Abstract
We previously reported that a combined myo-inositol, probiotics, and enriched micronutrient supplement (intervention) taken preconception and in pregnancy reduced postpartum blood loss (PBL) and major postpartum hemorrhage compared with a standard micronutrient supplement (control), as secondary outcomes of the NiPPeR trial. This study aimed to identify the intervention components that may contribute to this effect. Associations of plasma concentrations of myo-inositol and vitamins B2, B6, B12, and D at preconception (before and after supplementation), early (~7-weeks), and late pregnancy (~28-weeks) with PBL were assessed by multiple linear regression, adjusting for site, ethnicity, preconception BMI, parity, and previous cesarean section. Amongst 583 women, a higher concentration of myo-inositol in early pregnancy was associated with a PBL reduction [βadj −1.26 (95%CI −2.23, −0.29) mL per µmol/L myo-inositol increase, p = 0.011]. Applying this co-efficient to the increase in mean 7-week-myo-inositol concentration of 23.4 µmol/L with the intervention equated to a PBL reduction of 29.5 mL (~8.4% of mean PBL of 350 mL among controls), accounting for 84.3% of the previously reported intervention effect of 35 mL. None of the examined vitamins were associated with PBL. Therefore, myo-inositol may be a key intervention component mediating the PBL reduction. Further work is required to determine the mechanisms involved.
Keywords: postpartum hemorrhage, inositols, B-vitamins, vitamin D, prenatal nutritional supplement
1. Introduction
Postpartum hemorrhage (PPH) remains a leading cause of maternal mortality and morbidity. World Health Organization (WHO) statistics suggest that 60% of maternal deaths in developing countries are attributable to PPH, resulting in more than 100,000 maternal deaths worldwide annually [1]. Uterine atony (impaired uterine contraction) after childbirth is the most common etiology [2]. Risk factors of uterine atony include prolonged labor, previous cesarean section, high parity, multiple pregnancies, and maternal obesity [3].
Although administration of uterotonic agents is an established universal practice to promote uterine contractility post-delivery, they are not completely protective of atonic PPH [4,5]. Thus, there is a need to find complementary approaches acceptable to pregnant women, as part of a road map set out by the World Health Organization [6], to more effectively combat against PPH. One such additional strategy is prenatal nutritional supplementation. There is a further advantage if these are also affordable and easy to assimilate into existing cultures and systems operating in low-to-middle-income settings.
Studies suggest that several micronutrients are involved in promoting blood coagulation and myometrial contractility, and may have a role in reducing PPH. For instance, deficiencies in vitamin D [7,8,9] and zinc [10,11] are associated with impaired uterine contractility, leading to prolonged labor and PPH in human and animal studies. Indeed, antenatal vitamin D supplementation reportedly reduced the risk of severe PPH [12]. However, these findings were inconsistent between trials, and meta-analyses of studies have not found a significant difference in PPH with either vitamin D [13] or zinc supplementation [14]. Meanwhile, supplementation of multiparous dairy cows with folate and vitamin B12 lowered the incidence of labor dystocia [15], suggestive of improved uterine contractility.
Myo-inositol, a carbohydrate naturally present in cells and abundant in fruits, grains, and nuts, is another promising nutritional intervention [16]. Inositol is a component of many signaling pathways (e.g., phosphoinositides, inositol phosphoglycans) [16] and is being trialed in pregnancy for the prevention and treatment of gestational diabetes [17,18]. Studies in pre-clinical models demonstrated that myo-inositol could improve myometrial contractility partly by regulating calcium fluxes [19].
The NiPPeR (Nutritional Intervention Preconception and during Pregnancy to maintain healthy glucosE metabolism and OffspRing health) multi-center double-blinded randomized controlled trial previously reported that, compared with a standard micronutrient supplement, the intervention of a combined myo-inositol, probiotics, and enriched micronutrient supplement reduced postpartum blood loss (PBL) by 35 mL (95% confidence intervals [CI] −70.0, −3.5; 10% of the mean PBL among controls) [20] and the incidence of major PPH (adjusted risk ratio 0.44, 95%CI 0.20, 0.94) as secondary outcomes of the trial [21]. The current study aimed to identify the component(s) in the NiPPeR intervention that mediated the reduction in PBL.
2. Materials and Methods
2.1. Study Design and Participants
Approval was obtained from research ethics committees at each study site: United Kingdom, Singapore, and New Zealand. All participants provided written informed consent. This trial was prospectively registered at ClinicalTrials.gov NCT02509988, and oversight and monitoring were provided by an independent data and safety monitoring committee.
This current study is a secondary analysis of data collected in the NiPPeR trial. We have previously published the trial protocol and reported no difference in the primary outcome of the level of gestational glycemia at 28 weeks’ gestation with intervention compared with the control [21]. Briefly, between 2015 to 2017, the study recruited 1729 women who were planning conception, aged 18–38 years, from the community. Exclusion criteria were pregnant/lactating, assisted conception (apart from taking clomiphene or letrozole alone), serious food allergy, diabetes mellitus, taking hormonal contraception, metformin, systemic steroids, anticonvulsants or treatment for HIV or Hepatitis B or C in the past month. Participants were electronically randomized in a 1:1 ratio to be given a control supplement containing folic acid, iron, calcium, iodine, and β-carotene or an intervention supplement that additionally included myo-inositol (4 g daily), vitamin B2, vitamin B6, vitamin B12, vitamin D, zinc, and probiotics (Lactobacillus rhamnosus and Bifidobacterium animalis sp. Lactis) [22]. Supplements were consumed twice daily from preconception following randomization until delivery. Over 96% of participants achieved the pre-specified threshold of good adherence (>60% supplement intake) from recruitment to delivery [21].
2.2. Data Collection
Data on maternal age, ethnicity, preconception smoking, household income, parity, and history of previous cesarean section were collected from participants upon recruitment by interviewer-administered questionnaires. Pre-pregnancy body mass index (BMI) was calculated from measured pre-pregnancy weight divided by measured height squared (kg/m2). Details on the mode of delivery, total estimated blood loss at delivery, and birthweight (kg) were extracted from medical records. Gestational age at birth (weeks) was derived from a previously published algorithm using a combination of the last menstrual period date, cycle length and regularity, and early pregnancy ultrasound scan [20]. Neonates were defined as large-for-gestational-age (LGA; birthweight > 90th percentile standardized for gestation and sex) and small-for-gestational-age (SGA; birthweight < 10th percentile) using RCPCH 2009 UK-WHO growth charts [23].
2.3. Laboratory Analyses
Plasma obtained at four time points: baseline and after randomization and supplementation commencement at preconception (mostly 23–30 days [range 21–42]; median 28 days [IQR 22, 31]), in early pregnancy (7-weeks; median 7.4 weeks [IQR 7.1, 7.9]), and in late pregnancy (28-weeks; median 27.7 weeks [IQR 27.2, 28.3]), were batch-analyzed. Plasma concentrations of vitamins B2 (riboflavin), B6 (pyridoxal-5-phosphate), and D (25-hydroxyvitamin D3) were analyzed by targeted methods of liquid chromatography with tandem mass spectrometry (LC-MS-MS) while plasma concentrations of vitamin B12 (cobalamin) were measured by a microbiological assay; all at Bevital Laboratory (Bergen, Norway), as previously described [24,25]. A recently developed and validated ultra-high-performance-liquid chromatography tandem mass spectrometry (UHPLC-MS/MS; Neotron, Italy, in collaboration with Nestlé Research, Switzerland) method was used to quantify plasma and baseline urinary myo-inositol and scyllo-inositol concentrations [26].
2.4. Statistical Analysis
Data distribution was assessed through histograms and Kolmogorov–Smirnov tests. All nutrient variables were loge-transformed to achieve approximate normality and standardized across the whole dataset to derive standard deviation (SD) scores before regression analysis. This enabled the strength of the association with blood loss to be compared among different nutrients as well as across collection time points. Estimated blood loss was also loge-transformed for regression analyses. The resulting regression coefficients (beta; 95%CI) represent the % change in mL blood loss per increase of one standard deviation (SD) of loge nutrient concentration. For results of interest, these were then converted to mL per unit concentration of nutrient for reporting using the anti-loge equivalent of mean blood loss of the entire cohort (347.23 mL) and the anti-loge equivalent of one standard deviation of each nutrient. To derive an indicative estimate of blood loss (in mL), which could be ascribed to particular nutrients of interest, the coefficient (in mL per unit concentration of nutrient) was then applied to the mean increase in nutrient concentration with the NiPPeR intervention compared with the control (anti-loge mean nutrient in intervention group minus anti-loge mean nutrient in control group).
For examining each nutrient on a continuum, control and intervention data were analyzed as a combined group. Multiple linear regression modeling was conducted adjusting for (1) study site only (since estimations of blood loss are well-documented to vary between clinical settings) and (2) clinically important factors at recruitment that have prognostic influence on postpartum blood loss based on existing literature and that improved model-fit when assessed by stepwise forward regression: pre-pregnancy BMI (kg/m2), parity (nulliparous, parous), previous cesarean section, and age (year). Further adjustment for ethnicity and household income did not improve model fit. We then performed further analyses by mutually adjusting for the vitamins and myo-inositol components simultaneously within the same model. Potentially different nutrient-PBL associations between various groupings of parity, previous cesarean section, and across BMI, were also investigated, reporting any statistical interactions. To further confirm the identified relationships we conducted Pearson correlation analyses stratified by site, assessing the correlations between each nutrient of interest and residuals of pre-adjusted blood loss for preconception BMI and parity by linear regression.
Sensitivity analyses were performed to examine the robustness of associations. Firstly, we additionally adjusted for inherent variations in baseline inositol processing represented by inositol metabolism (plasma scyllo-inositol:myo-inositol ratio) and urinary excretion (urine:plasma myo-inositol ratio). Secondly, we excluded cases involving obstetric events known to be associated with increased blood loss, namely cesarean section delivery and large-for-gestational-age infants. These models additionally served as exploratory analyses to determine if these factors could potentially lie on the causal pathway.
There was no imputation for missing data. Results were considered statistically significant when the two-tailed probability was <0.05. Statistical analyses were carried out using Stata Software Release 15 (StataCorp, College Station, TX, USA).
3. Results
3.1. Participant Characteristics
Of the 1729 women recruited, 585 conceived and had a singleton live birth between April 2016 to January 2019 (Supplementary Figure S1). Of these, 583 (99.7%) provided peripartum data (290 control and 293 intervention) for this sub-study. The mean age of participants was 30.3 years with an average pre-pregnancy BMI of 23.7 kg/m2. The majority of participants were White Caucasian (59.2%), 79.8% never smoked, and 63.3% were nulliparous. At baseline, participants had a median plasma myo-inositol concentration of 21.9 µmol/L; 7.7%, 1.7%, 10.7%, and 41.9% had low status for vitamins B2, B6, B12, and D, respectively (Table 1).
Table 1.
Baseline characteristics of participants providing data for this study.
| Characteristics | Total (N = 583) | ||
|---|---|---|---|
| Maternal | |||
| Age [years; mean (SD)] | 30.3 (3.3) | ||
| Pre-pregnancy BMI [kg/m2; median (IQR)] | 23.7 (21.3–26.9) | ||
| Ethnicity, n (%) | |||
| White Caucasian | 345 (59.2) | ||
| Chinese | 146 (25.0) | ||
| South Asian (Indian, Pakistani, Bangladeshi) | 30 (5.2) | ||
| Malay | 23 (3.9) | ||
| Other (Mixed, Black, Polynesian) | 39 (6.7) | ||
| Site, n (%) | |||
| UK | 189 (32.4) | ||
| SG | 166 (28.5) | ||
| NZ | 228 (39.1) | ||
| Household income for country, n (%) | |||
| 1st quintile (lowest) | 7 (1.2) | ||
| 2nd quintile | 43 (7.4) | ||
| 3rd quintile | 124 (21.3) | ||
| 4th quintile | 202 (34.7) | ||
| 5th quintile (highest) | 184 (31.6) | ||
| Unavailable | 23 (3.8) | ||
| Preconception smoking, n (%) | |||
| Previous smoker | 93 (16.1) | ||
| Active smoker | 23 (4.0) | ||
| Nulliparous, n (%) | 369 (63.3) | ||
| Previous cesarean (denominator—all parous women), n (%) | 61 (28.5) | ||
| Mode of delivery, n (%) | |||
| Vaginal delivery | 414 (71.0) | ||
| Cesarean section in labor | 94 (16.1) | ||
| Cesarean section without labor | 75 (12.9) | ||
| Neonatal | |||
| Gestational age at birth [weeks; median (IQR)] | 39.4 (38.5–40.3) | ||
| Birthweight [kg; median (IQR)] | 3.3 (3.0–3.7) | ||
| Size at birth 1, n (%) | |||
| LGA > 90th centile | 43 (7.4) | ||
| AGA | 495 (84.9) | ||
| SGA < 10th centile | 45 (7.7) | ||
| Nutrient |
Median concentration at pre-pregnancy baseline (IQR) |
Low status at pre-pregnancy baseline, n (%) |
Low status
definition |
| Vitamin B2 (nmol/L) | 13.0 (8.0–20.9) | 45 (7.7%) | <5 |
| Vitamin B6 (nmol/L) | 60.4 (43.7–97) | 10 (1.7%) | <20 |
| Vitamin B12 (pmol/L) | 352.2 (276.1–435.5) | 62 (10.6%) | <221 |
| Vitamin D (nmol/L) | 53.8 (40.2–68.9) | 244 (41.9%) | <50 |
| Myo-inositol (µmol/L) | 21.9 (19.2–25.5) | NA | NA |
1 The Royal College of Paediatrics and Child Health (RCPCH) growth charts [23]. Abbreviations: AGA, appropriate-for-gestational-age; BMI, body mass index; IQR, interquartile range; LGA, large-for-gestational-age; n, number; NA, not applicable; NZ, New Zealand; SD, standard deviation; SG; Singapore, SGA, small-for-gestational-age; UK, the United Kingdom.
3.2. Associations between Nutrients and Postpartum Blood Loss
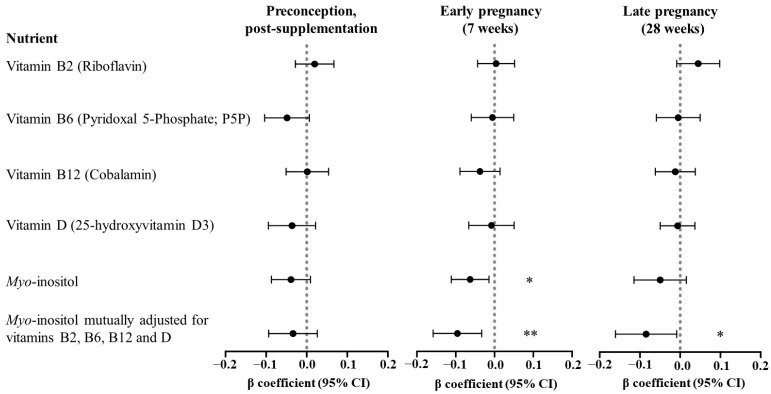
In analyses combining both intervention and control groups, adjusting for site only, higher concentrations of maternal preconception plasma myo-inositol and vitamin B6, and 7-week plasma myo-inositol and vitamin B12 were associated with reduced PBL (Supplementary Figure S2). However, following adjustment for the other covariates only maternal plasma myo-inositol remained associated with reduced PBL (Figure 1): 7-week myo-inositol [βadj −1.26 (95%CI −2.23, −0.29) mL per µmol/L myo-inositol increase, p = 0.011], 28-week myo-inositol [βadj −0.99 (−2.30, 0.31), p = 0.135]. There was a similar trend for myo-inositol measured at preconception post-supplementation [βadj −0.24 (−0.50, 0.03), p = 0.078].
Figure 1.
Associations of maternal plasma concentrations of vitamins and myo-inositol with postpartum blood loss. Plasma was collected at preconception post-supplementation (N = 581), 7 weeks of pregnancy (N = 579), and 28 weeks of pregnancy (N = 579). Loge-transformed and standardized; coefficients expressed as loge mL blood loss per SD loge increase of measured nutrient. Regression models were adjusted for site, preconception BMI, parity, previous cesarean section, and maternal age. Model fit (represented by R2): 7-week myo-inositol (adjusted: 0.206; mutually adjusted for other nutrients: 0.213) and 28-week myo-inositol (adjusted: 0.197; mutually adjusted for other nutrients: 0.207). Statistical significance: * p < 0.05, ** p < 0.01. Abbreviations: CI, confidence interval.
Taking the intervention supplement containing 4 g myo-inositol daily led to a 2-fold (23.4 µmol/L) increase in the mean 7-week myo-inositol concentration compared with control [26]. By applying the 7-week-myo-inositol-associated-PBL coefficient to this plasma concentration increase, a reduction in PBL by 29.5 mL could be predicted. This equates to 8.4% of the average PBL in the control group, which would account for approximately 84.3% of the earlier reported intervention effect of a decrease in PBL by 35 mL compared with the control [20]. The association between 7-week myo-inositol and reduced blood loss was similar in control and intervention groups (interaction term 7-week-myo-inositol*intervention-group, p = 0.839). In contrast, maternal plasma concentrations of vitamins B2, B6, B12, and D at all three time points were not associated with PBL in fully adjusted models.
When we accounted for concentrations of vitamins B2, B6, B12, and D by mutual adjustment in the same regression model as myo-inositol, the association between plasma myo-inositol concentrations at 7 weeks and at 28 weeks with a reduction in PBL became more apparent [7-week-myo-inositol: βadj −1.92 (95%CI −3.17, −0.66) mL per µmol/L, p = 0.003; 28-week-myo-inositol: −1.64 (−3.26, −0.20), p = 0.048] (Figure 1).
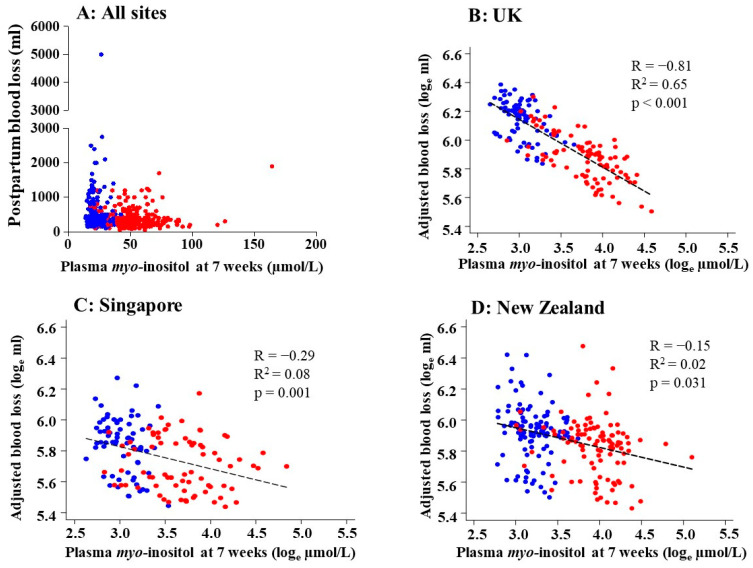
There was a consistent inverse relationship between 7-week myo-inositol and blood loss observed at all three sites, with the strongest correlation observed among UK participants (R −0.81, R2 0.65; p < 0.001) (Figure 2). Inverse associations between 7-week-myo-inositol and PBL were similarly observed across different groupings of parity (interaction-p = 0.63), previous cesarean section (interaction-p = 0.74), and preconception BMI (interaction-p = 0.80).
Figure 2.
Correlations between the maternal plasma concentration of myo-inositol at 7 weeks of gestation and postpartum blood loss. (A) All sites combined showing unadjusted raw data and (B–D) site-stratified plots using loge-transformed plasma myo-inositol data and loge-transformed blood loss adjusted for preconception BMI and parity generated using linear regression. Pearson’s correlation (R), R-squared (R2), and p-values are shown. The participants in the NiPPeR control group are represented by blue dots while those in the NiPPeR intervention group (taking the supplement containing myo-inositol) are represented by red dots. UK: n = 177, Singapore: n = 146, New Zealand: n = 214.
3.3. Sensitivity Analyses
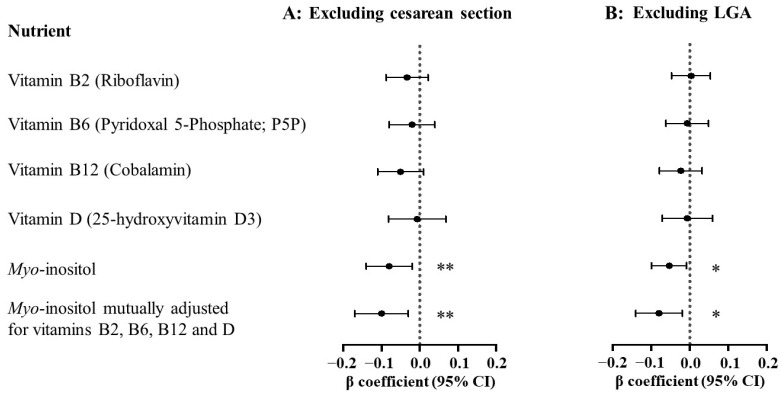
The association between 7-week myo-inositol and PBL reduction was robust in sensitivity analyses. With additional adjustment for individual inherent variations in inositol metabolism and excretion as a potential confounder, 7-week myo-inositol remained associated with PBL reduction, with no change in results [βadj −1.26 (95%CI −2.24, −0.28) mL per µmol/L, p = 0.012]. After excluding cases involving obstetric events that are known risk factors for increased PBL, 7-week-myo-inositol-PBL associations were not substantially altered either: excluding cesarean section delivery (n = 169 excluded) [βadj −1.77 (−2.88, −0.66), p = 0.002] and LGA cases (n = 43 excluded) [βadj −1.18 (−2.15, −0.20), p = 0.018] (Figure 3). Similar trends of association were also observed between 28-week myo-inositol and PBL. There remained no associations between plasma concentrations of vitamins B2, B6, B12, and D measured at any of the three time points and postpartum blood loss, following the exclusion of cesarean section and LGA cases.
Figure 3.
Sensitivity analyses examining associations between maternal plasma concentrations of vitamins and myo-inositol at 7 weeks of gestation with postpartum blood loss after excluding cases of (A) cesarean section delivery (n = 169 excluded), or (B) large-for-gestational-age neonate at birth (n = 43 excluded). Maternal plasma concentrations of vitamins and myo-inositol were loge-transformed and standardized while postpartum blood loss was loge-transformed. Coefficients expressed as % change in mL blood loss per standard deviation (SD) increase in loge nutrient concentration. Regression models were adjusted for site, preconception BMI, parity, previous cesarean section, and maternal age. Statistical significance: * p < 0.05, ** p < 0.01. Abbreviations: CI, confidence interval; LGA, large-for-gestational-age.
4. Discussion
This secondary analysis of the NiPPeR study data suggests that myo-inositol was a key component of the NiPPeR combined nutritional supplement that contributed substantially to the observed reduction in PBL previously reported with the NiPPeR intervention when compared with controls [20,21]. Of note, a higher plasma myo-inositol concentration was associated with reduced PBL across a continuum, even below the 500 mL blood loss threshold defining PPH. Our analyses also indicate that achieving a relatively higher plasma myo-inositol concentration from early pregnancy onward may be an important consideration for reducing PBL. Meanwhile, none of the other vitamins present in the NiPPeR intervention were associated with PBL. The relationship between plasma myo-inositol and reduced PBL remained evident even among cases not complicated by known risk factors for PPH, such as cesarean section deliveries or LGA infants. This finding suggests that the mechanism of the effect of myo-inositol does not necessarily involve the modulation of these particular risk factors. To our knowledge, this is the first report of a link between a higher maternal plasma myo-inositol concentration during pregnancy and reduced PBL.
4.1. Comparisons with Published Literature
Other trials of myo-inositol supplementation in pregnancy did not report on PBL nor PPH outcomes, likely because the data were not collected as they were not pre-specified outcomes. Notably, our results are inconsistent with a vitamin D trial that reported a reduction in major PPH [12]. Our finding of a lack of vitamin D effect on blood loss is, however, concordant with a meta-analysis of vitamin D trials [13] and a more recent vitamin D publication [27]. Furthermore, we previously reported that the NiPPeR intervention supplement did improve the maternal status of vitamins B2, B6, B12, and D during pregnancy leading to a wider range of plasma vitamin concentrations across the cohort [25]; despite this, no associations with PBL were observed. The initially observed links between higher plasma concentrations of preconception (post-supplementation) vitamin B6 and early pregnancy vitamin B12 with PBL reduction in the site-only adjusted models were no longer evident in the fully adjusted models. This finding suggests the strong influence of confounding factors rather than a direct causal effect in these relationships. Such factors may include a lower BMI, which is associated with improved myometrial contractility, and a healthier diet rich in vegetables, fruits, and grains, which can concurrently increase vitamin B6, vitamin B12, and myo-inositol plasma concentrations.
4.2. Clinical Implications
The estimation of a myo-inositol effect of 29.5 mL reduction in blood loss (with the increase in plasma myo-inositol at 7 weeks’ gestation with a 4 g daily supplement) amounts to approximately 84.3% of the mean difference of 35 mL in blood loss between the NiPPeR intervention and control groups that we reported previously [20]. This suggests that myo-inositol may predominantly account for the intervention effect. The potential roles of other components of the NiPPeR supplement, including zinc and probiotics, in regulating postpartum blood loss remain to be assessed. Although the magnitude of myo-inositol-related blood loss reduction of ~8.4% may seem small, its clinical significance must be interpreted in the context of a nutritional supplement study rather than a pharmacological-based intervention. By comparison, the anti-fibrinolytic drug, tranexamic acid, which is being trialed at delivery for PPH prophylaxis, has been estimated to reduce PBL by ~20% in clinical trials [28]. Every element that may reduce blood loss post-delivery would add to the cumulative effect of a constellation of variables, which could have a considerable overall influence.
Despite the NiPPeR study participants being generally healthy women delivering in high-resource settings with advanced medical care and widespread use of prophylactic oxytocin against PPH, it is remarkable that variations in plasma myo-inositol in pregnancy could still be associated with a discernible difference in PBL. Moreover, imprecisions in PBL estimations in healthcare are well-recognized, as is variation in the methods used for such estimations across clinical settings [29]. Yet, this did not obscure our finding of associations with myo-inositol, which was consistent across study sites. Further studies in more diverse populations, including in low-resource settings, are needed to determine if myo-inositol supplementation would have a similar or even more potent effect.
4.3. Postulated Mechanisms of Effect
Given that myo-inositol is a central component of many key players involved in intra- and inter-cellular signaling pathways (e.g., phosphoinositides, inositol phosphates, inositol phosphoglycans), as well as a component of important regulators of plasma membrane function (e.g., phosphatidylinositols), increased myo-inositol bioavailability through supplementation could influence many biological processes. There are several potential mechanisms by which myo-inositol may reduce PBL. A study on myometrial tissue from non-pregnant rats showed that inositol can promote uterine contractility through the regulation of calcium influxes [19]. Indeed, within the NiPPeR trial, we observed that participants who received the myo-inositol-containing NiPPeR intervention had a shorter second stage of labor duration and reduced operative delivery risk for delayed second stage of labor progress [20], events that are hugely influenced by uterine contractility. Enhanced uterine contractility may also promote more efficient occlusion of the uterine blood vessels once the placenta is detached from the uterine wall and prevent excessive PBL through these vessels.
If the early pregnancy concentration of myo-inositol is indeed most influential on PBL, as suggested by our findings, then underlying mechanisms may also include effects on early placental development. Deep trophoblastic invasion into the maternal uterine wall and blood vessels takes place in the first half of gestation, with critical transformation of maternal spiral arteries, facilitating blood flow through the placental bed to ensure adequate maternal-fetal exchange [30]. This is a tightly regulated process involving multiple hormonal, inflammatory, and metabolic elements, thus, it is conceivable that the facilitation of myo-inositol-related second messenger signals may optimize this entire process, with later consequences on PBL. Another mechanism by which myo-inositol may reduce PBL could be through effects on the maternal coagulation system by priming the clotting function of platelets. Inositol can be derivatized to form inositol pyrophosphate, which is important for the generation of polyphosphates that are released by activated platelets to promote clotting by enhancing the clotting cascade and strengthening the fibrin clot structure [31,32].
4.4. Strengths and Weaknesses
An advance over previous studies is our longitudinal measures of maternal plasma myo-inositol, vitamins B2, B6, B12, and D at preconception, and in early and late pregnancy. This approach may allow the identification of the periods of greatest potential impact of nutrient levels on PBL. Nonetheless, our resolution remains limited since nutrients were not measured between 7 weeks’ and 28 weeks’ gestation, nor later in pregnancy, as such, we cannot more precisely define the optimal gestational window for the achievement of a high myo-inositol level. Moreover, the preconception collection took place between weeks and up to a year before pregnancy and the actual periconception levels may have differed from those measured at our preconception study visit time point. Further studies evaluating the optimal timing and duration for myo-inositol supplementation and defining the ideal concentration range to achieve across pregnancy in order to impact PBL and other pregnancy outcomes are needed to fully unravel the therapeutic potential of myo-inositol. For example, the potential benefit of early myo-inositol supplementation on PBL reduction needs to be weighed against the potential side-effect of a higher early pregnancy myo-inositol marginally increasing post-prandial glycemia later in gestation [26]. Additionally, this exploratory study, even though conducted within the context of a randomized controlled trial, reported on adjusted associations between plasma myo-inositol and PBL reduction, which may not have accounted for yet-to-be-discovered confounders. The study may, therefore, have over- or under-estimated the effect of myo-inositol. Mechanistic studies and a new clinical trial of prenatal myo-inositol supplementation with PBL as the primary outcome would be needed to establish causation. Evidence from such studies would inform whether public health policies should be implemented to promote higher dietary intakes of myo-inositol-rich foods or myo-inositol supplementation from early pregnancy for the purposes of postpartum blood loss reduction.
5. Conclusions
In summary, our study demonstrates that a higher plasma concentration of myo-inositol, particularly in early pregnancy, is associated with reduced blood loss post-delivery. This may indicate the potential for myo-inositol as a nutrition-based approach to be incorporated into routine care to reduce the risk of PPH and, consequently, further lower maternal morbidity and mortality globally.
Acknowledgments
We thank the participants and their families for their enthusiastic involvement in the study; the study research staff and hospital clinical staff at participating centers, and operational support staff for their contributions to the trial; and the members of the Independent Data Monitoring and Safety Committee for their invaluable contributions and for overseeing the conduct of the trial. The NiPPeR Study Group authors for the Medline citation comprise the following: Aristea Binia, Mary Cavanagh, Ling Wei Chen, Mary F. Chong, Yap Seng Chong, Paula Costello, Vanessa Cox, Judith Hammond, Mrunalini Jagtap, Timothy Kenealy, See Ling Loy, Gernalia Satianegara, Karen M. L. Tan, Vicky Tay, Elizabeth Tham, Mark H. Vickers, Oliver C. Watkins, Gladys Woon.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16132054/s1, Figure S1: participant flow chart; Figure S2: Associations of plasma concentrations of vitamins and myo-inositol with postpartum blood loss adjusting for site only.
Author Contributions
W.S.C., K.M.G. and S.-Y.C. conceptualized, designed, conducted, obtained resources, and supervised the entire NiPPeR study. H.F.C., H.E.J.Y., H.Z. and S.-Y.C. performed the formal analysis for this substudy. J.-T.W., S.J.B., P.T., B.B.A., H.N., S.E.-H., J.O., L.L., J.M.R.-N., J.-P.G. and I.S.-Z. contributed to the methodology, investigation, validation, data curation, and project administration. H.F.C., H.E.J.Y. and S.-Y.C. prepared the original draft, and all authors reviewed and edited the paper for intellectual content. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Our trial was approved by the United Kingdom, Singapore, and New Zealand research ethics services at each site; Southampton: Health Research Authority NRES Committee South Central Research Ethics Committee (REC) reference 15/SC/0142, date of approval 22 April 2015. Singapore: the National Healthcare Group Domain Specific Review Board (NHG DSRB) Singapore reference 2015/00205, date of approval 11 June 2015. New Zealand: the Health and Disability Ethics Committee (HDEC) New Zealand reference 15/NTA/21), date of approval 30 June 2015. The trial was registered on 16 July 2015 at https://www.clinicaltrials.gov/ct2/show/NCT02509988.
Informed Consent Statement
Written informed consent was obtained from all participants involved in the study.
Data Availability Statement
Individual participant data may be shared with an appropriately qualified individual working in an appropriate institution where an institutional signatory can confirm the recipient’s adherence to relevant information safeguards stipulated in a formal Data Transfer Agreement. Reasonable requests can be made through Professor Nicholas Harvey (nch@mrc.soton.ac.uk), as Director of the MRC Lifecourse Epidemiology Centre.
Conflicts of Interest
S.-Y.C., W.S.C. and K.M.G. are part of an academic consortium that report grants from Société Des Produits Nestlé S.A. during the conduct of the study and are co-inventors on patent filings by Nestlé S.A. relating to the NiPPeR intervention or its components. S.-Y.C. has received reimbursement and honoraria into her research funds from Nestlé Nutrition Institute and EGOI (Experts Group on Inositols) for speaking at conferences. K.M.G. has received reimbursement for speaking at conferences sponsored by companies selling nutritional products. L.L., J.M.R.-N., J.-P.G. and I.S.-Z. are employees of Société Des Produits Nestlé S.A. All other authors declare no competing interests.
Funding Statement
Public good funding for this investigator-led study is through the UK Medical Research Council (as part of an MRC award to the MRC Lifecourse Epidemiology Unit (MC_UU_12011/4)); the Singapore National Research Foundation, National Medical Research Council (NMRC, NMRC/TCR/012-NUHS/2014); the National University of Singapore (NUS) and the Agency of Science, Technology and Research (as part of the Growth, Development and Metabolism Programme of the Singapore Institute for Clinical Sciences (SICS) (H17/01/a0/005); and as part of Gravida, a New Zealand Government Centre of Research Excellence. Funding for the provision of the intervention and control drinks and to cover aspects of the fieldwork for the study has been provided by Société Des Produits Nestlé S.A under a Research Agreement with the University of Southampton, Auckland UniServices Ltd., SICS, National University Hospital Singapore PTE Ltd. and NUS. KMG is supported by the National Institute for Health Research (NIHR Senior Investigator (NF-SI-0515-10042), NIHR Southampton 1000DaysPlus Global Nutrition Research Group (17/63/154), and NIHR Southampton Biomedical Research Center (IS-BRC-1215-20004)), British Heart Foundation (RG/15/17/3174) and the European Union (Erasmus+ Programme ImpENSA 598488-EPP-1-2018-1-DE-EPPKA2-CBHE-JP). SYC is supported by a Singapore NMRC Clinician Scientist Award (NMRC/CSA-INV/0010/2016; MOH-CSAINV19nov-0002).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ford J.B., Patterson J.A., Seeho S.K., Roberts C.L. Trends and outcomes of postpartum haemorrhage, 2003–2011. BMC Pregnancy Childbirth. 2015;15:334. doi: 10.1186/s12884-015-0788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill P., Patel A., Van Hook J.W. StatPearls. StatPearls Publishing LLC.; Treasure Island, FL, USA: 2022. Uterine Atony. [PubMed] [Google Scholar]
- 3.Kramer M.S., Dahhou M., Vallerand D., Liston R., Joseph K.S. Risk factors for postpartum hemorrhage: Can we explain the recent temporal increase? J. Obstet. Gynaecol. Can. 2011;33:810–819. doi: 10.1016/S1701-2163(16)34984-2. [DOI] [PubMed] [Google Scholar]
- 4.Parry Smith W.R., Papadopoulou A., Thomas E., Tobias A., Price M.J., Meher S., Alfirevic Z., Weeks A.D., Hofmeyr G.J., Gulmezoglu A.M., et al. Uterotonic agents for first-line treatment of postpartum haemorrhage: A network meta-analysis. Cochrane Database Syst. Rev. 2020;11:CD012754. doi: 10.1002/14651858.CD012754.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prata N., Bell S., Weidert K. Prevention of postpartum hemorrhage in low-resource settings: Current perspectives. Int. J. Womens Health. 2013;5:737–752. doi: 10.2147/IJWH.S51661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . A Roadmap to Combat Postpartum Haemorrhage between 2023 and 2030. World Health Organization; Geneva, Switzerland: 2023. [Google Scholar]
- 7.Li W.J., Chen K.H., Huang L.W., Tsai Y.L., Seow K.M. Low Maternal Serum 25-Hydroxyvitamin D Concentration Is Associated With Postpartum Hemorrhage: A Retrospective Observational Study. Front. Endocrinol. 2022;13:816480. doi: 10.3389/fendo.2022.816480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomsen C.R., Milidou I., Hvidman L., Khalil M.R., Rejnmark L., Uldbjerg N. Vitamin D and the risk of dystocia: A case-control study. PLoS ONE. 2020;15:e0240406. doi: 10.1371/journal.pone.0240406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tous M., Villalobos M., Iglesias L., Fernandez-Barres S., Arija V. Vitamin D status during pregnancy and offspring outcomes: A systematic review and meta-analysis of observational studies. Eur. J. Clin. Nutr. 2020;74:36–53. doi: 10.1038/s41430-018-0373-x. [DOI] [PubMed] [Google Scholar]
- 10.Dura Trave T., Puig Abuli M., da Cunha Ferreira R.M., Villa Elizaga I. Effect of zinc nutrition on parturition and postpartum in the rat. Gynecol. Obstet. Investig. 1984;18:275–280. doi: 10.1159/000299093. [DOI] [PubMed] [Google Scholar]
- 11.Lazebnik N., Kuhnert B.R., Kuhnert P.M., Thompson K.L. Zinc status, pregnancy complications, and labor abnormalities. Am. J. Obstet. Gynecol. 1988;158:161–166. doi: 10.1016/0002-9378(88)90802-2. [DOI] [PubMed] [Google Scholar]
- 12.Cooper C., Harvey N.C., Bishop N.J., Kennedy S., Papageorghiou A.T., Schoenmakers I., Fraser R., Gandhi S.V., Carr A., D’Angelo S., et al. Maternal gestational vitamin D supplementation and offspring bone health (MAVIDOS): A multicentre, double-blind, randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;4:393–402. doi: 10.1016/S2213-8587(16)00044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palacios C., Kostiuk L.K., Pena-Rosas J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019;7:CD008873. doi: 10.1002/14651858.CD008873.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carducci B., Keats E.C., Bhutta Z.A. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst. Rev. 2021;3:CD000230. doi: 10.1002/14651858.CD000230.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duplessis M., Girard C.L., Santschi D.E., Laforest J.P., Durocher J., Pellerin D. Effects of folic acid and vitamin B12 supplementation on culling rate, diseases, and reproduction in commercial dairy herds. J. Dairy Sci. 2014;97:2346–2354. doi: 10.3168/jds.2013-7369. [DOI] [PubMed] [Google Scholar]
- 16.Watkins O.C., Yong H.E.J., Sharma N., Chan S.Y. A review of the role of inositols in conditions of insulin dysregulation and in uncomplicated and pathological pregnancy. Crit. Rev. Food Sci. Nutr. 2022;62:1626–1673. doi: 10.1080/10408398.2020.1845604. [DOI] [PubMed] [Google Scholar]
- 17.Li C., Shi H. Inositol supplementation for the prevention and treatment of gestational diabetes mellitus: A meta-analysis of randomized controlled trials. Arch. Gynecol. Obstet. 2023;309:1959–1969. doi: 10.1007/s00404-023-07100-x. [DOI] [PubMed] [Google Scholar]
- 18.Motuhifonua S.K., Lin L., Alsweiler J., Crawford T.J., Crowther C.A. Antenatal dietary supplementation with myo-inositol for preventing gestational diabetes. Cochrane Database Syst. Rev. 2023;2:CD011507. doi: 10.1002/14651858.CD011507.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kavak E.Ç., Kacar E., Kavak S.B., Bulmus O., Serhatlioglu I., Tektemur A. Myoinositol Causes Myometrial Contractions in Isolated Non-Pregnant Rat Myometrium. East. J. Med. 2018;23:65–70. doi: 10.5505/ejm.2018.05925. [DOI] [Google Scholar]
- 20.Chan S.Y., Yong H.E.J., Chang H.F., Barton S.J., Galani S., Zhang H., Wong J.T., Ong J., Ebreo M., El-Heis S., et al. Peripartum outcomes after combined myo-inositol, probiotics, and micronutrient supplementation from preconception: The NiPPeR randomized controlled trial. Am. J. Obstet. Gynecol. MFM. 2022;4:100714. doi: 10.1016/j.ajogmf.2022.100714. [DOI] [PubMed] [Google Scholar]
- 21.Godfrey K.M., Barton S.J., El-Heis S., Kenealy T., Nield H., Baker P.N., Chong Y.S., Cutfield W., Chan S.Y., Ni P.S.G. Myo-Inositol, Probiotics, and Micronutrient Supplementation From Preconception for Glycemia in Pregnancy: NiPPeR International Multicenter Double-Blind Randomized Controlled Trial. Diabetes Care. 2021;44:1091–1099. doi: 10.2337/dc20-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godfrey K.M., Cutfield W., Chan S.Y., Baker P.N., Chong Y.S., Ni P.S.G. Nutritional Intervention Preconception and During Pregnancy to Maintain Healthy Glucose Metabolism and Offspring Health (“NiPPeR”): Study protocol for a randomised controlled trial. Trials. 2017;18:131. doi: 10.1186/s13063-017-1875-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole T.J., Williams A.F., Wright C.M., Group R.G.C.E. Revised birth centiles for weight, length and head circumference in the UK-WHO growth charts. Ann. Hum. Biol. 2011;38:7–11. doi: 10.3109/03014460.2011.544139. [DOI] [PubMed] [Google Scholar]
- 24.Midttun O., Hustad S., Ueland P.M. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass. Spectrom. 2009;23:1371–1379. doi: 10.1002/rcm.4013. [DOI] [PubMed] [Google Scholar]
- 25.Godfrey K.M., Titcombe P., El-Heis S., Albert B.B., Tham E.H., Barton S.J., Kenealy T., Chong M.F., Nield H., Chong Y.S., et al. Maternal B-vitamin and vitamin D status before, during, and after pregnancy and the influence of supplementation preconception and during pregnancy: Prespecified secondary analysis of the NiPPeR double-blind randomized controlled trial. PLoS Med. 2023;20:e1004260. doi: 10.1371/journal.pmed.1004260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan S.Y., Zhang H., Wong J.T., Chang H.F., Chen L.W., Barton S.J., Nield H., El-Heis S., Kenealy T., Lavalle L., et al. Higher early pregnancy plasma myo-inositol associates with increased postprandial glycaemia later in pregnancy: Secondary analyses of the NiPPeR randomized controlled trial. Diabetes Obes. Metab. 2024;26:1658–1669. doi: 10.1111/dom.15468. [DOI] [PubMed] [Google Scholar]
- 27.Moon R.J., D’Angelo S., Crozier S.R., Curtis E.M., Fernandes M., Kermack A.J., Davies J.H., Godfrey K.M., Bishop N.J., Kennedy S.H., et al. Does antenatal cholecalciferol supplementation affect the mode or timing of delivery? Post hoc analyses of the MAVIDOS randomized controlled trial. J. Public Health. 2023;45:738–747. doi: 10.1093/pubmed/fdac160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S.H., Kwek M.E., Tagore S., Wright A., Ku C.W., Teong A.C.A., Tan A.W.M., Lim S.W.C., Yen D.Y.T., Ang C.Y.X., et al. Tranexamic acid, as an adjunct to oxytocin prophylaxis, in the prevention of postpartum haemorrhage in women undergoing elective caesarean section: A single-centre double-blind randomised controlled trial. BJOG. 2023;130:1007–1015. doi: 10.1111/1471-0528.17445. [DOI] [PubMed] [Google Scholar]
- 29.Calvert C., Thomas S.L., Ronsmans C., Wagner K.S., Adler A.J., Filippi V. Identifying regional variation in the prevalence of postpartum haemorrhage: A systematic review and meta-analysis. PLoS ONE. 2012;7:e41114. doi: 10.1371/journal.pone.0041114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L., Liu J., Feng X., Lash G.E. Unraveling the mysteries of spiral artery remodeling. Placenta. 2023;141:51–56. doi: 10.1016/j.placenta.2023.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh S., Shukla D., Suman K., Lakshmi B.J., Manorama R., Kumar S., Bhandari R. Inositol hexakisphosphate kinase 1 maintains hemostasis in mice by regulating platelet polyphosphate levels. Blood. 2013;122:1478–1486. doi: 10.1182/blood-2013-01-481549. [DOI] [PubMed] [Google Scholar]
- 32.Morrissey J.H. One inositol ring to rule thrombosis. Blood. 2013;122:1331–1332. doi: 10.1182/blood-2013-07-511006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data may be shared with an appropriately qualified individual working in an appropriate institution where an institutional signatory can confirm the recipient’s adherence to relevant information safeguards stipulated in a formal Data Transfer Agreement. Reasonable requests can be made through Professor Nicholas Harvey (nch@mrc.soton.ac.uk), as Director of the MRC Lifecourse Epidemiology Centre.