Abstract
Endothelial cells (ECs) form a semi-permeable barrier between the interior space of blood vessels and the underlying tissues. Pulmonary endothelial barrier integrity is maintained through coordinated cellular processes involving receptors, signaling molecules, junctional complexes, and protein-regulated cytoskeletal reorganization. In acute lung injury (ALI) or its more severe form acute respiratory distress syndrome (ARDS), the loss of endothelial barrier integrity secondary to endothelial dysfunction caused by severe pulmonary inflammation and/or infection leads to pulmonary edema and hypoxemia. Pro-inflammatory agonists such as histamine, thrombin, bradykinin, interleukin 1β, tumor necrosis factor α, vascular endothelial growth factor, angiopoietin-2, and platelet-activating factor, as well as bacterial toxins and reactive oxygen species, cause dynamic changes in cytoskeletal structure, adherens junction disorganization, and detachment of vascular endothelial cadherin (VE-cadherin) from the actin cytoskeleton, leading to an increase in endothelial permeability. Endothelial interactions with leukocytes, platelets, and coagulation enhance the inflammatory response. Moreover, inflammatory infiltration and the associated generation of pro-inflammatory cytokines during infection cause EC death, resulting in further compromise of the structural integrity of lung endothelial barrier. Despite the use of potent antibiotics and aggressive intensive care support, the mortality of ALI is still high, because the mechanisms of pulmonary EC barrier disruption are not fully understood. In this review, we summarized recent advances in the studies of endothelial cytoskeletal reorganization, inter-endothelial junctions, endothelial inflammation, EC death, and endothelial repair in ALI and ARDS, intending to shed some light on the potential diagnostic and therapeutic targets in the clinical management of the disease.
Keywords: Lung, Endothelium, Pulmonary edema, Acute lung injury, Acute respiratory distress syndrome
Introduction
The pulmonary endothelium is a semi-permeable barrier that is critical for lung gas exchange at the alveolar–capillary interface and regulation of fluid and solute passage between the blood and interstitial compartments in the lung. Having initially been thought of as an inert, static structure, the lung endothelium is increasingly recognized as a dynamic, metabolically active organ that modulates several key regulatory functions including leukocyte diapedesis, intravascular coagulation, vasomotor tone, and solute and fluid trafficking via regulation of barrier permeability.1 Pulmonary endothelial barrier integrity is maintained through coordinated cell processes involving receptors, signaling molecules, junctional complexes, and protein-regulated cytoskeletal reorganization. Disruption of lung endothelial barrier function represents the most striking pathophysiological changes in acute lung injury (ALI), which in its most severe form manifests clinically as acute respiratory distress syndrome (ARDS).2 ARDS is characterized by dysfunction of the alveolar–capillary membrane barriers, which leads to hypoxia, hypercapnia, and pulmonary edema. In addition, inflammation of the pulmonary endothelium results in leukocyte adhesion, platelet aggregation, and coagulation activation, which, in turn, can lead to cell death, microthrombosis, and fibrin deposition, contributing to increased vascular permeability.3 This review will focus on recent advances in the pathophysiological mechanism of lung endothelium in ALI and ARDS.
Endothelial cytoskeletal reorganization in ARDS: actin microfilaments, microtubules, and intermediate microfilaments
The cytoskeleton consists of an interconnected network of three types of microfilaments: actin microfilaments, microtubules, and intermediate microfilaments.4 The actin microfilaments are made of filamentous polymerized actins (F-actin), which are arranged as a string of uniformly oriented globular actin (G-actin) subunits in a tight helix.5 Actin polymerization is required for the formation of F-actin, which drives actin-based cell locomotion.5 In lung endothelial cells (ECs), F-actin is found in three forms: membrane skeleton, cortical F-actin, and actin stress fibers.6 The membrane skeleton is composed of short F-actin microfilaments that are immediately adjacent to the plasma membrane. The membrane skeleton maintains plasma membrane organization, and interacts with the underlying cortical actin rim through their interaction with spectrin and its binding proteins. The cortical F-actin is located beneath the membrane skeleton. This dense F-actin rim is organized by actin-binding and cross-linking proteins, including spectrin, filamin, cortactin, Wiskott-Aldrich syndrome protein (WASP), vasodilator-stimulated phosphoprotein (VASP), gelsolin, cofilin, ezrin-radixin-moesin (ERM), and heat shock protein 27 (HSP27).7 The cortical F-actin provides structural stability and anchoring for multiple membrane-bound junctional complexes, which become activated, strengthening the assembly of ECs and EC-matrix adhesions. The stress fibers are prominent transcellular actomyosin bundle cables, which are linked to the cell membrane at focal adhesions.8 The actin stress fibers generate contractile forces through the association of actin with myosin. The dynamic changes in the actin cytoskeleton in ALI/ARDS are mediated by Ras homolog family member A (RhoA), Ras-related C3 botulinum toxin substrate 1 (Rac1), cell division cycle 42 (Cdc42), and Ras-related protein 1 (Rap1). A range of circulating (tumor necrosis factor [TNF], interleukin 6 [IL-6], lipopolysaccharide [LPS], vascular endothelial growth factor [VEGF], thrombin), released (reactive oxygen species [ROS], histamine), and physical (mechanical stretch) effectors activate RhoA in lung microvascular ECs.9 RhoA activation can also be induced by activated integrin in focal adhesions through binding to cleaved talin head and rod domains in LPS-induced ALI.10 The downstream events for Rho activation include activation of Rho kinase (ROCK) and increased formation of actin stress fibers.11 Over-expression of dominant-active RhoA mutant V14 increased formation of actin stress fibers, and over-expression of dominant-negative RhoA mutant N19 reduced formation of actin stress fibers in dermal microvascular ECs,12 and in lung ECs.11 RhoA also potentiates myosin light chain (MLC) phosphorylation by inhibiting MLC phosphatase activity through its downstream effector ROCK. The adenosine triphosphate (ATP)-dependent ratcheting of myosin heads against actin microfilaments generates contractile tension. The contraction of actin stress fibers causes EC shape change and intercellular gap formation leading to functional compromise of barrier integrity. Indeed, ROCK inhibition by the compound Y-27632 can alleviate pulmonary edema in animals after LPS challenge or re-expansion of the lung. Rac1, Cdc42, and Rap1 contribute to an intact barrier function.13,14 Over-expression of dominant-active Rac mutant V12 increased formation of cortical F-actin and over-expression of dominant-negative Rac mutant N17 reduced formation of cortical F-actin.15 Cdc42 directly regulates cortical actin organization as well as a host of proteins including cofilin, MLC kinase (MLCK), and neural-Wiskott-Aldrich syndrome protein (WASP) that affect actin organization and cell adhesion to the extracellular matrix (ECM).16 Rap1 enhances barrier function via inhibition of Rho and activation of Cdc42.17,18
Microtubules are long tubes made from tubulin heterodimers packed around a central core. The wall of microtubules is made of a helical array of repeating tubulin heterodimers of α-tubulin and β-tubulin. Microtubules are structures that can rapidly grow via polymerization or shrink via depolymerization in length, depending on how many tubulin molecules they contain.19 Changes in microtubule dynamics caused by vasoactive or inflammatory agonists and mechanical forces in ALI/ARDS regulate lung endothelial permeability via signaling crosstalk with actin cytoskeleton and endothelial junctions.20 Stabilizing microtubules enhances endothelial barrier integrity, while disassembly of the microtubule network is associated with an increase in endothelial permeability.21 Partial depolymerization of peripheral microtubules promoted, while stabilization prevented activation of the RhoA signaling pathway of endothelial permeability.21 Thrombin-induced peripheral microtubule depolymerization leading to RhoA activation and endothelial permeability was associated with increased microtubule instability caused by thrombin-induced phosphorylation of microtubule regulatory protein tau at Ser409 and Ser262.22 Partial dissolution of the peripheral microtubule network during endothelial permeability caused by TNF and ROS was linked to the activation of p38 mitogen-activated kinase and subsequent destabilization of microtubule structure.23, 24, 25 Microtubule depolymerization was shown to activate the inflammatory nuclear factor-κB (NF-κB) signaling pathway in ALI/ARDS and to upregulate the expression of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), which in turn increases Rho activation and endothelial permeability.25 In addition, destabilization of microtubules due to histone deacetylase 6 (HDAC6)-mediated deacetylation of α-tubulin in ARDS also contributes to LPS-induced endothelial barrier disruption in ALI.26,27 On the other hand, hepatocyte growth factor stimulates association of Rac-specific guanine nucleotide exchange factor Asef with adenomatous polyposis coli (APC) protein, a microtubule-associated protein, in peripheral microtubules leading to Rac-related endothelial barrier enhancement in ARDS.28,29
Intermediate microfilaments are the most stable of the three cytoskeletal microfilaments. They are tissue-specific with different protein expressions depending on the tissue type or developmental stage. The major intermediate filament protein in ECs is vimentin.30 Intermediate microfilaments are not necessary for cell viability but may contribute to mechanical stability of ECs, such as shear and contractile forces in blood vessels.31 The vimentin intermediate microfilaments link to VE-cadherin through desmosomal plakoglobin/desmoplakin or p0071 linker proteins. The adherens junction (AJ) protein VE-cadherin binds to F-actin via catenins and vimentin.32 Depolymerization of the vimentin intermediate microfilaments during inflammation can loosen the intercellular junctions and increase endothelial permeability.32 Phosphorylation of elements of the VE-cadherin complex disrupts the link between VE-cadherin and the vimentin intermediate microfilaments and causes endothelial barrier dysfunction.33 Increased phosphorylation of vimentin has been associated with the disassembly of microfilaments and lung endothelial barrier disruption in ARDS.34 Thus, blocking vimentin phosphorylation protects the endothelial barrier against endotoxin, implicating it as a target for drug development against ALI and ARDS.34
Inter-endothelial junctions in ARDS: AJs, tight junctions, and gap junctions
Inter-endothelial junctions are composed of AJs, tight junctions (TJs), and gap junctions (GJs).35 These junctions link ECs and are supported by cytoskeletal microfilaments to facilitate both maintenance of barrier function and modulation of signal transduction in response to tethering and contractile forces.1 Inter-endothelial junctions dynamically open to allow the passage of small molecules such as glucose, water, and ions, and inflammatory cells for tissue homeostasis and immune surveillance.35
AJs are composed of cadherins, primarily VE-cadherin, that bind intracellular catenin proteins including p120-catenin that in turn binds to other protein partners in the actin cytoskeleton.36,37 AJs (or VE-cadherin) are key regulators of paracellular permeability, which determines leucocyte transmigration and edema formation while cell membrane scaffolding proteins called caveolins regulate transendothelial trafficking (transcytosis) of macromolecules including albumin.38 EC barrier disruption is associated with a loss of AJ stability mediated through VE-cadherin/catenin signaling.3 In ALI/ARDS, pro-inflammatory agonists such as histamine, thrombin, bradykinin, IL-1β, TNF, VEGF, Ang2, and platelet-activating factor, as well as bacterial toxin LPS and ROS cause Src-dependent tyrosine phosphorylation of VE-cadherin, β-catenin, plakoglobin, and p120, resulting in disorganization of AJ proteins and detachment of VE-cadherin from the actin cytoskeleton, leading to increase in endothelial permeability.9,35,39, 40, 41 Downregulation of VE-cadherin by proinflammatory agonists in ALI causes AJ disassembly.42 Meanwhile, contraction of actin stress fiber generates a contractile force, which pulls VE-cadherin inward. This contraction forces VE-cadherin to dissociate from its adjacent partner causing inter-endothelial gaps and endothelial barrier disruption.9 Focal adhesion kinase (FAK), a non-receptor tyrosine kinase, regulates turnover of focal adhesion formation by binding to focal adhesion proteins as well as enhancing AJ formation. In experimental ARDS models (including conditional FAK deletion), decreased FAK expression was associated with lung edema, as well as with albumin and neutrophil influx.43,44
TJs are formed by the fusion of the outer layers of the plasma membranes and are comprised of occludin, claudins, and junctional adhesion molecules (JAMs).35 Claudins and junctional adhesion molecules are coupled to cytoplasmic proteins and are linked to the EC actin cytoskeleton by the zonula occludens (ZO).36 ECs predominantly express claudin-5, which binds to ZO-1 protein through its cytoplasmic domain. ZO-1 thus allows TJ proteins to interact with each other and with the actin cytoskeleton.36,45 The TJ protein ZO-1 also binds cadherins and thus provides a structural connection between TJ, AJ, and the cytoskeleton.36 VE-cadherin assembly in AJs also upregulates expression of claudin-5 through sequestration of the transcriptional repressor β-catenin in the cell membrane, thereby preventing β-catenin action in the nucleus.46 The inflammatory mediator histamine increases endothelial permeability through Src-dependent phosphorylation of TJ proteins that cause the dissociation of ZO-1 from occludin resulting in EC barrier dysfunction.35,47 TJ proteins such as claudin-5, occludin, and ZO-1 are down-regulated in lung endothelium in ARDS.48,49 It has been reported that RNA-binding motif protein 3 (RBM3) induces down-regulation of ZO-1 and occludins in lung ECs in ALI models.50
GJs provide a means to facilitate direct cell-to-cell transfer of signaling molecules, ions, current, and transmembrane potential. GJs are formed by interaction of two connexins (Cx) from opposite cells. Each connexin is made of six connexin subunits (hexamers) potential.51 Cx37, Cx40, and Cx43 are expressed in ECs.52 Cx40- and/or Cx43-based GJs are required to maintain the endothelial barrier function without altering the expression and localization of the tight-junction components.53 The expression of GJ protein Cx40 is suppressed by LPS in ALI, which leads to increased pulmonary vascular permeability.54,55
Endothelial inflammation in ARDS: interactions with leukocytes, platelets, and coagulation
Under physiological conditions, the lung endothelium adopts a predominantly inhibitory effect on inflammation and coagulation. The vascular endothelium maintains a negative transmural electrical charge, which prevents the adhesion of leukocytes and platelets. ECs release prostacyclin (PGI2), nitric oxide (NO), proteins C and S, and proteoglycans, which inhibit leukocyte and platelet adhesion and the coagulation cascade, as well as plasminogen activators, which activate the physiologic fibrinolytic pathway.56,57
Lung ECs are increasingly recognized as orchestrators of the inflammatory and coagulation response in ARDS.58 The pulmonary endothelium is a key regulator of innate cellular and cytokine responses, if not the major source of cytokine release.59 Endothelium-leukocyte and endothelium-platelet interactions resulting from inflammatory reactions play an important role in the pathogenesis of ALI and ARDS.60 ECs express various leukocyte adhesion molecules including ICAM-1, VCAM-1, and selectins, which mediate endothelium–leukocyte and endothelium–platelet interactions.61,62 Stimulated leukocytes transmigrate along chemotactic gradients into lung tissue across the endothelium en route to sites of pathogen invasion or tissue damage due to a toxic or physical insult.38 Binding of leukocyte surface integrins on their EC adhesion molecules tethers circulating leukocytes reversibly on the EC surface and facilitates rolling of leukocytes along the endothelium to the point of transmigration.63 Simultaneously, ligation of EC adhesion molecules by neutrophil binding triggers downstream signaling pathways in ECs that induce cytoskeletal contraction and junctional opening to allow leukocyte migration.64 The activated leukocytes that infiltrate the lungs and migrate into the airways release proteolytic enzymes (leukocyte elastase), which digest tissue, and pro-inflammatory cytokines such as TNF and IL-1β, which contribute to the endothelial injury and barrier disruption.65 The leukocytes also release large amounts of oxygen-based free radicals, which cause cell injury and upregulation of leukocyte adhesion molecule expression and release of neutrophil chemotactic factors.66 Platelet-bound endothelium releases P-selectin, thromboxane A2 (TXA2), and soluble CD40 ligand, which further enhance endothelium–platelet interactions and platelet–leukocyte complex, leading to EC and leukocyte activation.67,68 Blocking platelet and endothelial cell adhesion molecule 1 (PECAM) decreases leukocyte infiltration and the inflammation level in the lungs and protects lung injury in LPS-induced ALI mouse model.69 However, patients with decreased leukocytes and agranulocytosis complicated with ARDS have poor prognosis and a high overall mortality,70 suggesting leukocytes may help lung injury repair.71
The adhered and aggregated platelets on the endothelial surface provide an excellent platform for the activation of the coagulation cascade. In ARDS, intravascular micro-thrombi in lung capillaries can be formed via either the extrinsic pathway, which is initiated by tissue factor expression on ECs and other cells, or via the intrinsic pathway through the exposure of blood to denuded vascular wall matrix due to endothelial damage. Cytokines such as IL-6 induce tissue factor expression, while TNF-α blocks coagulation-inhibiting and fibrinolytic pathways.72,73 Initiation of coagulation leads to proteolytic cleavage of prothrombin and thrombin release, which has important downstream effects, including cleavage of fibrinogen to fibrin and platelet activation by binding to proteinase-activated receptors (PAR).74 Thrombin acts on ECs causing endothelial contraction and increased permeability.75
Lung endothelial cell death in ARDS
In ARDS, bacterial infection, inflammatory infiltration, and generation of pro-inflammatory cytokines may promote EC death resulting in further compromise of the structural integrity of the lung endothelial barriers.76, 77, 78, 79 The dead cells and debris may release toxic cellular components that may further augment inflammation.80 The dead cell detachment allows denuded vascular wall matrix exposure, activates blood coagulation and neutrophil infiltration and enhances inflammatory response. Lung endothelial death in ARDS can be induced through diverse cell death pathways, including apoptosis, necrosis, necroptosis, pyroptosis, and ferroptosis.78,80
Apoptosis is an ATP-dependent programmed cell death, morphologically characterized by cellular shrinkage, chromatin condensation, nuclear DNA fragmentation, cytosolic membrane blebbing, and apoptotic body formation.81 It is triggered by the extrinsic (death receptor pathway) pathway, or the intrinsic (mitochondrial pathway) pathway, involving a group of cysteinyl aspartate proteases (caspases) cleavage (activation).81 Endotoxin (LPS)-induced apoptosis occurs following its binding to toll-like receptor (TLR)-4. Downstream of TLR-4, adapter myeloid differentiation primary response protein 88 (MyD88) is associated with and activates IL-1 receptor-associated kinase-1 (IRAK-1). IRAK-1 then dissociates from MyD88 and interacts with TNF receptor-associated factor-6 (TRAF-6) leading to NF-κB and caspase activation.82 TNF-induced cell death is mainly mediated through the activation of the type I TNF receptor, which initiates TNF-related apoptosis-inducing ligand (TRAIL) signaling pathway. This death pathway recruits a dozen different signaling proteins involving tumor necrosis factor receptor-1-associated death domain (TRADD)–Fas-associating protein with a novel death domain (FADD)–receptor-interacting protein kinase 1 (RIPK1) intermediates. Depending on the ubiquitinylation status of RIPK1 and the activation status of caspase 8, TNF receptor 1 signaling may either mediate inflammation (RIPK1 ubiquitinylated), apoptosis (caspase 8 active), or necroptosis (caspase 8 inactive).83, 84, 85 Apart from TNF, the TRAIL pathway can stimulate a loss of mitochondrial membrane potential (Delta Psim) and cytochrome C release in an FADD/caspase-8 dependent manner.81 In ARDS, ROS regulate lung endothelial apoptosis through apoptosis-inducing factor (AIF)-dependent and caspase-dependent apoptotic pathways, and the latter involves mitochondria-, endoplasmic reticulum (ER) stress-, and Fas receptor-associated apoptotic pathways.86,87
In contrast to apoptosis, necrosis is an energy-independent process driven by mitochondrial permeability transition (MPT). Necrotic cell death occurs when the intracellular microenvironment of a cell is perturbed as during severe oxidative stress and following cytosolic Ca2+ overload due to lack of a blood supply or due to a toxin, resulting in rapid dissipation of mitochondrial transmembrane potential and osmotic breakdown of mitochondrial and cellular membranes.81 Necrotic cell death was observed in ALI induced by ischemic acute kidney injury88 and LPS.89
Necroptosis is a regulated inflammatory type of programmed necrosis mediated by RIPK1 and RIPK3-mixed lineage kinase domain-like (MLKL).90 In this necroptotic pathway, MLKL is recruited by the auto-phosphorylated RIPK3 and subsequently phosphorylated by RIPK3 at the threonine 357 and serine 358 residues of human MLKL (serine at positions 345, 347, and 352 and threonine at position 349 for mouse MLKL).90 Phosphorylated MLKL then oligomerizes and traffics to the plasma membrane, forming an MLKL pore, resulting in necroptosis.91 The occurrence of programmed necrosis causes a series of morphological alterations in cells including slight changes in the ultrastructure of the nucleus (especially the expansion of the nuclear membrane and the formation of small, irregular, and circumscribed patches by chromatin condensation), increases in lucent cytoplasm, and organelle swelling.78 The increased permeability of the cell membrane causes the cell to grow in size, resulting in the cell rupturing and the leak-out of intracellular contents and provoking the inflammatory response of the surrounding lung tissues.91 Various ALI/ARDS animal models have demonstrated evidence of necroptosis in endothelial barrier dysfunction.92,93
Pyroptosis is a type of programmed cell death that occurs in cells infected with certain viruses or bacteria.94 Pyroptosis is mediated by gasdermin D (GSDMD) that contains a specific cleavage site for inflammatory caspases, e.g., caspase 1/4/5/11).95 The cleavage of GSDMD by activated caspases 1/4/5/11 results in the formation of channels in the plasma membrane.94 Besides, inflammatory caspases cleave pro-IL-1β and pro-IL-18, converting them to the active IL-1β and IL-18, which are released through the membrane channels.96,97 Though pyroptosis mainly occurs in inflammatory cells such as macrophages and neutrophils, lung endothelial pyroptosis plays an important role in endothelial barrier disruption and pulmonary edema in ALI associated with ARDS.93,97
Ferroptosis is a form of regulated cell death initiated by severe toxic lipid peroxidation, which relies on ROS generation and iron accumulation.81,98 Ferroptosis occurs independent of caspases, necrosome components, and the molecular machinery for autophagy. Ferroptosis manifests with specific morphological features such as plasma membrane blebbing without rupture, shrunken mitochondria, increased mitochondrial membrane density, and disruption of mitochondria membrane, and lacks chromatin condensation and margination.99 In ARDS, cytokines of high levels activate the innate immune system. Activated neutrophils produce large amounts of proteases and ROS.100,101 Moreover, ROS can further increase the level of cytokines, exacerbating tissue damage and edema. Under oxidative stress, inactivation of cellular glutathione (GSH)-dependent antioxidant defenses or blocking the function of the GSH-dependent enzyme glutathione peroxidase 4 (GPX4) results in the accumulation of toxic lipid ROS and iron-dependent lipid peroxidation.102 Ferroptosis finally leads to the consistent release of immunostimulatory damage-associated molecular patterns (DAMPs) that not only damage lung cells but also recruit and activate the immune cells to amplify the lung damage. The release of the DAMPs triggered by ferroptosis may promote pyroptosis and necroptosis in ARDS.100
Lung endothelial repair in ARDS
Following the exudative phase of ARDS, the lung goes into recovery from lung injury that enables the restoration of an intact endothelial barrier and an efficient gas-exchange within the lung. The recovery processes require the removal of pulmonary edema fluid and inflammatory debris as well as lung vascular homeostasis and tissue–fluid balance.103,104 Restoration of the pulmonary microvascular barrier is dependent on the proliferation of ECs that generally involves two biological processes, referred to as angiogenesis and vasculogenesis.105
Angiogenesis involves the formation of new blood vessels via the migration and proliferation of the ECs of local endothelial niches of preexisting vessels and is required for neo-alveolarization.106 Stromal-cell-derived factor-1 and vascular endothelial growth factor signaling participate in this angiogenesis-related lung repair.107,108
Vasculogenesis is the de novo formation of blood vessels from endothelial progenitor cells (EPCs) from either local resident or bone-marrow-derived EPCs.105,109,110 Several studies found that local resident EPCs in the lung are highly proliferative and capable of reconstituting the entire proliferative hierarchy of lung microvascular ECs.111 The resident EPCs in their study possessed the capacity of integrating into various types of vessels, including blood and lymph vessels.112 Transplantation of murine bone marrow-derived EPCs suppressed lung inflammation and attenuated endothelial permeability and lung edema in ALI animal models.113 Clinically, levels of circulating EPCs are elevated in patients with ARDS and are associated with survival in ARDS.114 These results suggest that transplantation or mobilization of bone-marrow-derived EPCs can induce endothelial barrier protection in ARDS. Resident EPCs are major and bone-marrow-derived EPCs are complementary sources of new ECs in endothelial barrier repair, and both resident EPCs and bone-marrow-derived EPCs play important roles in endothelial barrier restoration following ARDS.110
Potential diagnostic and therapeutic targets in the clinical management of the disease
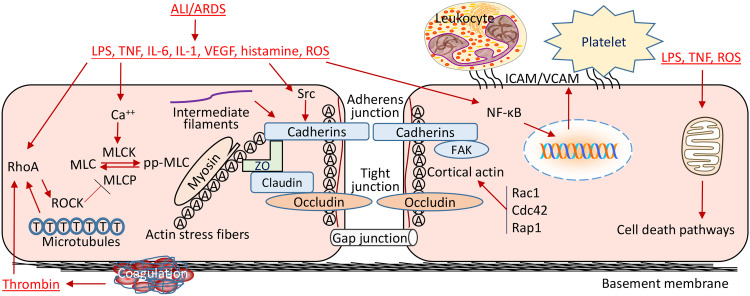
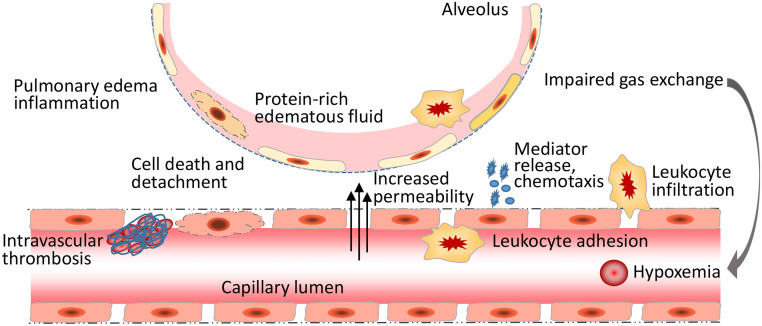
The pulmonary ECs are most important in the pathophysiological mechanism of ALI/ARDS (Fig. 1). The loss of endothelial barrier integrity caused by severe pulmonary inflammation leads to pulmonary edema and hypoxemia (Fig. 2). During the pathophysiological processes of ALI, a number of mediators are produced and could be used as biomarkers for the diagnosis and prognosis of ARDS.115 Inflammatory cytokines IL-6, IL-8, and TNF-α have been validated as promising biomarkers of ARDS.116,117 Biomarkers of endothelial injury can help the diagnosis and prognosis of ARDS.118 Elevated levels of endothelial biomarkers can also identify which patients with non-pulmonary sepsis will develop ARDS. Von Willebrand factor (vWF), angiopoietin-2 (Ang-2), and thrombomodulin are biomarkers for endothelial injury and were found to be significantly associated with mortality in ARDS with and without infection-related lung injury.118,119 ICAM-1 has been identified as a biomarker of endothelial inflammation in ARDS. An association between ICAM-1 expression and lung inflammation as well as ARDS progression has been observed in ARDS.120 Further studies are needed to translate these biomarkers into clinical use.121
Fig. 1.
Mechanisms of pulmonary endothelial barrier disruption in ALI/ARDS. Endothelial barrier disruption is resulted from actin cytoskeletal reorganization due to actin–myosin interaction after MLC-phosphorylation, which is regulated by MLCK and MLCP in ALI/ARDS. RhoA activation induced by pro-inflammatory mediators potentiates MLC phosphorylation by inhibiting MLC phosphatase activity leading to the increased formation of actin stress fibers and the dispersal of cortical actin bundles. Ratcheting of myosin heads against actin microfilaments generates a contractile tension, which pulls VE-cadherin inward and forces VE-cadherin to dissociate from its adjacent partner causing inter-endothelial gaps and endothelial barrier disruption. Src activation induced by pro-inflammatory mediators induces VE-cadherin phosphorylation leading to disorganization of AJ proteins and detachment of VE-cadherin from the actin cytoskeleton. Rac1, Cdc42, and Rap1 contribute to an intact barrier function by modifying the formation of cortical F-actin. FAK, a non-receptor tyrosine kinase, regulates the turnover of focal adhesion formation by binding to focal adhesion proteins as well as enhancing AJ formation. NF-κB activation promotes the expression of various leukocyte adhesion molecules including ICAM-1 and VCAM-1 and selectins, which mediate endothelium–leukocyte and endothelium–platelet interactions. Initiation of coagulation leads to proteolytic cleavage of prothrombin and release of thrombin. Bacterial infection and inflammatory infiltration and cytokines cause EC death, resulting in the compromise of structural integrity of lung endothelial barrier. AJ: Adherens junction; ALI: Acute lung injury; ARDS: Acute respiratory distress syndrome; Cdc42: Cell division cycle 42; EC: Endothelial cells; FAK: Focal adhesion kinase; ICAM: Intercellular adhesion molecule 1; IL: Interleukin; LPS: Lipopolysaccharide; MLC: Myosin light chain; MLCK: MLC kinase; MLCP: MLC phosphatase; NF-κB: Nuclear factor-κB; Rac1: Ras-related C3 botulinum toxin substrate 1; Rap1: Ras-related protein 1; RhoA: Ras homolog family member A; ROCK: Rho kinase; ROS: Reactive oxygen species; TNF: Tumor necrosis factor; VCAM: Vascular cell adhesion molecule 1; VE-cadherin: Vascular endothelial cadherin; VEGF: Vascular endothelial growth factor; ZO: Zonula occludens.
Fig. 2.
The loss of endothelial barrier integrity leads to pulmonary edema and hypoxemia in ALI/ARDS. The inflammatory mediator, bacterial toxins, and ROS cause dynamic changes in the cytoskeletal structure and inter-endothelial junctions, leading to an increase in endothelial permeability. Endothelial interactions with leukocytes, platelets, and coagulation enhance inflammatory response. Bacterial infection and inflammatory infiltration and cytokines induce EC death, causing the compromise of the structural integrity of lung endothelial barrier, eventually resulting in pulmonary edema, impaired gas exchange, and hypoxemia. ALI: Acute lung injury; ARDS: Acute respiratory distress syndrome; EC: Endothelial cells; ROS: Reactive oxygen species.
In ARDS, the inflammatory mediators, bacterial toxins, and ROS cause dynamic changes in cytoskeletal structure, AJ disorganization, and detachment of VE-cadherin from the actin cytoskeleton, leading to increase in endothelial permeability. Endothelial interactions with leukocytes, platelets, and coagulation enhance inflammatory response. Bacterial infection and inflammatory infiltration and cytokines cause EC death resulting in compromise of structural integrity of lung endothelial barrier (Fig. 2). From a clinical perspective, ARDS remains a challenging conundrum. Understanding the mechanisms of pathophysiological processes in pulmonary endothelium in ALI and ARDS has resulted in a number of novel potential therapeutic targets in preventing and treating experimental ALI. Modulating cytoskeleton reorganization in lung endothelium would provide a good opportunity for ARDS treatment. S1P and its analogue FTY720 reduced vascular endothelial leakage in small and large animal lung injury models,122 though the clinical application of S1P and its analogues is currently limited by systemic side effects. Inhibition of calpain attenuates talin cleavage, RhoA activation, pulmonary EC barrier disruption, and pulmonary edema in mouse ALI model.10 Modulating signal pathways that regulate inter-endothelial junctions provides alternative therapeutic strategies for ARDS. Natural products oxypeucedanin123 and forsythia124 have been found to enhance alveolar–capillary integrity by increasing the expression of TJs proteins in ALI models. Myosin II inhibitor lebbistatin downregulates the Wnt5a/β-catenin pathway and exerts a protective effect on lung injury.125 Verdiperstat, a myeloperoxidase inhibitor, enhances VE-cadherin stability by reducing the activation of myeloperoxidase-β-catenin signaling pathway in experimental ARDS.126 Targeting endothelial inflammation is another therapeutic opportunity for ARDS. An ICAM-1 inhibitor MMI-0100 has been shown to inhibit endothelial ICAM-1 expression and reduce lung injury and inflammation.127 Chitin derivatives, AVR-25 and AVR-48, have been shown to reduce the expression of lung adhesion molecules, decrease neutrophil recruitment and improve pulmonary endothelial barrier function and lung injury in the lungs of ALI mice.128 Several pharmacological compounds have been reported to mitigate pulmonary EC death in preclinical models of ARDS, of which safety and efficacy remain to be further examined in clinical studies.129,130 Finally, EPCs and mesenchymal stromal cells (MSCs) have therapeutic potential for vascular regeneration and may emerge as a novel strategy for the diseases that are associated with ALI/ARDS.131, 132, 133 In addition, MSCs also secrete a variety of biologically active factors that regulate related signal pathways such as PI3K/protein kinase B (AKT), Wnt, and NF-κB to reduce inflammation in ARDS.134 Of note, a number of preclinical studies on the manipulations of molecular pathways specifically in pulmonary endothelium have shown promising. Further clinical trials are needed to translate those findings into clinically useful tools to treat and/or improve the management of ARDS.
Funding
This work was supported, in whole or in part, by NIH grants HL134934 and HL158909 to YS, Augusta University intramural grant IGPCT00023 to YS, and by the Department of Veterans Affairs BX005350 to YS.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Edited by: Peifang Wei
References
- 1.Millar FR, Summers C, Griffiths MJ, Toshner MR, Proudfoot AG. The pulmonary endothelium in acute respiratory distress syndrome: insights and therapeutic opportunities. Thorax. 2016;71:462–473. doi: 10.1136/thoraxjnl-2015-207461. [DOI] [PubMed] [Google Scholar]
- 2.Kellner M, Noonepalle S, Lu Q, Srivastava A, Zemskov E, Black SM. ROS Signaling in the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) Adv Exp Med Biol. 2017;967:105–137. doi: 10.1007/978-3-319-63245-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vassiliou AG, Kotanidou A, Dimopoulou I, Orfanos SE. Endothelial damage in acute respiratory distress syndrome. Int J Mol Sci. 2020;21:8793. doi: 10.3390/ijms21228793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wongsawat M, Glaharn S, Srisook C, et al. Immunofluorescence study of cytoskeleton in endothelial cells induced with malaria sera. Malar J. 2024;23:10. doi: 10.1186/s12936-023-04833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ni J, Dong Z, Han W, Kondrikov D, Su Y. The role of RhoA and cytoskeleton in myofibroblast transformation in hyperoxic lung fibrosis. Free Radic Biol Med. 2013;61:26–39. doi: 10.1016/j.freeradbiomed.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasain N, Stevens T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res. 2009;77:53–63. doi: 10.1016/j.mvr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belvitch P, Htwe YM, Brown ME, Dudek S. Cortical actin dynamics in endothelial permeability. Curr Top Membr. 2018;82:141–195. doi: 10.1016/bs.ctm.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katoh K, Kano Y, Ookawara S. Role of stress fibers and focal adhesions as a mediator for mechano-signal transduction in endothelial cells in situ. Vasc Health Risk Manag. 2008;4:1273–1282. doi: 10.2147/vhrm.s3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzales JN, Lucas R, Verin AD. The acute respiratory distress syndrome: mechanisms and perspective therapeutic approaches. Austin J Vasc Med. 2015;2:1009. [PMC free article] [PubMed] [Google Scholar]
- 10.Song L, Shi X, Kovacs L, et al. Calpain promotes LPS-induced lung endothelial barrier dysfunction via cleavage of talin. Am J Respir Cell Mol Biol. 2023;69:678–688. doi: 10.1165/rcmb.2023-0009OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JG, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res. 2004;67:64–77. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Hoang MV, Whelan MC, Senger DR. Rho activity critically and selectively regulates endothelial cell organization during angiogenesis. Proc Natl Acad Sci U S A. 2004;101:1874–1879. doi: 10.1073/pnas.0308525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta D, Konstantoulaki M, Ahmmed GU, Malik AB. Sphingosine 1-phosphate-induced mobilization of intracellular Ca2+ mediates rac activation and adherens junction assembly in endothelial cells. J Biol Chem. 2005;280:17320–17328. doi: 10.1074/jbc.M411674200. [DOI] [PubMed] [Google Scholar]
- 14.Daneshjou N, Sieracki N, van Nieuw Amerongen GP, et al. Rac1 functions as a reversible tension modulator to stabilize VE-cadherin trans-interaction. J Cell Biol. 2015;208:23–32. doi: 10.1083/jcb.201409108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vouret-Craviari V, Boquet P, Pouyssegur J, Obberghen-Schilling E. Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function. Mol Biol Cell. 1998;9:2639–2653. doi: 10.1091/mbc.9.9.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barry DM, Xu K, Meadows SM, et al. Cdc42 is required for cytoskeletal support of endothelial cell adhesion during blood vessel formation in mice. Development. 2015;142:3058–3070. doi: 10.1242/dev.125260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ando K, Fukuhara S, Moriya T, Obara Y, Nakahata N, Mochizuki N. Rap1 potentiates endothelial cell junctions by spatially controlling myosin II activity and actin organization. J Cell Biol. 2013;202:901–916. doi: 10.1083/jcb.201301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pannekoek WJ, Post A, Bos JL. Rap1 signaling in endothelial barrier control. Cell Adh Migr. 2014;8:100–107. doi: 10.4161/cam.27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alieva IB, Zemskov EA, Kireev II, et al. Microtubules growth rate alteration in human endothelial cells. J Biomed Biotechnol. 2010;2010 doi: 10.1155/2010/671536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alieva IB, Zemskov EA, Smurova KM, Kaverina IN, Verin AD. The leading role of microtubules in endothelial barrier dysfunction: disassembly of peripheral microtubules leaves behind the cytoskeletal reorganization. J Cell Biochem. 2013;114:2258–2272. doi: 10.1002/jcb.24575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karki P, Birukova AA. Microtubules as major regulators of endothelial function: Implication for lung injury. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.758313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birukova AA, Birukov KG, Smurova K, et al. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J. 2004;18:1879–1890. doi: 10.1096/fj.04-2328com. [DOI] [PubMed] [Google Scholar]
- 23.Petrache I, Birukova A, Ramirez SI, Garcia JG, Verin AD. The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability. Am J Respir Cell Mol Biol. 2003;28:574–581. doi: 10.1165/rcmb.2002-0075OC. [DOI] [PubMed] [Google Scholar]
- 24.Kratzer E, Tian Y, Sarich N, et al. Oxidative stress contributes to lung injury and barrier dysfunction via microtubule destabilization. Am J Respir Cell Mol Biol. 2012;47:688–697. doi: 10.1165/rcmb.2012-0161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karki P, Ke Y, Tian Y, et al. Staphylococcus aureus-induced endothelial permeability and inflammation are mediated by microtubule destabilization. J Biol Chem. 2019;294:3369–3384. doi: 10.1074/jbc.RA118.004030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J, Ma Z, Shetty S, Ma M, Fu J. Selective HDAC6 inhibition prevents TNF-alpha-induced lung endothelial cell barrier disruption and endotoxin-induced pulmonary edema. Am J Physiol Lung Cell Mol Physiol. 2016;311:L39–L47. doi: 10.1152/ajplung.00051.2016. [DOI] [PubMed] [Google Scholar]
- 27.Kovacs L, Kovacs-Kasa A, Verin AD, Fulton D, Lucas R, Su Y. Histone deacetylases in vascular permeability and remodeling associated with acute lung injury. Vessel Plus. 2018;2:1–9. doi: 10.20517/2574-1209.2018.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian X, Tian Y, Gawlak G, et al. Asef controls vascular endothelial permeability and barrier recovery in the lung. Mol Biol Cell. 2015;26:636–650. doi: 10.1091/mbc.E14-02-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng F, Meliton A, Moldobaeva N, et al. Asef mediates HGF protective effects against LPS-induced lung injury and endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2015;308:L452–L463. doi: 10.1152/ajplung.00170.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franke WW, Schmid E, Osborn M, Weber K. Intermediate-sized filaments of human endothelial cells. J Cell Biol. 1979;81:570–580. doi: 10.1083/jcb.81.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helfand BT, Chang L, Goldman RD. The dynamic and motile properties of intermediate filaments. Annu Rev Cell Dev Biol. 2003;19:445–467. doi: 10.1146/annurev.cellbio.19.111401.092306. [DOI] [PubMed] [Google Scholar]
- 32.Bayir E, Sendemir A. Role of intermediate filaments in blood-brain barrier in health and disease. Cells. 2021;10:1400. doi: 10.3390/cells10061400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shasby DM, Ries DR, Shasby SS, Winter MC. Histamine stimulates phosphorylation of adherens junction proteins and alters their link to vimentin. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1330–L1338. doi: 10.1152/ajplung.00329.2001. [DOI] [PubMed] [Google Scholar]
- 34.Liu T, Ghamloush MM, Aldawood A, et al. Modulating endothelial barrier function by targeting vimentin phosphorylation. J Cell Physiol. 2014;229:1484–1493. doi: 10.1002/jcp.24590. [DOI] [PubMed] [Google Scholar]
- 35.Sukriti S, Tauseef M, Yazbeck P, Mehta D. Mechanisms regulating endothelial permeability. Pulm Circ. 2014;4:535–551. doi: 10.1086/677356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gu C, Liu M, Zhao T, Wang D, Wang Y. Protective role of p120-catenin in maintaining the integrity of adherens and tight junctions in ventilator-induced lung injury. Respir Res. 2015;16:58. doi: 10.1186/s12931-015-0217-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Maniatis NA, Kotanidou A, Catravas JD, Orfanos SE. Endothelial pathomechanisms in acute lung injury. Vascul Pharmacol. 2008;49:119–133. doi: 10.1016/j.vph.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maniatis NA, Orfanos SE. The endothelium in acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care. 2008;14:22–30. doi: 10.1097/MCC.0b013e3282f269b9. [DOI] [PubMed] [Google Scholar]
- 40.Dong W, He B, Qian H, et al. RAB26-dependent autophagy protects adherens junctional integrity in acute lung injury. Autophagy. 2018;14:1677–1692. doi: 10.1080/15548627.2018.1476811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong P, Angelini DJ, Yang S, et al. TLR4 signaling is coupled to SRC family kinase activation, tyrosine phosphorylation of zonula adherens proteins, and opening of the paracellular pathway in human lung microvascular endothelia. J Biol Chem. 2008;283:13437–13449. doi: 10.1074/jbc.M707986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gorbunov NV, Das DK, Goswami SK, Gurusamy N, Atkins JL. Spatial coordination of cell-adhesion molecules and redox cycling of iron in the microvascular inflammatory response to pulmonary injury. Antioxid Redox Signal. 2007;9:483–495. doi: 10.1089/ars.2006.1296. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt TT, Tauseef M, Yue L, et al. Conditional deletion of FAK in mice endothelium disrupts lung vascular barrier function due to destabilization of RhoA and Rac1 activities. Am J Physiol Lung Cell Mol Physiol. 2013;305:L291–L300. doi: 10.1152/ajplung.00094.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Infusino GA, Jacobson JR. Endothelial FAK as a therapeutic target in disease. Microvasc Res. 2012;83:89–96. doi: 10.1016/j.mvr.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jang AS, Concel VJ, Bein K, et al. Endothelial dysfunction and claudin 5 regulation during acrolein-induced lung injury. Am J Respir Cell Mol Biol. 2011;44:483–490. doi: 10.1165/rcmb.2009-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taddei A, Giampietro C, Conti A, et al. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 47.Chattopadhyay R, Dyukova E, Singh NK, Ohba M, Mobley JA, Rao GN. Vascular endothelial tight junctions and barrier function are disrupted by 15(S)-hydroxyeicosatetraenoic acid partly via protein kinase C epsilon-mediated zona occludens-1 phosphorylation at threonine 770/772. J Biol Chem. 2014;289:3148–3163. doi: 10.1074/jbc.M113.528190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia-Flores AE, Gross CM, Zemskov EA, et al. Loss of SOX18/CLAUDIN5 disrupts the pulmonary endothelial barrier in ventilator-induced lung injury. Front Physiol. 2022;13 doi: 10.3389/fphys.2022.1066515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Mu S, Li X, Liang Y, Wang L, Ma X. Unfractionated heparin alleviates sepsis-induced acute lung injury by protecting tight junctions. J Surg Res. 2019;238:175–185. doi: 10.1016/j.jss.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 50.Feng J, Pan W, Yang X, et al. RBM3 increases cell survival but disrupts tight junction of microvascular endothelial cells in acute lung injury. J Surg Res. 2021;261:226–235. doi: 10.1016/j.jss.2020.12.041. [DOI] [PubMed] [Google Scholar]
- 51.Goodenough DA, Paul DL. Beyond the gap: Functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 52.Van Rijen H, van Kempen MJ, Analbers LJ, et al. Gap junctions in human umbilical cord endothelial cells contain multiple connexins. Am J Physiol. 1997;272(1 Pt 1):C117–C130. doi: 10.1152/ajpcell.1997.272.1.C117. [DOI] [PubMed] [Google Scholar]
- 53.Nagasawa K, Chiba H, Fujita H, et al. Possible involvement of gap junctions in the barrier function of tight junctions of brain and lung endothelial cells. J Cell Physiol. 2006;208:123–132. doi: 10.1002/jcp.20647. [DOI] [PubMed] [Google Scholar]
- 54.Zhou HS, Li M, Sui BD, et al. Lipopolysaccharide impairs permeability of pulmonary microvascular endothelial cells via Connexin40. Microvasc Res. 2018;115:58–67. doi: 10.1016/j.mvr.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J, Wang W, Sun J, et al. Gap junction channel modulates pulmonary vascular permeability through calcium in acute lung injury: an experimental study. Respiration. 2010;80:236–245. doi: 10.1159/000274384. [DOI] [PubMed] [Google Scholar]
- 56.van Hinsbergh VW. Endothelium – role in regulation of coagulation and inflammation. Semin Immunopathol. 2012;34:93–106. doi: 10.1007/s00281-011-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jackson SP, Darbousset R, Schoenwaelder SM. Thromboinflammation: challenges of therapeutically targeting coagulation and other host defense mechanisms. Blood. 2019;133:906–918. doi: 10.1182/blood-2018-11-882993. [DOI] [PubMed] [Google Scholar]
- 58.Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83:536–545. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- 59.Teijaro JR, Walsh KB, Cahalan S, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gando S, Kameue T, Matsuda N, Sawamura A, Hayakawa M, Kato H. Systemic inflammation and disseminated intravascular coagulation in early stage of ALI and ARDS: role of neutrophil and endothelial activation. Inflammation. 2004;28:237–244. doi: 10.1023/b:ifla.0000049049.81688.fe. [DOI] [PubMed] [Google Scholar]
- 61.Wang L, Cao Y, Gorshkov B, et al. Ablation of endothelial Pfkfb3 protects mice from acute lung injury in LPS-induced endotoxemia. Pharmacol Res. 2019;146 doi: 10.1016/j.phrs.2019.104292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276:7614–7620. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 63.Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83:309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 64.Hordijk PL. Endothelial signalling events during leukocyte transmigration. FEBS J. 2006;273:4408–4415. doi: 10.1111/j.1742-4658.2006.05440.x. [DOI] [PubMed] [Google Scholar]
- 65.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boueiz A, Hassoun PM. Regulation of endothelial barrier function by reactive oxygen and nitrogen species. Microvasc Res. 2009;77:26–34. doi: 10.1016/j.mvr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 67.Zarbock A, Polanowska-Grabowska RK, Ley K. Platelet-neutrophil-interactions: Linking hemostasis and inflammation. Blood Rev. 2007;21:99–111. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Kiefmann R, Heckel K, Schenkat S, Dorger M, Goetz AE. Role of p-selectin in platelet sequestration in pulmonary capillaries during endotoxemia. J Vasc Res. 2006;43:473–481. doi: 10.1159/000095247. [DOI] [PubMed] [Google Scholar]
- 69.Blum E, Margalit R, Levy L, et al. A potent leukocyte transmigration blocker: GT-73 showed a protective effect against LPS-induced ARDS in mice. Molecules. 2021;26:4583. doi: 10.3390/molecules26154583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Q, Hu WT, Yin F, et al. The clinical characteristics of ARDS in children with hematological neoplasms. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.696594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ballesteros I, Rubio-Ponce A, Genua M, et al. Co-option of neutrophil fates by tissue environments. Cell. 2020;183:1282–1297.e18. doi: 10.1016/j.cell.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 72.Levi M, Schultz M. The inflammation-coagulation axis as an important intermediate pathway in acute lung injury. Crit Care. 2008;12:144. doi: 10.1186/cc6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ware LB, Camerer E, Welty-Wolf K, Schultz MJ, Matthay MA. Bench to bedside: targeting coagulation and fibrinolysis in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L307–L311. doi: 10.1152/ajplung.00157.2006. [DOI] [PubMed] [Google Scholar]
- 74.Wu CC, Wu SY, Liao CY, Teng CM, Wu YC, Kuo SC. The roles and mechanisms of PAR4 and P2Y12/phosphatidylinositol 3-kinase pathway in maintaining thrombin-induced platelet aggregation. Br J Pharmacol. 2010;161:643–658. doi: 10.1111/j.1476-5381.2010.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gonzales JN, Kim KM, Zemskova MA, et al. Low anticoagulant heparin blocks thrombin-induced endothelial permeability in a PAR-dependent manner. Vascul Pharmacol. 2014;62:63–71. doi: 10.1016/j.vph.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu Y, Wang Y, Gong S, et al. Ruscogenin alleviates LPS-induced pulmonary endothelial cell apoptosis by suppressing TLR4 signaling. Biomed Pharmacother. 2020;125 doi: 10.1016/j.biopha.2020.109868. [DOI] [PubMed] [Google Scholar]
- 77.Chen L, Li W, Qi D, Wang D. Lycium barbarum polysaccharide protects against LPS-induced ARDS by inhibiting apoptosis, oxidative stress, and inflammation in pulmonary endothelial cells. Free Radic Res. 2018;52:480–490. doi: 10.1080/10715762.2018.1447105. [DOI] [PubMed] [Google Scholar]
- 78.Zhu Z, Shi J, Li L, Wang J, Zhao Y, Ma H. Therapy targets SARS-CoV-2 infection-induced cell death. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.870216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zheng F, Wu X, Zhang J, Fu Z. Sevoflurane suppresses NLRP3 inflammasome-mediated pyroptotic cell death to attenuate lipopolysaccharide-induced acute lung injury through inducing GSK-3beta phosphorylation and activation. Int Immunopharmacol. 2022;109 doi: 10.1016/j.intimp.2022.108800. [DOI] [PubMed] [Google Scholar]
- 80.Deshpande R, Zou C. Pseudomonas aeruginosa induced cell death in acute lung injury and acute respiratory distress syndrome. Int J Mol Sci. 2020;21:5356. doi: 10.3390/ijms21155356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- 83.Dostert C, Grusdat M, Letellier E, Brenner D. The TNF family of ligands and receptors: communication modules in the immune system and beyond. Physiol Rev. 2019;99:115–160. doi: 10.1152/physrev.00045.2017. [DOI] [PubMed] [Google Scholar]
- 84.Wallach D. The TNF cytokine family: one track in a road paved by many. Cytokine. 2013;63:225–229. doi: 10.1016/j.cyto.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 85.Lucas R, Hadizamani Y, Enkhbaatar P, et al. Dichotomous role of tumor necrosis factor in pulmonary barrier function and alveolar fluid clearance. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.793251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ding W, Zhang X, Zhang Q, Dong Y, Wang W, Ding N. Adiponectin ameliorates lung injury induced by intermittent hypoxia through inhibition of ROS-associated pulmonary cell apoptosis. Sleep Breath. 2021;25:459–470. doi: 10.1007/s11325-020-02103-3. [DOI] [PubMed] [Google Scholar]
- 87.Kondrikov D, Fulton D, Dong Z, Su Y. Heat shock protein 70 prevents hyperoxia-induced disruption of lung endothelial barrier via caspase-dependent and AIF-dependent pathways. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zang D, Shao Y, Li X. Ultrastructural pathology of rat lung injury induced by ischemic acute kidney injury. Ultrastruct Pathol. 2013;37:433–439. doi: 10.3109/01913123.2013.833562. [DOI] [PubMed] [Google Scholar]
- 89.Widowati W, Wargasetia TL, Rahardja F, et al. hWJMSCs inhibit inflammation and apoptosis in an ARDS cell model. J Taibah Univ Med Sci. 2023;18:1519–1526. doi: 10.1016/j.jtumed.2023.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 91.Dhuriya YK, Sharma D. Necroptosis: a regulated inflammatory mode of cell death. J Neuroinflammation. 2018;15:199. doi: 10.1186/s12974-018-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Faust H, Mangalmurti NS. Collateral damage: necroptosis in the development of lung injury. Am J Physiol Lung Cell Mol Physiol. 2020;318:L215–L225. doi: 10.1152/ajplung.00065.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zheng Y, Huang Y, Xu Y, Sang L, Liu X, Li Y. Ferroptosis, pyroptosis and necroptosis in acute respiratory distress syndrome. Cell Death Discov. 2023;9:91. doi: 10.1038/s41420-023-01369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6:128. doi: 10.1038/s41392-021-00507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang K, Sun Q, Zhong X, et al. Structural mechanism for GSDMD targeting by autoprocessed caspases in pyroptosis. Cell. 2020;180:941–955.e920. doi: 10.1016/j.cell.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 96.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 97.Cheng KT, Xiong S, Ye Z, et al. Caspase-11-mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. J Clin Invest. 2017;127:4124–4135. doi: 10.1172/JCI94495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qu M, Zhang H, Chen Z, et al. The role of ferroptosis in acute respiratory distress syndrome. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.651552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zheng D, Liu J, Piao H, Zhu Z, Wei R, Liu K. ROS-triggered endothelial cell death mechanisms: focus on pyroptosis, parthanatos, and ferroptosis. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1039241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu X, Zhang J, Xie W. The role of ferroptosis in acute lung injury. Mol Cell Biochem. 2022;477:1453–1461. doi: 10.1007/s11010-021-04327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang X, Zhang X, Zhao DX, et al. Endothelial hypoxia-inducible factor-1alpha is required for vascular repair and resolution of inflammatory lung injury through forkhead box protein M1. Am J Pathol. 2019;189:1664–1679. doi: 10.1016/j.ajpath.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lv J, Zeng J, Guo F, et al. Endothelial Cdc42 deficiency impairs endothelial regeneration and vascular repair after inflammatory vascular injury. Respir Res. 2018;19:27. doi: 10.1186/s12931-018-0729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rafat N, Tonshoff B, Bierhaus A, Beck GC. Endothelial progenitor cells in regeneration after acute lung injury: do they play a role? Am J Respir Cell Mol Biol. 2013;48:399–405. doi: 10.1165/rcmb.2011-0132TR. [DOI] [PubMed] [Google Scholar]
- 106.Tsikis ST, Hirsch TI, Fligor SC, Quigley M, Puder M. Targeting the lung endothelial niche to promote angiogenesis and regeneration: a review of applications. Front Mol Biosci. 2022;9 doi: 10.3389/fmolb.2022.1093369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rafii S, Cao Z, Lis R, et al. Platelet-derived SDF-1 primes the pulmonary capillary vascular niche to drive lung alveolar regeneration. Nat Cell Biol. 2015;17:123–136. doi: 10.1038/ncb3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ding BS, Nolan DJ, Guo P, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li N, Xu X, Qi Z, et al. Lpar1-mediated effects in endothelial progenitor cells are crucial for lung repair in acute respiratory distress syndrome/acute lung injury. Am J Respir Cell Mol Biol. 2023;68:161–175. doi: 10.1165/rcmb.2021-0331OC. [DOI] [PubMed] [Google Scholar]
- 110.Mao SZ, Ye X, Liu G, Song D, Liu SF. Resident endothelial cells and endothelial progenitor cells restore endothelial barrier function after inflammatory lung injury. Arterioscler Thromb Vasc Biol. 2015;35:1635–1644. doi: 10.1161/ATVBAHA.115.305519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alvarez DF, Huang L, King JA, ElZarrad MK, Yoder MC, Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol. 2008;294:L419–L430. doi: 10.1152/ajplung.00314.2007. [DOI] [PubMed] [Google Scholar]
- 112.Schniedermann J, Rennecke M, Buttler K, et al. Mouse lung contains endothelial progenitors with high capacity to form blood and lymphatic vessels. BMC Cell Biol. 2010;11:50. doi: 10.1186/1471-2121-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao YD, Ohkawara H, Vogel SM, Malik AB, Zhao YY. Bone marrow-derived progenitor cells prevent thrombin-induced increase in lung vascular permeability. Am J Physiol Lung Cell Mol Physiol. 2010;298:L36–L44. doi: 10.1152/ajplung.00064.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med. 2005;172:854–860. doi: 10.1164/rccm.200410-1325OC. [DOI] [PubMed] [Google Scholar]
- 115.Ge R, Wang F, Peng Z. Advances in biomarkers for diagnosis and treatment of ARDS. Diagnostics (Basel) 2023;13:3296. doi: 10.3390/diagnostics13213296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peng W, Chang M, Wu Y, et al. Lyophilized powder of mesenchymal stem cell supernatant attenuates acute lung injury through the IL-6-p-STAT3-p63-JAG2 pathway. Stem Cell Res Ther. 2021;12:216. doi: 10.1186/s13287-021-02276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li L, Li J, Gao M, et al. Interleukin-8 as a biomarker for disease prognosis of coronavirus disease-2019 patients. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.602395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hendrickson CM, Matthay MA. Endothelial biomarkers in human sepsis: pathogenesis and prognosis for ARDS. Pulm Circ. 2018;8 doi: 10.1177/2045894018769876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sapru A, Calfee CS, Liu KD, et al. Plasma soluble thrombomodulin levels are associated with mortality in the acute respiratory distress syndrome. Intensive Care Med. 2015;41:470–478. doi: 10.1007/s00134-015-3648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Williams JG, Jones RL, Yunger TL, Lahni PM, Yehya N, Varisco BM. Comparison of 16 pediatric acute respiratory distress syndrome-associated plasma biomarkers with changing lung injury severity. Pediatr Crit Care Med. 2024;25:e31–e40. doi: 10.1097/PCC.0000000000003311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bime C, Casanova N, Oita RC, et al. Development of a biomarker mortality risk model in acute respiratory distress syndrome. Crit Care. 2019;23:410. doi: 10.1186/s13054-019-2697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang L, Bittman R, Garcia JG, Dudek SM. Junctional complex and focal adhesion rearrangement mediates pulmonary endothelial barrier enhancement by FTY720 S-phosphonate. Microvasc Res. 2015;99:102–109. doi: 10.1016/j.mvr.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Du L, Zhang J, Zhang X, et al. Oxypeucedanin relieves LPS-induced acute lung injury by inhibiting the inflammation and maintaining the integrity of the lung air-blood barrier. Aging (Albany NY) 2022;14:6626–6641. doi: 10.18632/aging.204235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang J, Luo L, Zhao X, et al. Forsythiae Fructuse extracts alleviates LPS-induced acute lung injury in mice by regulating PPAR-gamma/RXR-alpha in lungs and colons. J Ethnopharmacol. 2022;293 doi: 10.1016/j.jep.2022.115322. [DOI] [PubMed] [Google Scholar]
- 125.Zhang J, Pan Z, Zhou J, et al. The myosin II inhibitor, blebbistatin, ameliorates pulmonary endothelial barrier dysfunction in acute lung injury induced by LPS via NMMHC IIA/Wnt5a/beta-catenin pathway. Toxicol Appl Pharmacol. 2022;450 doi: 10.1016/j.taap.2022.116132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ren R, Xu Z, Wang X, Jiang W, Yu P. Verdiperstat attenuates acute lung injury by modulating MPO/mu-calpain/beta-catenin signaling. Eur J Pharmacol. 2022;924 doi: 10.1016/j.ejphar.2022.174940. [DOI] [PubMed] [Google Scholar]
- 127.He B, Geng S, Zhou W, et al. MMI-0100 ameliorates lung inflammation in a mouse model of acute respiratory distress syndrome by reducing endothelial expression of ICAM-1. Drug Des Devel Ther. 2018;12:4253–4260. doi: 10.2147/DDDT.S188095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shah D, Das P, Acharya S, et al. Small immunomodulatory molecules as potential therapeutics in experimental murine models of acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) Int J Mol Sci. 2021;22:2573. doi: 10.3390/ijms22052573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhuang R, Yang X, Cai W, et al. MCTR3 reduces LPS-induced acute lung injury in mice via the ALX/PINK1 signaling pathway. Int Immunopharmacol. 2021;90 doi: 10.1016/j.intimp.2020.107142. [DOI] [PubMed] [Google Scholar]
- 130.Huang Q, Le Y, Li S, Bian Y. Signaling pathways and potential therapeutic targets in acute respiratory distress syndrome (ARDS) Respir Res. 2024;25:30. doi: 10.1186/s12931-024-02678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sueblinvong V, Weiss DJ. Acute lung injury: endothelial progenitor cells to the rescue? Am J Med Sci. 2019;357:1–2. doi: 10.1016/j.amjms.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 132.Ju YN, Geng YJ, Wang XT, Gong J, Zhu J, Gao W. Endothelial progenitor cells attenuate ventilator-induced lung injury with large-volume ventilation. Cell Transplant. 2019;28:1674–1685. doi: 10.1177/0963689719874048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li H, Qiang Y, Wang L, et al. Repair of lipopolysaccharide-induced acute lung injury in mice by endothelial progenitor cells, alone and in combination with simvastatin. Chest. 2013;144:876–886. doi: 10.1378/chest.12-2429. [DOI] [PubMed] [Google Scholar]
- 134.Silva JD, Krasnodembskaya AD. Investigation of the MSC paracrine effects on alveolar-capillary barrier integrity in the in vitro models of ARDS. Methods Mol Biol. 2021;2269:63–81. doi: 10.1007/978-1-0716-1225-5_5. [DOI] [PubMed] [Google Scholar]