Abstract
Introduction
Communicating complex information about haemodialysis (HD) and ensuring it is well understood remains a challenge for clinicians. Informed consent is a high-impact checkpoint in augmenting patients’ decision awareness and engagement prior to HD. The aims of this study are to (1) develop a digital information interface to better equip patients in the decision-making process to undergo HD; (2) evaluate the effectiveness of the co-designed digital information interface to improve patient outcomes; and (3) evaluate an implementation strategy.
Methods and analysis
First, a co-design process involving consumers and clinicians to develop audio-visual content for an innovative digital platform. Next a two-armed, open-label, multicentre, randomised controlled trial will compare the digital interface to the current informed consent practice among adult HD patients (n=244). Participants will be randomly assigned to either the intervention or control group. Intervention group: Participants will be coached to an online platform that delivers a simple-to-understand animation and knowledge test questions prior to signing an electronic consent form. Control group: Participants will be consented conventionally by a clinician and sign a paper consent form. Primary outcome is decision regret, with secondary outcomes including patient-reported experience, comprehension, anxiety, satisfaction, adherence to renal care, dialysis withdrawal, consent time and qualitative feedback. Implementation of eConsent for HD will be evaluated concurrently using the Consolidation Framework for Implementation Research (CFIR) methodology. Analysis: For the randomised controlled trial, data will be analysed using intention-to-treat statistical methods. Descriptive statistics and CFIR-based analyses will inform implementation evaluation.
Ethics and dissemination
Human Research Ethics approval has been secured (Metro North Health Human Research Ethics Committee B, HREC/2022/MNHB/86890), and Dissemination will occur through partnerships with stakeholder and consumer groups, scientific meetings, publications and social media releases.
Trial registration number
Australian and New Zealand Clinical Trials Registry (ACTRN12622001354774).
Keywords: NEPHROLOGY, Patient Satisfaction, Clinical Trial, Dialysis, Patient Participation
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The consumer co-design approach increases the likelihood that the information presented in the digital information interface aligns closely with the target population’s health literacy capabilities and will enhance engagement and understanding throughout the consenting process.
The intervention effectiveness will be measured by patient-centred outcomes such as decision regret, participant-reported experiences, comprehension, anxiety, satisfaction and adherence to healthcare.
This research will evaluate factors that impact change in practice and will deliver a digital platform to support consenting for use in renal services settings and with integration into existing electronic medical records to ensure a seamless end-to-end user experience by clinicians and patients alike.
Study limitations include generalisability (factors such as health literacy and socioeconomic status can impact the effectiveness of digital interfaces and informed consent, and this study may not capture the full diversity of the haemodialysis (HD) patient population as it excludes individuals with vision and hearing impairments) and technological barriers (factors like digital literacy could limit eConsent HD applicability to certain patient populations).
Introduction
Chronic kidney disease (CKD) is a progressive condition that affects over 10% of the world’s general population.1 Millions of those with the most advanced stage of CKD are dependent on haemodialysis (HD) to extend their lives.2 The demographics of this group are shifting, with an increasing proportion of elderly, frail and ethnically diverse people.2 These patients pose increasing communication challenges when interacting with healthcare providers. A significant barrier is the legal and ethical process of obtaining informed consent, often hampered by patients’ ability to understand health information (health literacy), the complexity of the information, its implications on quality of life and time constraints.3 4 Health literacy is particularly challenging for those facing complex and life-limiting health conditions, such as kidney failure (KF) (previously termed end-stage kidney disease) and the decision regarding HD.5 Shortfalls in health literacy are associated with multiple negative outcomes5 6 and may explain why patients subsequently elect to stop HD. In 2021, over 30% of Australians who commenced HD withdrew from treatment, making this the most common cause of death in this group.7 8 The high rate of withdrawal underlines the importance of informed decision-making and person-centred care in the management of individuals with KF, particularly for vulnerable groups. Addressing health literacy challenges and promoting culturally sensitive communication during decision-making are essential to improve patient outcomes.
Current clinical practices, particularly in managing the transition to HD, have seen minimal changes over the past four decades, including the way in which patients are consented for dialysis.9 An informed decision to commence HD cannot be based solely on conveying knowledge of its mechanics, benefits and risks. While population statistics often guide prognostic conversations and recommendations, treating clinicians may not be fully aware of the personal circumstances and factors that may determine the patient’s ability to thrive on HD. This knowledge and perception gap may be the barrier that hinders comprehensive communication of the real demands of HD including (1) the significant investment of personal time required; (2) the impact of this on activities of daily living, especially those that may have given meaning to a person’s life before starting HD; (3) the impact and burden on family and caregivers; and (4) the potential intrusive restrictions on patients’ autonomy and independence while functional and cognitive decline progresses.4 7 10
Clinicians face challenges in communicating complex information in a simple to understand way within a time-limited clinical appointment.3 Wainstein et al conducted a comprehensive assessment of current consent processes for HD in Australia in 2018 and concluded that there is no standardised process.10 Checkpoints, such as evaluating patient comprehension, were inconsistently integrated. Additionally, critical information such as impact on quality of life, comparative survival with or without dialysis and alternative treatments, including conservative kidney management, were often inadequately addressed.4 10
A recent Cochrane review revealed that educational interventions can lead to improvements in knowledge about CKD.11 Patients undergoing procedures in different medical areas (eg, dermatology and vascular surgery) report video-assisted informed consent improved their experience in decision-making.12 13 Nevertheless, the benefit of educational and video-assisted interventions in the complex HD consent process is yet to be established.
We hypothesise that the current approach to obtaining consent for HD is suboptimal, and that video-assisted consent can help standardise the process to meet ethical and legal standards. First, this study will generate new knowledge to meet the evidence gap of the process of consenting for HD. Importantly, the effectiveness of the method will be measured by patient-centred outcomes. Second, this study will evaluate factors that impact change in practice. Third, this study is anticipated to deliver a market-ready product for use in all clinical settings with integrations into existing electronic medical records to ensure a seamless end-to-end user experience by clinicians and patients alike. Specific aims of this study are (figure 1):
Figure 1. A type 2 hybrid effectiveness-implementation design to test a digital information interface for informed consent at haemodialysis and implementation of electronic consent for haemodialysis in Australia.
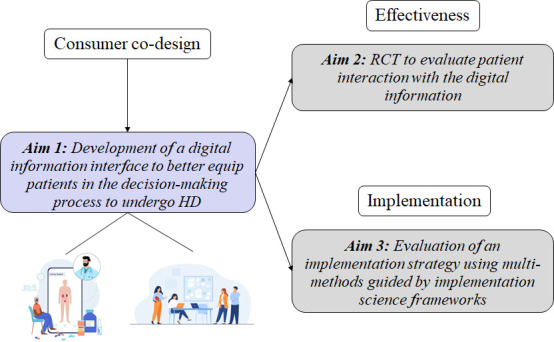
Aim 1
Development of a digital information interface to better equip patients in the decision-making process to undergo HD. We will engage with consumers in the co-design of audio-visual content loaded into an innovative digital eConsent platform. Content will be designed to accommodate for the real-world perspectives of patients, including those with limited health literacy capabilities. For culturally and linguistically diverse (CALD) groups, information will be presented in users’ preferred language.
Aim 2
Evaluation of patient interaction with the co-designed digital information interface. A randomised controlled trial (RCT) will evaluate the effectiveness of the information interface compared with the current informed consent practice for patients starting or continuing HD.
Aim 3
Evaluation of an implementation strategy using multimethods guided by implementation science frameworks. This phase will use the Consolidated Framework for Implementation Research (CFIR) methodology to evaluate the facilitators and barriers to the adoption of an evidence-based information interface into clinical practice.
Methods and analysis
This study (anticipated February 2024 to February 2028) will use a type 2 hybrid effectiveness-implementation design to address each of the aims. Methods for each aim are described below.
The first aim (aim 1) will focus on developing a digital information interface designed for patients consenting to HD. It will use a consumer co-design approach, structured into three sequential phases.
Phase 1 – needs assessment
Recruitment of participants
Consumer representatives (n=10) will be recruited from the catchment areas of five clinical sites across three Australian states, that is, Queensland—Metro North Health and Wide Bay Hospital and Health Service; New South Wales—John Hunter Hospital, St George and Sutherland Hospital; and Victoria—Monash Medical Centre. Consumer representatives will comprise of patients, family, carers and kidney patient advocates.
Consumer engagement
Consumer representatives will participate in forums, workshops and interviews (face-to-face and online) facilitated by a researcher experienced in co-design.
Interventions and data collection
A range of data will be collected, including demographic and socioeconomic data, the eHealth literacy scale – eHeals (a reliable and easy-to-use self-reporting tool to assess digital health literacy),14 meeting minutes, and observation and field notes. Semistructured interviews will be conducted with a sample of six consumer researchers (at least 50% of these will be people with CALD background) either face-to-face, via telephone or using platforms such as Microsoft Teams. The interview schedule containing semistructured questions can be found in the online supplemental material 1.
Analytical plan
Forums, workshops and interviews will be digitally recorded and professionally transcribed. Braun and Clarke’s thematic analysis technique15 16—a process of identifying and organising themes or patterns within the data to provide an in-depth understanding of participants’ experiences, perceptions and perspectives will be employed.
Phase 2: prototype development
An audio-visual animation will be developed by Consentic—our industry partner who brings excellence in digital capabilities and experience. Consentic is highly experienced in overseeing projects that assess digital advancements in enhancing the user experience during the informed consent process. Consentic has a track record in content development, translation functionality and hosting of the digital platform. A user-friendly animation will feature human figures role-playing an interaction between a patient and a doctor. The audio narrative will guide participants through the content presented in the video, while written subtitles and captions will further enhance the visual content. The script will cover various topics considered as priorities by the consumer researchers.
Phase 3: usability testing
Participants (not involved in development) (n=10 incident and n=10 prevalent HD patients) will test the refined product, rate its performance and report their satisfaction with the digital information interface.
Recruitment of participants
Participants will be identified in HD facilities across the five clinical sites. Written research consent will be obtained, and participants will be coached in navigation of the online digital eConsent HD. Participants will complete a knowledge questionnaire to assess and reinforce understanding and will have the option to go back and revisit specific points in the animation. The process finishes with signing of the electronic consent form which is date-stamped and time-stamped and is compliant with international requirements for electronic signatures (ie, the U.S. Food and Drug Administration Code of Federal Regulations Part 11 eSignature requirements). It is anticipated that navigation through the animation and questions will take up to 15 min. Participants will have the opportunity to discuss any questions or concerns they may have with their clinicians before giving their consent for HD treatment. This ensures that participants in both groups have the opportunity for clarification and to address any uncertainties prior to making their decision.
Inclusion criteria
The inclusion criteria are consecutive adult individuals (aged ≥18 years) who are either incident or prevalent HD patients and fulfil one of the following conditions: being fluent in English (the audio-visual content will be available only in English for phase 3). Incident HD patients are individuals who are scheduled to begin HD within the next month and prevalent HD patients are individuals who have been on HD for>3 months.
Exclusion criteria
Individuals with severe visual, hearing or cognitive impairment lack the capacity to provide informed consent (as per the attending clinician), have been on HD for <3 months and have previously provided written consent for HD (NB: in most Australian hospitals, only verbal or implied consent is obtained for HD).
Data collection
Demographic and socioeconomic data, the eHealth literacy scale – eHeals14; patient comprehension measure using an 18-item instrument,10 patient anxiety levels (six-item short form of the Spielberger State-Trait Anxiety Inventory)13; patient satisfaction (five-point Likert scale)13; time taken to consent (auto-timed in eConsent HD); and participants’ qualitative feedback (free text comment box available at the end of the post-consent survey). Additional data: pre-dialysis education (eg, number of sessions, type of education and educational materials provided) will be provided either by the participants prior to informed consent or extracted from clinical records.
Data analysis plan
Descriptive statistics will be employed to summarise the characteristics of study participants, providing a clear overview of their demographic and clinical characteristics. Two-sample t-tests and Mann–Whitney U-test will be used for the analysis of continuous variables between intervention and control groups, depending on distributions and appropriateness. Linear mixed models will be used to examine any group differences while accounting for potential confounding variables. This statistical approach allows for the analyses of repeated measures data, considering within-subject variability. Statistical significance between the intervention and control groups will be considered when p values are ≤0.05. Qualitative data, (written feedback from participants) will be analysed using Braun and Clarke’s thematic analysis.15 16
The second aim (aim 2) will centre on the evaluation of patient interaction with the co-designed digital information interface. It purposefully seeks to evaluate the differences between HD naïve (incident) patients and current HD (prevalent) patients.
Design
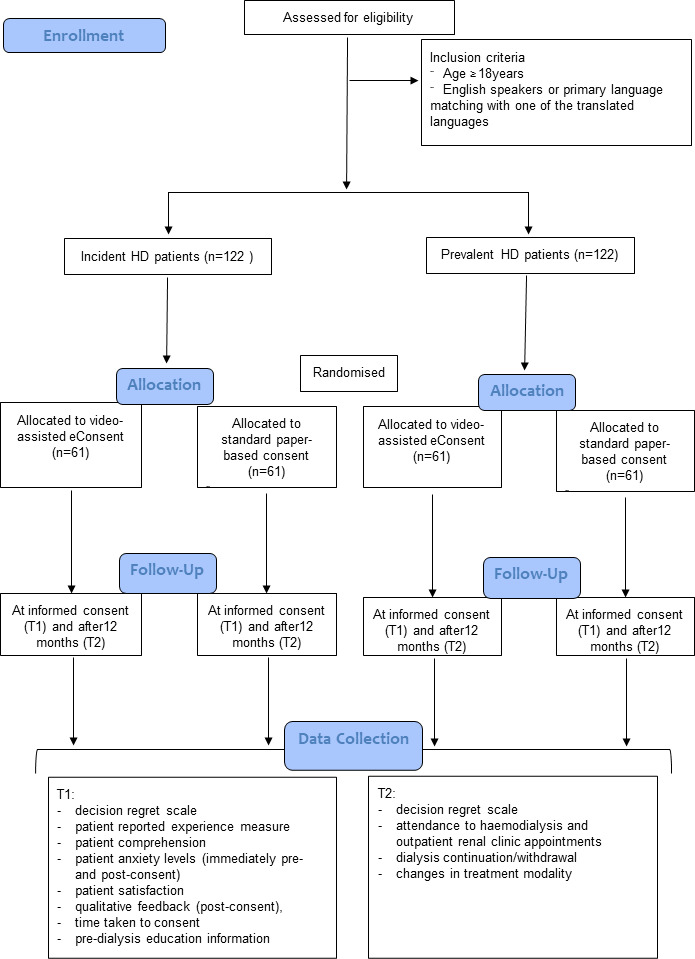
A parallel, two-armed, open-label, RCT will be conducted at five Australian clinical sites: Queensland—Metro North Health and Wide Bay Hospital and Health Service; New South Wales—John Hunter Hospital, St George and Sutherland Hospital; and Victoria— Monash Medical Centre. A flow chart of the RCT is shown in figure 2.
Figure 2. Flow diagram of the RCT protocol. Data collection will occur at informed consent (T1) and after 12 months (T2). RCT, randomised controlled trial.
Recruitment of participants
Participants will be identified in nephrology clinics and HD facilities across the five sites. Written research consent will be obtained, and participants randomised into either intervention or control groups.
Inclusion criteria
Adults (aged ≥18 years) who are either incident or prevalent HD patients and fulfil one of the following conditions: being fluent in English or having a primary language that aligns with one of the eConsent HD languages (currently Arabic, Cantonese, Farsi, Greek, Italian, Macedonian, Mandarin, Nepalese, Spanish and Vietnamese). Incident HD patients are individuals who are scheduled to begin HD within the next month and prevalent HD patients are individuals who have been on HD for>3 months.
Exclusion criteria
Individuals with severe visual, hearing or cognitive impairment who lack the capacity to provide informed consent independently (as per the attending clinician) or are currently on HD but have had it for <3 months.
Intervention group
Participants assigned to the intervention group will be coached to navigate the online digital eConsent HD. The consent animation will be a user-friendly animation featuring human figures role-playing an interaction between a patient and a doctor. The audio narrative will guide participants through the content presented in the video, while written subtitles and captions will further enhance the visual content. The script will cover various topics including treatment options for advanced kidney disease; what HD involves, its potential benefits and risks, likely survival with or without HD, and the process of making an informed choice about HD.
eConsent HD places control of uptake of information and consent with the participant. At the start of the animation, participants will select language and literacy level. Participants can alter the speed of navigation through the animation at any time. Participants will complete teach-back exercises, a cyclic process to assess and reinforce understanding, and will have the option to go back and revisit specific points in the animation. The process finishes with signing of the electronic consent form which is date-stamped and time-stamped and is compliant with international requirements for electronic signatures (ie, the U.S. Food and Drug Administration Code of Federal Regulations Part 11 eSignature requirements). It is anticipated that navigation through the animation and questions will take up to 15 min.
Control group
Participants assigned to the control group will undergo the usual method of obtaining informed consent at the trial site. The process may involve either a medical or nursing clinician consenting the participants, followed by signing a paper-based informed consent form.
Both groups
To ensure both groups have access to a minimum standard information, pre-consent information booklets will be provided to all participants. In cases where English is not the participants’ first language, interpreters will be accessed to facilitate communication, following usual practice at the trial site. Regardless of the assigned group, all participants will have the opportunity to discuss any questions or concerns they may have with their clinicians before giving their consent for HD treatment. This ensures that participants in both groups have the opportunity for clarification and to address any uncertainties prior to making their decision.
Primary outcome
Decision regret (assessed by a validated decision regret scale).17 18
Secondary outcomes
Patient reported experience measure (modified Kidney PREM);19 patient comprehension measure using an 18-item instrument;10 patient anxiety levels (six-item short form of the Spielberger State-Trait Anxiety Inventory)13 ; patient satisfaction (five-point Likert scale)13 ; adherence with kidney care (assessed by HD attendance, completion of prescribed hours of HD treatment, attendance at routine outpatient clinic appointments extracted from clinical records); dialysis continuation/withdrawal (extracted from data submitted by each site to the Australian and New Zealand Data Registry); changes in treatment modality (extracted from data submitted by each site to the Australian and New Zealand Data Registry); time taken to consent (auto-timed in eConsent HD or by the consenting clinician using a stopwatch); and participants’ qualitative feedback (free text comment box available at the end of the post-consent survey).
Data collection will occur at informed consent (T1) and after 12 months of HD treatments (T2) (figure 2). Additional baseline measures, including sociodemographic characteristics and any pre-dialysis education (eg, number of sessions, type of education and educational materials provided) will be provided either by the participants prior to informed consent or extracted from clinical records.
Sample size
eConsent HD is a distinctive first-of-its-kind study and there is no previous literature available to provide effect-size estimates for a similar intervention. Our sample size calculation was guided by a previous study that evaluated the effectiveness of a multimedia education format in assisting individuals with CKD to make treatment choices.20 In that study, the intervention group showed a significant reduction in decision regret. For our study, we aim to detect a 15% change in the primary outcome, which is decision regret, with a statistical power of 90% and a type 1 error rate of 5% (two-tailed). Based on these parameters, a total sample size of 244 participants will be required, with 61 participants in each arm for the incident and prevalent cohort, respectively.
Randomisation
To mitigate the bias of previous knowledge from exposure to HD, participants will be stratified into incident and prevalent HD participant groups. Once eligibility is confirmed, and written research consent provided, participants will be assigned (by computer random sequencing software) to either the intervention or the control group in a 1:1 stratified block randomisation. A research officer (or an investigator) will assign participants to one of the study groups and will use an opaque sealed envelopes for each enrolled participant. To ensure balanced and comparable groups, stratification based on language background will be employed, distinguishing between English speakers and non-English speakers as a surrogate for people with CALD backgrounds. This approach controls for the major sources of bias between the two groups.
Data analysis plan
Data analysis will be on an intention-to-treat basis. Incident and prevalent HD participants will be kept separate in the data analysis. Descriptive statistics will be employed to summarise the characteristics of study participants, providing a clear overview of their demographic and clinical characteristics. Two-sample t-tests and Mann–Whitney U-test will be used for the analysis of continuous variables between intervention and control groups, depending on distributions and appropriateness. Linear mixed models will be used to examine any group differences while accounting for potential confounding variables and repeated measurements over time. Statistical significance between the intervention and control groups will be considered when p values are ≤0.05. Qualitative data, (written feedback from participants), will undergo Braun and Clarke’s thematic analysis.15 16
In aim 3, we will focus on the evaluation of an implementation strategy using multimethods guided by an implementation science framework.
Design
The CFIR methodology will be used to systematically assess the implementation of a change to clinical practice.
Recruitment of participants
Two clinical sites—Metro North Health and John Hunter Hospital. The sites are metropolitan (Metro North Health) and regional (John Hunter Hospital) areas of Australia. Neither has implemented a standardised informed consent practice for HD. Participants from both sites will include patients on HD, caregivers, clinicians and stakeholders (eg, executive staff, head of departments, policymakers).
Intervention
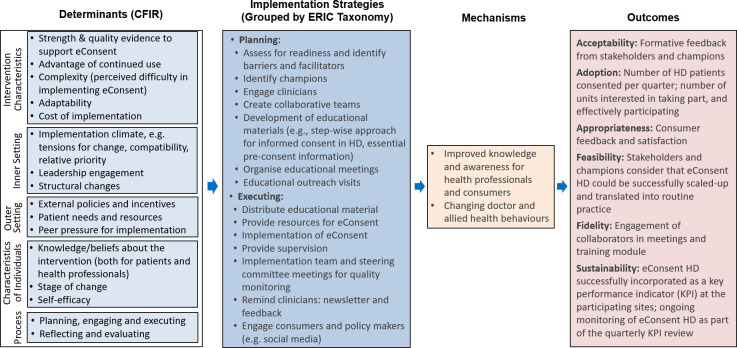
Data collection will use a range of methods, including e-surveys; acceptability, appropriateness and fidelity measures; in-depth interviews; stakeholder meeting minutes; observation and field notes. These methods will gather data on the five determinants of the CFIR framework (figure 3).
Figure 3. eConsent HD—Implementation Research Logic Model (IRLM). CFIR, Consolidated Framework for Implementation Research; ERIC, Expert Recommendations for Implementing Change;23 HD, haemodialysis.
Following written research consent, e-surveys will be distributed to participants. Surveys incorporate the validated 23-item NoMad instrument,21 with an additional open-ended field for general feedback. Participants will be asked to complete the e-surveys at 3 and 6 months following consent to HD for patient participants or at 3 and 6 months following recruitment of first participant into the RCT at the site for non-patient participants.
Stakeholders (n=36) will be invited to rate their perceived acceptability, appropriateness and fidelity of the process of consenting using the validated Acceptability of Intervention Measure, Intervention Appropriateness Measure and Feasibility of Intervention Measure instruments.22 Participant surveys will be conducted online and will be integrated into the eConsent HD activities for the intervention RCT group. All others will complete the survey online via REDCap.
Semistructured interviews will be conducted with a sample of 10 clinicians and 10 patient participants (at least 50% of these will be people with CALD backgrounds) pre-implementation and post-implementation of eConsent HD. Interviews will take place approximately 3 months and 9 months after consent to HD and will be conducted in various formats, including face-to-face, via telephone or using platforms such as Microsoft Teams. The interview schedule will be guided by the CFIR framework, ensuring that it encompasses all five domains of CFIR: process, characteristics of individuals, outer setting, inner setting and intervention characteristics (figure 3). Interviews will be audio-recorded, professionally transcribed and de-identified for qualitative analyses.
eConsent HD will apply validated concepts of adoption, acceptability, appropriateness, feasibility, fidelity and sustainability to further refine the constructs for progression into designing an implementation model in the future.23
Primary outcomes
Facilitators and barriers to implementation of the eConsent HD.
Secondary outcomes
Characteristics of the outcomes of the implementation and its feasibility. The outcomes of the implementation will be grouped into the five domains of CFIR: intervention characteristics, outer setting, inner setting, characterisation of individuals and processes. Five components of feasibility of implementing eConsent HD will be evaluated: (1) resource availability (eg, quality and quantity of materials resources and infrastructure); (2) staff availability (eg, number of clinicians opting in for eConsent HD roll out); (3) comprehensiveness and clarity (eg, ability of consumers to engage with the intervention and to understand the relevance of the intervention); (4) intervention functionality (eg, ease with which eConsent HD pathway is followed by clinicians); (5) time required for consumers to complete eConsent; and (6) user experience (eg, feedback from clinicians and consumers).
Data analysis
Quantitative data obtained from the implementation evaluation will be reported using descriptive statistics. Qualitative data from interviews will undergo a combination of inductive and deductive analyses using CFIR. These analyses focus on acceptability, appropriateness and feasibility in the first round of analyses. Then, the results inform additional code domains and their inclusion (or not), specifically to examine sustainability, barriers, enablers, and contextual factors.
Patient and public involvement
Healthcare professionals of the participating sites were involved in the study design via multidisciplinary meetings and electronic communication. Patients were not involved in the study design but will participate in phase 1 of aim 1. Regularly, the research team will share social media updates related to the project, along with other informative content generated by the initiative, such as success stories, updated literature and guidelines. The research team also seeks to identify consumer champions who will actively promote the project, both through word-of-mouth and, potentially, by advocating on their social media platforms.
Research governance
Research governance of eConsent HD is invested in the Steering Committee chaired by PFG. Membership will include consumers, representation from two subcommittees that report to the Steering Committee (the Advisory Team and the Data and Safety Monitoring Committees) and the Multidisciplinary Project Team. The Steering Committee will meet as required but no less than three times per year. Among the obligatory activities of the Steering Committee are compliance with Human Research Ethics Committee requirements.
The Advisory Team Committee will be chaired by AB and reports to the Steering Committee. Membership will include representatives from Consentic, The University of Queensland, Griffith University, one representative from Kidney Health Australia (Australia’s peak non-government patient advocacy group) and two consumer representatives. Meetings will occur as needed but at least two times per year.
The Data Monitoring and Safety Committee will be chaired by MW and reports to the Steering Committee. Membership will include clinician representatives from each research site and will be responsible for quarterly audits on data storage and collection. A safety data report will be prepared by the Project Manager and tabled at each Data Monitoring and Safety Committee meeting. Adverse events that may arise during the study period could include but are not limited to psychological distress or anxiety experienced by participants due to the content of the digital platform; technical issues or difficulties encountered by participants while navigating the online platform; and any unintended consequences or misunderstandings resulting from the intervention that may impact patient decision-making or outcomes. Meetings will occur two times per year and as needed.
The Multidisciplinary Project Team will be led by PFG and reports to the Steering Committee. Membership will include clinicians and research officers at each project site with knowledge of local healthcare settings for example, policies, procedures, etc. Meetings will occur monthly in the early phases of the project and subsequently bi-monthly.
Ethics and dissemination
Ethical considerations
This study was approved by the Metro North Hospital and Health Service Human Research Ethics Committee B (HREC/2022/MNHB/86890). Ethics approval has been secured for all participating sites through the Australian National Mutual Acceptance Scheme. This framework enables the acceptance of a single ethics review across multiple public health organisations, ensuring compliance and ethical standards are met uniformly across all sites.
Any modification of the study protocol will be subject to approval by the Steering Committee. A formal amendment request outlining the proposed modifications will be submitted for approval of the Metro North Hospital and Health Service Human Research Ethics committee. The Steering committee will ensure that the trial registration on Australian and New Zealand Clinical Trials Registry is up to date. Current and future participants affected by the modifications will be contacted. The research team will provide clear and concise explanations of the changes, their implications, and the reasons behind them.
A bi-monthly newsletter will be sent to clinical staff in the participating sites. Research officers at each site will actively search for eligible participants. The recruitment method has been shown to be acceptable and feasible in a previous study using eConsent for patients with kidney diseases undergoing kidney biopsies.
Study participants will consent to the study doctor and relevant research staff collecting and using personal and health information for the research project. Informed consent will be obtained from participants in each phase of the study at their respective study sites. The participant information and a copy of the consent form will be given to all participants. Any information obtained for this research project will remain confidential. Data collected will be individually coded and the access to a master code list will be restricted to the principal investigator. Re-identifiable (coded) data will be stored at each of the participating centres. eConsent will be undertaken using Consentic, a secure platform, where data is securely stored on servers in Australia, and kept for 15 years. Data will be collected using RedCap. All data will be managed in accordance with hospital policies on data confidentiality and security.
Participants may be consented for this study by either the clinical research nurse or an investigator. The research officer (or an investigator) will allow eligible participants to have the opportunity to read and consider the research information leaflet prior to consenting for this research. Participants will be allowed at least 24 hours to reflect on the implications of participating in the study. Participants will be given the option to waive the 24-hour consideration period. In case of conflicting decision-making or time constraint for decision-making (eg, when haemodialysis needs to be started urgently), eligible participants will not be included in this research.
We recognise the National Health and Medical Research Council Statement on Consumer and Community Involvement in Health and Medical research, and its requirement to appropriately remunerate consumers and community involved in research. Consumer researchers will be reimbursed for their participation in phase 1 (aim 1) according to approved health consumer honorarium payments in line with the Health Consumers Queensland position statement 2015 with annual inflation increases.
Participation in phase 3 (aim 1), aim 2 and aim 3 will be voluntary. Research participants have the right to withdraw from the project at any stage. Withdrawals will affect neither participants’ routine treatment nor their relationship with the treating staff.
Participants’ information will only be used for this research project and will only be disclosed with participants’ permission, except as required by law and/or regulatory authorities. Participants’ information may be obtained from health records held at this and other health services for this research. Participants will consent to the study team accessing health records if they are relevant to this research project.
Consentic is a digital health platform for eConsent of medical procedures and clinical trials. Data is stored on servers in Sydney, Australia. No data is shared with third parties. The data is encrypted in transit and at rest. Two-factor authentication is required by users. Data security/monitoring: Consentic has regular vulnerability scans, and penetration testing is undertaken following major updates and releases. All staff at Consentic have education/training on data security, and there are security systems architecture in place including disaster recovery plans.
Dissemination
Findings from this research will be disseminated via professional scientific meetings and publication in peer-reviewed journals. A short simple summary in each language will be available to all research participants at their respective HD units. A dedicated eConsent HD profile on Facebook, Twitter and Instagram will be accessible to consumers, clinicians, researchers and others, and we will regularly publish research updates on the project alongside other relevant HD information (eg, success stories, updated literature, guidelines) facilitating the dissemination and translation of our research findings. Additionally, our findings will be communicated back to consumer representatives to distribute to not-for-profit organisations and kidney patient support groups they are affiliated with.
supplementary material
Footnotes
Funding: This research received funding from the Royal Brisbane and Women’s Hospital Foundation (Project/initiative #62) for consumer engagement (Phase 1, Aim 1). Consentic will provide in-kind support for the development of the content of the digital platform. Funding is being sought for phases 2 and 3.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2023-081181).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Contributor Information
Pedro Henrique Franca Gois, Email: pedro.francagois@health.nsw.gov.au.
Rebecca B Saunderson, Email: rebecca.saunderson@consentic.com.
Marina Wainstein, Email: marinawainstein@outlook.com.
Chenlei Kelly Li, Email: ChenleiKelly.Li@health.nsw.gov.au.
Matthew J Damasiewicz, Email: matthew.damasiewicz@monash.edu.
Vera Y Miao, Email: veraymiao@gmail.com.
Martin Wolley, Email: martin.wolley@health.qld.gov.au.
Kirsten Hepburn, Email: kirsten.hepburn@health.qld.gov.au.
Clyson Mutatiri, Email: clyson.mutatiri@health.qld.gov.au.
Bobby Chacko, Email: bobby.chacko@health.nsw.gov.au.
Ann Bonner, Email: a.bonner@griffith.edu.au.
Helen Healy, Email: helen.healy@health.qld.gov.au.
References
- 1.Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022;12:7–11. doi: 10.1016/j.kisu.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bello AK, Okpechi IG, Osman MA, et al. Epidemiology of haemodialysis outcomes. Nat Rev Nephrol. 2022;18:378–95. doi: 10.1038/s41581-022-00542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brehaut JC, Carroll K, Elwyn G, et al. Informed consent documents do not encourage good-quality decision making. J Clin Epidemiol. 2012;65:708–24. doi: 10.1016/j.jclinepi.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Li KC, Brown MA. Consenting for dialysis or its alternative: systematic process is needed. Clin J Am Soc Nephrol. 2020;15:560–2. doi: 10.2215/CJN.09510819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessup RL, Osborne RH, Beauchamp A, et al. Health literacy of recently hospitalised patients: a cross-sectional survey using the health literacy questionnaire (HLQ) BMC Health Serv Res. 2017;17:52. doi: 10.1186/s12913-016-1973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muscat DM, Kanagaratnam R, Shepherd HL, et al. Beyond dialysis decisions: a qualitative exploration of decision-making among culturally and linguistically diverse adults with chronic kidney disease on haemodialysis. BMC Nephrol. 2018;19:339. doi: 10.1186/s12882-018-1131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ANZDATA Registry . 45th Report, Chapter 3: Mortality in Kidney Failure with Replacement Therapy. Adelaide, Australia: 2022. http://www.anzdata.org.au Available. [Google Scholar]
- 8.Chan S, Marshall MR, Ellis RJ, et al. Haemodialysis withdrawal in Australia and New Zealand: a binational registry study. Nephrol Dial Transplant. 2020;35:669–76. doi: 10.1093/ndt/gfz160. [DOI] [PubMed] [Google Scholar]
- 9.Brennan F, Stewart C, Burgess H, et al. Time to improve informed consent for dialysis: an international perspective. Clin J Am Soc Nephrol. 2017;12:1001–9. doi: 10.2215/CJN.09740916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wainstein M, Menzies AR, Brennan FP, et al. The legal doctrine of informed consent and renal dialysis - do patients really consent. J Law Med. 2018;25:992–1008. [PubMed] [Google Scholar]
- 11.Campbell ZC, Dawson JK, Kirkendall SM, et al. Interventions for improving health literacy in people with chronic kidney disease. Cochrane Database Syst Rev. 2022;12:CD012026. doi: 10.1002/14651858.CD012026.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowers N, Eisenberg E, Montbriand J, et al. Using a multimedia presentation to improve patient understanding and satisfaction with informed consent for minimally invasive vascular procedures. Surg. 2017;15:7–11. doi: 10.1016/j.surge.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Miao Y, Venning VL, Mallitt K-A, et al. A randomized controlled trial comparing video-assisted informed consent with standard consent for mohs micrographic surgery. JAAD Int. 2020;1:13–20. doi: 10.1016/j.jdin.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norman CD, Skinner HA. eHEALS: the eHealth literacy scale. J Med Internet Res. 2006;8:e27. doi: 10.2196/jmir.8.4.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saunders CH, Sierpe A, von Plessen C, et al. Practical thematic analysis: a guide for multidisciplinary health services research teams engaging in qualitative analysis. BMJ. 2023;381:e074256. doi: 10.1136/bmj-2022-074256. [DOI] [PubMed] [Google Scholar]
- 16.Virginia Braun VC. SAGE Publications; 2022. Thematic analysis.https://uk.sagepub.com/en-gb/eur/thematic-analysis/book248481 Available. [Google Scholar]
- 17.Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23:281–92. doi: 10.1177/0272989X03256005. [DOI] [PubMed] [Google Scholar]
- 18.Brown L, Gardner G, Bonner A. A randomized controlled trial testing a decision support intervention for older patients with advanced kidney disease. J Adv Nurs. 2019;75:3032–44. doi: 10.1111/jan.14112. [DOI] [PubMed] [Google Scholar]
- 19.Hawkins J, Wellsted D, Corps C, et al. Measuring patients' experience with renal services in the UK: development and validation of the kidney PREM. Nephrol Dial Transplant. 2022;37:1507–19. doi: 10.1093/ndt/gfac030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiou CP, Chung YC. Effectiveness of multimedia interactive patient education on knowledge, uncertainty and decision-making in patients with end-stage renal disease. J Clin Nurs. 2012;21:1223–31. doi: 10.1111/j.1365-2702.2011.03793.x. [DOI] [PubMed] [Google Scholar]
- 21.Finch TL, Rapley T, Girling M, et al. Improving the normalization of complex interventions: measure development based on normalization process theory (NoMAD): study protocol. Impl Sci. 2013;8:43. doi: 10.1186/1748-5908-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiner BJ, Lewis CC, Stanick C, et al. Psychometric assessment of three newly developed implementation outcome measures. Impl Sci. 2017;12:108. doi: 10.1186/s13012-017-0635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith JD, Li DH, Rafferty MR. The implementation research logic model: a method for planning, executing, reporting, and synthesizing implementation projects. Implement Sci. 2020;15:84. doi: 10.1186/s13012-020-01041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]