Abstract
Reverse transcription in hepatitis B viruses is initiated through a unique protein priming mechanism whereby the viral reverse transcriptase (RT) first assembles into a ribonucleoprotein (RNP) complex with its RNA template and then initiates DNA synthesis de novo using the RT itself as a protein primer. RNP formation and protein priming require the assistance of host cell factors, including the molecular chaperone heat shock protein 90 (Hsp90). To better understand the mechanism of RT activation by Hsp90, we have now mapped the minimal RT sequences of the duck hepatitis B virus that are required for chaperone binding, RNP formation, and protein priming. Furthermore, we have reconstituted in vitro both RNP formation and protein priming using purified RT proteins and host factors. Our results show that (i) Hsp90 recognizes two independent domains of the RT, both of which are necessary for RNP formation and protein priming; (ii) Hsp90 function is required not only to establish, but also to maintain, the RT in a state competent for RNA binding; and (iii) Hsp90 is not required during RT synthesis and can activate the RT posttranslationally. Based on these findings, we propose a model for Hsp90 function whereby the chaperone acts as an active interdomain bridge to bring the two RT domains into a poised but labile conformation competent for RNP formation. It is anticipated that the reconstitution system established here will facilitate the isolation of additional host factors required for RT functions and further elucidation of the mechanisms of RT activation.
Hepatitis B virus (HBV) infection is a major public health problem, with over 300 million chronically infected people worldwide (28). Chronic HBV infection carries a great risk of developing severe liver diseases such as cirrhosis and hepatocellular carcinoma (5, 10). HBV belongs to the family Hepadnaviridae, a group of small, hepatotropic DNA viruses that infect mammalian and avian hosts. All hepadnaviruses replicate their DNA genome through an RNA intermediate (the pregenomic RNA [pgRNA]) by reverse transcription carried out by a virally encoded reverse transcriptase (RT) (13, 40, 43).
The hepadnavirus RT has several unique functional and structural properties compared to all other known RTs (for a review, see reference 20). It can specifically recognize its RNA template via a short RNA signal ɛ, located at the 5′ end of the pgRNA, and can form a stable ribonucleoprotein (RNP) complex with ɛ (34, 48). The formation of this RT-ɛ complex triggers the assembly of viral core particles, leading to the selective encapsidation of both the RT and the pgRNA into replication-competent nucleocapsids (4, 34). Thus, ɛ functions as the signal for pgRNA packaging, a process also dependent on the RT (1, 18, 19, 25, 33). In addition, the formation of this RT-ɛ complex initiates viral reverse transcription through a unique protein priming mechanism. Thus, the RT is able to initiate viral DNA synthesis de novo, using the RT itself as a protein primer and ɛ as a specific RNA template (12, 26, 32, 46–49, 52). As a result of this protein priming mechanism, the RT becomes covalently attached to the viral minus-strand DNA (3, 14, 31). To carry out these functions, the hepadnavirus RT contains a unique N-terminal domain (the terminal protein [TP]), in addition to the central RT and C-terminal RNase H domains, which are homologous to those of other RTs. Both the TP and RT domains are required for RNP formation and protein priming (34, 39, 48).
It has proven difficult to study the hepadnavirus RT functions through biochemical and structural analyses, mainly due to problems in obtaining sufficient amounts of purified RT proteins. The RT is present only at very low levels in virions and in infected host cells; efforts to extract an active RT from either viral particles or infected cells for in vitro analysis have been unsuccessful (2, 37). Similarly, attempts to express and purify an active, recombinant RT using a variety of expression systems have had only limited success (for a summary, see reference 20). The most useful system to date has been a cell-free translation system developed using the duck hepatitis B virus (DHBV) RT. The DHBV RT, expressed in a rabbit reticulocyte lysate in vitro translation system, is active in both RNP formation and protein priming (46–48, 52). With the help of this cell-free system, we have recently shown that specific cellular factors, including components of the molecular chaperone complex, heat shock protein 90 (Hsp90), are required for the RT to carry out these functions (21, 23). However, only very small amounts of RT proteins can be produced in this cell-free translation system. Recombinant baculoviruses have also been used to express the HBV RT in insect cells at relatively high levels (26, 27, 45). However, it has not yet been possible to reconstitute RT-ɛ interaction and ɛ-dependent protein priming activity in vitro using the insect cell-expressed RT. These limitations have hampered a systematic search for additional factors required for RT functions, as well as biochemical and structural analyses of the mechanisms of RT activation by host factors.
Hsp90 is required for the activities of a specific subset of cellular proteins (so-called target or substrate proteins) which are diverse both in structure and function (9, 24, 35, 36). In facilitating the functions of its various target proteins, Hsp90 forms multicomponent chaperone complexes with different cofactors (cochaperones), which seem to vary depending on the particular target protein. The molecular basis of specific target recognition by Hsp90 is still not well understood. We have shown previously that both Hsp90 and one of its cofactors, p23, can bind specifically to the RT (21, 23) even though the RT does not share any apparent sequence similarities to other known Hsp90 target proteins. In addition, we proposed earlier that the role of Hsp90 in RT functions is to facilitate the conformational (and functional) maturation of the RT to a state that is competent for ɛ binding and protein priming (21, 23). This notion is consistent with the known functions of Hsp90 as a molecular chaperone; it is not clear, however, what such an RT conformation may be or how the chaperone may help to establish that conformation. It is also unknown when Hsp90 exerts its effect on the RT during RT synthesis and maturation. The fact that the RT preferentially interacts with and packages the pgRNA from which it is translated (cis preference) (1, 18) suggests that the RT may become competent for ɛ binding even while it is still being translated. This, in turn, implies that Hsp90 may activate the RT cotranslationally. On the other hand, Hsp90 is thought to act at a relatively late stage during the functional maturation of the steroid hormone receptors, the best-studied Hsp90 target proteins (9, 36). Furthermore, the chaperone components that we have identified so far are not sufficient for RT activation (21, 23), suggesting that an additional chaperone cofactor(s) required for RNP formation and protein priming remains to be identified.
To begin to address these questions, we have defined the RT sequences that are required for chaperone association, ɛ RNA binding, and protein priming. With this information, we were then able to express and purify two minimal RT proteins using bacterial as well as eukaryotic expression systems. Using the purified RT proteins, we were able to develop a cell-free, Hsp90-dependent RT activation system and to reconstitute both the RT-ɛ interaction and protein priming in vitro. We present here an initial analysis of the mechanisms of RT-Hsp90 interaction and RT activation using the newly established reconstitution system. Our results suggest a model for Hsp90 function whereby the chaperone acts as an active bridge to bring together the two domains of the RT necessary for ɛ binding, thus helping to establish and maintain a poised but unstable RT conformation competent for RNP formation and protein priming.
MATERIALS AND METHODS
Plasmids.
The DHBV RT, tagged with a synthetic hemagglutinin (HA) epitope inserted into the nonessential spacer region between the TP and RT domains, was expressed in vitro in the reticulocyte lysate translation system from plasmid pHTP (52). C-terminally truncated RT mutants were translated from pHTP that was linearized at various restriction sites within the RT coding sequences. The internal deletion mutant pHTP-dBX was derived from pHTP by removing codons 74 (at the unique BglII site) to 352 (XhoI site) of the RT coding sequence. pcDNA-miniRT1 encodes an RT polypeptide that has deletions from the N terminus (amino acids 1 to 74), the C terminus (amino acids 734 to 786), and the spacer region (amino acids 245 to 352). Similarly, pcDNA-miniRT2 encodes a short RT polypeptide that has the same N-terminal and spacer deletions as that encoded by pcDNA-miniRT1 but a more extensive C-terminal truncation (from amino acid 575 to 786). Both constructs contained c-Myc epitope tags fused to the C termini of the RT proteins, in addition to the internal HA tag derived from pHTP. Both of these were constructed using the pcDNA3 (Invitrogen) vector, and they were used to express the mini-RT proteins in vitro and in hepatoma cells.
For bacterial expression, the miniRT1 and miniRT2 cassettes were subcloned into pGEX-KT (16) in order to produce glutathione S-transferase (GST)–MiniRT1 and GST-MiniRT2, respectively, or into pQE30 (Qiagen) to produce His-MiniRT1 and His-MiniRT2, respectively. The N termini of the mini-RT proteins were fused in frame to either the GST encoded by pGEX-KT or to a stretch of six histidine residues encoded by pQE30.
The DHBV RT mutant CA29 harbors two amino acid substitutions that render the RT defective in ɛ RNA binding (39). The same substitutions were cloned into the mini-RT constructs to make MiniRT1/CA29 and MiniRT2/CA29.
Antibodies and reagents.
The monoclonal antibody (MAB) against Hsp90 (clone 3G3) was purchased from Affinity Bioreagents. The MAB against p23 (clone JJ3) was generously provided by David Toft (Mayo Clinic). The MABs against DnaK and GroEL were purchased from Stressgen. The goat anti-mouse immunoglobulin G (IgG), control mouse IgG, and anti-c-Myc epitope MAB (clone 9E10) were purchased from Sigma. The anti-HA MAB (clone 12CA5) was purchased from Berkeley Antibody. The antibiotic geldanamycin was obtained from the Drug Synthesis and Chemistry Branch, National Cancer Institute.
In vitro transcription and translation.
RNAs used for in vitro translation were transcribed from linearized plasmids using an in vitro transcription kit (MEGAscript; Ambion) and purified as described before (21, 47). Purified RNAs were then translated using the rabbit reticulocyte lysate in vitro translation system (Promega). Protein expression in the coupled transcription and translation reaction using the TnT rabbit reticulocyte lysate system (Promega) was carried out according to the manufacturer's instructions.
For the synthesis of the DHBV ɛ RNA, a synthetic DNA template containing the SP6 polymerase promoter and the ɛ coding sequences was transcribed with the in vitro transcription kit, as described before (21). A minimal ɛ RNA (44 nucleotides long, from nucleotide 2565 to 2609) (30), with a deletion of 6 bp and a single unpaired nucleotide from the bottom of the lower stem of the predicted ɛ secondary structure (22, 48), was used throughout this study. Preliminary results showed that this shorter ɛ RNA behaved identically to the longer ɛ RNA used in our previous studies (21, 23) with respect to RT binding and protein priming in vitro, as also observed by others (6).
Immunoprecipitation.
A mixture of two MAbs, anti-HA and anti-c-Myc, was bound to the protein A/G resin (Pierce) and used to immunoprecipitate the tagged mini-RT proteins from the in vitro translation reactions, as described before (21). The binding and wash buffer used was the radioimmunoprecipitation assay buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 0.05% NP-40, 10% glycerol, 2 mM dithiothreitol (DTT), and a cocktail of protease inhibitors [Protease Inhibitors Complete; Boehringer Mannheim]). Hsp90 or p23 with its associated RT proteins was immunoprecipitated from the in vitro translation reactions using Hsp90-specific MAB 3G3 or p23 MAB JJ3 under detergent-free conditions (homogenization buffer, 50 mM KPO3 [pH 7.4]–10 mM thioglycerol–10 mM sodium molybdate), as described previously (21).
Sucrose gradient centrifugation.
For sedimentation under standard conditions, the in vitro translation reaction mixtures were diluted 20-fold in TNMN buffer (50 mM Tris [pH 7.5], 100 mM NaCl, 5 mM MgCl2, 0.02% NP-40) and layered over a linear 20 to 40% sucrose gradient made in TNMN. The gradient was then centrifuged at 40,000 rpm at 4°C for 4 h in a Beckman SW41 rotor. To dissociate the RT complexes, the translation reaction mixtures were first diluted 20-fold into a high-salt and -detergent buffer (same as TNMN except with 1 M NaCl and 1% NP-40). The samples were then fractionated as described above except that the sucrose gradient was made in the high-salt and -detergent buffer. Individual fractions were then collected and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The 35S-labeled RT was detected by autoradiography.
Bacterial expression and protein purification.
Plasmids encoding GST-MiniRT1, GST-MiniRT2, His-MiniRT1, and His-MiniRT2 were transformed into protease-deficient bacterial strain BL21. The cells were grown to an optical density at 600 nm of 0.5 to 1, and mini-RT expression was then induced by adding isopropyl-β-d-thiogalactopyranoside to a final concentration of 0.5 to 1 mM. The cultures were continued for another hour to overnight at room temperature before harvest. The cells were then pelleted and lysed by sonication in lysis buffer (50 mM Tris [pH 8.0], 500 mM NaCl, 0.5% Triton X-100, 10% glycerol, 10 mM DTT, and a cocktail of protease inhibitors [Protease Inhibitors Complete; Boehringer Mannheim]). The mini-RT proteins were then purified from the soluble fraction (clarified lysate) by affinity purification. Glutathione-agarose beads (Sigma) were used for purifying the GST fusion proteins and Ni+-nitrilotriacetic acid resins (Qiagen) were used for purifying the six-histidine-tagged proteins, according to the manufacturer's instructions. To further separate the full-length fusion proteins from degradation products, the eluates from the glutathione or Ni+ beads were then subjected to a second immunoaffinity purification step by immunoprecipitation with the anti-c-Myc antibody. As the GST (or the six-histidine tag) and the c-Myc epitope were fused to the N and C termini of the mini-RT proteins, respectively, this sequential two-step purification could effectively separate the full-length fusion proteins from degradation products truncated from either end.
Reconstitution of protein priming and ɛ binding with purified mini-RT.
The purified mini-RT proteins used for in vitro reconstitution included the following: (i) immunoprecipitate from the in vitro translation reactions (still bound to the immunoaffinity resin), (ii) eluate from the glutathione or Ni+ affinity resins, and (iii) immunoprecipitate from the eluate described in item ii. In vitro protein priming and ɛ binding reactions were performed as previously described (21, 47), with the following modifications. The reaction buffer used for both the protein priming and RNA binding reactions was TMNK (20 mM Tris [pH 7.5], 2 mM MgCl2, 15 mM NaCl and 20 mM KCl, 2 mM DTT, 10 mM sodium molybdate). Following the RNA binding reaction, the 32P-labeled ɛ RNA was detected directly by resolving the ɛ-RT complex using SDS-PAGE (15% acrylamide) and autoradiography, omitting the RNA purification steps of phenol extraction and ethanol precipitation. We found it unnecessary to separate the RNA from the immunoprecipitates since the 32P-labeled ɛ ran towards the bottom of the gel and was well resolved by SDS-PAGE. Various supplements were added to the protein priming or RNA binding reactions; these included rabbit reticulocyte lysate and an ATP regenerating system consisting of 5 mM ATP, 10 mM creatine phosphate, and 50 μg of creatine phosphokinase/ml (21).
RESULTS
Mapping of RT sequences required for chaperone association, ɛ binding, and protein priming.
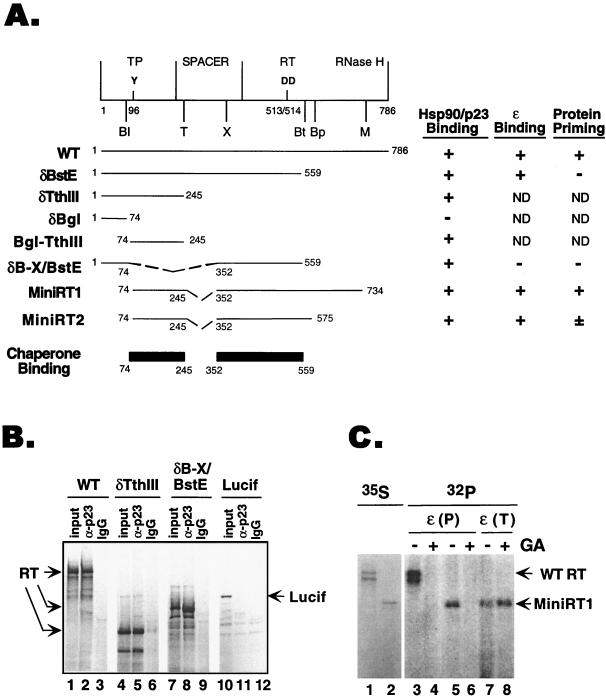
As a first step towards understanding the molecular basis for the RT-chaperone interaction, we sought to delineate the regions of the RT that can mediate chaperone binding. To this end, we translated the full-length RT and various RT deletion mutants in reticulocyte lysate and determined their association with the two components of the chaperone complex that we have previously shown to bind the RT, i.e., Hsp90 and p23 (21, 23). As shown in Fig. 1A and B, two segments of the DHBV RT could each independently mediate binding to the chaperone proteins, as determined by immunoprecipitation using Hsp90- and p23-specific MAbs. Both segments were approximately 200 residues long and were localized to the N-terminal TP and the central RT domains, respectively. We also assayed for the abilities of the various deletion mutants to bind ɛ RNA and carry out protein priming. Consistent with previous reports (34, 39, 48), ɛ binding required part of the TP and a short segment from the RT domain whereas protein priming required the TP and a longer sequence from the RT and RNase H domains.
FIG. 1.
RT sequences required for chaperone association, ɛ binding, and protein priming. (A) Schematic diagram (top) of the domain structure of the DHBV RT. The primer tyrosine (residue 96) in the TP domain and the two aspartic acids (residues 513 and 514) at the RT active site are indicated, as are the unique restriction sites used to construct the various deletion mutants: Bl, BglII; Bp, BspHI; Bt, BstEII; M, MscI; T, Tth111I; X, XhoI. Below are the activities of the wild-type (WT) RT and the various truncation and deletion RT mutants in chaperone binding, ɛ binding, and protein priming, as determined by coimmunoprecipitation and in vitro protein priming assays (see Materials and Methods). The two independent chaperone-binding regions of the RT are shown schematically. ND, not determined. (B) Examples of coimmunoprecipitation of full-length RT and deletion variants of the RT with p23. δTth111I harbored the N-terminal chaperone-binding region in the TP domain, and δB-X/BstE contained the central chaperone-binding region in the RT domain (A). The RT proteins, along with the luciferase protein (Lucif; used as a negative control for immunoprecipitation), were translated in reticulocyte lysate, and the translation reaction mixtures were subjected to immunoprecipitation with a MAB (clone JJ3) specific for p23 (α-p23; lanes 2, 5, 8, and 11) or a nonimmune control antibody (IgG; lanes 3, 6, 9, and 12). The immunoprecipitates, along with the translation reaction products (input; lanes 1, 4, 7, and 10), were resolved by SDS-PAGE and the 35S-labeled RT proteins were detected by autoradiography. (C) WT RT and MiniRT1 were translated in reticulocyte lysate, and their protein priming activities were assessed by the in vitro priming assay, with or without treatment with Hsp90 inhibitor geldanamycin (GA). Lanes 1 and 2, 35S-labeled translation product (lane 1, WT RT; lane 2, MiniRT1); lanes 3 to 8, 32P-labeled WT RT (lanes 3 and 4) and mini-RT (lanes 5 to 8) as a result of the protein priming reaction in the presence of [α-32P]dATP. Following translation but before the priming reaction, aliquots of the translation reaction mixture, as indicated, were treated with GA (100 μg/ml). ɛ was added during either the translation [i.e., before GA treatment; ɛ(T)] or priming [i.e., after GA treatment; ɛ(P)] step.
Based on these results and previous mutagenesis studies (34, 39, 48), we reasoned that it may be possible to express an RT polypeptide that is significantly shorter than full length while still retaining its ɛ binding and protein priming activities. Therefore, we constructed two mini-RT expression cassettes that produced N- and C-terminal truncations and internal deletions in the spacer region (Fig. 1A). MiniRT1 is 564 residues long (compared to 786 residues for the wild type), and MiniRT2 is 394 residues long, having a more extensive C-terminal truncation. As shown in Fig. 1C, MiniRT1 was as active as the wild-type RT in protein priming. The shorter MiniRT2 was fully active in ɛ binding but showed minimal activity (1 to 5% of that of the wild type) in protein priming. Both of these mini-RT proteins harbored the two chaperone-binding regions delineated above and, as expected, were associated with Hsp90 and p23 (Fig. 1A and 2B). Furthermore, like the full-length RT, they required the chaperone function for ɛ binding and protein priming. A specific inhibitor of Hsp90, geldanamycin (41, 50), diminished the protein priming activity of MiniRT1 when it was added before ɛ binding (Fig. 1C, lanes 3 to 6). However, when geldanamycin was added after ɛ had already bound to the mini-RT (during the translation reaction), it had no effect on protein priming (Fig. 1C, lanes 7 and 8). These results thus indicate that the drug specifically blocked the interaction between the ɛ RNA and the mini-RT but had no effect on DNA synthesis per se, just as we have observed previously with the full-length RT (21, 23).
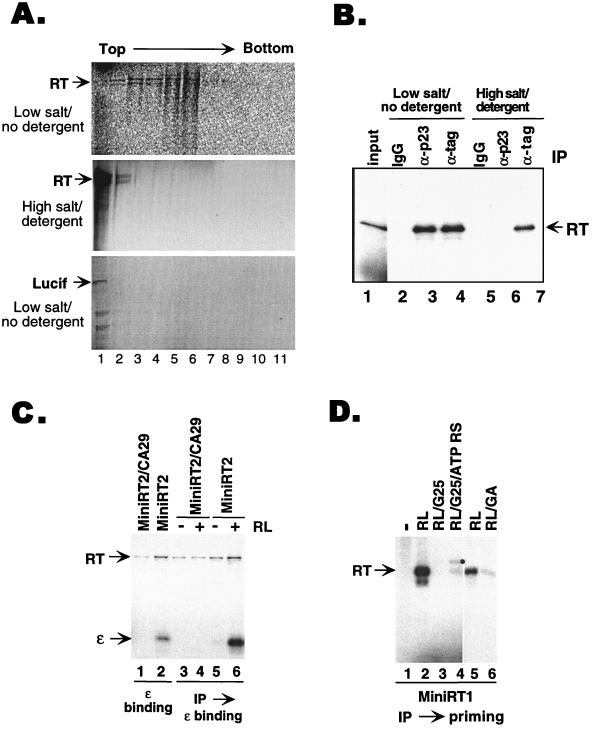
FIG. 2.
Purification of the RT following in vitro translation and reconstitution of ɛ binding and protein priming. (A) Dissociation of the RT complexes. 35S-labeled RT (upper and middle sections) and luciferase (Lucif) (lower section) were synthesized in reticulocyte lysate and centrifuged over a linear 20 to 40% sucrose gradient at 40,000 rpm for 4 h in an SW41 rotor. For the RT sample shown in the middle section, the translation reaction mixture was first treated with 1 M NaCl and 1% NP-40 before centrifugation over the sucrose gradient made in the same high-salt and -detergent buffer. Individual fractions were then collected and resolved by SDS-PAGE. The direction of centrifugation is indicated. (B) Dissociation of RT-p23 binding. MiniRT1, tagged with the c-Myc and HA epitopes, was expressed in TnT reticulocyte lysate (lane 1) and immunoprecipitated (IP) with either a control IgG (lanes 2 and 5), an anti-p23 MAb (lanes 3 and 6), or a mixture of anti-c-Myc and anti-HA (lanes 4 and 7) antibodies. The binding and washing buffers contained either 50 mM phosphate buffer–10 mM sodium molybdate (lanes 2 to 4) or 0.5 M NaCl–0.2% NP-40 (lanes 5 to 7). The immunoprecipitated, 35S-labeled mini-RT was then resolved by SDS-PAGE and detected by autoradiography. (C) Immunoaffinity purification of mini-RT proteins and reconstitution of ɛ binding. MiniRT2 and the corresponding ɛ-binding-defective mutant, MiniRT2/CA29, were expressed in TnT reticulocyte lysate supplemented with [35S]methionine and purified by immunoprecipitation under high-salt and -detergent conditions (as described for panel B). 32P-labeled ɛ RNA was then incubated with the purified RT proteins with (lanes 4 and 6) or without (lanes 3 and 5) the addition of reticulocyte lysate. Unbound RNA was washed away, and the bound ɛ was detected following SDS-PAGE (lanes 3 to 6). As controls, labeled ɛ RNA was added to the translation reaction mixtures and thus allowed to bind to RT during translation; the RT-ɛ complex was then immunoprecipitated and detected by SDS-PAGE and autoradiography (lanes 1 and 2). (D) Reconstitution of protein priming. In vitro-translated MiniRT1 was isolated by immunoprecipitation under high-salt and -detergent conditions. The ɛ RNA was then added to the purified RT to initiate protein priming. The 32P-labeled priming reaction products were detected by autoradiography following SDS-PAGE. The priming reactions were carried out with the following supplement: nonsupplemented control (lane 1), unfractionated reticulocyte lysate (RL; lanes 2 and 5), reticulocyte lysate desalted by passing through a Sephadex G-25 column (RL/G25; lane 3), desalted reticulocyte lysate supplemented with an ATP regenerating system (see Methods and Materials) (RL/G25/ATP RS; lane 4), reticulocyte lysate plus geldanamycin (100 μg/ml) (RL/GA; lane 6). Solid circle (lane 4) reaction product observed sometimes under the indicated conditions, the nature of which is currently unknown.
In summary, these results showed that the Hsp90 chaperone could recognize specifically two independent regions in the TP and RT domains. Furthermore, minimal RT proteins bearing only these two chaperone-binding regions retained full ɛ binding activity. Additional sequences outside the chaperone-binding regions (in the RT and RNase H domains) were required for protein priming, most likely due to the additional requirement for catalysis of DNA synthesis (39, 48).
Continuous requirement of Hsp90 for the maintenance of RT functions.
We reported previously that Hsp90 is required to establish an RT state that is active in ɛ binding and protein priming (21, 23). However, it was not known whether Hsp90 association and function would also be required to maintain such an RT state once it has been established. To address this issue, we attempted to separate the RT from associated chaperone proteins and determine the ability of the purified RT to carry out ɛ binding and protein priming.
As expected, most of the RT protein expressed in vitro fractionated on a sucrose gradient as high-molecular-weight complexes (Fig. 2A, upper section) due to its association with the Hsp90 chaperone complex (and possibly other yet to be identified high-molecular-weight complexes). To strip these complexes from the RT, we treated the in vitro-translated RT with high concentrations of salt and nonionic detergent (see Materials and Methods for details), conditions known to dissociate the Hsp90 complex from other target proteins (8, 38). Indeed, these treatments released the RT from its associated cellular proteins. Thus, the RT could no longer be immunoprecipitated with anti-p23 (Fig. 2B) or anti-Hsp90 antibodies (data not shown), and it sedimented on the sucrose gradient like the luciferase (61 kDa) (Fig. 2A, middle and lower sections). Importantly, the RT remained soluble upon dissociation from the chaperone and there was no evidence of RT aggregation under these conditions. In order to isolate the small amounts (in the nanogram range) of RT generated in vitro following dissociation from Hsp90, we attached two epitope tags to the mini-RT proteins, the HA epitope (inserted into the spacer region) and the c-Myc epitope (attached to the C terminus). By using a mixture of anti-HA and anti-c-Myc antibodies and the high-salt and -detergent conditions, we were able to efficiently immunoprecipitate (30 to 50% efficiency) the mini-RT proteins expressed in the reticulocyte lysate.
We then tested the ability of the purified mini-RT proteins to bind to ɛ RNA and to carry out protein priming. We found that the isolated RT proteins, free of their associated cellular factors, lost their ability to bind ɛ (Fig. 2C, lane 5) and to carry out protein priming (Fig. 2D, lane 1). However, by adding the reticulocyte lysate back to the purified mini-RT, we were able to reconstitute both ɛ binding (Fig. 2C, lane 6) and protein priming activities (Fig. 2D, lane 2), indicating that the RT proteins were not inactivated irreversibly by the purification process. In fact, reconstitution was efficient, as the ɛ binding and protein priming activities detected following reconstitution were at least as high as those assayed directly in the translation reaction without purification (Fig. 2C, lane 6 versus lane 2, and data not shown). We also purified the full-length RT and reconstituted its ɛ binding and protein priming activities using the same approach; immunoprecipitation of the full-length RT (approximately 5%) was not as efficient as that of the mini-RT proteins, possibly due to the masking of the inserted epitopes by the additional RT sequences (data not shown). To confirm that the ɛ binding activity detected in the reconstitution reaction was dependent on a functional RT, we introduced two amino acid substitutions (CA29) into MiniRT2 to make MiniRT2/CA29. These substitutions have been shown previously to abolish the ɛ binding activity of the RT (39) (Fig. 2C, lane 1). As expected, the purified MiniRT2/CA29 was unable to bind ɛ even after reconstitution with the reticulocyte lysate, indicating that the ɛ binding activity detected in our reconstitution assay indeed represented a specific RT-ɛ interaction. Furthermore, activation of the purified RT by reconstitution was dependent both on Hsp90 and on ATP. Thus, inhibition of Hsp90 function by geldanamycin (Fig. 2D, lane 6) or depletion of ATP (Fig. 2D, lane 3) diminished RT activation and supplementing the ATP-depleted lysate with an ATP regenerating system restored reconstitution (Fig. 2D, lane 4).
In summary, we were able to efficiently separate the RT expressed in vitro from its associated cellular factors. The purified RT lost its ability to bind ɛ and to carry out protein priming. Both of these activities could be restored upon reconstitution with the reticulocyte lysate in an Hsp90- and ATP-dependent fashion. These results thus indicate that an Hsp90-mediated, dynamic process is required not only to establish an ɛ-binding-competent state of the RT but also continuously to maintain this functional state.
Reconstitution of a functional RT following bacterial expression and purification: posttranslational RT activation by Hsp90.
Our success in reconstituting RT activities using the mini-RT proteins purified from the in vitro translation system prompted us to test whether it would be possible to purify the RT from bacteria and reconstitute its activities using similar approaches. To this end, we fused the N termini of the two mini-RT proteins to GST or a stretch of six histidines. The GST–mini-RT and six-His–mini-RT fusion proteins were then purified by using the glutathione and Ni+ affinity resins, respectively. Similar results were obtained with the mini-RT proteins purified using either method. However, the GST fusion proteins proved to be more easily purified, and the results reported below were obtained using the purified GST–mini-RT proteins.
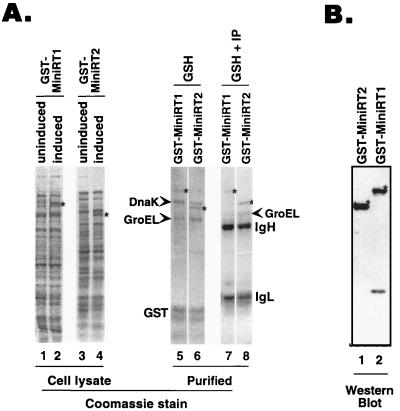
Following one-step purification using the glutathione resins, the full-length GST-RT fusion proteins (Fig. 3A, lanes 5 and 6, and B), some degradation products (particularly GST), and two prominent bacterial proteins (see below) were purified. We attempted to remove these degradation products and the bacterial proteins by a second step involving immunoprecipitation using antibodies against the C-terminal c-Myc epitope tag and were able to eliminate most but not all of the bacterial proteins (Fig. 3A, lanes 7 and 8).
FIG. 3.
Bacterial expression and purification of mini-RT. (A) Two GST mini-RT fusion proteins, GST-MiniRT1 and GST-MiniRT2, were expressed in BL21 cells as described in Materials and Methods. Uninduced (lanes 1 and 3) and induced (lanes 2 and 4) bacterial lysates were prepared. The fusion proteins were first purified using glutathione-agarose beads (lanes 5 and 6) and then further purified by immunoprecipitation (IP) with the anti-c-Myc antibody (lanes 7 and 8). The lysate and purified protein samples were resolved by SDS-PAGE and stained with Coomassie blue. Stars, full-length mini-RT fusion proteins; arrowheads, two major copurifying bacterial proteins (DnaK and GroEL; see Fig. 5). The Ig heavy (IgH) and light chains (IgL) and GST are also indicated. (B) GST-MiniRT1 (lane 2) and GST-MiniRT2 (lane 1) purified on glutathione-agarose beads were resolved by SDS-PAGE and detected by Western blot analysis using the anti-c-Myc antibody. Stars, full-length mini-RT fusion proteins.
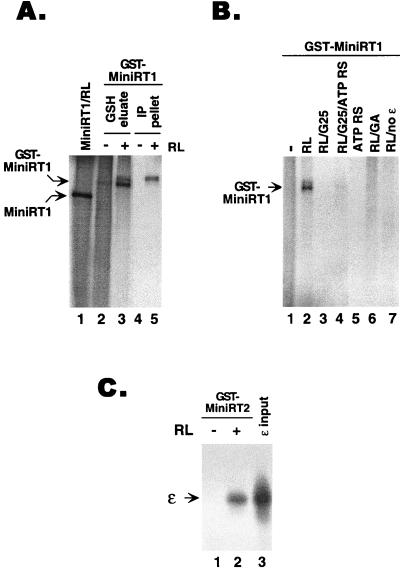
We anticipated that the mini-RT proteins purified from bacteria would be inactive in either ɛ binding or protein priming, due to the absence of a functional Hsp90 chaperone and possibly other eukaryotic cellular factors necessary for RT activities. Indeed, the purified GST–mini-RT proteins showed little or no ɛ binding or protein priming activity. Moreover, the addition of eukaryotic cellular factors from the reticulocyte lysate to the purified RT proteins could reconstitute both the ɛ binding (Fig. 4C) and the protein priming reactions (Fig. 4A and B). We routinely used 10 to 20 ng of purified RT proteins (as estimated by Coomassie blue staining and Western blotting) in the ɛ binding and protein priming assays. Based on quantitative analyses, we estimated that the specific activities, upon reconstitution, of the purified GST-RT fusion proteins in both ɛ binding and protein priming were approximately 20 to 30% of that of the RT expressed in reticulocyte lysate, indicating that reconstitution of RT activities was fairly efficient albeit incomplete. As would be expected for authentic RT activities, protein priming was dependent on the presence of ɛ RNA (Fig. 4B; compare lanes 2 and 7). Furthermore, RT activation also required the chaperone Hsp90, as specific Hsp90 inhibitor geldanamycin could block RT activation by reticulocyte lysate (Fig. 4B, lane 6). In addition, RT activation required ATP. Depletion of ATP from the reticulocyte lysate eliminated its ability to activate the RT (Fig. 4B, lane 3) and adding back ATP restored RT activation (Fig. 4B, lane 4). Interestingly, reconstitution of the desalted reticulocyte lysate with ATP only partially restored its ability to activate the RT (Fig. 4B, lane 4 versus lane 2, and 2D, lanes 2 and 4). This may suggest that an additional small molecule(s) removed by the desalting procedure may also be required for RT activation, and we are currently investigating this possibility.
FIG. 4.
Reconstitution of RNA binding and protein priming with mini-RT purified from bacteria. (A) GST-MiniRT1, purified either by glutathione-agarose beads (lanes 2 and 3) or by glutathione beads plus the second step of immunoaffinity purification (lanes 4 and 5), was used for the in vitro protein priming reaction in the presence of ɛ and [α-32P]dATP, with (lanes 3 and 5) or without (lanes 2 and 4) supplementation with reticulocyte lysate (RL). As a positive control, MiniRT1 (without the GST fusion) was translated in reticulocyte lysate and assayed in the priming reaction (lane 1). The 32P-labeled RT was then detected by SDS-PAGE and autoradiography. (B) Purified GST-MiniRT1 was used in the protein priming reaction as described for panel A, with the indicated supplements. Abbreviations are as defined in the Fig. 2D legend. RL/no ɛ, priming reaction mixture supplemented with reticulocyte lysate but without ɛ RNA. (C) Purified GST-MiniRT2 was used in the ɛ RNA binding assay as described for Fig. 2C, except that GST-MiniRT2 was bound to glutathione-agarose beads. 32P-labeled ɛ RNA precipitated by the RT-bound beads was then detected by SDS-PAGE and autoradiography. Reticulocyte lysate was added as indicated. Lane 3, RNA input.
In summary, we have successfully expressed two minimal RT proteins in bacteria and purified them using affinity tags. When reconstituted with eukaryotic cell extract, the purified RT proteins gained both specific ɛ RNA binding and protein priming activities. Reconstitution of the RT activities was an ATP-dependent process and required Hsp90 function. As bacteria do not have a functional eukaryotic Hsp90 chaperone system (9, 29), these results further indicated that Hsp90 function was not required during RT synthesis but rather could act posttranslationally to activate the RT.
Association between the RT and the bacterial chaperones GroEL and DnaK.
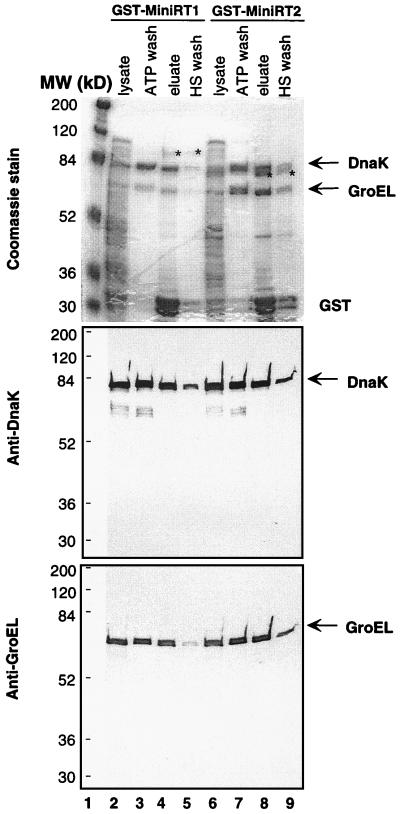
During the process of purifying the mini-RT proteins expressed in bacteria, we noticed that two prominent bacterial proteins copurified with the RT when the GST affinity and immunoaffinity beads were used (Fig. 3A, lanes 5 to 8, and Fig. 5). Their apparent sizes, approximately 70 and 57 kDa, and the fact that the RT associates with eukaryotic chaperones prompted us to determine whether these might be the two abundant bacterial chaperone proteins, the 70-kDa DnaK and the 57-kDa GroEL (11, 17). Immunoblot analysis using specific antibodies confirmed that these two proteins were indeed DnaK and GroEL (Fig. 5).
FIG. 5.
Association of DnaK and GroEL with RT. GST-MiniRT1 and -MiniRT2 were expressed in BL21 cells and purified by using glutathione beads. The bacterial lysate (lysate) and different fractions from the purification protocol were then analyzed by Coomassie staining (top) or Western blotting using anti-DnaK (middle) or anti-GroEL (bottom). ATP wash, proteins washed off the beads following a 30-min incubation with ATP (5 mM) at room temperature; eluate, proteins eluted from the beads by glutathione; HS (high salt) wash, proteins washed off the beads by 3 M NaCl (used to regenerate the affinity resin). Stars, mini-RT fusion proteins; arrows, DnaK and GroEL.
Several observations indicated that these two bacterial chaperone proteins were tightly associated with the mini-RT proteins rather than simply copurifying with the RT by chance. First, both DnaK and GroEL copurified with the mini-RT fusion proteins (but not the affinity tags alone) regardless of the affinity tags or resins used. Thus, the mini-RT proteins were tagged with GST, the six His residues, the HA and c-Myc epitopes, and the chitin binding domain. These different fusion proteins were purified using four different affinity resins (glutathione, Ni+, anti-HA and anti-myc, and chitin, respectively) under nondenaturing conditions (see Materials and Methods). In each case, DnaK and GroEL copurified with the mini-RT (Fig. 3A and 5 and data not shown). Second, when the GST–mini-RT fusion proteins were purified under conditions where the strong ionic detergent Sarkosyl was used, little or no DnaK or GroEL copurified (data not shown), most likely because their association with the RT was disrupted by the strong detergent. Third, substantial amounts of DnaK and GroEL associated with the RT could be released by a brief treatment with ATP (Fig. 5, lanes 3 and 7), which is known to disrupt the binding of these chaperones to other proteins (11, 17, 51). A more extensive wash with ATP could almost completely remove the two bacterial proteins from the mini-RT (data not shown). Together, these results indicate that bacterial chaperone proteins DnaK and GroEL associated with the mini-RT proteins tightly and, as discussed later, may play a role in RT folding and function.
DISCUSSION
Recent studies demonstrated that the hepadnavirus RT requires the assistance of specific host cofactors, including the Hsp90 chaperone and its cofactors, in order to interact with ɛ and to carry out protein priming (21, 23). The experiments reported here were carried out in order to gain a better understanding of the molecular basis for the specific interaction between the chaperone and the RT and the mechanisms of RT activation by the chaperone. Our results showed that (i) two RT segments located in the TP and the RT domains can each mediate Hsp90 and p23 binding independently; (ii) Hsp90 function is required not only to establish, but also to maintain, the RT in an ɛ-binding-competent state; and (iii) Hsp90 is not required during RT synthesis and can activate the RT posttranslationally. These findings were made possible largely due to the development of a biochemical reconstitution system for RT-ɛ interaction and protein priming, which we anticipate will facilitate future studies on these critical steps in hepadnavirus assembly and replication.
One of the important, unresolved issues in the Hsp90-RT interaction and in Hsp90 function in general is the molecular basis for the specific recognition by Hsp90 of the RT and other target proteins, including the steroid receptors and certain protein kinases (9, 24, 35). These various target proteins differ greatly in both structure and function. The two independent chaperone-binding regions of the RT that we have now defined do not have any apparent sequence similarity with other Hsp90 target proteins or, for that matter, with each other. One possibility arising from studies on the kinase targets is that distinct Hsp90 chaperone complexes exist in the cell, each with a specific “targeting” cofactor, which directs each distinct Hsp90 complex to its cognate substrate. For example, Hsp90 cofactor p50/CDC37 can specifically bind to certain protein kinases, e.g., CDK4 and Raf, and is thought to target a Hsp90–p50 complex to its protein kinase substrates (15, 42). If this is the case for Hsp90 targeting to the RT, two distinct targeting cofactors would presumably be required to target Hsp90 to the two distinct domains of the RT. Alternatively, the Hsp90 complex may recognize some as yet unknown structural properties shared by the various targets, including the two distinct domains of the RT, such as an intrinsic structural instability or some common folding characteristics. The resolution of this important issue will likely require the identification of all the essential components of each chaperone complex and high-resolution structural studies.
Although each of the two chaperone-binding regions of the RT can bind independently to the chaperone, both of these together are required for ɛ binding. Indeed, a minimal RT protein (MiniRT2) consisting only of these two segments (less than 400 amino acids long) can bind ɛ RNA as efficiently as the full-length RT in a chaperone-dependent manner. These findings lead us to suggest a plausible model for how Hsp90 may facilitate the RT-ɛ interaction (Fig. 6). We propose that the Hsp90 chaperone complex, by binding both to the TP and to the RT domains of the viral polymerase, acts to mediate interdomain interactions between these two regions. In doing so, the chaperone functions as a dynamic bridge to help bring the amino acid residues critical for ɛ recognition in both of these domains (34, 39, 48) into precise spatial arrangement for specific ɛ binding and protein priming.
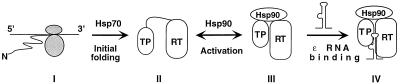
FIG. 6.
Model of RT folding and activation by cellular chaperones. The nascent RT polypeptide (I) exiting the translating ribosomes is first recognized by Hsp70 (or DnaK when expressed in bacteria). With Hsp70 assistance, the TP and RT domains can then partially fold (initial folding; II), independent of each other. However, the RT remains incompetent for ɛ binding. The Hsp90 complex then recognizes the partially folded TP and RT domains and brings them together to establish an RT conformation able to bind ɛ (activation; III). This RT conformation is intrinsically unstable and requires the continued assistance of Hsp90 to maintain it. Finally, ɛ binding stabilizes this RT conformation while Hsp90 remains bound to the RT (IV).
The definition of the minimal functional domains of the RT and the ability to strip the RT of its associated cellular proteins allowed us to purify mini-RT proteins using both in vitro translation and bacterial expression systems. Our results showed that the purified RT proteins, dissociated from the chaperone complex, lost their ability to recognize ɛ and to carry out protein priming and required reconstitution with the chaperone to regain these activities. We also attempted to express the same mini-RT proteins in hepatoma cells that can support efficient viral replication. Again, we found that the mini-RT proteins, even when expressed in authentic host cells, still could not maintain their activities when stripped of the necessary host factors and required in vitro reconstitution to recover the ɛ binding and protein priming activities (J. Hu, unpublished results). These results thus indicate that Hsp90 is required not only to establish but also to maintain the RT in a state competent for ɛ binding (Fig. 6). Also, since the RT dissociated from the chaperone showed no evidence of aggregation, Hsp90 apparently was not required to keep the RT in a soluble (and hence, at least partially folded) state. Furthermore, since the mini-RT proteins expressed in bacteria, in the absence of a functional Hsp90 chaperone system, could also be activated by Hsp90 following purification, the chaperone does not appear to have an essential cotranslational role in RT activation.
Taken together, these results point to a distinct, posttranslational role for Hsp90 in establishing and maintaining a specific functional conformation of the RT, rather than acting simply as a “generic chaperone” to keep the RT from aggregating. This notion agrees well with the interdomain bridging model proposed above (Fig. 6). Thus, the TP and RT domains may be able to fold to certain degrees independently of Hsp90, perhaps with the assistance of other host chaperones (see below). Subsequently, the Hsp90 complex may recognize the partially folded TP and RT domains and bring them together to establish a functional, ɛ-binding-competent conformation. This posttranslational, late-folding role for Hsp90 in RT function is consistent with the current view that Hsp90 mainly recognizes some late, metastable folding intermediates in the folding pathways of its target proteins (9, 35). Such folding intermediates, for Hsp90 target proteins such as the RT, are apparently labile and require the continuous assistance of the chaperone to maintain them in so-called “poised” states (7) ready for interaction with specific partners or ligands (e.g., the ɛ RNA). This proposed role for Hsp90 during a later stage in the RT folding and activation pathway in vitro does not contradict the cis preference phenomenon in the RT-ɛ interaction observed in vivo (1, 18). Thus, Hsp90 may interact with the RT and activate RT-ɛ binding in the cell immediately following translation (or even cotranslationally), as soon as the TP and RT domains are synthesized and partially folded. This would allow pgRNA packaging to proceed immediately following or during RT translation.
The task of keeping the RT in a soluble, albeit nonfunctional, state may instead fall on other chaperone proteins. We found that bacterial chaperones DnaK and GroEL tightly associated with the mini-RT proteins expressed in bacteria. DnaK, the homologue of the eukaryotic chaperone Hsp70, is known to bind short segments of hydrophobic peptides and is thought to act cotranslationally, keeping the elongating polypeptide chains from aggregating (11, 17). GroEL, on the other hand, is thought to act posttranslationally to provide a sequestered, folding-productive environment for its substrates by enclosing them in its central cavity (11, 17). Although the role of these chaperone proteins in RT folding and function remains to be investigated, it is possible that they may help to keep the RT in a soluble, activation-competent state, from which it can then be folded into its functional conformation with the assistance of the Hsp90 complex. Consistent with this notion is our observation that stripping the RT of its associated bacterial chaperones seemed to decrease the extent of RT activation through reconstitution and that the stripped RT tended to aggregate (J. Hu, unpublished results). These results, together with our previous evidence for a role of the eukaryotic Hsp70 in the RT functions (23), suggest that the bacterial DnaK may be able to substitute for the eukaryotic Hsp70 in the initial stage of RT folding (Fig. 6). In the absence of a functional Hsp90 chaperone in bacteria, some of the partially folded RT proteins may then be recognized by GroEL, which, however, is unable to establish the final RT conformation competent for ɛ binding. As a result, both DnaK and GroEL remain “trapped” by the incompletely folded, nonnative RT proteins.
Our results represent the first successful attempt to purify a recombinant hepadnavirus RT and reconstitute its ɛ binding and protein priming activities in vitro. This reconstitution system now provides the opportunity to analyze the requirement for RT functions in a systematic manner. The role of additional components of the Hsp90 complex known to be important in other substrate systems (9, 24, 36) may now be tested using purified, individual components. The system will also permit a systematic search for other host factors required for RT-ɛ interaction and protein priming that may or may not be related to the Hsp90 chaperone complex, such as the putative ɛ binding factor (34; J. Hu, unpublished results). The ability to purify a recombinant RT expressed in bacteria, in particular, raises the prospect that sufficient amounts of purified RT may be obtained so that high-resolution structural studies may be applied to this most unique RT.
ACKNOWLEDGMENTS
We thank S. Chaudhari and M. Luo for technical assistance; C. Seeger for encouragement and support during the early part of this work; David Toft for providing the anti-p23 antibody; and C. Seeger, G. Viglianti, and R. Corley for comments on the manuscript.
J. Hu is a Harcourt General Researcher and the recipient of an American Liver Foundation Liver Scholar Award. This work was supported by a Public Health Service grant (R01 AI43453) from the National Institutes of Health, a New Investigator Award of The Medical Foundation from the Harcourt General Charitable Foundation, and the American Liver Foundation.
REFERENCES
- 1.Bartenschlager R, Junker-Niepmann M, Schaller H. The P gene product of hepatitis B virus is required as a structural component for genomic RNA encapsidation. J Virol. 1990;64:5324–5332. doi: 10.1128/jvi.64.11.5324-5332.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartenschlager R, Kuhn C, Schaller H. Expression of the P-protein of the human hepatitis B virus in a vaccinia virus system and detection of the nucleocapsid-associated P-gene product by radiolabelling at newly introduced phosphorylation sites. Nucleic Acids Res. 1992;20:195–202. doi: 10.1093/nar/20.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartenschlager R, Schaller H. The amino-terminal domain of the hepadnaviral P-gene encodes the terminal protein (genome-linked protein) believed to prime reverse transcription. EMBO J. 1988;7:4185–4192. doi: 10.1002/j.1460-2075.1988.tb03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartenschlager R, Schaller H. Hepadnaviral assembly is initiated by polymerase binding to the encapsidation signal in the viral RNA genome. EMBO J. 1992;11:3413–3420. doi: 10.1002/j.1460-2075.1992.tb05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beasley R P, Lin C C, Hwang L Y, Chien C S. Hepatocellular carcinoma and hepatitis B virus. Lancet. 1981;ii:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 6.Beck J, Nassal M. A sensitive procedure for mapping the boundaries of RNA elements binding in vitro translated proteins defines a minimal hepatitis B virus encapsidation signal. Nucleic Acids Res. 1996;24:4364–4366. doi: 10.1093/nar/24.21.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohen S, Kralli A, Yamamoto K. Hold'em and fold'em: chaperones and signal transduction. Science. 1995;268:1303–1304. doi: 10.1126/science.7761850. [DOI] [PubMed] [Google Scholar]
- 8.Bresnick E, Dalman F, Pratt W. Direct stoichiometric evidence that the untransformed Mr 300000, 9S, glucocorticoid receptor is a core unit derived from a larger heteromeric complex. Biochemistry. 1990;29:520–527. doi: 10.1021/bi00454a028. [DOI] [PubMed] [Google Scholar]
- 9.Buchner J. Hsp90 & Co—a holding for folding. Trends Biochem Sci. 1999;24:136–141. doi: 10.1016/s0968-0004(99)01373-0. [DOI] [PubMed] [Google Scholar]
- 10.Buendia M A. Hepatitis B viruses and hepatocellular carcinoma. Adv Cancer Res. 1992;59:167–226. doi: 10.1016/s0065-230x(08)60306-1. [DOI] [PubMed] [Google Scholar]
- 11.Bukau B, Horwich A. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 12.Fallows D A, Goff S P. Mutations in the epsilon sequences of human hepatitis B virus affect both RNA encapsidation and reverse transcription. J Virol. 1995;69:3067–3073. doi: 10.1128/jvi.69.5.3067-3073.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganem D. Hapadnaviridae and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fundamental virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1199–1233. [Google Scholar]
- 14.Gerlich W, Robinson W S. Hepatitis B virus contains protein covalently attached to the 5′ terminus of its complete DNA strand. Cell. 1980;21:801–809. doi: 10.1016/0092-8674(80)90443-2. [DOI] [PubMed] [Google Scholar]
- 15.Grammatikakis N, Lin J-H, Grammatikakis A, Tsichlis P N, Cochran B H. p50cdc37 acting in concert with Hsp90 is required for Raf-1 function. Mol Cell Biol. 1999;19:1661–1672. doi: 10.1128/mcb.19.3.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakes D J, Dixon J E. New vectors for high level expression of recombinant proteins in bacteria. Anal Biochem. 1992;202:293–298. doi: 10.1016/0003-2697(92)90108-j. [DOI] [PubMed] [Google Scholar]
- 17.Hartl F. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch R C, Lavine J E, Chang L J, Varmus H E, Ganem D. Polymerase gene products of hepatitis B viruses are required for genomic RNA packaging as well as for reverse transcription. Nature. 1990;344:552–555. doi: 10.1038/344552a0. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch R C, Loeb D D, Pollack J R, Ganem D. cis-acting sequences required for encapsidation of duck hepatitis B virus pregenomic RNA. J Virol. 1991;65:3309–3316. doi: 10.1128/jvi.65.6.3309-3316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu J, Seeger C. Expression and characterization of hepadnavirus reverse transcriptases. Methods Enzymol. 1996;275:195–208. doi: 10.1016/s0076-6879(96)75013-9. [DOI] [PubMed] [Google Scholar]
- 21.Hu J, Seeger C. Hsp90 is required for the activity of a hepatitis B virus reverse transcriptase. Proc Natl Acad Sci USA. 1996;93:1060–1064. doi: 10.1073/pnas.93.3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu J, Seeger C. RNA signals that control DNA replication in hepadnaviruses. Semin Virol. 1997;8:205–211. [Google Scholar]
- 23.Hu J, Toft D O, Seeger C. Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids. EMBO J. 1997;16:59–68. doi: 10.1093/emboj/16.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson J, Craig E. Protein folding in vivo: unraveling complex pathways. Cell. 1997;90:201–204. doi: 10.1016/s0092-8674(00)80327-x. [DOI] [PubMed] [Google Scholar]
- 25.Junker-Niepmann M, Bartenschlager R, Schaller H. A short cis-acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO J. 1990;9:3389–3396. doi: 10.1002/j.1460-2075.1990.tb07540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanford R E, Notvall L, Beames B. Nucleotide priming and reverse transcriptase activity of hepatitis B virus polymerase expressed in insect cells. J Virol. 1995;69:4431–4439. doi: 10.1128/jvi.69.7.4431-4439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanford R E, Notvall L, Lee H, Beames B. Transcomplementation of nucleotide priming and reverse transcription between independently expressed TP and RT domains of the hepatitis B virus reverse transcriptase. J Virol. 1997;71:2996–3004. doi: 10.1128/jvi.71.4.2996-3004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee W. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 29.Lindquist S, Craig E. The heat shock proteins. Annu Rev Genet. 1988;22:631–637. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 30.Mandart E, Kay A, Galibert F. Nucleotide sequence of a cloned duck hepatitis B virus genome: comparison with woodchuck and human hepatitis B virus sequences. J Virol. 1984;49:782–792. doi: 10.1128/jvi.49.3.782-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molnar-Kimber K L, Summers J, Taylor J M, Mason W S. Protein covalently bound to minus-strand DNA intermediates of duck hepatitis B virus. J Virol. 1983;45:165–172. doi: 10.1128/jvi.45.1.165-172.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nassal M, Rieger A. A bulged region of the hepatitis B virus RNA encapsidation signal contains the replication origin for discontinuous first-strand DNA synthesis. J Virol. 1996;70:2764–2773. doi: 10.1128/jvi.70.5.2764-2773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollack J R, Ganem D. An RNA stem-loop structure directs hepatitis B virus genomic RNA encapsidation. J Virol. 1993;67:3254–3263. doi: 10.1128/jvi.67.6.3254-3263.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollack J R, Ganem D. Site-specific RNA binding by a hepatitis B virus reverse transcriptase initiates two distinct reactions: RNA packaging and DNA synthesis. J Virol. 1994;68:5579–5587. doi: 10.1128/jvi.68.9.5579-5587.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pratt W. The role of the hsp90-based chaperone system in signal transduction by nuclear receptors and receptors signaling via MAP kinase. Annu Rev Pharmacol Toxicol. 1997;37:297–326. doi: 10.1146/annurev.pharmtox.37.1.297. [DOI] [PubMed] [Google Scholar]
- 36.Pratt W, Toft D. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 37.Radziwill G, Zentgraf H, Schaller H, Bosch V. The duck hepatitis B virus DNA polymerase is tightly associated with the viral core structure and unable to switch to an exogenous template. Virology. 1988;163:123–132. doi: 10.1016/0042-6822(88)90239-5. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez E, Meshinchi S, Tienrungroj W, Schlesinger M, Toft D, Pratt W. Relationship of the 90-kDa murine heat shock protein to the untransformed and transformed states of the L cell glucocorticoid receptor. J Biol Chem. 1987;262:6986–6991. [PubMed] [Google Scholar]
- 39.Seeger C, Leber E H, Wiens L K, Hu J. Mutagenesis of a hepatitis B virus reverse transcriptase yields temperature-sensitive virus. Virology. 1996;222:430–439. doi: 10.1006/viro.1996.0440. [DOI] [PubMed] [Google Scholar]
- 40.Seeger C, Mason W S. Hepatitis B virus biology. Microbiol Mol Biol Rev. 2000;64:51–68. doi: 10.1128/mmbr.64.1.51-68.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stebbins C, Russo A, Schneider C, Rosen N, Hartl F, Pavletich N. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239–250. doi: 10.1016/s0092-8674(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 42.Stepanova L, Leng X, Parker S, Harper J. Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev. 1996;10:1491–1502. doi: 10.1101/gad.10.12.1491. [DOI] [PubMed] [Google Scholar]
- 43.Summers J, Mason W S. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell. 1982;29:403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- 44.Tavis J E, Massey B, Gong Y. The duck hepatitis B virus polymerase is activated by its RNA packaging signal, epsilon. J Virol. 1998;72:5789–5796. doi: 10.1128/jvi.72.7.5789-5796.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Urban M, McMillan D J, Canning G, Newell A, Brown E, Mills J S, Jupp R. In vitro activity of hepatitis B virus polymerase: requirement for distinct metal ions and the viral epsilon stem-loop. J Gen Virol. 1998;79:1121–1131. doi: 10.1099/0022-1317-79-5-1121. [DOI] [PubMed] [Google Scholar]
- 46.Wang G H, Seeger C. Novel mechanism for reverse transcription in hepatitis B viruses. J Virol. 1993;67:6507–6512. doi: 10.1128/jvi.67.11.6507-6512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang G H, Seeger C. The reverse transcriptase of hepatitis B virus acts as a protein primer for viral DNA synthesis. Cell. 1992;71:663–670. doi: 10.1016/0092-8674(92)90599-8. [DOI] [PubMed] [Google Scholar]
- 48.Wang G H, Zoulim F, Leber E H, Kitson J, Seeger C. Role of RNA in enzymatic activity of the reverse transcriptase of hepatitis B viruses. J Virol. 1994;68:8437–8442. doi: 10.1128/jvi.68.12.8437-8442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weber M, Bronsema V, Bartos H, Bosserhoff A, Bartenschlager R, Schaller H. Hepadnavirus P protein utilizes a tyrosine residue in the TP domain to prime reverse transcription. J Virol. 1994;68:2994–2999. doi: 10.1128/jvi.68.5.2994-2999.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitesell L, Mimnaugh E G, De Costa B, Myers C E, Neckers L M. Inhibition of heat shock proteins HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu-Sherman M, Goldberg A. Involvement of the chaperonin dnaK in the rapid degradation of a mutant protein in Escherichia coli. EMBO J. 1992;11:71–77. doi: 10.1002/j.1460-2075.1992.tb05029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zoulim F, Seeger C. Reverse transcription in hepatitis B viruses is primed by a tyrosine residue of the polymerase. J Virol. 1994;68:6–13. doi: 10.1128/jvi.68.1.6-13.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]