Abstract
Background/Objectives: Endoscopic ultrasound-guided hepaticogastrostomy (EUS-HGS) has emerged as an alternative option for biliary drainage in cases of failed endoscopic retrograde cholangiopancreatography (ERCP). Limited data exist on the safety and efficacy of EUS-HGS. In this comprehensive meta-analysis, we aim to study the safety and efficacy of EUS-HGS in cases of failed conventional ERCP. Methods: Embase, PubMed, and Web of Science databases were searched to include all studies that evaluated the efficacy and safety of EUS-HGS. Using the random effect model, the pooled weight-adjusted event rate estimate for clinical outcomes in each group were calculated with 95% confidence intervals (CIs). The primary outcomes were technical and clinical success rates. Secondary outcomes included overall adverse events (AEs), rates of recurrent biliary obstruction (RBO), and rates or re-intervention. Results: Our analysis included 70 studies, with a total of 3527 patients. The pooled technical and clinical success rates for EUS-HGS were 98.1% ([95% CI, 97.5–98.7]; I2 = 40%) and 98.1% ([95% CI, 97.5–98.7]; I2 = 40%), respectively. The pooled incidence rate of AEs with EUS-HGS was 14.9% (95% CI, 12.7–17.1), with bile leakage being the most common (2.4% [95% CI, 1.7–3.2]). The pooled incidence of RBO was 15.8% [95% CI, 12.2–19.4], with a high success rate for re-intervention (97.5% [95% CI, 94.7–100]). Conclusions: Our analysis showed high technical and clinical success rates of EUS-HGS, making it a feasible and effective alternative to ERCP. The ongoing development of dedicated devices and techniques is expected to make EUS-HGS more accessible and safer for patients in need of biliary drainage.
Keywords: endoscopic ultrasound-guided hepaticogastrostomy, endoscopic ultrasound, hepaticogastrostomy, endoscopic retrograde cholangiopancreatography, outcomes
1. Introduction
Endoscopic retrograde cholangiopancreatography (ERCP) is the gold standard therapy for biliary obstruction for a variety of benign and malignant pancreaticobiliary disorders, with a success rate reaching up to 95% [1,2,3]. However, in cases with surgically altered anatomy or malignant duodenal obstruction, it can be very challenging and has a failure rate ranging from 5–7% in achieving biliary drainage [4]. For years, the standard alternative in this situation was limited to percutaneous transhepatic biliary drainage (PTBD). However, in recent years, endoscopic ultrasound (EUS)-guided biliary interventions have emerged as effective alternate treatment options. EUS-guided biliary drainage (EUS-BD) techniques include EUS guided rendezvous ERCP, EUS-guided choledochoduodenostomy, and EUS-guided hepaticogastrostomy (EUS-HGS) [5].
Among the EUS-BD techniques, EUS-guided hepaticogastrostomy (EUS-HGS) has gained popularity as a novel drainage technique that provides biliary decompression from the left intrahepatic duct (IHD) to the stomach [6]. This method leverages the power of endoscopic ultrasound to access the biliary system, offering distinct advantages over other conventional techniques.
Despite its overall efficacy and safety, clinicians continue to have significant concerns for bile leak and stent migration with EUS-HGSs [7,8]. Therefore, EUS-guided antegrade stenting (EUS-AGS) has emerged as a valuable alternative to EUS-HGS, particularly for patients with an inaccessible ampulla, due to its potential to establish normal bile flow [9]. Despite the fact that these techniques have been around for almost a decade, there are concerns around the safety and efficacy of these modalities. Therefore, we have conducted a systematic review and meta-analysis to evaluate the efficacy and safety of EUS-HGS and EUS-AGS in cases of unsuccessful conventional ERCP.
2. Methods
2.1. Search Strategy and Study Eligibility
Two independent reviewers (S.A. and M.M.) identified studies published before 1 October 2023, that reported on the outcomes of EUS-HGS and EUS-AGS in cases of unsuccessful conventional ERCP. We systematically searched the online MEDLINE, Embase, Cochrane, and Scopus databases using key words in different combinations: (EUS, Endoscopic Ultrasound, Ultrasound) and (Hepaticogastrostomy, biliary drainage, anterograde stenting). Additionally, according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA), we screened the reference lists of the articles and corresponded with study investigators [10]. There was no restriction based on language as long as study outcomes were mentioned in the text. A third reviewer (O.T.) resolved any disagreement.
2.2. Study Inclusion and Exclusion
The inclusion criteria for studies in this analysis were as follows:
-
(1)
Prospective or retrospective studies with a study population comprising patients with biliary obstruction.
-
(2)
Studies involving the use of EUS-HGS or EUS-AGS as the primary intervention.
-
(3)
Evaluation of clinical safety and efficacy as the primary outcomes.
Studies were excluded if they were case reports, case series, animal studies, editorial articles, meta-analyses, review articles, or had sample sizes smaller than 10. Studies without relevant clinical data on clinical success or adverse events were also excluded.
2.3. Data Extraction and Quality Assessment
All relevant data were extracted according to a table independently predefined by S.A. and M.M. The following parameters were extracted: first author, year of publication, country, study design, patient demographics, stent type, cause of prior failed ERCP, stent patency time, technical success, functional success, and outcomes of interest. Using the Newcastle–Ottawa Scale, the methodological quality of the included cohort studies was assessed independently by two investigators (S.A. and O.T.). In the case of a discrepancy, a third independent individual (A.M) was consulted.
2.4. Definitions of Outcomes
The endpoint outcomes include stent patency, stent occlusion, and overall adverse events (AEs). The American Society for Gastrointestinal Endoscopy lexicon was used for the grading of the severity of procedural AEs with endoscopy [11]. Technical success of both EUS-HGS and EUS-AGS was generally defined as the successful biliary drainage as planned. Clinical success was defined as a reduction in serum bilirubin level by more than 50% at 2 to 4 weeks. Recurrent biliary obstruction was considered in case of stent migration, occlusion, or malignancy invasion of stent. Our primary outcome is technical and clinical success rate. Secondary outcomes include stent patency, stent occlusion, and adverse events.
2.5. Data Synthesis and Statistical Analysis
We used R version 3.2.3 (R Project for Statistical Computing) with Meta and Metaprop packages for all analyses. Using the Freeman–Tukey double arcsine transformation (FTT) method, the pooled, weight-adjusted event rate estimate for the clinical outcomes in each group was calculated using the Metaprop package. Continuity correction of 0.5 in studies with zero cell frequencies was used. Between-study heterogeneity was assessed using the Cochrane Q-statistic (I2), which represents the percentage of total between-study variation that cannot be attributed solely to chance. Between-study heterogeneity was rated as low if 25% < I2 ≤ 50%, moderate if 50% < I2 ≤ 75%, and high if I2 > 75%1. A leave-1-out meta-analysis was performed to assess the influence of the outcome by excluding each study and identifying influential studies that may contribute to heterogeneity. A subgroup analysis was performed for studies that reported the outcomes of EUS-HGS with anterograde stent. Statistical tests were 2-sided and used a significance threshold of p < 0.05. The assessment of publication bias was investigated by evaluation of funnel plot asymmetry and sensitivity analysis. In addition to the ethical standards of the competent institution for human subjects, this meta-analysis was conducted in compliance with the Helsinki Declaration [12].
3. Results
3.1. Literature Search and Study Characteristics
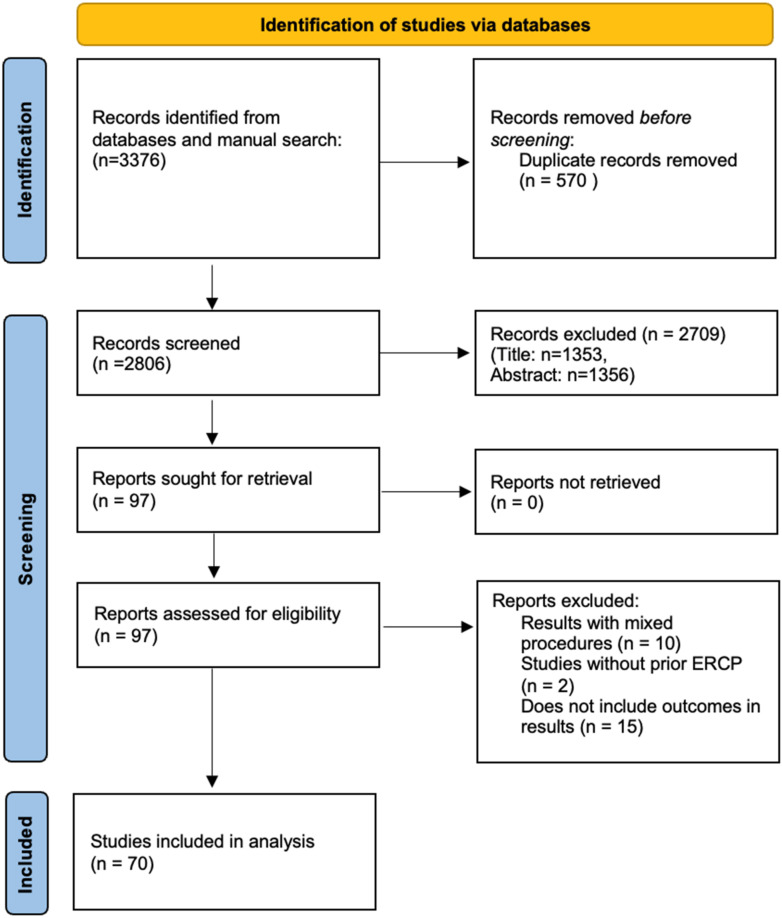
A total of 3276 unique records were identified according to the above search strategy. After title and abstract screening, 70 studies with a total of 3527 patients were included in the study. PRISMA flowchart illustrates our selection process as shown in Figure 1. Supplementary Table S1 shows the baseline characteristics of the included studies and their quality analysis. Of these studies, 53 were from Asia. The study design was prospective in 19 studies, and 23 were multi-center studies. Among the included studies, 43 were of good quality, 21 were of fair quality, and 6 were of poor quality. Supplementary Table S2.
Figure 1.
PRISMA flow chart.
3.2. Baseline Characteristics of Patients and Qualitative Procedure Outcomes
Table 1 shows the baseline characteristic of patients in the studies included and procedure outcomes including: gender, age, underlying cause of obstruction, reason for prior unsuccessful ERCP, overall survival, stent patency time, median procedural time, type of stent, and location of stricture. While 61 studies included only patients with malignant obstruction, 8 included mixed malignant and benign obstruction, and 1 study included only benign obstruction. The most common cause of obstruction was pancreatic cancer. Distal bile strictures were the most common location of stricture. Metal stents were the most commonly used type.
Table 1.
Comprehensive characteristics of included studies.
| Study Name | Malignant/Benign Number | Male Number | Age | Underlying Cause/Diagnosis | Reason for Prior Unsuccessful ERCP (Reason and Number) for Example: 4 Due to Inability to Puncture the Bile Duct …etc. | Location of the Bile Duct Stricture (e.g., Distal: 10, Proxima: 20) | Type of Stent (e.g., PS, FCMS, MS, CMS) and Number of Each if any | Median Procedural Time in Minutes with SD | Incidence of RBO, n | Number of Successful Reinterventions(i.e., Successful Endoscopic Reintervention for RBO) Number | Median Overall Survival (95% CI), Days | Stent Patency, Mean (d) ± SD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anderloni 2022 [13] | 22 malignant | 7 | Mean: 66.0 ± 10.0 | 18 Pancreatic Cancer; 2 Cholangiocarcinoma; 1 Gallbladder Cancer; 1 Duodenal Caner | 4 Infiltrated Papilla; 9 Unreachable Papila; 4 Altered Anatomy; 5 Incomplete Biliary Drainage | n/a | 22 Metal | Mean: 43.3 ± 26.8 | 2 | n/a | n/a | Mean: 10.8 ± 3.1 months |
| Artifon 2015 [14] | 25 malignant | 11 | Mean: 66.25 ± 14.28 | 16 Pancreatic Cancer; 5 Metatstatic Adenopathy; 2 Papillary Cancer; 1 Malignent Neuroendocrine Cancer; 1 Duodenal Cancer | n/a | 25 Distal | Metal | Mean: 47.8 | n/a | n/a | Mean: 75.08 (5.29) | n/a |
| Attasaranya 2012 [15] | 23 malignant | 14 | Mean: 58.03 ± 16.89 | 17 Periampullary or Pancreatic Cancer; 1 Gastric Cancer; 1 Duodenal Cancer; 1 Pancreatic Inflammatory Pseudotumor; 2 Metastatic Cancer; 3 Choledochojejunostomy Stenosis; 1 Gallstone with Cholecystitis; 1 Post-ERCP Cholecystitis; 1 CBD Stone; 1 Bile Leak; 1 Hilar Cholangiocarcinoma;1 Biloma with Postlaparoscopic Cholecystectomy | 14 Failed ERCP for Biliary Cannulation; 10 Inaccessible ERCP due to Luminal Stenosis Secondary to Tumor Invasion of Gastric Antrum or Duodenum; 4 Surgically Altered Anatomy; 2 Acute Cholecystitis with Unfit Condition for Surgery; 1 Biloma | n/a | Metal | n/a | 3 | 3/3 | n/a | n/a |
| Bories 2007 [16] | 8 malignant 3 benign | 7 | Mean (Range): 64 (47–80) | 4 Pancreatic Cancer; 2 Hilar Cholangiocarcinoama; 1 Duodenal Cancer; 1 Gastric Cancer; 3 Benign | Failed ERCP | n/a | 4 Plastic; 3 Metal | n/a | 3 | 3/3 | n/a | n/a |
| Cho 2017 [17] | 21 malignant | 16 | Median (Range): 66.3 (44–82) | 11 Cholangiocarcinoma; 3 Pancreatic Cancer; 3 Gallbladder Cancer; 4 Other Malignancy | 14 High Grade Biliary Stricture; 6 Duodenal Obstruction; 1 Previous Operation | n/a | 21 metal | Median (range): 18 (11–45) | 10 | 5/6 | Median (range): 173 (76.8–269.1) | Mean: 166.3 |
| Cho 2022 [18] | 106 malignant | 68 | Mean: 71.5 ± 11.2 | 28 Pancreatic Cancer; 42 Cholangiocarcinoma; 14 Gallbladder Cancer; 6 Ampullary Cancer; 16 Other Metastatic Disease | 19 Failed ERCP; 35 Insufficient Drainiage of IHD; 32 Gastric Outlet Obstruction; 20 Surgically Altered Anatomy | 41 Distal; 65 Hilar | Metal | Mean: 18.4 | 26 | n/a | Median (IQR): 178.0 (147.7–208.3) | Median (IQR): 138.0 (70.1–205.9) |
| Emmanuel 2020 [19] | 20 malignant | 16 | Mean: 71.8 ± 7.6 | 13 Pancreatic Cancer; 4 Periampullary Tumor; 2 Cholangiocarcinoma; 1 Metastatic Colon Cancer | 16 Inaccesible Papillae; 1 Surgical Anatomy; 3 Failed Cannulation | 19 Distal CBD; 1 Proximal CBD | 10 Metal | Mean: 39.9 ± 1.3 | 1 | 1/1 | n/a | n/a |
| Fujii 2022 [20] | 50 malignant | 28 | DGW Median (IQR): 69 (56–76) SGW Median (IQR): 68 (58–72) | 25 Pancreatic Cancer; 10 Biliary Cancer; 15 Other Malignancy; 4 Benign Stricture | 35 Duodenal Obstruction; 14 ERCP Failure; 5 Intractable Cholangitis | 34 Distal Bile Duct; 7 Perihilar Bile Duct; 13 Hepaticojejunostomy Anastomosis | 11 Plastic; 42 Metal | Metal Mean (range): 47 (32–62) Plastic Mean (range): 54 (44–65) | n/a | n/a | n/a | n/a |
| Harai 2022 [21] | 95 malignant | 50 | Median (IQR): 68 (58–75) | 38 Pancreatic Cancer; 20 Bile Duct Cancer; 37 Other Malignancy | 54 Duodenal Obstruction; 22 Surgical Anatomy; 19 Nonsuccessful ERCP | 66 Distal; 29 Hilar | 95 Metal | Median (IQR): 26 (17–37) | 10 | 10/10 | Median: 154 (95.0% CI 108–363) | n/a |
| Hashimoto 2022 [22] | 85 malignant | 48 | Median (Range): 72 (55–90) | 59 Pancreatobiliary Cancer; 26 Other Malignancy | 55 Inaccessible Papilla or Ileobillary Anastomosis; 30 Accessible Papilla but Inaccessible Target Bile Duct | Distal 61; Perihilar 24 | 28 Plastic; 57 Metal | Median (range): 41 (11–173) | 19 | n/a | Median: 88 (95% CI 62.8–113.2) | Metal Range: 72–329; Plastic Range: 89–272 |
| Hathorn 2022 [23] | 130 | 101 | Mean: 62.9 (14.7) | Cholangiocarcinoma 25, Gastric cancer 4, Pancreatic cancer 61, Ovarian cancer 1, Colorectal cancer 13, Lung cancer 5, Breast cancer 4, Ampullary carcinoma 2, Hepatocellular carcinoma 5, Pancreatic neuroendocrine tumor 2, Gallbladder 2, Vulvar cancer 1, Renal cell carcinoma 1, Duodenal adenocarcinoma 1, Malignant stricture NOS 3 | n/a | n/a | Metal | n/a | n/a | n/a | n/a | n/a |
| Hattori 2023 [24] | 37 malignant 12 benign | 30 | Drill Dilator Median (Range): 72 (59–92) Balloon Catheter Median (Range): 76 (48–91) | 21 Pancreatic Cancer; 12 Cholangiocarcinoma; 4 Duodenal Cancer; 3 Hepaticojejunostomy Stricture; 9 Other Malignancy | 27 Duodenal Obstruction; 20 Surgically Altered Anatomy; 2 Failed Biliary Cannulation | n/a | Plastic | Drill Dilator Mean: 22.7 ± 8.01; Balloon Catheter Mean: 11.1 ± 6.06 | 14 | 19/19 | n/a | n/a |
| Honjo 2018 [25] | 38 malignant | 35 | Mean: 68.9 ± 13.8 | 38 Malignant Biliary Stricture; 7 Bilioenteric Anastomosis Stricture; 4 Choledocolithiasis with Roux-en-Y | n/a | n/a | 56 Plastic; 6 Metal | Mean: 21.9 ± 10.2 | n/a | n/a | n/a | n/a |
| Imai 2017 [26] | 42 malignant | 24 | Mean: 67.3 ± 13.9 | 13 Pancreatic Cancer; 18 Bile Duct Cancer; 11 Lymph Node Metastasis | n/a | n/a | Metal | Mean: 73.5 ± 29.4 | n/a | n/a | 68 (5–185) | Mean: 68 (5–185) |
| Inoue 2023 [27] | 57 malignant | 34 | Median (IQR): 79 (69–85) | 57 Pancreatic Cancer | 44 Inability to Reach/Recognize the Ampulla; 13 Inability to Cannulate | 57 Distal | 57 Metal | Median (IQR): 25 (19–33) | 16 | 16/16 | Median: 167 (120–204) | n/a |
| Ishii 2023 [28] | 37 malignant | 22 | Median (IQR): 70 (62–76) | 20 Pancreatic Cancer; 6 Biliary Tract Cancer; 4 Gastric or Duodenal Cancer; 1 HCC; 6 Metastatic Lymph Node | 20 Duodenal Tumor Invasion; 7 Difficult to Approach Targt; 3 Altered Anatomy; 1 Unsuccessful Biliary Cannulation; 1 History of AE from ERCPs | 22 Distal; 15 Hilar | 37 Metal | Median (IQR): 18 (15–24) | 11 | 10/11 | Median: 4.0 (2.0–6.1) | n/a |
| Ishiwatari 2021 [29] | 96 malignant | 58 | Median (IQR): 70 (64–78) | 53 Pancreatic Cancer; 15 Bliary Cancer; 28 Other Malignancy | 51 MBO; 28 Surgical Anatomy | 78 Distal; 18 Hilar | 28 Plastic; 67 Metal | Median (IQR): 33(26–44) | n/a | n/a | n/a | n/a |
| Ishiwatari 2022 [30] | 58 malignant | 33 | Median (IQR): 71 (64–78) | 31 Pancreatic Cancer; 7 Biliary Cancer; 20 Others Malignancy | 44 Duodenal Obstruction; 8 Surgical Anatomy; 6 Others | B2:21; B3:37 | 6 Plastic; 52 Metal | Median (IQR): 30 (24–39) | 15 | 15/15 | Median: 123 | n/a |
| Iwashita 2017 [31] | 20 malignant | 10 | Median (Range): 69 (56–92) | 10 Dissemination; 5 Lymph Node Recurrent Malignancy; 4 Direction Invasion; 1 Anastomotic Recurrence | n/a | n/a | Metal | Median (range): 36.5 (10–80) | 3 | 2/3 | Median: 100.5 | n/a |
| Iwashita 2022 [32] | 21 malignant | 15 | Median (IQR): 71 (59.5–79) | 21 Malignant Bowel Obstruction; 3 Anastomosis Stricutre; 2 Biliary Stone | n/a | n/a | Plastic or Metal | Median (IQR): 32 (27.75–49.25) | n/a | n/a | n/a | n/a |
| Jagielski 2021 [33] | 53 malignant | 38 | Mean (Range): 74.66 (56–89) | 19 Pancreatic Cancer; 14 Cholangiocarcinoma; 6 Gallbladder Cancer; 3 Hepatocellular Carcinoma; 6 Major Duodenal Papillary Cancer; 1 Duodenal Cancer; 2 Metastatic Colorectal Cancer; 1 Metastatic Breast Caner; 1 Metastatic Cancer of Unknown Origin | 25 Duodenal Obstruction; 23 Periampullary Tumor Infiltration; 5 Failed Biliary Cannulation | n/a | Metal | Mean: 31.2 ± 15.0 | 3 | n/a | n/a | n/a |
| Kawakubo 2014 [34] | 20 malignant | 14 | Median (IQR): 72 (64–81) | 11 Pancreatic Cancer; 3 Bile Duct Cancer; 1 Gallbladder Cancer; 1 Ampullary Cancer; 4 Metastatic Lymph Node; 13 Previous Biliary Drainage | 14 Periamplullary Tumor Invasion; 2 Recurrent Ascending Cholangitis Due to Stent; 4 Altered GI Anatomy | n/a | Plastic and Metal | n/a | 6 | 6/6 | Median: 102 (61–262) | Mean: 51 |
| Khashab 2016 [35] | 61 malignant | 38 | Mean: 63.6 ± 13.8 | n/a | 18 Obscured Ampulla; 24 Distorted Anatomy; 14 Gastric Outlet Obstruction; 6 Others | Distal | 7 Plastic; 54 Metal | Mean: 45.3 ± 34.6 | 12 | n/a | Median: 142 (95% CI 82–256) | n/a |
| Kitagawa 2022 [36] | 21 malignant; 2 benign | 14 | Mean: 73 | 11 Pancreatic Cancer; 1 Uterine Cancer; 4 Bile Duct Cancer; 1 Gastric Cancer; 2 Gallbladder Cancer; 1 Duodenal Cancer; 1 Intrahepatic Stone; 2 Choledocojejunal Anastomosis Stenosis | n/a | n/a | Plastic | n/a | 8 | 4/4 | n/a | n/a |
| Kobori 2022 [37] | 20 malignant | 12 | Median (Range): 72 (47–90) | 9 Gastric Cancer; 6 Pancreatic Cancer; 3 Bile Duct Cancer; 2 Duodenal Caner; 1 Intrahepatic Gallstone | 12 Dificulty Reaching the Papilla; 7 Surgically Altered Anatomy; 3 Difficulty Cannulating the Bile Duct; 4 Presence of Cholantigis before EUS-HGS | 14 Distal; 5 Hilar; 3 Anastomosis | Plastic | Median (range): 45.5 (15–90) | 7 | n/a | n/a | n/a |
| Marx 2022 [38] | 205 malignant | 104 | Mean: 68 ± 12 | 64 Pancreatic Cancer; 8 Vaterian Ampuloma; 31 Cholangiocarcionma; 102 Metastasis | 76 Duodenal Infiltration; 29 Altered Anatomy; 9 Failed Papillary Cannulation; 91 Hilar Stenosis with Undrained Left Liver | n/a | FCMS | n/a | 47 | n/a | Median: 5.3 (2.9–7.5) | Mean: 153 |
| Marx 2022 [39] | 35 malignant | 28 | Mean: 64 ± 11.2 | n/a | n/a | n/a | Metal | n/a | 10 | n/a | n/a | n/a |
| Matsunami 2021 [40] | 57 benign | 28 | Median (Range): 68 (7–90) | 28 Bilioenteric Anastomotic Stricture; 8 Intrahepatic Biliary Stones; 15 Common Bile Duct Stones; 2 Alcoholic Chronic Pancreatitis; 1 Walled Off Necrosis; 1 Idiopathic Retroperitoneal Fibrosis; 1 Left Lobe Hepatic Injury; 1 Bile Duct Polyp | 51 Surgical Anatomy; 4 Gastric Outlet Obsruction; 2 Unsuccessful ERCP | n/a | Plastic or Metal | Median (range): 22 (7–71) | n/a | n/a | n/a | n/a |
| Minaga 2017 [41] | 30 malignant | 11 | Median (Range): 66 (52–87) | 12 Cholangiocarcinoma; 6 Gallbladder Cancer; 5 Pancreatic Cancer; 1 Hepatocellular Carcinoma; 5 Liver Mets; 1 Lypmh Node Metastasis | 4 Failed Duodenal Scope Insertion; 5 Failed Papilla Access After Duodenal Stent Insertion; 21 Failed Intrahepatic Biliary Drainage | 30 Hilar | Plastic and Metal | Median (Range): 39.5 (21–68) | 7 | 5/5 | Median (range): 64 (31–314) | Mean: 62.5 (31–210) |
| Minaga 2022 [42] | 33 malignant | 22 | Median (IQR): 72 (67–76) | 9 Gastric Cancer; 9 Bile Duct Cancer; 8 Pancreatic Cancer; 3 Hepatocellular Cancer; 4 Other Malignancy | 11 Failure of Duodenal Scope Insertion; 10 Surgically Altered Anatomy; 12 Failure of Biliary Cannulation | 18 Distal; 15 proximal | 33 Metal | Median (IQR): 27(20–40) | 33 | n/a | Median: 140 (95% CI, 70.8–209.2) | Mean: 394 days (95% CI, 85.7–702.3 days) |
| Miwa 2023 [43] | 52 malignant | 34 | Median (IQR): 73 (69–80) | 20 Pancreatic Cancer; 12 Biliary Cancer; 7 Colorectal Cancer; 13 Other Malignancy | 27 Duodenal Obstruction; 13 Hilar Biliary Obstruction; 9 Altered Anatomy; 3 Difficult Cannulation | n/a | 19 Plastic; 33 Metal | Median (IQR): 20.5 (17–30) | n/a | n/a | n/a | n/a |
| Miyano 2018 [44] | 27 malignant | 27 | Extra Scope Median (Range): 70 (57–82) Intra Scope Median (Range): 75 (57–88) | 13 Pancreatic Cancer; 14 Bile Duct Cancer; 14 Other Malignancy | 31 Duodenal Obstruction; 10 Surgical Anatomy | B2: 3; B3: 38 | Metal | n/a | na | n/a | Median: 132 (95% CI 69.3–196.3) | Extra Scope Mean: 107 days (95% CI 68.8 to 145.6); Intrascope Mean: 116 days (95% CI 57.1 to 1775.3 |
| Moryoussef 2017 [45] | 18 malignant | 11 | Mean: 68.8 ± 16.4 | 8 Pancreatic Cancer; 5 Hilar Cholangiocarcinoma; 3 Colorectal Cancer; 2 Gastric Cancer | 10 Surgical Anatomy; 7 Impassible Stricture; 1 Duodenal Obstruction | 18 Hilar | Metal | n/a | 3 | 3/3 | Median (range): 79 (5–390) | n/a |
| Nakai 2016 [46] | 33 malignant | 19 | Median (IQR): 70 (63–77) | 17 Pancreatic Cancer; 8 Biliary Tract Cancer; 2 Gastic Cancer; 2 Duodenal Cancer; 1 Hepatocellular Carcinoma; 3 Meastatic Lymph Nodes | 25 Gastric Outlet Obstruction; 5 Altered Anatomy; 3 HX of Adverse ERCP | 26 Distal; 7 Hilar | 33 Metal | Median (IQR): 45 (30–80) | 8 | 8/8 | Median: 8.7 months (95% CI 3.1–12.6) | n/a |
| Nakamura 2023 [47] | 166 malignant | 109 | Median (Range): 76 (20–94) | 59 Pancreatic Cancer; 24 Cholangiocarcinoma; 16 Hepaticojejunostomy Stricture; 26 Bile Duct Stone; 14 Gastric Cancer; 8 Duodenal Cancer; 7 Gallbladder Cancer; 3 Colon Cancer; 9 Other Malignancy | 84 Duodenal Invasion; 75 Surgical Altered Anatomy; 7 Failed ERCP | n/a | Plastic or Metal | Mean: 14.1 ± 8.5 | n/a | n/a | n/a | n/a |
| Ochiai 2021 [48] | 47 malignant | 30 | Median (IQR): 71 (50–93) | 24 Pancreatic Cancer; 8 Biliary Tract Cancer; 2 Gallbladder Cancer; 4 Gastric Cancer; 2 Hepatocellular Carcionoma; 8 Other | 27 Gastric Outlet Obstruction; 10 Alterd Anatomy; 10 Failed ERCP; 1 High Risk ERCP | 39 Distal; 9 Hilar | 47 SEMS | Median (IQR): 42(29–55) | n/a | n/a | n/a | n/a |
| Ogura 2016 [49] | 26 malignant | 13 | Mean: 70 ± 8.1 | 21 Pancreatobililliary Cancer; 5 Others | n/a | n/a | Metal | n/a | 2 | n/a | Median: 113 | Median: 113 |
| Ogura 2017 [50] | 49 malignant | 25 | Median (Range): 72 (43–96) | 19 Gastric Cancer; 13 Bile Duct Cancer; 11 Pancreatic Cancer; 6 Other Malignancy | 22 Duodenal Obstruction; 19 Surgical Anatomy; 8 Failed ERCP | 5 Left Hepatic Bile Duct; 9 Hepatic Hilum; 3 Upper Common Bile Duct; 13 Middle Common Bile Duct; 19 Lower Common Bile Duct | Metal | n/a | 7 | 6/6 | Median: 114 (95% C.I 73.012–154.988) | Mean: 320 days (95% CI, 269.899–772.037 days) |
| Ogura 2021 [51] | 14 malignant | 8 | Median (IQR): 3 (1–6) | 9 Pancreatic Cancer; 3 Gastric Cancer; 2 Bile Duct Cancer | 11 Duodenal Obstruction; 3 Surgically Altered Anatomy | n/a | 14 Metal | Median (IQR): 7 (5–10) | 1 | n/a | n/a | Mean: 101 days |
| Oh 2016 [52] | 113 malignant | 81 | Mean: 62.2 ± 13 | n/a | 52 Failure of the Guidewire Pass Across the Tight Stricture; 37 Surgically Altered Anatomy; 15 Obscured Ampulla Due to Metallic Enteral Stent; 13 Duodenal Obstruction; 10 Obscured Ampulla Due to Invasive Cancer; 2 Removal of Intrahepatic Duct Stones in Surgically Altered Anatomy | n/a | Plastic | Mean: 30.1 ± 13.1 | 6 | 5/6 | n/a | Mean: 137.1 ± 243.5 |
| Ohno 2022 [53] | 72 malignant | 42 | Dilation + Median (Range): 69 (36–93) Dilation- Median(Range): 73 (38–92) | 32 Pancreatic Cancer; 18 Biliary Tract; 8 Gastric Cancer; 14 Others Malignancy | 46 Surgically Alterd Anatomy; 22 Duodenal Obstruction; 4 Unsuccessful ERCP | n/a | Dilation + 35; Dilation- 3 | Dilation + Median (range): 72 (29–133); Dilation- Median (range): 44 (24–153) | 1 | 1/1 | n/a | n/a |
| Okuno 2018 [54] | 20 malignant | 12 | Median: 68 | 9 Gastric Cancer; 1 Colon Cancer; 2 Gallbladder Cancer; 7 Pancreatic Cancer; 1 Duodenal Cancer | 13 Duodenal Obstruction; 7 Altered Upper GI Anatomy | 20 Distal | 20 Metal | n/a | 1 | n/a | n/a | Mean: 87 days |
| Okuno 2022 [55] | 55 malignant 6 benign | 35 | Median (Range): 68 (38–87) | 28 Pancreatic Cancer; 5 Duodenal Cancer; 4 Gastric Cancer; 4 Gallbladder Cancer; 3 Colon Cancer; 3 Cholangiocellular Carcinoma; 8 Other; 6 Benign | 41 Primary Drainage; 20 Salvage Drainage | 7 Proximal | 44 FCEMS; 16 Plastic; 1 None | Median (range): 24 (8–70) | 0 | n/a | n/a | n/a |
| Okuno 2023 [56] | 18 malignant 2 benign | 12 | Median (Range): 70 (38–82) | 6 Pancreatic Cancer; 6 Biliary Tract Cancer; 2 Gastric Cancer; 2 Hepatocellular; 1 Cholangiocellular Carcinoma; 1 Colon Cancer; 2 Anastomosis Stricture | 12 Primary Drainage; 8 Salvage Drainage | n/a | Metal | Median (range): 13 (7–25) | n/a | n/a | n/a | n/a |
| Paik 2017 [57] | 16 malignant | 13 | Mean: 67.6 ± 9.3 | 7 Cholangiocarcinoma; 2 Pancreatic Cancer; 2 Ampulla of Vater; 2 Gallbladder Cancer; 1 Hepatocellular Carcinoma; 2 Peribilary Metastasis | n/a | Distal | Metal | Mean (SD): 33.4 (20.6) | n/a | n/a | n/a | Mean: 402 days |
| Paik 2018 [58] | 25 malignant 3 benign | 20 | Median (Range): 63 (29–87) | 10 Cholangiocarcinoma; 5 Pancreatic Cancer; 2 Gallbladder Cancer; 2 Gastric Cancer; 1 Ampulla of Vater Malignancy; 1 Colon Cancer; 1 Duodenal Cancer; 1 Hepatocellular Carcinoma; 1 Intraductal Papillary Neoplasm of Bile Duct; 1 Lymphoma; 3 Benign | n/a | n/a | Metal | Mean: 15.6 ± 5.8 | n/a | n/a | Median (range): 7.5 (5.0–12.0) | Mean: 150 (5–295) days |
| Park 2011 [59] | 51 malignant 6 benign | 35 | 61.7 (13) | Pancreatic cancer 12, Hilar cholangiocarcinoma 14, Ampulla of Vater cancer 5, Common bile duct cancer 3, Gallbladder cancer 2, Hepatocellular carcinoma 1, Duodenal cancer 2, Advanced gastric cancer 6, Metastatic lymph node 6 | n/a | n/a | FCMS | Mean: 132 | n/a | n/a | n/a | n/a |
| Park 2013 [7] | 45 malignant | 28 | Mean: 64.9 ± 13 | 10 Pancreatic Cancer; 6 Hilar Cholangiocarcinoma; 6 Amplulla Cancer; 3 Common Bile Duct Cancer; 3 Gallbladder Cancer; 2 Hepatocellular Carcinoma; 1 Colon Cancer; 3 Lymphoma; 4 Advanced Gastric Cancer; 1 Breast Malignancy; 6 Bengin | N/A | n/a | n/a | Median: 50 | n/a | n/a | n/a | n/a |
| Park 2015 [60] | 32 malignant | 20 | DH Mean: 66.2 ± 11 FC Mean: 68.8 ± 13 | 11 Pancreatic Cancer; 13 Hilar Cholangiocarcinoma; 2 Distal Common Bile Duct Malignancy; 6 Other Malignancy | 7 Surgical Anatomy; 13 High Grade Hilar Obstruction; 12 Duodenal Invastion | n/a | Metal | Median (range): 13 (10–21) | 2 | 2/2 | n/a | Mean: 121 ± 11.2 days |
| Poincloux 2015 [61] | 98 malignant | 58 | Mean (Range): 70 (38–91) | 51 Pancreatic Cancer; 12 Cholangiocarcinoma; 8 Ampulla Carcinoma; 3 Gallbladder Cancer; 2 Hepatocellular Carcinoma; 2 Duodenal Caner; 5 Gastric Cancer; 4 Colorectal Cancer; 3 Breast Cancer; 3 Ovarian Cancer; 2 Unknown Adenocarcinoma; 1 Pulmonary Malignancy; 1 Renal Malignancy; 3 Benign | 25 Duodenal Stenosis; 7 Surgical Anatomy; 40 Periampulary Tumor Infiltration; 1 Altered Ampula Position; 1 Biliary Fistula; 27 Incomplete Draininge of High Grade Hilar Tumors | n/a | Plastic and Metal | n/a | 4 | n/a | n/a | n/a |
| Prachayakul 2013 [62] | 21 malignant | 10 | Mean (Range): 62.8 (46–84) | 9 Pancreatic Cancer; 4 Cholangiocarcinoma; 4 Gallbladder Cancer; 4 Other Malignancy | 20 Obstrucive Jaundice | n/a | 21 Metal | n/a | n/a | n/a | n/a | Mean: 93 days |
| Ragab 2023 [63] | 91 malignant | 59 | Median (IQR): 61 (55–69) | 75 Ampullary Tumor; 7 Altered Anatomy; 5 Cholangiocarcinoma; 4 Undiferentiated Common Bile Duct Malignancy | 55 Inability to Achieve Deep Cannulation; 13 Duodenal Infiltration; 15 Gastric Outlet Obstruction; 8 Altered Anatomy | 91 Distal | Metal, Plastic, Half to Half, Partially Covered, Fully Covered | Median (Range): 20 (15–27) | n/a | n/a | n/a | n/a |
| Samanta 2023 [64] | 43 malignant 6 benign | 23 | Median (Range): 52.0 (28–76) | 20 Pancreatic Cancer; 13 Gallbladder Cancer; 8 Periampullary Carcinoma; 2 Other Malignancy; 6 Benign Causes | 25 Duodenal Obstruction/Inaccessible Papilla; 4 Altered Anatomy; 20 Failed ERCP | 19 Hilar; 30 Distal | Metal | n/a | 9 | n/a | 3 Month Mortality 11/49 | n/a |
| Sassatelli 2019 [65] | 36 malignant | 15 | Mean: 69.3 ± 12.4 | 25 Pancreatic Adnocarcinoma; 3 Metastatasis; 3 Cholangiocarcionma; 3 Gastric Cancer; 2 Gallbladder Cancer | 13 Ampulary Obstruction by Invasive Cancer; 12 Postsurgical Anatomy; 10 Hepaticojejunostomy Stricture or Duodenal Obstruction | n/a | 9 Plastic; 24 Metal | n/a | n/a | n/a | Median: 49 ± 156.7 | TG-BD Mean: 72.7 ± 136.4 days TD-BD Mean: 128.5 ± 176.8 days |
| Schoch 2022 [66] | 34 malignant | 17 | Median (IQR): 76 (67–83) | 25 Perihilar Cholangiocarcinoma; 9 Gallbladder Cancer | 22 ERCP Failure; 8 Duodenal Stricture; 2 Altered Anatomy; 2 Isolated Left Hepatic Duct Dilation | 34 Perihilar | Metal | n/a | 9 | n/a | Median (IQR): 91 (31–263) | Mean (IQR): 145 (30–222) |
| Sekine 2022 [67] | 144 malignant | 54 | B2 Mean (Range): 66.9 (32–90) B3 Mean (Range): 68.6 (32–87) | 66 Pancreatic Cancer; 42 Biliary Tract; 27 Gastroduodenal Cancer; 9 Malignant Disease; 4 Bile Duct Stone; 13 Benign Disease | n/a | Distal 89; Perihilar 65; 3 Anastomosis; 1 Ampulla of Vater 1; 3 No Stenosis | 114 Plastic; 47 Metal | B2 Mean (Range): 35.2 (8–110); B3 Mean (Range): 47.0 (9–187) | n/a | n/a | n/a | n/a |
| Shibuki 2023 [68] | 154 malignant | 102 | Plastic Median (Range): 70 (32–85) Metal Median (Range): 69 (32–90) | 62 Pancreatic Cancer; 41 Bile Duct Cancer; 28 Gastric Cancer; 21 Other Malignancy | 55 Inaccessible Papilla; 33 Isolated Intrahepatic Bile Duct Obstruction; 21 Recurrent Ascenting Cholangitis; 22 Surgically Altered Anatomy; 21 Failed Biliary Cannulation | 89 Distal; 63 Perihilar | 109 Plastic; 43 Metal | Plastic Median (range): 30 (8–187); Metal Median (range): 41 (15–150) | 47 | plastic 30/35, metal 12/12 | Plastic Median (range): 189 (99–270); Metal Median (range): 164 (95–281) | n/a |
| Shin 2023 [69] | 24 malignant | 7 | Median (IQR): 67 (61–76) | 16 Cholangiocarcinoma; 2 Pancreatic Cancer; 4 Gallbladder Cancer; 2 Ampullary Cancer | 12 Failed ERCP; 7 Surgical Anatomy; 5 Gastric Outlet Obstruction | n/a | Metal | Median (IQR): 19.3 (18.4–21.2) | 7 | 7/7 | n/a | Mean: 6.7 months |
| Song 2014 [70] | 27 malignant | 13 | Median (Range): 67 (29–86) | 2 Pancreatic Cancer; 8 Hilar Cholangiocarcinoma; 2 Pancreatic Cancer Neuroendocrine Tumors; 2 Gallbladder Cancer; 1 Ampulla of Vater Cancer; 1 Advanced Gastric Cancer; 1 Rectal Cancer | 11 Pyloric or Duodenal Obstruction; 9 High Grade Biliary Stricture; 7 Periampullary Tumor Infiltration | n/a | Metal | Median (range): 22 (14–35) | 2 | 2 | n/a | n/a |
| Sportes 2017 [71] | 31 malignant | 17 | Mean: 69.2 | 22 Pancreatic Cancer; 5 Metatstatic Lymphadenopathy; 3 Cholangiocarcinoma; 1 Periampullary Cancer | 13 Prior Surgery; 9 Duodenal Stenosis; 5 Periampullary Tumor Infiltration; 4 Impassable Stricture | n/a | Metal | n/a | 2 | 2 | Median (IQR): 71 (30–95) | n/a |
| Takenaka 2022 [72] | 45 malignant | 33 | Median (IQR): 73 (65–77) | 15 Pancreatic Cancer; 10 Gastric Cancer; 6 Cholangiocarcinoma; 6 Hepatocellular Carcinoma; 8 Other Malignancy | 21 Failed Biliary Cannulation; 18 Surgical Anatomy; 6 Duodenal Obstruction | n/a | Plastic or Metal | Median (IQR): 15.8 (11.7–19.7) | 9 | 9/9 | n/a | n/a |
| Tyberg 2022 [73] | 89 malignant | 52 | Mean: 69.9 ± 12.7 | 1 Ampullary Adenocarcionma; 5 Gallbladder Cancer; 19 Cholangiocarcinoma; 42 Pancreatic Cancer; 6 Colorectal Cancer; 16 Other Malignancy; 1 Choledocolithiasis | 75 Obstructive Jaundice; 25 Cholangitis | n/a | 8 Plastic; 82 Metal | n/a | n/a | 12 | n/a | n/a |
| Umeda 2015 [74] | 15 malignant | 15 | Median: 77 | 5 Common Bile Duct Stone; 2 Ampullary Cancer; 2 Post Op Stricture; 9 Pancreatic Cancer; 1 Metastatic Lymph Nodes; 1 Bile Duct Cancer; 1 Duodenal Caner | 9 Periampullary Tumor Invasion; 7 Altered Anatomy; 3 Failed Duodenal Intubation; 4 Prior ERCP Failure | n/a | Plastic | Median: 22.8 | n/a | n/a | n/a | Median (Range): 4 months (0.5–9) |
| Vila 2012 [75] | 34 malignant | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Yagi 2022 [76] | 27 malignant | 24 | Median (Range): 69 (36–84) | 18 Pancreatic Cancer; 9 Billiary Cancer | n/a | 26 Distal; 9 Hilar; 3 Postoperative Anastomosis | 38 Metal | Median (range): 35.5 (17–80) | 6 | 6/6 | n/a | n/a |
| Yamamoto 2018 [77] | 23 malignant | 14 | Median: 69 ± 12.2 | 11 Pancreatic Cancer; 2 Gastric Cancer; 2 Ampullary Cancer; 1 Duodenal Cancer; 1 Bile Duct Cancer; 6 Metastasis of Other Cancer | 3 Failed ERCP | n/a | 23 Plastic | n/a | 0 | n/a | Median (Range): 96 (36–656) | Mean (Range): 66 (36–462) |
| Yamamura 2022 [78] | 31 malignant | 23 | Median (range): 74 (55–87) | 20 Pancreatic Cancer; 9 Bile Duct Cancer; 2 Gastric Cancer | 16 Duodenal Obstruction; 15 Surgically Altered Anatomy | 31 Segment 3 | Metal | Mean: 17.7 ± 3.76 | n/a | n/a | n/a | Median: 97 (95% CI, 88–99) |
| Yane 2023 [79] | 36 malignant | 21 | Median (Range): 71 (40–88) | 17 Pancreatic Cancer; 10 Gastric Cancer; 2 Gallbladder Cancer; 2 Bile Duct Cancer; 5 Other Malignancy; 1 Choledocolithiasis | 20 Surgical Anatomy; 10 Duodenal Obstruction; 2 Obscured Ampulla due to Invasive Cancer; 5 Segmental Cholangitis Difficult to Control with ERCP | 27 Distal; 6 Hilar; 2 Choledocojejunal Anastomosis; 1 Distal plus Hilar; 1 n/a | 7 Plastic; 6 Metal; 24 Both | Median (range): 35 (16–125) | 0 | 0 | n/a | n/a |
| Yasuda 2023 [80] | 10 malignant | 6 | Median (Range): 66.5 (58–77) | 3 Pancreatic Cancer; 5 Gastric Cancer; 1 Metastatic Colorectal Cancer; 1 Metastatic Cervical Cancer | 2 Failed Biliary Cannulation | n/a | 10 Metal | Median (range): 20 (15–44) | 3 | 3/3 | n/a | Mean (Range): 43 (13–215) |
| Zhang 2022 [81] | 24 malignant | 4 | Mean: 69.3 ± 6.8 | n/a | 19 Surgically Altered Anatomy; 5 Gastrointestinal Obstruction | n/a | 24 Plastic | n/a | 1 | 1/1 | n/a | Mean: 141.0 ± 73.6 |
3.3. Clinical and Technical Success
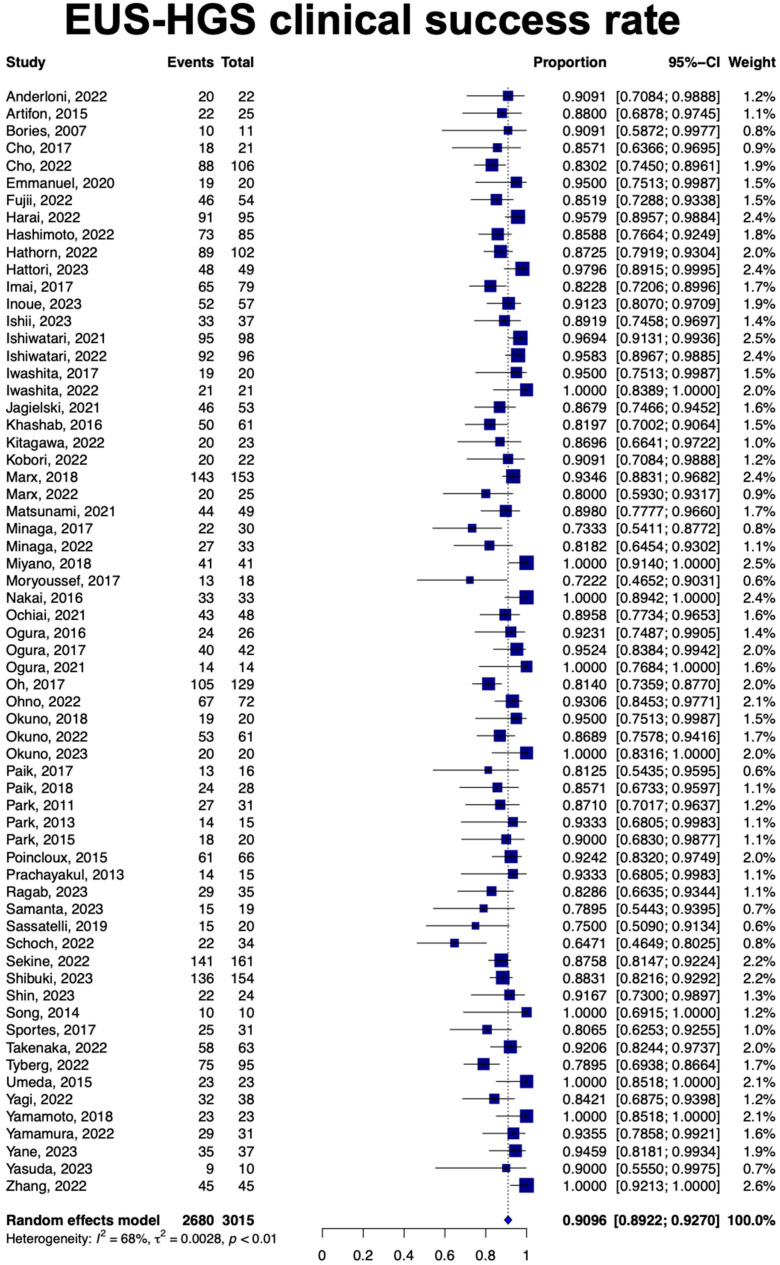
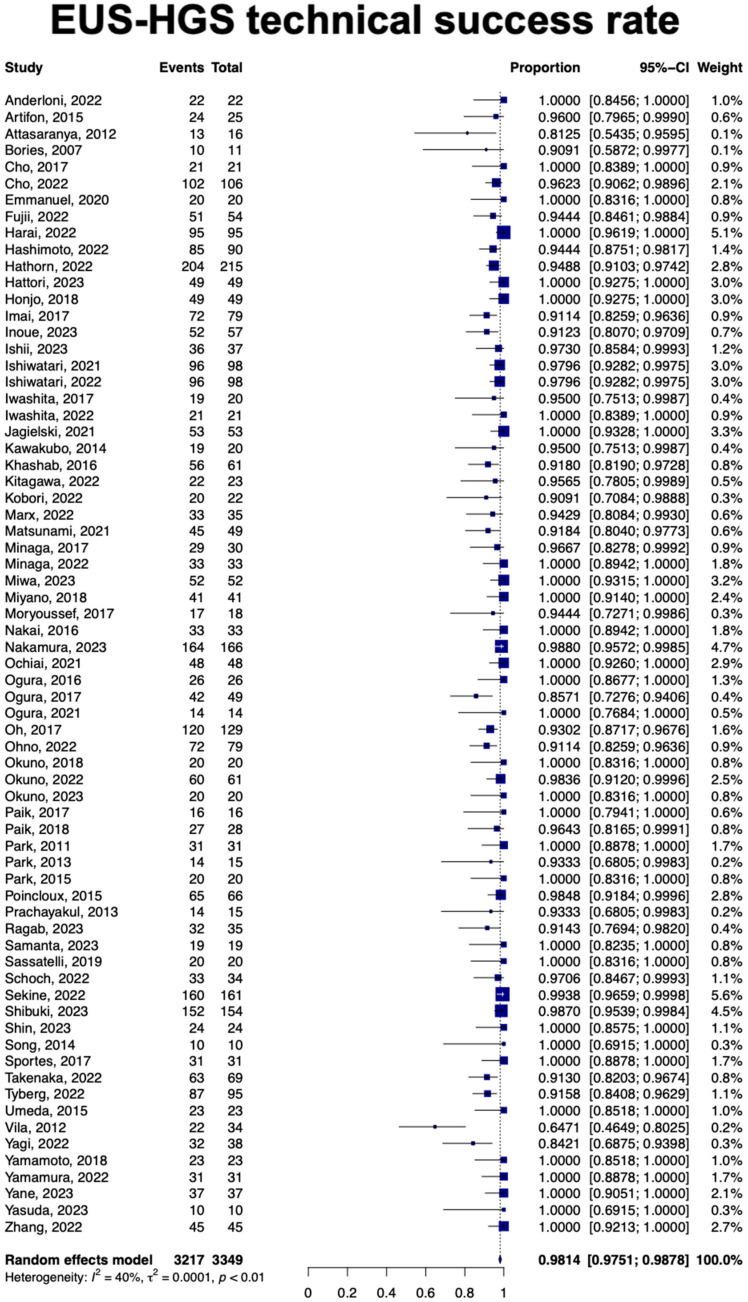
A total of 64 studies with 3015 patients showed the pooled clinical success rate for EUS-HGS was 90.9% (95% Confidence Interval [CI], 89.2–92.7; I2 = 68%) (Figure 2). Data from 3349 patients showed a pooled technical success rate of 98.1% [95% CI, 97.5–98.7]; I2 = 40% (Figure 3). On subgroup analysis, the reported pooled clinical and technical success rates of HGAS were 95.2% [95% CI, 91.7–98.9] and 93.8% [95% CI, 89.3–98.2], respectively Table 2.
Figure 2.
Forest plot of clinical success rat [7,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81].
Figure 3.
Forest plot of technical success rate [7,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81].
Table 2.
Details of clinical and technical success rates and adverse events associated with EUS-HGS and HGAS.
| EUS-HGS | HGAS | |
|---|---|---|
| Success rate | ||
| Clinical success | 90.9 (89.2–92.7) | 95.2 (91.7–98.9) |
| Technical success | 98.1 (97.5–98.7) | 93.8 (89.3–98.2) |
| Adverse events | ||
| Overall adverse events | 14.9 (12.7–17) | 10.8 (6.6–15.0) |
| Bile leakage | 2.4 (1.7–3.2) | 0.1 (0.0–1.1) |
| Bleeding | 1.3 (0.8–1.8) | 1.6 (0.5–2.7) |
| Peritonitis | 1.27 (0.7–1.8) | 1.1 (0.6–1.6) |
| Cholangitis | 0.5 (0.1–0.8 | 0.5 (0–2.5) |
| Mortality | 0.1 (0.0–0.3) | 0 (0.0–0.5) |
| Abdominal pain | 0.13 (0.0–0.4) | 0 (0.0–1.2) |
| Stent migration | 0.3 (0.1–0.6) | 0 (0.0–1.5) |
| Sepsis | 0.5 (0.1–0.8) | 0 (0.0–1.3) |
| Pneumoperitoneum | 0.1 (0.0–0.4) | 0 (0.0–1.0) |
| Perforation | 0.1 (0.0–0.3) | 0 (0.0–1.1) |
| Cholecystitis | 0.1 (0.0–0.6) | 0 (0.0–0.9) |
| ASGE lexicon classification of adverse events severity | ||
| Mild | 7 (4.3–9.7) | NA |
| Moderate | 2.7 (1–4.5) | NA |
| Severe | 0.9 (0.1–1.7) | NA |
| Fatal | 0.03 (0.0–4.6) | NA |
| Recurrent obstruction and reintervention success rate | ||
| RBO | 15.8 (12.2–19.4) | NA |
| Reintervention success | 97.5 (94.7–100) | NA |
Values are percentages (%) with the corresponding (95% confidence interval) EUS-HGS: Endoscopic Ultrasound Hepaticogastostomy. HGAS: EUS-guided antegrade stenting.
3.4. Overall Adverse Events
Overall, a total of 68 studies (3454 patients) reported the total number of AEs related to EUS-HGS. The pooled incidence rate of AEs with EUS-HGS was 14.9% (95% CI, 12.7–17.1; I2= 71%). A total of 20 studies reported the severity of AEs according to the ASGE Lexicon classification system. The results were as the following: mild: 7% [95% CI, 4.3–9.7]; moderate: 2.7 [95% CI, 1–4.5]; severe: 0.9% [95% CI, 0.1–1.7]; fatal 0.03% [95% CI, 0.0–4.6]. For the HGAS group, the pooled incidence of total AEs was 10.8% [95% CI, 6.6–15.0].
3.5. Individual Adverse Events
Table 1 shows the number of studies and patients and pooled incidence rate of individual AEs. The most common reported AE was bile leakage (2.4% [95% CI, 1.7–3.2]), followed by bleeding and peritonitis, with pooled incidences of 1.30% [95% CI, 0.8–1.8] and 1.27% [95% CI, 0.7–1.8], respectively. A pooled incidence of 0.5% [95% CI, 0.1–0.8] was reported for cholangitis. The pooled incidence of mortality related to the procedure was low at 0.1% [95% CI, 0.0–0.3]. The most common reported symptom was abdominal pain, with a pooled incidence of 0.13% [95% CI, 0.0–0.4]. Stent migration was reported at rate of 0.3% [95% CI, 0.1–0.6]. AEs were less frequent in HGAS group, with the most frequent reported AEs being pancreatitis with a pooled incidence of 4.7% [95% CI, 0.6–8.8].
3.6. Recurrent Biliary Obstruction (RBO) and Re-Intervention
A total of 43 studies (1919 patients) reported the rate of RBO after EUS-HGS. The pooled incidence was 15.8% [95% CI, 12.2–19.4]. However, the success rate for reintervention was high with a pooled rate of 97.5% [95% CI, 94.7–100].
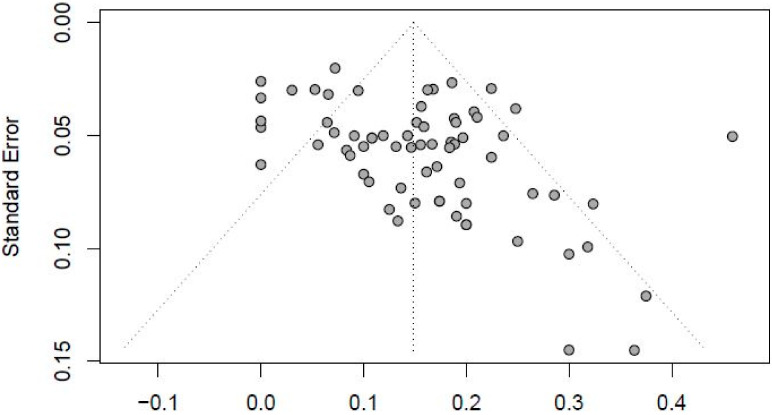
3.7. Assessment of Publication Bias and Sensitivity Analysis
A funnel plot of included studies is shown in Figure 4, which indicates no publication bias. The symmetry of the plot around the central line suggests an even distribution of study results, implying that both positive and negative outcomes were equally likely to be published. This balanced spread of effect sizes across studies of varying sizes supports the conclusion that there is no selective publication bias affecting the meta-analysis results. Additionally, the influence of a single study on the overall meta-analysis estimate was investigated by omitting one study at a time. The omission of any study resulted in no significant difference, indicating that our results were statistically reliable.
Figure 4.
Funnel plot.
4. Discussion
In this comprehensive systematic review and metanalysis of 70 studies with 3643 patients, the pooled clinical success of EUS-HGS after unsuccessful ERCP was 90.9% and the technical success rate was 98.1%. Our analysis showed that the pooled incidence of AEs with EUS-HGS was 14.9% with bile leak being the most common AE at 2.4%. To our knowledge, this is the largest analysis including 70 studies focused on EUS-HGS. Our analysis provides valuable insights into the efficacy and safety of EUS-HGS with or without EUS-AGS when conventional ERCP fails or is not feasible.
Biliary obstruction is a challenging medical condition that can often necessitate a wide variety of interventions via endoscopic retrograde cholangiopancreatography (ERCP). When ERCP is unsuccessful, endoscopic ultrasound-guided hepaticogastrostomy (EUS-HGS) emerges as a promising alternative for biliary drainage [82]. Numerous studies have provided insights into the efficacy and safety of EUS-HGS as an option for biliary drainage [83,84,85]. The high success rates make EUS-HGS a viable alternative when conventional ERCP is not feasible, particularly in cases involving surgically altered anatomy or inaccessible papilla. Our meta-analysis showed results further supporting this expanding body of evidence, with a pooled clinical success rate of 90.9%, and a pooled technical success rate of 98.1%. Compared to endoscopic retrograde biliary drainage (ERBD) and percutaneous transhepatic biliary drainage (PTBD), EUS-HGS shows comparable success rates [86,87]. Moreover, EUS-HGS has the added advantage of being accessible in cases where ERBD is not feasible due to anatomical challenges.
Stent patency in EUS-HGS is a crucial aspect of its long-term effectiveness. Although theoretically, stent patency might be longer in EUS-HGS than in ERBD, various factors influence the duration of patency [88]. Reported stent patency durations for EUS-HGS have varied widely, ranging from 62 to 402 days. While EUS-HGS may have fewer instances of tumor ingrowth or overgrowth, it can be more susceptible to stent migration and clogging, potentially shortening stent patency. In our review which included 70 studies, there was a significant variation in the stent patency duration, ranging from 31 to 771 days with a pooled average stent patency of 155 days. The location and degree of biliary stricture, presence of gastric or duodenal obstruction, type and length of the stent used, presence of liver metastasis, and other factors all contribute to the stent patency of EUS-HGS.
In the studies included in our meta-analysis, we observed a notable variation in the reported types of stents utilized. Out of the 70 total studies, 36 (51.4%) mentioned the deployment of metal stents, while 8 studies (11.4%) specifically indicated the use of plastic stents. Additionally, 24 studies (34.3%) scrutinized the utilization of both metal and plastic stents. In contrast, 2 studies (2.9%) did not provide information regarding the type of stent used.
A recent prospective study compared stent patency between EUS-guided biliary drainage (EUS-HGS and EUS-choledochoduodenostomy) and ERBD, showing that EUS-guided drainage had significantly longer stent patency [6]. However, it is essential to consider patient survival when interpreting these results, as many patients with biliary obstruction have a short survival time. Shorter survival may reduce the likelihood of observing stent dysfunction because patients may not live long enough for the stent to fail. This distinction is crucial for understanding the actual efficacy and reliability of the stent.
EUS-HGS has been shown to produce fewer procedure-related adverse events than PTBD, making it a safer alternative [6]. The overall previously reported rate of adverse events in EUS-HGS is approximately 18%, with common adverse events including abdominal pain, self-limiting pneumoperitoneum, bile leak, cholangitis, and bleeding. However, in rare cases, serious adverse events like perforation, intraperitoneal stent migration, and mediastinitis have been reported. Notably, our analysis indicates a pooled adverse events incidence rate of 14.89%, with the most frequently encountered adverse events being bile leakage (2.4%), bleeding (1.3%), and peritonitis (1.27%).
Compared to EUS-HGS, EUS-choledochoduodenostomy (EUS-CDS) was shown to result in less early adverse events and shorter procedure time [20]. This suggests that EUS-CDS might be a safer option for novice practitioners, while EUS-HGS should be reserved for experienced operators [89]. The learning curve for EUS-HGS is steep, and it is a technically challenging procedure. Studies suggest that achieving proficiency in EUS-HGS may require a significant number of cases, with some reports indicating that more than 33 cases may be needed to reach a plateau in the learning curve [52]. While EUS-HGS is still technically challenging, one approach to mitigate the learning curve is the conversion of PTBD to EUS-HGS for beginners [90]. This transition can offer several advantages, including the ability to identify the optimal puncture site in the intrahepatic duct via opacification through a PTBD catheter. Furthermore, it allows practitioners to become more familiar with EUS-HGS while potentially reducing the risk of adverse events, such as cholangitis or bile leak.
Despite its advantages, EUS-HGS has several limitations and challenges that need to be considered [6]. Some of these limitations include the technical complexity of the procedure, the lack of dedicated devices for EUS-HGS, the risk of serious adverse events, and the need for skilled practitioners. Additionally, there are technical challenges in draining the right liver in cases of bilateral stenosis and difficulties in patients with a non-dilated biliary system [91].
It is imperative to address the limitations inherent in our meta-analysis. A substantial portion of the studies incorporated in our analysis adopted a retrospective design. This approach has the potential to result in an overestimation of technical and clinical success rates, particularly when populations of patients initially assigned to EUS-HGS or EUS-AGS were subsequently transitioned to an alternative method, such as EUS-choledochoduodenostomy, and labeled as such. Furthermore, several essential parameters that might be of interest, such as the stratification of adverse events and outcomes based on common bile duct size or stent dimensions, were frequently omitted in the studies we reviewed. In recent years, various new devices, including dedicated stent systems, have been developed to make EUS-HGS more accessible [36]. These innovations aim to simplify the procedure, increase its success rate, and decrease the risk of adverse events. Continued development in this area is essential to improve the safety and feasibility of EUS-HGS.
5. Conclusions
EUS-HGS has emerged as a valuable alternative for biliary drainage when conventional ERCP is not feasible. The technique has shown high technical and clinical success rates and potentially longer stent patency compared to ERBD [6]. However, it is not without its challenges, including a steep learning curve, the need for skilled practitioners, and potential risks of adverse events. The ongoing development of dedicated devices and techniques is expected to address these challenges, making EUS-HGS more accessible and safer for patients in need of biliary drainage.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13133883/s1, Table S1: Baseline characteristics of the included studies and their quality level; Table S2: Quality assessment of included studies using Newcastle-Ottawa Scale.
Author Contributions
Conception and design: S.A., M.Y.M., D.S.D., H.G., M.B. and W.K. Administrative support: S.A., M.Y.M., D.S.D. and W.K. Provision, collection, and assembly of data: S.A., M.Y.M., D.S.D., F.J., Y.K., M.A. (Mohammad Ahmed), A.B., H.G., M.B. and W.K. Statistical analysis: S.A. and A.B. Review of literature: All authors. Drafting the manuscript: All Authors. Revision of key components of manuscript: All authors. Final approval of manuscript: All Authors. Agreement to be accountable for all aspects of the work: all authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The data utilized in this study are available publicly. Hence, an analysis did not require Institutional Review Board (IRB) approval as per guideline put forth by our institutional IRB.
Informed Consent Statement
The data utilized in this study is available publicly. Hence, patient consent was not required.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gravito-Soares E., Gravito-Soares M., Gomes D., Almeida N., Tomé L. Clinical applicability of Tokyo guidelines 2018/2013 in diagnosis and severity evaluation of acute cholangitis and determination of a new severity model. Scand. J. Gastroenterol. 2018;53:329–334. doi: 10.1080/00365521.2018.1430255. [DOI] [PubMed] [Google Scholar]
- 2.Smith A.C., Dowsett J.F., Russell R.C., Hatfield A.R., Cotton P.B. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet. 1994;344:1655–1660. doi: 10.1016/S0140-6736(94)90455-3. [DOI] [PubMed] [Google Scholar]
- 3.EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J. Hepatol. 2016;65:146–181. doi: 10.1016/j.jhep.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Coté G.A., Singh S., Bucksot L.G., Lazzell-Pannell L., Schmidt S.E., Fogel E., McHenry L., Watkins J., Lehman G., Sherman S. Association between volume of endoscopic retrograde cholangiopancreatography at an academic medical center and use of pancreatobiliary therapy. Clin. Gastroenterol. Hepatol. 2012;10:920–924. doi: 10.1016/j.cgh.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Lesmana C.R.A., Paramitha M.S., Gani R.A. Therapeutic interventional endoscopic ultrasound in pancreato-biliary disorders: Does it really replace the surgical/percutaneous approach? World J. Gastrointest. Surg. 2021;13:537–547. doi: 10.4240/wjgs.v13.i6.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paik W.H., Park D.H. Outcomes and limitations: EUS-guided hepaticogastrostomy. Endosc. Ultrasound. 2019;8((Suppl. S1)):S44–S49. doi: 10.4103/eus.eus_51_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park D.H., Jeong S.U., Lee B.U., Lee S.S., Seo D.W., Lee S.K., Kim M.H. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video) Gastrointest. Endosc. 2013;78:91–101. doi: 10.1016/j.gie.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 8.Ogura T., Kurisu Y., Masuda D., Imoto A., Hayashi M., Malak M., Umegaki E., Uchiyama K., Higuchi K. Novel method of endoscopic ultrasound-guided hepaticogastrostomy to prevent stent dysfunction. J. Gastroenterol. Hepatol. 2014;29:1815–1821. doi: 10.1111/jgh.12598. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen-Tang T., Binmoeller K.F., Sanchez-Yague A., Shah J.N. Endoscopic ultrasound (EUS)-guided transhepatic anterograde self-expandable metal stent (SEMS) placement across malignant biliary obstruction. Endoscopy. 2010;42:232–236. doi: 10.1055/s-0029-1243858. [DOI] [PubMed] [Google Scholar]
- 10.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotton P.B., Eisen G.M., Aabakken L., Baron T.H., Hutter M.M., Jacobson B.C., Mergener K., Nemcek A., Petersen B.T., Petrini J.L., et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest. Endosc. 2010;71:446–454. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 12.World Medical Association World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 13.Anderloni A., Fugazza A., Spadaccini M., Colombo M., Capogreco A., Carrara S., Maselli R., Ferrara E., Galtieri P., Pellegatta G., et al. Feasibility and safety of a new dedicated biliary stent for EUS-guided hepaticogastrostomy: The FIT study (with video) Endosc. Ultrasound. 2023;12:59. doi: 10.4103/EUS-D-22-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Artifon E.L., Marson F.P., Gaidhane M., Kahaleh M., Otoch J.P. Hepaticogastrostomy or choledochoduodenostomy for distal malignant biliary obstruction after failed ERCP: Is there any difference? Gastrointest. Endosc. 2015;81:950–959. doi: 10.1016/j.gie.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 15.Attasaranya S., Netinasunton N., Jongboonyanuparp T., Sottisuporn J., Witeerungrot T., Pirathvisuth T., Ovartlarnporn B. The spectrum of endoscopic ultrasound intervention in biliary diseases: A single center’s experience in 31 cases. Gastroenterol. Res. Pr. 2012;2012:680753. doi: 10.1155/2012/680753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bories E., Pesenti C., Caillol F., Lopes C., Giovannini M. Transgastric endoscopic ultrasonography-guided biliary drainage: Results of a pilot study. Endoscopy. 2007;39:287–291. doi: 10.1055/s-2007-966212. [DOI] [PubMed] [Google Scholar]
- 17.Cho D.H., Lee S.S., Oh D., Song T.J., Park D.H., Seo D.W., Lee S.K., Kim M.-H. Long-term outcomes of a newly developed hybrid metal stent for EUS-guided biliary drainage (with videos) Gastrointest. Endosc. 2017;85:1067–1075. doi: 10.1016/j.gie.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Cho J.H., Park S.W., Kim E.J., Park C.H., Park D.H., Lee K.J., Lee S.S. Long-term outcomes and predictors of adverse events of EUS-guided hepatico-gastrostomy for malignant biliary obstruction: Multicenter, retrospective study. Surg. Endosc. 2022;36:8950–8958. doi: 10.1007/s00464-022-09346-z. [DOI] [PubMed] [Google Scholar]
- 19.Emmanuel J., Omar H., See L.T. Endoscopic ultrasound-guided hepaticogastrostomy using a partially covered metal stent in patients with malignant biliary obstruction after failedEndoscopic retrograde cholangiopancreatography. JGH Open. 2020;4:1059–1064. doi: 10.1002/jgh3.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujii Y., Kato H., Himei H., Ueta E., Ogawa T., Terasawa H., Yamazaki T., Matsumoto K., Horiguchi S., Tsutsumi K., et al. Double guidewire technique stabilization procedure for endoscopic ultrasound-guided hepaticogastrostomy involving modifying the guidewire angle at the insertion site. Surg. Endosc. 2022;36:8981–8991. doi: 10.1007/s00464-022-09350-3. [DOI] [PubMed] [Google Scholar]
- 21.Harai S., Hijioka S., Nagashio Y., Ohba A., Maruki Y., Sone M., Saito Y., Okusaka T., Fukasawa M., Enomoto N. Usefulness of the laser-cut, fully covered, self-expandable metallic stent for endoscopic ultrasound-guided hepaticogastrostomy. J. Hepato-Biliary-Pancreatic Sci. 2022;29:1035–1043. doi: 10.1002/jhbp.1165. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto S., Iwashita Y., Taguchi H., Tanoue S., Ohi T., Shibata R., Haraguchi T., Kamikihara Y., Toyodome K., Kojima I., et al. Comparison of recurrent biliary obstruction with the use of metal and plastic stents in EUS-guided biliary drainage: A propensity score-matched analysis. Endosc. Ultrasound. 2023;12:64–73. doi: 10.4103/EUS-D-21-00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hathorn K.E., Canakis A., Baron T.H. EUS-guided transhepatic biliary drainage: A large single-center U.S. experience. Gastrointest. Endosc. 2022;95:443–451. doi: 10.1016/j.gie.2021.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Hattori N., Ogura T., Ueno S., Okuda A., Nishioka N., Miyano A., Yamamoto Y., Bessho K., Uba Y., Tomita M., et al. Clinical evaluation of a novel drill dilator as the first-line tract dilation technique during EUS-guided biliary drainage by nonexpert hands (with videos) Gastrointest. Endosc. 2023;97:1153–1157. doi: 10.1016/j.gie.2023.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Itoi T., Honjo M., Tsuchiya T., Tanaka R., Tonozuka R., Mukai S., Sofuni A., Nagakawa Y., Iwasaki H., Kanai T. Safety and efficacy of ultra-tapered mechanical dilator for EUS-guided hepaticogastrostomy and pancreatic duct drainage compared with electrocautery dilator (with video) Endosc. Ultrasound. 2018;7:376–382. doi: 10.4103/eus.eus_2_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imai H., Takenaka M., Omoto S., Kamata K., Miyata T., Minaga K., Yamao K., Sakurai T., Nishida N., Watanabe T., et al. Utility of endoscopic ultrasound-guided hepaticogastrostomy with antegrade stenting for malignant biliary obstruction after failed endoscopic retrograde cholangiopancreatography. Oncology. 2017;93((Suppl. S1)):69–75. doi: 10.1159/000481233. [DOI] [PubMed] [Google Scholar]
- 27.Inoue T., Kitano R., Ibusuki M., Sakamoto K., Kimoto S., Kobayashi Y., Sumida Y., Nakade Y., Ito K., Yoneda M. Endoscopic ultrasound-guided hepaticogastrostomy with antegrade stenting without dilation device application for malignant distal biliary obstruction in pancreatic cancer. Dig. Dis. Sci. 2022;68:2090–2098. doi: 10.1007/s10620-022-07749-5. [DOI] [PubMed] [Google Scholar]
- 28.Isayama H., Ishii S., Sasahira N., Matsubara S., Nakai Y., Fujisawa T., Tomishima K., Sasaki T., Ishigaki K., Kogure H., et al. A pilot study of spring stopper stents: Novel partially covered self-expandable metallic stents with anti-migration properties for EUS-guided hepaticogastrostomy. Endosc. Ultrasound. 2023;12:266–272. doi: 10.4103/EUS-D-22-00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishiwatari H., Satoh T., Sato J., Kaneko J., Matsubayashi H., Yabuuchi Y., Kishida Y., Yoshida M., Ito S., Kawata N., et al. Bile aspiration during EUS-guided hepaticogastrostomy is associated with lower risk of postprocedural adverse events: A retrospective single-center study. Surg. Endosc. 2021;35:6836–6845. doi: 10.1007/s00464-020-08189-w. [DOI] [PubMed] [Google Scholar]
- 30.Ishiwatari H., Ishikawa K., Niiya F., Matsubayashi H., Kishida Y., Yoshida M., Kawata N., Imai K., Hotta K., Ono H. Endoscopic ultrasound-guided hepaticogastrostomy versus hepaticogastrostomy with antegrade stenting for malignant distal biliary obstruction. J. Hepato-Biliary-Pancreatic Sci. 2022;29:703–712. doi: 10.1002/jhbp.1118. [DOI] [PubMed] [Google Scholar]
- 31.Iwashita T., Yasuda I., Mukai T., Iwata K., Doi S., Uemura S., Mabuchi M., Okuno M., Shimizu M. Endoscopic ultrasound-guided antegrade biliary stenting for unresectable malignant biliary obstruction in patients with surgically altered anatomy: Single-center prospective pilot study. Dig. Endosc. 2017;29:362–368. doi: 10.1111/den.12800. [DOI] [PubMed] [Google Scholar]
- 32.Iwashita T., Ogura T., Ishiwatari H., Nakai Y., Iwata K., Mukai T., Shimizu M., Isayama H., Yasuda I., Itoi T. Utility of dedicated bougie dilator for a 0.018-inch guidewire during EUS-guided biliary drainage: A multi-center retrospective cohort study. J. Hepato-Biliary-Pancreatic Sci. 2022;29:810–816. doi: 10.1002/jhbp.1021. [DOI] [PubMed] [Google Scholar]
- 33.Jagielski M., Zieliński M., Piątkowski J., Jackowski M. Outcomes and limitations of endoscopic ultrasound-guided hepaticogastrostomy in malignant biliary obstruction. BMC Gastroenterol. 2021;21:202. doi: 10.1186/s12876-021-01798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawakubo K., Isayama H., Kato H., Itoi T., Kawakami H., Hanada K., Ishiwatari H., Yasuda I., Kawamoto H., Itokawa F., et al. Multicenter retrospective study of endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction in Japan. J. Hepato-Biliary-Pancreatic Sci. 2014;21:328–334. doi: 10.1002/jhbp.27. [DOI] [PubMed] [Google Scholar]
- 35.Khashab M.A., Messallam A.A., Penas I., Nakai Y., Modayil R.J., De la Serna C., Hara K., El Zein M., Stavropoulos S.N., Perez-Miranda M., et al. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. choledochoduodenostomy approaches. Endosc. Int. Open. 2016;4:E175–E181. doi: 10.1055/s-0041-109083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitagawa K., Mitoro A., Minami R., Nagamatsu S., Ozutsumi T., Fujinaga Y., Nishimura N., Sawada Y., Namisaki T., Akahane T., et al. Efficacy of a dedicated plastic stent in endoscopic ultrasound-guided hepaticogastrostomy during the learning curve: Cumulative multi-center experience. Scand. J. Gastroenterol. 2023;58:296–303. doi: 10.1080/00365521.2022.2118557. [DOI] [PubMed] [Google Scholar]
- 37.Kobori I., Hashimoto Y., Shibuki T., Okumura K., Sekine M., Miyagaki A., Sasaki Y., Takano Y., Katayama Y., Kuwada M., et al. Safe performance of track dilation and bile aspiration with ERCP catheter in eus-guided hepaticogastrostomy with plastic stents: A retrospective multicenter study. J. Clin. Med. 2022;11:4986. doi: 10.3390/jcm11174986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marx M., Caillol F., Sfumato P., Romero J., Ratone J.-P., Pesenti C., Godat S., Hoibian S., Dahel Y., Boher J.M., et al. EUS-guided hepaticogastrostomy in the management of malignant biliary obstruction: Experience and learning curve in a tertiary referral center. Dig. Liver Dis. 2022;54:1236–1242. doi: 10.1016/j.dld.2022.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Marx M., Caillol F., Autret A., Ratone J.-P., Zemmour C., Boher J., Pesenti C., Bories E., Barthet M., Napoléon B., et al. EUS-guided hepaticogastrostomy in patients with obstructive jaundice after failed or impossible endoscopic retrograde drainage: A multicenter, randomized phase II Study. Endosc. Ultrasound. 2022;11:495–502. doi: 10.4103/EUS-D-21-00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itoi T., Matsunami Y., Sofuni A., Tsuchiya T., Ishii K., Tanaka R., Tonozuka R., Honjo M., Mukai S., Nagai K., et al. EUS-guided hepaticoenterostomy with using a dedicated plastic stent for the benign pancreaticobiliary diseases: A single-center study of a large case series. Endosc. Ultrasound. 2021;10:294–304. doi: 10.4103/EUS-D-20-00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minaga K., Takenaka M., Kitano M., Chiba Y., Imai H., Yamao K., Kamata K., Miyata T., Omoto S., Sakurai T., et al. Rescue EUS-guided intrahepatic biliary drainage for malignant hilar biliary stricture after failed transpapillary re-intervention. Surg. Endosc. 2017;31:4764–4772. doi: 10.1007/s00464-017-5553-6. [DOI] [PubMed] [Google Scholar]
- 42.Minaga K., Kitano M., Uenoyama Y., Hatamaru K., Shiomi H., Ikezawa K., Miyagahara T., Imai H., Fujimori N., Matsumoto H., et al. Feasibility and efficacy of endoscopic reintervention after covered metal stent placement for EUS-guided hepaticogastrostomy: A multicenter experience. Endosc. Ultrasound. 2022;11:478–486. doi: 10.4103/EUS-D-22-00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miwa H., Sugimori K., Matsuoka Y., Endo K., Oishi R., Nishimura M., Tozuka Y., Kaneko T., Numata K., Maeda S. Loop technique for guidewire manipulation during endoscopic ultrasound-guided hepaticogastrostomy. JGH Open. 2023;7:358–364. doi: 10.1002/jgh3.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyano A., Ogura T., Yamamoto K., Okuda A., Nishioka N., Higuchi K. clinical impact of the intra-scope channel stent release technique in preventing stent migration during EUS-guided hepaticogastrostomy. J. Gastrointest. Surg. 2018;22:1312–1318. doi: 10.1007/s11605-018-3758-1. [DOI] [PubMed] [Google Scholar]
- 45.Moryoussef F., Sportes A., Leblanc S., Bachet J.B., Chaussade S., Prat F. Is EUS-guided drainage a suitable alternative technique in case of proximal biliary obstruction? Ther. Adv. Gastroenterol. 2017;10:537–544. doi: 10.1177/1756283X17702614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakai Y., Isayama H., Yamamoto N., Matsubara S., Ito Y., Sasahira N., Hakuta R., Umefune G., Takahara N., Hamada T., et al. Safety and effectiveness of a long, partially covered metal stent for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Endoscopy. 2016;48:1125–1128. doi: 10.1055/s-0042-116595. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura J., Ogura T., Ueno S., Okuda A., Nishioka N., Uba Y., Tomita M., Bessho K., Hattori N., Nishikawa H. Liver impaction technique improves technical success rate of guidewire insertion during EUS-guided hepaticogastrostomy (with video) Ther. Adv. Gastroenterol. 2023;16:17562848231188562. doi: 10.1177/17562848231188562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ochiai K., Fujisawa T., Ishii S., Suzuki A., Saito H., Takasaki Y., Ushio M., Takahashi S., Yamagata W., Tomishima K., et al. Risk factors for stent migration into the abdominal cavity after endoscopic ultrasound-guided hepaticogastrostomy. J. Clin. Med. 2021;10:3111. doi: 10.3390/jcm10143111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogura T., Chiba Y., Masuda D., Kitano M., Sano T., Saori O., Yamamoto K., Imaoka H., Imoto A., Takeuchi T., et al. Comparison of the clinical impact of endoscopic ultrasound-guided choledochoduodenostomy and hepaticogastrostomy for bile duct obstruction with duodenal obstruction. Endoscopy. 2016;48:156–163. doi: 10.1055/s-0034-1392859. [DOI] [PubMed] [Google Scholar]
- 50.Ogura T., Kitano M., Takenaka M., Okuda A., Minaga K., Yamao K., Yamashita Y., Hatamaru K., Noguchi C., Gotoh Y., et al. Multicenter prospective evaluation study of endoscopic ultrasound-guided hepaticogastrostomy combined with antegrade stenting (with video) Dig. Endosc. 2018;30:252–259. doi: 10.1111/den.12976. [DOI] [PubMed] [Google Scholar]
- 51.Ogura T., Ueno S., Okuda A., Nishioka N., Yamada M., Matsuno J., Ueshima K., Yamamoto Y., Higuchi K. Technical feasibility and safety of one-step deployment of EUS-guided hepaticogastrostomy using an 8-mm diameter metal stent with a fine-gauge stent delivery system (with video) Endosc. Ultrasound. 2021;10:355–360. doi: 10.4103/EUS-D-20-00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oh D., Park D.H., Song T.J., Lee S.S., Seo D.-W., Lee S.K., Kim M.-H. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Ther. Adv. Gastroenterol. 2017;10:42–53. doi: 10.1177/1756283X16671671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohno A., Fujimori N., Kaku T., Takamatsu Y., Matsumoto K., Murakami M., Teramatsu K., Takeno A., Hijioka M., Kawabe K., et al. Feasibility and efficacy of endoscopic ultrasound-guided hepaticogastrostomy without dilation: A propensity score matching analysis. Dig. Dis. Sci. 2022;67:5676–5684. doi: 10.1007/s10620-022-07555-z. [DOI] [PubMed] [Google Scholar]
- 54.Okuno N., Hara K., Mizuno N., Kuwahara T., Iwaya H., Ito A., Kuraoka N., Matsumoto S., Polmanee P., Niwa Y. Efficacy of the 6-mm fully covered self-expandable metal stent during endoscopic ultrasound-guided hepaticogastrostomy as a primary biliary drainage for the cases estimated difficult endoscopic retrograde cholangiopancreatography: A prospective clinical study. J. Gastroenterol. Hepatol. 2018;33:1413–1421. doi: 10.1111/jgh.14112. [DOI] [PubMed] [Google Scholar]
- 55.Hara K., Okuno N., Mizuno N., Haba S., Kuwahara T., Kuraishi Y., Tajika M., Tanaka T., Onishi S., Yamada K., et al. B2 puncture with forward-viewing EUS simplifies EUS-guided hepaticogastrostomy (with video) Endosc. Ultrasound. 2022;11:319–324. doi: 10.4103/EUS-D-21-00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okuno N., Hara K., Haba S., Kuwahara T., Kuraishi Y., Yanaidani T., Ishikawa S., Yasuda T., Yamada M., Fukui T. Novel drill dilator facilitates endoscopic ultrasound-guided hepaticogastrostomy. Dig. Endosc. 2022;35:389–393. doi: 10.1111/den.14447. [DOI] [PubMed] [Google Scholar]
- 57.Paik W.H., Lee N.K., Nakai Y., Isayama H., Oh D., Song T.J., Lee S.S., Seo D.-W., Lee S.K., Kim M.-H., et al. Conversion of external percutaneous transhepatic biliary drainage to endoscopic ultrasound-guided hepaticogastrostomy after failed standard internal stenting for malignant biliary obstruction. Endoscopy. 2017;49:544–548. doi: 10.1055/s-0043-102388. [DOI] [PubMed] [Google Scholar]
- 58.Paik W.H., Park D.H., Choi J.H., Choi J.H., Lee S.S., Seo D.W., Lee S.K., Kim M.H. Lee Simplified fistula dilation technique and modified stent deployment maneuver for EUS-guided hepaticogastrostomy. World J. Gastroenterol. 2014;20:5051–5059. doi: 10.3748/wjg.v20.i17.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park D.H., Jang J.W., Lee S.S., Seo D.-W., Lee S.K., Kim M.-H. EUS-guided biliary drainage with transluminal stenting after failed ERCP: Predictors of adverse events and long-term results. Gastrointest. Endosc. 2011;74:1276–1284. doi: 10.1016/j.gie.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 60.Park D.H., Lee T.H., Paik W.H., Choi J., Song T.J., Lee S.S., Seo D., Lee S.K., Kim M. Feasibility and safety of a novel dedicated device for one-step EUS-guided biliary drainage: A randomized trial. J. Gastroenterol. Hepatol. 2015;30:1461–1466. doi: 10.1111/jgh.13027. [DOI] [PubMed] [Google Scholar]
- 61.Poincloux L., Rouquette O., Buc E., Privat J., Pezet D., Dapoigny M., Bommelaer G., Abergel A. Endoscopic ultrasound-guided biliary drainage after failed ERCP: Cumulative experience of 101 procedures at a single center. Endoscopy. 2015;47:794–801. doi: 10.1055/s-0034-1391988. [DOI] [PubMed] [Google Scholar]
- 62.Prachayakul V. A novel technique for endoscopic ultrasound-guided biliary drainage. World J. Gastroenterol. 2013;19:4758–4763. doi: 10.3748/wjg.v19.i29.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ragab K., Abdel-Hameed M., Gouda M., Katamish H., Madkour A., Atalla H., Hamed H., Shiha G., Abdallah O., Agwa R., et al. Endoscopic ultrasound-guided biliary drainage for distal malignant biliary obstruction: A prospective 3-year multicenter Egyptian study. Acta Gastro Enterol. Belg. 2023;86:26–35. doi: 10.51821/86.1.10828. [DOI] [PubMed] [Google Scholar]
- 64.Samanta J., Sundaram S., Dhar J., Mane K., Gupta P., Gupta V., Patil P., Sinha S.K., Kochhar R., Mehta S. EUS-guided biliary drainage in patients with moderate–severe cholangitis is safe and effective: A multi-center experience. Surg. Endosc. 2023;37:298–308. doi: 10.1007/s00464-022-09495-1. [DOI] [PubMed] [Google Scholar]
- 65.Sassatelli R., Cecinato P., Lupo M., Azzolini F., Decembrino F., Iori V., Sereni G., Tioli C., Cavina M., Zecchini R., et al. Endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction after failed ERCP in low performance status patients. Dig. Liver Dis. 2020;52:57–63. doi: 10.1016/j.dld.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 66.Napoléon B., Schoch A., Lisotti A., Walter T., Fumex F., Leblanc S., Artru P., Desramé J., Brighi N., Marsot J., et al. Efficacy of EUS-guided hepaticogastrostomy in prolonging survival of patients with perihilar cholangiocarcinoma. Endosc. Ultrasound. 2022;11:487–494. doi: 10.4103/EUS-D-22-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sekine M., Hashimoto Y., Shibuki T., Okumura K., Kobori I., Miyagaki A., Sasaki Y., Takano Y., Matsumoto K., Mashima H. A retrospective multicenter study comparing the punctures to B2 and B3 in endoscopic ultrasound–guided hepaticogastrostomy. DEN Open. 2023;3:e201. doi: 10.1002/deo2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shibuki T., Okumura K., Sekine M., Kobori I., Miyagaki A., Sasaki Y., Takano Y., Hashimoto Y. Covered self-expandable metallic stents versus plastic stents for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Gastrointest. Endosc. 2023;56:802–811. doi: 10.5946/ce.2022.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shin I.S., Moon J.H., Lee Y.N., Myeong J.H., Lee T.H., Yang J.K., Cho Y.D., Park S.-H., Giovannini M. Preliminary feasibility study of a new partially covered self-expandable metal stent with an anchoring flange for EUS-guided hepaticogastrostomy (with videos) Gastrointest. Endosc. 2023;98:848–856. doi: 10.1016/j.gie.2023.07.020. [DOI] [PubMed] [Google Scholar]
- 70.Song T.J., Lee S.S., Park D.H., Seo D.W., Lee S.K., Kim M.-H. Preliminary report on a new hybrid metal stent for EUS-guided biliary drainage (with videos) Gastrointest. Endosc. 2014;80:707–711. doi: 10.1016/j.gie.2014.05.327. [DOI] [PubMed] [Google Scholar]
- 71.Sportes A., Camus M., Greget M., Leblanc S., Coriat R., Hochberger J., Chaussade S., Grabar S., Prat F. Endoscopic ultrasound-guided hepaticogastrostomy versus percutaneous transhepatic drainage for malignant biliary obstruction after failed endoscopic retrograde cholangiopancreatography: A retrospective expertise-based study from two centers. Ther. Adv. Gastroenterol. 2017;10:483–493. doi: 10.1177/1756283X17702096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takenaka M., Rehani M.M., Hosono M., Yamazaki T., Omoto S., Minaga K., Kamata K., Yamao K., Hayashi S., Nishida T., et al. Comparison of Radiation Exposure between Endoscopic Ultrasound-Guided Hepaticogastrostomy and Hepaticogastrostomy with Antegrade Stenting. J. Clin. Med. 2022;11:1705. doi: 10.3390/jcm11061705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kahaleh M., Tyberg A., Napoleon B., Robles-Medranda C., Shah J., Bories E., Kumta N., Yague A., Vazquez-Sequeiros E., Lakhtakia S., et al. Hepaticogastrostomy versus choledochoduodenostomy: An international multicenter study on their long-term patency. Endosc. Ultrasound. 2022;11:38–43. doi: 10.4103/EUS-D-21-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Umeda J., Itoi T., Tsuchiya T., Sofuni A., Itokawa F., Ishii K., Tsuji S., Ikeuchi N., Kamada K., Tanaka R., et al. A newly designed plastic stent for EUS-guided hepaticogastrostomy: A prospective preliminary feasibility study (with videos) Gastrointest. Endosc. 2015;82:390–396.e2. doi: 10.1016/j.gie.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 75.Vila J.J., Pérez-Miranda M., Vazquez-Sequeiros E., Abadia M.A.-S., Pérez-Millán A., González-Huix F., Gornals J., Iglesias-Garcia J., De la Serna C., Aparicio J.R., et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: A Spanish national survey. Gastrointest. Endosc. 2012;76:1133–1141. doi: 10.1016/j.gie.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 76.Yagi S., Kurita Y., Sato T., Hasegawa S., Hosono K., Kobayashi N., Endo I., Saigusa Y., Kubota K., Nakajima A. Utility of Fine-Gauge Balloon Catheter for EUS-Guided Hepaticogastrostomy. J. Clin. Med. 2022;11:5681. doi: 10.3390/jcm11195681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Itoi T., Yamamoto K., Tsuchiya T., Tanaka R., Tonozuka R., Honjo M., Mukai S., Fujita M., Asai Y., Matsunami Y., et al. EUS-guided antegrade metal stenting with hepaticoenterostomy using a dedicated plastic stent with a review of the literature (with video) Endosc. Ultrasound. 2018;7:404–412. doi: 10.4103/eus.eus_51_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamamura M., Ogura T., Ueno S., Okuda A., Nishioka N., Yamada M., Ueshima K., Matsuno J., Yamamoto Y., Higuchi K. Partially covered self-expandable metal stent with antimigratory single flange plays important role during EUS-guided hepaticogastrostomy. Endosc. Int. Open. 2022;10:E209–E214. doi: 10.1055/a-1729-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yane K., Yoshida M., Imagawa T., Morita K., Ihara H., Hanada K., Hirokawa S., Tomita Y., Minagawa T., Okagawa Y., et al. Usefulness of endoscopic ultrasound-guided transhepatic biliary drainage with a 22-gauge fine-needle aspiration needle and 0.018-inch guidewire in the procedure’s induction phase. DEN Open. 2024;4:e297. doi: 10.1002/deo2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yasuda T., Hara K., Mizuno N., Haba S., Kuwahara T., Okuno N., Kuraishi Y., Yanaidani T., Ishikawa S., Yamada M., et al. Safety of endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction and ascites. Clin. Endosc. 2024;57:246–252. doi: 10.5946/ce.2023.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y., Wang X., Sun K., Chen J., Zhang Y., Shi L., Fan Z., Liu L., Chen B., Ding Y. Application of endoscopic ultrasound-guided hepaticogastrostomy combined with antegrade stenting in patients with malignant biliary obstruction after failed ERCP. Surg. Endosc. 2022;36:5930–5937. doi: 10.1007/s00464-022-09117-w. [DOI] [PubMed] [Google Scholar]
- 82.Fugazza A., Colombo M., Spadaccini M., Vespa E., Gabbiadini R., Capogreco A., Repici A., Anderloni A. Relief of jaundice in malignant biliary obstruction: When should we consider endoscopic ultrasonography-guided hepaticogastrostomy as an option? Hepatobiliary Pancreat. Dis. Int. 2022;21:234–240. doi: 10.1016/j.hbpd.2022.03.003. [DOI] [PubMed] [Google Scholar]
- 83.Gupta K., Perez-Miranda M., Kahaleh M., Artifon E.L., Itoi T., Freeman M.L., De-Serna C., Sauer B., Giovannini M., InEBD Study Group Endoscopic ultrasound-assisted bile duct access and drainage: Multicenter, long-term analysis of approach, outcomes, and complications of a technique in evolution. J. Clin. Gastroenterol. 2014;48:80–87. doi: 10.1097/MCG.0b013e31828c6822. [DOI] [PubMed] [Google Scholar]
- 84.Giovannini M., Moutardier V., Pesenti C., Bories E., Lelong B., Delpero J.R. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 85.Facciorusso A., Mangiavillano B., Paduano D., Binda C., Crinò S.F., Gkolfakis P., Ramai D., Fugazza A., Tarantino I., Lisotti A., et al. Methods for Drainage of Distal Malignant Biliary Obstruction after ERCP Failure: A Systematic Review and Network Meta-Analysis. Cancers. 2022;14:3291. doi: 10.3390/cancers14133291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mao K., Hu B., Sun F., Wan K. Choledochoduodenostomy versus Hepaticogastrostomy in Endoscopic Ultrasound-guided Drainage for Malignant Biliary Obstruction: A Meta-analysis and Systematic Review. Surg. Laparosc. Endosc. Percutaneous Tech. 2021;32:124–132. doi: 10.1097/SLE.0000000000000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nennstiel S., Weber A., Frick G., Haller B., Meining A., Schmid R.M., Neu B. Drainage-related Complications in Percutaneous Transhepatic Biliary Drainage: An Analysis over 10 Years. J. Clin. Gastroenterol. 2015;49:764–770. doi: 10.1097/MCG.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 88.Ogura T., Higuchi K. Endoscopic Ultrasound-Guided Hepaticogastrostomy: Technical Review and Tips to Prevent Adverse Events. Gut Liver. 2021;15:196–205. doi: 10.5009/gnl20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li J., Tang J., Liu F., Fang J. Comparison of Choledochoduodenostomy and Hepaticogastrostomy for EUS-Guided Biliary Drainage: A Meta-Analysis. Front. Surg. 2022;9:811005. doi: 10.3389/fsurg.2022.811005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morita S., Sugawara S., Suda T., Hoshi T., Abe S., Yagi K., Terai S. Conversion of percutaneous transhepatic biliary drainage to endoscopic ultrasound-guided biliary drainage. DEN Open. 2021;1:e6. doi: 10.1002/deo2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chantarojanasiri T., Ratanachu-Ek T., Pausawasdi N. What You Need to Know before Performing Endoscopic Ultrasound-guided Hepaticogastrostomy. Clin. Endosc. 2021;54:301–308. doi: 10.5946/ce.2021.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.