Abstract
Recombinant adeno-associated virus (rAAV) is capable of directing long-term, high-level transgene expression without destructive cell-mediated immune responses. However, traditional packaging methods for rAAV vectors are generally inefficient and contaminated with replication-competent AAV (rcAAV) particles. Although wild-type AAV is not associated with any known human diseases, contaminating rcAAV particles may affect rAAV gene expression and are an uncontrolled variable in many AAV gene transfer studies. In the current study, a novel strategy was designed to both optimize AAV rep gene expression and increase vector yield, as well as simultaneously to diminish the potential of generating rcAAV particles from the helper plasmid. The strategy is based on the insertion of an additional intron in the AAV genome. In the AAV infectious clone, the intron insertion had no effects on the properties of Rep proteins expressed. Normal levels of both Rep and Cap proteins were expressed, and the replication of the AAV genome was not impaired. However, the generation of infectious rcAAV particles using intronized AAV helper was greatly diminished, which was due to the oversized AAV genome caused by the insertion of the artificial introns. Moreover, the rAAV packaging was significantly improved with the appropriate choice of intron and insertion position. The intron is another element that can regulate the rep and cap gene expression from the helper plasmid. This study provides for a novel AAV packaging system which is highly versatile and efficient. It can not only be combined with other AAV packaging systems, including rep-containing cell lines and herpes simplex virus hybrid packaging methods, but also be used in other vector systems as well.
Adeno-associated virus type 2 (AAV-2) is a nonpathogenic human parvovirus with a single-stranded DNA genome which consists of approximately 4.7 kb (24). All characterized AAV serotypes share three key features, including two copies of AAV terminal repeats (ITRs), one rep region and one cap region. The ITRs are capable of forming T-shape secondary structure and are the only cis elements that are required for AAV replication, packaging, integration, and rescue. In recombinant AAV (rAAV) vectors, the ITRs are generally the only viral element preserved. When rAAV vectors transduce target cells, the ITRs are believed to play a role in mediating rAAV concatemerization (9, 33). This property has been utilized in a variety of strategies to increase the gene expression from rAAV vector and to overcome the size limitation of the AAV packaging capacity (10, 25, 28). The rep region encodes four overlapping proteins designated as Rep78, Rep68, Rep52, and Rep40, according to the apparent molecular mass of the protein. In addition to their well-defined roles in AAV replication, Rep proteins also regulate AAV packaging and site-specific integration. The cap region encodes three structural proteins, VP1, VP2, and VP3. These three proteins share the same reading frame. The ratio of VP1, VP2, and VP3 in AAV virion is ca. 1:1:10. Besides these features, there is a single intron of 321 bp in the AAV genome. In AAV-2, this intron is located at positions 1907 to 2228. When the RNA is not spliced, the intron serves as codons for Rep78 and Rep52. In mRNA for Rep68 and Rep40, this intron is removed. The regulation of the expression of these two classes of proteins appears to control AAV replication and packaging (2).
AAV has increasingly become an important gene therapy vector, which is largely due to the fact that wild-type AAV (wtAAV) is not related to any known human diseases and has the capability to integrate site specifically into the human genome at chromosome 19 qter13.3 (20–22, 27). Although the rAAV vector can no longer integrate in a site-specific manner since the rep gene is removed from rAAV genome to accommodate the transgene, rAAV genomes can persist in vivo as concatemers either in the host chromosome or the episome. The ability of rAAV vectors to direct long-term transgene expression in many tissues has been demonstrated in numerous animal experiments (11, 13, 18, 32). The lack of devastating cell-mediated immune responses is another key reason for the prolonged gene expression in vivo. Most recently, some of the promise of gene therapy may finally be realized with the exciting data obtained in the phase I clinical trial of hemophilia B using AAV, further suggesting the potential of this parvovirus (19).
In general, rAAV vectors are generated by transfection of a vector plasmid and a helper plasmid in the presence of helper virus infection (26). The vector plasmid is constructed by replacing the whole coding region of AAV genome with a transgene expression cassette. The helper plasmid contains the AAV coding region except the ITRs. Although there is no overlapping sequence between the vector plasmid and the helper plasmid, the probability of generation of replication-competent AAV (rcAAV) particles through nonhomologous recombination is quite high (1). In some cases, these particles appear to affect transgene expression (15). Moreover, such undesired particles are also a safety concern in applications of AAV vectors for human gene therapy.
Previous studies have addressed in part the issue of generation of the rcAAV particles in rAAV production (1, 16, 29). Wang et al. reported that a truncated AAV terminal repeat D10 was effective in reducing the probability of generation wtAAV particles (29). They reported that the first 10 nucleotides in the D sequence proximal to the AAV hairpin structures are essential for successful replication and encapsidation of the viral genome and that the 10 nucleotides in the AAV D sequence distal to viral hairpin structures are involved in recombination events leading rcAAV generation. The combined use of rAAV plasmids lacking the distal 10 nucleotides in the D sequence and helper plasmids lacking the adenovirus ITRs led to complete elimination of replication-competent wtAAV-like particles in recombinant vector stocks. Another approach using heterologous promoters for driving the rep gene and the cap gene can also be employed to control rcAAV contamination. However, it is also reported that the rcAAV particles can still be detected in large-scale preparations by using more sensitive assays (1, 12). Splitting the rep gene and the cap gene into different vectors is yet another approach to reducing the rcAAV. Gao et al. reported that the AAV rep and cap cell line B50 can reduce the generation of rcAAV significantly, which may result from considerably lower copies of integrated helper genome. Generally, these approaches led to the decrease in rAAV packaging efficiency. We conducted here a study to explore a new approach to reduce the generation of rcAAV particles using intron-mediated mutagenesis of AAV genome. The additional intron in the AAV coding region sufficiently increased AAV genome to a size beyond that associated with effective packaging. In some of these new helper plasmids, the size of the rep gene alone was beyond the limit of AAV packaging, which effectively reduced the potential of generating rcAAV particles. All of these new helper plasmids exhibited at least the same efficiency in supporting rAAV replication and packaging. In addition, the rAAV yield could be improved through controlling the gene expression profile by the selection of appropriate introns and the insertion positions.
MATERIALS AND METHODS
Cell culture.
Human 293 cell lines were obtained from the American Type Culture Collection and maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 μg of streptomycin per ml, and 100 U of penicillin per ml (all purchased from Sigma). Cells were maintained in a humidified 37°C incubator with 5% CO2.
Transfection.
Transfections were carried out using Lipofectamine or calcium phosphate precipitation. Lipofectamine was purchased from GIBCO-BRL. The transfections were performed as recommended by the manufacturers. For transfection using calcium phosphate precipitation, the method used was as described previously (30).
Immunohistochemical staining.
To examine cells expressing β-galactosidase, the cells were fixed on plates by incubation for 5 min in ice-cold 2% formaldehyde and 0.2% glutaraldehyde in phosphate-buffered saline (PBS). After three washes with PBS, the β-galactosidase activity was detected by staining the cells for 4 h in PBS containing 5 mM K4Fe(CN)6, 1 mM MgCl2, and 1 mg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) per ml. The reaction was stopped by removing the staining solution and replacing it with PBS containing 10% glycerol.
Plasmid construction and DNA manipulation.
The 850-bp human β-globin intron 2 was amplified by PCR from human genomic DNA using primers INS1 (5′-GTT TTG GGA CGT TTC CTG AGT CAG GTG AGT CTA TGG GAC CCT TGA TG-3′) and INA2 (5′-CAG TTT TTC GCG AAT CTG TGG GAG GAA GAT AAG AGG TAT G-3′). An AAV fragment was amplified with primer VS1 (5′-CCG TGG CCG AGA AGC TGC AGC GCG ACT TTC-3′) and INA1 (5′-CAT CAA GGG TCC CAT AGA CTC ACC TGA CTC AGG AAA CGT CCC AAA AC-3′). The obtained intron fragment and AAV fragment were linked together by PCR using primer VS1 and INA2. The resulting fragment was digested with SfiI and NruI and cloned into pSub201 at the same sites to obtain piAAV. The resulting plasmid piAAV has β-globin intron at position 654. The intron cloned into this position maintained the consensus sequence of the splice donor site and the splice acceptor site. The helper plasmid pCLR1 was cloned by swapping the SfiI-NruI fragment of piAAV850 to pAd/AAV. The helper plasmids pCLR0, pCLR2, pCLV1, pCLV2, and pCLV3 were cloned in a similar way by inserting the 850-bp human β-globin intron into AAV genome at positions 302, 1529, 2309, 2728, and 2916, respectively. These sites correspond to the position in RNA for 5′-untranslated region, Rep52 and Rep40, VP1, VP2, and VP3. All of these insertions maintained the consensus sequences for the splice donor sites and acceptor sites. To generate pCLR-C3k, the human collagen intron was amplified by 5′-CGG AGA AGC AGT GGA TCC AGG TGA GTA ATT GAC AAA GCC A-3′ and 5′-GAT GTA TGA GGC CTG GTC CTC CTG TGA GCA AGA AGG AAG TG-3′ and then cloned into pAd/AAV at position 1052. The 1.5-, 2.0-, and 3.5-k bp lambda DNA fragments (EcoRI/HindIII digestion) were cloned into the MfeI site in the globin intron in pCLR1 to generate pCLR1-1.5k, pCLR1-2.0k, and pCLR1-3.5k, respectively. All PCRs were performed using the Expand Long Template PCR System (Roche) according to the manufacturer's instructions. The construction of p5E18 was as described previously (30, 31).
Total cellular DNA was extracted from cells according to the protocol described in The Current Protocols in Molecular Biology (2a). Specifically, the cells were harvested, washed once with PBS, and digested with proteinase K in the presence of 150 mM NaCl, 10 mM Tris, and 100 mM EDTA at 37°C overnight. After two extractions with phenol-chloroform, the DNA was precipitated with two times the volume of ethanol and used for PCR analysis.
AAV production and purification.
The rAAV vectors were produced as described previously (31). The rAAV vectors were purified using heparin affinity chromatography as described by Clark et al. (4).
rcAAV assay and wtAAV titer determination.
The infectious rcAAV or wtAAV was assayed using a modified method described by Clark et al. (6). In detail, the AAV or rAAV preparations in 10-fold dilutions were used to infect 293 cells in the presence of adenovirus infection at a multiplicity of infection (MOI) of 10. The cells were harvested at 36 h postinfection, and total cellular DNA was extracted. The amount of rcAAV or wtAAV was determined by PCR analysis of total cellular DNA for the presence AAV rep region using the primers 5′-CCG TGG CCG AGA AGC TGC AGC GCG ACT TTC-3′ and 5′-CCC CTC CTC CCA CCA GAT CAC CAT C-3′. The last dilution with positive signal was used to calculate the amount of infectious rcAAV and wtAAV particles.
The AAV virion titer was determined by enzyme-linked immunosorbent assay (ELISA) using a Progen kit (15). The procedures were carried out as described by the manufacturer.
AAV genome titer was determined by dot blot. The procedures were as described previously (14).
rAAV titer determination.
The rAAV infectious titer was determined using either green fluorescent protein (GFP) or lacZ as the reporter gene. For rAAV-lacZ, each blue cell after X-Gal staining represents one infectious unit (IU). For rAAV-GFP, each green cell under fluorescence microscopy represents one IU.
AAV genome titer was determined by dot blot or quantitative PCR. The dot blot procedures were described previously (14). Quantitative PCR was carried out using PRISM/7700 Sequence Detector (PE Applied Biosystems). The AAV-tet-GCSF, the primers were designed to amplify the 72-bp GCSF sequences. The forward and reverse primers were 5′-GTG CTT AGA GCA AGT GAG GAA GAT C-3′ and 5′-GCA CAC TCA CTC ACC AGC TTC T-3′, respectively. The reactions were performed according to the instructions of the manufacturer using the SYBR Green PCR Core Reagents Kit (PE Biosystems). A DNA plasmid standard curve was set up using 103 to 107 AAV genome equivalents. The virus DNA samples were prepared by digesting DNase-resistant virus with proteinase K in 1× PCR buffer at 50°C for 1 h, followed by boiling for 20 min.
Western blot.
The harvested cells were lysed with radioimmunoprecipitation assay buffer (10 mM Tris, pH 8.2; 1% Triton X-100; 1% sodium dodecyl sulfate; 0.15 M NaCl). About 10 μg of protein for each sample was electrophoresed on 10% polyacrylamide gels. Proteins were transferred to nitrocellulose membranes, and the Rep and capsid proteins were detected with anti-Rep (259.5) and anti-Cap monoclonal antibodies. All of these antibodies were purchased from Research Diagnostics, Inc. (Flanders, N.J.). An enhanced chemiluminescence kit (Amersham) was used to develop the final pictures.
RESULTS
The additional intron rendered AAV inefficient for packaging.
In the wtAAV genome, there is only a single native intron. The alternative splicing from this native intron gives rise to mRNA for Rep68, Rep40, and VP1. The small intron itself encodes amino acid residues for Rep78 and Rep52. Due to the size restraint of the AAV virion, nonessential introns cannot be accommodated in the AAV genome. To explore the biological effects of additional artificial introns on AAV replication and gene expression, we introduced the 850-bp human β-globin intron into the AAV genome at position 654 (Fig. 1A) and obtained an infectious AAV clone piAAV (“i” stands for intron). This insertion was located in the coding region of the gene driven by the p5 promoter. Being upstream, it was predicted to have no effects on the transcripts from p19 and p40 promoters. We reasoned that the overall AAV gene expression should remain unchanged if the artificial intron could be spliced out efficiently. The replication of AAV genome should not be impaired. One major effect would be the increase in the AAV genome size. Since parvoviruses, including AAV, can generally accommodate no more than an additional 5% of their genome, we anticipated that the modified oversized virus would be a lot less efficient for packaging even with the normal level of rep and cap gene expression and virus DNA replication.
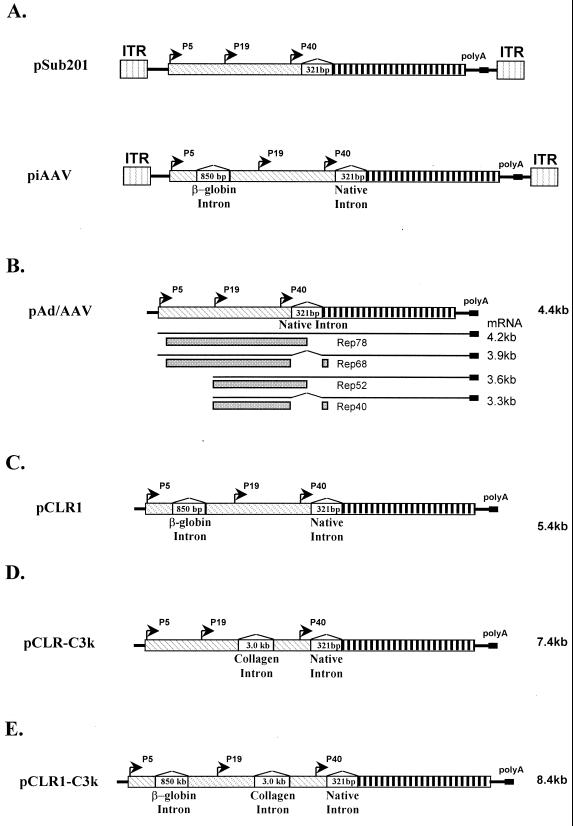
FIG. 1.
Illustration of constructs. (A) Infectious clones pSub201 and piAAV. Key features were identified in the figure. (B) Helper plasmid pAd/AAV. Note that there are no ITRs in pAd/AAV. The transcripts and Rep proteins expressed are identified in the figure. (C) pCLR1 with non-native β-globin intron. (D) pCLR-3K with non-native collagen intron. (E) pCLR1-3k with both β-globin and collagen introns.
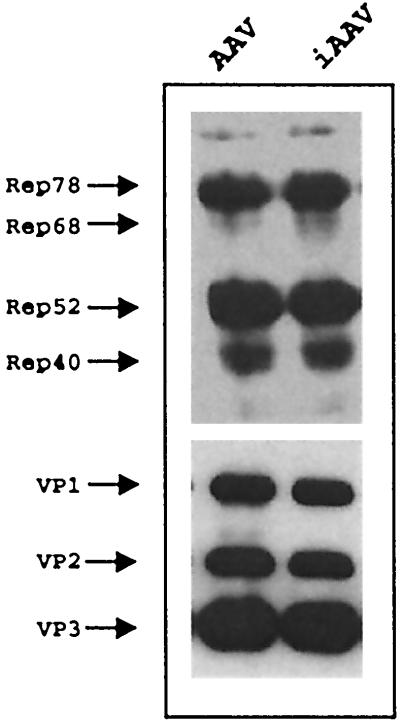
We performed several experiments to test the above hypothesis. The replication and packaging of pSub201 and piAAV were compared after equal amounts of each plasmid were transfected into 293 cells in the presence of helper virus adenovirus infection. The AAV rep and cap gene expression profile of piAAV was almost identical to that of pSub201 (Fig. 2). The rescue and replication of AAV and iAAV (intronized AAV) were indistinguishable from each other since the plasmid backbone and AAV ITRs are identical in these two constructs. The packaged virion particles assayed by ELISA were almost identical between pSub201 and piAAV (Table 1). However, there was a distinct difference in the genomic titer measured by dot blot. About 50% of virus produced from pSub201 contained DNA, while <20% of virus generated from piAAV contained DNA. A comparison of infectious particles revealed even more dramatic differences. One of approximately one-hundred virus particles generated from pSub201 was infectious and was capable of replication. Less than one of ten thousand particles generated from piAAV was capable of replication. These data suggested that the majority of particles generated from piAAV were defective particles which cannot replicate.
FIG. 2.
Western blot of rep and cap gene expression from AAV and iAAV. The human 293 cells were transfected with 10 μg of plasmid DNA for pSub201 or piAAV and infected with adenovirus at an MOI of 10. The cells were harvested 36 h postinfection. The Western blot for Rep and Cap proteins was carried out as described in Materials and Methods. A total of 10 μg of protein was used in each lane for Western blot.
TABLE 1.
Comparison of wtAAV generation from pSub201 and piAAVa
| AAV | Virion titer (pts/ml) as determined by ELISA | Genome titer (genomes/ml) as determined by dot blot | Infectious titer (IU/ml) as determined by infectious assay | Genomes/pts (%) | IU/pts |
|---|---|---|---|---|---|
| wtAAV | 2.7 × 1012 | 1.35 × 1012 | 2 × 1010 | 50 | 1:135 |
| piAAV | 3.8 × 1012 | 6.75 × 1011 | 2 × 108 | 17.8 | 1:19,000 |
The human 293 cells were transfected with 5 μg of pSub201 or piAAV in the presence of adenovirus infection at an MOI of 10. The virus was harvested at 48 h postinfection, and the virus titer was determined according to the procedures described in Materials and Methods. pts, particle(s).
Helper plasmids with introns supported rAAV production.
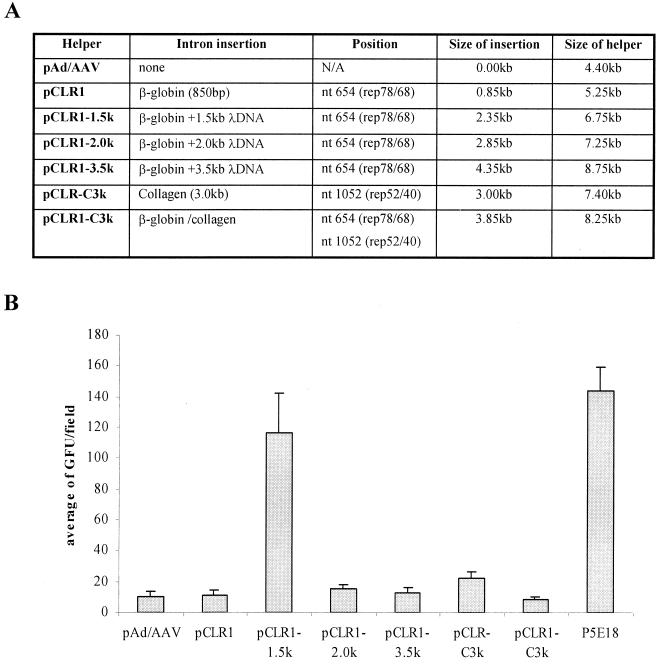
The helper plasmids for rAAV production with non-native introns were constructed as shown in Fig. 1C to E. The plasmid pCLR1 carried one human β-globin intron. Plasmids pCLR1-1.5k, pCLR1-2.0k, and pCLR1-3.0k carried the same β-globin intron with additional phage λ DNA sequences inserted within the intron. The size of the λ DNA sequences were 1.5, 2.0, and 3.0 kb, respectively. The λ DNA sequences were used simply to increase the size of the intron. The plasmid pCLR-C3k carried the 3.0-kb human collagen intron in p19 transcripts. The pCLR1-C3k carried both the β-globin intron and the collagen intron. These helper plasmids were then examined for their ability to support rAAV production. The result is presented in Fig. 3. These new helper plasmids were at least as efficient in supporting rAAV production as the original helper plasmid pAd/AAV. These observations were confirmed by using two reporter vector plasmids rAAV-CMV-lacZ and rAAV-CMV-GFP.
FIG. 3.
(A) Properties of the helper plasmids used for rAAV generation. (B) rAAV yield comparison using various helper plasmids. The human 293 cells were transfected with each helper plasmid, vector plasmid pAAV-EGFP, and adenovirus helper plasmid in a ratio of 1:1:2. The cells were harvested at 96 h posttransfection. Equal amount of cell lysates was used to infect 293 cells in presence of adenovirus infection at an MOI of 10. The relative rAAV titer was reported as IU/field under microscopy. See Materials and Methods for details on rAAV production and titer determination.
Helper plasmids with additional introns reduce the generation of rcAAV particles.
Based on the observation that increases in the size of the AAV genome would decrease AAV packaging, we hypothesized that these new helper plasmids would be efficient in reducing the generation of rcAAV particles. It is unlikely that the AAV virion will accommodate the oversized AAV genome with additional large, non-native introns. Since the introns are scattered within the coding region, this new approach makes it very hard for nonhomologous recombinant mutants to be packaged. To confirm these hypotheses, the amount of rcAAV particles in our rAAV preparations was assayed. Approximately 1 of 10 of our vector preparations from 107 cells (∼1010 rAAV particles) was used to infect 106 293 cells in the presence of helper adenovirus infection at an MOI of 10. The total cellular DNA was extracted at 36 h post-adenovirus and -rAAV infections. The replicated rcAAV genome was detected by PCR using 500 ng of extracted total cellular DNA. As shown in Table 2 (except the last column), the rAAV vector produced by pAd/AAV generated detectable rcAAV at a 1:100 dilution. But none of the helper plasmids with additional non-native introns produced detectable rcAAV even at a 1:10 dilution. It is also interesting to see that the helper plasmid p5E18 also generated detectable rcAAV at a dilution of 1:10 but not at a dilution of 1:100. In p5E18, there is a 3.0-kb plasmid backbone inserted between the p5 promoter and Rep initiation codons. It is clear that the previous approach using spacers reduced the generation of rcAAV but did not completely eliminate it.
TABLE 2.
rcAAV contamination in rAAV preparations using various helper plasmidsa
| Helper plasmid | rcAAV contamination at dilution:
|
rcAAV contamination of vectors (large scale, second round) | ||
|---|---|---|---|---|
| 1:10 | 1:100 | 1:1,000 | ||
| pAd/AAV | + | + | − | + |
| pCLR1 | − | − | − | − |
| pCLR1-1.5k | − | − | − | − |
| pCLR1-2.0k | − | − | − | ND |
| pCLR-3.5k | − | − | − | ND |
| pCLR-C3k | − | − | − | ND |
| pCLR1-C3k | − | − | − | − |
| p5E18 | + | − | − | ND |
The experiments were carried out as described in Fig. 2. Columns 2 to 4 are based on virus preparations from ∼2 × 107 cells. The viruses were diluted as indicated. The last column shows the vectors prepared from 2 × 108 cells. The vectors were not diluted and were subjected to two rounds of amplification for rcAAVs. ND, not determined. See Materials and Methods for more details on the rcAAV determinations.
To further confirm that the new approach can significantly reduce the generation of rcAAVs, we tested rcAAVs from several larger-scale rAAV preparations using approximately 2 × 108 293 cells. The vector plasmid was pAAV-tet-GCSF. After the viruses were purified using a heparin column, all viruses (approximately 1012) were used to infect 108 293 cells in the presence of adenovirus infection at an MOI of 10. The cells were harvested when full cytopathic effects were observed. The obtained cell lysates were used for the second-round infection of 293 cells. The cells were harvested 36 h later, and the total cellular DNA was used for wtAAV detection. The result is shown in the last column of Table 2. Since no rcAAV could be detected even after two rounds of amplification, we concluded that the contaminated rcAAV was less than one out of 1011 to 1012 rAAV genomes using this new strategy.
The new helper plasmids with additional introns can improve rAAV yield.
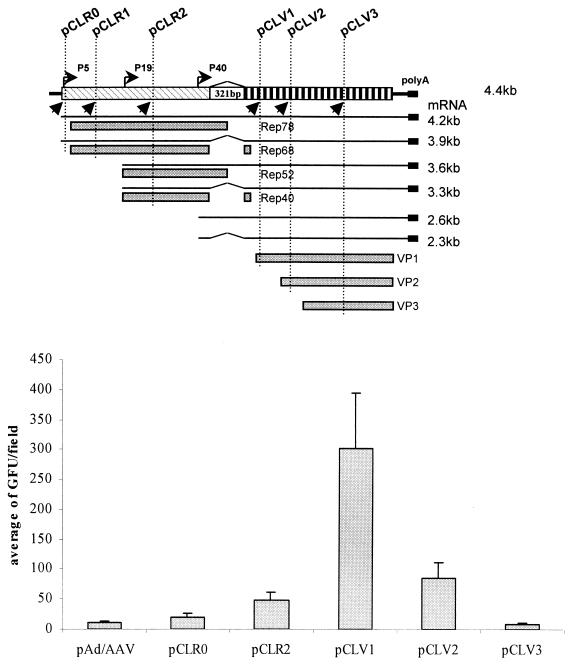
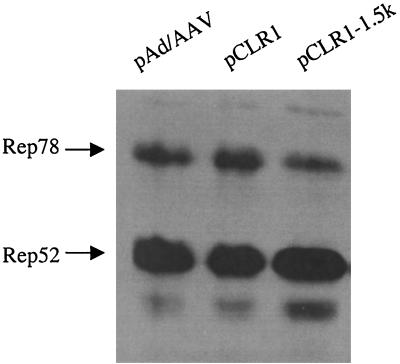
Introns play a very important role in regulating gene expression. The properties of introns can affect the level of gene expression either positively or negatively. We attempted to regulate rep and cap gene expression by inserting the human β-globin at various positions in the AAV genome. These mutant helpers are listed in Fig. 4A. As expected, all of these helper plasmids were capable of supporting rAAV production (Fig. 4B) using either lacZ or GFP as the reporter gene. It is worth noting that the rAAV yields using pCLR1-λ1.5k and pCLV1 as helpers are considerably higher than with the other helper plasmids (Fig. 3 and 4). The increase in the rAAV titer was about 5- to 10-fold. The Western blot revealed that there was an increase in the ratio of Rep52 and Rep40 to Rep78 and Rep68 in pCLR1-λ1.5k (Fig. 5). For pCLV1, there may be several possible mechanisms to explain the observed increase in rAAV titer. We did not observe in Western blots a clear difference in the ratios of Rep proteins and Cap proteins. We speculate that some subtle changes in VP1 may contribute to the increase in vector yield.
FIG. 4.
rAAV production using various helper plasmids with intron in different positions. (A) Illustration of position of additional introns relative to AAV genome. (B) rAAV titer comparison using various helper plasmids. GFP was used as the reporter gene. The experiments were carried out as described in Fig. 3. Each construct was tested using two independent clones.
FIG. 5.
Western blot of rep gene expression from pAd/AAV, pCLR1, and pCLR1-1.5k. The human 293 cells were transfected with 10 μg of DNA and infected with adenovirus at an MOI of 10. The cells were harvested at 36 h postinfection. The Western blot for Rep proteins was carried as described in Materials and Methods. A total of 10 μg of protein was used in each lane for the Western blot.
DISCUSSION
The quality of rAAV vector stocks is a critical issue in developing rAAV for human clinical trials. rcAAV contaminants in an AAV vector stock is a safety concern and is likely to decrease the efficiency of gene transfer and transgene expression. Previous studies have attempted to solve this problem. Gao et al. showed that integrated helper functions can significantly decrease rcAAV generation. There are only limited copies of rep and cap sequences in the cell line. But for the transfection-mediated rAAV production method, each cell usually receives thousands of copies of helper plasmid and vector plasmid. The probability of generating rcAAV through nonhomologous recombination is significantly higher. Additional approaches employed include (i) modifying the AAV terminal repeat, (ii) mutating the AAV p5 promoter, and (iii) splitting the AAV rep and cap into different plasmids (1, 16, 29). These methods all successfully reduce rcAAV contamination. However, it is still unknown if the modification of AAV terminal repeats will have any effect on AAV integration or other aspects of rAAV transduction. In addition, the p5 promoter seems to be more responsive to activation by helper virus transactivators. Helper plasmids containing the p5 promoter are generally more efficient in rAAV production than are helper plasmids containing heterologous promoters (16, 23). We tried to preserve the AAV elements as much as possible while reducing the generation of rcAAV particles. The intron insertion into AAV genome serves for this purpose well.
The evolution of introns is important for eukaryotic cells. The majority of the human genome has been shown to be intronic sequences. For example, the factor VIII gene spans almost 186 kb even though the mRNA is only 9 kb. However, AAV only keeps a simple intron due to its limited virion size. The increase in size caused by the intron insertion had a dramatic effect on AAV packaging. This observation is in consistent with previous studies (8, 17). Most particles generated from AAV with non-native intron were noninfectious defective particles (Table 1). The ratio of infectious particles to physical particles measured by ELISA was decreased by 100-fold with the addition of an 850-bp intron. These observations were very useful in designing the current AAV packaging system. Assuming the probability of nonhomologous events leading to the ligation between the AAV helper genome and ITRs remains unchanged in rAAV preparations, the insertion of an 850-bp intron would be expected to decrease the number of rcAAV particles by 2 logs. It is interesting to note that the actual decreases were larger than this estimation in our rAAV production experiment. A longer intron or multiple introns are highly likely to eliminate rcAAV completely since the intron insertion functions to block the packaging step. As demonstrated in Table 2, this approach is very efficient in reducing rcAAV contamination beyond the detection limit of our assays.
In present study, the rcAAVs assayed by PCR amplification of the total cellular DNA from the infection of rAAV vectors and adenovirus. This assay is quite sensitive. Previous reports have shown that as little as one IU can be detected in this way (4, 14). On the other hand, this assay requires the wild-type-like particle to have both functional ITRs and an intact rep gene to be detected. Since adoption of intronized AAV helper genome has no effects on the frequency of nonhomologous reaction that links the AAV ITR and rep gene, we are not surprised to see approximately 0.02% of the total packaged particles which contained at least some rep sequences. This is in accordance with the observation by Srivastava's group that there exists such particles in optimal condition with pD10 as vector and helper without adenovirus terminal repeats (29). It is interesting that all those particles have lost their ability to replicate and therefore they can no longer be categorized as rcAAVs.
We did not eliminate the rcAAVs completely by simply increasing the helper plasmid size. In the case of p5E18, it contains a 3-kb spacer fragment between the p5 promoter and rep initiation codon and the total size of the sequence from the p5 promoter to the cap gene is 7.5 kb (31). However, rcAAV can still be detected in rAAV preparations generated using p5E18 as helper plasmid, although at a 10-fold-lower amount than that of pAd/AAV. It is clear that reversion mutants can be easily generated even with p5E18 as the helper plasmid. This phenomenon is in agreement with previous observations that additional λ DNA can be deleted from the AAV genome when it was inserted in the nonessential region (17). The insertion of an intron is less likely to be removed without affecting AAV genome integrity since it is in the coding region. This is the key factor leading to the rcAAV reduction.
Introns often increase the level of gene expression. In some cases, gene expression can be increased by as much as 500-fold (3). However, a less-efficient intron may also decrease gene expression. Previous studies have suggested that a reduction in rep gene expression is associated with an increase in rAAV yield (23). We observed the same phenomenon with pCLR1-λ1.5k. The 1.5k λ DNA inadvertently disrupted the efficacy of the intron and led to a decrease in Rep78 and Rep68 expression, thereby increasing the rAAV packaging efficiency. Another interesting phenomenon was observed with pCRV1. Although we did not observe clear differences in rep and cap gene expression (data not shown), the two independent clones we used were capable of generating high-titer rAAV vectors. The intron was in the VP1 region but not in VP2 and VP3. Moreover, AAV uses a different RNA transcript for VP1. We speculate that some subtle changes in the expression of VP1 are likely to be the primary cause for the observed increase in rAAV titer. This result suggests an alternative method for improving rAAV yield.
Intron insertions offer a new approach for improving the yield of rAAV production with the potential for complete elimination of rcAAV contamination. In addition, this approach offers unique flexibility and can be combined with other established rAAV production systems to further improve the rAAV yield. For example, it can easily be adopted in cell lines or carried by other viral vectors such as herpes simplex virus, Epstein-Barr virus, or baculovirus (5, 7, 14). Multiple intron insertions and further optimization of this system is currently under way. Since almost any virus has a limitation in terms of packaging capacity, the addition of introns into helper genome in a key region may be a versatile and universal method for reducing or eliminating wild-type-like virus contamination in recombinant viral vector preparations.
ACKNOWLEDGMENT
This study was supported in part by NIH grant NS39144 to M.J.D.
REFERENCES
- 1.Allen J M, Debelak D J, Reynolds T C, Miller A D. Identification and elimination of replication-competent adeno-associated virus (AAV) that can arise by nonhomologous recombination during AAV vector production. J Virol. 1997;71:6816–6822. doi: 10.1128/jvi.71.9.6816-6822.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berns K I, Giraud C. Biology of adeno-associated virus. Curr Top Microbiol Immunol. 1996;218:1–23. doi: 10.1007/978-3-642-80207-2_1. [DOI] [PubMed] [Google Scholar]
- 2a.Brent R, Moore D D, Kingston R, Ausubel F M, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1993. [Google Scholar]
- 3.Brinster R L, Allen J M, Behringer R R, Gelinas R E, Palmiter R D. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci USA. 1988;85:836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark K R, Liu X, McGrath J P, Johnson P R. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum Gene Ther. 1999;10:1031–1039. doi: 10.1089/10430349950018427. [DOI] [PubMed] [Google Scholar]
- 5.Clark K R, Voulgaropoulou F, Fraley D M, Johnson P R. Cell lines for the production of recombinant adeno-associated virus. Hum Gene Ther. 1995;6:1329–1341. doi: 10.1089/hum.1995.6.10-1329. [DOI] [PubMed] [Google Scholar]
- 6.Clark K R, Voulgaropoulou F, Johnson P R. A stable cell line carrying adenovirus-inducible rep and cap genes allows for infectivity titration of adeno-associated virus vectors. Gene Ther. 1996;3:1124–1132. [PubMed] [Google Scholar]
- 7.Conway J, Rhys C, Zolotukhin I, Zolotukhin S, Muzyczka N, Hayward G, Byrne B. High-titer recombinant adeno-associated virus production utilizing a recombinant herpes simplex virus type I vector expressing AAV-2 Rep and Cap. Gene Ther. 1999;6:986–993. doi: 10.1038/sj.gt.3300937. [DOI] [PubMed] [Google Scholar]
- 8.Dong J Y, Fan P D, Frizzell R A. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther. 1996;7:2101–2112. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- 9.Duan D, Yan Z, Yue Y, Engelhardt J F. Structural analysis of adeno-associated virus transduction circular intermediates. Virology. 1999;261:8–14. doi: 10.1006/viro.1999.9821. [DOI] [PubMed] [Google Scholar]
- 10.Duan D, Yue Y, Yan Z, Engelhardt J F. A new dual-vector approach to enhance recombinant adeno-associated virus-mediated gene expression through intermolecular cis activation. Nat Med. 2000;6:595–598. doi: 10.1038/75080. [DOI] [PubMed] [Google Scholar]
- 11.During M J, Xu R, Young D, Kaplitt M G, Sherwin R S, Leone P. Peroral gene therapy of lactose intolerance using an adeno-associated virus vector. Nat Med. 1998;4:1131–1135. doi: 10.1038/2625. [DOI] [PubMed] [Google Scholar]
- 12.Flotte T R, Barraza-Ortiz X, Solow R, Afione S A, Carter B J, Guggino W B. An improved system for packaging recombinant adeno-associated virus vectors capable of in vivo transduction. Gene Ther. 1995;2:29–37. [PubMed] [Google Scholar]
- 13.Flotte T R, Carter B J. Adeno-associated virus vectors for gene therapy of cystic fibrosis. Methods Enzymol. 1998;292:717–732. doi: 10.1016/s0076-6879(98)92055-9. [DOI] [PubMed] [Google Scholar]
- 14.Gao G P, Qu G, Faust L Z, Engdahl R K, Xiao W, Hughes J V, Zoltick P W, Wilson J M. High-titer adeno-associated viral vectors from a Rep/Cap cell line and hybrid shuttle virus. Hum Gene Ther. 1998;9:2353–2362. doi: 10.1089/hum.1998.9.16-2353. [DOI] [PubMed] [Google Scholar]
- 15.Grimm D, Kern A, Pawlita M, Ferrari F, Samulski R, Kleinschmidt J. Titration of AAV-2 particles via a novel capsid ELISA: packaging of genomes can limit production of recombinant AAV-2. Gene Ther. 1999;6:1322–1330. doi: 10.1038/sj.gt.3300946. [DOI] [PubMed] [Google Scholar]
- 16.Grimm D, Kern A, Rittner K, Kleinschmidt J A. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther. 1998;9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- 17.Hermonat P L, Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc Natl Acad Sci USA. 1984;81:6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herzog R W, Yang E Y, Couto L B, Hagstrom J N, Elwell D, Fields P A, Burton M, Bellinger D A, Read M S, Brinkhous K M, Podsakoff G M, Nichols T C, Kurtzman G J, High K A. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 19.Kay M A, Manno C S, Ragni M V, Larson P J, Couto L B, McClelland A, Glader B, Chew A J, Tai S J, Herzog R W, Arruda V, Johnson F, Scallan C, Skarsgard E, Flake A W, High K A. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 20.Kotin R M, Linden R M, Berns K I. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 1992;11:5071–5078. doi: 10.1002/j.1460-2075.1992.tb05614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotin R M, Menninger J C, Ward D C, Berns K I. Mapping and direct visualization of a region-specific viral DNA integration site on chromosome 19q13-qter. Genomics. 1991;10:831–834. doi: 10.1016/0888-7543(91)90470-y. [DOI] [PubMed] [Google Scholar]
- 22.Kotin R M, Siniscalco M, Samulski R J, Zhu X D, Hunter L, Laughlin C A, McLaughlin S, Muzyczka N, Rocchi M, Berns K I. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Samulski R J, Xiao X. Role for highly regulated rep gene expression in adeno-associated virus vector production. J Virol. 1997;71:5236–5243. doi: 10.1128/jvi.71.7.5236-5243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 25.Nakai H, Storm T A, Kay M A. Increasing the size of rAAV-mediated expression cassettes in vivo by intermolecular joining of two complementary vectors. Nat Biotechnol. 2000;18:527–532. doi: 10.1038/75390. [DOI] [PubMed] [Google Scholar]
- 26.Samulski R J, Chang L S, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samulski R J, Zhu X, Xiao X, Brook J D, Housman D E, Epstein N, Hunter L A. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991;10:3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. . (Erratum, 11:1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L, Li J, Xiao X. Overcoming adeno-associated virus vector size limitation through viral DNA heterodimerization. Nat Med. 2000;6:599–602. doi: 10.1038/75087. [DOI] [PubMed] [Google Scholar]
- 29.Wang X S, Khuntirat B, Qing K, Ponnazhagan S, Kube D M, Zhou S, Dwarki V J, Srivastava A. Characterization of wild-type adeno-associated virus type 2-like particles generated during recombinant viral vector production and strategies for their elimination. J Virol. 1998;72:5472–5480. doi: 10.1128/jvi.72.7.5472-5480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao W, Berta S C, Lu M M, Moscioni A D, Tazelaar J, Wilson J M. Adeno-associated virus as a vector for liver-directed gene therapy. J Virol. 1998;72:10222–10226. doi: 10.1128/jvi.72.12.10222-10226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao W, Chirmule N, Berta S C, McCullough B, Gao G, Wilson J M. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang J, Zhou W, Zhang Y, Zidon T, Ritchie T, Engelhardt J F. Concatamerization of adeno-associated virus circular genomes occurs through intermolecular recombination. J Virol. 1999;73:9468–9477. doi: 10.1128/jvi.73.11.9468-9477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]