Abstract
The confused flour beetle, Tribolium confusum du Val, is one of the cosmopolitan and polyphagous storage insect pests. The frequent application of chemical insecticides has resulted in several side effects, including threats to human health and non-target organisms and the resistance of insect pests. In the current study, the fumigant toxicity and feeding deterrence potential of Artemisia fragrans Willd. essential oil on T. confusum adults were investigated. The essential oil was rich in terpenic compounds, in which α-thujone (27.8%) and 1,8-cineole (22.8%) were dominant. The essential oil displayed significant fumigant toxicity on T. confusum, where a concentration of 35.3 μL/L caused 100% mortality of the treated adults after 48 h. The LC30 and LC40 values (lethal concentrations to kill 30% and 40% of tested insects: 15.1 and 18.4 μL/L, respectively) significantly decreased the nutritional indices of the pest, including the consumption index, relative consumption rate, and relative growth rate. The feeding deterrence index of the essential oil were calculated as being 62.29 and 48.66% for the concentrations of 15.1 and 18.4 μL/L after 5 days, respectively. Accordingly, A. fragrans essential oil can be considered an efficient, available, and natural alternative to detrimental chemical pesticides in the management of T. confusum.
Keywords: chemical composition, essential oil, feeding deterrence, fumigant toxicity, wormwood
1. Introduction
The confused flour beetle, Tribolium confusum du Val (Coleoptera: Tenebrionidae), is a cosmopolitan and polyphagous insect pest of stored products, including cereal, legumes, flour, pasta, chocolate, dried fruits, and animal collections [1]. Along with direct damage caused by feeding, contamination of stored products with feces and shells of different stages of the pest leads to significant indirect damage [1]. Although the utilization of chemical insecticides is the main method used to manage such an insect pest, their frequent application results in numerous side effects, such as environmental pollution, threats to human health and non-target organisms, and the development of pest resistance [2,3]. Therefore, it is necessary to introduce efficient and low-risk agents in the management of insect pests.
The search for green strategies as a valid alternative to synthetic chemical pesticides has been active for many decades in the agricultural, pharmaceutical, food, and cosmetic industries [4,5,6], as well as in the conservation of cultural and landscape heritage [7,8,9]. Plant-derived essential oils are a complex mixture of active and volatile compounds, which are usually seen as aromatic and aliphatic compounds [10]. Terpenes, such as monoterpenes, sesquiterpenes, and diterpenes, are the main group of essential oil composition. In other words, monoterpenes and monoterpenoids (oxygenated monoterpenes), such as α-pinene and 1,8-cineole, are the dominant components of most essential oils [11]. Previous studies revealed that the essential oils isolated from several aromatic plants can be used as bio-pesticides against different groups of insect pests [12,13,14]. According to the findings of recent studies, T. confusum is sensitive to plant-derived essential oils. For example, the toxicity of Artemisia dracunculus L., Ocimum basilicum L., and Rosmarinus officinalis L. essential oils against the adults of T. confusum was demonstrated [15], in which the exposure of insects to a concentration of 153.8 μL/L of essential oils resulted in 93.3%, 98.3%, and 98.3% mortalities after 96 h, respectively. The adults of T. confusum were also susceptible to the essential oils of Ricinus communis L., Eucalyptus globulus Labill, and Eruca sativa Mill., and the 24 h LC50 values (lethal concentration to kill 50% of tested insects after 24 h) of the essential oils were 25.3, 33.5, and 38.0 mg/L, respectively [16]. The fumigant toxicity of the essential oil of Myrtus communis L. with a 24 h LC50 value of 247.0 μL/L was also reported against the adults of T. confusum [17]. Along with lethal effects, the essential oils can show sub-lethal activities against insect pests. For instance, the essential oil of R. officinalis displayed lethal fumigant toxicity and sub-lethal antifeedant effects against the adults of T. confusum [18].
There are about 500 aromatic species in the Artemisia L. genus, which is the most prominent member of the Asteraceae family, known as wormwood, and widely distributed throughout the world [19]. Artemisia fragrans Willd. has aromatic leaves and flowers and is about 45 cm tall. The leaves of this aromatic plant tend to be white at first due to the presence of numerous trichomes, which are lost as the plant continues to grow [20]. A. fragrans has spread widely in Iran and is one of the dominant plants in the north of Ardabil province, especially in the rangelands of the Mughan region [21]. Although aliphatic compounds can be detected, the essential oil of A. fragrans contains various terpenic compounds from hydrocarbon monoterpenes and monoterpenoids to sesquiterpenes [22]. The terpenes 1,8-cineole, α-thujone, α-pinene, β-pinene, camphor, and camphene are among the dominant compounds in A. fragrans essential oil [23,24,25]. Different biological effects of the essential oil of A. fragrans, including antioxidant, antibacterial, antimalarial, antileishmanial, and even herbicidal activities, were reported in previous studies [25,26,27,28].
Although the possibility of insect pest management by essential oils extracted from several species of Artemisia genus has been reported in recent studies [29,30,31], the insecticidal potential of A. fragrans essential oil has not been investigated yet. Accordingly, the fumigant toxicity of the essential oil extracted from the aerial parts of A. fragrans as an available natural agent was studied against T. confusum. In addition to the acute toxicity, the nutritional indices of the pest treated with essential oil were also investigated. The chemical composition of the essential oil was analyzed, and the relationship between the identified components and the pesticidal properties of the essential oil was discussed.
2. Results
2.1. Chemical Analysis of Essential Oil
The A. fragrans essential oil was analyzed by GC-MS (gas chromatography–mass spectrometry). A total of 54 compounds were identified in the essential oil, which accounted for 92.7% of the total composition (Table 1). The essential oil was dominated by two oxygenated monoterpenoids, 1,8-cineole (22.8%) and α-thujone (27.8%). The yield of essential oil extraction was 1.23 ± 0.14 (w/w).
Table 1.
Chemical analysis of the essential oil isolated from the aerial parts of Artemisia fragrans Willd.
| RIcalc | RIdb | Compounds | % | RIcalc | RIdb | Compounds | % |
|---|---|---|---|---|---|---|---|
| 844 | 847 | (Z)-Salvene | 0.3 | 1237 | 1238 | Cuminal | 1.2 |
| 929 | 932 | α-Pinene | 2.0 | 1242 | 1239 | Carvone | 0.8 |
| 945 | 946 | Camphene | 1.9 | 1244 | 1244 | Carvotanacetone | 0.2 |
| 969 | 969 | Sabinene | tr | 1249 | 1249 | Piperitone | 0.8 |
| 978 | 974 | β-Pinene | 0.3 | 1251 | 1255 | Carvenone | 0.2 |
| 985 | 979 | Octan-3-one | 0.1 | 1254 | 1253 | trans-Sabinene-hydrate acetate | 0.3 |
| 991 | 988 | Myrcene | 0.6 | 1283 | 1287 | Bornyl acetate | 1.0 |
| 1029 | 1025 | p-Cymene | 1.3 | 1294 | 1289 | p-Cymen-7-ol | 3.5 |
| 1034 | 1026 | 1,8-Cineole | 22.8 | 1298 | 1299 | Terpin-1-en-4-yl acetate | 0.4 |
| 1053 | 1054 | γ-Terpinene | 0.4 | 1304 | 1298 | Carvacrol | 1.4 |
| 1100 | 1101 | α-Thujone | 27.8 | 1315 | 1316 | δ-Terpinyl acetate | 0.4 |
| 1118 | 1112 | β-Thujone | 2.8 | 1322 | 1324 | Myrtenyl acetate | 0.2 |
| 1122 | 1118 | cis-p-Menth-2-en-1-ol | 0.8 | 1345 | 1346 | α-Terpinyl acetate | 0.4 |
| 1134 | 1139 | Camphor | 1.1 | 1352 | 1356 | Eugenol | 0.2 |
| 1136 | 1136 | trans-p-Menth-2-en-1-ol | 2.3 | 1380 | 1376 | Methyl (E)-cinnamate | 0.6 |
| 1140 | 1140 | trans-Verbenol | 1.5 | 1398 | 1392 | (Z)-Jasmone | 0.8 |
| 1150 | 1154 | Sabina ketone | 0.4 | 1416 | 1417 | (E)-β-Caryophyllene | 0.7 |
| 1154 | 1160 | Pinocarvone | 0.7 | 1445 | 1454 | Geranyl acetone | 0.1 |
| 1159 | 1155 | iso-Borneol | 1.3 | 1449 | 1452 | α-Humulene | 0.1 |
| 1166 | 1165 | Borneol | 2.7 | 1483 | na | p Menthane-1,2,4-triol | 0.2 |
| 1178 | 1174 | Terpinen-4-ol | 2.9 | 1574 | 1574 | γ-Undecalactone | 0.2 |
| 1182 | 1179 | p-Methylacetophenone | 0.3 | 1583 | 1577 | Spathulenol | 1.0 |
| 1185 | 1183 | Cryptone | 0.3 | 1587 | 1582 | Caryophyllene oxide | 0.6 |
| 1191 | 1185 | p-Cymen-8-ol | 1.0 | 1662 | 1668 | 14-Hydroxy-9-epi-(E)-Caryophyllene | 0.2 |
| 1195 | 1186 | α-Terpineol | 0.9 | Monoterpene hydrocarbons | 4.5 | ||
| 1198 | 1195 | cis-Piperitol | 1.0 | Oxygenated monoterpenoids | 83.8 | ||
| 1202 | 1194 | Myrtenol | 0.5 | Sesquiterpene hydrocarbons | 0.9 | ||
| 1220 | 1207 | trans-Piperitol | 1.3 | Oxygenated sesquiterpenoids | 1.6 | ||
| 1223 | 1204 | Verbenone | 0.5 | Benzenoid aromatics | 1.2 | ||
| 1234 | 1227 | p-Cumenol | 0.3 | Others | 1.7 | ||
| Total identified | 92.7 |
RIcalc—retention index calculated with respect to a homologous series of n-alkanes on an HP-5ms column. RIdb—reference retention indices from the databases. tr—trace (<0.05%). na—reference retention index not available.
2.2. Fumigant Toxicity of Essential Oil
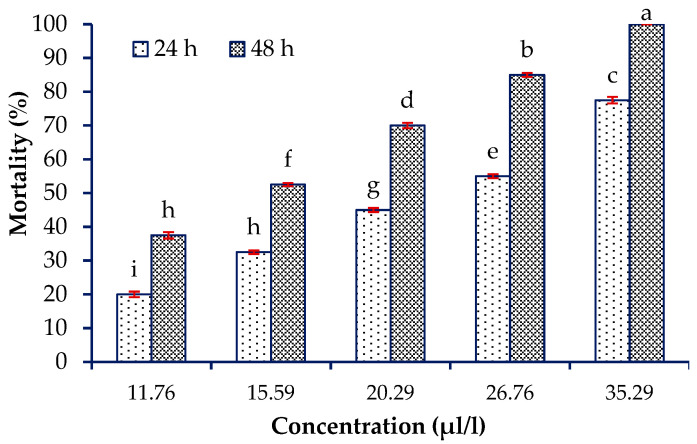
The data obtained from the fumigant toxicity of the A. fragrans essential oil on the adults of T. confusum had a normal distribution according to the Kolmogorov–Smirnov test (Z = 0.56 and sig. (two-tailed) = 0.91). There was no mortality in the control group after 24 and 48 h exposure times. The effects of different concentrations of the A. fragrans essential oil (F = 93.62; df = 4, 30; p < 0.001) and 24 and 48 h exposure times (F = 113.36; df = 1, 30; p < 0.001) were significant to the mortality of the insect pests. However, the interaction effect of the essential oil concentration and exposure time to the pest was not significant (F = 0.99; df = 4, 30; p = 0.427). The Artemisia fragrans essential oil had a high fumigant toxicity on the T. confusum, where the concentration of 35.3 µL/L from the essential oil caused 100% mortality of the treated adults after 48 h (Figure 1).
Figure 1.
Mean mortality percentage (±SE) of the Tribolium confusum du Val exposed to different concentrations of Artemisia fragrans Willd. essential oil after 24 and 48 h. Different letters display significant differences between the corresponding means.
According to the results of the Probit analysis shown in Table 2, the LC50 value of the A. fragrans essential oil was decreased from 22.13 μL/L at 24 h of exposure time to 14.70 μL/L after 48 h. The LC30 and LC40 values were used to evaluate the antinutritional effects of the essential oil. The values of the correlation coefficients (0.98 and 0.99 for 24 and 48 h exposure times) indicate a positive and direct relationship between the pest mortality and the essential oil concentrations.
Table 2.
Probit analysis of the mortality of Tribolium confusum du Val adults treated by Artemisia fragrans Willd. essential oil after 24 and 48 h of exposure time.
| Time (h) |
Lethal Concentrations with 95% Confidence Limits (μL/L) | Intercept | Slope | χ2 (df = 3) |
Sig.* | R2 | |||
|---|---|---|---|---|---|---|---|---|---|
| LC30 | LC40 | LC50 | LC90 | ||||||
| 24 | 15.09 (13.23–16.61) |
18.40 (16.69–20.02) |
22.13 (20.34–24.27) |
56.42 (46.14–76.97) |
−4.24 | 3.15 | 1.91 | 0.59 | 0.98 |
| 48 | 11.30 (9.92–12.42) |
12.94 (11.69–14.01) |
14.70 (13.52–15.74) |
27.96 (25.47–31.73) |
−5.36 | 4.59 | 7.33 | 0.06 | 0.99 |
* Since the significance level was greater than 0.05, no heterogeneity factor was used in the calculation of the confidence limits. LC30, LC40, LC50, and LC90 values are the lethal concentrations to kill 30, 40, 50, and 90% of tested insects, respectively. Sig.: significant.
2.3. Antifeedant Effects of Essential Oil
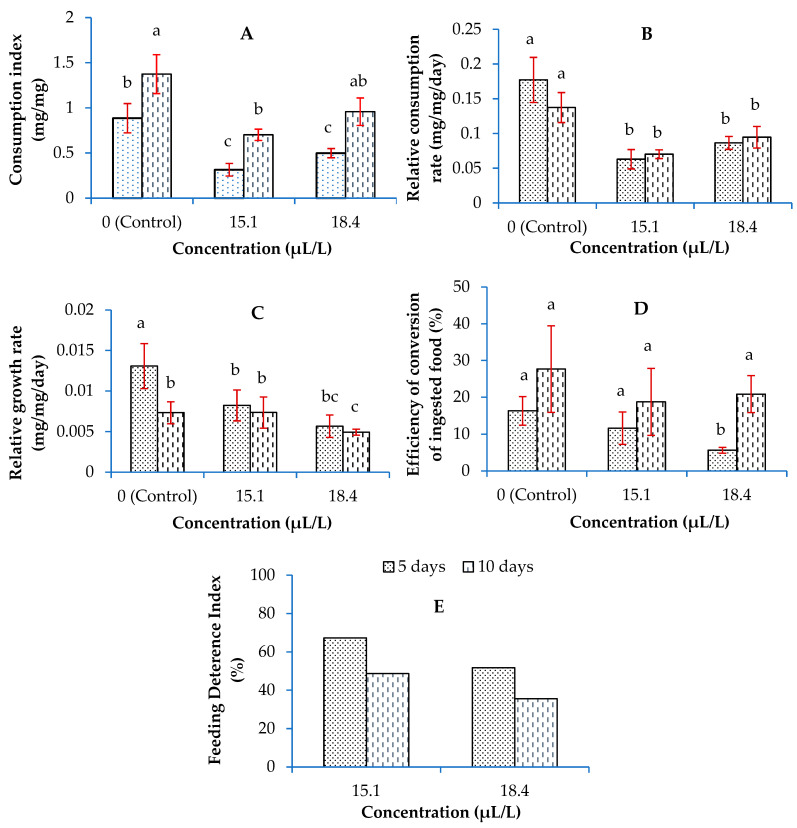
The effect of the 24 h LC30 and LC40 values of the A. fragrans essential oil (15.1 and 18.4 μL/L, respectively) on the nutritional indices of the T. confusum adults, including the consumption index, relative consumption rate, relative growth rate, and efficiency of conversion of ingested food after 5 and 10 days, are shown in Figure 2. The consumption index was significantly affected by the essential oil concentrations (F = 9.93; df = 2, 24; p < 0.05) and exposure times (F = 14.82; df = 1, 24; p < 0.05), where it was significantly decreased for the insects treated with 15.1 and 18.4 μL/L of essential oil after 5 days compared with the control group (Figure 2A).
Figure 2.
Effects of the LC30 and LC40 values of the Artemisia fragrans Willd. essential oil on the nutritional indices, including the consumption index (A), relative consumption rate (B), relative growth rate (C), efficiency of conversion of ingested food (D), and feeding deterrence index (E) of Tribolium confusum du Val after 5 and 10 days of exposure time. Different letters designate significant differences between the corresponding means according to Tukey’s test at the probability level of 5%.
The relative consumption rate of T. confusum adults was also decreased by treating with LC30 and LC40 values of the A. fragrans essential oil. Even though the increase in time did not affect the relative consumption rate (F = 0.26; df = 1, 24; p = 0.62), the value of this index was significantly reduced by the tested concentrations at both 5 and 10 days of exposure time (F = 11.39; df = 2, 24; p < 0.05) (Figure 2B).
The use of the studied concentrations of A. fragrans essential oil reduced the relative growth rate of the pest compared with the control group (F = 3.43; df = 2, 24; p = 0.04); the lowest value was seen for the adults treated by the highest tested concentration. However, it was not affected by the exposure time (F = 2.56; df = 1, 24; p = 0.12) (Figure 2C). The effect of the essential oil concentrations (F = 0.08; df = 2, 24; p = 0.46) and exposure time of insect pest (F = 3.60; df = 1, 24; p = 0.07) on the efficiency of converting the eaten food were not significant (Figure 2D).
The feeding deterrence index of the A. fragrans essential oil at a concentration of 15.1 μL/L was calculated as being 62.29 and 51.75% after 5 and 10 days, respectively. The corresponding values with the concentration of 18.4 µL/L were 48.66 and 35.58%, respectively (Figure 2E).
3. Discussion
The insecticidal effects of essential oils isolated from different Artemisia species were demonstrated in previous studies. For example, the Artemisia annua L. essential oil showed significant fumigant toxicity, with a 24 h LC50 of 3.34 μL/L against the fourth-instar larvae of the mulberry pyralid Glyphodes pyloalis Walker [31]. The essential oils of Artemisia absinthium L. and Artemisia dracunculus L. displayed noticeably fumigant toxicity, with LC50 values of 2.60 and 1.08 µL/L, respectively, against eggs of the potato tuber moth Phthorimaea operculella (Zeller) [32]. Toxicity of essential oils isolated from four Artemisia species, including A. dalai-lamae Krasch., A. tangutica Pampanini, A. tanacetifolia L., and A. ordosica Krasch., where 24 LC50 values of 25.7, 17.4, 41.9, and 21.7 µg/insect, respectively, against the red flour beetle Tribolium castaneum Herbst were documented [33]. However, in the present study, the insecticidal effects of A. fragrans essential oil was evidenced for the first time, in which the essential oil with a 24 h LC50 of 22.13 μL/L had noteworthy fumigant toxicity against the adults of T. confusum. The different lethal concentrations in the abovementioned and present studies may be justified by differences in the tested insect pests and Artemisia species.
Along with acute toxicity, based on the results of the present study, the A. fragrans essential oil had anti-nutritional effects on the adults of T. confusum: A significant reduction in consumption index, relative consumption rate, and relative growth rate, and a 62.29% feeding deterrence index at a concentration of 15.1 μL/L after 5 days. There was also a decrease in the consumption index and relative consumption rate of the pest treated with A. fragrans essential oil actually shows that the insect refused to eat food. The inhibitory compounds in the essential oil probably interfered with the functioning of the pest’s feeding stimulation signals. It may also be said that given the reduction in the relative growth rate of insects, which can be related to a decrease in the relative consumption rate and conversion efficiency of the digested food, the utilization quantity of food also decreased [34]. In general, decreasing nutritional indices result in low biological performance of the pest, especially in terms of food absorption and growth. In consonance with the results of the present study, nutritional indices falling in the insect pests treated by Artemisia essential oils was documented in recent years: The relative growth rate and efficiency of conversion of ingested food of Egyptian cotton leaf worm Spodoptera littoralis (Boisduval) larvae treated with essential oil of Artemisia monosperma Del. decreased [35]. In the other study, a 53.4% feeding deterrence index was displayed for the S. littoralis larvae treated by 100 μg/cm2 of A. dracunculus essential oil [36].
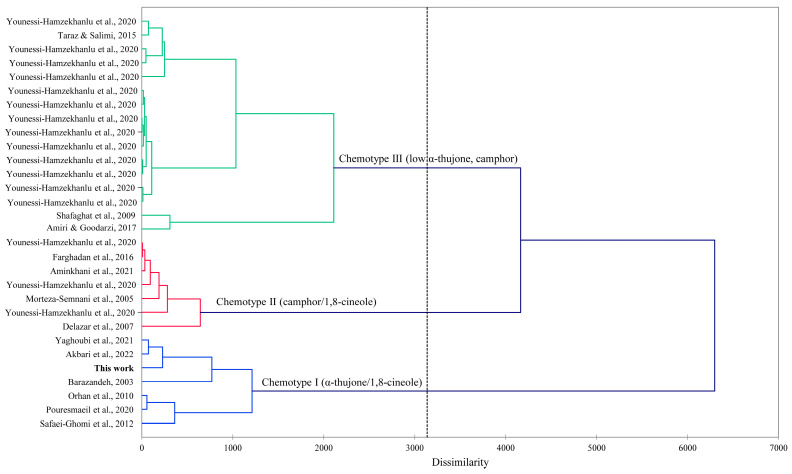
The terpenes comprising α-thujone (27.8%), 1,8-cineole (22.8%), p-cymen-7-ol (3.5%), terpinen-4-ol (2.9%), β-thujone (2.8%), borneol (2.7%), trans-p-menth-2-en-1-ol (2.3%), and α-pinene (2.0%) were identified as the main compounds in the A. fragrans essential oil in the present study. The chemical profile of the A. fragrans essential oil was explored in previous studies. Morteza-Semnani et al. [37] revealed that the terpenes camphor (46.0%), 1,8-cineole (23.7%), camphene (7.9%), borneol (4.9%), and chrysanthenone (3.4%) were the prominent compounds in the essential oil isolated from the flowering aerial parts of A. fragrans. In the present study, camphor (1.1%), camphene (1.9%), and borneol (2.7%) with different quantities were also identified, but no trace of chrysanthenone was seen. It was also found that the chemical profile of the A. fragrans essential oil can be varied based on the chemotypes and different parts of the plant. For example, according to the results of Saedi et al. [38], there were two different chemotypes among the essential oils of populations of A. fragrans in East Azerbaijan province, Iran. Artemisyl acetate (20.9%), 1,8-cineole (17.2%), borneol (8.0%), β-ocimene (7.8%), camphor (7.2%), bornyl acetate (4.1%), and camphene (2.5%) were prominent in the first chemotype. In the second chemotype, the chemical profile was significantly different, in which chrysanthenone (47.5%) had the highest amount, followed by 1,8-cineole (6.0%), β-thujone (5.2%), caryophyllene oxide (4.1%), and pinocarvone (3.2%). According to the study of Aminkhani et al. [24], camphor (33.9%), 1,8-cineole (23.5%), terpinene-4-ol (3.1%), artemisyl acetate (3.0%), and camphene (2.9%) in the essential oil of leaves, and camphor (27.3%), 1,8-cineole (27.0%), terpinene-4-ol (4.0%), borneol (3.1%), and carvacrol (3.0%) in the essential oil isolated from flowers of A. fragrans were the main compounds. In another investigation, the chemical composition of the A. fragrans essential oil from three provinces of Ardabil, East Azerbaijan, and West Azerbaijan of Iran were investigated. The results showed that camphor (9.9–34.4%), α-thujone (19.2–42.6%), and 1,8-cineole (12.6–31.9%) were the dominant compounds [23]. Three chemotypes based on the chemical compositions were identified in this study: chemotype I, α-thujone/1,8-cineole; chemotype II, camphor/1,8-cineole; and chemotype III, which showed relatively low concentrations of α-thujone or camphor. In order to place the essential oil composition of A. fragrans in this work with previously reported compositions, a hierarchical cluster analysis was carried out based on the major essential oil components (Figure 3). The cluster analysis was in excellent agreement with Younessi-Hamzekhanlu et al. [23], and firmly placed the essential oil in this current work into the α-thujone/1,8-cineole chemotype. In general, the chemical composition of plant essential oils may be changed by various exogenous and endogenous factors, such as climate conditions, geographical location, plant growth stage and ecotype, and the method of essential oil extraction [39]. Therefore, the differences observed in the identified compounds of A. fragrans essential oil in the present study and the abovementioned studies could have originated due to such factors.
Figure 3.
Dendrogram based on hierarchical cluster analysis (HCA) of Artemisia fragrans Willd. essential oil compositions. Akbari et al. 2022 [25], Aminkhani et al. 2021 [24], Amiri and Goodarzi 2017 [40], Barazandeh 2003 [41], Delazar et al. 2007 [42], Orhan et al. 2010 [26], Farghadan et al. 2016 [43], Morteza-Semnani et al. 2005 [37], Pouresmaeil et al. 2020 [27], Safaei-Ghomi et al. 2012 [44], Shafaghat et al. 2009 [45], Taraz and Salimi 2015 [46], Yaghoubi et al. 2021 [47], and Younessi-Hamzekhanlu et al. 2020 [23].
Recent research results show that the insecticidal properties of Artemisia essential oils are directly related to their chemical compositions [30,36,48]. Additionally, several reports are on the insecticidal potential of the identified compounds in the A. fragrans essential oil. For example, based on the toxicity against the larvae, pupae, and adult stages, Kheloul et al. [17] stated that 1,8-cineole or essential oils containing large amounts of this monoterpenoid may have high insecticidal potential against T. confusum. In another study, the fumigant toxicity of 1,8-cineole, as one of the main compounds of Artemisia nakaii Pamp., with a 24 h LC50 value of 7.00 μL/L determined for third-instar larvae of Spodoptera litura Fab., gave A. nakaii essential oil insecticidal properties [30]. Xie et al. [49] indicated that among the main compounds of Seriphidium brevifolium (Wall. ex DC.) essential oil, (α + β) thujone and 1,8-cineole had considerable fumigant toxicity against workers of the red imported fire ant Solenops isinvicta Buren, with LC50 values of 17.7 and 30.7 μL/L after 12 h of exposure. Different insecticidal modes of action were also reported in this research: inhibitory effects on acetylcholinesterase and carboxylesterase activity by 1,8-cineole and (α + β) thujone, respectively. They concluded that S. brevifolium essential oil and the monoterpenes (α + β) thujone and 1,8-cineole could be developed as eco-friendly agents for managing red imported fire ants. The insecticidal potential of borneol, bornyl acetate, camphene, camphor, carvacrol, p-cymene, spathulenol, terpinene-4-ol, and α-pinene, as well as some other compounds recognized in the A. fragrans essential oil, were also documented [50,51,52,53]. It can be concluded that the observed insecticidal effects of A. fragrans essential oil may be due to the presence of such pesticidal compounds and their interactions.
The results of the present study showed that the fumigation of the A. fragrans essential oil could cause high mortality in the adults of T. confusum. Moreover, if the pest was affected by the LC30 and LC40 values, its nutritional indices, including the consumption index, relative consumption rate, and relative growth rate, diminished. The reduction in these indices may result in critical disruption in the biological processes of the pest, especially in food absorption and growth. Recent studies showed that Artemisia essential oils displayed other modes of action, such as the inhibition of digestive (α-amylases, proteases, lipases, and α- and β-glucosidases) and detoxifying enzymes (acetylcholinesterase and glutathione-s-transferase) activity; reductions in protein, glucose, triglyceride amounts, and hemocyte numbers; the deterioration of digestive cells of the larval midgut; and a decrease in yolk spheres in the ovaries of emerging adults of the insect pests [31,35,54]. Indeed, according to recent studies [55,56], insect pests can detoxify essential oils by increasing the activities of detoxifying enzymes, such as esterases and glutathione-S-transferase. However, due to the multiple modes of action of essential oils and their compounds, insect pests are susceptible and their chance of developing resistance against these natural agents will be low. In contrast, the possibility of pest resistance to chemical pesticides has been high due to simple modes of action.
4. Materials and Methods
4.1. Plant Materials and Essential Oil Extraction
Aerial parts of Artemisia fragrans were collected from the pastures of the Khoroslu region, Ardabil province, Iran (39°15′21.0″ N 48°01′13.0″ E), in May–June 2023. The plant species was identified according to the keys described by Asri [57]. The collected specimens were dried in the shade in the laboratory within one week and powdered using an electric grinder (IKA®, M20, Königswinter, Germany). Essential oil extraction was performed using a glass Clevenger apparatus with 50 g of dry plant powder and 1000 mL of distilled water within 180 min. The extracted essential oil was dehydrated with sodium sulfate and stored in glass containers with aluminum coating at 4 °C until use. The yield of essential oil extraction was calculated using the following equation [58]:
| Yield (%) = weight of obtained essential oil/weight of dry plant × 100 |
4.2. Chemical Analysis of Essential Oil
The chemical composition of the A. fragrans essential oil was analyzed using a gas chromatograph (Agilent 7890B, Santa Clara, CA, USA) connected to a mass spectrometer (Agilent 5977A). The length, diameter, and thickness of the gas chromatography column (HP-5ms) were 30 m, 0.25 mm, and 0.25 µm, respectively. The essential oil solution was prepared by diluting in methanol (1:10). The solution (1 µL) was injected at 250 °C, and helium was used as a carrier gas at a rate of 0.1 mL per minute. Retention index (RI) values were determined using a homologous series of n-alkanes [59]. The essential oil compositions were ascertained by a comparison of their RI values and MS fragmentation patterns with those reported in the databases [60,61].
4.3. Insect Rearing
The initial population of T. confusum was gathered from infected flour in the Khoroslu region, Ardabil province, Iran. Rearing of the pest was carried out inside cylindrical containers whose opening was covered with net fabric for ventilation. The pest was reared in the laboratory for at least 3 generations. Adults (100 insects) were released into the containers on 200 g wheat flour (Zagros) to obtain synchronized insects. The containers were kept in the growth chamber with a temperature of 28 ± 2 °C, relative humidity of 65 ± 5%, and 24 h darkness. The adult insects were separated from the breeding containers, and the flour containing the pest eggs was kept. Synchronized adult insects (1–7 days old) were used for the experiments.
4.4. Fumigant Toxicity of Essential Oil
To evaluate the fumigation toxicity of the A. fragrans essential oil, ten adult insects (1–7 days old) were transferred to 340 mL glass containers with a diameter of 7.5 cm and a height of 9.2 cm as a fumigation chamber. Adult insects were treated with concentrations that were responsible for about 25–75% insect mortality, which were calculated according to the results of the primary experiment based on logarithmic distance (11.76, 15.59, 20.29, 26.76, and 35.29 µL/L). Concentrations were poured on filter paper pieces with a diameter of 3 cm. The treated filter papers were glued to the inner surface of the fumigant containers, and the screw lids of the containers were closed in an air-tight manner. Then, the containers comprising treated insects were maintained in a growth chamber at a 28 ± 2 °C temperature, 65 ± 5% relative humidity, and 24 h darkness. The experiments were repeated four times and the insect mortalities were recorded after 24 and 48 h. In the control group, all steps were repeated, except for the addition of essential oil concentrations.
4.5. Antifeedant Effects of Essential Oil
To assess the effects of the A. fragrans essential oil on the nutritional indices of T. confusum, two hundred adults were treated with LC30 (15.09 µL/L) and LC40 (18.40 µL/L) values (lethal concentrations to kill 40% and 50% of tested insects, respectively) of essential oil, which were calculated based on fumigant bioassays. After 24 h, the surviving insects were separated into five replicates of 10 adults separately for each concentration and the control and transferred inside 6 cm Petri dishes containing 2 g wheat flour. The weight of the insects before and after feeding, the weight of the given food, and the weight of the remaining food at the end of the experiment were documented after 5 and 10 days using a digital scale (Sartorius AG, GCA803S, Göttingen, Germany). To measure the percentage of dry weight of adult insects and flour, the studied samples were weighed, dried in an oven at 60 °C for 48 h, and then weighed again [56]. The nutritional indices, including the consumption index (CI), relative consumption rate (RCR), relative growth rate (RGR), and efficiency of conversion of ingested food (ECI) was measured using the following formulae [62]:
| CI = F/A |
| RCR = F/TA |
| RGR = G/TA |
| ECI = G/F |
where F is the dry weight of the food eaten (mg), A is the mean dry weight of the insects during the feeding period (mg), T is the feeding period (days), and G is the dry weight obtained during the feeding period (mg). Also, the feeding deterrence index (FDI) was calculated using the following formula [63]: FDI = [(C − T)/C] × 100, in which C and T are the mean weights of the food eaten in the control and treatment groups, respectively.
4.6. Statistical Analysis
The normality of the data related to the fumigant toxicity of A. fragrans essential oil was tested using the Kolmogorov–Smirnov test. The results of all experiments were analyzed by variance analysis and compared using Tukey’s test at the probability level of 5%. A probit analysis was performed to achieve lethal concentrations and associated regression lines. The statistical analyses were performed using SPSS version 16 software.
HCA (hierarchical cluster analysis) was carried out using XLSTAT v. 2018.1.1.62926 (Addinsoft, Paris, France). The concentrations of the 10 most abundant components (camphene, p-cymene, 1,8-cineole, filifolone, α-thujone, β-thujone, chrysanthenone, camphor, filifolide A, and (E)-β-caryophyllene) from this study, as well as previously reported compositions from the literature [23,24,25,26,27,37,40,41,42,43,44,45,46,47], were used for this analysis. Dissimilarity was used to determine the clusters considering the Euclidean distance, and Ward’s method was used to define agglomeration.
5. Conclusions
The promising fumigant toxicity and antifeedant effects of A. fragrans essential oil, which is rich in insecticide terpenes, such as 1,8-cineole and α-thujone, against T. confusum were realized in the present study. It should also be noted that the plant samples in this study were abundantly grown in the north of Ardabil province, Iran, and it is possible to obtain a large amount of it if needed. Therefore, A. fragrans essential oil can be introduced as an effective and available natural control agent for T. confusum. The investigation of the other insecticidal activities of A. fragrans essential oil, such as the effect on demographic parameters and its modes of action, can be effective in its application. Also, the preparation of new formulations of the essential oil, such as nanocapsules and nanoemulsions, with controlled-release capability in the application of current findings may be effective. Furthermore, for the development of A. fragrans essential oil as a natural insecticide, further research should be focused on its safety in humans.
Author Contributions
Conceptualization, A.E.; methodology, A.E.; formal analysis, A.E.; investigation, A.E. and W.N.S.; validation, A.E.; writing—original draft preparation, A.E., W.N.S., and F.P.; writing—review and editing, A.E., W.N.S. and F.P. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work project was financially supported by the University of Mohaghegh Ardabili, Iran.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pai A., Bucher G. Tribolium. In: Choe J.C., editor. Encyclopedia of Animal Behavior. 2nd ed. Volume 3. Elsevier Science & Technology; San Diego, CA, USA: 2019. pp. 231–241. [Google Scholar]
- 2.Nicolopoulou-Stamati P., Maipas S., Kotampasi C., Stamatis P., Hens L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health. 2016;4:148. doi: 10.3389/fpubh.2016.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scorza F.A., Beltramim L., Bombardi L.M. Pesticide exposure and human health: Toxic legacy. Clinics. 2023;78:100249. doi: 10.1016/j.clinsp.2023.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrovska B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012;6:1–5. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassanien M.F.R., Assiri A.M.A., Alzohairy A.M., Oraby H.F. Health-promoting value and food applications of black cumin essential oil: An overview. J. Food Sci. Technol. 2015;52:6136–6142. doi: 10.1007/s13197-015-1785-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hossain F., Mostofa M.G., Alam A.K. Traditional uses and pharmacological activities of the genus Leea and its phytochemicals: A review. Heliyon. 2021;7:e06222. doi: 10.1016/j.heliyon.2021.e06222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palla F., Bruno M., Mercurio F., Tantillo A. Essential oil as natural biocides in conservation of cultural heritage. Molecules. 2020;25:730. doi: 10.3390/molecules25030730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palla F., Bucchini A.E.A., Giamperi L., Marino P., Raimondo F.M. Extracts as antimicrobial agents in sustainable conservation of Erythrina caffra (Fabaceae) historical trees. Antibiotics. 2023;12:1098. doi: 10.3390/antibiotics12071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russo R., Palla F. Plant essential oils as biocides in sustainable strategies for the conservation of cultural heritage. Sustainability. 2023;15:8522. doi: 10.3390/su15118522. [DOI] [Google Scholar]
- 10.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 11.De Sousa D.P., Damasceno R.O., Amorati R., Elshabrawy H.A., De Castro R.D., Bezerra D.P., Nunes V.R., Gomes R.C., Lima T.C. Essential oils: Chemistry and pharmacological activities. Biomolecules. 2023;13:1144. doi: 10.3390/biom13071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebadollahi A., Jalali Sendi J. A review on recent research results on bio-effects of plant essential oils against major Coleopteran insect pests. Toxin Rev. 2015;34:76–91. doi: 10.3109/15569543.2015.1023956. [DOI] [Google Scholar]
- 13.Ebadollahi A., Ziaee M., Palla F. Essential oils extracted from deferent species of the Lamiaceae plant family as prospective bioagents against several detrimental pests. Molecule. 2020;25:1556. doi: 10.3390/molecules25071556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isman M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020;19:235–241. doi: 10.1007/s11101-019-09653-9. [DOI] [Google Scholar]
- 15.Gokturk T., Kordali S., Ak K., Kesdek M., Usanmaz Bozhuyuk A. Insecticidal effects of some essential oils against Tribolium confusum (du Val.) and Acanthoscelides obtectus (Say), (Coleoptera: Tenebrionidae and Bruchidae) adults. Int. J. Trop. Insect Sci. 2020;40:637–643. doi: 10.1007/s42690-020-00113-y. [DOI] [Google Scholar]
- 16.Zaka S.M., Iqbal N., Saeed Q., Akrem A., Batool M., Khan A.A., Anwar A., Bibi M., Azeem S., Rizvi D.E., et al. Toxic effects of some insecticides, herbicides, and plant essential oils against Tribolium confusum Jacquelin du Val (Insecta: Coleoptera: Tenebrionidae) Saudi J. Biol. Sci. 2019;26:1767–1771. doi: 10.1016/j.sjbs.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kheloul L., Anton S., Bréard D., Kellouche A. Fumigant toxicity of some essential oils and eucalyptol on different life stages of Tribolium confusum (Coleoptera: Tenebrionidae) Bot. Lett. 2023;170:3–14. doi: 10.1080/23818107.2021.1982767. [DOI] [Google Scholar]
- 18.Ebadollahi A. Fumigant toxicity and antifeedant effects of rosemary essential oil against the flour beetle, Tribolium confusum. Plant Pest Res. 2024;14:19–33. doi: 10.22124/iprj.2024.26642.1560. [DOI] [Google Scholar]
- 19.Bora K.S., Sharma A. The genus Artemisia: A comprehensive review. Pharm. Boil. 2011;49:101–109. doi: 10.3109/13880209.2010.497815. [DOI] [PubMed] [Google Scholar]
- 20.Batsatsashvili K., Mehdiyeva N.P., Fayvush G., Kikvidze Z., Khutsishvili M., Maisaia I., Sikharulidze S., Tchelidze D., Aleksanyan A., Alizade V.M., et al. Artemisia annua L. Artemisia fragrans Willd. Asteraceae. In: Bussmann R., editor. Ethnobotany of the Caucasus. European Ethnobotany. 1st ed. Springer; Cham, Switzerland: 2017. pp. 117–122. [DOI] [Google Scholar]
- 21.Sharifi J., Shahmoradi A., Nori A., Azimi Motam F. The study of vegetation dynamics in Moqan rangelandse of Ardabil Province-Iran (Case study: Boran winter rangelands) IJRDR. 2018;24:719–729. doi: 10.22092/ijrdr.2017.114063. [DOI] [Google Scholar]
- 22.Younessi-Hamzekhanlu M., Farmani B., Alirezalu K., Fathizadeh O., Sabzi Nojadeh M. Study of phytochemical composition and antibacterial effects of Artemisia fragrans Willd. essential oil in different seasons. J. Food Sci. Technol. 2019;91:357–367. [Google Scholar]
- 23.Younessi-Hamzekhanlu M., Sanjari S., Dejahang A., Sheidai Karkaj E., Sabzi Nojadeh M., Mert Gönenç T., Ozturk M. Evaluation of essential oil from different Artemisia fragrans Willd. populations: Chemical composition, antioxidant, and antibacterial activity. J. Essent. Oil-Bear. Plants. 2020;23:1218–1236. doi: 10.1080/0972060X.2020.1854129. [DOI] [Google Scholar]
- 24.Aminkhani A., Sharifi S., Hosseinzadeh P. Chemical constituent, antimicrobial activity, and synergistic effect of the stem, leaf, and flower essential oil of the Artemisia fragrans Willd. From Khoy. Chem. Biodivers. 2021;18:e2100241. doi: 10.1002/cbdv.202100241. [DOI] [PubMed] [Google Scholar]
- 25.Akbari P., Asnaashari S., Rahimpour Y., Asgharian P. In Vitro antimalarial activity and phytochemical analysis of aerial parts of Artemisia fragrans Willd. Jundishapur. J. Nat. Pharm. Prod. 2022;17:e117597. doi: 10.5812/jjnpp.117597. [DOI] [Google Scholar]
- 26.Orhan I.E., Belhattab R., Şenol F.S., Gülpinar A.R., Hoşbaş S., Kartal M. Profiling of cholinesterase inhibitory and antioxidant activities of Artemisia absinthium, A. herba-alba, A. fragrans, Marrubium vulgare, M. astranicum, Origanum vulgare subsp. glandulossum and essential oil analysis of two Artemisia species. Ind. Crops Prod. 2010;32:566–571. doi: 10.1016/j.indcrop.2010.07.005. [DOI] [Google Scholar]
- 27.Pouresmaeil M., Nojadeh M.S., Movafeghi A., Maggi F. Exploring the bio-control efficacy of Artemisia fragrans essential oil on the perennial weed Convolvulus arvensis: Inhibitory effects on the photosynthetic machinery and induction of oxidative stress. Ind. Crops Prod. 2020;155:112785. doi: 10.1016/j.indcrop.2020.112785. [DOI] [Google Scholar]
- 28.Najm M., Hadighi R., Heidari-Kharaji M., Alipour M., Hajizadeh M., Rafiei Sefiddashti R., Heidari A., Badirzadeh A. Anti-Leishmanial activity of Artemisia persica, A. spicigera, and A. fragrance against Leishmania major. Iran J. Parasitol. 2021;16:464–473. doi: 10.18502/ijpa.v16i3.7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao S., Zhang K., Wei L., Wei G., Xiong W., Lu Y., Zhang Y., Gao A., Li B. Insecticidal activity of Artemisia vulgaris essential oil and transcriptome analysis of Tribolium castaneum in response to oil exposure. Front. Genet. 2020;11:589. doi: 10.3389/fgene.2020.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J., Hua J., Qu B., Guo X., Wang Y., Shao M., Luo S. Insecticidal terpenes from the essential oils of Artemisia nakaii and their inhibitory effects on acetylcholinesterase. Front. Plant Sci. 2021;12:720816. doi: 10.3389/fpls.2021.720816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oftadeh M., Sendi J.J., Ebadollahi A., Setzer W.N., Krutmuang P. Mulberry protection through flowering-stage essential oil of Artemisia annua against the lesser mulberry pyralid, Glyphodes pyloalis Walker. Foods. 2021;10:210. doi: 10.3390/foods10020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naghizadeh S., Rafiee-Dastjerdi H., Naseri B., Golizadeh A., Esmaielpour B. Insecticidal activity of essential oils from Artemisia absinthium L., Artemisia dracunculus L. and Achillea millefolium L. against Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae) JCP. 2019;8:479–489. [Google Scholar]
- 33.Zhang J., Li B., Lu X., Zheng Y., Wang D., Zhang Z., Zeng D., Du S. Chemical diversity and anti-insect activity evaluation of essential oils extracted from five Artemisia species. Plants. 2022;11:1627. doi: 10.3390/plants11131627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikawati S., Himawan T., Abadi A.L., Tarno H. Fumigant and feeding deterrent activity of essential oils against Cryptolestes ferrugineus (Stephens) (Coleoptera: Laemophloeidae) Biodiversitas. 2020;21:4301–4308. doi: 10.13057/biodiv/d210948. [DOI] [Google Scholar]
- 35.Abdelgaleil S.A.M., El-Sabrout A.M. Anti-nutritional, antifeedant, growth-disrupting and insecticidal effects of four plant essential oils on Spodoptera littoralis (Lepidoptera: Noctuidae) JCP. 2018;7:135–150. [Google Scholar]
- 36.Valcárcel F., Olmeda A.S., González M.G., Andrés M.F., Navarro-Rocha J., González-Coloma A. Acaricidal and insect antifeedant effects of essential oils from selected aromatic plants and their main components. Front. Agron. 2021;3:662802. doi: 10.3389/fagro.2021.662802. [DOI] [Google Scholar]
- 37.Morteza-Semnani K., Akbarzadeh M., Moshiri K. Essential oil composition of Artemisia fragrans Willd. From Iran. Flav. Frag. J. 2015;20:330–331. doi: 10.1002/ffj.1431. [DOI] [Google Scholar]
- 38.Saedi K., Azarnivand H., Jalili A., Sefidkon F., Jafari M. Essential oil studies in eight populations of Artemisia L. species in Azarbaijan-e-Gharbi, Iran. J. Iranian Nat. Res. 2008;61:501–512. [Google Scholar]
- 39.Moghaddam M., Mehdizadeh L. Chemistry of essential oils and factors influencing their constituents. In: Grumezescu A.M., Holban A.M., editors. Handbook of Food Bioengineering. 1st ed. Academic Press; New York, NY, USA: 2017. pp. 379–419. [DOI] [Google Scholar]
- 40.Amiri H., Goodarzi M. Screening chemical composition of essential oils and antioxidant activities of two Artemisia species from Iran. Iran. J. Plant Physiol. 2017;7:2017–2025. [Google Scholar]
- 41.Barazandeh M.M. Essential oil composition of Artemisia fragrans Willd. from Iran. J. Essent. Oil Res. 2003;15:414–415. doi: 10.1080/10412905.2003.9698626. [DOI] [Google Scholar]
- 42.Delazar A., Naseri M., Nahar L., Moghadam S.B., Esnaashari S., Nazemiyeh H., Saker S.D. GC-MS analysis and antioxidant activities of essential oils of two cultivated Artemisia species. Chem. Nat. Compd. 2007;43:112–114. doi: 10.1007/s10600-007-0047-8. [DOI] [Google Scholar]
- 43.Farghadan M., Ghafoori H., Vakhshiteh F., Shahzadeh Fazeli S.A., Farzaneh P., Kokhaei P. The Effect of Artemisia fragrans Willd: Essential oil on inducible nitric oxide synthase gene expression and nitric oxide production in lipopolysaccharide-stimulated murine macrophage cell line. Iran J. Allergy Asthma Immunol. 2016;15:515–524. [PubMed] [Google Scholar]
- 44.Safaei-Ghomi J., Ahmadi T., Batooli H., Kashi F.J. Antioxidant and antimicrobial activity of Artemisia fragrans Willd essential oil and methanol extracts. Chemija. 2012;23:100–107. [Google Scholar]
- 45.Shafaghat A., Noormohammadi Y., Zaifizadeh M. Composition and antibacterial activity of essential oils of Artemisia fragrans Willd. leaves and roots from Iran. Nat. Prod. Commun. 2009;4:279–282. doi: 10.1177/1934578X0900400223. [DOI] [PubMed] [Google Scholar]
- 46.Taraz A., Salimi F. Chemical composition and antimicrobial activity of essential oil of Artemisia fragrans Willd. in north-west of Iran. Am. J. Essent. Oils Nat. Prod. 2015;3:07–09. [Google Scholar]
- 47.Yaghoubi M., Ayaseh A., Alirezalu K., Nemati Z., Pateiro M., Lorenzo J.M. Effect of chitosan coating incorporated with Artemisia fragrans essential oil on fresh chicken meat during refrigerated storage. Polymers. 2021;13:716. doi: 10.3390/polym13050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mojarab-Mahboubkar M., Sendi J.J., Mahmoodi N. The sweet wormwood essential oil and its two major constituents are promising for a safe control measure against fall webworm. Pestic. Biochem. Phys. 2022;184:105124. doi: 10.1016/j.pestbp.2022.105124. [DOI] [PubMed] [Google Scholar]
- 49.Xie F., Rizvi S.A.H., Zeng X. Fumigant toxicity and biochemical properties of (α+β) thujone and 1, 8-cineole derived from Seriphidium brevifolium volatile oil against the red imported fire ant Solenopsis invicta (Hymenoptera: Formicidae) Rev. Bras. Farmacogn. 2019;29:720–727. doi: 10.1016/j.bjp.2019.04.013. [DOI] [Google Scholar]
- 50.Rozman V., Kalinovic I., Korunic Z. Toxicity of naturally occurring compounds of Lamiaceae and Lauraceae to three stored-product insects. J. Stored Prod. Res. 2007;43:349–355. doi: 10.1016/j.jspr.2006.09.001. [DOI] [Google Scholar]
- 51.You C.X., Guo S.S., Zhang W.J., Yang K., Wang C.F., Geng Z.F., Du S.S., Deng Z.W., Wang Y.Y. Chemical constituents and activity of Murraya microphylla essential oil against Lasioderma serricorne. Nat. Prod. Commun. 2015;10:1635–1638. doi: 10.1177/1934578X1501000936. [DOI] [PubMed] [Google Scholar]
- 52.Kordali Ş., Usanmaz A., Bayrak N., Çakır A. Fumigation of volatile monoterpenes and aromatic compounds against adults of Sitophilus granarius (L.) (Coleoptera: Curculionidae) Rec. Nat. Prod. 2017;11:362–373. [Google Scholar]
- 53.Ortiz de Elguea-Culebras G., Sánchez-Vioque R., Berruga M.I., Herraiz-Peñalver D., Santana-Méridas O. Antifeedant effects of common terpenes from Mediterranean aromatic plants on Leptinotarsa decemlineata. J. Soil Sci. Plant Nutr. 2017;17:475–485. doi: 10.4067/S0718-95162017005000034. [DOI] [Google Scholar]
- 54.Oftadeh M., Sendi J.J., Valizadeh B., Ebadollahi A. Hemocytic cell line from the moth Glyphodes pyloalis (Lepidoptera: Crambidae) response to essential oils from Artemisia annua (Asterales: Asteraceae) In Vitro Cell Dev. Biol. Anim. 2022;58:14–20. doi: 10.1007/s11626-021-00643-w. [DOI] [PubMed] [Google Scholar]
- 55.Ebadollahi A., Naseri B., Abedi Z., Setzer W.N., Changbunjong T. Promising insecticidal efficiency of essential oils isolated from four cultivated Eucalyptus species in Iran against the lesser grain borer, Rhyzopertha dominica (F.) Insects. 2022;13:517. doi: 10.3390/insects13060517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ebadollahi A., Naseri B., Abedi Z., Setzer W.N. Chemical profiles and insecticidal potential of essential oils isolated from four Thymus species against Rhyzopertha dominica (F.) Plants. 2022;11:1567. doi: 10.3390/plants11121567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asri Y. Range Plants of Iran, Vil. 2: Dicotyledons. 1st ed. Research Institute of Forests and Rangelands; Tehran, Iran: 2012. pp. 575–1107. [Google Scholar]
- 58.Jaimand K., Rezaee M.B. Investigation on chemical constituents of essential oils from Achillea millefolium L. subsp. millefolium by distillation methods. J. Med. Arom. Plants. 2004;20:181–190. [Google Scholar]
- 59.Van den Dool H., Kratz P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 60.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing; Carol Stream, IL, USA: 2007. [Google Scholar]
- 61.NIST . NIST17. National Institute of Standards and Technology; Gaithersburg, MD, USA: 2017. [(accessed on 12 May 2024)]. Available online: https://webbook.nist.gov/chemistry/ [Google Scholar]
- 62.Waldbauer G.P. The consumption and utilization of food by insects. Adv. Insect Physiol. 1968;5:229–288. doi: 10.1016/S0065-2806(08)60230-1. [DOI] [Google Scholar]
- 63.Isman M.B., Koul O., Luczynski A., Kaminski J. Insecticidal and antifeedant bioactivities of neem oils and their relationship to azadirachtin content. J. Agric. Food Chem. 1990;38:1406–1411. doi: 10.1021/jf00096a024. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request.