Abstract
Introduction
Patients with clinically significant portal hypertension (CSPH) are recommended to be treated with non-selective beta-blockers (ie, carvedilol) to prevent the first hepatic decompensation event by the renewing Baveno VII consensus. CSPH is defined by hepatic venous pressure gradient (HVPG)≥10 mm Hg; however, the HVPG measurement is not widely adopted due to its invasiveness. Liver stiffness (LS)≥25 kPa can be used as a surrogate of HVPG≥10 mm Hg to rule in CSPH with 90% of the positive predicting value in majority aetiologies of patients. A compelling argument is existing for using LS≥25 kPa to diagnose CSPH and then to initiate carvedilol in patients with compensated cirrhosis, and about 5%–6% of patients under this diagnosis criteria may not be benefited from carvedilol and are at risk of lower heart rate and mean arterial pressure. Randomised controlled trial on the use of carvedilol to prevent liver decompensation in CSPH diagnosed by LS remains to elucidate. Therefore, we aimed to investigate if compensated cirrhosis patients with LS≥25 kPa may benefit from carvedilol therapy.
Methods and analysis
This study is a randomised, double-blind, placebo-controlled, multicentre trial. We will randomly assign 446 adult compensated cirrhosis patients with LS≥25 kPa and without any previous decompensated event and without high-risk gastro-oesophageal varices. Patients are randomly divided into two groups, with 223 subjects in group A and 223 subjects in group B. Group A is a carvedilol intervention group, while group B is a placebo group. All patients in both groups will receive aetiology therapies and are followed up at an interval of 6 months. The 3-year incidences of decompensated events of cirrhosis-related and liver-related death are the primary outcome. The secondary outcomes include development of each complication of portal hypertension individually (ascites, variceal bleeding or overt hepatic encephalopathy), development of spontaneous bacterial peritonitis and other bacterial infections, development of new varices, growth of small varices to large varices, delta changes in LS and spleen stiffness, change in hepatic dysfunction assessed by Child-Pugh and model for end-stage liver disease score, change in platelet count, development of hepatocellular carcinoma, development of portal vein thrombosis and adverse events with a 3-year follow-up. A predefined interim analysis will be performed to ensure that the calculation is reasonable.
Ethics and dissemination
The study protocol has been approved by the ethics committees of the Sixth People’s Hospital of Shenyang (2023-05-003-01) and independent ethics committee for clinical research of Zhongda Hospital, affiliated to Southeast University (2023ZDSYLL433-P01). The results from this trial will be submitted for publication in peer-reviewed journals and will be presented at international conferences.
Trial registration number
ChiCTR2300073864.
Keywords: Hepatobiliary disease, Hepatology, Gastroduodenal disease
Strengths and limitations of this study.
The study is a randomised, double-blind, placebo-controlled, multicentre trial, with a sample size of 446.
Patients with compensated cirrhosis and liver stiffness (LS)≥25 kPa is expected to become a new alternative or criterion for precisely assessing whether patients compensated cirrhosis need carvedilol therapy.
The standard of LS≥25 kPa measured by transient elastography cannot rule in all patients with clinically significant portal hypertension, and the study results are not applicable to any other elastography technology.
Introduction
In PREDESCI Study, 201 patients with clinically significant portal hypertension (CSPH) but without high-risk gastro-oesophageal varices (GEV) were randomised to use non-selected beta-blockers (NSBBs) in 101 patients and placebo in 100 patients.1 The primary endpoint was the occurrence of decompensated events or death. The study has shown that NSBBs significantly reduce the risk of decompensation in compensated cirrhosis patients with CSPH.1
A portal pressure≥10 mm Hg, usually estimated by hepatic venous pressure gradient (HVPG), defines CSPH.2,5 CSPH is the strongest predictor of clinical decompensation.2 The patients with CSPH are at high risk of variceal haemorrhage, overt clinical decompensation with a reduced median survival of 2 years.34 6,8 CSPH is an important driving factor for decompensation of cirrhosis, and treatment with carvedilol should be considered in preventing the first hepatic decompensation event.3 4 8
In a competing-risk meta-analysis, carvedilol was shown that it reduces the risk of decompensation and mortality in patients with compensated cirrhosis.9 However, HVPG measurement is limited in clinical practice due to its complexity, invasiveness and preoperative anxiety of patients.2 8 10 Transient elastography is widely used in clinical practice to grade liver disease.2 In order to avoid the invasive nature of HVPG there is growing interest in identifying non-invasive methods to detect the presence of CSPH, liver stiffness (LS) by transient elastography≥25 kPa was sufficient to rule in CSPH in patients with various aetiologies, such as alcoholic liver disease, hepatitis B and C and non-obese non-alcoholic steatohepatitis, with positive predicting value higher than 90% and without the need of additional non-invasive parameters.11 Recent studies by Liu et al and Dajti et al showed that patients with LS≥25 kPa had a high risk of liver decompensation.12 13 Non-invasive methods such as LS to diagnose CSPH is more widely accepted by physicians and patients.14,17
Although these findings strongly support the use of LS≥25 kPa as a substitute for CSPH diagnosis to initiate carvedilol in patients with compensated cirrhosis, approximately 5%–6% of patients may not benefit from carvedilol under this diagnostic criterion and are at risk of reducing heart rate and mean arterial pressure.1 8 11 18 Meanwhile, the performance of LS in specific setting such as in obese patients with non-alcoholic steatohepatitis cirrhosis is another concern.11
As far as the data is concerned, the renewing Baveno VII consensus recommends that CSPH is an important driving factor for decompensation of cirrhosis, and treatment with carvedilol should be considered in preventing the first decompensation event.8 And LS≥25 kPa can be applied to the diagnosis of CSPH.8 However, using LS≥25 kPa as a surrogate of HVPG≥10 mm Hg to diagnose CSPH and then to initiate carvedilol among patients with compensated cirrhosis require solid evidence. These Baveno VII consensus statements on the use of carvedilol to prevent decompensation in CSPH diagnosed by LS≥25 kPa remain to elucidate.
Methods and analysis
Objectives
The purpose of this trial is to test the efficacy and safety of carvedilol for the prevention of hepatic decompensation in patients with CSPH defined by LS≥25 kPa measured with transient elastography. The study will begin in 2024 and is expected to conclude in 2028.
Trial design and setting
This is a randomised, double-blind, placebo-controlled multicentre trial to compare the incidence of first episode of hepatic decompensation between patients treated with carvedilol (starting with 6.25 mg/day and increase up to 12.5 mg/day) and placebo. Patients with LS≥25 kPa and without high-risk GEV19 will be randomly assigned in a 1:1 ratio to receive carvedilol or placebo. Regular follow-up will be performed every 6 months to track hepatic decompensation as defined by the following criteria: ascites, variceal bleeding, overt hepatic encephalopathy and liver-related death.
Participant selection
Participants who meet the following selection criteria will be included in the study: (1) age 18–75 years; (2) with liver cirrhosis diagnosed by previous biopsy or clinical criteria and analytical image; (3) without any previous decompensation of cirrhosis and without high-risk GOV (ie, no small varices with red signs, no small varices with Child-Pugh C and no medium or large varices; all three <3 months before randomisation); (4) absence of ascites demonstrated by a recent ultrasound (<3 months before the randomisation); (5) without hepatic encephalopathy; and (6) informed consent. We will exclude patients who meet any of the following criteria: (1) previous decompensation associated with portal hypertension; (2) LS<25 kPa; (3) portal axis thrombosis affecting the portal trunk or main hepatic branches, or the splenic or mesenteric vein; (4) hepatocellular carcinoma demonstrated by imaging tests; (5) bilirubin>3 mg/dL, platelets<30×109/L or Quick prothrombin time test<30%; (6) presence of renal insufficiency (serum creatinine>2 mg/dL); (7) any comorbidity involving a therapeutic limitation and/or a prognosis of life<12 months; (8) absolute contraindication to treatment with beta-blockers (severe bronchospasm, stenosis aortic A-V block, intermittent claudication, severe psychosis, bronchial asthma, etc); (9) hypersensitivity to beta-blockers; (10) pregnancy or lactation; (11) receive anticoagulant treatment; (12) ALT (alanine aminotransferase)>5 ULN or AST (aspartate transaminase)>5 ULN; (13) past treatment with nitrates or beta-blockers in the 2 weeks prior inclusion; and (14) non-alcoholic steatohepatitis related cirrhosis with body mass index≥30 kg/m2.
Sample size calculation
It is well documented that the cumulative rate of developing first episode of decompensated events in our target population is around 21%.16 We have assumed that the carvedilol treatment would reduce this rate to 10% from 21%,1 which would be considered clinically relevant. In total, 223 patients per arm (446 in total) would be sufficient to detect such a difference with 90% power at a significance level of 0.05, while accounting for a 10% of loss to follow-up/withdrawal from the trial.
Recruitment, randomisation and blinding
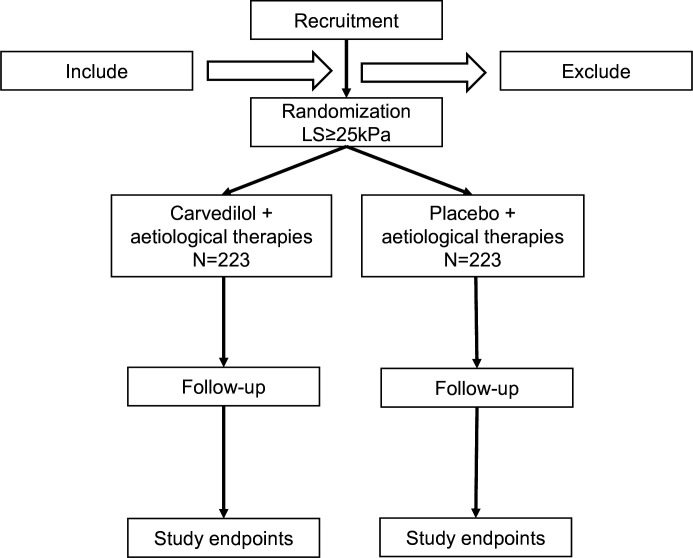
We will enrol 446 compensated cirrhotic patients with LS≥25 kPa without high-risk GOV according to the process with the inclusion and exclusion criteria (figure 1). Written informed consents will be obtained from the patients or their representatives. We will collect the demographic and clinical characteristics of the 446 patients, including age, gender, nation, height, weight, clinical diagnosis, etc. Medical histories (eg, liver cirrhosis treatment, liver cancer and other tumours, other common diseases) are also collected.
Figure 1. Flow diagram of the study.
Randomisation was executed at a 1:1 ratio through Interactive Web Response System (a centralised, web-based system), employing a double-blinded mechanism via electronically generated codes in fixed, random permuted blocks. Distinct blocks were delineated for each centre, categorised into placebo and carvedilol strata.
The trial maintained a double-blind design, whereby participants, outcome assessors and investigators remained unaware of allocation and intervention specifics. Study medication, comprising either carvedilol or a matched placebo, was compounded by investigational pharmacist’s privy to the randomisation code through a password-protected system. These pharmacists ensured the carvedilol and placebo pills were indistinguishable, maintaining the double-blind integrity of the study. An independent allocator, knowledgeable only of participant study ID numbers and not their identities, was tasked with treatment assignment. This allocator, excluded from participant screening, inclusion, follow-up, data management and treatment processes, ensured the preservation of the double-blind status. In the instance of adverse events, the principal investigator was empowered to initiate emergency unblinding protocols. The decision to unblind, along with the consequential actions and outcomes, was to be thoroughly documented in an adverse reaction report, with the principal investigator adjudicating on the necessity of unblinding study personnel or participants.
Patients in both groups will receive aetiological therapies and are followed up at an interval of 6 months. To increase the reliability of the conclusions deduced from the results of the study, the blind method was applied to patients and care providers.
Study protocol
Eligible patients with compensated cirrhosis and LS≥25 kPa will be randomly divided into two groups—223 subjects in group A (carvedilol therapy) and 223 subjects in group B (placebo therapy). All patients who participated in the study undergo the examination and record the baseline value of number including laboratory examination, endoscopy and transient elastography, and we will also record Child-Pugh value. All patients in both groups will receive regular follow-up at an interval of 6 months and record decompensation events and adverse events. At each follow-up visit, investigators will record the examination value and the decompensation events and assess the heart rate, pill count and occurrence of adverse events.
The oral dose of carvedilol (or placebo) to be used in the study is individually determined during an open label titration period. Patients receive carvedilol, starting with 6.25 mg/day and increased up to 12.5 mg/day. The dose is titrated against clinical tolerance, keeping heart rate above 55 beats per minute and systolic blood pressure greater than 90 mm Hg. The titration period lasts up to 2 weeks and patients are randomly assigned once the daily dose of carvedilol has been determined.
Study endpoints
Primary endpoint: The primary outcome is composite endpoint defined by occurrence of decompensated events (overt ascites, oesophagogastric variceal bleeding or overt hepatic encephalopathy), or liver-related death. Non-liver-related death and transplantation were defined as competing events.
Secondary endpoint: The secondary outcomes include development of each complication of portal hypertension individually (ascites, variceal bleeding or overt hepatic encephalopathy), development of spontaneous bacterial peritonitis and other bacterial infections, development of new varices, growth of small varices to large varices, delta changes in LS and spleen stiffness, change in hepatic dysfunction assessed by Child-Pugh and model for end-stage liver disease score, change in platelet count, development of hepatocellular carcinoma, development of portal vein thrombosis and adverse events with a 3-year follow-up.
Ascites identification relied on compatible physical examination signs, validated by ultrasonography or paracentesis; however, intraperitoneal fluid detectable exclusively by ultrasonography or the mere presence of ankle oedema did not qualify as an endpoint.1 Variceal diagnosis was established through endoscopy, categorising varices as large (non-collapsible by insufflation) or small (collapsible by insufflation). Large varices, alongside small varices exhibiting red wale marks or presenting in patients with Child-Pugh class C, were designated as high risk according to Baveno criteria.4 Diagnoses of variceal bleeding, overt hepatic encephalopathy and spontaneous bacterial peritonitis adhered to established guidelines.20 Encephalopathy was defined in line with West Haven criteria, categorising symptoms and signs indicative of a grade exceeding level II.
Safety assessments
All analyses were done in the intention-to-treat population, which included all patients who underwent randomisation. Adverse event refers to an adverse medical condition or a sign of deterioration of an existing medical condition after receiving an examination, which is not necessarily related to the examination. This adverse medical condition can be abnormal laboratory tests, symptoms (eg, fatigue, shortness of breath, dizziness, hypotension, nausea, constipation, sexual dysfunction and erectile dysfunction) and diseases. The investigator will record the adverse reaction, duration (start and end date), severity, course, outcome, significance and any action taken in relation to the adverse event.
Data management
All raw data will be recorded accordingly on the case report form and signed by the direct investigator. The complete case report form will be sent to the lead investigator. Data collation and entry into Excel are conducted by experts in data collation and entry. All processes related to data access and analysis will be overseen by the ethics committees of all study sites.
Statistical analysis
Categorical variables are compared with Fisher’s exact test and continuous variables with the Student’s t-test. The Wilcoxon rank-sum test is used for skewed or ordinal data. Continuous variables measure repeatedly over time are analysed by the mixed models repeated measure. HRs and 95% CI are estimated by Cox regression model. Data are reviewed at death, liver transplant, last visit or the end of the follow-up period, whichever occur earliest. Liver transplant patients are reviewed as alive. Patients who lost follow-up, withdrew consent and withdrew antiviral therapy are reviewed as if they have not shown any results since the last recorded visit. The log-rank test is used to compare the two study groups in terms of primary endpoints. Cumulative probabilities of development of secondary outcomes are estimated using cumulative incidence functions, and comparisons rely on Gray’s test.
Patient and public involvement
The study is a multicentre trial. Patients participate voluntarily and are not involved in the trial design. During the study, patients should report any adverse reactions in time. At the end of the trial, the final results will be reported back to the patient.
Ethics and dissemination
The study protocol has been approved by the ethics committees of the Sixth People’s Hospital of Shenyang (2023-05-003-01) and independent ethics committee for clinical research of Zhongda Hospital, affiliated to Southeast University (2023ZDSYLL433-P01).
Each patient must give written informed consent prior to participating in the practice. Investigators must provide qualified candidates with detailed information about the study and must let each participant know that he or she has the right to withdraw at any time during the study. All participants will be informed that their personal information will be used for this study only and that all such information will not be used for other purposes. The whole process will be conducted in accordance with the Chinese Good Clinical Practice Guidelines. The results will be submitted to peer-reviewed academic journals and will be presented at national and international conferences.
Acknowledgements
The authors would like to thank Gao-Jun Teng, Shiv Kumar Sarin, Annalisa Berzigotti, Terry Yip and Mengyi Lu for their constructive comments and criterial revision of the protocol.
Footnotes
Funding: This work was supported by fundings for clinical trials from Zhongda Hospital, School of Medicine, Southeast University (ZDYYGSPLCYJ02).
Prepublication history for this paper is available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2023-081623).
Patient consent for publication: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Chuan Liu, Email: liuchuan101z@163.com.
Liting Zhang, Email: lcheneye@163.com.
Shuairan Zhang, Email: cmuzsr@outlook.com.
Xiaoguo Li, Email: 2569043400@qq.com.
Yu-Jun Wong, Email: Eugene.wong.y.j@singhealth.com.sg.
Xuan Liang, Email: tb10ywk@163.com.
Yan Wang, Email: 1102605829@qq.com.
Xiaofeng Wu, Email: xiaofengwu70@163.com.
Wei Gou, Email: gouwei_qd@126.com.
Jiaojian Lv, Email: lslvjiaojian@163.com.
Shengjuan Hu, Email: hsj.judy@163.com.
Junliang Fu, Email: fjunliang@163.com.
Ju Huang, Email: huangju501@163.com.
Guohong Ge, Email: geguohong@126.com.
Mingxing Huang, Email: huangmx5@mail.sysu.edu.cn.
Fang Wang, Email: kaixin919@163.com.
Qingge Zhang, Email: zqgys321@163.com.
Tao Ren, Email: rentaotibet@163.com.
Zhongji Meng, Email: zhongji.meng@163.com.
Deping Ding, Email: dingdp2009@163.com.
Basang Zhuoga, Email: 936231547@qq.com.
Cidan Zhuoga, Email: cdzgwai@qq.com.
Jian Fan, Email: 1084270084@qq.com.
Dianjie Dang, Email: dang656@163.com.
Liang Miao, Email: miaoliang202202@126.com.
Zhaomin Song, Email: 15930030030@163.com.
Xingguo Xiao, Email: xiaoxingguo200810@126.com.
Huili Wu, Email: wuhuili616161@126.com.
Kai Jiang, Email: 415345552@qq.com.
Tianyu Liu, Email: lty1395@sns120.com.
Youfang Gao, Email: bzgyf301@163.com.
Lan Ma, Email: 15255952116@163.com.
Tao Fang, Email: 13757980130@139.com.
Yuehua Wang, Email: jhwyh793@yeah.net.
Qianhua Zhang, Email: stassiazh@163.com.
Da Zhu, Email: 529735749@qq.com.
Dong Ji, Email: jidg302@126.com.
Zhujun Cao, Email: estherlucifer@163.com.
Qing-Lei Zeng, Email: zengqinglei2009@163.com.
Jie Li, Email: lijier@sina.com.
Ping Chen, Email: pingchendoctor@zju.edu.cn.
Yufang Wei, Email: 18961326126@189.cn.
Zhaowei Tong, Email: hztongzhaowei@163.com.
Zhongsi Hong, Email: hongzhs@mail.sysu.edu.cn.
Xiao Liang, Email: srrshlx@zju.edu.cn.
Yiling Li, Email: lyl-72@163.com.
Yuemin Nan, Email: nanyuemin@163.com.
Xiaolong Qi, Email: qixiaolong@vip.163.com.
References
- 1.Villanueva C, Albillos A, Genescà J, et al. Β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597–608. doi: 10.1016/S0140-6736(18)31875-0. [DOI] [PubMed] [Google Scholar]
- 2.Qi X, Berzigotti A, Cardenas A, et al. Emerging non-invasive approaches for diagnosis and monitoring of portal hypertension. Lancet Gastroenterol Hepatol. 2018;3:708–19. doi: 10.1016/S2468-1253(18)30232-2. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–35. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 4.de Franchis R, Baveno VI Faculty Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–52. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Bosch J, Abraldes JG, Berzigotti A, et al. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573–82. doi: 10.1038/nrgastro.2009.149. [DOI] [PubMed] [Google Scholar]
- 6.Tripathi D, Stanley AJ, Hayes PC, et al. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015;64:1680–704. doi: 10.1136/gutjnl-2015-309262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shung DL, Garcia-Tsao G. Liver capsule: portal hypertension and varices: pathogenesis, stages, and management. Hepatology. 2017;65:1038. doi: 10.1002/hep.29026. [DOI] [PubMed] [Google Scholar]
- 8.de Franchis R, Bosch J, Garcia-Tsao G, et al. Renewing consensus in portal hypertension. J Hepatol. 2022;76:959–74. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villanueva C, Torres F, Sarin SK, et al. Carvedilol reduces the risk of decompensation and mortality in patients with compensated cirrhosis in a competing-risk meta-analysis. J Hepatol. 2022;77:1014–25. doi: 10.1016/j.jhep.2022.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Sun J-H, Zhao H, Zhang H, et al. Tolerance and acceptance of hepatic venous pressure gradient measurement in cirrhosis (Chess1904): an international multicenter study. Port Hypertens Cirrhos. 2022;1:7–14. doi: 10.1002/poh2.4. [DOI] [Google Scholar]
- 11.Pons M, Augustin S, Scheiner B, et al. Noninvasive diagnosis of portal hypertension in patients with compensated advanced chronic liver disease. Am J Gastroenterol. 2021;116:723–32. doi: 10.14309/ajg.0000000000000994. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Liu C, Li J, et al. Risk stratification of decompensation using liver stiffness and platelet counts in compensated advanced chronic liver disease (Chess2102) J Hepatol. 2022;76:248–50. doi: 10.1016/j.jhep.2021.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Dajti E, Ravaioli F, Marasco G, et al. A combined Baveno VII and spleen stiffness algorithm to improve the noninvasive diagnosis of clinically significant portal hypertension in patients with compensated advanced chronic liver disease. Am J Gastroenterol. 2022;117:1825–33. doi: 10.14309/ajg.0000000000001887. [DOI] [PubMed] [Google Scholar]
- 14.You M-W, Kim KW, Pyo J, et al. A meta-analysis for the diagnostic performance of transient elastography for clinically significant portal hypertension. Ultrasound Med Biol. 2017;43:59–68. doi: 10.1016/j.ultrasmedbio.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 15.Berzigotti A. Non-invasive evaluation of portal hypertension using ultrasound elastography. J Hepatol. 2017;67:399–411. doi: 10.1016/j.jhep.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Abraldes JG, Bureau C, Stefanescu H, et al. “Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: the "anticipate" study”. Hepatology. 2016;64:2173–84. doi: 10.1002/hep.28824. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Cao Z, Yan H, et al. A novel SAVE score to stratify decompensation risk in compensated advanced chronic liver disease (Chess2102): an international multicenter cohort study. Am J Gastroenterol. 2022;117:1605–13. doi: 10.14309/ajg.0000000000001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarzer R, Kivaranovic D, Paternostro R, et al. Carvedilol for reducing portal pressure in primary prophylaxis of Variceal bleeding: a dose-response study. Aliment Pharmacol Ther. 2018;47:1162–9. doi: 10.1111/apt.14576. [DOI] [PubMed] [Google Scholar]
- 19.Bosch J. Carvedilol as best Β-blocker for secondary prophylaxis of variceal bleeding: are we there, or not yet. Clin Gastroenterol Hepatol. 2023;21:2195–6. doi: 10.1016/j.cgh.2022.08.026. [DOI] [PubMed] [Google Scholar]
- 20.European Association for the Study of the Liver EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]