Lancet. 2004;364:597–602. doi: 10.1016/S0140-6736(04)16852-9. [DOI] [PubMed] [Google Scholar]
- 45.Puljic A, Kim E, Page J, Esakoff T, Shaffer B, LaCoursiere DY, et al. The risk of infant and fetal death by each additional week of expectant management in intrahepatic cholestasis of pregnancy by gestational age. Am J Obstet Gynecol. 2015;212:e6671–5. doi: 10.1016/j.ajog.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 46.Lo JO, Shaffer BL, Allen AJ, Little SE, Cheng YW, Caughey AB. Intrahepatic cholestasis of pregnancy and timing of delivery. J Matern Fetal Neonatal Med. 2015;28:2254–8. doi: 10.3109/14767058.2014.984605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are not publicly available due to privacy but are available from the corresponding author on reasonable request.