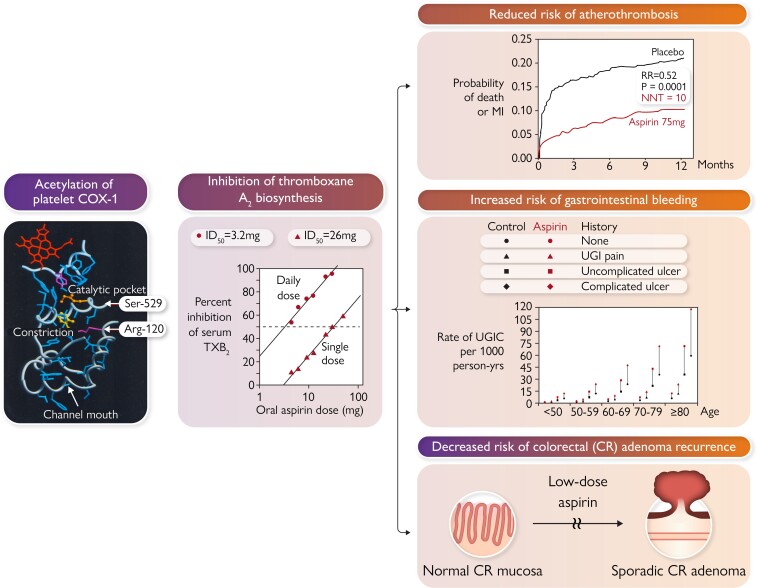
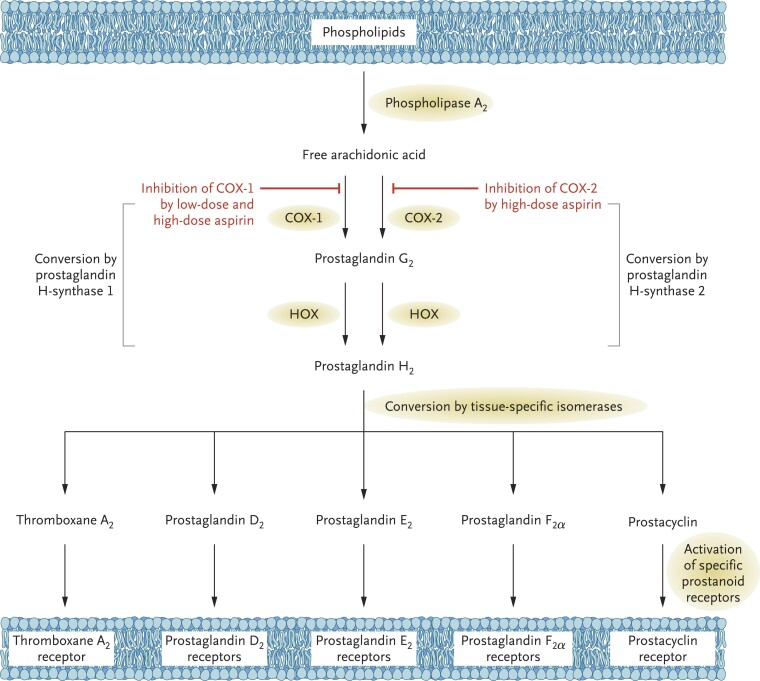
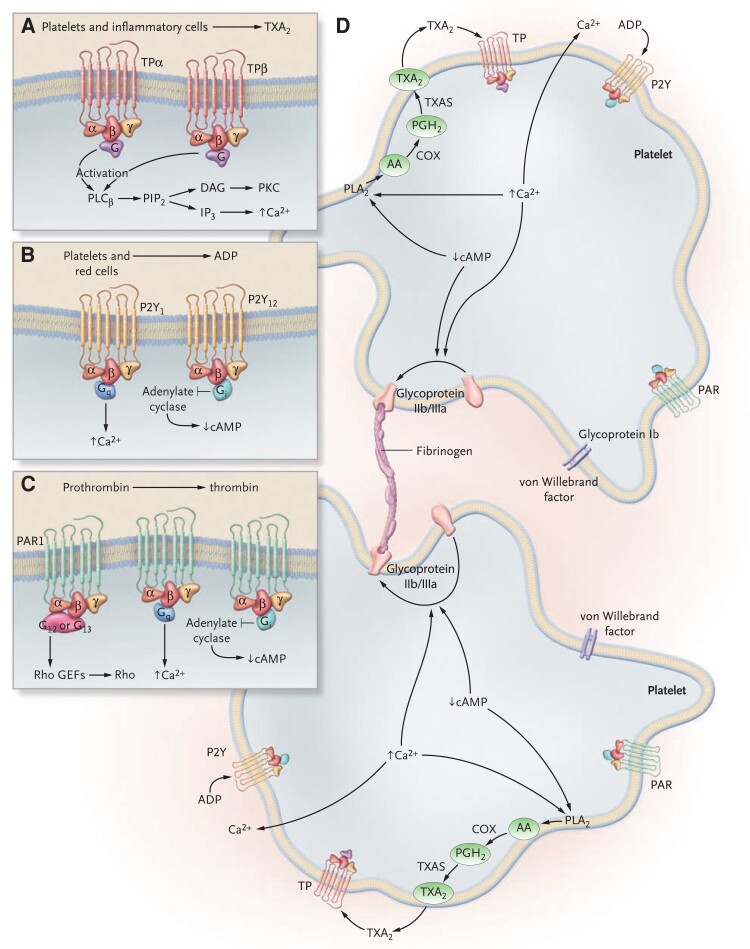
Graphical Abstract
Graphical Abstract.
Acetylation of serine-529 (Ser-529) of platelet prostaglandin H synthase-1 by low micromolar concentrations of aspirin permanently blocks the cyclooxygenase (COX)-1 channel near the catalytic pocket. Inactivation of platelet COX-1 is cumulative upon repeated daily dosing, because of the irreversible nature of enzyme acetylation, ensuring virtually complete suppression of thromboxane (TX) A2 biosynthesis at very low daily doses and limited interindividual variability. In randomized, placebo-controlled clinical trials, suppression of TXA2-dependent platelet activation by low-dose aspirin reduces risk of coronary atherothrombosis and its recurrence, increases risk of gastrointestinal bleeding from pre-existing mucosal lesions, and decreases risk of sporadic colorectal (CR) adenoma recurrence. Arg-120, arginine-120; ID50 = 50% inhibitory dose; MI, myocardial infarction; NNT, number needed to treat; RR, rate ratio; UGIC, upper gastrointestinal complication.
Keywords: Aspirin, P2Y12 inhibitors, Antiplatelet therapy, Clopidogrel, Ticagrelor, Atherothrombosis, Atherosclerotic cardiovascular disease, Bleeding, Colorectal cancer
Abstract
During the past 30 years, several developments have occurred in the antiplatelet field, including the role of aspirin in primary prevention of atherosclerotic cardiovascular disease. There have been several attempts to develop antiplatelet drugs more effective and safer than aspirin and a shift in emphasis from efficacy to safety, advocating aspirin-free antiplatelet regimens after percutaneous coronary intervention. Evidence supporting a chemopreventive effect of low-dose aspirin against colorectal (and other digestive tract) cancer has also strengthened. The aim of this article is to revisit the role of aspirin in the prevention of atherothrombosis across the cardiovascular risk continuum, in view of developments in the antiplatelet field. The review will offer a clinical perspective on aspirin’s mechanism of action, pharmacokinetics, and pharmacodynamics. This will be followed by a detailed discussion of its clinical efficacy and safety.
Introduction
Thirty years ago, the Antiplatelet Trialists’ Collaboration published the first individual participant data (IPD) overview of 145 randomized trials of prolonged antiplatelet therapy for prevention of death, myocardial infarction (MI), and stroke (vascular events) in various categories of patients.1 The most widely tested antiplatelet regimen was ‘medium dose’ (75–325 mg/day) aspirin, and the Antiplatelet Trialists’ Collaboration found no evidence that either a higher aspirin dose or any other antiplatelet regimen was more effective than medium-dose aspirin in preventing vascular events.1 At about the same time, I published a review article on aspirin as an antiplatelet drug that concluded ‘The present recommendation of a single loading dose of 200 to 300 mg followed by a daily dose of 75 to 100 mg is based on findings that this dose is as clinically efficacious as higher doses and is safer than higher doses’.2 Moreover, I added ‘the efficacy of a cheap drug such as aspirin in preventing one fifth to one third of all important cardiovascular events should not discourage the pharmaceutical industry from attempting to develop more effective antithrombotic drugs, since a sizable proportion of these events continue to occur despite currently available therapy’.2
During the past 30 years, we have witnessed several developments in the antiplatelet field, including (i) a large number of trials exploring the role of aspirin in primary prevention of atherosclerotic cardiovascular disease;3 (ii) several attempts by the pharmaceutical industry to develop antiplatelet drugs, more effective and safer than aspirin;4 (iii) a shift in emphasis from efficacy to safety leading to a strong opinion movement favouring aspirin-free antiplatelet regimens after percutaneous coronary intervention;5 and (iv) an IPD meta-analysis comparing P2Y12 inhibitor monotherapy with aspirin monotherapy for secondary prevention of coronary events.6 Moreover, evidence supporting a chemopreventive effect of low-dose aspirin against colorectal (and other digestive tract) cancer has strengthened, as recently reviewed.7
The aim of this article is to revisit the role of aspirin in the prevention of atherothrombosis across the cardiovascular risk continuum, in view of developments in the antiplatelet field. The review will offer a clinical perspective on aspirin’s mechanism of action, pharmacokinetics, and pharmacodynamics. This will be followed by a detailed discussion of its clinical efficacy and safety (Graphical Abstract).
Mechanism of action
The best characterized molecular mechanism of action of low-dose (i.e. 75 to 100 mg once daily) aspirin in preventing atherothrombosis is related to permanent inactivation of a bifunctional enzyme, prostaglandin (PG)G/H synthase-1, that catalyses the first committed step in prostanoid biosynthesis, i.e. the sequential conversion of arachidonic acid into PGG2 and PGH2 through its cyclooxygenase (COX) and peroxidase activities (Figure 1).8 Blockade of platelet COX-1 activity, through selective acetylation of a critical serine residue (Ser-529) located just below the catalytic pocket of the enzyme, deprives the next biosynthetic step of the substrate PGH2 for its further conversion to thromboxane (TX) A2, a potent platelet agonist and vasoconstrictor.8 Thromboxane A2 and its interaction with a specific prostanoid receptor, TP, on the platelet membrane represents one of the three important and largely independent pathways of platelet activation, which include the adenosine diphosphate (ADP)–P2Y12 and the thrombin–PAR-1 agonist–receptor interactions (Figure 2), susceptible to pharmacological modulation by approved antiplatelet drugs.9
Figure 1.
Mechanism of action of aspirin. Arachidonic acid, a 20-carbon fatty acid containing 4 double bonds, is liberated from the sn2 position of membrane phospholipids by several forms of phospholipase A2, which are activated by diverse stimuli. Arachidonic acid is converted by cytosolic prostaglandin H synthases, which have both cyclooxygenase and hydroperoxidase activity, to the unstable intermediates PGG2 and PGH2, respectively. The synthases are colloquially termed cyclooxygenases and exist in two forms, COX-1 and COX-2. Low-dose aspirin selectively inhibits COX-1, whereas high-dose aspirin inhibits both COX-1 and COX-2. PGH2 is converted by tissue-specific isomerases to multiple prostanoids. These bioactive lipids activate specific cell membrane receptors of the superfamily of G-protein–coupled receptors, such as the thromboxane A2 receptor (TP), the PGD2 receptors (DPs), the PGE2 receptors (EPs), the PGF2α receptors (FPs), and the prostacyclin (PGI2) receptor (IP). COX, cyclooxygenase; HOX, hydroperoxidase. Reproduced from Patrono et al.,8 with permission from the Massachusetts Medical Society
Figure 2.
Agonists, receptors, and effector systems in platelet activation. The activation of platelets is induced by the interaction of several agonists with receptors expressed on the platelet membrane. Panels A, B, and C depict outside-in signalling mediated by thromboxane A2, adenosine diphosphate, and thrombin, respectively. Thromboxane A2 is synthesized by activated platelets from arachidonic acid through the cyclooxygenase pathway (A). Once formed, thromboxane A2 can diffuse across the membrane and activate other platelets. In platelets, there are two splice variants of the TXA2 receptor, TPα and TPβ, which differ in their cytoplasmic tail. TPα and TPβ couple to the proteins Gq and G12 or G13, all of which activate phospholipase C. Adenosine diphosphate is released from platelets and red cells. Platelets express at least two adenosine diphosphate receptors, P2Y1 and P2Y12, which couple to Gq and Gi, respectively (B). The activation of P2Y12 inhibits adenylate cyclase, causing a decrease in the cyclic adenosine monophosphate level, and the activation of P2Y1 causes an increase in the intracellular Ca2+ level. The P2Y12 receptor is the major receptor able to amplify and sustain platelet activation in response to adenosine diphosphate. Thrombin is rapidly generated at sites of vascular injury from circulating prothrombin and, besides mediating fibrin generation, represents the most potent platelet activator (C). Platelet responses to thrombin are largely mediated through G-protein–linked protease-activated receptors, which are activated after thrombin-mediated cleavage of their N-terminal exodomain. Human platelets express PAR1 and PAR4. PAR1 couples to members of the G12/13, Gq, and Gi protein families. Panel D depicts inside-out signalling. The effects of agonists mediated by the decrease in cAMP levels and increase in intracellular Ca2+ levels lead to platelet aggregation through the change in the ligand-binding properties of the glycoprotein IIb/IIIa (αIIbβ3), which acquires the ability to bind soluble adhesive proteins such as fibrinogen and von Willebrand factor. The release of adenosine diphosphate and thromboxane A2 induces further platelet activation and aggregation. AA, arachidonic acid; ADP, adenosine diphosphate; cAMP, cyclic adenosine monophosphate; COX, cyclooxygenase; PAR, protease-activated receptors; PGH2, prostaglandin H2; PLA2, phospholipase A2; PLC, phospholipase C; TXAS, thromboxane synthase; TXA2, thromboxane A2. Reproduced from Davì and Patrono,9 with permission from the Massachusetts Medical Society
In particular, the TXA2–TP and ADP–P2Y12 outside-in signalling provides platelet activation with two amplification loops, inasmuch as any membrane perturbation triggers the immediate platelet synthesis of TXA2 and release of ADP, in turn inducing further platelet activation.9 Blockade of one or the other would be expected to yield similar protection against atherothrombosis as well as comparable impairment of primary haemostasis, with less-than-additive effects when blocking both, because of some redundancy of these amplification loops of the platelet activation signal.9 The results of randomized clinical trials (RCTs) of low-dose aspirin and P2Y12 inhibitors, given as monotherapy or combined as dual antiplatelet therapy (DAPT), are largely consistent with this mechanistic expectation.4,8,9
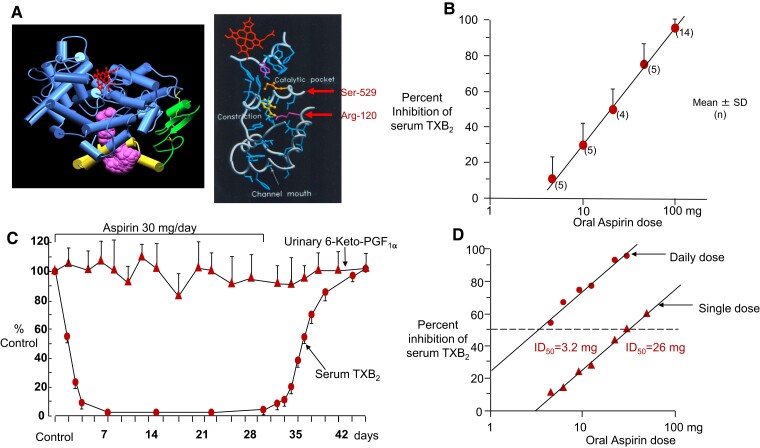
The clinical implications of the irreversible nature of platelet COX-1 inactivation by aspirin are related to an eight-fold shift in potency due to cumulative inactivation of the enzyme upon repeated daily dosing10 and to COX isozyme–selective inhibition at low doses (Figure 3).11 The former is reflected by the fact that the dose of aspirin required to inhibit platelet COX-1 by 50% (i.e. its ID50) is shifted from 26 mg after single oral dosing to approximately 3 mg upon repeated daily dosing.10 The latter implies that, when given once daily at low doses, aspirin completely blocks platelet COX-1 activity while largely sparing clinically relevant sites of COX-2 activity (e.g. endothelial cells of the vasculature and renal cells), not because of a differential affinity of the drug for the two COX isozymes, but because of its short half-life (see below) and resynthesis of any acetylated COX-2 in nucleated cells within a few hours.8
Figure 3.
Aspirin antiplatelet pharmacodynamics in healthy subjects. (A) Tridimensional model of human PGG/H synthase-1. Acetylation of Ser-529 by aspirin permanently blocks the COX-1 channel near the catalytic pocket. (B) Inhibition of platelet thromboxane A2 production by oral aspirin in healthy subjects. Thromboxane A2 production during whole blood clotting was measured before and 24 h after a single aspirin ingestion. The results are expressed as per cent inhibition, each subject serving as his or her own control. Mean values ± 1 SD are plotted. Numbers in parentheses indicate the number of subjects for each dose of aspirin. Reproduced from Patrignani et al., J Clin Invest 1982, with permission from the American Society for Clinical Investigation. (C) Long-term effects of low-dose (0.45 mg/kg per day) aspirin on platelet thromboxane A2 and renal PGI2 synthesis. Serum thromboxane A2 concentrations and urinary excretion of 6-keto-PGFIα were measured in three healthy subjects before, during, and after aspirin therapy. Mean values ± SEM are plotted. Reproduced from Patrignani et al., J Clin Invest 1982, with permission from the American Society for Clinical Investigation. (D) Dose dependence of the inhibition of platelet thromboxane B2 production by aspirin. Serum thromboxane B2 was measured before and after single (▴) or daily (●) dosing with aspirin in four healthy subjects. Individual data are expressed as per cent inhibition, with each subject serving as his or her own control. Daily dosing values represent measurements obtained at steady-state inhibition. ID50 = 50% inhibitory dose. Reproduced from Patrono et al.,10 with permission from Wolters Kluwer Health, Inc. SD, standard deviation; ID50, 50% inhibitory dose; TXB2, thromboxane B2
While P2Y12 inhibitors permanently modify (clopidogrel and prasugrel) or reversibly inhibit (ticagrelor) the platelet ADP receptor and consequently block ADP signalling, with no specific biochemical or functional changes that can be detected in vivo (though measurable ex vivo), aspirin inhibits the production of a platelet product, TXA2, that can be measured both ex vivo and in vivo.4 The assessment of TXA2 biosynthesis in vivo, through the measurement of the urinary excretion of its stable enzymatic metabolites (e.g. 11-dehydro-TXB2), provides a non-invasive biomarker of platelet activation9 and can help identify clinical settings in which low-dose aspirin may be particularly effective.12
Pharmacokinetics
Aspirin has a relatively simple pharmacokinetics, with no requirement for metabolic activation, ∼50% oral bioavailability, and 15 to 20 min half-life.4 The drug is rapidly absorbed in the stomach and upper intestine without active transporters, by virtue of its weak acidic properties, and undergoes first-pass metabolism in the liver resulting in partial deacetylation of acetylsalicylic acid to salicylic acid. The portal blood represents an important pre-systemic compartment for antiplatelet pharmacodynamics, as platelets are exposed early to approximately two-fold higher aspirin concentrations than in the systemic circulation.13 Pharmacokinetic parameters, such as Cmax and Tmax, depend on the aspirin formulation and influence the rate at which inhibition of platelet TXA2 production becomes detectable, with enteric-coated formulations requiring up to 4 to 5 h to reach a peak antiplatelet effect.13 The irreversible mechanism of action and short half-life of aspirin allow a ‘hit-and-run’ modality of antiplatelet action and limit any extra-platelet effects, thereby reducing vascular and gastrointestinal (GI) toxicity vis-à-vis traditional non-steroidal anti-inflammatory drugs (tNSAIDs).14 A simple pharmacokinetics limits the potential for drug–drug interactions. In fact, the only clinically relevant interaction of low-dose aspirin is with some tNSAIDs, such as ibuprofen and naproxen, which when present in the patient’s blood stream may limit access of aspirin to Ser-529, because of higher affinity for a common docking site (arginine-120) within the COX-1 channel (Figure 3).15 The concomitant administration of ibuprofen, but not celecoxib, paracetamol, or diclofenac, prevents the irreversible platelet inhibition induced by low-dose aspirin and may limit its cardioprotective effects.15,16 Moreover, failure to suspect or detect this drug–drug interaction may have been responsible for numerous past reports of the so-called aspirin ‘resistance’.17
The lack of requirement for conversion of acetylsalicylic acid to an active metabolite and pharmacokinetics that is not influenced by genetic polymorphisms of cytochrome P450 isozymes ensure highly reproducible antiplatelet pharmacodynamics.4,17 However, lower bioavailability of some enteric-coated preparations of low-dose aspirin and poor absorption from the higher pH environment of the small intestine may result in inadequate platelet inhibition, particularly in heavier subjects.18 Therefore, plain rather than enteric-coated aspirin formulation should be preferred when used as monotherapy in patients with body mass index >35 kg/m2 or body weight >120 kg.18 In a post hoc secondary analysis of the ADAPTABLE secondary prevention trial, enteric-coated aspirin was not associated with a statistically significant efficacy or safety advantage compared with uncoated aspirin, regardless of dose,19 and it is unclear under which circumstances the former should be preferred over the latter.
Pharmacodynamics
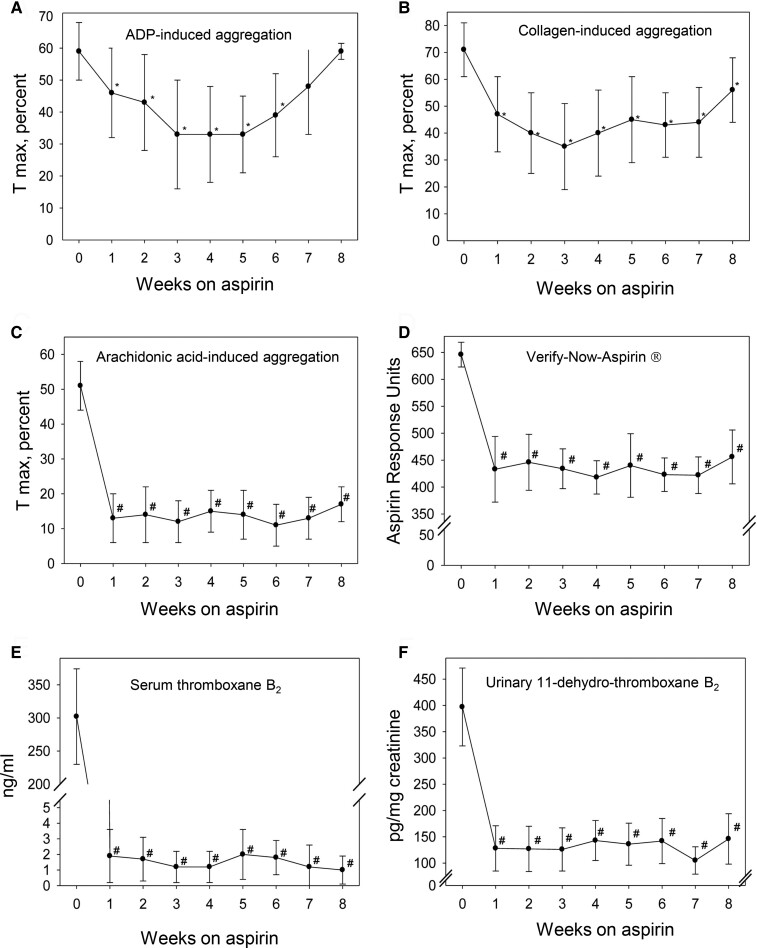
Traditionally, dose-finding studies of antiplatelet drugs were guided by measurement of platelet function based on the principle of optical aggregometry.20 This technique led to the discovery of the first aggregation inhibitors, i.e. ATP and adenosine,20 but suffers from major limitations primarily related to its recording a non-specific signal (light transmission) in response to the addition of a specific platelet agonist (e.g. ADP or arachidonic acid) to platelet-rich plasma.20 At variance with optical aggregometry, measurement of TXA2 production during whole blood clotting in response to endogenously formed thrombin, as reflected by serum TXB2,21 provides a mechanism-based, specific biomarker of aspirin’s antiplatelet pharmacodynamics that was used to investigate its time and dose dependence, in health10,11 and disease.22 In a study of 48 healthy volunteers randomized to receive aspirin 100 mg daily for 1 to 8 weeks, with weekly measurements of 6 functional and biochemical assays, serum TXB2 had the highest signal-to-noise ratio and the lowest interindividual and intraindividual variabilities (Figure 4).23
Figure 4.
Platelet biochemical and functional assays before and during aspirin intake in healthy subjects. Maximal aggregation (Tmax) values of adenosine diphosphate (A), collagen-induced (B), and arachidonic acid–induced (C) aggregation; aspirin response units of VerifyNow Aspirin (D); and absolute values of serum thromboxane B2 (E) and urinary 11-dehydro-thromboxane B2 (F) at baseline and during aspirin intake. Values are mean ± standard deviation of baseline [week 0 (n = 48), week 1 (n = 47), week 2 (n = 42), week 3 (n = 34), week 4 (n = 28), week 5 (n = 23), week 6 (n = 17), week 7 (n = 11), and week 8 (n = 6)]. *P < .01 vs. baseline. #P < .001 vs. baseline. Reproduced from Santilli et al.,23 with permission from Elsevier
Based on measurements of serum TXB2, aspirin is a potent (i.e. acting at very low doses) and highly effective (i.e. virtually inhibiting platelet TXA2 production by >99%) inhibitor of platelet COX-1 activity. However, it is often erroneously described as a ‘weak’ antiplatelet agent because of its limited effects on platelet aggregation induced by high concentrations of ADP or collagen (Figure 4). This is largely explained by the fact that these agonists, different from arachidonic acid, activate platelets through both TXA2-dependent and TXA2-independent pathways.9
While initial evaluation of aspirin as an antiplatelet agent was based on functional assays predicting the requirement of relatively high doses (975–1300 mg) and repeated daily dosing (e.g. t.i.d. or q.i.d.) regimens,24,25 further development of low-dose aspirin was largely guided by biochemical assays reflecting its mechanism of action.11,26,27 The latter successfully predicted two important features of the drug, that is, an optimal once-daily regimen and saturability of the antithrombotic effect at low doses.11,26,27
The antiplatelet pharmacodynamics of low-dose aspirin is largely independent of systemic bioavailability, because of pre-systemic (i.e. occurring in the portal blood) acetylation of platelet COX-1.13,28 Inactivation of platelet COX-1 is cumulative upon repeated daily dosing,11 because of the irreversible nature of enzyme acetylation,26 ensuring virtually complete suppression of TXA2 production and limited interindividual variability.23 Systemically available aspirin also acetylates megakaryocyte COX-1, ensuring long-lasting (i.e. >24 h) suppression of platelet TXA2 production.23,26
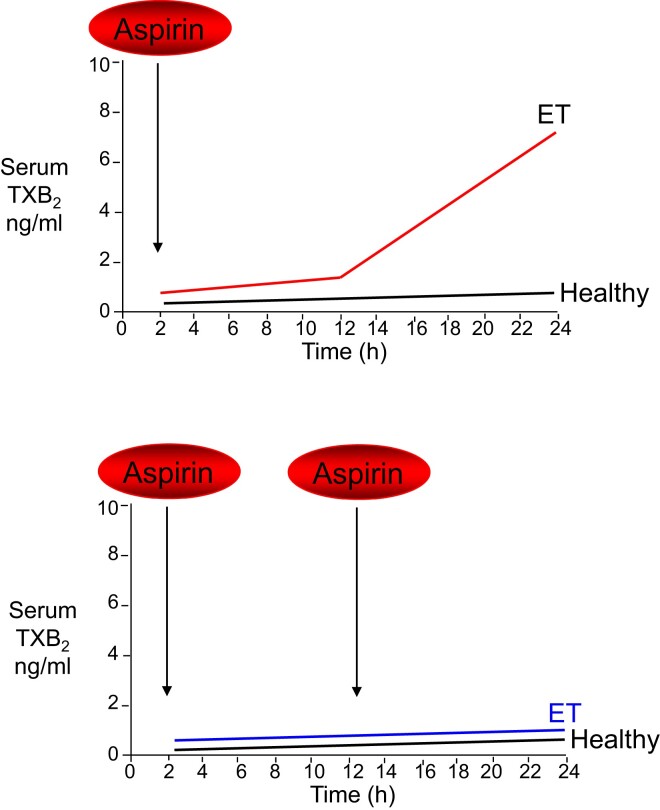
The rate of platelet turnover is a major determinant of the duration of the antiplatelet effect of aspirin, as suggested by the study of myeloproliferative neoplasms29 and modelled in silico.30 While physiological megakaryopoiesis and platelet production ensure at least 24–48 h of complete suppression of platelet TXA2 production, under conditions of enhanced platelet turnover, the duration of the antiplatelet effect of aspirin is shortened because of the accelerated renewal of the drug target (Figure 5).29 This may occur transiently, as observed following on-pump cardiac surgery,31 or persistently, as reported in patients with essential thrombocythemia.32 Shortening the dosing interval from 24 to 12 h, but not doubling the once-daily dose of aspirin, rescues the impaired antiplatelet effect of low-dose aspirin (Figure 5) and prevents platelet activation associated with acute inflammation31 and enhanced platelet turnover.31,32
Figure 5.
Model of altered aspirin pharmacodynamics in essential thrombocythemia. Upper panel: under conditions of normal megakaryopoiesis (healthy subjects), low-dose aspirin acetylates cyclooxygenase isozymes in both circulating platelets and bone marrow megakaryocytes, and negligible amounts of unacetylated enzymes are resynthesized within the 24 h dosing interval. This pharmacodynamic pattern is associated with virtually complete suppression of platelet thromboxane A2/B2 production in clotted peripheral blood throughout the dosing interval. Under conditions of abnormal megakaryopoiesis such as in essential thrombocythemia, an accelerated rate of cyclooxygenase isozyme resynthesis occurs in bone marrow megakaryocytes and platelet precursors, accompanied by faster peripheral release of immature platelets with unacetylated enzyme(s) during the aspirin dosing interval and in particular between 12 and 24 h after dosing. This pharmacodynamic pattern is associated with incomplete suppression of platelet thromboxane A2 production in peripheral blood and time-dependent recovery of thromboxane A2–dependent platelet function during the 24 h dosing interval. Lower panel: when low-dose aspirin is administered more frequently (i.e. twice daily), the daily platelet thromboxane A2 production in the peripheral blood of essential thrombocythemia patients is steadily inhibited. ET, essential thrombocythemia; TXB2, thromboxane B2
Similar considerations may apply to thienopyridines (ticlopidine, clopidogrel, and prasugrel),33 which, like aspirin, are characterized by a short-lived active moiety (the active metabolite) permanently inactivating its platelet drug target (i.e. P2Y12),4 with a long-lasting antiplatelet effect related to the variable platelet turnover.33
Clinical efficacy
Versus placebo
The clinical efficacy of aspirin as an antithrombotic agent has been evaluated in placebo-controlled RCTs addressing the whole spectrum of atherosclerosis, from apparently healthy, middle-aged, low-risk individuals to patients presenting with acute MI or acute ischaemic stroke. Moreover, aspirin trials have used variable follow-up, from as short as a few weeks to as long as 10 years.8,34
In the Second International Study of Infarct Survival, once-daily dosing with low-dose aspirin (162.5 mg) started within 24 h of the onset of symptoms of a suspected MI produced highly significant reductions in 5 week vascular mortality (the primary endpoint) by 23%, non-fatal reinfarction by 49%, and non-fatal stroke by 46% in over 17 000 patients.35 Three smaller but longer-term (12 weeks to 2 years) placebo-controlled RCTs in ∼2600 patients with unstable coronary artery disease consistently showed that aspirin therapy (75 to 1300 mg daily) halved the probability of death or MI, independent of the aspirin dose.36–38 Two RCTs with a similar protocol, the International Stroke Trial39 and the Chinese Acute Stroke Trial,40 randomized approximately 40 000 patients within 48 h of the onset of symptoms of an acute ischaemic stroke to 2 to 4 weeks of daily aspirin therapy (300 and 160 mg, respectively) or placebo and showed more modest reductions in death or non-fatal stroke than reported in acute MI (Figure 6).
Figure 6.
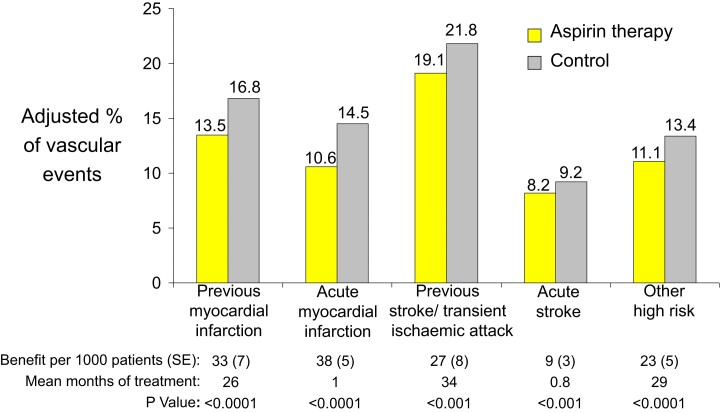
Absolute effects of antiplatelet therapy with aspirin on the risk of vascular events (non-fatal myocardial infarction, non-fatal stroke, or death from vascular causes) in five groups of high-risk patients. The figure is based on an analysis of data from the Antithrombotic Trialists’ Collaboration.34 Reproduced from Patrono et al.,8 with permission from the Massachusetts Medical Society
Based on a large number of placebo-controlled RCTs, long-term (2 to 3 year) aspirin therapy confers conclusive net benefit on the risk of subsequent MI, stroke, or vascular death among subjects at high risk of vascular complications (Figure 6).8,34 The proportional effects of long-term aspirin therapy on vascular events in these different clinical settings are rather homogeneous, ranging between 20% and 25% odds reduction based on an overview of all RCTs.34 The absolute benefits of antiplatelet prophylaxis in different categories of patients are detailed in Figure 6.8
The lowest effective daily dose of aspirin for long-term antiplatelet prophylaxis ranges between 50 and 100 mg, with no evidence that higher doses are more effective, consistent with saturability of platelet COX-1 inactivation at low doses.8 This evidence is based on indirect comparisons of RCTs employing different aspirin dosing regimens,34 as well as on a limited number of head-to-head randomized comparisons of a lower vs. a higher dose, both in acute coronary syndromes41 and stable patients with atherosclerotic cardiovascular disease.42
There is also no convincing evidence that the dose requirement for the antithrombotic effect of aspirin varies in different clinical settings or as a function of body weight.8,18,41,42 Similarly, there is no evidence that the effect of aspirin on major vascular events is influenced by gender, age, concomitant treatments, or primary vs. secondary prevention. In the ASPREE trial involving 19 114 healthy elderly persons (median age, 74 years) who did not have known cardiovascular disease (CVD), the use of low-dose aspirin did not result in a significantly lower risk of CVD than placebo [hazard ratio (HR) 0.95; 95% confidence interval (CI) 0.83–1.08].43 However, the widely cited message from ASPREE that low-dose aspirin is ineffective in old age is potentially misleading, for the following reasons: (i) CVD was not the primary endpoint of ASPREE; (ii) CVD, a pre-specified secondary endpoint, was a composite of platelet-dependent as well as platelet-independent events, i.e. fatal coronary heart disease, non-fatal MI, fatal or non-fatal stroke, or hospitalization for heart failure; and (iii) when the analysis was restricted to a traditional composite of fatal coronary heart disease, non-fatal MI, or fatal or non-fatal ischaemic stroke, the rate of this endpoint was 7.8 events per 1000 person-years in the aspirin group and 8.8 events per 1000 person-years in the placebo group (HR 0.89; 95% CI 0.77–1.03).43 Such a moderate treatment effect is identical to that reported for younger subjects (<65 years) in a setting of primary prevention,44 an effect that ASPREE failed to detect as statistically significant in a population at low cardiovascular risk (<1.0% per year) because it was largely underpowered. Despite serious limitations, these results were published as a separate, stand-alone paper in the New England Journal of Medicine43 and named as one of the 12 ‘game changers’ of 2018.
The apparent numerical difference in relative risk reductions observed in primary (typically, 10% to 12% reduction) and secondary (typically, 20% to 25% reduction) prevention trials is most likely explained by the much longer duration of the former (6.9 years) than the latter (2.5 years)44 and time-dependent loss of compliance with trial medication.
Strengths of the evidence for the antithrombotic efficacy of low-dose aspirin are related to the number, sample size, and duration of placebo-controlled RCTs across the whole spectrum of atherosclerotic cardiovascular disease risk. Potential limitations are represented by the time frame over which this evidence accumulated, prior to the development of coronary revascularization and other pharmacological preventive strategies (e.g. statins). Such improvements in treatment and prevention would be expected to reduce the baseline risk of serious vascular events and therefore the absolute benefits of aspirin addition. However, revascularization and statin therapy are unlikely to modify the relative risk reduction associated with aspirin use, if its benefits are additive to those of other strategies, as would be expected by their different mechanisms of action.
Versus other antiplatelet drugs
Additional evidence for the efficacy (and safety) of aspirin can be derived from a number of head-to-head RCTs in over 80 000 high-risk patients, which were designed to test the superiority of novel antiplatelet drugs developed during the past 30 years to replace aspirin as the mainstay of antithrombotic therapy (Table 1).45–50 These include oral inhibitors of the platelet ADP receptor, P2Y12; of the fibrinogen receptor, glycoprotein IIb/IIIa; and of the TXA2 receptor, TP. None of the Phase 3 pivotal trials or post-marketing studies of these agents provided unequivocal evidence for superiority vs. low-dose aspirin, and none of these drugs was approved by regulatory authorities with a superiority claim.
Table 1.
Superiority trials of other antiplatelet drugs vs. aspirin
| Trial (year) | Randomized comparison | Clinical setting | Sample size | Primary endpoint | Main result |
|---|---|---|---|---|---|
| CAPRIE45 (1996) | Clopidogrel (75 mg o.d.) vs. aspirin (325 mg o.d.) | ASCVD | 19 185 | Ischaemic stroke, MI, or vascular death | RR 0.91 (P = .043) |
| SYMPHONY46 (2000) | Sibrafiban (3.0, 4.5, or 6.0 mg) vs. aspirin (80 mg b.i.d.) | ACS | 9233 | Death, non-fatal MI, or recurrent ischaemia | OR 1.03, 95% CI 0.87–1.21 |
| PERFORM47 (2011) | Terutroban (30 mg o.d.) vs. aspirin (100 mg o.d.) | Stroke or TIA | 19 110 | Ischaemic stroke, MI, or vascular death | HR 1.02, 95% CI 0.94–1.12 |
| SOCRATES48 (2016) | Ticagrelor (90 mg b.i.d.) vs. aspirin (100 mg o.d.) | Acute stroke or TIA | 13 199 | Stroke, MI, or death | HR 0.89, 95% CI 0.78–1.01 |
| GLOBAL LEADERS49 (2018) | Ticagrelor-based (90 mg b.i.d.) vs. aspirin-based (75–100 mg o.d.) strategy | PCI for stable CAD or ACS | 15 968 | All-cause death or new Q-wave MI | RR 0.87, 95% CI 0.75–1.01 |
| HOST-EXAM50 (2021) | Clopidogrel (75 mg o.d.) vs. aspirin (100 mg o.d.) | After PCI | 5530 | All-cause death, MI, stroke, readmission due to ACS, and BARC bleeding ≥3 | HR 0.73, 95% CI 0.59–0.90 |
ACS, acute coronary syndrome; ASCVD, atherosclerotic cardiovascular disease; BARC, Bleeding Academic Research Consortium; CAD, coronary artery disease; CI, confidence interval; HR, hazard ratio; MI, myocardial infarction; PCI, percutaneous coronary intervention; RR, rate ratio; TIA, transient ischaemic attack.
However, because of the number of marketed P2Y12 inhibitors (ticlopidine, clopidogrel, prasugrel, and ticagrelor) and head-to-head RCTs against aspirin, two recent meta-analyses with a different design have addressed the superiority issue. In the tabular data meta-analysis of Chiarito et al.,51 9 RCTs were included with 42 108 patients randomly allocated to a P2Y12 inhibitor (ticlopidine, clopidogrel, or ticagrelor) or aspirin. Patients who received a P2Y12 inhibitor had a borderline reduction in the risk of MI compared with those who received aspirin (odds ratio 0.81; 95% CI 0.66–0.99). Risks of stroke, all-cause death, and vascular death did not differ between patients who received a P2Y12 inhibitor and those who received aspirin.51 Based on these results, the authors concluded that the potential advantage of P2Y12 inhibitor monotherapy over aspirin is of debatable clinical relevance, in view of the high number needed to treat (NNT = 244) to prevent one MI and the absence of any effect on all-cause and vascular mortality.51
In the IPD meta-analysis of the PANTHER Collaboration,6 7 RCTs were included with 24 325 participants with established CAD, and ticlopidine trials were excluded. In both meta-analyses, a single trial published 30 years ago, i.e. CAPRIE (Table 1),45 accounted for ∼60% of the total weight. However, the PANTHER meta-analysis included a sub-group of the CAPRIE trial [8446 patients with previous MI, including the original category of recent MI patients (n = 6302) plus patients with recent stroke or symptomatic atherosclerotic peripheral arterial disease with a previous MI at any time (n = 2144)] and a sub-group of the GLOBAL LEADERS trial (Table 1),49 published as GLASSY,52 represented by 7585 patients (out of 15 968) from the 20 top-enrolling participating sites.6 The primary outcome (a composite of cardiovascular death, MI, and stroke) occurred less frequently with P2Y12 inhibitor monotherapy compared with aspirin over 2 years (HR 0.88; 95% CI 0.79–0.97; P = .012), mainly due to less MI (HR 0.77; 95% CI 0.66–0.90; P < .001), with identical rates of cardiovascular and all-cause death.6 This difference was largely driven by the HOST-EXAM trial (Table 1),50 the only one among the seven studies included in the PANTHER meta-analysis whose 95% CI did not cross the identity line for the comparison vs. aspirin.6 In fact, the substantially lower point estimate of HOST-EXAM (HR 0.63; 95% CI 0.46–0.87), a trial exclusively conducted in South Korea, as compared with the other trials in non-Asian countries (HR 0.91; 95% CI 0.82–1.02) resulted in a treatment-by-sub-group interaction with geographical regions (Pinteraction = .034), suggesting that the difference between P2Y12 inhibitor and aspirin monotherapy was confined to an Asian population.6 The absolute difference in the calculated annual rate of the primary outcome (3.58% vs. 4.07%) would correspond to an NNT value of 204 patients to prevent 1 vascular event. Based on these results, the authors concluded that P2Y12 inhibitor monotherapy might be preferred over aspirin monotherapy for long-term secondary prevention in patients with established CAD,6 an interpretation of the available evidence not shared by the writing groups of the most recent ESC and AHA/ACC guidelines for the management of patients with acute coronary syndrome53 and those with chronic coronary disease.54
Safety
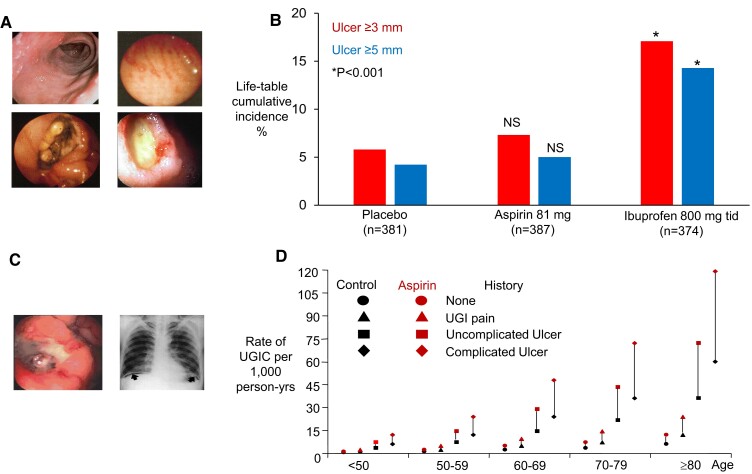
Traditional NSAIDs, including aspirin at analgesic–antipyretic doses (325 to 1000 mg every 4 to 6 h), cause GI lesions, such as mucosal erosions and ulcers, which are endoscopically detectable in 30% to 50% of tNSAID users (Figure 7).55 These lesions develop rapidly, are often asymptomatic, and tend to heal spontaneously.55 The GI toxicity of tNSAIDs is mostly attributed to inhibition of COX-1 and COX-2 in the GI mucosa, which impairs the physiological role of prostanoids (PGE2 in particular) in mucosal cytoprotection and tissue repair.55 Local irritation of the GI mucosa by the acidic nature of these drugs does not appear to play a clinically relevant role in ulcer complications, as enteric-coated formulations are not safer than plain formulations of tNSAIDs.55 Serious complications (bleeding, perforation, and obstruction) occur in 1% to 2% of tNSAID users (Figure 7).55 The major risk factors for upper GI bleeding are older age and a previous history of GI disorders (Figure 7).8
Figure 7.
Inducing new gastroduodenal lesions versus enhancing bleeding from pre-existing lesions. (A) Gastroduodenal erosions and ulcers induced by non-steroidal anti-inflammatory drugs. (B) Twelve-week cumulative incidences of gastroduodenal ulcers in osteoarthritis patients treated with low-dose aspirin, placebo, or ibuprofen. The figure was drawn with data from Laine et al., Gastroenterology 2004. (C) Gastrointestinal complications induced by non-steroidal anti-inflammatory drugs. (D) Estimated rates of upper gastrointestinal complications in men, according to age and the presence or absence of a history of such complications and regular treatment with low-dose aspirin. The vertical lines connecting each pair of black and red symbols depict the absolute excess of complications related to aspirin therapy. Reproduced from Patrono et al.,8 with permission from the Massachusetts Medical Society
Low-dose aspirin appears to spare COX-1 and COX-2 activity in the GI mucosa, by virtue of its short half-life and rapid resynthesis of the acetylated COX isozymes in nucleated epithelial cells. This is reflected by a 12 week cumulative ulcer incidence of 7% associated with low-dose (81 mg daily) aspirin, which was not significantly higher than the 6% placebo incidence in a large endoscopic RCT of elderly osteoarthritic patients (Figure 7).56 Because of the important role of platelet activation in tissue repair,57 pre-existing mucosal lesions throughout the GI tract will heal more slowly and bleed more easily in subjects on antiplatelet therapy. This is reflected by increased risk of upper and lower GI bleeding associated with low-dose aspirin and other antiplatelet drugs.4,8
In RCTs, the relative risk of a serious GI bleed in low-dose aspirin-treated subjects as compared with controls is ∼1.5, while in population-based observational studies, the corresponding value is ∼2.0.8 As shown in Figure 7, the annual rate of upper GI complications in the general population increases linearly with age and exponentially with the severity of prior history of GI disturbances within each decade, ranging from as low as 0.6 per 1000 to as high as 60 per 1000. A doubling of these rates due to chronic use of low-dose aspirin will yield an absolute excess of such complications ranging from as low as 6 per 10 000 (number needed to harm [NNH], 1667) in a young person with no prior GI history to as high as 600 per 10 000 (NNH, 17) in a very old person with a prior complicated ulcer (Figure 7).8
The GI toxicity of aspirin is dose-related, but differences in relatively rare GI bleeding complications among doses may require a very large sample size and a randomized comparison to be reliably demonstrated. In the CURRENT–OASIS 7 trial of 25 086 patients with acute coronary syndrome randomly assigned to either higher-dose (300 to 325 mg daily) or lower-dose (75 to 100 mg daily) aspirin for 30 days, there was a significant increase in the incidence of major GI bleeding among patients who received higher-dose aspirin, as compared with those who received lower-dose aspirin [47 patients (0.4%) vs. 29 patients (0.2%); P = .04], with no detectable difference in less frequent intracranial bleeding.41 The absolute excess of any intracranial haemorrhage due to aspirin is in the order of 1 to 2 per 10 000 treated per year.34
In the meta-analysis of Chiarito et al.,51 the risk of any bleeding and major bleeding was similar in patients who received a P2Y12 inhibitor and those who received aspirin. However, the risk for GI bleeding was 40% lower in patients who received a P2Y12 inhibitor than those who received aspirin, a difference largely driven by the CAPRIE trial.45 It should be emphasized that CAPRIE compared a standard dose of clopidogrel with aspirin 325 mg daily and reported rates of severe GI haemorrhage of 0.5% and 0.7%, respectively (P < .05),45 i.e. the same absolute excess of 2 per 1000 that was found in the CURRENT–OASIS 7 trial when comparing a higher with a lower dose of aspirin.41 In the PANTHER meta-analysis, the risk of major bleeding was similar in patients treated with a P2Y12 inhibitor or aspirin.6
Given the rather convincing evidence for comparable bleeding risk associated with the use of P2Y12 inhibitors and low-dose aspirin, it is surprising and scientifically unjustified that a number of relatively small RCTs, aimed at improving haemostatic safety shortly after percutaneous coronary intervention, recently tested the largely expected safety advantage of stopping aspirin vs. continuing DAPT,5 with inadequate statistical power to detect any plausible loss in cardioprotection. The overall risk of bleeding was reduced by ∼50% in these trials,5 a figure quite similar to the 40% reduction associated with earlier discontinuation of clopidogrel in older trials.54 However, these studies did not address the clinically more relevant question of the benefit and potential hazard of stopping the P2Y12 inhibitor vs. stopping aspirin as compared with continuing DAPT.
Further evidence supporting the concept that GI bleeding caused by low-dose aspirin is largely due to its antiplatelet effect is provided by the results of the PERFORM trial,47 a head-to-head randomized comparison of terutroban, a TP receptor antagonist, vs. low-dose aspirin (Table 1). Terutroban does not inhibit COX-1 or COX-2 and therefore does not impair the cytoprotective function of GI prostanoids. Antagonism of the platelet TXA2 receptor by terutroban yielded no significant difference in major or life-threatening bleedings vs. inactivation of platelet COX-1 by aspirin 100 mg daily. Intracranial haemorrhage occurred in <2% of the 19 100 patients with cerebral ischaemic events, with no statistically significant difference between groups (terutroban 1.54% vs. aspirin 1.28%; HR 1.20; 95% CI 0.94–1.53). Most importantly, no difference in the occurrence of GI bleedings was reported, based on 621 events during a 28 month follow-up (3.2% vs. 3.3%; HR 0.97; 95% CI 0.83–1.13).47
Although gastroprotectant agents, in particular proton-pump inhibitors, reduce the risk of peptic ulcer disease and its complications in a wide range of clinical circumstances,58 their use in patients taking antiplatelet drugs in recent trials is still quite limited. More recently, the HEAT trial showed that Helicobacter pylori eradication protects against aspirin-associated peptic ulcer bleeding, but this might not be sustained in the long term.59
The absolute excess of major bleeds caused by low-dose aspirin in one of the most recent placebo-controlled RCTs for primary prevention, ASCEND, is of the same order of magnitude as the absolute reduction in major vascular events,60 confirming a substantially uncertain balance of benefits and risks,61 as previously reported by an IPD meta-analysis of the six earliest primary prevention trials.44 However, when making a similar comparison of NNH and NNT values in contemporary RCTs assessing other antithrombotic strategies added on top of low-dose aspirin,62–64 a NNH/NNT ratio of ∼1 is not uncommon (Table 2).
Table 2.
Benefit/risk ratio in contemporary randomized controlled trials of antithrombotic therapy for primary and secondary prevention
| Trial | NNT | NNH | NNH/NNT |
|---|---|---|---|
| ASCEND60 | 91 | 112 | 1.2 |
| COMPASS62 | 77 | 83 | 1.1 |
| PEGASUS-TIMI 5463 | 79 | 81 | 1.0 |
| THEMIS64 | 138 | 93 | 0.7 |
NNT, number needed to treat; NNH, number needed to harm.
Aspirin could cause cognitive impairment through an increased risk of intracranial haemorrhage and cerebral micro-bleeds.65 However, in the ASCEND trial, allocation to low-dose aspirin for a mean of 7.4 years was not associated with any statistically significant differences vs. placebo in the risk of dementia (rate ratio 0.91; 95% CI 0.81–1.02; P = .11).65 A tabular data meta-analysis of three primary prevention trials, which included ASCEND65 and ASPREE,66 yielded a combined rate ratio of 0.92 (95% CI 0.84–1.01; P = .09).65
Traditional NSAIDs and coxibs share COX-2-dependent renal effects, which may acutely reduce renal function and impair blood pressure control.67 Moreover, an IPD meta-analysis of tNSAID and coxib RCTs showed that all COX-2 inhibitors roughly doubled the risk of hospitalization due to heart failure.14 Low-dose aspirin, by virtue of its relative COX-1 selectivity, does not impair renal function or blood pressure control68 and does not increase the risk of heart failure.43,60
Aspirin and cancer
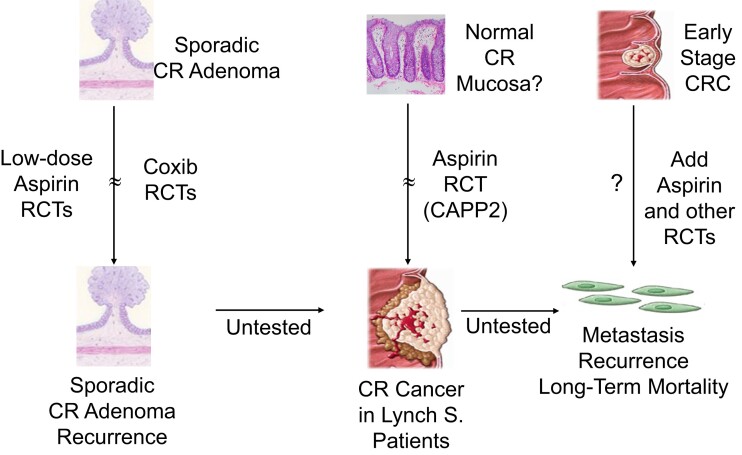
Multiple lines of evidence support a chemopreventive effect of aspirin against cancer, particularly tumours of the GI tract.7,69 These include (i) ∼70 observational studies suggesting a consistent association between regular aspirin use and reduced risk of oesophageal, gastric, and colorectal cancer; (ii) meta-analyses of post hoc, long-term follow-up of aspirin RCTs for primary and secondary prevention of CVD, suggesting a comparable protection against the development of GI cancers as reported from observational studies; (iii) prospective, long-term follow-up of the largest aspirin RCT for primary prevention in which colorectal cancer was a pre-specified, secondary endpoint displaying similar time-dependent protection; and (iv) a limited number of RCTs in which colorectal adenoma recurrence (in individuals with a history of these lesions) and cancer development (in patients with Lynch syndrome) were the primary endpoints, significantly reduced by aspirin treatment (Figure 8).7,69 While it was originally assumed that this was due to the anti-inflammatory effect of aspirin,70,71 more recent evidence points to platelet inhibition as the most likely mechanism explaining its chemopreventive effect.72,73 The sequential involvement of abnormal TXA2-dependent platelet activation at sites of intestinal mucosal injury, in turn triggering a local COX-2-driven inflammatory milieu in the early stages of colorectal carcinogenesis,72,73 has been suggested to explain these clinical benefits of aspirin that appear largely independent of the daily dose.70 Moreover, a review of this evidence suggested that even a 10% reduction in overall cancer incidence beginning during the first 10 years of treatment could tip the balance of benefits and risks in favour of using aspirin in average-risk populations.71
Figure 8.
Stages of colorectal carcinogenesis and aspirin chemopreventive effects. The figure depicts the three clinical settings in which aspirin and other cyclooxygenase inhibitors have been or are being evaluated by randomized clinical trials. CR, colorectal; CRC, colorectal cancer; Lynch S., Lynch syndrome; RCT, randomized controlled trial. Reproduced from Patrono,7 with permission from the American Society for Pharmacology and Experimental Therapeutics
A number of ongoing RCTs are testing the potential efficacy and safety of aspirin in the prevention of cancer recurrence and metastasization, when used in the early stages of several solid cancers.7
Conclusions and perspective
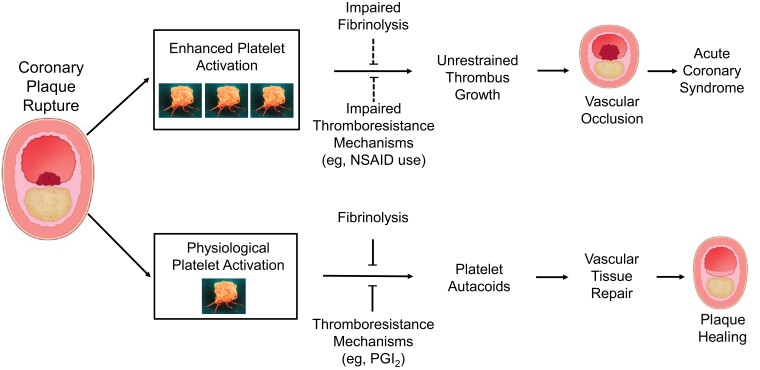
Basic and clinical research during the last 30 years has provided considerable insight in the role of TXA2-dependent platelet activation in primary haemostasis, tissue repair,57 atherothrombosis,9 intestinal inflammation,72 and colorectal cancer,7 through manipulation of the COX-1 gene,72,73 measurement of TXA2 biosynthesis in murine72,73 and human models,74,75 and use of low-dose aspirin.3,4,8 Appreciation of the role of platelet activation in vascular tissue repair and plaque healing76,77 warrants a reconsideration of the intensity and duration of antiplatelet therapy, given that the vast majority of episodes of coronary plaque erosion or fissure evolve towards lesion repair and healing (Figure 9).76,77
Figure 9.
Role of platelet activation in vascular tissue repair and atherothrombosis. The intensity and duration of the haemostatic response to coronary plaque erosion or rupture is controlled by a local balance between stimuli to platelet activation and counter-regulatory thromboresistance mechanisms (e.g. endothelial prostacyclin synthesis) of the vessel wall and fibrinolysis. Increased thromboxane A2–dependent platelet activation, in concert with impaired endothelial thromboresistance mechanisms (e.g. because of non-steroidal anti-inflammatory drug use), may favour uncontrolled progression towards vascular occlusion over physiological tissue repair and plaque healing
The role of aspirin in the current therapeutic armamentarium is unlikely to be replaced by currently available P2Y12 inhibitors, because of unconvincing demonstration of their superiority vs. low-dose aspirin, as reflected by the lack of a regulatory superiority claim and by recommendations of current cardiovascular treatment guidelines.53,54 The available evidence for comparable antithrombotic efficacy and bleeding liability of P2Y12 inhibitors and low-dose aspirin has far-reaching implications, when switching from DAPT to single antiplatelet therapy, inasmuch as no single trial has directly compared stopping one vs. the other in non-Asian populations.78
Given the very large body of evidence for the efficacy and safety of low-dose aspirin as an antithrombotic agent, there is still largely insufficient use of aspirin for secondary prevention, particularly in low-income countries.79 Efforts to address this unmet therapeutic need would have a much larger impact on global CVD prevention than substituting one antiplatelet agent for the other. Similarly, inadequate compliance or withdrawal of aspirin treatment is associated with increased risk of cardiovascular events in subjects with or at moderate-to-high CVD risk,80,81 and patients with a guideline-based indication should be encouraged to stay on antiplatelet treatment as long as the estimated benefit/risk profile remains favourable.53,54
Novel druggable therapeutic targets and compounds for antiplatelet therapy are currently in pre-clinical development, and some appear to have a more favourable safety profile than currently approved drugs with regard to bleeding risk.82 Further development of non-invasive biomarkers of platelet activation, and research on disutility and decision analysis,83,84 may improve the uncertain balance of benefits and risks of antiplatelet therapy in a primary prevention setting.3
Finally, the results of ongoing adjuvant trials of aspirin for cancer treatment and prevention7 may lead to further understanding of the role of platelet activation in cancer development and progression and widen the scope of long-term antiplatelet therapy.
Acknowledgements
The author is grateful to Andrea Bacigalupo, Francesca Santilli, Rocco Vergallo, and Andrea Zito for critically reviewing the first draft of this article.
Supplementary data
Supplementary data are not available at European Heart Journal online.
Declarations
Disclosure of Interest
The author reports personal consulting and lecture fees from AbbVie, Eli Lilly, and Tremeau and grant support (to the Institution) for investigator-initiated research from Bayer and Cancer Research UK; he chaired the Scientific Advisory Board of the International Aspirin Foundation.
Data Availability
No data were generated or analysed for this manuscript.
Funding
The author’s original studies that form the basis of this review article were funded by Agenzia Italiana del Farmaco (AIFA), Bayer, Cancer Research UK, and European Commission.
Ethical Approval
Ethical approval was not required.
Pre-registered Clinical Trial Number
None supplied.
References
- 1. Collaborative overview of randomised trials of antiplatelet therapy–I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ 1994;308:81–106. 10.1136/bmj.308.6921.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patrono C. Aspirin as an antiplatelet drug. N Engl J Med 1994;330:1287–94. 10.1056/NEJM199405053301808 [DOI] [PubMed] [Google Scholar]
- 3. Patrono C, Baigent C. Role of aspirin in primary prevention of cardiovascular disease. Nat Rev Cardiol 2019;16:675–86. 10.1038/s41569-019-0225-y [DOI] [PubMed] [Google Scholar]
- 4. Patrono C, Morais J, Baigent C, Collet JP, Fitzgerald D, Halvorsen S, et al. Antiplatelet agents for the treatment and prevention of atherothrombosis. J Am Coll Cardiol 2017;70:1760–76. 10.1016/j.jacc.2017.08.037 [DOI] [PubMed] [Google Scholar]
- 5. Capodanno D, Baber U, Bhatt DL, Collet JP, Dangas G, Franchi F, et al. P2y12 inhibitor monotherapy in patients undergoing percutaneous coronary intervention. Nat Rev Cardiol 2022;19:829–44. 10.1038/s41569-022-00725-6 [DOI] [PubMed] [Google Scholar]
- 6. Gragnano F, Cao D, Pirondini L, Franzone A, Kim HS, von Scheidt M, et al. P2y12 inhibitor or aspirin monotherapy for secondary prevention of coronary events. J Am Coll Cardiol 2023;82:89–105. 10.1016/j.jacc.2023.04.051 [DOI] [PubMed] [Google Scholar]
- 7. Patrono C. Cyclooxygenase inhibitors and cancer: the missing pieces. J Pharmacol Exp Ther 2023;386:181–9. 10.1124/jpet.122.001631 [DOI] [PubMed] [Google Scholar]
- 8. Patrono C, García Rodríguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med 2005;353:2373–83. 10.1056/NEJMra052717 [DOI] [PubMed] [Google Scholar]
- 9. Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med 2007;357:2482–94. 10.1056/NEJMra071014 [DOI] [PubMed] [Google Scholar]
- 10. Patrono C, Ciabattoni G, Patrignani P, Pugliese F, Filabozzi P, Catella F, et al. Clinical pharmacology of platelet cyclooxygenase inhibition. Circulation 1985;72:1177–84. 10.1161/01.CIR.72.6.1177 [DOI] [PubMed] [Google Scholar]
- 11. Patrignani P, Filabozzi P, Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J Clin Invest 1982;69:1366–72. 10.1172/JCI110576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G, Patrono C, et al. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med 2004;350:114–24. 10.1056/NEJMoa035572 [DOI] [PubMed] [Google Scholar]
- 13. Patrignani P, Tacconelli S, Piazuelo E, Di Francesco L, Dovizio M, Sostres C, et al. Reappraisal of the clinical pharmacology of low-dose aspirin by comparing novel direct and traditional indirect biomarkers of drug action. J Thromb Haemost 2014;12:1320–30. 10.1111/jth.12637 [DOI] [PubMed] [Google Scholar]
- 14. Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013;382:769–79. 10.1016/S0140-6736(13)60900-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Catella-Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, Tournier B, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med 2001;345:1809–17. 10.1056/NEJMoa003199 [DOI] [PubMed] [Google Scholar]
- 16. Renda G, Tacconelli S, Capone ML, Sacchetta D, Santarelli F, Sciulli MG, et al. Celecoxib, ibuprofen, and the antiplatelet effect of aspirin in patients with osteoarthritis and ischemic heart disease. Clin Pharmacol Ther 2006;80:264–74. 10.1016/j.clpt.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 17. Rocca B, Patrono C. Determinants of the interindividual variability in response to antiplatelet drugs. J Thromb Haemost 2005;3:1597–602. 10.1111/j.1538-7836.2005.01380.x [DOI] [PubMed] [Google Scholar]
- 18. Rocca B, Fox KAA, Ajjan RA, Andreotti F, Baigent C, Collet J-P, et al. Antithrombotic therapy and body mass: an expert position paper of the ESC Working Group on Thrombosis. Eur Heart J 2018;39:1672–91. 10.1093/eurheartj/ehy066 [DOI] [PubMed] [Google Scholar]
- 19. Sleem A, Effron MB, Stebbins A, Wruck LM, Marquis-Gravel G, Muñoz D, et al. Effectiveness and safety of enteric-coated vs uncoated aspirin in patients with cardiovascular disease: a secondary analysis of the ADAPTABLE randomized clinical trial. JAMA Cardiol 2023;8:1061–9. 10.1001/jamacardio.2023.3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Born G, Patrono C. Antiplatelet drugs. Br J Pharmacol 2006;147:S241–51. 10.1038/sj.bjp.0706401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patrono C, Ciabattoni G, Pinca E, Pugliese F, Castrucci G, De Salvo A, et al. Low dose aspirin and inhibition of thromboxane B2 production in healthy subjects. Thromb Res 1980;17:317–27. 10.1016/0049-3848(80)90066-3 [DOI] [PubMed] [Google Scholar]
- 22. De Caterina R, Giannessi D, Boem A, Bernini W, Battaglia D, Michelassi C, et al. Equal antiplatelet effects of aspirin 50 or 324 mg/day in patients after acute myocardial infarction. Thromb Haemost 1985;54:528–32. 10.1055/s-0038-1657890 [DOI] [PubMed] [Google Scholar]
- 23. Santilli F, Rocca B, De Cristofaro R, Lattanzio S, Pietrangelo L, Habib A, et al. Platelet cyclooxygenase inhibition by low-dose aspirin is not reflected consistently by platelet function assays: implications for aspirin “resistance”. J Am Coll Cardiol 2009;53:667–77. 10.1016/j.jacc.2008.10.047 [DOI] [PubMed] [Google Scholar]
- 24. Chesebro JH, Clements IP, Fuster V, Elveback LR, Smith HC, Bardsley WT, et al. A platelet-inhibitor-drug trial in coronary-artery bypass operations: benefit of perioperative dipyridamole and aspirin therapy on early postoperative vein-graft patency. N Engl J Med 1982;307:73–8. 10.1056/NEJM198207083070201 [DOI] [PubMed] [Google Scholar]
- 25. Canadian Cooperative Study Group . A randomized trial of aspirin and sulfinpyrazone in threatened stroke. N Engl J Med 1978;299:53–9. 10.1056/NEJM197807132990201 [DOI] [PubMed] [Google Scholar]
- 26. Burch JW, Stanford N, Majerus PW. Inhibition of platelet prostaglandin synthetase by oral aspirin. J Clin Invest 1978;61:314–9. 10.1172/JCI108941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. FitzGerald GA, Oates JA, Hawiger J, Maas RL, Roberts LJ II, Lawson JA, et al. Endogenous biosynthesis of prostacyclin and thromboxane and platelet function during chronic administration of aspirin in man. J Clin Invest 1983;71:676–88. 10.1172/JCI110814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pedersen AK, FitzGerald GA. Dose-related kinetics of aspirin. Presystemic acetylation of platelet cyclooxygenase. N Engl J Med 1984;311:1206–11. 10.1056/NEJM198411083111902 [DOI] [PubMed] [Google Scholar]
- 29. Patrono C, Rocca B, De Stefano V. Platelet activation and inhibition in polycythemia vera and essential thrombocythemia. Blood 2013;121:1701–11. 10.1182/blood-2012-10-429134 [DOI] [PubMed] [Google Scholar]
- 30. Giaretta A, Rocca B, Di Camillo B, Toffolo GM, Patrono C. In silico modeling of the antiplatelet pharmacodynamics of low-dose aspirin in health and disease. Clin Pharmacol Ther 2017;102:823–31. 10.1002/cpt.694 [DOI] [PubMed] [Google Scholar]
- 31. Cavalca V, Rocca B, Veglia F, Petrucci G, Porro B, Myasoedova V, et al. On-pump cardiac surgery enhances platelet renewal and impairs aspirin pharmacodynamics: effects of improved dosing regimens. Clin Pharmacol Ther 2017;102:849–58. 10.1002/cpt.702 [DOI] [PubMed] [Google Scholar]
- 32. Pascale S, Petrucci G, Dragani A, Habib A, Zaccardi F, Pagliaccia F, et al. Aspirin-insensitive thromboxane biosynthesis in essential thrombocythemia is explained by accelerated renewal of the drug target. Blood 2012;119:3595–603. 10.1182/blood-2011-06-359224 [DOI] [PubMed] [Google Scholar]
- 33. Rocca B, Patrono C. Platelet progenitors: the hidden drug target. Eur Heart J 2015;36:3211–3. 10.1093/eurheartj/ehv366 [DOI] [PubMed] [Google Scholar]
- 34. Antithrombotic Trialists’ Collaboration . Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high-risk patients. BMJ 2002;324:71–86. 10.1136/bmj.324.7329.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet 1988;2:349–60. 10.1016/S0140-6736(88)92833-4 [DOI] [PubMed] [Google Scholar]
- 36. Lewis HD Jr, Davis JW, Archibald DG, Steinke WE, Smitherman TC, Doherty JE III, et al. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study. N Engl J Med 1983;309:396–403. 10.1056/NEJM198308183090703 [DOI] [PubMed] [Google Scholar]
- 37. Cairns JA, Gent M, Singer J, Finnie KJ, Froggatt GM, Holder DA, et al. Aspirin, sulfinpyrazone, or both in unstable angina. Results of a Canadian multicenter trial. N Engl J Med 1985;313:1369–75. 10.1056/NEJM198511283132201 [DOI] [PubMed] [Google Scholar]
- 38. Wallentin LC. Aspirin (75 mg/day) after an episode of unstable coronary artery disease: long-term effects on the risk for myocardial infarction, occurrence of severe angina and the need for revascularization. J Am Coll Cardiol 1991;18:1587–93. 10.1016/0735-1097(91)90489-v [DOI] [PubMed] [Google Scholar]
- 39. The International Stroke Trial (IST): a randomized trial of aspirin, subcutaneous heparin, both, or neither among 19,435 patients with acute ischemic stroke. International Stroke Trial Collaborative Group. Lancet 1997;349:1569–81. 10.1016/S0140-6736(97)04011-7 [DOI] [PubMed] [Google Scholar]
- 40. CAST: randomized placebo-controlled trial of early aspirin use in 20,000 patients with acute ischemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet 1997;349:1641–9. 10.1016/S0140-6736(97)04010-5 [DOI] [PubMed] [Google Scholar]
- 41. CURRENT-OASIS 7 Investigators; Mehta SR, Bassand JP, Chrolavicius S, Diaz R, Eikelboom JW, et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 2010;363:930–42. 10.1056/NEJMoa0909475 [DOI] [PubMed] [Google Scholar]
- 42. Jones WS, Mulder H, Wruck LM, Pencina MJ, Kripalani S, Muñoz D, et al. Comparative effectiveness of aspirin dosing in cardiovascular disease. N Engl J Med 2021;384:1981–90. 10.1056/NEJMoa2102137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med 2018;379:1509–18. 10.1056/NEJMoa1805819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Antithrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–60. 10.1016/S0140-6736(09)60503-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. CAPRIE Steering Committee . A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348:1329–39. 10.1016/S0140-6736(96)09457-3 [DOI] [PubMed] [Google Scholar]
- 46. Comparison of sibrafiban with aspirin for prevention of cardiovascular events after acute coronary syndromes: a randomised trial. The SYMPHONY investigators. Sibrafiban versus aspirin to yield maximum protection from ischemic heart events post-acute coronary syndromes. Lancet 2000;355:337–45. 10.1016/S0140-6736(99)11179-6 [DOI] [PubMed] [Google Scholar]
- 47. Bousser M-G, Amarenco P, Chamorro A, Fisher M, Ford I, Fox KM, et al. Terutroban versus aspirin in patients with cerebral ischaemic events (PERFORM): a randomised, double-blind, parallel-group trial. Lancet 2011;377:2013–22. 10.1016/S0140-6736(11)60600-4 [DOI] [PubMed] [Google Scholar]
- 48. Johnston SC, Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med 2016;375:35–43. 10.1056/NEJMoa1603060 [DOI] [PubMed] [Google Scholar]
- 49. Vranckx P, Valgimigli M, Jüni P, Hamm C, Steg PG, Heg D, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet 2018;392:940–9. 10.1016/S0140-6736(18)31858-0 [DOI] [PubMed] [Google Scholar]
- 50. Koo BK, Kang J, Park KW, Rhee TM, Yang HM, Won KB, et al. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet 2021;397:2487–96. 10.1016/S0140-6736(21)01063-1 [DOI] [PubMed] [Google Scholar]
- 51. Chiarito M, Sanz-Sánchez J, Cannata F, Cao D, Sturla M, Panico C, et al. Monotherapy with a P2Y12 inhibitor or aspirin for secondary prevention in patients with established atherosclerosis: a systematic review and meta-analysis. Lancet 2020;395:1487–95. 10.1016/S0140-6736(20)30315-9 [DOI] [PubMed] [Google Scholar]
- 52. Franzone A, McFadden E, Leonardi S, Piccolo R, Vranckx P, Serruys PW, et al. Ticagrelor alone versus dual antiplatelet therapy from 1 month after drug-eluting coronary stenting. J Am Coll Cardiol 2019;74:2223–34. 10.1016/j.jacc.2019.08.1038 [DOI] [PubMed] [Google Scholar]
- 53. Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J 2023;44:3720–826. 10.1093/eurheartj/ehad191 [DOI] [PubMed] [Google Scholar]
- 54. Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, Demeter SH, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease. J Am Coll Cardiol 2023;82:833–955. 10.1016/j.jacc.2023.04.003 [DOI] [PubMed] [Google Scholar]
- 55. Patrono C. Nonsteroidal antiinflammatory drugs. In: Hochberg MC, Gravallese EM, Smolen JS, van der Heijde D, Weinblatt ME, Weisman MH (eds.), Rheumatology. 8th edn. Elsevier, 2022, 715–22. [Google Scholar]
- 56. Laine L, Maller ES, Yu C, Quan H, Simon T. Ulcer formation with low-dose enteric-coated aspirin and the effect of COX-2 selective inhibition: a double-blind trial. Gastroenterology 2004;127:395–402. 10.1053/j.gastro.2004.05.001 [DOI] [PubMed] [Google Scholar]
- 57. Gawaz M, Vogel S. Platelets in tissue repair: control of apoptosis and interactions with regenerative cells. Blood 2013;122:2550–4. 10.1182/blood-2013-05-468694 [DOI] [PubMed] [Google Scholar]
- 58. Scally B, Emberson JR, Spata E, Reith C, Davies K, Halls H, et al. Effects of gastroprotectant drugs for the prevention and treatment of peptic ulcer disease and its complications: a meta-analysis of randomised trials. Lancet Gastroenterol Hepatol 2018;3:231–41. 10.1016/S2468-1253(18)30037-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hawkey C, Avery A, Coupland CAC, Crooks C, Dumbleton J, Hobbs FDR, et al. Helicobacter pylori eradication for primary prevention of peptic ulcer bleeding in older patients prescribed aspirin in primary care (HEAT): a randomised, double-blind, placebo-controlled trial. Lancet 2022;400:1597–606. 10.1016/S0140-6736(22)01843-8 [DOI] [PubMed] [Google Scholar]
- 60. ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018;379:1529–39. 10.1056/NEJMoa1804988 [DOI] [PubMed] [Google Scholar]
- 61. Patrono C, Baigent C, Hirsh J, Roth G. Antiplatelet drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest 2008;133:199S–233S. 10.1378/chest.08-0672 [DOI] [PubMed] [Google Scholar]
- 62. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–30. 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 63. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015;372:1791–800. 10.1056/NEJMoa1500857 [DOI] [PubMed] [Google Scholar]
- 64. Steg PG, Bhatt DL, Simon T, Fox K, Mehta SR, Harrington RA, et al. Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med 2019;381:1309–20. 10.1056/NEJMoa1908077 [DOI] [PubMed] [Google Scholar]
- 65. Parish S, Mafham M, Offer A, Barton J, Wallendszus K, William Stevens W, et al. Effects of aspirin on dementia and cognitive function in diabetic patients: the ASCEND trial. Eur Heart J 2022;43:2010–19. 10.1093/eurheartj/ehac179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McNeil JJ, Woods RL, Nelson MR, Reid CM, Kirpach B, Wolfe R, et al. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med 2018;379:1499–508. 10.1056/NEJMoa1800722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med 2001;345:433–42. 10.1056/NEJM200108093450607 [DOI] [PubMed] [Google Scholar]
- 68. Zanchetti A, Hansson L, Leonetti G, Rahn KH, Ruilope L, Warnold I, et al. Low-dose aspirin does not interfere with the blood pressure-lowering effects of antihypertensive therapy. J Hypertens 2002;20:1015–22. 10.1097/00004872-200205000-00038 [DOI] [PubMed] [Google Scholar]
- 69. Elwood P, Morgan G, Watkins J, Protty M, Mason M, Adams R, et al. Aspirin and cancer treatment: systematic reviews and meta-analyses of evidence: for and against. Br J Cancer 2024;130:3–8. 10.1038/s41416-023-02506-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Patrignani P, Patrono C. Aspirin and cancer. J Am Coll Cardiol 2016;68:967–76. 10.1016/j.jacc.2016.05.083 [DOI] [PubMed] [Google Scholar]
- 71. Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol 2012;9:259–67. 10.1038/nrclinonc.2011.199 [DOI] [PubMed] [Google Scholar]
- 72. Sacco A, Bruno A, Contursi A, Dovizio M, Tacconelli S, Ricciotti E, et al. Platelet-specific deletion of cyclooxygenase-1 ameliorates dextran sulfate sodium-induced colitis in mice. J Pharmacol Exp Ther 2019;370:416–26. 10.1124/jpet.119.259382 [DOI] [PubMed] [Google Scholar]
- 73. Bruno A, Contursi A, Tacconelli S, Sacco A, Hofling U, Mucci M, et al. The specific deletion of cyclooxygenase-1 in megakaryocytes/platelets reduces intestinal polyposis in ApcMin/+ mice. Pharmacol Res 2022;185:106506. 10.1016/j.phrs.2022.106506 [DOI] [PubMed] [Google Scholar]
- 74. Petrucci G, Buck G, Rocca B, Parish S, Baigent C, Hatem D, et al. Thromboxane biosynthesis and future events in diabetes: the ASCEND trial. Eur Heart J 2024;45:1355–1367. 10.1093/eurheartj/ehad868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Joharatnam-Hogan N, Hatem D, Cafferty FH, Petrucci G, Cameron DA, Ring A, et al. Thromboxane biosynthesis in cancer patients and its inhibition by aspirin. Br J Cancer 2023;129:706–20. 10.1038/s41416-023-02310-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vergallo R, Crea F. Atherosclerotic plaque healing. N Engl J Med 2020;383:846–57. 10.1056/NEJMra2000317 [DOI] [PubMed] [Google Scholar]
- 77. Baaten CCFMJ, Nagy M, Bergmeier W, Spronk HMH, van der Meijden PEJ. Platelet biology and function: plaque erosion vs. rupture. Eur Heart J 2024; 45:18–31. 10.1093/eurheartj/ehad720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rocca B, Patrono C. Aspirin at 120: retiring, recombining, or repurposing? Res Pract Thromb Haemost 2021;5:e12516. 10.1002/rth2.12516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yoo SGK, Chung GS, Bahendeka SK, Sibai AM, Damasceno A, Farzadfar F, et al. Aspirin for secondary prevention of cardiovascular disease in 51 low-, middle-, and high-income countries. JAMA 2023;330:715–24. 10.1001/jama.2023.12905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Biondi-Zoccai GGL, Lotrionte M, Agostoni P, Abbate A, Fusaro M, Burzotta F, et al. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50 279 patients at risk for coronary artery disease. Eur Heart J 2006;27:2667–74. 10.1093/eurheartj/ehl334 [DOI] [PubMed] [Google Scholar]
- 81. Cea Soriano L, Bueno H, Lanas A, García Rodríguez LA. Cardiovascular and upper gastrointestinal bleeding consequences of low-dose acetylsalicylic acid discontinuation. Thromb Haemost 2013;110:1298–304. 10.1160/TH13-04-0326 [DOI] [PubMed] [Google Scholar]
- 82. Gawaz M, Geisler T, Borst O. Current concepts and novel targets for antiplatelet therapy. Nature Rev Cardiol 2023;20:583–99. 10.1038/s41569-023-00854-6 [DOI] [PubMed] [Google Scholar]
- 83. Lackey LG, Garnett CE, Senatore F. Applying decision analysis to inform the US Food and Drug Administration’s benefit-risk assessment of ticagrelor for primary prevention of myocardial infarction or stroke based on THEMIS. Circulation 2021;144:655–8. 10.1161/CIRCULATIONAHA.120.053294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dehmer SP, O'Keefe LR, Evans CV, Guirguis-Blake JM, Perdue LA, Maciosek MV. Aspirin use to prevent cardiovascular disease and colorectal cancer: updated modeling study for the US Preventive Services Task Force. JAMA 2022;327:1598–607. 10.1001/jama.2022.3385 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were generated or analysed for this manuscript.