Abstract
Background and Aims
Homozygous familial hypercholesterolaemia (HoFH) is a rare genetic disorder characterized by severely elevated LDL cholesterol (LDL-C) and premature atherosclerotic cardiovascular disease. In the pivotal Phase 3 HoFH trial (NCT03399786), evinacumab significantly decreased LDL-C in patients with HoFH. This study assesses the long-term safety and efficacy of evinacumab in adult and adolescent patients with HoFH.
Methods
In this open-label, single-arm, Phase 3 trial (NCT03409744), patients aged ≥12 years with HoFH who were evinacumab-naïve or had previously received evinacumab in other trials (evinacumab-continue) received intravenous evinacumab 15 mg/kg every 4 weeks with stable lipid-lowering therapy.
Results
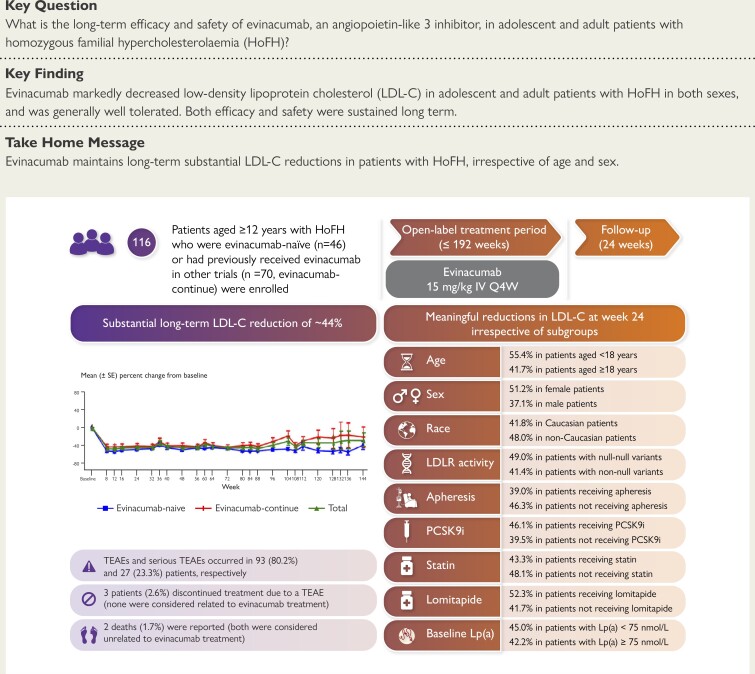
A total of 116 patients (adults: n = 102; adolescents: n = 14) were enrolled, of whom 57 (49.1%) were female. Patients were treated for a median (range) duration of 104.3 (28.3–196.3) weeks. Overall, treatment-emergent adverse events (TEAEs) and serious TEAEs were reported in 93 (80.2%) and 27 (23.3%) patients, respectively. Two (1.7%) deaths were reported (neither was considered related to evinacumab). Three (2.6%) patients discontinued due to TEAEs (none were considered related to evinacumab). From baseline to Week 24, evinacumab decreased mean LDL-C by 43.6% [mean (standard deviation, SD), 3.4 (3.2) mmol/L] in the overall population; mean LDL-C reduction in adults and adolescents was 41.7% [mean (SD), 3.2 (3.3) mmol/L] and 55.4% [mean (SD), 4.7 (2.5) mmol/L], respectively.
Conclusions
In this large cohort of patients with HoFH, evinacumab was generally well tolerated and markedly decreased LDL-C irrespective of age and sex. Moreover, the efficacy and safety of evinacumab was sustained over the long term.
Keywords: Atherosclerosis, Cholesterol, Homozygous familial hypercholesterolaemia
Structured Graphical Abstract
Structured Graphical Abstract.
HoFH, homozygous familial hypercholesterolaemia; IV, intravenous; LDL-C, LDL cholesterol; LDLR, LDL receptor; Lp(a), lipoprotein(a); PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor; Q4W, every 4 weeks; SE, standard error; TEAE, treatment-emergent adverse event.
See the editorial comment for this article ‘ANGPTL3 as a therapeutic target for treating homozygous familial hypercholesterolaemia: a shot in the arm for evinacumab’, by G.F. Watts et al., https://doi.org10.1093/eurheartj/ehae321.
Introduction
Homozygous familial hypercholesterolaemia (HoFH) is a rare genetic lipid disorder that is associated with a high burden of cardiovascular outcomes and premature mortality.1,2 Homozygous familial hypercholesterolaemia is often caused by genetic variants in the LDL receptor (LDLR), apolipoprotein B (APOB), and proprotein convertase subtilisin/kexin type 9 (PCSK9) genes, resulting in severely impaired LDL cholesterol (LDL-C) clearance from the circulation.2–4 Some patients with HoFH have complete loss-of-function (‘null–null’) LDLR variants, resulting in higher LDL-C levels compared with other variants.2 Homozygous familial hypercholesterolaemia causes early, accelerated onset of atherosclerotic cardiovascular disease (ASCVD),4 and patients with null–null LDLR variants are at greater risk for premature ASCVD than those with non-null LDLR variants.2 Given that untreated patients with HoFH are not expected to live past their third decade,5 early treatment initiation is essential for young patients with HoFH, as effective management of LDL-C levels reduces the risk of subsequent cardiovascular events.6,7
Treatment guidelines recommend the use of lipid-lowering therapy (LLT), including statins,8 PCSK9 inhibitors,9 and lipoprotein apheresis,10,11 to lower LDL-C levels in patients with HoFH.1,3,11 Recent European Society of Cardiology and European Atherosclerosis Society guidelines for patients with HoFH recommend LDL-C levels of <1.8 mmol/L (<70 mg/dL) and <1.4 mmol/L (<55 mg/dL) for adults with and without ASCVD, respectively.12 In children and adolescents, an LDL-C level of <3 mmol/L (<115 mg/dL) is recommended if LLTs are initiated before the age of 18 years, and imaging assessment does not indicate the presence of ASCVD.12 For children and adolescents with established ASCVD, a lower LDL-C goal is recommended.12 Treatments that increase LDLR expression, such as statins and PCSK9 inhibitors, have limited to no effect in patients with null–null LDLR variants.13–15 Lomitapide and lipoprotein apheresis are treatment options for HoFH that work independently of the LDLR pathway.16–18 However, lomitapide is not approved for patients aged <18 years and is associated with gastrointestinal adverse events and hepatic fat accumulation.15,16,19–21 Consequently, patients are advised to follow a low-fat diet with adequate supplementation of essential fatty acids and fat-soluble vitamins.19,20,22 Lipoprotein apheresis is considered a safe and effective LDL-C-lowering therapy that should be started as soon as possible in children with HoFH, ideally by 3 years of age and no later than 8 years of age, depending on appropriate venous access.12,23 However, despite its therapeutic value, the use of apheresis is not universal due to variable access, particularly as apheresis is limited to highly specialized centres in many countries.12,23–25 Concerns regarding the use of apheresis include the depletion of apolipoprotein E HDL and pre-β1-HDL particles by apheresis, suggesting that cellular cholesterol efflux may be impaired in the immediate post-apheresis period.26 With current standard-of-care LLTs, guideline-recommended LDL-C treatment goals are rarely met.8,25,27 Given that the extent of LDL-C reduction is strongly associated with survival outcomes, many patients with HoFH remain at high risk for cardiovascular mortality.28 Therefore, an urgent unmet need exists for effective and tolerable lipid-lowering treatments in patients with HoFH.4,29
Evinacumab is a fully human monoclonal antibody that specifically binds to and inhibits angiopoietin-like 3, a protein that inhibits the activity of lipoprotein and endothelial lipase and thereby reduces the concentration of plasma lipids.30–32 Evinacumab was approved as an adjunct to other LLTs for the treatment of HoFH in adult and adolescent patients aged ≥12 years by the US Food and Drug Administration (FDA) in February 2021 and the European Medicines Agency (EMA) in June 2021.33,34 These approvals were based on the findings of a pivotal, double-blind, placebo-controlled, Phase 3 study (NCT03399786) in which evinacumab significantly decreased LDL-C levels by ∼50% in patients with HoFH aged ≥12 years after 24 weeks when administered with maximally tolerated LLT, irrespective of apheresis status.35 Subsequently, in a single-arm, open-label, Phase 3 trial (NCT04233918), the addition of evinacumab to aggressive baseline LLTs reduced LDL-C by 48% in paediatric patients with HoFH aged 5–11 years after 24 weeks of treatment.36 As a result, the initial approval of evinacumab was extended to patients with HoFH aged 5–11 years by the FDA (in March 2023) and the EMA (in December 2023).34,36 In September 2023, evinacumab was approved by Health Canada for the treatment of patients with HoFH aged ≥5 years.37
The long-term effects of evinacumab are yet to be described. In this study, we present data from an open-label, Phase 3 trial, the Evinacumab Lipid Studies in Patients with Homozygous Familial Hypercholesterolaemia extension study, which evaluated the long-term safety and efficacy of evinacumab in adult and adolescent patients with HoFH.
Methods
Study design
This single-arm, open-label, Phase 3 study (NCT03409744) evaluated the long-term safety and efficacy of evinacumab in adult and adolescent patients with HoFH. This study comprised a run-in period (≤10 weeks), a screening period (2 weeks), an open-label treatment period (≤192 weeks; OLTP), and a follow-up period (24 weeks) after the last dose of the study drug (see Supplementary data online, Figure S1). The run-in period was established for patients who had an unconfirmed diagnosis of HoFH or background LLT/apheresis schedules that were not stable for ≥8 weeks prior to the start of the study. Patients who were evinacumab-naïve or had not entered the study within 7 days of completing a previous evinacumab study were required to enter the 2-week screening period. At baseline (Day 1) and every 4 weeks (Q4W), patients received intravenous evinacumab 15 mg/kg. Patients were required to maintain a stable regimen of LLT for the duration of the study. Study duration varied for each patient, ranging from months to ∼4 years. The date of the final database lock for this open-label study was 22 May 2023, and the analysis reported herein is based on the data from this final database lock.
Trial oversight
This study was conducted at 38 sites across 12 countries (see Supplementary data online, Tables S1 and S2). The principal investigators and the sponsor (Regeneron Pharmaceuticals, Inc.) designed the trial protocol and selected the participating sites (see Supplementary data online, Table S2). The study protocol was approved by the institutional review board and/or ethics committee at each site.
This study was conducted in accordance with the principles of the Declaration of Helsinki and was consistent with Good Clinical Practices of the International Conference on Harmonisation and applicable regulatory requirements. Monitoring and site supervision were performed with oversight by the sponsor. The sponsor also participated in the collection, analysis, and interpretation of the data and checked the information provided in the manuscript. All authors had access to the data, contributed to the drafting of the initial version of the manuscript, participated in revisions, and agreed with the decision to submit the manuscript for publication. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol.
Patients
Patients may or may not have participated in previous evinacumab studies. Patients who participated in the Phase 2 proof-of-concept study (NCT02265952) or the pivotal Phase 3 evinacumab HoFH study (NCT03399786) were eligible for participation in this open-label study. Eligible patients were aged ≥12 years and diagnosed with HoFH by genetic or clinical criteria. Genetic diagnosis was defined as a documented pathogenic variant in both LDLR alleles, or homozygous or compound heterozygous variants in APOB or PCSK9. Patients who were double heterozygotes (i.e. pathogenic variants on different genes, e.g. LDLR and PCSK9) and patients with compound heterozygous or homozygous variants in the LDLR adaptor protein 1 (LDLRAP1) gene were also eligible. Clinical diagnosis was defined as an untreated total cholesterol level >12.9 mmol/L (>500 mg/dL) with a documentation of cutaneous or tendinous xanthoma before 10 years of age, or a total cholesterol level >6.5 mmol/L (>250 mg/dL) in both parents. Patients with null–null variants had <15% LDLR activity based on in vitro assays, as previously reported.38 In addition, patients were required to be receiving stable LLT. All patients provided written informed consent. All patient eligibility criteria are provided in the Supplementary data online, Appendix S1.
Outcomes
The primary outcome was the incidence and severity of treatment-emergent adverse events (TEAEs) and other safety analyses during the OLTP. Efficacy outcomes were per cent and absolute change from baseline over time in LDL-C, apolipoprotein B (Apo B), non-HDL cholesterol (non-HDL-C), total cholesterol, and fasting triglycerides.
Statistical analysis
As this was an open-label, single-arm study, no calculation of sample size was performed. The aim was to enrol 120 patients, 14 of whom were to be adolescents, in order to study the effect of evinacumab in adolescent patients with HoFH.
Safety and efficacy analyses were performed in the safety analysis set, which included all patients who were enrolled and received at least one dose or a partial dose of open-label treatment with evinacumab 15 mg/kg in this study. Safety data were summarized descriptively, and TEAEs were reported and coded using the Medical Dictionary for Regulatory Activities version 26.0.
Per cent and absolute change from baseline in LDL-C, as well as other lipid and lipoprotein parameters, were summarized using descriptive statistics. Per cent and absolute change from baseline in LDL-C were analysed by evinacumab treatment status (evinacumab-naïve and evinacumab-continue) and age (adults and adolescents) up to Week 184. At Week 24, per cent and absolute change from baseline in LDL-C were assessed by age, sex, race, LDLR genotype (null–null and non-null), lipoprotein apheresis status, triple therapy status, and quadruple therapy status at baseline. For patients who had received evinacumab in the previous Phase 2 study or who had not previously received evinacumab, baseline was defined as the last obtained value before the first dose of evinacumab in this open-label study. For patients who had previously received evinacumab in the pivotal Phase 3 study, baseline was defined as the last obtained value before the first dose of double-blind evinacumab in the Phase 3 study. Triple therapy was defined as treatment with a statin, ezetimibe, and a PCSK9 inhibitor, and quadruple therapy was defined as treatment with a statin, ezetimibe, a PCSK9 inhibitor, and lomitapide. Note that the subgroups indicated do not exclude the use of other concomitant LLTs (e.g. bile acid sequestrants, nicotinic acid and derivatives, phytosterols, probucol, and omega-fatty acids). Per cent change from baseline in other lipid and lipoprotein parameters was assessed in the overall population, and by evinacumab treatment status, at Week 24. Missing data were not imputed. All statistical analyses were performed using SAS Linux 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
A total of 116 patients (evinacumab-naïve group: n = 46; evinacumab-continue group: n = 70) were enrolled in the study (see Supplementary data online, Figure S2). All patients received at least one dose of intravenous evinacumab 15 mg/kg Q4W and were included in the safety analysis set.
The baseline demographics and clinical characteristics of all patients are presented in Table 1 and by sex in Supplementary data online, Table S3. Overall, the mean (standard deviation, SD) age was 38.8 (15.9) years. Fifty-seven (49.1%) patients were female, 85 (73.3%) patients had a genetically confirmed diagnosis of HoFH, and 31 (26.7%) patients had their HoFH diagnosed clinically. Thirty-seven (31.9%) patients had null–null variants (<15% LDLR activity) in either LDLR (n = 36, 31.0%) or LDLRAP1 (n = 1, 0.9%). Genotype data are provided in Supplementary data online, Table S4. The mean (SD) baseline LDL-C level was 6.8 (4.1) mmol/L [261.0 (160.1) mg/dL]. Almost all patients (n = 107; 92.2%) were receiving a statin at baseline. The median (range) duration of evinacumab exposure was 104.3 (28.3–196.3) weeks.
Table 1.
Patient demographics and baseline clinical characteristics
| Evinacumab-naïve (n = 46) | Evinacumab-continue (n = 70) | Total (n = 116) | |
|---|---|---|---|
| Age, years, mean (SD) | 35.2 (16.5) | 41.2 (15.2) | 38.8 (15.9) |
| Male, n (%) | 26 (56.5) | 33 (47.1) | 59 (50.9) |
| Race, n (%) | |||
| White | 31 (67.4) | 49 (70.0) | 80 (69.0) |
| Black or African American | 2 (4.3) | 2 (2.9) | 4 (3.4) |
| Asian | 3 (6.5) | 9 (12.9) | 12 (10.3) |
| Not reported | 7 (15.2) | 4 (5.7) | 11 (9.5) |
| Other | 3 (6.5) | 6 (8.6) | 9 (7.8) |
| BMI, kg/m2, mean (SD) | 24.5 (5.2) | 26.4 (6.1) | 25.7 (5.8) |
| HoFH diagnosis, n (%) | |||
| Genotyping | 33 (71.7) | 52 (74.3) | 85 (73.3) |
| Clinical diagnosis | 13 (28.3) | 18 (25.7) | 31 (26.7) |
| Genotype state, n (%) | |||
| Homozygous | 22 (47.8) | 33 (47.1) | 55 (47.4) |
| Compound heterozygous | 18 (39.1) | 23 (32.9) | 41 (35.3) |
| Double heterozygous | 0 | 1 (1.4) | 1 (0.9) |
| Other (heterozygous or undetermined) | 5 (10.9) | 12 (17.1) | 17 (14.7) |
| No mutations | 0 | 1 (1.4) | 1 (0.9) |
| Missing | 1 (2.2) | 0 | 1 (0.9) |
| Baseline LLT, n (%) | |||
| Statin | 44 (95.7) | 63 (90.0) | 107 (92.2) |
| High-intensity statina | 37 (80.4) | 55 (78.6) | 92 (79.3) |
| Ezetimibe | 36 (78.3) | 57 (81.4) | 93 (80.2) |
| PCSK9 inhibitorb | 22 (47.8) | 50 (71.4) | 72 (62.1) |
| Lomitapide | 8 (17.4) | 14 (20.0) | 22 (19.0) |
| Lipoprotein apheresis | 22 (47.8) | 23 (32.9) | 45 (38.8) |
| Calculated LDL-C, mmol/L, mean (SD)c | 7.0 (3.2) | 6.6 (4.7) | 6.8 (4.1) |
| Apolipoprotein B, g/L, mean (SD) | 1.8 (0.7) | 1.7 (0.9) | 1.7 (0.8) |
| HDL-C, mmol/L, mean (SD) | 1.1 (0.4) | 1.1 (0.4) | 1.1 (0.4) |
| Non-HDL-C, mmol/L, mean (SD) | 7.5 (3.1) | 7.2 (4.8) | 7.3 (4.2) |
| Total cholesterol, mmol/L, mean (SD) | 8.6 (3.0) | 8.3 (4.7) | 8.4 (4.1) |
| Fasting triglycerides, mmol/L, median (Q1, Q3) | 1.0 (0.7, 1.6) | 1.0 (0.6, 1.6) | 1.0 (0.7, 1.6) |
| Lipoprotein(a), nmol/L, median (Q1, Q3) | 50.0 (23.0, 113.0) | 73.5 (32.0, 168.0) | 66.0 (28.0, 166.0) |
BMI, body mass index; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; LLT, lipid-lowering therapy; PCSK9, proprotein convertase subtilisin/kexin type 9.
aHigh-intensity statin corresponds to atorvastatin 40 or 80 mg daily or rosuvastatin 20–40 mg daily.
bAlirocumab or evolocumab.
cLDL-C was calculated using the Friedewald formula.
Patient demographics and clinical characteristics were generally similar between the evinacumab-naïve and evinacumab-continue groups. The median (range) duration of evinacumab exposure was 97.3 (31.9–196.3) and 109.6 (28.3–188.1) weeks in the evinacumab-naïve and evinacumab-continue groups, respectively.
Safety
Overall, TEAEs during the OLTP were reported in 93 (80.2%) patients (Table 2). For most patients, TEAEs were mild (n = 27; 23.3%) or moderate (n = 48; 41.4%) in severity. The most common TEAEs were nasopharyngitis (n = 23; 19.8%), coronavirus disease 2019 (COVID-19; n = 19, 16.4%) headache (n = 19; 16.4%), and influenza-like illness (n = 16; 13.8%). Ten (8.6%) patients experienced TEAEs that were considered related to evinacumab treatment by the investigator: asthenia (n = 2), oral pigmentation (n = 1), face oedema (n = 1), feeling hot (n = 1), infusion site erythema (n = 1), abnormal hepatic function (n = 1), upper respiratory tract infection (n = 1), blood glucose decreased (n = 1), transaminases increased (n = 1), muscle spasms (n = 1), headache (n = 2), hypoesthesia (n = 1), paraesthesia (n = 1), acne (n = 1), pruritus (n = 1), and swelling face (n = 1). Treatment-emergent serious adverse events (SAEs) occurred in 27 (23.3%) patients (Table 3); however, none were considered related to evinacumab treatment. Three (2.6%) TEAEs (pregnancy, n = 2; headache, n = 1) led to a discontinuation of study treatment.
Table 2.
Summary of treatment-emergent adverse events during the open-label treatment period
| n (%) of patients | Evinacumab-naïve (n = 46) | Evinacumab-continue (n = 70) | Total (n = 116) |
|---|---|---|---|
| Any TEAE | 33 (71.7) | 60 (85.7) | 93 (80.2) |
| Treatment-emergent SAEs | 5 (10.9) | 22 (31.4) | 27 (23.3) |
| TEAEs leading to treatment discontinuation | 0 | 3 (4.3)a | 3 (2.6) |
| TEAEs leading to death | 1 (2.2) | 1 (1.4) | 2 (1.7) |
| TEAEs occurring in ≥5% of patients by preferred term | |||
| Nasopharyngitis | 11 (23.9) | 12 (17.1) | 23 (19.8) |
| Coronavirus disease 2019 | 5 (10.9) | 14 (20.0) | 19 (16.4) |
| Headache | 7 (15.2) | 12 (17.1) | 19 (16.4) |
| Influenza-like illness | 4 (8.7) | 12 (17.1) | 16 (13.8) |
| Arthralgia | 6 (13.0) | 9 (12.9) | 15 (12.9) |
| Back pain | 4 (8.7) | 10 (14.3) | 14 (12.1) |
| Nausea | 4 (8.7) | 10 (14.3) | 14 (12.1) |
| Cough | 6 (13.0) | 6 (8.6) | 12 (10.3) |
| Diarrhoea | 3 (6.5) | 7 (10.0) | 10 (8.6) |
| Gastroenteritis | 7 (15.2) | 3 (4.3) | 10 (8.6) |
| Pyrexia | 3 (6.5) | 7 (10.0) | 10 (8.6) |
| Urinary tract infection | 3 (6.5) | 7 (10.0) | 10 (8.6) |
| Pain in extremity | 3 (6.5) | 5 (7.1) | 8 (6.9) |
| Upper respiratory tract infection | 3 (6.5) | 5 (7.1) | 8 (6.9) |
| Abdominal pain | 4 (8.7) | 3 (4.3) | 7 (6.0) |
| Dizziness | 0 | 7 (10.0) | 7 (6.0) |
| Myalgia | 2 (4.3) | 5 (7.1) | 7 (6.0) |
| Toothache | 2 (4.3) | 5 (7.1) | 7 (6.0) |
| Vomiting | 3 (6.5) | 4 (5.7) | 7 (6.0) |
| Contusion | 2 (4.3) | 4 (5.7) | 6 (5.2) |
| Gastro-oesophageal reflux disease | 1 (2.2) | 5 (7.1) | 6 (5.2) |
| Oropharyngeal pain | 5 (10.9) | 1 (1.4) | 6 (5.2) |
| Immunization reaction | 1 (2.2) | 4 (5.7) | 5 (4.3) |
| Alanine aminotransferase increased | 3 (6.5) | 1 (1.4) | 4 (3.4) |
| Aspartate aminotransferase increased | 3 (6.5) | 1 (1.4) | 4 (3.4) |
| Angina pectoris | 0 | 4 (5.7) | 4 (3.4) |
| Blood creatine phosphokinase increased | 3 (6.5) | 1 (1.4) | 4 (3.4) |
| Chest pain | 0 | 4 (5.7) | 4 (3.4) |
| Acne | 3 (6.5) | 0 | 3 (2.6) |
| Paraesthesia | 3 (6.5) | 0 | 3 (2.6) |
SAE, serious adverse event; TEAE, treatment-emergent adverse event.
aTEAEs leading to treatment discontinuation were due to pregnancy in two patients and headache in one patient.
Table 3.
Summary of serious treatment-emergent adverse events during the open-label treatment period
| n (%) of patients | Evinacumab-naïve n = 46 | Evinacumab-continue n = 70 | Total n = 116 |
|---|---|---|---|
| Angina pectoris | 0 | 2 (2.9) | 2 (1.7) |
| Angina unstable | 0 | 2 (2.9) | 2 (1.7) |
| Aortic valve disease | 0 | 2 (2.9) | 2 (1.7) |
| Chest pain | 0 | 2 (2.9) | 2 (1.7) |
| Coronary artery disease | 0 | 2 (2.9) | 2 (1.7) |
| Acute myocardial infarction | 0 | 1 (1.4) | 1 (0.9) |
| Aortic stenosis | 0 | 1 (1.4) | 1 (0.9) |
| Arteriosclerosis | 0 | 1 (1.4) | 1 (0.9) |
| Arteriovenous fistula-site complication | 1 (2.2) | 0 | 1 (0.9) |
| Atrial fibrillation | 1 (2.2) | 0 | 1 (0.9) |
| Cardiac arrest | 1 (2.2) | 0 | 1 (0.9) |
| Cardiac failure acute | 0 | 1 (1.4) | 1 (0.9) |
| Cardiac failure chronic | 1 (2.2) | 0 | 1 (0.9) |
| Cardiac valve disease | 1 (2.2) | 0 | 1 (0.9) |
| Cataract | 0 | 1 (1.4) | 1 (0.9) |
| Cervical vertebral fracture | 0 | 1 (1.4) | 1 (0.9) |
| Coronary artery occlusion | 0 | 1 (1.4) | 1 (0.9) |
| Coronary artery stenosis | 0 | 1 (1.4) | 1 (0.9) |
| Food allergy | 0 | 1 (1.4) | 1 (0.9) |
| Gastroenteritis | 1 (2.2) | 0 | 1 (0.9) |
| Glaucoma | 0 | 1 (1.4) | 1 (0.9) |
| Hepatitis acute | 0 | 1 (1.4) | 1 (0.9) |
| Intestinal ischaemia | 0 | 1 (1.4) | 1 (0.9) |
| Ischaemic stroke | 0 | 1 (1.4) | 1 (0.9) |
| Mental status changes | 0 | 1 (1.4) | 1 (0.9) |
| Myocardial infarction | 0 | 1 (1.4) | 1 (0.9) |
| Oesophageal candidiasis | 0 | 1 (1.4) | 1 (0.9) |
| Ovarian cyst ruptured | 1 (2.2) | 0 | 1 (0.9) |
| Peripheral artery stenosis | 1 (2.2) | 0 | 1 (0.9) |
| Pneumonia | 1 (2.2) | 0 | 1 (0.9) |
| Prostate cancer | 0 | 1 (1.4) | 1 (0.9) |
| Renal infarct | 0 | 1 (1.4) | 1 (0.9) |
| Rib fracture | 0 | 1 (1.4) | 1 (0.9) |
| Spinal epidural haematoma | 0 | 1 (1.4) | 1 (0.9) |
| Scapula fracture | 0 | 1 (1.4) | 1 (0.9) |
| Supravalvular aortic stenosis | 0 | 1 (1.4) | 1 (0.9) |
| Vascular pseudoaneurysm | 1 (2.2) | 0 | 1 (0.9) |
SAE, serious adverse event; TEAE, treatment-emergent adverse event.
Two (1.7%) patients experienced TEAEs that resulted in death. First, a 54-year-old male patient, with a significant medical history that was consistent with underlying HoFH disease (xanthomas, corneal arcus, coronary artery disease, aortic valve disease, angina, hypertension, and coronary artery bypass graft procedure), experienced a sudden cardiac arrest and died on study Day 235, 6 days after the eighth dose of evinacumab. Second, a 54-year-old female patient, with a medical history significant for HoFH (multiple myocardial infarctions, coronary artery bypass, carotid artery occlusion, carotid endarterectomy, and carotid bruit) died from a presumed acute myocardial infarction on study Day 366, 21 days after receiving the eighth dose of evinacumab. Consistent with the occurrence of a myocardial infarction, troponin levels were elevated [16 773 ng/L (normal value: <0.04 ng/L)]. Both of these fatal adverse events were considered unrelated to evinacumab treatment.
The incidence of TEAEs and SAEs was higher in the evinacumab-continue group compared with the evinacumab-naïve group (85.7% vs. 71.7% and 31.4% vs. 10.9%, respectively). Exposure-adjusted TEAEs are presented in Supplementary data online, Table S5. During the study, two (1.7%) patients developed treatment-emergent anti-evinacumab antibodies (evinacumab-naïve group: n = 1; evinacumab-continue group: n = 1). However, the anti-evinacumab antibody responses for both patients were characterized as transient, with low (<1000) titres. Pre-existing anti-evinacumab antibodies were detected in five (4.3%) patients (evinacumab-naïve group: n = 1; evinacumab-continue group: n = 4).
Overall, there were no clinically relevant changes in liver function parameters from baseline to Week 24 (see Supplementary data online, Table S6). Eight (6.9%) patients in the overall population [evinacumab-naïve group (n = 3); evinacumab-continue group (n = 5)] experienced TEAEs related to liver function, including abnormal hepatic function (n = 1; 0.9%), acute hepatitis (n = 1; 0.9%), increase in alanine aminotransferase (n = 4; 3.4%), increase in aspartate aminotransferase (n = 4; 3.4%), increase in blood creatine phosphokinase (n = 1; 0.9%), and an increase in transaminases (n = 1; 0.9%; see Supplementary data online, Table S7). Two TEAEs related to liver function [abnormal hepatic function (n = 1; 0.9%) and increase in transaminases (n = 1; 0.9%)] were considered related to evinacumab treatment and possibly related to concomitant LLTs (lomitapide or rosuvastatin). All other TEAEs related to liver function were considered unrelated to evinacumab treatment. One (0.9%) patient experienced a serious TEAE of acute hepatitis of moderate intensity on study Day 1120, 27 days after the 40th dose of evinacumab. Laboratory tests showed the patient to have elevated levels of aspartate aminotransferase [2624 U/L (normal range: 9–34 U/L)] and alanine aminotransferase [680 U/L (normal range: 6–41 U/L)]. The patient was admitted to hospital on study Day 1121. Although asymptomatic, the patient reportedly had an alcohol intake more than the usual a few days prior. An elevated level of cytomegalovirus immunoglobulin M antibody was noted, and the patient was diagnosed with possible hepatitis virus. The patient received no treatment, but administration of the study drug was interrupted. On study Day 1127, the event of acute hepatitis was considered resolved, and the patient was discharged from the hospital. The study drug was resumed with the patient receiving evinacumab on study Day 1149. No patient experienced a liver-related TEAE leading to a permanent discontinuation of study treatment.
Efficacy
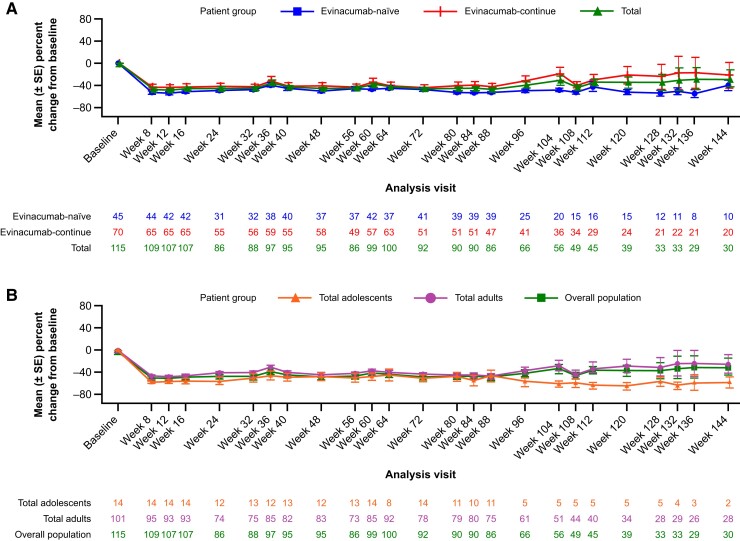
The mean per cent reduction in LDL-C level from baseline to Week 24 was 43.6% in the overall patient population (n = 86), with a mean (SD) absolute difference of 3.4 (3.2) mmol/L [132.0 (124.4) mg/dL; Figure 1). Mean LDL-C reductions were maintained at Week 48 [43.9%, n = 95; mean (SD): 3.4 (3.5) mmol/L]. When patients were assessed by evinacumab treatment status at Week 24, the mean per cent reduction in LDL-C level was 47.8% [mean (SD): 3.8 (2.7) mmol/L] and 41.3% [mean (SD): 3.2 (3.5) mmol/L] in the evinacumab-naïve and evinacumab-continue groups, respectively (Figure 1A). In adult and adolescent patients, LDL-C was reduced by 41.7% [mean (SD): 3.2 (3.3) mmol/L] and 55.4% [mean (SD): 4.7 (2.5) mmol/L], respectively, at Week 24 (Figure 1B). Although patient numbers varied at later time points due to the COVID-19 pandemic, the observed reductions in LDL-C were maintained through at least Week 120 (Figure 1).
Figure 1.
Per cent change in LDL cholesterol from baseline to Week 144 by (A) treatment status and (B) age. LDL-C, LDL cholesterol; SE, standard error
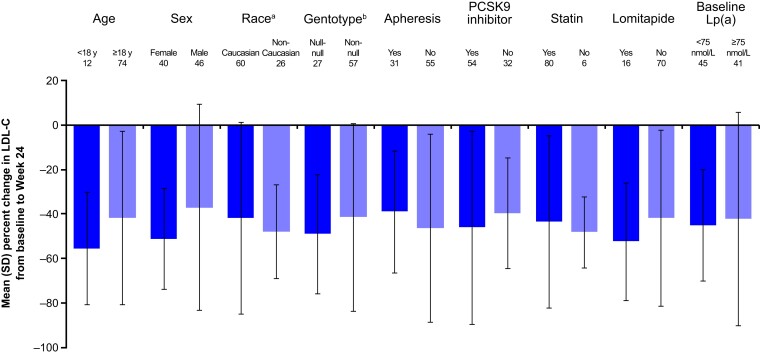
The mean per cent change in LDL-C from baseline to Week 24 was analysed by patient subgroups (Figure 2). In patients <18 years of age and ≥18 years of age, the mean per cent reduction in LDL-C from baseline to Week 24 was 55.4% [mean (SD): 4.7 (2.5) mmol/L] and 41.7% [mean (SD): 3.2 (3.3) mmol/L], respectively. Among female and male patients, the mean per cent reduction in LDL-C from baseline to Week 24 was 51.2% [mean (SD): 3.9 (3.6) mmol/L] and 37.1% [mean (SD): 3.0 (2.8) mmol/L], respectively. In Caucasian and non-Caucasian patients, the mean per cent reduction in LDL-C from baseline to Week 24 was 41.8% [mean (SD): 3.4 (3.5) mmol/L] and 48.0% [mean (SD): 3.4 (2.5) mmol/L], respectively. The mean per cent reduction in LDL-C from baseline to Week 24 was 49.0% [mean (SD): 5.1 (3.4) mmol/L] and 41.4% [mean (SD): 2.7 (2.9) mmol/L] in patients with null–null and non-null variants, respectively. For patients receiving and not receiving lipoprotein apheresis, the mean per cent reduction in LDL-C from baseline at Week 24 was 39.0% [mean (SD): 3.3 (2.7) mmol/L] and 46.3% [mean (SD): 3.5 (3.5) mmol/L], respectively. The mean per cent reduction in LDL-C from baseline to Week 24 was 47.1% [mean (SD): 3.4 (3.0) mmol/L] and 27.5% [mean (SD): 3.4 (4.4) mmol/L] in patients who were receiving ezetimibe and those who were not, respectively. Among patients receiving and not receiving a PCSK9 inhibitor, the mean per cent reduction in LDL-C from baseline to Week 24 was 46.1% [mean (SD): 3.6 (3.5) mmol/L] and 39.5% [mean (SD): 3.1 (2.7) mmol/L], respectively. The mean per cent reduction from baseline to Week 24 was 43.3% [mean (SD): 3.5 (3.3) mmol/L] and 48.1% [mean (SD): 2.9 (2.6) mmol/L] in patients who were receiving statin and those who were not, respectively. For patients receiving and not receiving lomitapide, the mean per cent reduction in LDL-C from baseline to Week 24 was 52.3% [mean (SD): 2.9 (2.6) mmol/L] and 41.7% [mean (SD): 3.5 (3.4) mmol/L], respectively. Finally, the mean per cent reduction in LDL-C from baseline to Week 24 was 45.0% [mean (SD): 3.2 (3.0) mmol/L] and 42.2% [mean (SD): 3.7 (3.5) mmol/L] for patients with baseline lipoprotein(a) [Lp(a)] <75 and ≥75 nmol/L, respectively.
Figure 2.
Mean per cent change in LDL cholesterol from baseline to Week 24 by patient subgroup. aNon-Caucasian group comprised patients who self-reported as African American or Black, Asian, and other (including those who did not self-report as African American or Black, Asian, American Indian, or Alaska Native, Native Hawaiian or other Pacific Islander). The non-Caucasian group also included patients for whom race was not reported. bNull–null genotype is defined as <15% LDLR activity. LDL-C, LDL cholesterol; LDLR, LDL receptor; Lp(a), lipoprotein(a); PCSK9, proprotein convertase subtilisin/kexin type 9; SD, standard deviation
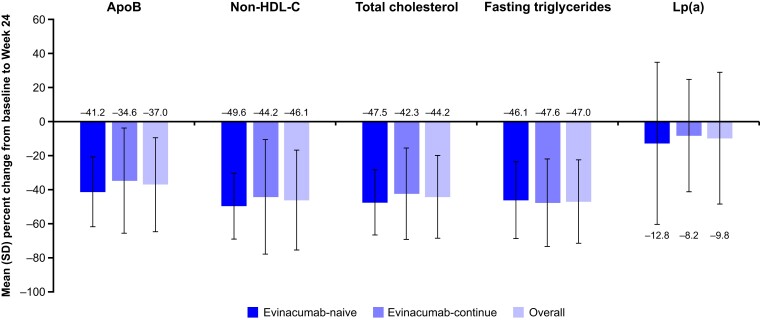
The effect of evinacumab on other lipid and lipoprotein parameters for the overall population and by treatment group is presented in Figure 3 and Table 4. As was observed with LDL-C level, evinacumab substantially decreased levels of Apo B, non-HDL-C, total cholesterol, fasting triglycerides, and Lp(a) in the overall population and in both treatment groups after 24 weeks. These observed reductions in lipid and lipoprotein parameters remained stable until at least Week 120 (see Supplementary data online, Figure S3A–E).
Figure 3.
Mean per cent change in lipid and lipoprotein parameters from baseline to Week 24. Apo B, apolipoprotein B; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; Lp(a), lipoprotein(a); SD, standard deviation
Table 4.
Absolute and per cent change in lipid and lipoprotein parameters from baseline to Week 24
| Parameter | Evinacumab-naïve | Evinacumab-continue | Total |
|---|---|---|---|
| (n = 46) | n = 70 | n = 116 | |
| LDL-C, mmol/L, mean (SD) | |||
| Baseline | 7.0 (3.2) | 6.6 (4.7) | 6.8 (4.1) |
| Absolute change from baseline | −3.8 (2.7) | −3.2 (3.5) | −3.4 (3.2) |
| Per cent change from baseline | −47.8 (23.6) | −41.3 (43.7) | −43.6 (37.7) |
| Apolipoprotein B, g/L, mean (SD) | |||
| Baseline | 1.8 (0.7) | 1.7 (0.9) | 1.7 (0.8) |
| Absolute change from baseline | −0.8 (0.6) | −0.6 (0.6) | −0.7 (0.6) |
| Per cent change from baseline | −41.2 (20.4) | −34.6 (30.8) | −37.0 (27.6) |
| Non-HDL-C, mmol/L, mean (SD) | |||
| Baseline | 7.5 (3.1) | 7.2 (4.8) | 7.3 (4.2) |
| Absolute change from baseline | −4.1 (2.6) | −3.5 (3.6) | −3.7 (3.2) |
| Per cent change from baseline | −49.6 (19.3) | −44.2 (33.5) | −46.1 (29.2) |
| Total cholesterol, mmol/L, mean (SD) | |||
| Baseline | 8.6 (3.0) | 8.3 (4.7) | 8.4 (4.1) |
| Absolute change from baseline | −4.5 (2.7) | −3.8 (3.6) | −4.1 (3.3) |
| Per cent change from baseline | −47.5 (19.0) | −42.3 (26.7) | −44.2 (24.2) |
| Fasting triglycerides, mmol/L, median (Q1, Q3) | |||
| Baseline | 1.0 (0.7, 1.6) | 1.0 (0.6, 1.6) | 1.0 (0.7, 1.6) |
| Absolute change from baseline | −0.4 (−0.8, −0.2) | −0.6 (−0.9, −0.2) | −0.5 (−0.9, −0.2) |
| Per cent change from baseline | −50.8 (−58.3, −35.5) | −51.8 (−65.9, −36.8) | −51.8 (−62.7, −36.2) |
| Lipoprotein(a), nmol/L, median (Q1, Q3) | |||
| Baseline | 50.0 (23.0, 113.0) | 73.5 (32.0, 168.0) | 66.0 (28.0, 166.0) |
| Absolute change from baseline | −8.0 (−22.0, −1.0) | −9.0 (−29.0, 2.0) | −8.5 (25.0, 0.0) |
| Per cent change from baseline | −18.2 (−33.3, −4.4) | −12.4 (−31.8, 4.7) | −14.6 (31.8, 0.0) |
HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; Q1, first quartile; Q3, third quartile; SD, standard deviation.
The achievement of relevant LDL-C goals during the study was also investigated as post hoc analyses. At Week 24, the proportion of patients achieving an LDL-C reduction ≥50% from baseline was 42.2% of the overall cohort, consisting of 39.2% of adults and 64.3% of adolescents; at Week 48, the proportions were 45.7%, 45.1%, and 50.0%, respectively (see Supplementary data online, Figure S4). For the overall cohort, the proportion of patients achieving LDL-C <1.4, <1.8, and <2.6 mmol/L was 14.7%, 19.8%, and 33.6% at Week 24, respectively (see Supplementary data online, Figure S5A). For those with ASCVD (n = 61), the proportion of patients achieving LDL-C <1.4 and <1.8 mmol/L at Week 24 was 18.0% and 27.9%, respectively (see Supplementary data online, Figure S5A). The proportion of patients achieving LDL-C goals for adults and adolescents is summarized in Supplementary data online, Figure S5A, and goal attainment results at Week 48 are summarized in Supplementary data online, Figure S5B.
Discussion
This study assessed the long-term safety and efficacy of evinacumab 15 mg/kg Q4W in a large cohort of adult and adolescent patients with HoFH. The results of this open-label study showed that evinacumab was generally well tolerated for up to 2.5 years. The mean per cent reduction in LDL-C level at Week 24 was 43.6% in the overall population, and this effect was maintained after 48 weeks. To our knowledge, this study comprised the largest cohort of patients with HoFH in a clinical study, and specifically female patients with HoFH. Among female patients, the mean per cent reduction in LDL-C level at Week 24 was 51.2% vs. 37.1% for male patients. Marked reductions in LDL-C from baseline to Week 24 were also observed irrespective of age, race, LDLR genotype, background LLT, or baseline Lp(a).
The reason for a higher mean LDL-C reduction among female patients with HoFH is unclear but might in part be due to higher mean LDL-C levels at baseline among female patients compared with male patients. The higher baseline LDL-C among female patients is consistent with previous studies that show female patients with FH have higher LDL-C levels compared with male patients.39,40
Evinacumab concomitantly decreased Apo B, non-HDL-C, total cholesterol, fasting triglycerides, and Lp(a) at Week 24 and this remained stable until at least Week 120. Lp(a) levels are frequently elevated in patients with HoFH and are associated with an increased risk of adverse cardiovascular outcomes.41,42 Thus, a reduction in Lp(a) might provide ASCVD benefit; however, this finding requires further verification in large clinical outcome trials.
Evinacumab was generally well tolerated in both adult and adolescent patients with HoFH, as was observed in the pivotal Phase 3 study.35 In the present study, most TEAEs were mild or moderate in severity, and no clinically significant changes were identified during the first 24 weeks of the OLTP. The safety profile of evinacumab was consistent with that of the pivotal Phase 3 study, as the incidence of TEAEs was ∼66% and the most common TEAEs were nasopharyngitis, influenza-like illness, and headache.35
The magnitude of LDL-C reduction was similar in the evinacumab-naïve and evinacumab-continue groups, suggesting that the effects of evinacumab were independent of treatment duration. Furthermore, the mean per cent reduction in LDL-C level was 47.8% and 41.3% in the evinacumab-naïve and evinacumab-continue groups, respectively, which was comparable with that of the pivotal Phase 3 study (47.1%).35 Together, these results suggest that evinacumab demonstrates a durable response in patients with HoFH.
Findings from this analysis suggest that evinacumab may fulfil an important unmet need. Evinacumab substantially decreased LDL-C levels in patients with null–null LDLR variants, who generally do not respond or respond suboptimally to currently accessible lipid-lowering medications.43 Moreover, LDL-C reductions were comparable in evinacumab-treated adult and adolescent patients. As early treatment initiation is imperative,4 evinacumab may assist young patients in achieving and maintaining LDL-C goals (Structured Graphical Abstract).
These findings have broad implications that are also promising. The prognosis of patients with HoFH is poor, given that many fail to respond adequately to available treatments.44,45 As LDL-C reduction is a key determinant of survival, sustained LDL-C reduction in evinacumab-treated patients has the potential to decrease the risk of cardiovascular events and mortality.
Different angiopoietin-like three inhibition strategies have been evaluated in clinical trials for their lipid-lowering potential. Evinacumab is a monoclonal antibody that binds to and inhibits angiopoietin-like 3 activity, leading to an increase in lipoprotein and endothelial lipase activity, and a decrease in the concentration of plasma lipids independent of LDLR activity.30–32 Conversely, vupanorsen, an N-acetyl galactosamine–conjugated antisense oligonucleotide, selectively inhibits hepatic translation of angiopoietin-like 3 mRNA, preventing hepatic synthesis and secretion of the angiopoietin-like 3 protein.46 Thus, the site of ANGPTL3 inhibition with vupanorsen is the nucleus of the hepatocyte vs. the plasma with evinacumab.47 In a placebo-controlled, double-blind, randomized, Phase 2b trial, that was designed to evaluate the effect of escalating doses of vupanorsen on non-HDL-C levels in statin-treated adults with hyperlipidaemia (TRANSLATE-TIMI 70), vupanorsen produced modest placebo-adjusted reductions in non-HDL-C ranging from 22.0% to 27.7%, at 24 weeks.48 Moreover, vupanorsen was associated with dose-dependent increases in hepatic fat fraction, and higher doses were associated with elevations in alanine aminotransferase and aspartate aminotransferase.48,49 Based on the results from the TRANSLATE-TIMI 70 study, the vupanorsen clinical development programme was discontinued on 31 January 2022.49 It is not yet understood whether the dose-dependent increases in hepatic fat fraction and liver enzymes occurred due to a specific metabolic effect of vupanorsen or an off-target effect as a result of intrahepatic ANGPTL3 inhibition.48 Therapeutic approaches to inhibition of intrahepatic ANGPTL3 are not necessarily expected to lead to an increase in hepatic adverse events.50 In mice, the intrahepatic inhibition of ANGPTL3 mRNA by an antisense oligonucleotide led to a decrease in liver triglyceride content.51 Furthermore, in individuals with loss-of-function mutations in ANGPTL3, complete or partial ANGPTL3 deficiency was not associated with hepatic steatosis.50 Notably, over the course of our study, the extracellular inhibition of ANGPTL3 with evinacumab was not associated with clinically meaningful changes in liver function parameters.
This study had several limitations, including its open-label design and the lack of a placebo control. Furthermore, patient retention was affected by the COVID-19 pandemic and accounted for the decline in patient numbers during the study. However, the effects of evinacumab on LDL-C level were sustained at Week 48, suggesting that this observation had little impact on the observed efficacy of evinacumab.
Conclusions
The results from this single-arm, open-label, Phase 3 study showed that evinacumab treatment for up to 4 years effectively decreased levels of LDL-C and other lipids and lipoproteins in patients with HoFH, with a safety profile that was consistent with previous clinical trials. Findings from this study extend those of the pivotal Phase 3 study and support the long-term safety and efficacy of evinacumab in reducing LDL-C levels in patients with HoFH irrespective of treatment duration, age, sex, race, LDLR genotype, and background LLT.
Supplementary Material
Acknowledgements
The authors would like to thank the patients, their families, and all the investigators involved in this study. Medical writing assistance and editorial support, under the direction of the authors, was provided by Stephanie Agbu, PhD, and Caryn Trbovic, PhD, of Regeneron Pharmaceuticals, Inc.; and Rebecca Mottram, PhD, of Prime (Knutsford, UK), funded by Regeneron Pharmaceuticals, Inc. according to Good Publication Practice guidelines.
Contributor Information
Daniel Gaudet, Clinical Lipidology and Rare Lipid Disorders Unit, Community Gene Medicine Center, Department of Medicine, Université de Montréal and ECOGENE-21, 930 Jacques-Cartier, Suite 210-B, Chicoutimi, Québec G7H 7K9, Canada.
Susanne Greber-Platzer, Division of Pediatric Pulmonology, Allergology and Endocrinology, Department of Pediatrics and Adolescent Medicine, Medical University of Vienna, Vienna, Austria.
Laurens F Reeskamp, Department of Vascular Medicine, Amsterdam University Medical Center, Location AMC, University of Amsterdam, Amsterdam, The Netherlands.
Gabriella Iannuzzo, Department of Clinical Medicine and Surgery, University of Naples, Naples, Italy.
Robert S Rosenson, Metabolism and Lipids Unit, Mount Sinai Heart, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Samir Saheb, LDL-Apheresis Unit, Department of Endocrinology, Hôpital de la Pitié-Salpêtrière, Université Paris Diderot, Sorbonne Paris, Paris, France.
Claudia Stefanutti, Department of Molecular Medicine, Extracorporeal Therapeutic Techniques Unit, Lipid Clinic and Atherosclerosis Prevention Centre, Regional Centre for Rare Diseases, Immunohematology and Transfusion Medicine, Umberto I Hospital, ‘Sapienza’ University of Rome, Rome, Italy.
Erik Stroes, Department of Vascular Medicine, Amsterdam University Medical Center, Location AMC, University of Amsterdam, Amsterdam, The Netherlands.
Albert Wiegman, Department of Paediatrics, Amsterdam University Medical Centers, Location University of Amsterdam, The Netherlands.
Traci Turner, Medpace Reference Laboratories, Cincinnati, OH, USA.
Shazia Ali, Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA.
Poulabi Banerjee, Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA.
Tiera Drewery, Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA.
Jennifer McGinniss, Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA.
Alpana Waldron, Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA.
Richard T George, Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA.
Xue-Qiao Zhao, Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA.
Robert Pordy, Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA.
Jian Zhao, Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA.
Eric Bruckert, Department of Endocrinology, Hôpital de la Pitié-Salpêtrière, Université Paris Diderot, Sorbonne Paris, Paris, France.
Frederick J Raal, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
D.G. reports grants and personal fees from Regeneron Pharmaceuticals, Inc. during the conduct of the study; and research grants, honoraria, or consulting fees for professional input and/or delivered lectures from Amgen, Amryt Pharma, Arrowhead, Eli Lilly, Esperion, Pfizer, Sanofi, Novartis, Ultragenyx, and Verve Therapeutics outside the submitted work. S.G.-P. reports receiving research support from Akcea Therapeutics, Amgen, Regeneron Pharmaceuticals, Inc., and Sanofi; lecture fees from Akcea Therapeutics, Chiesi, and Ultragenyx; support for attending meetings and/or travel from Akcea Therapeutics, Chiesi, and Ultragenyx; and receipt of equipment from Abbott Laboratories. L.F.R. reports investigational fees from Regeneron Pharmaceuticals, Inc., and speaker fees from Ultragenyx, Daiichi Sankyo, and Novartis. G.I. reports research grants from Amgen, Sanofi, and Regeneron Pharmaceuticals, Inc.; consulting fees from Akcea Therapeutics, Amryt Pharma, Daiichi Sankyo, Novartis, Sanofi, and Ultragenyx; fees for educational events from Amryt Pharma and Ultragenyx; advisory board fees from Daiichi Sankyo and Ultragenyx; and support for attending meetings and/or travel from Lusofarmaco and Sanofi. R.S.R. reports research grants and/or personal fees outside the submitted work from Regeneron Pharmaceuticals, Inc., Amgen, Arrowhead, Avilar Therapeutics, CRISPR Therapeutics, Kowa, Lilly, Lipigon, Meda Pharma, Merck, Novartis, Precision BioSciences, UpToDate, UltraGenyx, and Verve Therapeutics; and stock holdings in MediMergent, LLC. S.S. reports consulting fees from Regeneron Pharmaceuticals, Inc., Octapharma France, Argenx France, and Vifor Pharma France. C.S. reports research grants from Akcea Therapeutics, Amgen, Amryt Pharma, B. Braun Avitum, Fresenius Medical Care, Kaneka Pharma Europe, Regeneron Pharmaceuticals, Inc., and Sanofi. E.S. reports grants and/or personal fees outside the submitted work from Amgen, AstraZeneca, Daiichi Sankyo, Ionis Pharmaceuticals, Novo Nordisk, Novartis, and Sanofi. A.W. reports payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Novartis and Algorithm; participation in a data safety monitoring board or advisory board for Chiesi; leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid, for Novartis; and research support for pharmaceutical trials from Amgen, Regeneron Pharmaceuticals, Inc., Novartis, Silence Therapeutics, Esperion, Sanofi, and Ultragenyx. T.T. reports no conflicts of interest. S.A., P.B., J.M., R.T.G., X.-Q.Z., R.P., and J.Z. are employees of and shareholders in Regeneron Pharmaceuticals, Inc. T.D. is a former employee of Regeneron Pharmaceuticals, Inc. A.W. is an employee of and shareholder in Regeneron Pharmaceuticals, Inc.; and reports being a shareholder in Bristol Myers Squibb. E.B. reports consulting fees from Aegerion, Akcea Therapeutics, Amarin, Amgen, Genfit, Ionis Pharmaceuticals, Lilly, Merck Sharp & Dohme, Mylan, Novartis, Regeneron Pharmaceuticals, Inc., Sanofi, and Servier. F.J.R. has received research grants, honoraria, or consulting fees for professional input and/or delivered lectures from Amgen, Sanofi-Aventis, Regeneron Pharmaceuticals, Inc., Novartis, LIB Therapeutics, and Ultragenyx.
Data Availability
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, and statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the product and indication has been approved by major health authorities (e.g. FDA, EMA, PMDA, etc.), if there is legal authority to share the data, and if there is not a reasonable likelihood of participant re-identification. Submit requests to https://vivli.org/.
Funding
This study was funded by Regeneron Pharmaceuticals, Inc.
Ethical Approval
The study protocol was approved by the institutional review board and/or ethics committee at each site. The study was conducted in accordance with the principles of the Declaration of Helsinki and was consistent with Good Clinical Practices of the International Conference on Harmonisation and applicable regulatory requirements. All patients provided written informed consent.
Pre-registered Clinical Trial Number
The pre-registered clinical trial number is NCT03409744 (ClinicalTrials.gov).
References
- 1. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111–88. 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 2. Moorjani S, Roy M, Torres A, Betard C, Gagne C, Lambert M, et al. Mutations of low-density-lipoprotein-receptor gene, variation in plasma cholesterol, and expression of coronary heart disease in homozygous familial hypercholesterolaemia. Lancet 1993;341:1303–6. 10.1016/0140-6736(93)90815-X [DOI] [PubMed] [Google Scholar]
- 3. Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur Heart J 2013;34:3478–90. 10.1093/eurheartj/eht273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J 2014;35:2146–57. 10.1093/eurheartj/ehu274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bélanger AM, Akioyamen L, Alothman L, Genest J. Evidence for improved survival with treatment of homozygous familial hypercholesterolemia. Curr Opin Lipidol 2020;31:176–81. 10.1097/MOL.0000000000000686 [DOI] [PubMed] [Google Scholar]
- 6. Braamskamp M, Kastelein JJP, Kusters DM, Hutten BA, Wiegman A. Statin initiation during childhood in patients with familial hypercholesterolemia: consequences for cardiovascular risk. J Am Coll Cardiol 2016;67:455–6. 10.1016/j.jacc.2015.11.021 [DOI] [PubMed] [Google Scholar]
- 7. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e1082–143. 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raal FJ, Hovingh GK, Catapano AL. Familial hypercholesterolemia treatments: guidelines and new therapies. Atherosclerosis 2018;277:483–92. 10.1016/j.atherosclerosis.2018.06.859 [DOI] [PubMed] [Google Scholar]
- 9. Roth EM, Davidson MH. PCSK9 inhibitors: mechanism of action, efficacy, and safety. Rev Cardiovasc Med 2018;19:S31–46. 10.3909/ricm19S1S0002 [DOI] [PubMed] [Google Scholar]
- 10. Kayikcioglu M. LDL apheresis and Lp (a) apheresis: a clinician’s perspective. Curr Atheroscler Rep 2021;23:15. 10.1007/s11883-021-00911-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stefanutti C, Julius U, Watts GF, Harada-Shiba M, Cossu M, Schettler VJ, et al. Toward an international consensus—integrating lipoprotein apheresis and new lipid-lowering drugs. J Clin Lipidol 2017;11:858–71.e3. 10.1016/j.jacl.2017.04.114 [DOI] [PubMed] [Google Scholar]
- 12. Cuchel M, Raal FJ, Hegele RA, Al-Rasadi K, Arca M, Averna M, et al. 2023 Update on European Atherosclerosis Society Consensus Statement on Homozygous Familial Hypercholesterolaemia: new treatments and clinical guidance. Eur Heart J 2023;44:2277–91. 10.1093/eurheartj/ehad197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. France M, Rees A, Datta D, Thompson G, Capps N, Ferns G, et al. HEART UK statement on the management of homozygous familial hypercholesterolaemia in the United Kingdom. Atherosclerosis 2016;255:128–39. 10.1016/j.atherosclerosis.2016.10.017 [DOI] [PubMed] [Google Scholar]
- 14. Gaudet D, Gipe DA, Pordy R, Ahmad Z, Cuchel M, Shah PK, et al. ANGPTL3 inhibition in homozygous familial hypercholesterolemia. N Engl J Med 2017;377:296–7. 10.1056/NEJMc1705994 [DOI] [PubMed] [Google Scholar]
- 15. Rosenson RS. Existing and emerging therapies for the treatment of familial hypercholesterolemia. J Lipid Res 2021;62:100060. 10.1016/j.jlr.2021.100060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alonso R, Cuevas A, Mata P. Lomitapide: a review of its clinical use, efficacy, and tolerability. Core Evid 2019;14:19–30. 10.2147/CE.S174169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stefanutti C, Thompson GR. Lipoprotein apheresis in the management of familial hypercholesterolaemia: historical perspective and recent advances. Curr Atheroscler Rep 2015;17:465. 10.1007/s11883-014-0465-6 [DOI] [PubMed] [Google Scholar]
- 18. Stefanutti C. Lomitapide—a microsomal triglyceride transfer protein inhibitor for homozygous familial hypercholesterolemia. Curr Atheroscler Rep 2020;22:38. 10.1007/s11883-020-00858-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. European Medicines Agency . Lojuxta [summary of product characteristics]. https://www.ema.europa.eu/en/documents/product-information/lojuxta-epar-product-information_en.pdf (3 July 2023, date last accessed).
- 20. US Food and Drug Administration . JUXTAPID™ (lomitapide) prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/203858s019lbl.pdf (15 March 2024, date last accessed).
- 21. Cuchel M, Meagher EA, du Toit Theron H, Blom DJ, Marais AD, Hegele RA, et al. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet 2013;381:40–6. 10.1016/S0140-6736(12)61731-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blom DJ, Raal FJ, Santos RD, Marais AD. Lomitapide and mipomersen—inhibiting microsomal triglyceride transfer protein (MTP) and apoB100 synthesis. Curr Atheroscler Rep 2019;21:48. 10.1007/s11883-019-0809-3 [DOI] [PubMed] [Google Scholar]
- 23. Watts GF, Gidding SS, Hegele RA, Raal FJ, Sturm AC, Jones LK, et al. International Atherosclerosis Society guidance for implementing best practice in the care of familial hypercholesterolaemia. Nat Rev Cardiol 2023;20:845–69. 10.1038/s41569-023-00892-0 [DOI] [PubMed] [Google Scholar]
- 24. Wang A, Richhariya A, Gandra SR, Calimlim B, Kim L, Quek RG, et al. Systematic review of low-density lipoprotein cholesterol apheresis for the treatment of familial hypercholesterolemia. J Am Heart Assoc 2016;5:e003294. 10.1161/JAHA.116.003294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tromp TR, Hartgers ML, Hovingh GK, Vallejo-Vaz AJ, Ray KK, Soran H, et al. Worldwide experience of homozygous familial hypercholesterolaemia: retrospective cohort study. Lancet 2022;399:719–28. 10.1016/S0140-6736(21)02001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Orsoni A, Saheb S, Levels JHM, Dallinga-Thie G, Atassi M, Bittar R, et al. LDL-apheresis depletes apoE-HDL and pre-beta1-HDL in familial hypercholesterolemia: relevance to atheroprotection. J Lipid Res 2011;52:2304–13. 10.1194/jlr.P016816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alonso R, Diaz-Diaz JL, Arrieta F, Fuentes-Jimenez F, de Andres R, Saenz P, et al. Clinical and molecular characteristics of homozygous familial hypercholesterolemia patients: insights from SAFEHEART registry. J Clin Lipidol 2016;10:953–61. 10.1016/j.jacl.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 28. Thompson GR, Blom DJ, Marais AD, Seed M, Pilcher GJ, Raal FJ. Survival in homozygous familial hypercholesterolaemia is determined by the on-treatment level of serum cholesterol. Eur Heart J 2018;39:1162–8. 10.1093/eurheartj/ehx317 [DOI] [PubMed] [Google Scholar]
- 29. Santos RD, Stein EA, Hovingh GK, Blom DJ, Soran H, Watts GF, et al. Long-term evolocumab in patients with familial hypercholesterolemia. J Am Coll Cardiol 2020;75:565–74. 10.1016/j.jacc.2019.12.020 [DOI] [PubMed] [Google Scholar]
- 30. Fujimoto K, Koishi R, Shimizugawa T, Ando Y. Angptl3-null mice show low plasma lipid concentrations by enhanced lipoprotein lipase activity. Exp Anim 2006;55:27–34. 10.1538/expanim.55.27 [DOI] [PubMed] [Google Scholar]
- 31. Shimamura M, Matsuda M, Yasumo H, Okazaki M, Fujimoto K, Kono K, et al. Angiopoietin-like protein3 regulates plasma HDL cholesterol through suppression of endothelial lipase. Arterioscler Thromb Vasc Biol 2007;27:366–72. 10.1161/01.ATV.0000252827.51626.89 [DOI] [PubMed] [Google Scholar]
- 32. Adam RC, Mintah IJ, Alexa-Braun CA, Shihanian LM, Lee JS, Banerjee P, et al. Angiopoietin-like protein 3 governs LDL-cholesterol levels through endothelial lipase-dependent VLDL clearance. J Lipid Res 2020;61:1271–86. 10.1194/jlr.RA120000888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. European Medicines Agency . Evkeeza 150 mg/mL concentrate for solution for infusion [summary of product characteristics]. https://www.ema.europa.eu/en/documents/product-information/evkeeza-epar-product-information_en.pdf (5 March 2024, date last accessed).
- 34. US Food and Drug Administration . EVKEEZA™ (evinacumab-dgnb) prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761181s001lbl.pdf (5 March 2024, date last accessed).
- 35. Raal FJ, Rosenson RS, Reeskamp LF, Hovingh GK, Kastelein JJP, Rubba P, et al. Evinacumab for homozygous familial hypercholesterolemia. N Engl J Med 2020;383:711–20. 10.1056/NEJMoa2004215 [DOI] [PubMed] [Google Scholar]
- 36. Regeneron Pharmaceuticals Inc . FDA approves first-in-class EVKEEZA® (evinacumab-dgnb) for young children with ultra-rare form of high cholesterol. https://investor.regeneron.com/news-releases/news-release-details/fda-approves-first-class-evkeezar-evinacumab-dgnb-young-children (15 March 2024, date last accessed).
- 37. Ultragenyx Pharmaceutical Inc . EVKEEZA® (evinacumab) for injection (150 mg/mL concentrate for solution for infusion). https://pdf.hres.ca/dpd_pm/00072602.PDF (14 December 2023, date last accessed).
- 38. Banerjee P, Chan KC, Tarabocchia M, Benito-Vicente A, Alves AC, Uribe KB, et al. Functional analysis of LDLR (low-density lipoprotein receptor) variants in patient lymphocytes to assess the effect of evinacumab in homozygous familial hypercholesterolemia patients with a spectrum of LDLR activity. Arterioscler Thromb Vasc Biol 2019;39:2248–60. 10.1161/ATVBAHA.119.313051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johansen AK, Bogsrud MP, Christensen JJ, Rundblad A, Narverud I, Ulven S, et al. Young women with familial hypercholesterolemia have higher LDL-cholesterol burden than men: novel data using repeated measurements during 12-years follow-up. Atheroscler Plus 2023;51:28–34. 10.1016/j.athplu.2023.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Agarwala A, Deych E, Jones LK, Sturm AC, Aspry K, Ahmad Z, et al. Sex-related differences in premature cardiovascular disease in familial hypercholesterolemia. J Clin Lipidol 2023;17:150–6. 10.1016/j.jacl.2022.11.009 [DOI] [PubMed] [Google Scholar]
- 41. Ellis KL, Pang J, Chieng D, Bell DA, Burnett JR, Schultz CJ, et al. Elevated lipoprotein(a) and familial hypercholesterolemia in the coronary care unit: between Scylla and Charybdis. Clin Cardiol 2018;41:378–84. 10.1002/clc.22880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Perrot N, Verbeek R, Sandhu M, Boekholdt SM, Hovingh GK, Wareham NJ, et al. Ideal cardiovascular health influences cardiovascular disease risk associated with high lipoprotein(a) levels and genotype: the EPIC-Norfolk prospective population study. Atherosclerosis 2017;256:47–52. 10.1016/j.atherosclerosis.2016.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ito MK, Watts GF. Challenges in the diagnosis and treatment of homozygous familial hypercholesterolemia. Drugs 2015;75:1715–24. 10.1007/s40265-015-0466-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bruckert E. Recommendations for the management of patients with homozygous familial hypercholesterolaemia: overview of a new European Atherosclerosis Society consensus statement. Atheroscler Suppl 2014;15:26–32. 10.1016/j.atherosclerosissup.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 45. Nohara A, Tada H, Ogura M, Okazaki S, Ono K, Shimano H, et al. Homozygous familial hypercholesterolemia. J Atheroscler Thromb 2021;28:665–78. 10.5551/jat.RV17050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kosmas CE, Bousvarou MD, Sourlas A, Papakonstantinou EJ, Pena Genao E, Echavarria Uceta R, et al. Angiopoietin-like protein 3 (ANGPTL3) inhibitors in the management of refractory hypercholesterolemia. Clin Pharmacol 2022;14:49–59. 10.2147/CPAA.S345072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brandts J, Ray KK. Familial hypercholesterolemia: JACC focus seminar 4/4. J Am Coll Cardiol 2021;78:1831–43. 10.1016/j.jacc.2021.09.004 [DOI] [PubMed] [Google Scholar]
- 48. Bergmark BA, Marston NA, Bramson CR, Curto M, Ramos V, Jevne A, et al. Effect of vupanorsen on non-high-density lipoprotein cholesterol levels in statin-treated patients with elevated cholesterol: TRANSLATE-TIMI 70. Circulation 2022;145:1377–86. 10.1161/CIRCULATIONAHA.122.059266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pfizer . Pfizer and Ionis Announce Discontinuation of Vupanorsen Clinical Development Program. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-ionis-announce-discontinuation-vupanorsen (12 December 2023, date last accessed).
- 50. D'Erasmo L, Di Martino M, Neufeld T, Fraum TJ, Kang CJ, Burks KH, et al. ANGPTL3 deficiency and risk of hepatic steatosis. Circulation 2023;148:1479–89. 10.1161/CIRCULATIONAHA.123.065866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu W, Peralta R, et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med 2017;377:222–32. 10.1056/NEJMoa1701329 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, and statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the product and indication has been approved by major health authorities (e.g. FDA, EMA, PMDA, etc.), if there is legal authority to share the data, and if there is not a reasonable likelihood of participant re-identification. Submit requests to https://vivli.org/.