Abstract
Hemorrhagic cystitis (HC) caused by viral infections such as BK virus, cytomegalovirus, and/or adenovirus after allogeneic hematopoietic stem cell transplantation (allo-HCT) causes morbidity and mortality, affects quality of life, and poses a substantial burden to the health care system. At present, HC management is purely supportive, as there are no approved or recommended antivirals for virus-associated HC. The objective of this retrospective observational study was to compare the economic burden, health resource utilization (HRU), and clinical outcomes among allo-HCT recipients with virus-associated HC to those without virus-associated HC using a large US claims database. Claims data obtained from the Decision Resources Group Real-World Evidence Data Repository were used to identify patients with first (index) allo-HCT procedure from January 1, 2012, through December 31, 2017. Outcomes were examined 1 year after allo-HCT and included total health care reimbursements, HRU, and clinical outcomes for allo-HCT patients with virus-associated HC versus those without. Further, a generalized linear model was used to determine adjusted reimbursements stratified by the presence or absence of any acute or chronic graft-versus-host disease (GVHD) after adjusting for age, health plan, underlying disease, stem cell source, number of comorbidities, baseline reimbursements, and follow-up time. Of 13,363 allo-HCT recipients, 759 (5.7%) patients met the prespecified criteria for virus-associated HC. Total unadjusted mean reimbursement was $632,870 for patients with virus-associated HC and $340,469 for patients without virus-associated HC. In a multivariable model, after adjusting for confounders, the adjusted reimbursements were significantly higher for virus-associated HC patients with and without GVHD compared to patients without virus-associated HC (P < .0001). Patients with virus-associated HC stayed 7.9 additional days in the hospital (P < .0001) and 6.1 additional days (P = .0009) in the intensive care unit (ICU) for the index hospitalization, as compared to patients without virus-associated HC. The hospital readmission rate was higher for allo-HCT patients with versus without virus-associated HC (P < .0001), resulting in 12.9 more days in the hospital (P < .0001) and 7.3 more days in the ICU (P < .0001) after the index hospitalization. Among patients with GVHD, those with virus-associated HC had significantly higher all-cause mortality as compared to those without virus-associated HC (23.2% versus 18.4%; P = .0035). In an adjusted analysis, patients with virus-associated HC had a significantly higher risk of mortality, regardless of the presence of GVHD. When stratified by GVHD, there were no significant differences in the baseline risk for renal impairment; virus-associated HC was associated with increased risk for renal impairment in the follow-up period in patients with or without GVHD (P < .0001 for both). After allo-HCT, patients with virus-associated HC have significantly higher health care reimbursements and HRU, with worse clinical outcomes, including renal impairment, irrespective of the presence of GVHD and significantly higher all-cause mortality in the presence of GVHD. Our results highlight the unmet clinical need for effective strategies to prevent and treat virus-associated HC in HCT recipients that may also reduce costs among these patients.
Keywords: Allogeneic HCT, Virus-associated hemorrhagic, cystitis
Allogeneic hematopoietic stem cell transplantation (allo-HCT) is often the only curative option for both malignant and nonmalignant diseases [1,2]. During the period of immune recovery after allo-HCT, viral infections, which are normally controlled by T cell immunity, are a major cause of morbidity and mortality [3–6]. A recent retrospective study of 404 allo-HCT recipients showed that detection of multiple viruses was common, including BK virus (BKV), cytomegalovirus (CMV), adenovirus (AdV), Epstein-Barr virus (EBV), and human herpesvirus-6 (HHV-6) and demonstrated an association between the cumulative burden of virus exposure and increased mortality [7,8].
Hemorrhagic cystitis (HC) is the primary clinical manifestation associated with BKV viremia, occurring in 8% to 25% and 7% to 54% of pediatric and adult patients, respectively, following allo-HCT [9]. Virus-associated HC occurs between 2 and 8 weeks (range, 1 week to 6 months) after allo-HCT and is defined by lower urinary tract symptoms that typically include severe, often debilitating, abdominal pain (marked by use of pain medications, often requiring continuous narcotic infusions), bladder pressure, urinary frequency, urinary urgency, dysuria, nocturia, and the presence of gross hematuria with clots that may lead to urinary obstruction and renal impairment [9–14]. HC can result in rapidly deteriorating health-related quality of life, prolonged hospitalization, and increasing health care reimbursements [9,13]. Recent prospective studies have highlighted the importance of BKV-specific T cell reconstitution by demonstrating the relationship between low levels of BKV-specific T cells and severe manifestations of HC [11,15]. While BKV causes the majority of HC cases, HC has also been associated with AdV and CMV infection in a smaller number of cases [16,17].
Moreover, virus-associated HC may increase post-HCT mortality. In a large cohort study of 1321 patients, 219 patients (16.6%) developed virus-associated HC, and severe grade HC (grades 3 to 4) was associated with increased treatment-related mortality at 1 year [18]. A recent prospective, multicenter trial of the natural history of BKV after allo-HCT showed that 22% of patients developed grade 2 or higher HC, resulting in a 2-fold increased risk of death [15].
There are currently no therapies approved by the US Food and Drug Administration or European Medicines Agency for virus-associated HC [19]. The current standard of care relies on supportive measures to address the symptoms and manifestations of HC, including urinary bladder irrigation to avoid blood clot obstructions, red blood cell and platelet transfusions for bleeding, narcotics for pain, cystectomy in cases with uncontrollable bleeding, and dialysis for acute renal failure [14,16]. Furthermore, no multicenter, population-based study that specifically examines the economic and clinical burden of virus-associated HC has been performed in the United States. The objective of this study was to describe the economic burden, health resource utilization (HRU), and clinical outcomes among allo-HCT recipients with virus-associated HC in the United States compared to those without virus-associated HC using a large real-world claims database.
MATERIALS AND METHODS
The study identified an allo-HCT patient cohort by utilizing the claims database from the Decision Resources Group (DRG) Real-World Data Repository. DRG’s deidentified claims database is an open-source claims database that tracks >300 million longitudinal patient lives covering multiple commercial and government-funded health plans from across the United States. The database includes epidemiologic, formulary, and enrollment data from a comprehensive geographical representation across the US population. The open-source nature of the database provides the ability to track patients as they change payers.
Inclusion/Exclusion Criteria
The inclusion criterion was defined as the first (index) allo-HCT identified through the International Classification of Diseases (ICD-9 or ICD-10 procedure code, Healthcare Common Procedure Coding System (HCPCS) code, or a Current Procedural Terminology (CPT®) code observed for a patient during the study period, defined as January 1, 2012, through December 31, 2017. Subsequent allo-HCTs for the same patient were not considered in order to avoid double counting. There were no limits on age or underlying conditions that would prevent patients from being included in this study. Patients were excluded if they had a previous allo-HCT ICD-9/ICD-10, HCPCS, or CPT procedure code between January 1, 2011, through December 31, 2011, or if their index procedure was performed in an outpatient setting.
Study Baseline and Follow-up
The baseline period for assessment of demographics and comorbidities was defined as the 1-year period prior to the index allo-HCT procedure. Patients were followed for a maximum of 1 year after the index allo-HCT procedure, or date of death, whichever occurred first.
Study Variables
Baseline characteristics
The following demographic information was included to characterize the study population: age at the time of allo-HCT procedure, sex, health insurance plan type, and geographic region. In addition, the number of comorbidities at baseline was identified through the ICD-9 and ICD-10 diagnosis codes for different comorbidities observed during the baseline period. These comorbidities were based on the comorbidities included in the Hematopoietic Cell Transplantation-Comorbidity Index and include the following: arrhythmia, cardiovascular disease (eg, coronary artery disease, congestive heart failure, ejection fraction, and shortening fraction), inflammatory bowel disease, diabetes, cerebrovascular disease, psychiatric conditions, liver disease, infectious disease, rheumatologic disease, peptic ulcer disease, renal disease, pulmonary disease, prior solid tumor(s), and heart valve disease.
Transplant characteristics
The underlying disease condition for the allo-HCT procedure was defined by searching specific diagnostic disease codes within the patient’s record during the baseline period closest to the date of the index allo-HCT procedure and categorized as malignant, nonmalignant immunodeficient, nonmalignant immunocompetent, and inherited metabolic disorders. The stem cell source (eg, bone marrow, peripheral blood, or cord blood) was identified from the ICD-9 and ICD-10 procedure code for the index allo-HCT procedure. The stem cell source was considered unknown for those whose index procedure was identified through HCPCS or CPT codes and for those patients who reported >1 type of stem cell source, possibly due to miscoding (1.02% of total patients).
Virus-associated HC
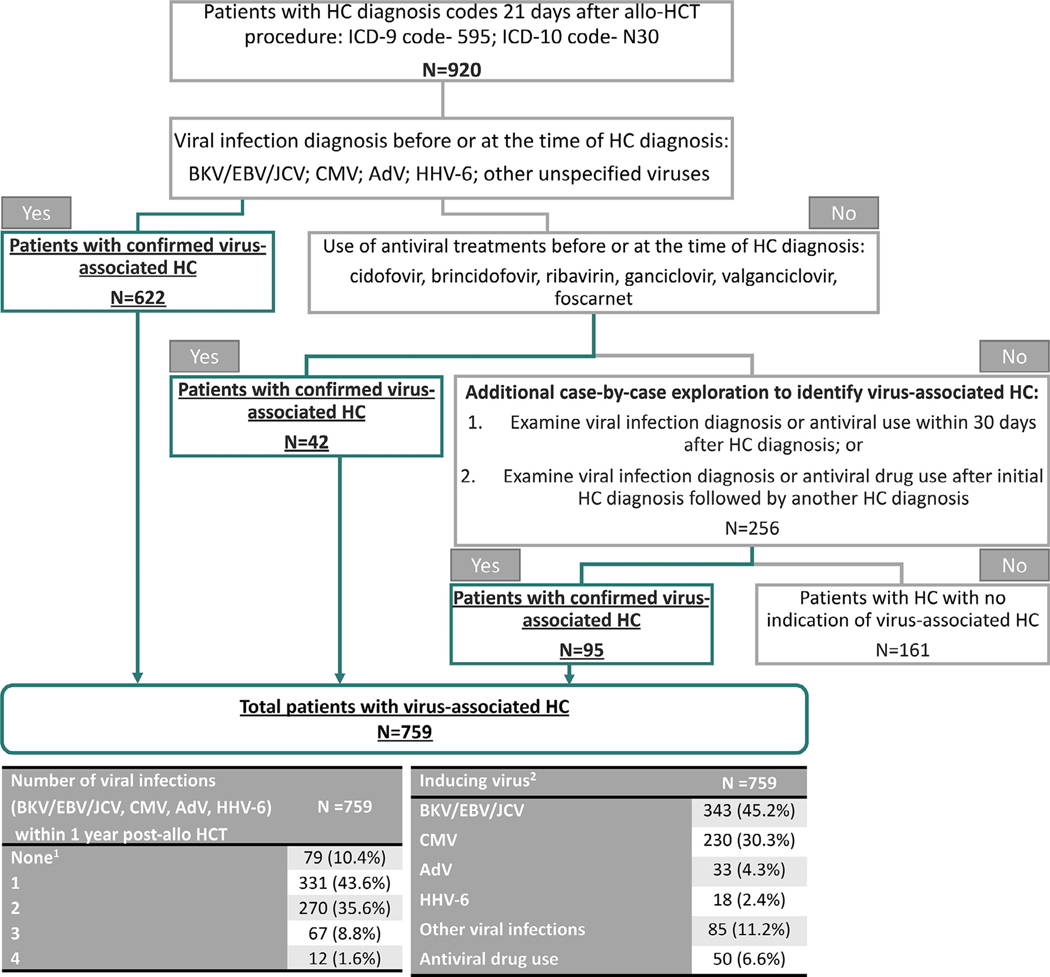
There are no direct ICD-9 or ICD-10 diagnosis codes to identify virus-associated HC; however, diagnosis codes are available to identify HC (ICD-9 code, 595; ICD-10 code, N30). The presence of HC attributable to viral reactivation usually occurs >2 weeks after the allo-HCT procedure; therefore, a base case of 21 days was chosen to identify HC [20]. Sensitivity analyses were conducted using 14 and 28 days after allo-HCT procedure to determine the diagnosis of HC. Based on these results, the base case definition of 21 days was chosen. After identification of the first HC diagnosis between 21 days and 1 year after the allo-HCT procedure, an algorithm was developed through clinical guidance to identify patients with virus-associated HC (Figure 1).
Figure 1.
Algorithm for identification of patients who had allo-HCT with virus-associated HC within 1 year. Algorithm used to determine cases of virus-associated HC after allo-HSCT. 1Patients were identified as virus-associated HC by other viral infection or antiviral drug. 2The identification of inducing virus follows the hierarchy: BKV/EBV/JCV > CMV > AdV > HHV-6 > other virus.
In this algorithm, among the patients diagnosed with HC (n = 920), 622 patients with diagnosis codes for BKV/EBV/JC virus (JCV; grouped together due to nonspecific diagnosis codes for these infections), CMV, AdV, HHV-6, and other unspecified viruses and an additional 42 patients who had antiviral use before or at the time of the HC diagnosis code were considered patients with confirmed virus-associated HC. Among the remaining patients, 95 patients who had either a viral infection diagnosis or antiviral use 30 days after HC diagnosis, or viral infection diagnosis or antiviral use after the initial HC diagnosis followed by another HC diagnosis, were identified as patients with confirmed virus-associated HC. A total of 759 allo-HCT patients met the criteria for virus-associated HC. Inducing virus for the virus-associated HC patients with >1 viral infection diagnosis was identified by applying the hierarchy of BKV/EBV/JCV > CMV > AdV > HHV-6 > other viruses. The category “other viruses” encompassed coding for all other viruses and included, but was not limited to, unspecified viral infections, herpes simplex, and infectious mononucleosis.
Economic outcomes
The economic outcome of total health care reimbursement per patient was estimated through total charges in the DRG Real-World Data Repository and presented in 2019 US dollars, adjusted using the medical care component of the US Consumer Price Index [21]. The total health care charges were first identified as the total submitted charges for claims in the 1-year follow-up period after allo-HCT procedure. These charges were then winsorized at the 1st and 99th percentiles in each subgroup of interest (patients with and without virus-associated HC) to adjust for outliers. The total reimbursed amount was then calculated through the ~20% of submitted claims in the DRG Real-World Claims Database where reimbursed amounts were available. Using these overlapping claims, a reimbursement-to-charge ratio was calculated as 0.425 and applied to the submitted charges to generate an estimated reimbursement amount.
Health resource utilization
The major HRU elements included in the analysis for all allo-HCT patients were hospitalization length of stay (LOS), divided into intensive care unit (ICU) and general ward stays. The study also examined hospital readmissions, emergency room visits, outpatient visits, and physician office visits.
Clinical outcomes
All-cause mortality was identified by discharge status code of “expired” or “hospice” (with transfer to hospice presumed to be a proxy for mortality) or death-related ICD/HCPCS codes. In addition, other clinical outcomes in the follow-up period identified through diagnosis codes included renal impairment and urinary retention. Furthermore, patients with diagnosis codes for any acute or chronic graft-versus-host disease (GVHD) during the follow-up period were also identified, and clinical outcomes were examined separately in these 2 groups.
Statistical Analyses
Descriptive analyses were conducted for baseline patient demographics, transplant characteristics, and comorbidities to describe the allo-HCT patient cohort with and without virus-associated HC. For all descriptive analyses, categorical variables were summarized by the frequency and percentages with rounding to 1 decimal place based on available observations. Continuous variables were summarized using descriptive statistics (n, mean with SD rounded to 1 decimal place), median, and interquartile range.
Outcomes in the allo-HCT patients with and without virus-associated HC were compared. Total health care charges and reimbursement, HRU, and clinical outcomes were descriptively summarized and compared. Clinical outcomes were examined separately for patients with and without GVHD during the follow-up periods in order to distinguish the impact of virus-associated HC and GVHD.
Statistical comparisons between continuous variables were conducted using 2-sample t tests, Wilcoxon-Mann-Whitney tests, or analysis of variance tests as appropriate. Statistical comparisons between categorical variables were conducted using χ2 or Fisher exact tests. All statistical tests were 2-sided with a 5% level of significance. A multivariate analysis for total health care reimbursements was also conducted through a generalized linear model and a negative binomial distribution. Covariates were retained in the multivariate model through backward selection if P < .10. The final covariates included in the model were virus-associated HC, age, health insurance plan, underlying disease, stem cell source, number of comorbidities at baseline, health care reimbursements at baseline, GVHD during follow-up, interaction between virus-associated HC and GVHD, and follow-up duration. The use of an interaction term between virus-associated HC and GVHD in this model allowed for assessment of the joint impact of virus-associated HC and GVHD on total health care reimbursement as well as determining the impact of virus-associated HC alone by stratifying patients with and without GVHD. The final multivariate model was then used to derive the observed-margin least mean square estimates and 95% confidence interval (CI) of the total adjusted health care reimbursements for patients with and without virus-associated HC. The interaction between GVHD and virus-associated HC was statistically significant in the model, and thereby, the results of the adjusted reimbursements were stratified by the presence or absence of GVHD.
A Cox proportional hazards model for time to mortality was built with all-cause mortality as the event of interest, and patients were followed from the date of allo-HCT until date of death or until 1 year after allo-HCT, whichever was earlier. Virus-associated HC and any GVHD were assessed as time-varying covariates based on their date of diagnosis, in addition to the baseline covariates of age at index, sex, underlying disease, stem cell source, and number of comorbidities at baseline. There were 5 patients with unknown age who were excluded from this analysis. Covariates with P ≤ .10 were retained in the model through backward selection.
Separate subgroup analyses on pediatric (aged <18 years at index) and adult (aged ≥ 18 years at index) patients are presented in the supplementary material.
All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) or later.
RESULTS
Study Population
The study population consisted of 13,363 allo-HCT patients who met the inclusion criteria during the time period of January 1, 2012, through December 31, 2017. Of the 920 (6.9%) allo-HCT patients with HC diagnosis codes 21 days after the allo-HCT procedure, 759 (5.7%) patients were identified as patients with virus-associated HC (Figure 1). Among these 759 patients, 270 (35.6%) were coded with 2 infections, and 79 (10.5%) were coded with ≥3 infections among BKV/EBV/JCV, CMV, AdV, and HHV-6 within the follow-up period. In addition, BKV/EBV/JCV was identified as the inducing virus for 343 (45.2%) patients.
The demographic and transplant characteristics for patients with and without virus-associated HC are summarized in Table 1. The patients who had allo-HCT with virus-associated HC were younger (39.4 20.1 versus 46.9 20.7; P < .0001) and more likely to be male (63% versus 56.8%; P = .0008) than allo-HCT patients without virus-associated HC, which is in line with this sex difference having previously been identified as a risk factor for the development of HC (Table 1) [22]. The geographic patient distribution was representative across the United States and the vast majority of patients with and without virus-associated HC in this data extract have commercial health insurance (>85%) (Table 1). Although this number is higher than national US estimates, it is a common observation of claims databases. Additionally, some patients had multiple health insurance plans for the index allo-HCT procedure; 69% of the patients had only commercial insurance.
Table 1.
Demographic and Transplant Characteristics of the Allo-HCT Patients with or without Virus-Associated HC
| Characteristic | Patients with Virus-Associated HC (n = 759) | Patients without Virus-Associated HC (n = 12,604) | P Value | |
|---|---|---|---|---|
| Age | Mean ± SD | 39.4 ± 20.1 | 46.9 ± 20.7 | <.0001 |
| Sex, n (%) | Female | 281 (37.0) | 5443 (43.2) | .0008 |
| Male | 478 (63.0) | 7155 (56.8) | ||
| Health insurance plan type at time of allo-HCT procedure,* n (%) | Commercial | 661 (87.1) | 10,826 (85.9) | .3574 |
| Medicaid | 68 (9.0) | 745 (5.9) | .0006 | |
| Managed Medicaid | 31 (4.1) | 413 (3.3) | .2280 | |
| Medicare | 64 (8.4) | 1877 (14.9) | <.0001 | |
| Managed Medicare | 8 (1.1) | 249 (2.0) | .0726 | |
| VA/other | 64 (8.4) | 1228 (9.7) | .2353 | |
| Unknown | 39 (5.1) | 592 (4.7) | .5777 | |
| Region of the United States, n (%) | Northeast | 164 (21.6) | 3061 (24.3) | .0039 |
| Midwest | 166 (21.9) | 2769 (22.0) | ||
| South | 291 (38.3) | 4029 (32.0) | ||
| West | 90 (11.9) | 1799 (14.3) | ||
| Unknown | 48 (6.3) | 946 (7.5) | ||
| Underlying disease, n (%) | Malignant | 685 (90.3) | 11,157 (88.5) | .0004 |
| Nonmalignant immunodeficient | 7 (0.9) | 167 (1.3) | ||
| Nonmalignant immunocompetent | 61 (8.0) | 941 (7.5) | ||
| Inherited metabolic disorders | † | 31 (0.3) | ||
| Unknown | † | 308 (2.4) | ||
| Stem cell source, n (%) | Bone marrow | 94 (12.4) | 1703 (13.5) | .0002 |
| Peripheral blood | 542 (71.4) | 8862 (70.3) | ||
| Cord blood | 68 (9.0) | 727 (5.8) | ||
| Unknown | 55 (7.3) | 1312 (10.4) | ||
| Number of baseline comorbidities, mean ± SD | 2.1 ± 1.5 | 2.0 ± 1.6 | .2479 | |
VA indicates Veterans Administration.
Nonexclusive categories.
Cells with ≤5 observations are not reported.
Health care reimbursements
The total submitted health care charges for allo-HCT patients with and without virus-associated HC showed a mean difference of $686,478 ($1,481,699 versus $795,221; P < .0001; Table 2). Furthermore, the unadjusted total health care reimbursement for allo-HCT patients with and without virus-associated HC showed a mean difference of $292,401 ($632,870 versus $340,469; P < .0001; Table 2). For the unadjusted total inpatient reimbursement during the index allo-HCT hospitalization, the mean difference was $57,151 ($174,881 versus $117,730; P < .0001; Table 2) while the mean difference in the unadjusted total inpatient reimbursement for hospital readmissions after the index allo-HCT hospitalization was $97,463 ($207,149 versus $109,686; P < .0001; Table 2). Additionally, a mean difference of $28,905 ($88,436 versus $59,531; P < .0001; Table 2) was observed for unadjusted total outpatient reimbursement in patients with virus-associated HC as compared to patients without virus-associated HC. When patients were subdivided based on the presence of CMV diagnosis code, patients with virus-associated HC in both groups had significantly higher health care reimbursement than those without HC. The mean difference in patients with CMV was $220,047 ($667,362 versus $447,315; P < .0001), and the mean difference in patients without CMV was $286,085 ($586,059 versus $299,974; P < .0001; Supplementary Table S1). A sensitivity analysis was run on the total reimbursement to exclude patients who died within 30 and 100 days after allo-HCT and found that the mean reimbursement was comparable to the overall group in both patients with and without virus-associated HC (Supplementary Table S2).
Table 2.
Economic Burden and HRU in the First Year after Allo-HCT for Patients with or without Virus-Associated HC
| Characteristic | Patients with Virus-Associated HC (n = 759) | Patients without Virus-Associated HC (n = 12,604) | P Value |
|---|---|---|---|
| Total health care submitted charges (2019 USD) | |||
| Mean ± SD | 1,481,699 ± 1,508,451 | 795,221 ± 885,877 | <.0001 |
| Median [Q1; Q3] | 1,020,571 [538,421; 1,912,975] | 516,861 [224,627; 1,028,482] | |
| Total health care reimbursement (2019 USD) | |||
| Mean ± SD | 632,870 ± 640,815 | 340,469 ± 377,591 | <.0001 |
| Median [Q1; Q3] | 435,420 [229,588; 815,979] | 222,494 [97,746; 441,018] | |
| Overall LOS (index hospitalization and readmissions, d) | |||
| Mean ± SD | 76.8 ± 57.4 | 49.7 ± 47.4 | <.0001 |
| Median [Q1; Q3] | 62.0 [38.0; 98.0] | 35.0 [22.0; 61.0] | |
| Index hospitalization | |||
| Total inpatient reimbursement (2019 USD) | |||
| Mean ± SD | 174,881 ± 278,682 | 117,730 ± 175,660 | <.0001 |
| Median [Q1; Q3] | 89,571 [6,614; 207,154] | 67,410 [4,573; 155,405] | |
| LOS, d | |||
| Mean ± SD | 35.5 ± 31.5 | 27.6 ± 25.9 | <.0001 |
| Median [Q1; Q3] | 27.0 [19.0; 39.0] | 23.0 [16.0; 31.0] | |
| ICU stay during index hospitalization, n (%) | 223 (29.4) | 2942 (23.3) | .0001 |
| Time in ICU, d | |||
| Mean ± SD | 34.3 ± 26.6 | 28.2 ± 23.0 | .0009 |
| Median [Q1; Q3] | 28.0 [21.0; 39.0] | 25.0 [17.0; 33.0] | |
| Readmissions after index hospitalization, n (%) | 627 (82.6) | 7507 (59.6) | <.0001 |
| Total inpatient reimbursement (2019 USD) | |||
| Mean ± SD | 207,149 ± 314,649 | 109,686 ± 174,644 | <.0001 |
| Median [Q1; Q3] | 95,758 [267; 2,075,607] | 39,986 [48; 1,000,873] | |
| LOS after index, d | |||
| Mean ± SD | 50.0 ± 49.3 | 37.1 ± 44.0 | <.0001 |
| Median [Q1; Q3] | 35.0 [17.0; 68.0] | 21.0 [9.0; 49.0] | |
| ICU stays after index hospitalization, n (%) | 332 (43.7) | 3480 (27.6) | <.0001 |
| Time in ICU, d | |||
| Mean ± SD | 25.8 ± 31.7 | 18.5 ± 24.9 | <.0001 |
| Median [Q1; Q3] | 13.5 [4.0; 34.5] | 9.0 [4.0; 23.0] | |
| Readmission rate (per person year) (95% CI) | 3.47 (3.34–3.62) | 1.92 (1.90–1.95) | <.0001 |
| Outpatient visits, n (%) | 752 (99.1) | 12,040 (95.5) | <.0001 |
| Total outpatient reimbursement (2019 USD) | |||
| Mean ± SD | 88,436 ± 71,593 | 59,531 ± 55,349 | <.0001 |
| Median [Q1; Q3] | 70,638 [34,981; 124,388] | 45,350 [20,096; 82,718] | |
| Number of outpatient visits | |||
| Mean ± SD | 42.6 ± 30.2 | 33.7 ± 25.5 | <.0001 |
| Median [Q1; Q3] | 39.0 [19.0; 59.0] | 29.0 [14.0; 47.0] | |
| ER visits, n (%) | 437 (57.6) | 5717 (45.4) | <.0001 |
| Number of ER visits | |||
| Mean ± SD | 3.2 ± 5.7 | 2.5 ± 2.3 | .0058 |
| Median [Q1; Q3] | 2.0 [1.0; 4.0] | 2.0 [1.0; 3.0] | |
| Physician office visits, n (%) | 417 (54.9) | 6843 (54.3) | .7276 |
| Number of physician office visits | |||
| Mean ± SD | 16.0 ± 19.0 | 14.7 ± 16.9 | .1718 |
| Median [Q1; Q3] | 9.0 [2.0; 24.0] | 7.0 [2.0; 23.0] | |
Q1 indicates first quartile; Q3, third quartile; ER, emergency room.
Impact of HC on hospital LOS
During the index allo-HCT hospitalization, the mean hospital LOS was significantly longer in the virus-associated HC group compared to those without virus-associated HC (35.5 days versus 27.6 days; P < .0001) (Table 2). ICU stays were seen in 29.4% of patients with virus-associated HC, significantly higher than the 23.3% of patients without virus-associated HC (P = .0001). Furthermore, among those with an ICU stay, mean ICU days were significantly longer in the virus-associated HC cohort compared to the cohort without virus-associated HC (34.3 versus 28.2; P = .0009). The hospital readmission rate (per person year) was higher for allo-HCT patients with virus-associated HC compared to those without virus-associated HC (3.47 versus 1.92; P < .0001), resulting in 12.9 more days in mean hospital LOS in the postindex period (50.0 days versus 37.1 days; P < .0001; Table 2). The most common diagnosis requiring hospital readmission was due to complications associated with the allo-HCT procedure, at 11.4% of patients with virus-associated HC compared to 9.4% of patients without virus-associated HC. Emergency room visits were greater in allo-HCT patients with virus-associated HC (3.2 versus 2.5; P = .0058) compared to those without virus-associated HC (Table 2).
Multivariable model for reimbursement
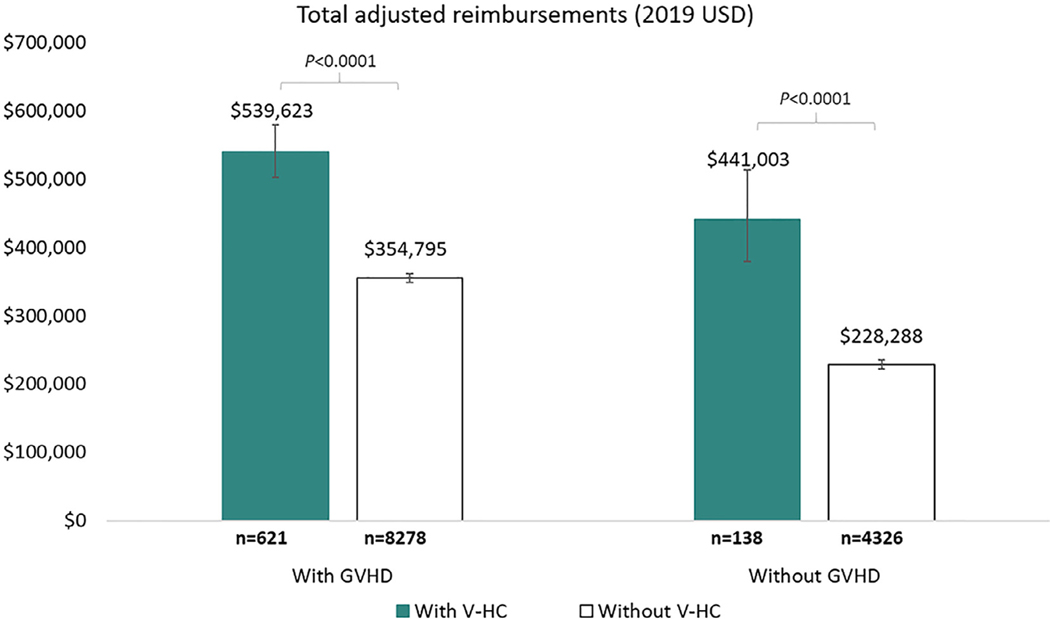
Further, the multivariate model showed that after adjusting for age, health insurance plan, underlying disease, stem cell source, number of comorbidities at baseline, health care reimbursements at baseline, and follow-up duration, patients with virus-associated HC had significantly higher total health care reimbursements as compared to patients without virus-associated HC (P < .0001) irrespective of the presence or absence of GVHD (Figure 2).
Figure 2.
Adjusted total reimbursements within 1 year of undergoing allo-HCT for patients with and without virus-associated HC, stratified by GVHD. Least square means and 95% CIs for the adjusted reimbursements for each patient group were derived from a generalized linear model. The model provided estimates adjusted for age, health insurance plan, underlying disease, stem cell source, number of comorbidities, reimbursements at baseline, GVHD during follow-up, interaction between presence of virus-associated HC and GVHD, and follow-up time. V-HC, virus-associated HC.
Among patients with GVHD, the mean adjusted total reimbursement was assessed and found to be $539,623 (95% CI, $502,280 to $579,742) for patients with virus-associated HC and $354,795 (95% CI, $347,885 to $361,842) for patients without virus-associated HC, resulting in a $184,828 difference between the groups (Figure 2). Among patients without GVHD, the mean adjusted total reimbursement was $441,003 (95% CI, $378,980 to $513,177) for patients with virus-associated HC and $228,288 (95% CI, $222,053 to $234,697) for patients without virus-associated HC, resulting in a $212,715 difference between the groups (Figure 2).
To explore potential differences between adult and pediatric patients, we compared the unadjusted total health care reimbursement for patients with and without virus-associated HC. This analysis showed a mean difference of $336,571 ($913,843 versus $577,272; P < .0001) and $258,316 ($561,931 versus $303,615; P < .0001) in the pediatric and adult allo-HCT subgroups, respectively (Supplementary Table S3). The mean adjusted total reimbursement was $269,576 higher for pediatric patients with GVHD and $236,362 higher for those without GVHD between the 2 groups of patients with versus without virus-associated HC (Supplementary Figure S1). The mean adjusted total reimbursement was $171,904 higher for adult patients with GVHD and $213,678 higher for those without GVHD between the 2 groups of patients with versus without virus-associated HC (Supplementary Figure S1). Both pediatric and adult patients with virus-associated HC had significantly higher risk of mortality (Supplementary Figure S2).
Impact of HC on clinical outcomes
Among allo-HCT patients with GVHD, all-cause mortality was significantly higher for patients with virus-associated HC as compared to patients without virus-associated HC (23.2% versus 18.4%; P = .0035). The presence of renal impairment during baseline was comparable between allo-HCT patients with and without virus-associated HC, irrespective of the presence or absence of GVHD. However, among allo-HCT patients with GVHD, a significantly higher proportion of patients with virus-associated HC had renal impairment during follow-up and not during baseline compared to patients without virus-associated HC (43.2% versus 24.1%; P < .0001). In addition, a significantly higher proportion of patients with virus-associated HC had urinary retention (5.3% versus 2.1%; P < .0001) compared to patients without virus-associated HC among the allo-HCT patients with GVHD (Table 3). Among allo-HCT patients without GVHD, a significantly higher proportion of patients with virus-associated HC had renal impairment during follow-up and not during baseline (the year before allo-HCT) compared to patients without virus-associated HC (36.2% versus 15.8%; P < .0001). However, there was no statistically significant difference in all-cause mortality (23.9% versus 20.5%; P = .3297; Table 3). New diagnoses of renal impairment remained significantly higher in patients with virus-associated HC as compared to patients without virus-associated HC, irrespective of use of cidofovir in these patients (P < .01) (Supplementary Table S4).
Table 3.
Clinical Outcomes in the First Year after Allo-HCT for Patients with or without Virus-Associated HC, Stratified by the Presence or Absence of GVHD
| Characteristic | Patients with Virus-Associated HC (n = 759) | Patients without Virus-Associated HC (n = 12,604) | P Value |
|---|---|---|---|
| Allo-HCT patients with GVHD, n (%) | 621 (81.8) | 8278 (65.7) | <.0001 |
| All-cause mortality | 144 (23.2) | 1527 (18.4) | .0035 |
| Renal impairment during follow-up | 360 (58.0) | 3129 (37.8) | <.0001 |
| Renal impairment during baseline | 128 (20.6) | 1782 (21.5) | .5922 |
| Renal impairment during follow-up and not during baseline | 268 (43.2) | 1997 (24.1) | <.0001 |
| Urinary retention during follow-up | 33 (5.3) | 177 (2.1) | <.0001 |
| Allo-HCT patients without GVHD, n (%) | 138 (18.2) | 4326 (34.3) | <.0001 |
| All-cause mortality | 33 (23.9) | 887 (20.5) | .3297 |
| Renal impairment during follow-up | 78 (56.5) | 1150 (26.6) | <.0001 |
| Renal impairment during baseline | 37 (26.8) | 916 (21.2) | .1116 |
| Renal impairment during follow-up and not during baseline | 50 (36.2) | 682 (15.8) | <.0001 |
| Urinary retention during follow-up | * | 48 (1.1) | — |
Renal impairment was defined through ICD-9 and ICD-10 diagnosis codes for glomerular diseases, renal tubulo-interstitial diseases, acute kidney failure and chronic kidney disease, urolithiasis, other disorders of the kidney and the ureter, kidney injury, and dialysis.
Cells with ≤5 observations are not reported.
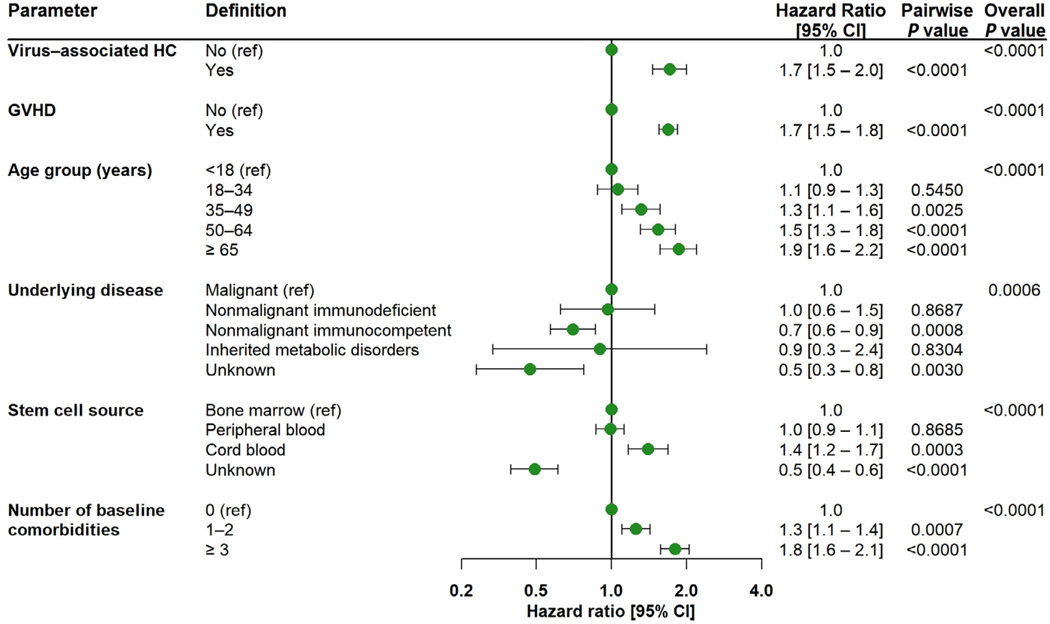
To assess the impact of HC on all-cause mortality, we performed the Cox proportional hazards model for time to all-cause mortality. This assessment showed that after adjusting for confounders, patients with virus-associated HC had a 70% higher risk of mortality as compared to patients without virus-associated HC (hazard ratio, 1.7; 95% CI, 1.5 to 2.0). In addition, diagnosis of GVHD, higher age, higher number of comorbidities, and type of underlying disease and stem cell source were significant predictors of mortality (Figure 3).
Figure 3.
Cox proportional hazards model for time to all-cause mortality as determined by parameter. Virus-associated HC and any GVHD were assessed as time-varying covariates based on their date of diagnosis, in addition to the baseline covariates of age at index, sex, underlying disease, stem cell source, and number of comorbidities at baseline.
DISCUSSION
Infections and GVHD have been reported to be major cost drivers in the allogeneic HCT setting [8,23]. In this retrospective observational database study assessing the economic burden of virus-associated HC in the setting of allo-HCT, patients identified as having virus-associated HC utilized significantly more health care resources than those without virus-associated HC, as evidenced by prolonged hospitalization, a greater proportion of patients with any ICU days, longer ICU stays, higher hospital readmission rates, and increased reimbursements. Irrespective of the presence or absence of GVHD, virus-associated HC had a significant association with increased health care reimbursements even after adjusting for age, health plan, underlying disease, stem cell source, number of comorbidities, baseline reimbursements, and follow-up time. The mean overall hospital LOS (index hospitalization and readmissions after index) was prolonged by 27.1 days (76.8 versus 49.7; P < .0001), or 55% greater, for allo-HCT patients with virus-associated HC versus patients without virus-associated HC. This finding is consistent with other studies of allo-HCT patients with virus-associated HC that have reported significantly prolonged hospitalizations, although this study is unique in that it also reports an increase in the number of days the patients were required to be in the ICU [13].
Multiple retrospective and prospective clinical studies have demonstrated the significant and independent association between BK viral infection, HC, and subsequent kidney function decline and worse patient survival [15,24,25]. In a recent prospective multicenter study of 193 allo-HCT recipients, those with viremia >10,000 copies/mL in the first 3 months after their transplantation had significantly higher risk of receiving dialysis independent of the presence of symptoms [15]. In our study, virus-associated HC was associated with increased incidence of renal impairment in all allo-HCT patients. When stratified by GVHD, there were no significant differences in baseline incidence for renal impairment. However, virus-associated HC was associated with increased incidence for renal impairment in the follow-up period in patients with or without GVHD (P < .0001 for both). The etiology for renal impairment following HCT is likely multifactorial. HC with severe hematuria may cause urinary tract obstruction resulting in renal insufficiency [24]. Use of nephrotoxic medications [24], BK nephropathy [15], and other complications of HCT, such as GVHD [18,24] and transplant-associated thrombotic microangiopathy [24], may contribute to short- and long-term renal impairment.
Importantly, virus-associated HC was associated with increased all-cause mortality in patients with GVHD but not in patients without GVHD. However, when examining time to all-cause mortality as an adjusted analysis through a Cox proportional hazards model, virus-associated HC was significantly (P < .0001) associated with a higher risk of all-cause mortality. In particular, patients with virus-associated HC had a 70% increased risk of mortality, a statistically significant (P < .0001) result seen even after adjusting for presence of GVHD as well as other baseline factors. Laskin et al. [15] recently reported that the risk of death was doubled in patients with grade ≥ 2 BK virus-associated HC. Other retrospective, single-center studies have reported an association between grade 3 to 4 BK virus-associated HC and decreased overall survival [25] or increased treatment-related mortality [18] in multivariate analyses.
Treatment for these patients remains palliative; cidofovir has been used to treat patients, but its use is limited because of renal toxicity and the lack of clinical trials evaluating its efficacy [9,19]. Studies have been conducted with ciprofloxacin to evaluate its prophylactic prevention of BK virus-associated HC, but these studies were inconclusive [9]. Intravesicular administration of cidofovir has been used in the treatment of this disease, but it will require clinical trials to verify its efficacy and safety [26]. Therefore, there remains a substantial unmet medical need for new safe and efficacious therapies for these patients [19].
One emerging therapy that aims at reducing this burden is infusion of virus-specific T cells (VSTs), targeted to help restore virus-specific immunity in allo-HCT patients. This therapy is created from healthy seropositive donors and can be stored until needed, making it immediately available. In a study of allo-HCT patients with BKV-associated HC, treatment with VSTs led to rapid resolution of gross hematuria and symptomatic improvement with no significant treatment-related toxicities observed. The data from this study suggest that VSTs could provide a safe and effective therapy in the treatment of virus-associated HC in allo-HCT patients [27]. Additional therapies with other mechanisms of action are also being investigated to address the unmet need in the treatment of BKV-associated HC [19].
Our study has several limitations, consistent with any study using administrative claims data. First, clinical outcomes within 1 year of an allo-HCT procedure may also be under-represented due to coding limitations, especially with the ICD-9 coding used prior to 2015. Second, there was no specific code for the identification of virus-associated HC, and hence, an algorithm based on clinical guidance was defined to identify these patients. Because of this, it is likely that the number of patients with virus-associated HC is underestimated in this study. Future assessments of these data may benefit from additional internal validation verifying correct capture of patients with virus-associated HC. Third, longitudinal claims database studies requiring continuous enrollment are often hampered by the transition of patients into and out of US health plans every few years, although the open claims nature of the database used in this study may account for such transitions. Fourth, the claims data do not include information concerning events outside the hospital, such as out-of-hospital deaths. In addition to the limitations of the coding system and the claims database, the total reimbursed amount in our study was estimated through a reimbursement-to-charge ratio of 0.425 derived from ~20% of submitted-remitted overlapping claims in the DRG Real-World Claims Database, which is an estimated reimbursement and not the real reimbursement. Additionally, there were some patients with missing information on the submitted charges for the index allo-HCT hospitalizations, and hence, the total reimbursements of these patients are underestimated.
In addition, in this study, 5.7% of allo-HCT patients were identified as having virus-associated HC. The observed low incidence compared to the higher range reported in the literature may reflect the stringent and focused diagnostic algorithm applied in order to identify allo-HCT patients with a high certainty of virus-associated HC, in addition to the under-reporting of certain diagnoses that is commonly seen in claims databases. While acknowledging these limitations, the strengths of this work lie in the nature of the study as the first of its kind to assess the burden of viral-associated HC in the United States through the use of a comprehensive multivariate modeling system.
CONCLUSIONS
Allo-HCT patients with virus-associated HC incurred significantly greater costs (as measured through health care reimbursements) and increased HRU, when adjusting for additional variables using this multivariate model. Clinical outcomes significantly associated with virus-associated HC in allo-HCT patients included renal impairment and urinary retention, irrespective of the presence or absence of GVHD. The adjusted analyses showed that the total reimbursement and mortality were higher for allo-HCT patients with virus-associated HC, regardless of the presence of GVHD. Our results suggest that improved treatment and prevention strategies for virus-associated HC can potentially reduce the associated costs and resources required for treatment and improve clinical outcomes for allo-HCT patients.
Supplementary Material
ACKNOWLEDGMENTS
This project represents a work for hire by Certara from AlloVir. Medical writing assistance and revision of the manuscript, under the direction of the authors, was provided by PRECISIONscientia and compensated by AlloVir.
Financial disclosure:
This project represents a work for hire by Certara from AlloVir.
DECLARATIONS OF INTEREST
JM reports grants and personal fees from AlloVir, grants and personal fees from Juno Therapeutics, Inc, grants and personal fees from Gilead-Kite Pharmaceuticals, grants and other from Magenta Therapeutics, outside the submitted work. CD reports personal fees from Omeros, personal fees from Novartis, personal fees from Takeda, outside the submitted work. SHM reports other from AlloVir, during the conduct of the study; other from AlloVir, outside the submitted work. AC and ZZ report other support from AlloVir, during the conduct of the study. GP reports grants and personal fees from Merck & Co, grants and personal fees from Astellas Pharma, other from Octapharma, other from AlloVir, personal fees from ADMA biologics, personal fees from Partner Therapeutics, personal fees from Cidara, personal fees from Shionogi, personal fees from Siemens Healthineers, outside the submitted work.
Financial disclosure:
See Acknowledgments on page 505.e9.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jtct.2021.02.021.
REFERENCES
- 1.D’Souza A, Fretham C, Lee SJ, et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2020;26:e177–e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kedia S, Acharya P, Mohammad F, et al. Infectious complications of hematopoietic stem cell transplantation. Stem Cell Res Ther. 2013;S3(002):1–8. [Google Scholar]
- 3.Kodad S SW, Elsayed A, Goubran H, Elemary M. Viral infections in allogeneic hematopoietic stem cell transplant recipients: literature review. OBM Transplant. 2019;3:1–25. [Google Scholar]
- 4.Leen AM, Bollard CM, Mendizabal AM, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121:5113–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Aalderen MC, Heutinck KM, Huisman C, ten Berge IJ. BK virus infection in transplant recipients: clinical manifestations, treatment options and the immune response. Neth J Med. 2012;70:172–183. [PubMed] [Google Scholar]
- 6.Abudayyeh A, Hamdi A, Abdelrahim M, et al. Poor immune reconstitution is associated with symptomatic BK polyomavirus viruria in allogeneic stem cell transplant recipients. Transpl Infect Dis. 2017;1:19. [DOI] [PubMed] [Google Scholar]
- 7.Hill JA, Mayer BT, Xie H, et al. The cumulative burden of double-stranded DNA virus detection after allogeneic HCT is associated with increased mortality. Blood. 2017;129:2316–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YT, Kim SJ, Lee YJ, et al. Co-infections by double-stranded DNA viruses after ex vivo T cell-depleted, CD34(+) selected hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2017;23:1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cesaro S, Dalianis T, Hanssen Rinaldo C, et al. ECIL guidelines for the prevention, diagnosis and treatment of BK polyomavirus-associated haemorrhagic cystitis in haematopoietic stem cell transplant recipients. J Antimicrob Chemother. 2018;73:12–21. [DOI] [PubMed] [Google Scholar]
- 10.Ambalathingal GR, Francis RS, Smyth MJ, Smith C, Khanna R. BK polyomavirus: clinical aspects, immune regulation, and emerging therapies. Clin Microbiol Rev. 2017;30:503–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Espada E, Cheng MP, Kim HT, et al. BK virus-specific T-cell immune reconstitution after allogeneic hematopoietic cell transplantation. Blood Adv. 2020;4:1881–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gargiulo G, Orlando L, Alberani F, et al. Haemorrhagic cystitis in haematopoietic stem cell transplantation (HSCT): a prospective observational study of incidence and management in HSCT centres within the GITMO network (Gruppo Italiano Trapianto Midollo Osseo). Ecancermedicalscience. 2014;8:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilis L, Morisset S, Billaud G, et al. High burden of BK virus-associated hemorrhagic cystitis in patients undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2014;49:664–670. [DOI] [PubMed] [Google Scholar]
- 14.Imlay H, Xie H, Leisenring WM, et al. Presentation of BK polyomavirus-associated hemorrhagic cystitis after allogeneic hematopoietic cell transplantation. Blood Adv. 2020;4:617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laskin BL, Denburg MR, Furth SL, et al. The natural history of BK polyomavirus and the host immune response after stem cell transplantation. Clin Infect Dis. 2019;71:3044–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han SB, Cho B, Kang JH. BK virus-associated hemorrhagic cystitis after pediatric stem cell transplantation. Korean J Pediatr. 2014;57:514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heath JA, Mishra S, Mitchell S, Waters KD, Tiedemann K. Estrogen as treatment of hemorrhagic cystitis in children and adolescents undergoing bone marrow transplantation. Bone Marrow Transplant. 2006;37:523–526. [DOI] [PubMed] [Google Scholar]
- 18.Lunde LE, Dasaraju S, Cao Q, et al. Hemorrhagic cystitis after allogeneic hematopoietic cell transplantation: risk factors, graft source and survival. Bone Marrow Transplant. 2015;50:1432–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Najafabadi M, Soleimani M, Ahmadvand M, Zomorrod M, Mousavi S. Treatment protocols for BK virus associated hemorrhagic cystitis after hematopoietic stem cell transplantation. Am J Blood Res. 2020;10: 217–230. [PMC free article] [PubMed] [Google Scholar]
- 20.Silva Lde P, Patah PA, Saliba RM, et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplants is the complex result of BK virus infection, preparative regimen intensity and donor type. Haematologica. 2010;95:1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Department of Labor, US Bureau of Labor Statistics. Measuring price change in the CPI: medical care. 2020. Available at: https://www.bls.gov/news.release/cpi.toc.htm. Accessed October 30, 2020.
- 22.Asano Y, Kanda Y, Ogawa N, et al. Male predominance among Japanese adult patients with late-onset hemorrhagic cystitis after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;32:1175–1179. [DOI] [PubMed] [Google Scholar]
- 23.Majhail NS, Mothukuri JM, Brunstein CG, Weisdorf DJ. Costs of hematopoietic cell transplantation: comparison of umbilical cord blood and matched related donor transplantation and the impact of posttransplant complications. Biol Blood Marrow Transplant. 2009;15:564–573. [DOI] [PubMed] [Google Scholar]
- 24.Haines HL, Laskin BL, Goebel J, et al. Blood, and not urine, BK viral load predicts renal outcome in children with hemorrhagic cystitis following hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1512–1519. [DOI] [PubMed] [Google Scholar]
- 25.Kerbauy LN, Kerbauy MN, Bautzer V, et al. Severe hemorrhagic cystitis caused by the BK polyomavirus is associated with decreased survival post-allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2019;21:e13101. [DOI] [PubMed] [Google Scholar]
- 26.Tooker GM, Stafford KA, Nishioka J, Badros AZ, Riedel DJ. Intravesicular cidofovir in the treatment of BK virus-associated hemorrhagic cystitis following hematopoietic stem cell transplantation. Ann Pharmacother. 2020;54:547–553. [DOI] [PubMed] [Google Scholar]
- 27.Tzannou I, Papadopoulou A, Naik S, et al. Off-the-shelf virus-specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, Epstein-Barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J Clin Oncol. 2017;35:3547–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.