Abstract
Background
Glucagon-like peptide-1 (GLP-1) plays a crucial role in metabolic disorders by enhancing insulin secretion, inhibiting glucagon release, and slowing gastric emptying, thereby improving glycemic control. In recent years, GLP-1 role in neuronal pathways has expanded its therapeutic potential. We aim to comprehensively evaluate the relevance of GLP-1 in headache and pain disorders.
Methods
A systematic literature search was conducted on PubMed and Embase (Ovid) databases using the search terms “GLP-1” and “pain”. Animal and human studies published in English language were included. Abstracts, reviews, and articles on other disorders than “pain” were excluded.
Results
The search strategy identified 833 hits, of which 42 studies were included in the final review. The studies were categorized into four groups: inflammatory pain and osteoarthritis, headaches, neuropathic pain and diabetic neuropathy, and visceral pain and irritable bowel syndrome. GLP-1 receptor (GLP-1R) agonists, like liraglutide, have shown analgesic effects by modulating pain hypersensitivity in animal models of inflammatory and neuropathic pain. GLP-1 is involved in migraine mechanisms and GLP-1R agonists are beneficial in individuals with idiopathic intracranial hypertension. Additionally, GLP-1R agonists reduce visceral hypersensitivity and ameliorate symptoms in patients with irritable bowel syndrome.
Conclusions
The therapeutic scope of GLP-1R agonists is expanding beyond traditional metabolic targets, highlighting its potential for headache and pain disorders. Engineering bimodal molecules that integrate GLP-1R agonism with specific pain-related mechanisms may offer innovative therapeutic options.
Keywords: Diabetic neuropathy, Irritable bowel syndrome, Migraine, Neuropathic pain, Osteoarthritis
Background
Glucagon-like peptide-1 (GLP-1) is a peptide hormone known for its role in regulating glucose homeostasis and satiety. Synthesized in the intestinal L-cells, GLP-1 is secreted in response to food intake, stimulating insulin secretion and inhibiting glucagon release [1]. GLP-1 enhances glucose-dependent insulin secretion from pancreatic beta cells, inhibits glucagon release from alpha cells, and slows gastric emptying, thereby aiding in blood glucose regulation. The GLP-1 receptor (GLP-1R) is a G-protein-coupled receptor widely expressed in various tissues, including the pancreas, brain, and gastrointestinal tract. Upon binding to GLP-1, GLP-1R activates intracellular signaling pathways, such as cyclic adenosine monophosphate (cAMP) and protein kinase A (PKA), which mediate its physiological effects. Due to its potent effects on glucose homeostasis and appetite regulation, GLP-1R agonists are treatments for type 2 diabetes and obesity [2, 3]. In addition, GLP-1 has significant effects on the nervous system [4, 5]. GLP-1R is expressed in various brain regions, including the hypothalamus, cortex, and hippocampus, as well as peripheral nervous tissues [6]. The widespread distribution implicates GLP-1 in several neural processes, including neuroprotection, synaptic plasticity, and modulation of neuroinflammation [7]. These properties have sparked interest in GLP-1R agonists as potential treatments for neurological disorders, such as Parkinson’s disease (PD) and Alzheimer’s disease (AD). PD leads to debilitating motor and non-motor symptoms and is characterized by the progressive degeneration of dopaminergic neurons in the substantia nigra. Neuroinflammation and oxidative stress are key pathological features of PD [8, 9]. Pre-clinical studies have demonstrated anti-PD effects of GLP-1R agonists and clinical trials are underway to evaluate the efficacy of GLP-1R agonists in slowing disease progression and alleviating symptoms in PD patients [10, 11]. Similarly, GLP-1R agonists are candidate therapies for AD due to their role in modulating neuroinflammation and β-amyloid accumulation. Experimental models have shown that GLP-1R activation can improve cognitive function and reduce amyloid plaque burden, suggesting potential disease-modifying effects [12, 13]. GLP-1R agonists may influence other neurological pathways, particularly in pain conditions. This systematic review aims to comprehensively evaluate the role of GLP-1 in headache and pain disorders. By emphasizing the expanding therapeutic scope of GLP-1 beyond traditional metabolic targets, we elucidate the current evidence and potential mechanisms of GLP-1R agonists in neurological conditions other than PD and AD.
Methods
We performed a systematic literature search identifying articles reporting original data on GLP-1 and pain, including headaches. We conducted the literature search on PubMed and Embase (Ovid) in December 2023 and updated it in June 2024. We used the following search terms: “GLP-1” and “pain”. Both animal and human studies published in English were included. Original studies that did not investigate pain as a primary and/or secondary outcome were excluded. Abstracts, reviews, editorials, and other articles without original data were also excluded. Additionally, we considered articles from the reference lists of relevant studies and literature known to be pertinent by the authors.
Data extraction
Titles and abstracts of retrieved records were screened independently and separately for obvious exclusions. Exclusion of records was performed using voting based on a majoritarian system. Data extraction was conducted independently by LP and WH/YAD, who reviewed full texts of selected articles to extract relevant data on study design, population, interventions, outcomes, and results. Discrepancies were resolved through collegial discussions. We used Endnote (Endnote 21) software to manage references, identify duplicate records, and remove them. We followed PRISMA guidelines for systematic reviews to ensure transparency and completeness in our reporting [14].
Results
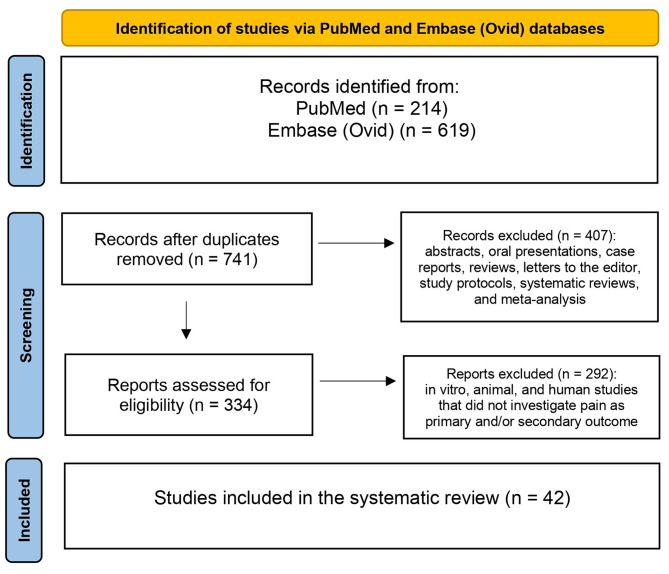
Our search strategy identified 833 hits of which 42 studies were included in the final review. After excluding 92 hits as duplicates, another 282 hits were excluded because they were reviews, abstracts, oral presentations and letters to the editors. We also excluded case reports (n = 97), study protocols, systematic reviews and meta-analysis (n = 28). After full-text assessment, we excluded studies on diabetes mellitus (n = 154), studies on Parkinson or neurodegenerative conditions (n = 8), and in vitro, animal and human studies that did not investigate pain as primary and/or secondary outcome (n = 130). In total, 42 animal and human studies were included in the final review (Fig. 1). The identified studies were further divided in four categories: (1) inflammatory pain and osteoarthritis, (2) headaches, (3) neuropathic pain and diabetic neuropathy, and (4) visceral pain and irritable bowel syndrome (IBS) (Fig. 2). Experimental compounds used to test GLP-1 role in headache and pain disorders are listed in Table 1. Human and animal experimental techniques are described in Table 2.
Fig. 1.
Flowchart of the systematic review
Fig. 2.
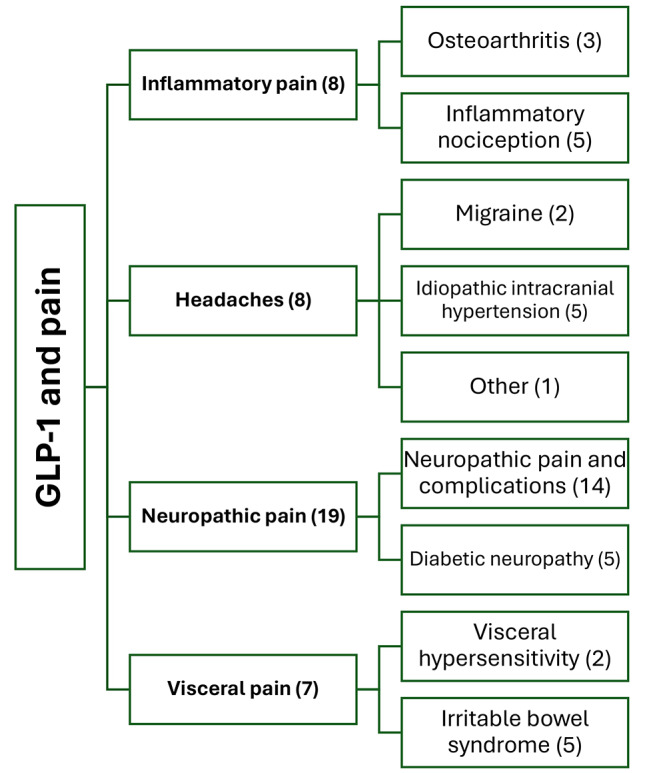
Overview and categorization of the studies evaluating the role of glucagon-like peptide 1 (GLP-1) in headache and pain disorders
Table 1.
Experimental compounds used to test glucagon-like peptide 1 (GLP-1) role in headache and pain disorders
| GLP-1 and GLP-1 analogs | Exendin 4, GLP-1, GLP-1 (7–36 amide) and ROSE-010 |
|---|---|
| GLP-1 receptor agonists | Exenatide, liraglutide, semaglutide and WB4-24 |
| Dipeptidyl peptidase 4 (DPP-4) inhibitors | Evogliptin tartrate, diprotin A, PKF275-055, teneligliptin and vildagliptin |
| Natural products with a GLP-1 receptor agonistic activity | Morroniside, geniposide, Lamiophlomis rotata and its principle effective iridoid glycoside (shanzhiside methylester) |
Table 2.
Human and animal experimental techniques used to study the role of glucagon-like peptide (GLP-1) in headache and pain disorders
| Animal studies | Human studies | |
|---|---|---|
| Inflammatory pain | Mouse model of sodium monoiodoacetate osteoarthritis | Randomized, placebo-controlled trial |
| Carrageenan-induced, formalin-induced and Complete Freund´s Adjuvant (CFA)-induced peripheral inflammation | Prospective observational study | |
| Headaches | Hydrocephalus rat model with raised intracranial pressure | Randomized, placebo-controlled trial |
| Nitroglycerin-induced model of migraine | Case-control study | |
| Neuropathic pain | Formalin tests | |
| Bone cancer pain model | ||
| Spared nerve injury, partial sciatic nerve transection and spinal nerve ligation models | ||
| Nicotinamide- and streptozotocin-induced model of diabetic pain | ||
| Visceral pain | Model of visceral hypersensitivity induced by lipopolysaccharide, repeated water avoidance stress and intra-colonic infusion of acetic acid | Randomized, placebo-controlled trial |
| Model of irritable bowel syndrome |
Inflammatory pain and osteoarthritis
We identified eight studies exploring the interplay between GLP-1 and inflammatory pain [15–22]. Liraglutide, a GLP-1 analog, emerges as a promising therapeutic agent in this context. In a murine model of osteoarthritis, intra-articular injection of liraglutide mitigated pain-associated behaviors induced by sodium monoiodoacetate [15]. In vitro investigations revealed its capacity to downregulate inflammatory gene expression and suppress the synthesis of interleukin 6 (IL-6), prostaglandin E2 and nitric oxide in chondrocytes and macrophages in a dose-dependent manner. Moreover, liraglutide elicited a phenotypic shift in macrophages from proinflammatory M1 to anti-inflammatory M2, while also inhibiting the expression of enzymes implicated in cartilage degradation. Another study in rats showcased the efficacy of liraglutide in alleviating acute peripheral inflammation induced by carrageenan, either alone or in combination with tramadol [16]. Mechanistic insights revealed reductions in swelling and paw temperature, alongside elevations in anti-inflammatory interleukin 10 (IL-10) and total antioxidant status within inflamed tissues. WB4-24, a non-peptide GLP-1 receptor agonist, demonstrated dose-dependent attenuation in mouse models of acute and chronic inflammatory nociception via the release of β-endorphins from spinal microglia [17]. Further investigations involving dipeptidyl peptidase 4 (DPP-4) inhibitors such as evogliptin tartrate, diprotin A, and vildagliptin underscored the analgesic properties of GLP-1 analogs through modulation of the endogenous opioid system [18–20]. Human studies exploring the analgesic efficacy of liraglutide in knee osteoarthritis yielded mixed results, suggesting that clinical benefits may derive from weight loss rather than direct analgesic mechanisms [21, 22]. A randomized, placebo-controlled trial was conducted in people with knee osteoarthritis and a Body Mass Index (BMI) > 27 [21]. A total of 156 trial participants were randomized to receive daily liraglutide (30 mg) (n = 80) or placebo (n = 76) for 52 weeks. Pain was measured using the Knee Injury and Osteoarthritis Outcome Score measurement scale. Compared to placebo, patients treated with liraglutide lost weight but did not experience less knee pain. A prospective, observational, multicenter study was conducted in more than 40,000 Chinese adults with knee osteoarthritis and type 2 diabetes mellitus [22]. Patients taking GLP-1R agonists lost weight and had a lower risk of knee surgery than patients not taking GLP-1R agonists. The use of GLP-1R agonists was associated with fewer intra-articular injections of steroids and a lower cartilage loss velocity from the medial femoral joint, but the direct effects of GLP-1R agonist, apart from the weight loss-mediated pathway, did not reach statistical significance.
Headaches
Seven studies were identified concerning headache, migraine, and idiopathic intracranial hypertension (IIH) [23–29]. In rats, GLP-1R expression is found in microglial cells within the trigeminocervical complex [23]. In a rat migraine model, liraglutide mitigated nitroglycerin-induced sensitization of the trigeminocervical complex, leading to reduced expression of pain mediators such as calcitonin gene-related peptide and c-Fos [23]. Moreover, liraglutide suppressed the production of pro-inflammatory molecules interleukin 1β (IL-1β) and tumor necrosis factor alpha (TNF-α) via the phosphoinositide 3kinase/serine-threonine kinase (PI3K/Akt) signaling pathway. A second study in rats highlighted liraglutide´s role in promoting the release of IL-10, an anti-inflammatory cytokine that was effective in relieving migraine-associated pain [24]. GLP-1R is present in the human choroid plexus, where GLP-1R analogs reduces cerebrospinal fluid secretion and intracranial pressure by increasing the intracellular concentration of cyclic adenosine monophosphate and inhibiting the Na+/K+ ATPase pump in the choroid plexus [25]. GLP-1R agonists have been tested for their ability to promote weight loss and reduce headache in patients with IIH. A single-center, case-control pilot study included 39 participants with a BMI ≥ 30 kg/m² [26]. Participants (n = 13) were treated with semaglutide or liraglutide in addition to standard body weight management, while a control group (n = 26) received standard body weight management. The group treated with GLP-1R agonists achieved significantly greater weight loss after six months compared to the control group (-12.0% vs. -2.8%). Furthermore, the group treated with GLP-1-R agonists experienced fewer headache days than the other group while the dose of acetazolamide was reduced. A double-blind, placebo-controlled trial allocated women with active IIH to 12-week dosing of either placebo (n = 7) or exenatide (n = 7), a GLP-1R agonist [27]. The primary outcome was the difference in intracranial pressure between exenatide and placebo at 2.5 h, 24 h, and 12 weeks. A meaningful reduction in intracranial pressure has been reported at all time points in the exenatide group as compared to the placebo group. Mean monthly headache days reduced significantly in the exenatide arm (− 7.7 days) compared to the placebo arm (-1.5 days), with no significant difference between exenatide and placebo groups at 12 weeks. There was no significant change in BMI within the exenatide group at 12 weeks, indicating that the effect on intracranial pressure was not driven by reduction in body weight, but likely by direct effect of exenatide to modulate intracranial pressure at the choroid plexus. A placebo-controlled exploratory study showed that exenatide treatment for 12 weeks did not affect cognitive function in women with IIH [28]. The abrupt cessation of GLP-1R agonists in individuals with metabolic disorders may lead to adverse effects, as seen in a case where a patient developed IIH after discontinuing duraglutide [29]. A study in healthy volunteers explored the vasodilatory and headache-inducing properties of GLP-1, revealing no significant differences in post-infusion headache between GLP-1 and placebo [30].
Neuropathic pain and diabetic neuropathy
We identified 19 animal studies evaluating the role of GLP-1 in neuropathic pain [31–49]. Intrathecal administration of GLP-1R agonists such as GLP-1(7–36) and exenatide alleviated hypersensitivity in models of formalin-induced, peripheral nerve injury-induced, bone cancer-induced, and diabetes-induced pain in mice and rats [31]. GLP-1(7–36) and exenatide activated GLP-1Rs expressed on microglial cells in the spinal dorsal horn, which were significantly upregulated following peripheral nerve injury. These effects were completely prevented by GLP-1R antagonism and GLP-1R gene knockdown, although acute nociceptive responses were unaffected. Electroacupuncture demonstrated similar findings, relieving pain hypersensitivity in rats with spared nerve injury [32]. In the ipsilateral dorsal horn, electroacupuncture reduced ionized calcium-binding adapter molecule 1 (Iba-1) and glial fibrillary acidic protein (GFAP) levels while increasing the expression levels of GLP-1 and GLP-1R. In a rat spinal nerve ligation model, exenatide exhibited antiallodynic effects on neuropathic pain [33]. Differential gene expression analysis indicated that exenatide could normalize the aberrant expression of 591 genes in the spinal dorsal horn caused by nerve injury, particularly those related to inflammatory signaling via TNF-α and Toll-like receptors. In a similar model, intrathecal injections of exenatide inhibited thermal hyperalgesia and mechanical allodynia by enhancing spinal microglial expression of β-endorphins, IL-10 and interleukin 4 (IL-4) [34, 35]. Teneligliptin (TEN), a DPP-4 inhibitor, demonstrated mild analgesic effects against acute pain and significant effects against neuropathic pain in a rat model induced by partial transection of the sciatic nerve [36]. By preventing the breakdown of GLP-1 and prolonging its circulation, TEN antinociceptive action was partially counteracted by the GLP-1R antagonist exendin-3, suggesting GLP-1R-independent mechanisms. TEN significantly reduced glial fibrillary acidic protein immunoreactivity and astrocyte activation, implying that its analgesic effects are associated with suppression of spinal astrocytes and neuroinflammation [36, 37]. Morroniside, a GLP-1R agonist, reduced mechanical allodynia and thermal hyperalgesia in a dose-dependent manner in a rat model of neuropathic pain, with peak effects within 1 h and lasting over 4 h [38]. Repeated daily injections for seven days did not induce tolerance to its analgesic effects. In a more detailed study, morroniside enhanced IL-10 and β-endorphin gene expression in the spinal lumbar enlargements and cultured microglia of neuropathic rats [39]. Neutralization of spinal IL-10 or β-endorphin, or blockade of the µ-opioid receptor, completely reversed morroniside-induced mechanical antiallodynia. Geniposide, a major iridoid glycoside of Gardenia jasminoides and a GLP-1R agonist, dose-dependently reduced formalin-induced pain in rats without generating antinociceptive tolerance [40]. Lamiophlomis rotata, a Tibetan herb containing iridoid glycosides with GLP-1R agonist activity, blocked formalin-induced hyperalgesia, peripheral nerve injury- and bone cancer-induced mechanical allodynia [41]. The herb reduced pain by 50 to 80% at doses between 130 and 250 mg/kg without leading to antiallodynic tolerance. Shanzhiside methyl ester (SM), the major iridoid glycoside of Lamiophlomis rotata, demonstrated dose-dependent and long-lasting anti-allodynic effects in neuropathic rats without inducing tolerance [42]. SM significantly induced β-endorphin expression and activated p38 mitogen-activated protein kinase signaling in the spinal dorsal horn and primary microglia. Regarding neuropathic pain-related complications, exenatide attenuated memory deficits induced by neuropathic pain in rats [43, 44]. Neuropathic pain may impair memory by reducing GLP-1R levels in the hippocampus [45]. Immunohistochemical staining and western blot assays revealed increased microglial cells and activated astrocytes in the dentate gyrus of the hippocampus in rats with neuropathic pain, along with elevated expression of TNF-α, IL-1β, and IL-6 [43, 44]. Activation of GLP-1R ameliorated these memory deficits through regulation of adenosine monophosphate-activated protein kinase (AMPK) and nuclear factor-κB (NF-κB) pathway, reducing hippocampal neuroinflammation. Five studies focused specifically on the role of GLP-1 in diabetic neuropathy [46–50]. Liraglutide improved nociceptive thresholds and mitigated histopathological damage of the sciatic nerve in a rat model of diabetic neuropathy induced by nicotinamide and streptozotocin [46]. Liraglutide normalized the content of malondialdehyde, nitric oxide, IL-6, and matrix metalloproteinases-2 and − 9 while increasing superoxide dismutase and IL-10 in the sciatic nerve. Another study found that liraglutide decreased diabetic neuropathy-induced microglial cell activation in the cerebral cortex and thalamus of rats by reducing the expression of the NLR family pyrin domain containing 3 (NLRP3) protein in brain microglia [47]. The analgesic effects of liraglutide were not observed in mice with constitutively active glycogen synthase kinase-3 beta (GSK3β), as activation of GSK3β promotes the activation of the NLRP3 inflammasome [48]. In another model of diabetic neuropathy in rats, significant improvements in pain, oxidative stress, and inflammatory markers of the sciatic nerve were demonstrated following oral administration of amitriptyline and subcutaneous liraglutide, as well as with concurrent oral administration of amitriptyline and liraglutide loaded into proniosomal formulations [49]. PKF275-055, an analogue of vildagliptin and a DPP-4 inhibitor, counteracted changes in Na⁺/K⁺-ATPase activity, nerve conduction velocity, and nociceptive thresholds in diabetic rats [50]. PKF275-055 restored mechanical sensitivity thresholds by approximately 50% and progressively improved thermal responsiveness, suggesting its potential as a therapeutic agent for diabetic neuropathy.
Visceral pain and irritable bowel syndrome
Seven studies investigated the role of GLP-1 in visceral pain and IBS [51–57]. GLP-1R-like immunoreactivity is present in the innervation of the human colon and is increased in biopsies from individuals with IBS [51]. Administration of GLP-1 and exendin-4 promoted neurite outgrowth in cultured dorsal root ganglion (DRG) neurons, presumably explaining the increased nerve fibers observed in biopsies of IBS individuals [51]. While adenosine triphosphate (ATP) signaling was enhanced, capsaicin sensitivity was unaffected by the acute application of exendin-4 in cultured DRG neurons, suggesting GLP-1R role in modulating gut motility rather than pain signaling. In a rat model of IBS, intraperitoneal administration of exendin-4 normalized stress-induced defecation and visceral pain sensitivity by modulating enteric neuronal function through GLP-1 receptors in submucosal and myenteric ganglion neurons [52]. These benefits appeared to stem from the modulation of enteric neuronal function and tight junction expression. Exendin-4 administration did not affect anxiety-like behaviors regulated by the central nervous system. Another study examined exendin-4 effect on visceral hypersensitivity in colon-sensitized rats [53]. Rats treated with colonic acetic acid infusions exhibited low plasma GLP-1 levels and high serotonin levels in plasma and colonic tissues. Exendin-4 administration reduced visceral hypersensitivity in a dose-dependent manner and decreased serotonin levels in the colon. Post-treatment, the expression of the serotonin reuptake transporter (SERT) significantly increased, while tryptophan hydroxylase-1 (TPH-1) expression significantly decreased in colonic tissue, suggesting exendin-4 mitigates visceral hypersensitivity by upregulating SERT and downregulating TPH-1. In a model of lipopolysaccharide (LPS)-induced visceral hypersensitivity and repeated water deprivation stress in rats, liraglutide reduced visceral allodynia by inhibiting proinflammatory cytokine production and attenuating intestinal permeability [54]. Specifically, liraglutide blocked increased IL-6 levels in colonic mucosa via a nitric oxide-mediated response. In rectosigmoid biopsies from patients with constipation-predominant IBS and healthy controls, GLP-1R was significantly downregulated in IBS individuals compared to controls [55]. Serum GLP-1 levels were significantly lower in IBS individuals and negatively correlated with abdominal pain scores. ROSE-010, a GLP-1 analog, was tested in a randomized, placebo-controlled clinical trial for treating acute pain in IBS patients [56]. As a neuronal GLP-1R agonist, ROSE-010 increases smooth muscle motility in the gastrointestinal tract. Subcutaneous administration of ROSE-010 (100 µg and 300 µg) was well-tolerated and more effective than placebo [56]. Pain relief was dose-dependent, with the best effect observed at 120 min after a 300-µg injection. Patients with at least four abdominal pain attacks per month, each lasting at least two hours, experienced significant pain relief as early as 20 min post-administration. The most pronounced improvements were in patients with frequent constipation compared to those with frequent diarrhea. More patients were satisfied with ROSE-010 and considered it superior to previous treatments for IBS. Female participants responded more positively than males, while age and BMI did not influence treatment response [57].
Discussion
Our systematic review of GLP-1 role in headache and pain disorders reveals a promising landscape for its application beyond traditional metabolic uses. The potential of GLP-1R agonists as multi-faceted therapeutic agents extends their benefits to various pain conditions, including inflammatory, neuropathic, visceral pain, and headaches (Table 3). GLP-1R activation can downregulate pro-inflammatory mediators and shift macrophage phenotypes toward anti-inflammatory profiles [15–17]. However, the translation to clinical efficacy in human osteoarthritis remains ambiguous, as weight loss appears to play a substantial role in pain alleviation [21, 22]. While the mechanistic insights are encouraging, further clinical investigations are needed to isolate the direct analgesic effects of GLP-1R agonists from those mediated by weight loss. GLP-1 appears to modulate inflammatory pathways and reduce intracranial pressure through direct actions on the choroid plexus in the context of migraine and IIH [23–25]. A clinical trial and a case-control study highlighted the effectiveness of GLP-1R agonists in reducing headache frequency and intracranial pressure in individuals with IIH, independent of weight loss [26, 27]. These findings suggest a novel therapeutic pathway for IIH management, where GLP-1R agonists may offer dual benefits in weight reduction and headache relief [58]. An international multicenter trial is currently underway to validate the effects of GLP-1R activation in active IIH (NCT05347147). The role of GLP-1R in neuropathic pain, particularly diabetic neuropathy, is supported by preclinical evidence. GLP-1R agonists exert their effects by modulating microglial activity and inflammatory cytokine production in the spinal cord. Following peripheral nerve injury, GLP-1R expression is upregulated in microglial cells of the spinal dorsal horn [31]. The activation of GLP-1R promotes the release of β-endorphins, which mediate analgesic effects through µ receptors at the neuronal level [39, 42]. The reduction of inflammatory signaling may play an additional role in the analgesic effects of GLP-1R agonists against neuropathic pain [32, 34, 35]. Notably, these effects are more effective for chronic rather than acute pain and are achieved without the development of tolerance [31, 36, 38, 40–42]. Furthermore, the ability of GLP-1R agonists to ameliorate neuropathy-related complications, such as cognitive deficits, underscores their broader neuroprotective benefits [43, 44]. GLP-1R agonists impact visceral pain, with specific relevance to IBS. The modulation of enteric neuronal function and serotonin signaling by GLP-1R agonists contributes to reduced visceral hypersensitivity and pain [52, 53]. The clinical trial with ROSE-010 demonstrated its efficacy in acute pain relief for IBS patients and correlation between serum GLP-1 levels and abdominal pain severity further supports the role of GLP-1R agonists in managing visceral pain. Despite promising research, it is important to exercise caution when considering the potential benefits of GLP-1 agonists beyond diabetes and obesity. The current trend of uncontrolled use of these medications may obscure potential harms to patients. Our understanding of the long-term safety of GLP-1 agonists remains limited, a concern that is particularly pertinent for chronic conditions such as pain and headaches. Some populations, such as adolescents with migraine who suffer from comorbid obesity, may be of interest for further study, as GLP-1 agonists could potentially relieve pain while also addressing obesity [59]. Properly designed randomized controlled trials are necessary to elucidate these aspects and ensure that the use of GLP-1 agonists in these contexts is both safe and beneficial.
Table 3.
Human studies evaluating the analgesic effects of glucagon-like peptide 1 receptor (GLP-1R) agonists in headache and pain disorders
| Study | Year | Study design | Study drug | Study duration | Disease | Study participants | Main findings |
|---|---|---|---|---|---|---|---|
| Gudbergsen et al. [21] | 2021 | Randomized controlled trial | Liraglutide 3 mg/daily | 52 weeks | Knee osteoarthritis | 156 | Liraglutide did not reduce knee pain compared to placebo |
| Zhu et al. [22] | 2023 | Observational multicentre study | GLP-1R agonists | At least 5 years | Knee osteoarthritis | > 40 000 | Cartilage loss velocity and incidence of knee surgery was lower in individuals treated with GLP-1R agonists |
| Krajnc et al. [26] | 2023 | Case-control study | Semaglutide and liraglutide | 6 months | Idiopathic intracranial hypertension | 39 | Reduction in monthly headache days and acetazolamide dosage were higher in individuals treated with GLP-1R agonists |
| Mitchell et al. [27] | 2023 | Randomized controlled trial | Exenatide 10 µg twice daily | 12 weeks | Idiopathic intracranial hypertension | 15 | Exenatide lowered intracranial pressure compared to placebo |
| Hellström et al. [55] | 2009 | Randomized controlled trial | 100 and 300 µg ROSE-010 | 2 years and 9 months | Irritable bowel syndrome | 99 | Twice as many patients were responders in the primary efficacy endpoint after both ROSE-010 injections compared to placebo |
| Touny et al. [56] | 2022 | Substudy of earlier data | 100 and 300 µg ROSE-010 | 2 years and 9 months | Irritable bowel syndrome | 166 | Female participants are more likely than males to respond to ROSE-010 to achieve meaningful pain relief |
Limitations
In our systematic review, we did not prioritize the risk of bias evaluation. Our focus was on mapping existing research, identifying trends, and discussing potential directions for further studies. This approach aligns with the goals of a systematic review into a novel research area, where the primary aim is to gather and describe existing knowledge rather than to rigorously assess study quality or synthesize outcomes quantitatively. Another limitation is the broad search strategy employed, using the terms “GLP-1” and “pain”. We did not incorporate more specific keywords related to distinct pain disorders, such as “headache” or “diabetic neuropathy”. While this approach ensured a comprehensive inclusion of relevant articles and provided a general overview of the role of GLP-1 in pain, it may have limited the depth of evidence on specific pain conditions. Future studies should focus on targeted searches to obtain more refined evidence on the relationship between GLP-1 and individual pain disorders.
Future directions
Future research directions for the use of GLP-1R agonists in the treatment of pain and headache disorders should focus on conducting human trials to evaluate their efficacy and safety, particularly in individuals with comorbid obesity [60]. In addition, detailed mechanistic studies are necessary to elucidate the specific pathways through which GLP-1R agonists exert their analgesic effects, independent of weight loss and metabolic improvements. Exploring the synergistic potential of GLP-1R agonists with other analgesic agents could enhance therapeutic outcomes and reduce the necessity for high-dose monotherapy. Investigating differential responses based on patient demographics, genetic profiles, and specific pain conditions will also be crucial in developing personalized treatment strategies, thereby optimizing the therapeutic benefits of GLP-1R agonists for diverse patient populations. The engineering of bimodal molecules that integrate GLP-1R agonism with other pain-related mechanisms, such as N-methyl-D-aspartate (NMDA) receptor antagonism, may be especially beneficial for pain management [61].
Conclusions
GLP-1R analogs represent a promising frontier in the treatment of various pain disorders. Their diverse biological effects, coupled with a growing body of preclinical and clinical evidence, support the potential for GLP-1R agonists to be integrated into multidisciplinary pain management protocols. Continued research and clinical trials will be pivotal in fully realizing their therapeutic potential and establishing their role in pharmacological practice.
Acknowledgements
Not applicable.
Abbreviations
- AD
Alzheimer’s disease
- AMPK
adenosine monophosphate-activated protein kinase
- ATP
adenosine triphosphate
- BMI
body mass index
- cAMP
cyclic adenosine monophosphate
- DRG
dorsal root ganglion
- DPP-4
dipeptidyl peptidase 4
- GFAP
glial fibrillary acidic protein
- GLP-1
glucagon-like peptide 1
- GLP-1R
glucagon-like peptide 1 receptor
- GSK3β
glycogen synthase kinase-3 beta
- Iba-1
ionized calcium-binding adapter molecule 1
- IBS
irritable bowel syndrome
- IIH
idiopathic intracranial hypertension
- IL-1β
interleukin 1β
- IL-4
interleukin 4
- IL-6
interleukin 6
- IL-10
interleukin 10
- LPS
lipopolysaccharide
- NF-κB
nuclear factor-κB
- NLRP3
NLR family pyrin domain containing 3
- NMDA
N-methyl-D-aspartate
- PD
Parkinson’s disease
- PI3K/Akt
phosphoinositide 3kinase/serine-threonine kinase
- PKA
protein kinase A
- SERT
serotonin reuptake transporter
- SM
shanzhiside methyl ester
- TNF-α
tumor necrosis factor alpha
- TEN
teneligliptin
- TPH-1
tryptophan hydroxylase-1
Author contributions
LP designed and directed the project. WH, YAD and LP performed the systematic search. WH, YAD, DB and LP analysed the data and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
DB received presentation fees (TEVA, Novartis and Pfizer), travel fees (Allergan, TEVA, Pfizer, Abbvie and Lundbeck), advisory board fees (Novartis, Lilly, Teva, Lundbeck, Pfizer and Abbvie) and participated in clinical trials (TEVA, Lundbeck, Novartis, Lilly and Novo Nordisk Foundation) outside of the submitted work. LP has been employed by Lundbeck in the past two years. WH and YAD declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gribble FM, Reimann F. Metabolic messengers: glucagon-like peptide 1. Nat Metab. 2021;3(2):142–148. doi: 10.1038/s42255-020-00327-x. [DOI] [PubMed] [Google Scholar]
- 2.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 3.Lafferty RA, Flatt PR, Irwin N. GLP-1/GIP analogs: potential impact in the landscape of obesity pharmacotherapy. Expert Opin Pharmacother. 2023;24(5):587–597. doi: 10.1080/14656566.2023.2192865. [DOI] [PubMed] [Google Scholar]
- 4.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 5.Sandoval DA, D’Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev. 2015;95(2):513–548. doi: 10.1152/physrev.00013.2014. [DOI] [PubMed] [Google Scholar]
- 6.Smith NK, Hackett TA, Galli A, Flynn CR. GLP-1: molecular mechanisms and outcomes of a complex signaling system. Neurochem Int. 2019;128:94–105. doi: 10.1016/j.neuint.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopp KO, Glotfelty EJ, Li Y, Greig NH. Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: implications for neurodegenerative disease treatment. Pharmacol Res. 2022;186:106550. doi: 10.1016/j.phrs.2022.106550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imbriani P, Martella G, Bonsi P, Pisani A. Oxidative stress and synaptic dysfunction in rodent models of Parkinson’s disease. Neurobiol Dis. 2022;173:105851. doi: 10.1016/j.nbd.2022.105851. [DOI] [PubMed] [Google Scholar]
- 9.Heidari A, Yazdanpanah N, Rezaei N. The role of toll-like receptors and neuroinflammation in Parkinson’s disease. J Neuroinflammation. 2022;19(1):135. doi: 10.1186/s12974-022-02496-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today. 2016;21:802–818. doi: 10.1016/j.drudis.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Kim DS, Choi HI, Wang Y, Luo Y, Hoffer BJ, Greig NH. A new treatment strategy for Parkinson’s disease through the gut-brain axis: the glucagon-like peptide-1 receptor pathway. Cell Transpl. 2017;26:1560–1571. doi: 10.1177/0963689717721234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng J, Xie Y, Ren L, Qi L, Wu L, Pan X, et al. GLP-1 improves the supportive ability of astrocytes to neurons by promoting aerobic glycolysis in Alzheimer’s disease. Mol Metab. 2021;47:101180. doi: 10.1016/j.molmet.2021.101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du H, Meng X, Yao Y, Xu J. The mechanism and efficacy of GLP-1 receptor agonists in the treatment of Alzheimer’s disease. Front Endocrinol (Lausanne) 2022;13:1033479. doi: 10.3389/fendo.2022.1033479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;29:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meurot C, Martin C, Sudre L, Breton J, Bougault C, Rattenbach R, et al. Liraglutide, a glucagon-like peptide 1 receptor agonist, exerts analgesic, anti-inflammatory and anti-degradative actions in osteoarthritis. Sci Rep. 2022;12(1):1567. doi: 10.1038/s41598-022-05323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mert I, Cetinkaya A, Gurler M, Turel CA, Celik H, Torun IE, et al. Anti-inflammatory potential of liraglutide, a glucagon-like peptide-1 receptor agonist, in rats with peripheral acute inflammation. Inflammopharmacology. 2022;30(3):1093–1105. doi: 10.1007/s10787-022-00978-0. [DOI] [PubMed] [Google Scholar]
- 17.Fan H, Gong N, Li TF, Ma AN, Wu XY, Wang MW, et al. The non-peptide GLP-1 receptor agonist WB4-24 blocks inflammatory nociception by stimulating β-endorphin release from spinal microglia. Br J Pharmacol. 2015;172(1):64–79. doi: 10.1111/bph.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho PG, Jang JH, Ko S, Shin DA, Chung S, Chang MC. The effect of evogliptin tartrate on controlling inflammatory pain. Biomedicines. 2023;11(11):2990. doi: 10.3390/biomedicines11112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balogh M, Varga BK, Karádi DÁ, Riba P, Puskár Z, Kozsurek M, et al. Similarity and dissimilarity in antinociceptive effects of dipeptidyl-peptidase 4 inhibitors, Diprotin A and vildagliptin in rat inflammatory pain models following spinal administration. Brain Res Bull. 2019;147:78–85. doi: 10.1016/j.brainresbull.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Aykan DA, Kesim M, Ayan B, Kurt A. Anti-inflammatory and antinociceptive activities of glucagon-like peptides: evaluation of their actions on serotonergic, nitrergic, and opioidergic systems. Psychopharmacology. 2019;236(6):1717–1728. doi: 10.1007/s00213-018-5154-7. [DOI] [PubMed] [Google Scholar]
- 21.Gudbergsen H, Overgaard A, Henriksen M, Wæhrens EE, Bliddal H, Christensen R, et al. Liraglutide after diet-induced weight loss for pain and weight control in knee osteoarthritis: a randomized controlled trial. Am J Clin Nutr. 2021;113(2):314–323. doi: 10.1093/ajcn/nqaa328. [DOI] [PubMed] [Google Scholar]
- 22.Zhu H, Zhou L, Wang Q, Cai Q, Yang F, Jin H, et al. Glucagon-like peptide-1 receptor agonists as a disease-modifying therapy for knee osteoarthritis mediated by weight loss: findings from the Shanghai Osteoarthritis Cohort. Ann Rheum Dis. 2023;82(9):1218–1226. doi: 10.1136/ard-2023-223845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jing F, Zou Q, Wang Y, Cai Z, Tang Y. Activation of microglial GLP-1R in the trigeminal nucleus caudalis suppresses central sensitization of chronic migraine after recurrent nitroglycerin stimulation. J Headache Pain. 2021;22(1):86. doi: 10.1186/s10194-021-01302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jing F, Zou Q, Pu Y. GLP-1R agonist liraglutide attenuates pain hypersensitivity by stimulating IL-10 release in a nitroglycerin-induced chronic migraine mouse model. Neurosci Lett. 2023;812:137397. doi: 10.1016/j.neulet.2023.137397. [DOI] [PubMed] [Google Scholar]
- 25.Botfield HF, Uldall MS, Westgate CSJ, Mitchell JL, Hagen SM, Gonzalez AM, et al. A glucagon-like peptide-1 receptor agonist reduces intracranial pressure in a rat model of hydrocephalus. Sci Transl Med. 2017;9(404):eaan0972. doi: 10.1126/scitranslmed.aan0972. [DOI] [PubMed] [Google Scholar]
- 26.Krajnc N, Itariu B, Macher S, Marik W, Harreiter J, Michl M, et al. Treatment with GLP-1 receptor agonists is associated with significant weight loss and favorable headache outcomes in idiopathic intracranial hypertension. J Headache Pain. 2023;24(1):89. doi: 10.1186/s10194-023-01631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell JL, Lyons HS, Walker JK, Yiangou A, Grech O, Alimajstorovic A, et al. The effect of GLP-1RA exenatide on idiopathic intracranial hypertension: a randomized clinical trial. Brain. 2023;146(5):1821–1830. doi: 10.1093/brain/awad003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grech O, Mitchell JL, Lyons HS, Yiangou A, Thaller M, Tsermoulas G, et al. Effect of glucagon like peptide-1 receptor agonist exenatide, used as an intracranial pressure lowering agent, on cognition in idiopathic intracranial hypertension. Eye (Lond) 2024;38(7):1374–1379. doi: 10.1038/s41433-023-02908-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heckel B. Idiopathic intracranial hypertension after Abrupt Cessation of Medication: a Case Report of Abrupt Glucagon-Like Peptide-1 (GLP-1) receptor Agonist Cessation and Review of the literature. Curr Pain Headache Rep. 2024 doi: 10.1007/s11916-024-01215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghanizada H, Christensen RH, Al-Karagholi MA, Elbahi FA, Coskun H, Ashina M. Arterial responses to infusion of glucagon-like peptide-1 in humans: a randomized trial study. Peptides. 2022;150:170736. doi: 10.1016/j.peptides.2022.170736. [DOI] [PubMed] [Google Scholar]
- 31.Gong N, Xiao Q, Zhu B, Zhang CY, Wang YC, Fan H, et al. Activation of spinal glucagon-like peptide-1 receptors specifically suppresses pain hypersensitivity. J Neurosci. 2014;34(15):5322–5334. doi: 10.1523/JNEUROSCI.4703-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong K, Long X, Wan YQ, Zhou Y. Electroacupuncture inhibited the spinal glial activation in Neuropathic Pain via Glucagon-like Peptide-1/Glucagon-like Peptide-1 receptor signaling. Neurol Sci Neurophysiol. 2024;41(1):23–33. [Google Scholar]
- 33.Ma L, Ju P, Wang W, Wei J, Wang W, Zhao M. Microglial activation of GLP-1R signaling in Neuropathic Pain promotes Gene expression adaption involved in inflammatory responses. Neural Plast. 2021;2021:9923537. doi: 10.1155/2021/9923537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma L, Peng S, Wei J, Zhao M, Ahmad KA, Chen J, et al. Spinal microglial β-endorphin signaling mediates IL-10 and exenatide-induced inhibition of synaptic plasticity in neuropathic pain. CNS Neurosci Ther. 2021;27(10):1157–1172. doi: 10.1111/cns.13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu HY, Tang XQ, Mao XF, Wang YX. Autocrine Interleukin-10 mediates Glucagon-Like Peptide-1 receptor-Induced spinal microglial β-Endorphin expression. J Neurosci. 2017;37(48):11701–11714. doi: 10.1523/JNEUROSCI.1799-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuthati Y, Rao VN, Busa P, Wong CS. Teneligliptin exerts Antinociceptive effects in Rat Model of partial sciatic nerve Transection Induced Neuropathic Pain. Antioxid (Basel) 2021;10(9):1438. doi: 10.3390/antiox10091438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang YR, Mao XF, Wu HY, Wang YX. Liposome-encapsulated clodronate specifically depletes spinal microglia and reduces initial neuropathic pain. Biochem Biophys Res Commun. 2018;499(3):499–505. doi: 10.1016/j.bbrc.2018.03.177. [DOI] [PubMed] [Google Scholar]
- 38.Xu M, Wu HY, Liu H, Gong N, Wang YR, Wang YX. Morroniside, a secoiridoid glycoside from Cornus officinalis, attenuates neuropathic pain by activation of spinal glucagon-like peptide-1 receptors. Br J Pharmacol. 2017;174(7):580–590. doi: 10.1111/bph.13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang X, Wu H, Mao X, Li X, Wang Y. The GLP-1 receptor herbal agonist morroniside attenuates neuropathic pain via spinal microglial expression of IL-10 and β-endorphin. Biochem Biophys Res Commun. 2020;530(3):494–499. doi: 10.1016/j.bbrc.2020.05.080. [DOI] [PubMed] [Google Scholar]
- 40.Gong N, Fan H, Ma AN, Xiao Q, Wang YX. Geniposide and its iridoid analogs exhibit antinociception by acting at the spinal GLP-1 receptors. Neuropharmacology. 2014;84:31–45. doi: 10.1016/j.neuropharm.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Zhu B, Gong N, Fan H, Peng CS, Ding XJ, Jiang Y, et al. Lamiophlomis Rotata, an orally available tibetan herbal painkiller, specifically reduces pain hypersensitivity states through the activation of spinal glucagon-like peptide-1 receptors. Anesthesiology. 2014;121(4):835–851. doi: 10.1097/ALN.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 42.Fan H, Li TF, Gong N, Wang YX. Shanzhiside methylester, the principle effective iridoid glycoside from the analgesic herb Lamiophlomis Rotata, reduces neuropathic pain by stimulating spinal microglial β-endorphin expression. Neuropharmacology. 2016;101:98–109. doi: 10.1016/j.neuropharm.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 43.Cui SS, Feng XB, Zhang BH, Xia ZY, Zhan LY. Exendin-4 attenuates pain-induced cognitive impairment by alleviating hippocampal neuroinflammation in a rat model of spinal nerve ligation. Neural Regen Res. 2020;15(7):1333–1339. doi: 10.4103/1673-5374.272620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang LQ, Zhang W, Li T, Yang T, Yuan X, Zhou Y, et al. GLP-1R activation ameliorated novel-object recognition memory dysfunction via regulating hippocampal AMPK/NF-κB pathway in neuropathic pain mice. Neurobiol Learn Mem. 2021;182:107463. doi: 10.1016/j.nlm.2021.107463. [DOI] [PubMed] [Google Scholar]
- 45.Chen S, Zhou M, Sun J, Guo A, Fernando RL, Chen Y, et al. DPP-4 inhibitor improves learning and memory deficits and AD-like neurodegeneration by modulating the GLP-1 signaling. Neuropharmacology. 2019;157:107668. doi: 10.1016/j.neuropharm.2019.107668. [DOI] [PubMed] [Google Scholar]
- 46.Moustafa PE, Abdelkader NF, El Awdan SA, El-Shabrawy OA, Zaki HF. Liraglutide ameliorated peripheral neuropathy in diabetic rats: involvement of oxidative stress, inflammation and extracellular matrix remodeling. J Neurochem. 2018;146(2):173–185. doi: 10.1111/jnc.14336. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Q, Li Q, Liu S, Zheng H, Ji L, Yi N, et al. Glucagon-like peptide-1 receptor agonist attenuates diabetic neuropathic pain via inhibition of NOD-like receptor protein 3 inflammasome in brain microglia. Diabetes Res Clin Pract. 2022;186:109806. doi: 10.1016/j.diabres.2022.109806. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Zhang Q, Bai Y, Zheng H, Ji L, Zhu X, et al. Glycogen synthesis kinase-3β involves in the analgesic effect of liraglutide on diabetic neuropathic pain. J Diabetes Complications. 2023;37(3):108416. doi: 10.1016/j.jdiacomp.2023.108416. [DOI] [PubMed] [Google Scholar]
- 49.Eissa RG, Eissa NG, Eissa RA, Diab NH, Abdelshafi NA, Shaheen MA, et al. Oral proniosomal Amitriptyline and liraglutide for management of diabetic neuropathy: exceptional control over hyperglycemia and neuropathic pain. Int J Pharm. 2023;647:123549. doi: 10.1016/j.ijpharm.2023.123549. [DOI] [PubMed] [Google Scholar]
- 50.Bianchi R, Cervellini I, Porretta-Serapiglia C, Oggioni N, Burkey B, Ghezzi P, et al. Beneficial effects of PKF275-055, a novel, selective, orally bioavailable, long-acting dipeptidyl peptidase IV inhibitor in streptozotocin-induced diabetic peripheral neuropathy. J Pharmacol Exp Ther. 2012;340(1):64–72. doi: 10.1124/jpet.111.181529. [DOI] [PubMed] [Google Scholar]
- 51.Anand U, Yiangou Y, Akbar A, Quick T, MacQuillan A, Fox M, et al. Glucagon-like peptide 1 receptor (GLP-1R) expression by nerve fibres in inflammatory bowel disease and functional effects in cultured neurons. PLoS ONE. 2018;13(5):e0198024. doi: 10.1371/journal.pone.0198024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Brien R, O’Malley D. The glucagon-like peptide-1 receptor agonist, exendin-4, ameliorated gastrointestinal dysfunction in the Wistar Kyoto rat model of irritable bowel syndrome. Neurogastroenterol Motil. 2020;32(2):e13738. doi: 10.1111/nmo.13738. [DOI] [PubMed] [Google Scholar]
- 53.Yang Y, Cui X, Chen Y, Wang Y, Li X, Lin L, et al. Exendin-4, an analogue of glucagon-like peptide-1, attenuates hyperalgesia through serotonergic pathways in rats with neonatal colonic sensitivity. J Physiol Pharmacol. 2014;65(3):349–357. [PubMed] [Google Scholar]
- 54.Nozu T, Miyagishi S, Kumei S, Nozu R, Takakusaki K, Okumura T. Glucagon-like peptide-1 analog, liraglutide, improves visceral sensation and gut permeability in rats. J Gastroenterol Hepatol. 2018;33(1):232–239. doi: 10.1111/jgh.13808. [DOI] [PubMed] [Google Scholar]
- 55.Li ZY, Zhang N, Wen S, Zhang J, Sun XL, Fan XM, et al. Decreased glucagon-like peptide-1 correlates with abdominal pain in patients with constipation-predominant irritable bowel syndrome. Clin Res Hepatol Gastroenterol. 2017;41(4):459–465. doi: 10.1016/j.clinre.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 56.Hellström PM, Hein J, Bytzer P, Björnssön E, Kristensen J, Schambye H. Clinical trial: the glucagon-like peptide-1 analogue ROSE-010 for management of acute pain in patients with irritable bowel syndrome: a randomized, placebo-controlled, double-blind study. Aliment Pharmacol Ther. 2009;29(2):198–206. doi: 10.1111/j.1365-2036.2008.03870.x. [DOI] [PubMed] [Google Scholar]
- 57.Touny AA, Kenny E, Månsson M, Webb DL, Hellström PM. Pain relief and pain intensity response to GLP-1 receptor agonist ROSE-010 in irritable bowel syndrome; clinical study cross-analysis with respect to patient characteristics. Scand J Gastroenterol. 2022;57(7):783–791. doi: 10.1080/00365521.2022.2041084. [DOI] [PubMed] [Google Scholar]
- 58.Peng MG, Gokoffski KK. Updates on recent developments in idiopathic intracranial hypertension. SN Compr Clin Med. 2021;3:1031–1041. [Google Scholar]
- 59.Westgate CSJ, Israelsen IME, Jensen RH, Eftekhari S. Understanding the link between obesity and headache- with focus on migraine and idiopathic intracranial hypertension. J Headache Pain. 2021;22(1):123. doi: 10.1186/s10194-021-01337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bruijn N, van Lohuizen R, Boron M, Fitzek M, Gabriele F, Giuliani G, et al. Influence of metabolic state and body composition on the action of pharmacological treatment of migraine. J Headache Pain. 2024;25(1):20. doi: 10.1186/s10194-024-01724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petersen J, Ludwig MQ, Juozaityte V, Ranea-Robles P, Svendsen C, Hwang E, et al. GLP-1-directed NMDA receptor antagonism for obesity treatment. Nature. 2024;629(8014):1133–1141. doi: 10.1038/s41586-024-07419-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.