Abstract
The identification of TVBS3, a cellular receptor for the cytopathic subgroups B and D of avian leukosis virus (ALV-B and ALV-D), as a tumor necrosis factor receptor-related death receptor with a cytoplasmic death domain, provides a compelling argument that viral Env-receptor interactions are linked to cell death (4). However, other TVB proteins have been described that appear to have similar death domains but are cellular receptors for the noncytopathic subgroup E of ALV (ALV-E): TVBT, a turkey subgroup E-specific ALV receptor, and TVBS1, a chicken receptor for subgroups B, D, and E ALV. To begin to understand the role of TVB receptors in the cytopathic effects associated with infection by specific ALV subgroups, we asked whether binding of a soluble ALV-E surface envelope protein (SU) to its receptor can lead to cell death. Here we report that ALV-E SU-receptor interactions can induce apoptosis in quail or turkey cells. We also show directly that TVBS1 and TVBT are functional death receptors that can trigger cell death by apoptosis via a mechanism involving their cytoplasmic death domains and activation of the caspase pathway. These data demonstrate that ALV-B and ALV-E use functional death receptors to enter cells, and it remains to be determined why only subgroups B and D viral infections lead specifically to cell death.
Cytopathic retroviruses have been shown to induce cell death (cytopathic effect [CPE]) upon infection of their target cells. Such viruses include avian leukosis viruses (ALVs), avian reticuloendotheliosis viruses (REVs), avian hemangioma viruses (AHVs), feline leukemia viruses (FeLVs), human and simian immunodeficiency viruses (HIVs, and SIVs), visna viruses, equine infectious anemia viruses, and spumaviruses (12, 16, 23). We are using ALV as a model system to understand how cytopathic retroviruses kill their target cells. ALVs are divided into different subgroups (designated A through J), and three of these viral subgroups (ALV-B, ALV-D, and ALV-F) induce CPEs upon infection of cultured avian cells (24, 25). This CPE is manifested during the acute phase of infection when up to 40% of the target cells are killed (24, 25). In addition, the genomic DNA contained within the dying cells is fragmented into nucleosomal ladders (24), suggesting that the cells have undergone apoptosis (8, 18).
It has been proposed that viral superinfection may lead directly to cell death in this system since the dying cells contain multiple (on average, 300 to 400) copies of unintegrated viral DNA (UVD) (24, 25). High levels of UVD are also associated with the CPE induced by other retroviruses including REV, visna virus, HIV type 1 and FeLV (23). However, at least for HIV-1, accumulation of UVD is not required for the viral CPE (3, 10). Thus, the role played by viral superinfection in the CPE induced by different retroviruses remains in question.
Viral determinants required for the CPE have been mapped to the Env proteins of ALV-B (7), HIV (5), Cas-Br-MLV (15), AHV (17), and FeLV (6), indicating that viral Env-receptor interactions are linked to retroviral CPEs. Indeed, the determinants on the ALV-B surface (SU) Env protein that are required for cell killing appear to be the same as those needed for receptor recognition (7). In addition, the cellular receptor for ALV-B and ALV-D, encoded by the s3 allele of the chicken tvb gene, appears to be a death receptor of the tumor necrosis factor receptor (TNFR) family (4, 21). The TVBS3 protein contains a putative cytoplasmic death domain which, in other TNFR-related receptors, is known to promote cell death following receptor activation by ligand binding or antibody binding (19).
The fact that binding of an ALV-B surface envelope (SU)-immunoglobulin fusion protein (an immunoadhesin) to TVBS3 can mediate cell death by apoptosis (4) gives additional support to the model that ALV-B/D Env-receptor interactions are involved in ALV-induced cell death. However, cell killing by the immunoadhesin only occurs when cells are incubated with cycloheximide to prevent new rounds of protein synthesis (4). In the case of TNFR-1, the protein synthesis inhibitor cycloheximide is thought to prevent expression of cellular survival factors that would otherwise protect cells from apoptosis (19). Expression of these cellular survival factors appears to be regulated by the transcription factor NF-κB (19).
Despite the compelling evidence that viral Env-receptor interactions play a role in ALV-induced cell death, it is curious that receptors for the noncytopathic subgroup E ALV are TVB proteins with putative cytoplasmic death domains: the turkey TVBT protein (formerly designated as SEAR) (1) and TVBS1 encoded by chicken s1 allele of tvb (2). To begin to understand why ALV-B infections can lead to cell death while ALV-E infections are unable to do so, we have asked whether subgroup E ALV SU-receptor interactions are capable of triggering cell death. We have also tested whether TVBS1 and TVBT are functional death receptors such as TVBS3 and whether cell death induction by these receptors requires their putative cytoplasmic death domains. Here we show that subgroup E ALV SU-receptor interactions are capable of inducing the death of avian cell types. We also show that each TVB receptor is a functional death receptor that can kill cells through the caspase pathway by a mechanism that is dependent upon the cytoplasmic death domains. We discuss the possible implications of these findings for understanding ALV-induced CPEs.
MATERIALS AND METHODS
Cell lines, viruses, and immunoadhesins.
Quail QT6 cells, primary turkey embryo fibroblasts (TEFs), and human 293 cells were described elsewhere (1, 4). QT6:TVBS3 cells expressing TVBS3 were described previously (4). The subgroup B-specific and subgroup E-specific SU-immunoglobulin fusion proteins (SUB-rIgG and SUE-rIgG, respectively) were described elsewhere (1). The subgroup A-specific RCASH(A) virus and the subgroup E-specific RCASE-Hgr virus, both encoding hygromycin B phosphotransferase, were described previously (4, 26). The subgroup B-specific RCASBP(B)-EGFP virus encoding the enhanced green fluorescent protein (EGFP) was kindly provided by C. Chang (Stratagene).
Plasmids.
Plasmids pBK7.6-2 encoding TVBS3, pBKTEF24 encoding TVBT, and pHA1 encoding TVBS1 were as described previously (1, 2, 4). The pJJ2 plasmid encoding TVBS3-ΔDD was derived from plasmid pBK7.6-2, which contains the complete tvbs3 cDNA clone in the pBK cloning vector (4). Plasmid pBK7.6-2 was digested with EcoRI, which cuts 77 nucleotides upstream of the first base of the ATG start codon of this clone, and with SalI, which cuts at a position that is 840 nucleotides downstream of that base. The resultant 917-bp EcoRI-SalI fragment encodes a truncated form of TVBS3 that lacks amino acids 281 to 369, thus eliminating the death domain (residues 281 to 353) (4). This EcoRI-SalI fragment was subcloned into a modified form of the pBK vector (Stratagene) that had a SalI site followed by two in-frame stop codons engineered into the EcoRI site by insertion of a double-stranded oligonucleotide. Plasmid pHA1 is a pCI-neo (Promega) derivative encoding TVBS1-ΔDD, a truncated receptor bearing the same deletion as TVBS3-ΔDD but with an added C-terminal FLAG epitope tag (DYKDDDDK), and was described elsewhere (2). Plasmid pHA3 is also a pCI-neo derivative that encodes TVBT-ΔDD, a truncated form of the TVBT receptor in which the amino acid residues after residue 280 were also replaced with a single copy of the FLAG epitope tag.
Plasmid pBK7.6-2 was used as a template for site-directed mutagenesis, which was performed by PCR amplification using overlapping mutagenic oligonucleotide primers. The DNA sequence of each mutant construct was confirmed by DNA sequencing (performed by the core DNA sequencing facility in the Department of Microbiology and Molecular Genetics, Harvard Medical School). Because the DNA sequences encompassing the cytoplasmic tail domains of TVBS1 and TVBS3 are identical (2), TVBS1 mutants were generated by replacing a BglII/MluI DNA fragment of the wild-type tvbs1 cDNA clone in plasmid pHA1 (encompassing the cytoplasmic death domain) with BglII/MluI DNA fragments derived from the mutant TVBS3 DNA constructs. Plasmid pJJ8 encodes TVBS3-L298A, plasmid pJJ10 encodes TVBS3-E315A, plasmid pJJ11 encodes TVBS3-W324A, plasmid pJJ15 encodes TVBS1-L298A, plasmid pJJ17 encodes TVBS1-E315A, and plasmid JJ18 encodes TVBS1-W324A. The sequences of all of the oligonucleotide primers that were used for mutagenesis are available upon request.
Cell death assays. (i) Immunoadhesin-mediated killing.
Approximately 105 QT6 cells were incubated in medium that contained 5 μg of cycloheximide per ml and either no immunoadhesin or 100 ng of purified SUB-rIgG or SUE-rIgG. After 5 days, the cells were replated, and the number of remaining adherent cells was determined 2 days later. Similar numbers of TEF cells were treated in the same way except they were incubated in medium containing or lacking immunoadhesin with 10 μg of cycloheximide per ml for 7 days, and the adherent cells were counted 4 days after being replated.
(ii) Infected cell killing.
Approximately 5 × 104 QT6:TVBS3 cells were infected at a multiplicity of infection of approximately 1 with the ALV vectors. Seven days after infection, the cells challenged with RCASH(A) or RCASE-Hgr were selected in medium containing 300 μg of hygromycin B per ml for an additional 10 days. The cells challenged with RCASBP(B)-EGFP were judged to be completely infected as visualized by fluorescence microscopy. Approximately 105 uninfected/infected QT6:TVBS3 cells were incubated in medium that contained 5 μg of cycloheximide per ml for 5 days, and the adherent cells were counted.
Human 293 cells.
Approximately 1.5 × 105 human 293 cells were transfected with 3 μg of plasmid DNA encoding the different TVB proteins or instead with 3 μg of a control plasmid pBK-CMV (Stratagene). Approximately 36 h after transfection (with or without 20 μM zVAD-fmk), the cells were incubated for 30 min in medium that contained 1 ng of Hoechst 33342 stain (Sigma) per ml and washed twice with phosphate-buffered saline (PBS), and the number of apoptotic nuclei associated with each cell population was determined by fluorescence microscopy using a Nikon TE-200 microscope.
Immunoblotting.
Approximately 2 × 106 human 293 cells were transfected with 10 μg of the plasmids encoding wild-type or mutant Tvb receptors or, for control purposes, with 10 μg of the empty vector pBK (Stratagene). Approximately 48 h after transfection, cells on each plate were lysed in 500 μl of NP-40 lysis buffer supplemented with protease inhibitors (1). Then, 100-μg protein samples, or 10 μg of protein in the case of TVBS1(ΔDD), were subjected to electrophoresis under reducing conditions on a 10% polyacrylamide gel containing sodium dodecyl sulfate. The proteins were then transferred to a nitrocellulose membrane (MSI) and subjected to immunoblotting using SUB-rIgG (crude extracellular supernatant diluted 1:3 in TBST). The membranes were probed with a horseradish peroxidase (HRP)-conjugated donkey antibody specific for rabbit immunoglobulins (Amersham) diluted 1:3,000 in TBST. Bound antibodies were detected by enhanced chemiluminescence.
Flow cytometry.
Human 293 cells were transfected as described above and, approximately 60 h after transfection, cells were harvested in Ca2+-Mg2+-free PBS containing 1 mM EDTA and prepared for flow cytometry as described previously (1, 27). Briefly, this involved incubating 105 cells with 1 ml of medium containing SUB-rIgG (for TVBS1-based and TVBS3-based constructs) or SUE-rIgG (for TVBT-based constructs) and then with a fluoresceinated secondary antibody (1, 27). Samples of five-thousand cells were then analyzed on a Coulter Epics XL Flow Cytometer in the Department of Hematologic Oncology at the Dana-Farber Cancer Institute and on a FACSCaliber Flow Cytometer at the McArdle laboratory.
RESULTS
ALV-E SU-receptor interactions can lead to cell death.
Previously, we have demonstrated that subgroup B ALV SU-receptor interactions can lead to the death of avian cells that express TVBS3: these cells were induced to die in the presence of a subgroup B-specific ALV SU-immunoglobulin protein (SUB-rIgG) in a cycloheximide-dependent manner (4). To determine whether ALV-E SU-receptor interactions can also lead to cell death, we tested the effect of adding an ALV-E SU-immunoglobulin protein (SUE-rIgG) (1) in the presence of cycloheximide to TEFs and quail QT6 cells. TEFs express TVBT, a subgroup E-specific ALV receptor, and lack any receptors for subgroups B and D ALV (1). QT6 cells are also permissive for infection only by ALV-E, presumably because they express a TVB receptor, which confers susceptibility to subgroup E viruses.
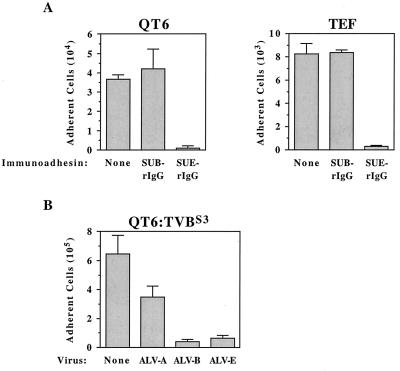
QT6 cells and TEF cells were induced to die in the presence of SUE-rIgG but not when incubated with SUB-rIgG or without immunoadhesin (Fig. 1A). Previously, we had shown that QT6 cells are resistant to cell killing induced by SUB-rIgG because they do not express subgroup B viral receptors (4). These data demonstrate that QT6 and TEF cells are killed specifically by the subgroup E-specific SU-immunoglobulin fusion protein, indicating that ALV-E SU-receptor interactions can trigger cell death. This is further supported by experiments that employed stably transfected QT6 cells that express TVBS3 (QT6:TVBS3), chronically infected by different ALV subgroups. In contrast to uninfected QT6:TVBS3 cells, cells infected by ALV-B and ALV-E vectors were specifically induced to die when incubated with cycloheximide, and only a moderate level of cell death was observed in an ALV-A-infected cell population (Fig. 1B). These data support the idea that cells which are chronically infected by subgroup B and E viruses are kept alive, at least in part, by the action of cellular survival factors that can counteract a TVB-associated death signal.
FIG. 1.
(A) Avian cells that express subgroup E viral receptors die specifically when incubated with an ALV-E SU-IgG fusion protein. Quail QT6-cells and primary TEFs were incubated in medium containing cycloheximide and either no immunoadhesin (None), SUB-rIgG, or SUE-rIgG. The numbers of adherent cells that survived this treatment are shown from a representative experiment performed in triplicate with the standard deviations indicated. (B) ALV-B- and ALV-E-infected cells are induced to die specifically in the presence of cycloheximide. Transfected QT6 cells expressing TVBS3 (QT6:TVBS3) were infected with subgroup A, B, and E ALV vectors and then incubated with cycloheximide. The average numbers of adherent cells surviving this treatment are shown from a representative experiment that was performed in triplicate, and the standard deviations of the data are indicated with error bars.
TVBS1 and TVBT are functional death receptors.
To test directly whether TVBS1 and TVBT are functional death receptors, we used a human 293 cell transient-transfection system that is commonly used to monitor the function of TNFR-related death receptors (9, 13, 14, 19, 20). Overexpression of TNFR-related death receptors in these cells leads to cell death in the absence of ligand binding, presumably because the cytoplasmic domains of these receptors become activated by “clustering” when expressed at a sufficiently high concentration (19).
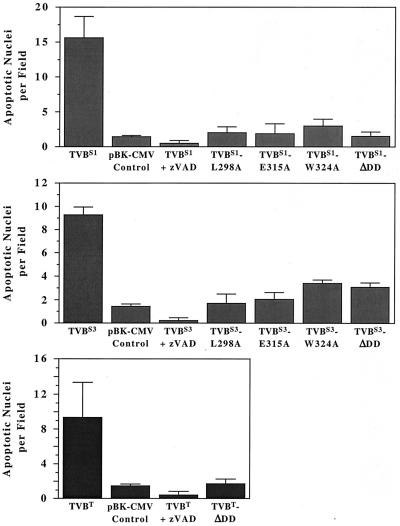
These studies reveal that, like TVBS3, the TVBS1 and TVBT proteins are functional death receptors (Fig. 2). Transfected cell populations expressing these proteins contained approximately 3.5- to 5-fold more apoptotic nuclei than were found associated with cells transfected with a control plasmid vector (pBK-CMV) (Fig. 1). Furthermore, cell killing induced by each of the TVB proteins was inhibited by z-VAD-fmk, a general caspase inhibitor (Fig. 2). Therefore, the TVBS1, TVBS3, and TVBT proteins are functional death receptors that can trigger apoptosis by a mechanism involving the caspase pathway (11).
FIG. 2.
TVBS1, TVBS3, and TVBT proteins induce apoptosis via the caspase pathway by a mechanism involving their cytoplasmic death domains. Human 293 cells were transfected with plasmid DNA encoding wild-type and mutant TVB proteins or instead with a control plasmid pBK-CMV (Stratagene). Cells were incubated with or without the caspase inhibitor zVAD-fmk (zVAD). The numbers of apoptotic nuclei associated with each cell population was determined by fluorescence microscopy after Hoechst staining by using a Nikon TE-200 microscope. Ten fields of each cell population were studied. The experiments were performed in triplicate, and the standard deviations of the data obtained are shown.
TVB-induced cell death requires the cytoplasmic death domain.
By comparison of TVB proteins with other known TNFR-related death receptors, it seemed likely that the cell-killing activities associated with each TVB receptor required the activity of their cytoplasmic candidate death domains. To test this possibility, the candidate death domains of these proteins were removed by truncation. Three truncated receptors lacking the death domains were generated and were designated TVBS1-ΔDD (2), TVBS3-ΔDD, and TVBT-ΔDD. In addition, site-specific mutations that were predicted to interfere with the cell-killing function were introduced into full-length versions of the TVBS1 and TVBS3 receptors. Specific mutations introduced into these proteins led to single amino acid substitutions in which either residue Leu-298, Glu-315, or Trp-324 was changed to an alanine. These amino acid substitutions were chosen because similar changes introduced at corresponding positions in the death domain of TNFR-1 abrogated the cell-killing activity of that receptor (22).
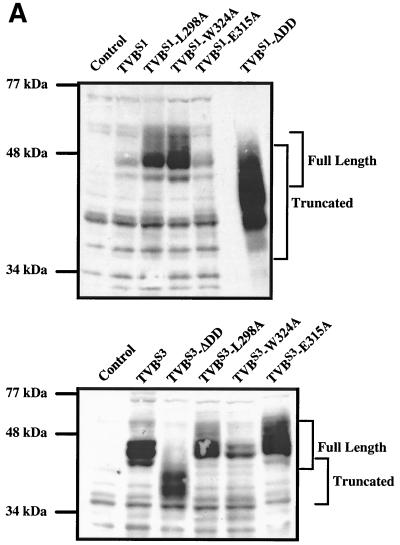
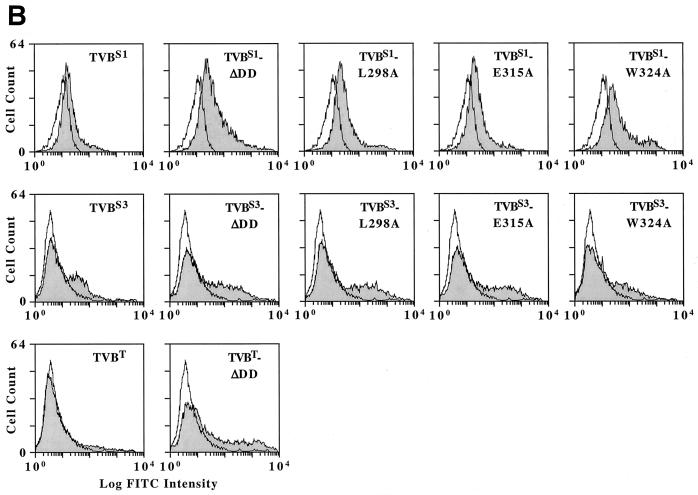
Expression of mutant TVB receptors was confirmed by immunoblotting of cellular protein lysates using an ALV-B SU Env-immunoglobulin fusion protein (SUB-rIgG) for detection. These data demonstrated that the altered TVBS1 and TVBS3 receptors were expressed in 293 cells (Fig. 3A). The highest level of expression obtained was with the TVBS1-ΔDD protein (Fig. 3A). Cell surface expression of each mutant TVBS1 and TVBS3 receptor was confirmed by flow cytometric analysis using the SUB-rIgG protein and a fluoresceinated secondary antibody as binding probes (Fig. 3B). Similarly, cell surface expression of TVBT-ΔDD was confirmed by flow cytometry using SUE-rIgG as a binding probe (Fig. 3B).
FIG. 3.
Wild-type and mutant TVB proteins are expressed in transfected human 293 cells. (A) Human 293 cells were transfected with plasmids encoding wild-type or mutant TVB receptors or, for control purposes, with pBK vector (Stratagene). Protein lysates from these cells were subjected to immunoblotting using SUB-rIgG and an HRP-coupled secondary antibody as binding probes. The positions of the full-length and truncated (ΔDD) receptors are indicated. (B) Human 293 cells expressing wild-type and mutant TVB receptors were incubated with SUB-rIgG (TVBS1-based and TVBS3-based constructs) or with SUE-rIgG (TVBT-based constructs) and then with a fluoresceinated secondary antibody (1, 26). The cells were then analyzed by flow cytometry. Open histograms represent data from cells that were not transfected, and the shaded histograms represent data from cells expressing the different TVB proteins as indicated.
In contrast to the wild-type TVB receptors, which induced apoptosis when overexpressed in human 293 cells, each of the mutant TVB receptors was unable to elicit cell death (Fig. 2). In each case, the level of cell death observed with each mutant receptor was similar to that seen by transfection with a control pBK-CMV plasmid vector (Fig. 2). These data demonstrate that the cytoplasmic death domains of each TVB receptor are required for cell killing.
DISCUSSION
Taken together, the data presented here provide evidence that ALV-E SU-receptor interactions are capable of causing the death of avian cells, at least when the expression of putative cellular survival factors is inhibited. In addition, we provide direct evidence that the TVB receptors for cytopathic and noncytopathic subgroups of ALV are functional TNFR-related death receptors capable of killing cells via the caspase pathway, by a mechanism involving their cytoplasmic death domains. Presumably, these death domains engage other death-domain-containing proteins such as TRADD, FADD, caspase-8, etc. (19), to elicit cell death. Although these data suggest that the TVB receptors for subgroups B, D, and E ALV are functionally equivalent, ALV-B/D Env-receptor interactions are linked to virus-induced cell death, whereas ALV-E Env-receptor interactions are not (7, 24, 25).
Several models can be envisaged to explain the differences in cytopathogenicity associated with these viruses. First, the TVB receptors might play no actual role in the CPE associated with subgroup B and D viral infections. However, this possibility seems unlikely because of the previous links established between ALV Env-receptor interactions and cell killing (7) and because the TVB receptors for these viruses are functional TNFR-related death receptors that trigger cell death by apoptosis (4), the very mechanism of cell death that seems to be associated with cytopathic ALV infections (24).
A second model implies that native interactions between ALV-B/D and ALV-E Env proteins and their receptors are necessary but not sufficient for cell killing. In support of this idea, cell killing induced by the SUB-rIgG and SUE-rIgG proteins occurs only in the presence of cycloheximide (reference 4 and the present study), which is thought to block the expression of protective cellular factors (19). This is further supported by the selective death of ALV-B- and ALV-E-infected QT6 cells that express TVBS3 when these cells are incubated with cycloheximide. Therefore, extinguishing the expression of putative protective factors may also be necessary for the viral CPE, and this might be a property specific to the acute phase of subgroup B and D viral infections, although this idea remains to be tested. Another line of evidence supporting the idea that ALV Env-receptor interactions are not sufficient for virus-induced cell killing comes from the fact that cell death is not observed during the first round of viral infection but instead appears to be associated with repeated rounds of subgroup B viral superinfection (24, 25). However, it remains to be shown if superinfection is involved in the CPE.
A third model suggests that ALV-B Env and ALV-E Env interact with the shared TVBS1 receptor in fundamentally different ways: these viruses exhibit a nonreciprocal receptor interference pattern in which infection of cells by ALV subgroups B or D interferes with the receptors for ALV-B, ALV-D, and ALV-E, whereas infection of cells by subgroup E viruses only interferes with the receptor for ALV-E. Furthermore, our recent studies have shown that the interaction of TVBS1 with ALV-E Env requires the presence of a putative disulfide bond between residues Cys-46 and Cys-59 of the receptor, whereas ALV-B Env can interact with receptors lacking these two cysteine residues (2). It remains to be determined if these differences in subgroup-specific ALV Env-receptor interactions have consequences for virus-induced cell killing. However, it is possible that ALV-B infections preferentially activate the receptor-associated cell death pathway, whereas ALV-E infections may preferentially activate a receptor-associated cell survival pathway (19). Our experiments performed with cycloheximide do not exclude this possibility since, under these conditions, the expression of putative cellular survival factors would be inhibited (19). The actual role played by ALV-B and ALV-D Env-receptor interactions in virus-induced cell death is currently under investigation.
ACKNOWLEDGMENTS
We thank members of the Young lab for many helpful discussions. We also thank John Daly and Janet Lewis for assistance with the flow cytometry and Cathy Chang for providing the RCASBP(B)-EGFP virus.
This work was supported by NIH grant CA62000.
REFERENCES
- 1.Adkins H B, Brojatsch J, Naughton J, Rolls M M, Pesola J M, Young J A T. Identification of a cellular receptor for subgroup E avian leukosis virus. Proc Natl Acad Sci USA. 1997;94:11617–11622. doi: 10.1073/pnas.94.21.11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adkins H B, Brojatsch J, Young J A T. Identification and characterization of a shared TNFR-related receptor for subgroups B, D, and E avian leukosis viruses reveals cysteine residues required specifically for subgroup E viral entry. J Virol. 2000;74:3572–3578. doi: 10.1128/jvi.74.8.3572-3578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergeron L, Sodroski J. Dissociation of unintegrated viral DNA accumulation from single-cell lysis induced by human immunodeficiency virus type 1. J Virol. 1992;66:5777–5787. doi: 10.1128/jvi.66.10.5777-5787.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brojatsch J, Naughton J, Rolls M M, Zingler K, Young J A T. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell. 1996;87:845–855. doi: 10.1016/s0092-8674(00)81992-3. [DOI] [PubMed] [Google Scholar]
- 5.Chirmule N, Pahwa S. Envelope glycoproteins of human immunodeficiency virus type 1: profound influences on immune functions. Microbiol Rev. 1996;60:386–406. doi: 10.1128/mr.60.2.386-406.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donahue P R, Quackenbush S L, Gallo M V, deNoronha C M, Overbaugh J, Hoover E A, Mullins J I. Viral genetic determinants of T-cell killing and immunodeficiency disease induction by the feline leukemia virus FeLV-FAIDS. J Virol. 1991;65:4461–4469. doi: 10.1128/jvi.65.8.4461-4469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorner A J, Coffin J M. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell. 1986;45:365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- 8.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 9.Kitson J, Raven T, Jiang Y P, Goeddel D V, Giles K M, Pun K T, Grinham C J, Brown R, Farrow S N. A death-domain-containing receptor that mediates apoptosis. Nature. 1996;384:372–375. doi: 10.1038/384372a0. [DOI] [PubMed] [Google Scholar]
- 10.Laurent-Crawford A G, Hovanessian A G. The cytopathic effect of human immunodeficiency virus is independent of high levels of unintegrated viral DNA accumulated in response to superinfection of cells. J Gen Virol. 1993;74:2619–2628. doi: 10.1099/0022-1317-74-12-2619. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Yuan J. Deciphering the pathways of life and death. Curr Opin Cell Biol. 1999;11:261–266. doi: 10.1016/s0955-0674(99)80035-0. [DOI] [PubMed] [Google Scholar]
- 12.Linial M L. Foamy viruses are unconventional retroviruses. J Virol. 1999;73:1747–1755. doi: 10.1128/jvi.73.3.1747-1755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan G, Ni J, Wei Y F, Yu G, Gentz R, Dixit V M. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 14.Pan G, O'Rourke K, Chinnaiyan A M, Gentz R, Ebner R, Ni J, Dixit V M. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 15.Paquette Y, Hanna Z, Savard P, Brousseau R, Robitaille Y, Jolicoeur P. Retrovirus-induced murine motor neuron disease: mapping the determinant of spongiform degeneration within the envelope gene. Proc Natl Acad Sci USA. 1989;86:3896–3900. doi: 10.1073/pnas.86.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasty S, Dhruva B R, Schiltz R L, Shih DS, Issel C J, Montelaro R C. Proviral DNA integration and transcriptional patterns of equine infectious anemia virus during persistent and cytopathic infections. J Virol. 1990;64:86–95. doi: 10.1128/jvi.64.1.86-95.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resnick-Roguel N, Burstein H, Hamburger J, Panet A, Eldor A, Vlodavsky I, Kotler M. Cytocidal effect caused by the envelope glycoprotein of a newly isolated avian hemangioma-inducing retrovirus. J Virol. 1989;63:4325–4330. doi: 10.1128/jvi.63.10.4325-4330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 19.Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter M E. Apoptosis signaling by death receptors. Eur J Biochem. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- 20.Sheridan J P, Marsters S A, Pitti R M, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray C L, Baker K, Wood W I, Goddard A D, Godowski P, Ashkenazi A. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 21.Smith E J, Brojatsch J, Naughton J, Young J A T. The CAR1 gene encoding a cellular receptor specific for subgroup B and D avian leukosis viruses maps to the chicken tvb locus. J Virol. 1998;72:3501–3503. doi: 10.1128/jvi.72.4.3501-3503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tartaglia L A, Ayres T M, Wong G H, Goeddel D V. A novel domain within the 55-kDa TNF receptor signals cell death. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 23.Temin H M. Mechanisms of cell killing/cytopathic effects by nonhuman retroviruses. Rev Infect Dis. 1988;10:399–405. doi: 10.1093/clinids/10.2.399. [DOI] [PubMed] [Google Scholar]
- 24.Weller S K, Temin H M. Cell killing by avian leukosis viruses. J Virol. 1981;39:713–721. doi: 10.1128/jvi.39.3.713-721.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weller S K, Joy A E, Temin H M. Correlation between cell killing and massive second-round superinfection by members of some subgroups of avian leukosis virus. J Virol. 1980;33:494–506. doi: 10.1128/jvi.33.1.494-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young J A T, Bates P, Varmus H E. Isolation of a chicken gene that confers susceptibility to infection by subgroup A avian leukosis and sarcoma viruses. J Virol. 1993;67:1811–1816. doi: 10.1128/jvi.67.4.1811-1816.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zingler K, Young J A T. Residue Trp-48 of Tva is critical for viral entry but not for high-affinity binding to the SU glycoprotein of subgroup A avian leukosis and sarcoma viruses. J Virol. 1996;70:7510–7516. doi: 10.1128/jvi.70.11.7510-7516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]