Abstract
Background
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in reproductive age and the most common cause of infertility due to anovulation. PCOS in adolescents is concerning. Nigella sativa is effective in improving gonadotropins and sex hormones. The current study was designed to investigate the effect of Nigella sativa supplementation on PCOS symptoms and their severity in adolescents.
Methods
The current randomized clinical trial was conducted on 114 adolescents with PCOS who were referred to gynecologist offices and clinics in Gonabad, Iran from March 2022 to March 2023. Participants were randomly allocated to the intervention (Nigella sativa 1000 mg/day) and control (10 mg/day medroxyprogesterone from the 14th day of the cycle for 10 nights) groups. The study duration was 16 weeks. Ovarian volume (measured by ultrasound), anthropometric and blood pressure; serum testosterone, dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), luteinizing hormone (LH), hirsutism severity (Ferriman–Gallwey score) levels were evaluated before and after the study.
Results
Data from 103 participants (control group = 53, intervention group = 50) were analyzed. The mean age of participants was 17.0 (Interquartile range [IQR]:2.0). The mean difference in hirsutism score changes (p < 0.001), right (p = 0.002), and left (p = 0.010) ovarian volume, serum LH (p < 0.001) and testosterone (p = 0.001) were significantly higher in the intervention group compared to the control group. The frequency of oligomenorrhea, menometrorrhagia, and amenorrhea, were significantly reduced after the study in the intervention group compared to the control group (ps < 0.001).
Conclusions
Short-term Nigella sativa supplementation may be effective in reducing ovarian volume and improving hormonal balance, and menstrual irregularities in adolescents with PCOS. Further research and long-term studies are warranted to validate the potential therapeutic effects of Nigella sativa in adolescents with PCOS.
IRCT registration number
IRCT20221017056209N1 Registration date: 2022-11-22.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13048-024-01460-x.
Keywords: Polycystic ovary syndrome, Nigella sativa, Medroxyprogesterone, Adolescent
Background
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder among women of reproductive age and is the most common cause of infertility due to anovulation [1, 2]. In 2022, the global prevalence of PCOS based on Rotterdam criteria ranged between 2.2 and 22.5% [3]. In Iran, the prevalence of PCOS based on Rotterdam criteria and sonographic methods were estimated to be 19.5 and 41.1% [6]. PCOS is a complex multifactorial, polygenic, and hereditary disorder and is characterized by hyperandrogenism, polycystic ovaries, and ovarian dysfunction [4]. Short and long-term complications of PCOS impose a great health and economic burden on countries [5].
In recent years, teenagers have been recognized as a susceptible age group to PCOS [1]. Different prevalence rates have been reported for PCOS among teenagers. The reason for this difference may be related to the use of different diagnostic criteria [1]. In a meta-analysis study published in 2019, the global prevalence of PCOs based on Rotterdam criteria among adolescents was 11.4% [1]. Early diagnosis and treatment of adolescents with PCOS can prevent long-term reproductive, cardio-metabolic, and emotional consequences associated with this syndrome [6, 7]. PCOS can lead to the cessation of the menstrual cycle or primary or secondary amenorrhea and secondary infertility [8, 9]. PCOS is also associated with the risk of depression, anxiety, obsessive-compulsive disorder, and physical abuse in adolescents [10].
Several treatment methods have been suggested for adult women with PCOS. Combined hormonal contraceptive (CHC) is suggested for the treatment of menstrual disorders and hyperandrogenism in PCOS [11]. Weight loss is the first non-pharmacological treatment for infertility in obese women with PCOS, while clomiphene citrate (anti-estrogen), aromatase inhibitors (estrone and estradiol reducing), gonadotropins (stimulating ovulation) and metformin (lowering blood sugar) can be effective pharmacological treatments for infertility due to PCOS [12]. However, there is limited information on the efficacy of these treatments in improving the manifestations of PCOS in adolescence [12].
The side effects of pharmacological agents have resulted in the desire to use herbal medicines, especially among women [13]. Medicinal plants are cheap, accessible, and have fewer side effects compared to chemical drugs [14]. Many herbal medicines, including vitex, Cichorium intybus, beetroot, green tea, Foeniculum vulgare Mill, Carum carvi, aloe vera, Stachys lavandulifolia, Nigella sativa, and licorice, have been studied in the treatment of PCOS [15].
Nigella sativa Linn, commonly known as black seed, is a member of the Ranunculaceae family. Nigella sativa has been used for therapeutic purposes in India, Arab countries, Europe and Iran. Nigella sativa compounds include estrone, saponin, phenolic compounds, alkaloids, fatty acids, and volatile oils. Eight types of amino acids are known in the proteins found in Nigella sativa. Nigella sativa contains monosaccharides in the form of glucose, rhamnose, xylose, and arabinose [16]. Nigella sativa has relaxant, fat-lowering, antioxidant, anti-inflammatory, antidiabetic, antihypertensive, antibacterial, analgesic, antiparasitic, anti-flatulent, diuretic, lactating, and antiparasitic, and liver, kidney, blood vessels protective properties [17–19]. It has also been used as a spice and in the treatment of asthma, hypertension, diabetes, inflammation, cough, bronchitis, headache, eczema, fever, dizziness and flu [19]. Some studies have evaluated the effects of Nigella sativa in the treatment of PCOS in adults [16, 17, 19, 20]. Based on the findings of these studies, Nigella sativa can have therapeutic effects on PCOS due to its antioxidant, and insulin resistance-lowering properties [16, 17, 19, 20].
To the best of our knowledge, the effects of Nigella sativa have not been studied among adolescents with PCOS. Considering the known effects of Nigella sativa on adults with PCOS and PCOS-related infertility, the current study was designed to compare the effects of Nigella sativa with medroxyprogesterone acetate on the symptoms of PCOS and their severity in adolescent girls.
Methods
Study design, participants, and sampling
The current study was a randomized controlled clinical trial, registered in the Iranian Registry of Clinical Trials (Code: IRCT20221017056209N1. Registration date: 2022-11-22). The study design was based on the Consolidated Standards of Reporting Trials (CONSORT) checklist [21].
The sample size for the study was calculated using the G*Power 3.1.9.2 software considering the type 1 error of 0.01 and the power of 0.99 and the difference in ovarian volume reported among women with PCOS (15.15 ± 5.6 cm3) reported in a previous study [22]. The calculated sample size (49 participants in each group) was increased to 57 participants in each group considering 15% dropout.
Participants were selected using a convenience sampling technique from adolescent girls referred to private or governmental Obstetrics and Gynecology clinics in Gonabad, Iran, from March 2022 to March 2023. The inclusion criteria were age range of 12 to 18 years, menstruation history for at least two years, willingness to participate in the study, documented diagnosis of PCOS based on the recent guidelines for the diagnosis of PCOS among adolescents [23] (diagnosis of PCOS was confirmed by a gynecologist), absence of other causes of hyperandrogenism, including non-classic congenital adrenal hyperplasia, body mass index (BMI) between 19 and 25 kg/m2, no history of consuming hormonal medications in the past three months, no history of consuming medications that interfere with medroxyprogesterone, including amphetamines, thyroid medications, diphenhydramine, ciprofloxacin, duloxetine, estradiol, omega-3 fatty acids, ecitalopram, pregabalin, levonorgestrel, montelukast, testosterone, cyanocobalamin, ascorbic acid, multivitamins, sertraline, and cetirizine, no allergy to Nigella sativa or medroxyprogesterone based on self-report, no history for hypothyroidism, hyperprolactinemia, or renal, cardiac, hepatic, or bone disorders, hypophysis tumors, cancer or diabetes based on self-report and history taking, no history for smoking or drug abuse, no history of surgery on one or both ovaries, and no history for incidents that trigger psychologic tensions, including death of a relative, accidents, sexual insult, or rubbery, in the past three months. The exclusion criteria were refusal to continue the study, allergic reactions to the intervention medication based on self-report or family member report, lack of adherence to the prescribed medication or supplement (not using the capsules or pills for more than four weeks consecutively or 30% of the capsules or pills during the study).
Randomization and blinding
Participants were randomly assigned to the intervention group (Nigella sativa) or the control group (medroxyprogesterone) using permutation blocks of sizes 2 and 4. The random allocation sequence was generated using the online website (https://www.sealedenvelope.com) and concealed in sequentially numbered, opaque, sealed envelopes. These envelopes were opened sequentially only after the participant provided informed consent and entered the study.
Only the statistical analyzer was blinded. Treatments were coded as A and B in the dataset to maintain confidentiality and prevent bias during analysis.
Study instruments
Demographic and menstrual questionnaire: A researcher-made questionnaire was used to obtain the demographic characteristics of the participants. These characteristics included age, education level, maternal education, maternal occupation, paternal education, paternal occupation, child order in the family, and number of siblings. Menstrual history information was obtained based on interviews with the participants and their parents. Menstrual history data included age at menarche, history of dysmenorrhea, duration of menstrual cycle, duration of menstrual bleeding, volume of menstrual bleeding, presence of menstrual disorders, and duration of PCOS diagnosis.
Ultrasound assessment: Ovarian volume was assessed trans-abdominally using an ultrasound device by a radiologist. Ovarian volume was measured in the longitudinal plane by measuring the distance between the inner and outer margins of the ovaries. The ovarian volume was separately recorded for each side. All ultrasound assessments were done by one radiologist.
Laboratory measurements: Serum dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), serum testosterone, and luteinizing hormone (LH) were measured using the quantitative luminescence method (LIAISON Assay, Diasorin, Dietzenbach, Germany).
Anthropometric measurements: The height, waist, and hip circumference of the participants were measured using a measuring tape to the nearest 0.1 centimeters. The weight of the participants was measured using a digital weighing scale to the nearest 100 g. BMI was then calculated by dividing the weight in kg by the square of height in meters. Waist-hip ratio (WHR) was calculated by dividing the waist circumference to the waist circumference.
The Ferriman–Gallwey score: The Ferriman-Gallwey score was utilized to assess hirsutism among the participants in the study. This scoring system is based on the evaluation of hair density in nine different body areas, such as the upper lip, chin, chest, upper and lower abdomen, thighs, and upper and lower back. The score can range from 0 (indicating no hair growth) to 4 (indicating extensive hair growth) in each area. A total score of 8 or above was indicative of hirsutism [24].
Study procedure
After obtaining informed consent, the study questionnaires were filled in an interview and the anthropometric measurements were performed in a private, calm, and stress-free environment. The ovarian volume was measured by ultrasound scan, and venous blood samples were taken for laboratory measurements after filling out the questionnaires. Then the participants were allocated to the intervention (Nigella sativa) and control (medroxyprogesterone).
The participants in the intervention group received 1000 mg capsules containing Nigella sativa extract (Barij Essence Pharmaceutical Company, Kashan, Iran) daily for 16 weeks (Supplementary file 1) (55). The product was commercially available in the pharmaceutical market of Iran and has approval from the Iran Food and Drug Organization. The participants in the control group received 10 mg medroxyprogesterone tablets (Aburaihan Pharmaceutical company, Iran) 10 nights per month from the 14th day of their menstrual cycle for 16 weeks [3].
Participants were asked to fill out a logbook for medication consumption and report any side effects to the primary researcher. Participants in both groups were reminded of regular consumption of the drugs through short messaging system (SMS) reminders daily.
At the end of the study (at the end of the 16th week), Ferriman–Gallwey scoring, ovarian volume, laboratory tests, anthropometric measurements, and blood pressure were re-evaluated in participants in both groups.
Statistical analysis
The study data was analyzed using the statistical package for social sciences (SPSS) software version 21 (SPSS Inc, Chicago, IL). The normality of the quantitative variables was determined using the Kolmogorov-Smirnov test. Normal quantitative variables were described using mean and standard deviation, and non-normal quantitative variables were described using median and interquartile range. Frequency and percentage were also used to describe qualitative data. The Fisher exact test was used to compare qualitative variables between groups and the Mann-Whitney test was used for the comparison of quantitative variables between groups.
Unfortunately, the questionnaires of patients who refused cooperation, or incomplete supplement consumption were mistakenly thrown away. As a result, the data for those patients were lost. This made it impossible to conduct an intention-to-treat (ITT) analysis which includes all randomized participants regardless of protocol deviations or incomplete follow-up. Therefore, a per-protocol analysis was performed. To assess the treatment’s impact on the outcomes, linear regression models were employed, incorporating the effects of treatment, time, and their interaction. This method facilitated a more precise analysis of the treatment’s true effects on the response variable by accounting for potential confounding variables. The assumptions of regression analysis, including the normality of the distribution of the response variable, homogeneity of variance, and independence of observations, were checked respectively by checking the normality of the distribution of the residuals, the graph of the residuals against the fitted values, and the graph of the residuals against time. The level of significance was considered 0.05.
Ethical considerations
The study was approved by the Ethics Committee of the Gonabad University of Medical Sciences (Code: IR.GMU.REC.1401.080). All participants and their parents or guardians were briefed about the study’s aims and procedures. Then, the willing participants and their parents or guardians gave written informed consent before entering the study.
Results
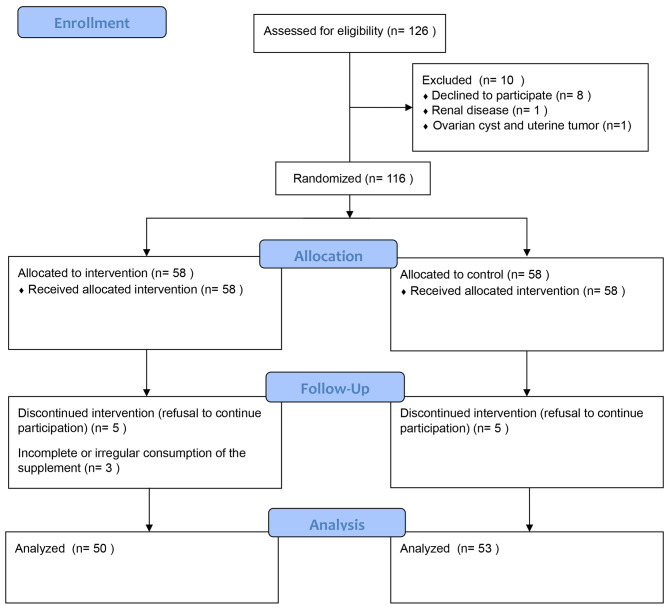
In the current study, of the primary identified 126 adolescent girls with PCOS, 116 entered the study and were randomly assigned to the intervention (n = 58) and control (n = 58) groups. During the study, five participants from the control group and 8 participants from the intervention group were excluded; therefore, data on 50 participants in the intervention group and 53 participants in the control group were analyzed (Fig. 1).
Fig. 1.
The CONSORT flow diagram of the study
The demographic and menstrual characteristics of the participants are presented in Table 1. Overall, there were no significant differences between the groups in terms of demographic characteristics, except for paternal education level (p = 0.013). These findings indicated a higher percentage of high school diplomas in the control group compared to the intervention group. There were no significant differences between the groups in terms of menstrual characteristics.
Table 1.
Comparison of the demographic and menstrual characteristics between Nigella sativa and Medroxyprogesterone groups
| Variable | Nigella sativa (n = 50) | Medroxyprogesterone (n = 53) | p |
|---|---|---|---|
| Age (years), Median (Q1, Q3) | 17.0 (16.0, 18.0) | 17.0 (16.0, 18.0) | 0.418† |
| Number of siblings, Median (Q1, Q3) | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 0.386† |
| Education level, n (%) | 0.135# | ||
| Literacy | 0 (0.0) | 2 (3.8) | |
| Primary | 5 (10.0) | 1 (1.8) | |
| Secondary | 32 (64.0) | 32 (60.4) | |
| High school diploma | 13 (26.0) | 18 (34.0) | |
| Maternal occupation, n (%) | 0.407# | ||
| Housewife | 32 (64.0) | 30 (56.6) | |
| Employee | 12 (24.0) | 20 (37.7) | |
| Worker | 5 (10.0) | 2 (3.8) | |
| Retired | 1 (2.0) | 1 (1.9) | |
| Maternal education, n (%) | 0.207# | ||
| Primary | 18 (36.0) | 24 (45.3) | |
| Secondary | 18 (36.0) | 10 (18.9) | |
| High school diploma | 4 (8.0) | 6 (11.3) | |
| Diploma | 8 (16.0) | 13 (24.5) | |
| Bachelor | 1 (2.0) | 0 (0.0) | |
| Master | 1 (2.0) | 0 (0.0) | |
| Paternal occupation, n (%) | 0.284# | ||
| Unemployed | 2 (4.0) | 5 (9.4) | |
| Employee | 28 (56) | 32 (60.4) | |
| Worker | 15 (30.0) | 12 (22.6) | |
| Retired | 5 (10.0) | 2 (3.8) | |
| Freelance | 0(0.0) | 2 (3.8) | |
| Paternal education, n (%) | 0.013 # | ||
| Primary | 11 (22.0) | 9 (17.0) | |
| Secondary | 9 (18.0) | 9 (17.0) | |
| High school diploma | 9 (18.0) | 22 (41.5) | |
| Diploma | 17 (34.0) | 6 (11.3) | |
| Bachelor | 2 (6.0) | 7 (13.2) | |
| Master | 1 (2.0) | 0 (0.0) | |
| Birth order, n (%) | 0.437# | ||
| 1 | 4 (8.0) | 1 (1.9) | |
| 2 | 14 (28.0) | 14 (26.4) | |
| 3 | 20 (40.0) | 20 (37.7) | |
| 4 | 9 (18.0) | 16 (30.2) | |
| 5 | 3 (6.0) | 2 (3.8) | |
| Age at menarche (years), Median (Q1, Q3) | 13.0 (12.0, 14.0) | 13.0 (12.0, 14.0) | 0.555† |
| Menstrual cycle duration (days), Median (Q1, Q3) | 40.0 (40.0, 45.0) | 40.0 (40.0, 45.0) | 0.742† |
| Menstrual bleeding duration (days), Median (Q1, Q3) | 3.0 (2.0, 4.0) | 2.0 (2.0, 3.0 ) | 0.136† |
| Menstrual bleeding severity, Median (Q1, Q3) | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) | 0.349† |
| PCOS diagnosis duration, Median (Q1, Q3) | 6.0 (4.0, 7.0) | 5.0 (4.0, 7.0) | 0.184† |
| History of dysmenorrhea | 0.233## | ||
| Yes | 2 (4.0) | 0 (0.0) | |
| No | 48 (96.0) | 53 (100.0) |
Q1, 1st quartile; Q3, 3rd quartile; † The Mann-Whitney test; # the chi-square test not exact test; ## The Fisher exact test
A comparison of imaging and laboratory measurements between groups is presented in Table 2. A significant treatment effect was observed for endometrial thickness (p < 0.001), LH (p = 0.013), BMI (p < 0.001), and systolic pressure (p = 0.035), indicating a significant difference between the groups in these outcomes before interventions.
Table 2.
Comparison of imaging and laboratory measurements between Nigella sativa and Medroxyprogesterone groups
| Variable | Nigella sativa Mean (SD) |
Medroxyprogesterone Mean (SD) |
Treatment | Time | Time* Treatment | |
|---|---|---|---|---|---|---|
| Right ovarian volume (cc) | Before | 11.96 (2.94) | 11.95 (2.34) | < 0.770 | 0.213 | 0.002* |
| After | 9.40 (2.12) | 11.17 (2.09) | ||||
| Left ovarian volume (cc) | Before | 11.28 (3.67) | 11.91 (3.07) | 0.316 | 0.343 | 0.010* |
| After | 8.36 (2.68) | 10.84 (3.29) | ||||
| Endometrial thickness (cm) | Before | 10.24 (3.15) | 9.01 (1.48) | < 0.001* | 0.317 | 0.002* |
| After | 7.30 (2.04) | 7.78 (1.55) | ||||
| DHEA (ng/dL) | Before | 123.74 (36.49) | 125.02 (29.07) | 0.736 | 0.232 | 0.074 |
| After | 113.91 (28.11) | 126.44 (28.88) | ||||
| DHEA-S (ng/dL) | Before | 128.79 (39.65) | 133.98 (35.44) | 0.628 | 0.559 | 0.120 |
| After | 113.89 (31.10) | 131.96 (34.28) | ||||
| Testosterone (ng/dL) | Before | 0.56 (0.49) | 0.66 (0.67) | 0.369 | 0.040* | 0.001* |
| After | 0.33 (0.27) | 0.65 (0.69) | ||||
| Hirsutism severity score | Before | 3.98 (1.56) | 3.75 (1.40) | 0.111 | 0.001* | < 0.001* |
| After | 1.36 (0.85) | 3.38 (1.79) | ||||
| LH (mU/dL) | Before | 15.72 (3.19) | 14.29 (3.40) | 0.013* | 0.004* | < 0.001* |
| After | 9.90 (2.06) | 13.57 (2.87) | ||||
| BMI (kg/m 2 ) | Before | 25.56 (3.63) | 23.84 (2.61) | < 0.001* | 0.517 | 0.252 |
| After | 24.57 (2.36) | 23.72 (2.47) | ||||
| WHR | Before | 0.92 (0.06) | 0.89 (0.06) | 0.064 | 0.768 | 0.814 |
| After | 0.92 (0.07) | 0.90 (0.07) | ||||
| SBP (mmHg) | Before | 105.10 (5.67) | 103.30 (5.37) | 0.035* | 0.700 | 0.610 |
| After | 105.04 (4.69) | 103.28 (4.24) | ||||
| DBP (mmHg) | Before | 71.10 (2.73) | 71.51 (2.87) | 0.548 | 0.433 | 0.330 |
| After | 71.66 (2.27) | 71.55 (2.51) | ||||
DHEA: dehydroepiandrosterone, DHEA-S: dehydroepiandrosterone sulfate, LH: Luteinizing Hormone, BMI: Body Mass Index, WHR: Waist-hip ratio, SBP: Systolic Blood Pressure, DBP: Diastolic Blood Pressure
The repeated measures analysis of variance was used for the analysis
* Significant effect
Furthermore, a significant effect was noted for the interaction of time and treatment regarding right ovarian volume (p = 0.002), left ovarian volume (p = 0.010), endometrial thickness (p = 0.002), Testosterone (p = 0.001), hirsutism severity score (p < 0.001), and LH (p < 0.001). This suggests a significant difference between the groups in terms of these outcomes from before to after the interventions, after adjusting for differences before the intervention.
Overall, a significantly higher reduction in mean ovarian volume on both sides, endometrial thickness, serum testosterone, hirsutism score, and serum LH were identified in the intervention group compared to the control group.
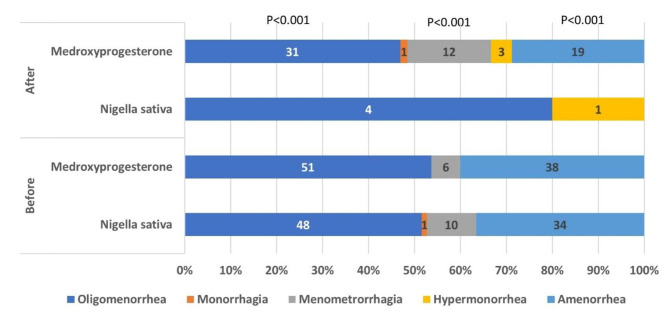
Comparison of the frequency of menstrual disorders between the study groups before and after the study is presented in Fig. 2. (Fig. 2). There was no significant difference between groups in terms of the frequency of menstrual disorders before the study (ps > 0.05). There was a significant difference in the frequency of oligomenorrhea, menometrorrhagia, and amenorrhea (ps < 0.001) between groups after the study. This finding indicated that the frequency of these menstrual disorders was significantly lower in the intervention group compared to the control group.
Fig. 2.
Frequency of menstrual disorders in study groups before and after the study (The percentage of each menstrual disorder in each study group is presented in the horizontal axis. The frequency of each menstrual disorder in the study group is presented on the bar with the same color. There was a significant difference in the frequency of oligomenorrhea, menometrorrhagia, and amenorrhea between groups after the study (p < 0.001 for each disorder))
Discussion
The current study was conducted to compare the effects of 16 weeks of Nigella sativa supplementation on ovarian volume, hormonal, and menstrual disorders with those of medroxyprogesterone administration in adolescent girls with PCOS. The results of the current study showed that Nigella sativa supplementation resulted in a significantly higher reduction in ovarian volume, endometrial thickness, serum testosterone, and LH; as well as some menstrual disorders compared to the medroxyprogesterone group.
Similar to the results of the current study in terms of ovarian volume reduction, an animal study showed that intraperitoneal administration of Nigella sativa for three weeks decreased the number of primordial follicles, primary and secondary follicles, and ovarian weight compared to the control group [25].
The results of the current study in terms of the effect of Nigella sativa supplementation on serum testosterone were in line with the findings of previous studies. In a study on rats with PCOS, the combination of Nigella sativa and honey significantly reduced serum testosterone [20]. Another animal study on letrozole-induced PCOS in mice model showed that Nigella sativa administration for 8 weeks significantly reduced serum testosterone levels [26].
To the best of our knowledge, no study has yet investigated the effect of Nigella sativa supplementation on hirsutism. However, similar to the present study, some studies have shown that progesterone administration reduces hirsutism [27, 28]. Women with PCOS often suffer from skin disorders, including hirsutism due to excessive environmental androgens [15]. It seems that the mechanism of Nigella sativa effect on hirsutism might be related to the male sex hormone-reducing effects of Nigella sativa.
The results of the current study in terms of the effects of Nigella sativa supplementation on serum LH levels were similar to the findings of previous studies. A study conducted on rats with PCOS, that showed a high dose of Nigella sativa and honey administration significantly reduced serum LH [20]. The mechanism for this effect can be attributed to the effects of Nigella sativa on insulin resistance. Previous evidence has shown that increased insulin levels and insulin resistance intensify the production of sex steroids stimulated by ovarian gonadotropins and cause abnormal LH secretion [20].
The results of the current study in terms of the effects of Nigella sativa supplementation on oligomenorrhea, menometrorrhagia, and amenorrhea were in line with the findings of some previous studies. For instance, a previous study showed that 16-week Nigella sativa supplementation significantly reduced menstrual cycle intervals and increased menstruation frequency in women with PCOS [29]. Another study showed that oral Nigella sativa supplementation (1000 mg) reduced the severity of physical symptoms of premenstrual syndrome [30]. The exact mechanism of this effect is not known; however, it seems that these effects are due to the phytoestrogen compounds in Nigella sativa extract [31]. Phytoestrogens are weak estrogenic compounds that bind to estrogen receptors and present their agonistic and antagonistic effects, both of which can affect menstrual disorders in PCOS [29]. Another mechanism of the effect of Nigella sativa might be related to its unsaturated fatty acid content, including linoleic acid and oleic acid. The estrogenic effects of these unsaturated fatty acids have been reported in previous studies [32]. This mechanism was hypothesized based on the findings of previous animal studies. A previous study on postmenopausal rats reported that the methanolic extract of Nigella sativa had similar effects to conjugated estrogen on vaginal epithelial recovery [33]. Other animal studies showed that Nigella sativa administration increased the weight of the uterus and serum level of estradiol [33, 34].
Other mechanisms for the potential effect of Nigella sativa on the improvement of oligomenorrhea include the anti-inflammatory and antioxidant properties of this plant compound [35]. Recent studies have emphasized the important role of oxidative stress in the pathogenesis of PCOS and suggested the effectiveness of antioxidant consumption in improving these symptoms [36]. The antioxidant mediators of Nigella sativa have been studied using enzymatic methods in previous studies [37]. On the other hand, oxidative stress can result in the production of inflammatory mediators, including interleukin-6 and tumor necrosis factor-alpha, and affect the ovary and endothelium causing anovulation and premature atherosclerosis in PCOS [38]. Inflammatory mediators are produced in the initial responses of the immune system to obesity and insulin resistance in patients with PCOS, which may be reduced by antioxidants [39].
The results of previous studies in terms of the effects of Nigella sativa supplementation on BMI and WHR were contradictory. For instance, a previous study showed that Nigella sativa supplementation along with exercise decreased BMI, waist circumference, and WHR in young overweight women [40]. A systematic review showed that Nigella sativa supplementation reduced BMI but not WHR [41]. These contradictory results seem to be due to the difference in the study population (overweight women compared to PCOS adolescents in our study) and the difference in the intervention (Nigella sativa with exercise compared to Nigella sativa alone in our study).
The results of the previous studies in terms of the effects of Nigella sativa supplementation on systolic and diastolic blood pressure were contradictory. A previous study showed that hydroalcoholic extract of Nigella sativa for 8 weeks significantly decreased systolic and diastolic blood pressure in a dose-dependent manner among patients with mild hypertension [42]. Furthermore, in another study on hypertensive patients, Nigella sativa supplementation for 12 weeks significantly reduced blood pressure [43]. The reason for the contradiction in the findings can be due to the inclusion of hypertensive patients in the mentioned study compared to normotensive adolescents in the current study.
The current study showed that although the mean DHEA and DHEA-S decreased after the intervention, but the difference was not statistically significant. In this regard, the findings of the previous studies were contradictory. For instance, an animal study showed that the administration of Nigella sativa combined with honey to male rats increased DHEA and androstenedione [44]. In addition to the fact that this study is animal-based, the contradiction in the findings can be caused by the consumption of the combination of honey and Nigella sativa. It is also possible that increasing the dosage of black seeds can cause different therapeutic effects.
The study faced a limitation of missing data for patients who were lost to follow-up due to discarded questionnaires when they discontinued participation or had irregular supplement consumption. This resulted in the inability to perform an intention-to-treat (ITT) analysis, which could have provided a more comprehensive assessment of the intervention’s effectiveness. So, the impact of missing data on the study results should be considered when interpreting the findings. Further studies are needed to confirm the findings of our study.
Conclusion
The results indicated that Nigella sativa supplementing could reduce ovarian volume, improve hormonal balance, and alleviate menstrual irregularities in adolescents with PCOS compared to medroxyprogesterone administration. However, these findings should be confirmed by further studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The current report is part of a Master’s degree thesis in Midwifery. The authors would like to thank the Vice Chancellor of Research and Technology of Gonabad University of Medical Sciences for providing financial support for the study.
Abbreviations
- PCOS
Polycystic ovary syndrome
- DHEA
Dehydroepiandrosterone
- DHEA-S
Dehydroepiandrosterone sulfate
- LH
Luteinizing hormone
- BMI
Body mass index
- WHR
Waist-hip ratio
- SPSS
Statistical package for social sciences
- SBP
Systolic Blood Pressure
- DBP
Diastolic Blood Pressure
Author contributions
Study concept and design: A. A., and N. B.; analysis and interpretation of data: F. M., and S. D.; drafting of the manuscript: A. M., and R. R.; critical revision of the manuscript for important intellectual content: N. B., S. D., and F. M. statistical analysis: F.M.
Funding
The study was financially supported by Gonabad University of Medical Sciences. (Grant number: 1195).
Data availability
The datasets generated and analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Gonabad University of Medical Sciences (Code: IR.GMU.REC.1401.080). All participants and their parents or guardians were briefed about the study’s aims and procedures. Then, the willing participants and their parents or guardians gave written informed consent before entering the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Naz M, Tehrani F, Majd H, Ahmadi F, Ozgoli G, Fakari F, et al. The prevalence of polycystic ovary syndrome in adolescents: a systematic review and meta-analysis. Int J Reprod Biomed. 2019;17(8):533–42. doi: 10.18502/ijrm.v17i8.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palomba S. Is fertility reduced in ovulatory women with polycystic ovary syndrome? An opinion paper. Hum Reprod. 2021;36(9):2421–8. doi: 10.1093/humrep/deab181. [DOI] [PubMed] [Google Scholar]
- 3.Ghazanfarpour M, Dolatabadi Z, Bamorovat Z, Mahmoodabadi M, Salari Nasab J, Basari S, et al. Prevalence of symptoms of polycystic ovary syndrome and some Associated factors in medical students. J Babol Univ Med Sci. 2022;24(1):215–23. [Google Scholar]
- 4.Pourahmadi G. The success of other organizations (raising the quality of working life of women employed in the oil industry) Q J Nurs Manage. 2019;7(4):21–4. [Google Scholar]
- 5.Moran L, Tassone E, Boyle J, Brennan L, Harrison C, Hirschberg A, et al. Evidence summaries and recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome: Lifestyle management. Obes Rev. 2020;21(10):e13046. doi: 10.1111/obr.13046. [DOI] [PubMed] [Google Scholar]
- 6.Goodman N, Cobin R, Futterweit W, Glueck J, Legro R, Carmina E. American Association of Clinical Endocrinologists, American College of Endocrinology, and androgen excess and PCOS Society Disease State Clinical Review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome–part 1. Endocr Pract. 2015;21(11):1291–300. doi: 10.4158/EP15748.DSC. [DOI] [PubMed] [Google Scholar]
- 7.Palomba S, Piltonen TT, Giudice LC. Endometrial function in women with polycystic ovary syndrome: a comprehensive review. Hum Reprod Update. 2021;27(3):584–618. doi: 10.1093/humupd/dmaa051. [DOI] [PubMed] [Google Scholar]
- 8.Deligeoroglou E, Athanasopoulos N, Tsimaris P, Dimopoulos KD, Vrachnis N, Creatsas G. Evaluation and management of adolescent amenorrhea. Ann N Y Acad Sci. 2010;1205:23–32. doi: 10.1111/j.1749-6632.2010.05669.x. [DOI] [PubMed] [Google Scholar]
- 9.Palomba S, Daolio J, La Sala GB. Oocyte competence in women with polycystic ovary syndrome. Trends Endocrinol Metabolism. 2017;28(3):186–98. doi: 10.1016/j.tem.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Adone A, Fulmali DG. Polycystic ovarian syndrome in adolescents. Cureus. 2023;15(1):e34183. doi: 10.7759/cureus.34183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramezani Tehrani F, Amiri M. Polycystic ovary syndrome in adolescents: challenges in diagnosis and treatment. Int J Endocrinol Metab. 2019;17(3):e91554. doi: 10.5812/ijem.91554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fauser B, Tarlatzis B, Rebar R, Legro R, Balen A, Lobo R. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 13.Mirazi N, Mosalsal S, Izadi Z, Nourian A. Protective effect of Hydroethanolic Extract of Lippia citrodora L. Leaf and vitamin D3 on Estradiol Valerate-Induced Polycystic Ovary Syndrome in mice. Qom Univ Med Sci J. 2020;14(9):16–27. doi: 10.52547/qums.14.9.16. [DOI] [Google Scholar]
- 14.Grant P. Spearmint herbal tea has significant anti-androgen effects in polycystic ovarian syndrome: a randomized controlled trial. Phytother Res. 2010;24(2):186–8. doi: 10.1002/ptr.2900. [DOI] [PubMed] [Google Scholar]
- 15.Shabani Azim F, Dashti N. Review on clinical manifestations and long-term consequences of polycystic ovary syndrome (PCOS) Labdiag. 2017;9(36):19–27. [Google Scholar]
- 16.Kohzadi R, Nejati V, Razi M, Najafi G. Effects Hydro-alcoholic extract of (Nigella sativa L.) on the level of malondialdehyde (MDA) and total antioxidant capacity (TAC) of the ovary tissue in a rat model of PCOS. J Anim Environ. 2017;9(3):85–92. [Google Scholar]
- 17.Ashraf SS, Rao MV, Kaneez FS, Qadri S, Al-Marzouqi AH, Chandranath IS, et al. Nigella sativa extract is a potent antioxidant for petrochemical-induced oxidative stress. J Chromatogr Sci. 2011;49(4):321–6. doi: 10.1093/chrsci/49.4.321. [DOI] [PubMed] [Google Scholar]
- 18.Kooti W, Hasanzadeh-Noohi Z, Sharafi-Ahvazi N, Asadi-Samani M, Ashtary-Larky D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa) Chin J Nat Med. 2016;14(10):732–45. doi: 10.1016/S1875-5364(16)30088-7. [DOI] [PubMed] [Google Scholar]
- 19.Niazi A, Rahimi V, Hatami H, Shirazinia R, Esmailzadeh-Dizaji R, Askari N, et al. Effective Medicinal plants in the treatment of the cyclic Mastalgia (breast Pain): a review. J Pharmacopunct. 2019;22(3):131–9. doi: 10.3831/KPI.2019.22.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naseran SN, Mokhtari M, Abedinzade M, Shariati M. Evaluation of the Effect of Nigella Sativa Hydro-alcoholic Extract and Honey on gonadotropins and Sex hormones Level in the polycystic ovarian syndrome model of Wistar Rat. SJKU. 2020;25(1):117–29. doi: 10.52547/sjku.25.1.117. [DOI] [Google Scholar]
- 21.Plint AC, Moher D, Morrison A, Schulz K, Altman DG, Hill C, et al. Does the CONSORT checklist improve the quality of reports of randomized controlled trials? A systematic review. Med J Aust. 2006;185(5):263–7. doi: 10.5694/j.1326-5377.2006.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 22.Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. The age of adolescence. Lancet Child Adolesc Health. 2018;2(3):223–8. doi: 10.1016/S2352-4642(18)30022-1. [DOI] [PubMed] [Google Scholar]
- 23.Trent M, Gordon CM. Diagnosis and management of polycystic ovary syndrome in adolescents. Pediatrics. 2020;145(Supplement2):S210–8. doi: 10.1542/peds.2019-2056J. [DOI] [PubMed] [Google Scholar]
- 24.Ramezani Tehrani F, Minooee S, Simbar M, Azizi F. A simpler diagnostic method to assess Hirsutism in the Iranian Population: based on modified ferriman- Gallwey Scoring System (Tehran lipid and glucose study) Iran J Endocrinol Metabolism. 2013;15(3):303–10. [Google Scholar]
- 25.Ghalandari R, Ghassemi F, Kargar Jahromi H, Gholamzadeh N. Effects of hydro-alcoholic extract of Nigella Sativa L. on ovarian function factors in female rats. Iran J Med Aromatic Plants Res. 2016;32(5):915–23. [Google Scholar]
- 26.Anwar N, Hamid S, Nadeem A, Asad A, Waseem N, Mehmood N. Effect of Nigella Sativa on serum testosterone levels in Letrozole Induced Polycystic Ovarian Syndrome in mice. Pakistan Armed Forces Med J. 2021;71(1):338–42. doi: 10.51253/pafmj.v71i1.3693. [DOI] [Google Scholar]
- 27.Rizwan M, Hameed A. Treatment of idiopathic facial hirsutism with medroxyprogesterone acetate iontophoresis. J Pakistan Association Dermatologists. 2016;19(2):90–4. [Google Scholar]
- 28.Schmidt JB, Huber J, Spona J. Medroxyprogesterone acetate therapy in hirsutism. Br J Dermatol. 1985;113(2):161–5. doi: 10.1111/j.1365-2133.1985.tb02059.x. [DOI] [PubMed] [Google Scholar]
- 29.Naeimi S, Tansaz M, Hajimehdipoor H, Saber S. Comparing the effect of Nigella Sativa oil soft gel versus placebo on oligomenorrhea, amenorrhea, and laboratory characteristics in patients with polycystic ovarian syndrome, a randomized clinical trial. Res J Pharmacogn. 2019;7(1):49–58. [Google Scholar]
- 30.Maskani S, Tafazoli M, Rakhshandeh H, Esmaily H. Effect of Nigella sativa seeds on the severity of symptoms of premenstrual syndrome: a randomized clinical trial. Koomesh J. 1398;22(1):33–40. [Google Scholar]
- 31.Latiff L, Hanachi P, Rahman S. A randomized control trial on the effect of black seeds (Nigella sativa) on climacteric symptoms and quality of life of perimenopausal women in Rawang, Selangor, Malaysia. Volume 44. Clinical Biochemistry - CLIN BIOCHEM; 2011.
- 32.Suzuki KM, Isohama Y, Maruyama H, Yamada Y, Narita Y, Ohta S, et al. Estrogenic activities of fatty acids and a sterol isolated from royal jelly. Evid Based Complement Alternat Med. 2008;5(3):295–302. doi: 10.1093/ecam/nem036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parhizkar S, Latiff L, Rahman S, Aziz M, Hanachi P. Assessing estrogenic activity of Nigella sativa in ovariectomized rats using vaginal cornification assay. Afr J Pharm Pharmacol. 2011;5.
- 34.Shafie I, Ibrahim R, Latiff L, Dollah M. Effect of short-term supplementation of Nigella sativa on estrogen level in ovariectomized rabbits2007.
- 35.Ismail M, Al-Naqeep G, Chan KW. Nigella sativa thymoquinone-rich fraction greatly improves plasma antioxidant capacity and expression of antioxidant genes in hypercholesterolemic rats. Free Radic Biol Med. 2010;48(5):664–72. doi: 10.1016/j.freeradbiomed.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Amini L, Tehranian N, Movahedin M, Ramezani Tehrani F, Ziaee S. Antioxidants and management of polycystic ovary syndrome in Iran: a systematic review of clinical trials. Iran J Reprod Med. 2015;13(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 37.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14(5):323–8. doi: 10.1002/1099-1573(200008)14:5<323::AID-PTR621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 38.Blagojević IP, Ignjatović S, Macut D, Kotur-Stevuljević J, Božić-Antić I, Vekić J, et al. Evaluation of a Summary score for Dyslipidemia, oxidative stress and inflammation (the Doi score) in women with polycystic ovary syndrome and its relationship with obesity. J Med Biochem. 2018;37(4):476–85. doi: 10.2478/jomb-2018-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heshmati J, Namazi N. Effects of black seed (Nigella sativa) on metabolic parameters in diabetes mellitus: a systematic review. Complement Ther Med. 2015;23(2):275–82. doi: 10.1016/j.ctim.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 40.Alamdar S, Avandi M. The Effect of high-intensity interval training with Nigella sativa supplementation on lipid profile, fasting blood sugar and body composition of overweight young women. J Sport Exerc Physiol. 2023;16:35–45. doi: 10.52547/joeppa.16.1.35. [DOI] [Google Scholar]
- 41.Naghsh N, Moridpour AH, Kavyani Z, Musazadeh V, Jafarzadeh J, Safaei E, et al. The effect of Nigella sativa (black seed) supplementation on body weight and body composition: a GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. J Funct Foods. 2023;105:105565. doi: 10.1016/j.jff.2023.105565. [DOI] [Google Scholar]
- 42.Dehkordi FR, Kamkhah AF. The antihypertensive effect of Nigella sativa seed extract in patients with mild hypertension. Fundam Clin Pharmacol. 2008;22(4):447–52. doi: 10.1111/j.1472-8206.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 43.Siddiqui A, Khan N, Naseer S. Anti-hypertensive effect of Nigella Sativa Seeds in patients with hypertension. PJMHS. 2022;16(2).
- 44.Poorzal P, MOKHTARI M, SHARIATI M. The effect of the combination of black seed and honey (docin) on the level of sex steroids and spermatogenesis following induction of hypothyroidism in adult male rats. J Anim Physiol Dev (QUARTERLY J Biol SCIENCES) 2022;15:80–96. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available but are available from the corresponding author on reasonable request.