Public awareness of this common form of cancer needs to be increased because despite important advances in treatment, prognosis still largely depends on the stage of presentation
More than 90% of tumours in the head and neck are squamous carcinomas. Cancer of the head and neck, which can arise in several places, is often preventable, and if diagnosed early is usually curable. Unfortunately, patients often present with advanced disease that is incurable or requires aggressive treatment, which leaves them functionally disabled. We have reviewed current practice and potential future advances in the referral, diagnosis, and management of head and neck cancer.
Summary points
Squamous cell cancer of the head and neck is common worldwide (4% of all cancers in the United States; 5% in the United Kingdom)
The prognosis for early stage disease is good, but for patients with advanced disease it has altered little in the past 20 years
Multidisciplinary teams are essential for optimum management
Combinations of treatments can offer preservation of organs and function
Improved reporting of morbidity and quality of life is essential
Increased public awareness about the association with smoking and alcohol and the importance of early detection is needed
Methods
We gathered information from several sources, including personal experience of treating head and neck cancer in a multidisciplinary tertiary referral centre and the Medline and Cochrane databases.
Incidence
Squamous cell cancer of the head and neck is one of the most common cancers worldwide, with incidences of more than 30 per 100 000 population in India (oral cancer) and in France and Hong Kong (nasopharyngeal cancer). It constitutes about 4% of all cancers in the United States and 5% in the United Kingdom. A total of 2940 new cases of lip, mouth, and pharyngeal cancer in men were reported in the United Kingdom in 1996: an incidence of 10.2 per 100 000 population.1 People in their 40s and 50s are most susceptible. The 3:1 ratio of prevalence in men to women is decreasing: in the past 10 years the incidence in Scotland has risen by 19.4% in men and 28.7% in women.2 In the United Kingdom incidence and mortality are greater in deprived populations, most notable in carcinoma of the tongue.2
Causes
Smoking tobacco, drinking alcohol, and having a poor diet are important risk factors in the West, and chewing betel or areca nuts, smoking bidis, and taking snuff are important in the Indian subcontinent. Epstein-Barr virus has been implicated in nasopharyngeal carcinoma, and hypopharyngeal carcinoma in elderly women has been associated with a pre-existing postcricoid web. A total of 70% of tumours show loss of heterozygosity near genome 9p21, which may indicate loss of a gene that suppresses tumours.3
Presentation
Most head and neck cancers present with symptoms from the primary site—for example, hoarseness, difficulty in swallowing, or pain in the ear. Enlargement of a cervical lymph node as the first presenting feature is not uncommon, particularly with certain “silent” sites—the tongue base, supraglottis, and nasopharynx. Systemic metastases are uncommon at presentation (10%),4 however, synchronous or metachronous tumours of the upper aerodigestive tract occur in 10-15% of patients.5 Guidelines have been written for general medical and dental practitioners for referring patients with suspected malignancies of the head and neck (box B1), and most head and neck units have an open access clinic to see these patients urgently.6 Removing the node before referral to a specialist centre without first identifying the primary tumour is associated with increased morbidity and poorer long term outcome.7
Box 1.
Head and neck cancer: guidelines for urgent referral
- Hoarseness persisting for >6 weeks
- Ulceration of oral mucosa persisting for >3 weeks
- Oral swellings persisting for >3 weeks
- All red or red and white patches on the oral mucosa
- Dysphagia persisting for >3 weeks
- Unilateral nasal obstruction, particularly when associated with purulent discharge
- Unexplained tooth mobility not associated with periodontal disease
- Unresolved neck masses for >3 weeks
- Cranial neuropathies
- Orbital masses
Screening and early diagnosis
Primary prevention—stopping smoking and drinking less alcohol—is the most effective way to reduce mortality. Early detection should be a priority, given the excellent prognosis of early stage disease compared with the poor results in advanced stages. In Indian screening programmes, community health workers have been trained in primary prevention and early detection of oral cancer and premalignant lesions, but no evidence suggests that this reduces mortality. Screening is most cost effective when targeted at high risk groups—for example, heavy drinkers and smokers.
In the United Kingdom there is relatively little public awareness of head and neck cancer, although individual centres have taken local initiatives. Dentists largely carry the responsibility for examining the oral mucosa in the self selected population that attends for treatment.
Chemoprevention
Retinoids, vitamin A, N-acetyl-cysteine, and other agents may prevent recurrence in patients at risk or prevent malignant transformation in precancerous conditions such as leukoplakia, but no evidence suggests that these treatments are effective in routine clinical practice.8
Investigation
Diagnosis is confirmed by biopsy of the primary site and fine needle aspiration of any enlarged lymph nodes. A full panendoscopy allows full assessment of the extent of the tumour and exclusion of tumours at other sites within the head and neck. Most centres in the United Kingdom recommend computed tomography of the chest to pick up synchronous early lung tumours or metastases.
Imaging of the head and neck
Imaging is crucial in assessing the site, extent, and relationships of a histologically proved primary tumour and to detect the presence of enlarged lymph nodes. After imaging, the staging of the tumour or node is upgraded in at least 30% of cases. Computed tomography is the mainstay of assigning advanced head and neck malignancy a stage because it is generally available. Magnetic resonance imaging is the preferred tool for investigating the primary tumour in all head and neck sites, particularly for assessing cartilage, bone, perineural, and perivascular invasion. A combination of neck ultrasonography and fine needle aspiration improves the specificity of staging of cervical lymph nodes. Although not widely available, positron emission tomography is useful for detecting recurrent disease in the head and neck.
Staging
Staging is done according to the International Union Against Cancer's (UICC) classification system for oral cancer.9
Stage I—T1 N0 M0
Stage II—T2 N0 M0
Stage III—T3 N0, T1-3 N1, and M0
Stage IV—T4 any N, T1-3 N2-3, any T any N M1
(T=tumour; N=node; M=metastasis.)
Multidisciplinary team
Head and neck tumours can occur at a large number of subsites, often invading more than one. Each has its own particular problems regarding management. Patients are often in poor general health and may have appreciable comorbidities or psychosocial problems. Different members of the multidisciplinary team need to collaborate to devise the best management plan for each patient. Guidelines recommend that teams include at least clinical oncologists, otorhinlaryngologists, oromaxillofacial surgeons, and plastic surgeons.10 Ideally, a radiologist and a pathologist with specialist interests should be included. The contributions of clinical nurse specialists, speech and language therapists, dieticians, and prosthetics technicians are indispensable to optimal outcome.
Management
Management of squamous cell head and neck tumours has to be considered in respect to both the primary site and potential cervical lymph node metastases. Radiotherapy and surgery offer equally good long term results in small early head and neck cancers (fig 1). The particular subsite of the disease and the likely long term morbidity usually determine the decision on management. Generally, function is better after radiotherapy than after surgery, but treatment time for surgery is shorter. The performance status and ability of patients to cope with anaesthetic or to attend daily for 4-6 weeks of radiotherapy is also taken into account. Patients themselves may have strong preferences. Traditionally, more advanced head and neck cancer is best managed surgically, providing the tumour is resectable, with postoperative radiotherapy for poor prognostic situations (box B2).
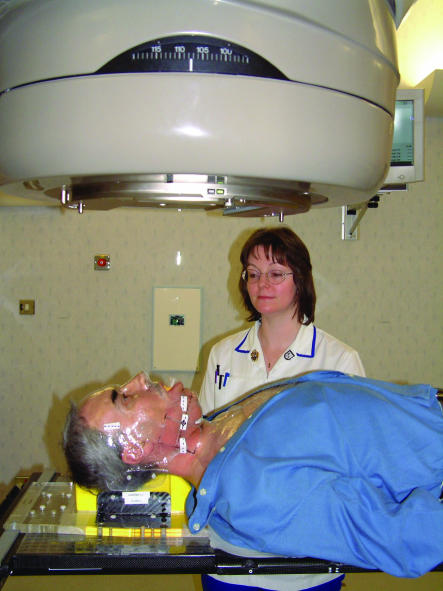
Figure 1.
Patients undergoing head and neck irradiation are immobilised in a beam direction shell which has been vacuum formed over a plaster mould of the patient's head and neck. This allows for accuracy and reproducibility of the treatment set up, and where possible irradiation to normal tissues is kept to a minimum by a system of multileaf collimators (lead shields)
Box 2.
Indications for postoperative radiotherapy
- Close or involved margins of excision
- Extranodal spread of tumour
- Multiple nodes
- Poorly differentiated pathology with perineural or perivascular spread
With large tumours, the defect from excision is often considerable. The ability to close large defects of the head and neck has improved greatly over the past 25 years, with the introduction of pedicled myocutaneous flaps and more recently free flaps. Cosmetic disfigurement and the time a patient spends in hospital has lessened considerably. Unfortunately, the increased capacity for reconstruction has not been accompanied by an increase in survival, and some substantial reconstructions are not entirely functionally satisfactory. Large tumours that were previously unresectable because of their location, such as tumours at the skull base—for example, nasopharyngeal carcinoma or tumours in the neck extending into the mediastinum—can now, with the advent of new surgical approaches, often be resected. These techniques sometimes require the input of other surgical disciplines such as neurosurgery and cardiothoracic surgery.
Inoperable disease may be treated with combinations of chemotherapy and radiotherapy, but outcomes generally remain poor, and in some cases of advanced disease only patients' symptoms can be treated.
Management of the neck
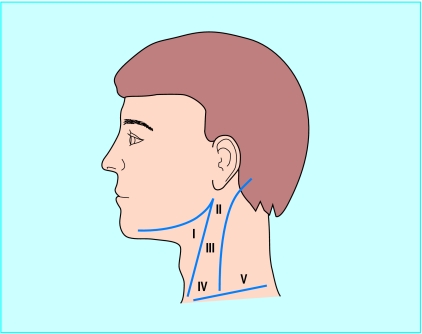
Surgery is the mainstay of treatment for cervical lymph node metastases, which are grouped into five levels (fig 2). With clinical evidence of nodal disease it is clear that the neck requires treatment, traditionally in the form of a neck dissection. Surgery has moved away from radical neck dissections towards modified and selective neck dissections (see box A on bmj.com). This preserves function, especially in relation to the accessory nerve, which if sacrificed usually gives rise to a stiff and painful shoulder. If clinical evidence of the presence of enlarged cervical nodes is lacking, but the expected incidence of node metastases is greater than 20%, it is common practice to treat the neck (see table A on bmj.com). The incidence of involved cervical lymph nodes for different sites and stages of tumour is known from retrospective studies.w2 Watching and waiting, to see if a node appears, is also practised, and no prospective randomised trials compare the two approaches. Prophylactic treatment of the neck may reduce the rate of systemic metastatic disease.11
Figure 2.
Nodal groups
Strategies to improve outcomes
Laser treatment
Using lasers, especially in early laryngeal disease, yields long term survival results equivalent to radiotherapy.12 Although most patients with early laryngeal cancer are treated with radiotherapy in the United Kingdom, lasers are used increasingly, as the patients may often be treated as a day case, and radiotherapy can be held in reserve for metachronous tumours or recurrence. The laser is used increasingly for larger lesions and different sites in the head and neck, with encouraging results relating to survival and function, although there is little data on voice quality.13
Organ preservation in operable disease
In two large studies, chemotherapy and then radiotherapy for responding patients or surgery for non-responding patients gaves equal results for locoregional control compared with immediate surgery and then radiotherapy. Survival rates did not differ between the two groups, but this approach allowed a number of patients to retain their larynx.14,15
These results have led to a trend towards preserving organs by giving chemotherapy during radiotherapy in advanced disease. Mostly, these strategies have scheduled chemoradiotherapy to the primary and neck, followed by a neck dissection six weeks later provided there is a complete response of the primary tumour.16 An alternative for an inoperable primary tumour or potentially functionally debilitating surgery is neck surgery followed by chemoirradiation to the primary. A prime example of this is in advanced tongue base tumours, where surgical management would involve a total glossolaryngectomy.
A patient's perspective
It started with difficulty clearing my throat, then my voice began to fade. After several appointments with my general practitioner I was sent to an ear, nose, and throat specialist. He put a camera up my nose and said, “There is something nasty down there.” I was sent away, recalled for a biopsy, and sent away again. Eventually I was summoned back to the department, where a doctor with detached bedside manner announced, “It is cancer,” and then asked me to wait outside while arrangements were made for treatment. This abrupt statement was the first indication of just how serious my condition was, and as I sat alone in that corridor my spirits were low and my thoughts were black.
I received a course of radiotherapy, attending every day for treatment. The treatment was successful and my voice returned: I was a happy man. Sadly, seven months later my voice faded again, and I had trouble breathing. A visit to the oncology unit resulted in me being admitted to hospital, where the consultant brusquely announced that he would perform a tracheotomy to relieve my breathing immediately, and a larger operation to remove my voice box was also necessary. This would have to wait, however, as the consultant was abroad on holiday over Christmas and the New Year. I would lose my voice forever in the year 2000; just the news you need to hear at Christmas time.
I woke up after surgery on 10 January 2000 and gradually the awful realisation that my voice, which I had had for 66 years and which my wife and children knew so well, had gone and nothing was left. I have never fully discovered exactly what was wrong with my larynx. I know it was cancerous, but where and why? Was the disease caused by smoking? I hadn't smoked in almost 30 years.
I have no doubt that my surgeon was good at his job, but in the days after my operation it seemed his only concern was how the flesh wounds were healing. Anything else (like feelings) was obviously someone else's job.
As healing progressed, I began speech therapy and was assured, “You will speak again.” Sure enough, after a short difficult period of learning techniques, I was delighted to be able to greet the gaggle at doctors' rounds with, “Good morning everyone.”
Progress has been good, and as my general wellbeing improved I was introduced to several new speaking techniques and I can now use a new hands-free system which allows me to speak apparently normally without using fingers or buttons.
I am always pleased when asked to speak with other patients who are waiting for the same operation. I try and give them some insight into what lies ahead and some hope that life in the future can be pretty good again.
Edward Martin, Edinburgh
Addition of chemotherapy to locoregional treatment
A meta-analysis showed that chemotherapy administered during radiotherapy (concurrent chemotherapy) gave an absolute benefit at five years of 8%.17 A number of randomised controlled trials have been published since, including the United Kingdom head and neck study of 971 patients.18 Several of these trials have consistently shown an overall survival benefit to concomitant chemoirradiation compared with radiotherapy alone, and a systematic review of this group showed an overall reduction in mortality of 11%.19
These gains in survival come at the expense of increased acute morbidity and might be equally produced by an increase in the radiation dose and potentially therefore not a true improvement in therapeutic index.20 Interest focuses on the future use of radiation protectants such as amifostine and growth factors (rhGM-CSF).21
The optimum chemotherapy regimen is not yet known. Platinum combinations, in particular cisplatin and fluorouracil, are generally regarded as the “gold standard,” but low dose chemotherapy may be equally effective as full dose,22 and radiation sensitisers such as nimorazole have shown similar results.23
Altered radiation fractionation schedules
Conventional radiotherapy consists of one daily treatment (fraction) Monday to Friday for three to seven weeks, varying between centres in the United Kingdom. Total doses vary from 50 Gy to 70 Gy. In the United States and Europe 60 Gy to 70 Gy are standard. These schedules are assumed to have the same overall radiobiological effect, which depends on the relation between overall time, total dose, and the number of fractions. They developed through clinical experience and training, however, randomised controlled trials have never been used to compare these different “conventional” fractionation schedules.
In the 1980s focus centred on time-fractionation schedules; low doses per fraction could give reduced late morbidity.24 This led to trials of hyperfractionation in which the dose per fraction was small—that is, divided up into two or three treatments per day instead of one. With increasing overall treatment time the total dose had to be increased to achieve the same effect. Accelerated regimens with shortened overall duration were therefore investigated, with the aim of reducing the time in which tumour cell repopulation could occur. These regimens have been studied by groups at Mount Vernon, United Kingdom, the Danish head and neck cancer group, radiation therapy, and oncology group in the United States, the European Organization for Research and Treatment of Cancer, and others with improvements in disease specific survival and locoregional control (see box B on bmj.com).
Brachytherapy
Brachytherapy is the implantation of radioactive sources in soft tissues or body cavities. Some are removed after a specified number of days—for example, iridium wires or hairpins; others, where the half life of the isotope is short, are left in place—for example, gold or iodine seeds (see fig A on bmj.com). This technique delivers high doses of radiation to the tumour while sparing healthy surrounding tissues. Brachytherapy has a number of useful applications (box B3).
Box 3.
Applications of brachytherapy
- Primary treatment of early tumours
- Boosting to the primary tumour after locoregional external beam radiotherapy
- Boosting to the tumour bed after surgery: catheters can be placed at the time of operation and active wires loaded when patient has recovered from anaesthetic
- Treatment of recurrent disease within a previously irradiated field
Low dose rate radiotherapy has the disadvantage of exposing staff to radiation. Patients are nursed in special lead protected rooms and visiting time is limited while implants are in place. High dose rate remote afterloading brachytherapy, which involves considerable reduction in overall treatment times for the patient and provides protection for staff, is increasingly being used. No controlled trial has compared its efficacy with low dose brachytherapy.
Intensity modulated radiotherapy
Intensity modulated radiotherapy is a developing new technology which can produce an even distribution of radiation dose within a target volume which follows the contours of an irregularly shaped tumour. It spares normal tissues close to or even within a concavity of a tumour and gives scope for escalation of radiation dose.25
Quality of life
Quality of life issues in head and neck cancer are crucial given the nature of the disease and its treatment, which can affect function in vital areas such as speech, swallowing, breathing, and facial appearance. This may have enormous sociopsychological impact and cause physical disability. Despite the importance of quality of life issues in comparisons of treatments, few clinical trials report meaningful quality of life data for long term outcome.
A recent large longitudinal study of 357 patients from Norway and Sweden found that patients with hypopharyngeal cancer had the worst health related quality of life score, compared with tumours at other sites within the head and neck, and that stage had the strongest impact. Women scored worse in emotional functioning and older patients scored better for emotional and social functioning but worse for physical functioning. At 12 months, quality of life tended to recover except for senses, dry mouth, and sexuality.26,27
Additional educational resources
Useful publications
DeVita VT, Hellman S, Rosenberg SA, eds. Cancer: principles and practice of oncology. 6th ed. Philadelphia: Lippincott Williams and Wilkins, 2000—Reflects developments in every aspect of oncology, from molecular biology, to multimodality treatment, to new data on cancer prevention by drugs and diet
British Association of Otorhinolaryngologists Head and Neck Surgeons. Effective head and neck cancer management. London: BAOHNS, 2000. www.baoms.org.uk/download/cancer/baorl-hns/hnc.pdf—Covers initial diagnosis, primary treatment, rehabilitating speech and swallowing, and management of airways
British Association of Otolaryngologists (www.orl-baohns.org)—General information about a range of conditions treated by the specialty
Information for patients
British Dental Association (www.bda-dentistry.org.uk)—Information and guidelines about oral cancer
CancerBACUP (www.cancerbacup.org.uk)—Support, information, and campaigning for people with cancer
National Association of Laryngectomee Clubs (www.laryngectomees.inuk.com)—Information, support, links, and contact for people who have had laryngectomies
Let's Face It (www.nas.com/∼letsfaceit/)—Resources for people with facial disfigurement
Palliation
Although a tracheostomy or peg tube can restore vital functions, a patient with slowly advancing incurable head and neck cancer can present enormous challenges. The palliative care team and Macmillan services have a pivotal role in controlling the symptoms of advanced head and neck malignancy. Palliative radiotherapy should be used judiciously to avoid a painful radiation mucositis causing further distress with little therapeutic gain. Epistaxis, stomal recurrence, or proptosis might be controlled with a short course of radiotherapy, and electron therapy or brachytherapy can be helpful for recurrence of tumours in the neck.
Untreated head and neck cancer is often chemosensitive, but response rates tend to be lower in recurrent disease. Cisplatin and infusional 5-fluorouracil in combination is the standard to which new combinations are compared. Docetaxel in combination with cisplatin shows response rates of around 40%, but so far does not seem to offer any survival advantage and its toxicity can be considerable.28 Oral agents such as fluoropyrimidines—for example, capecitabine—are under investigation.
Prognosis
Prognosis depends largely on the stage of presentation, with the single most important factor being the presence of neck node metastases, which reduces long term survival by 50%. Overall survival is considerably different from disease specific survival. These patients have serious cardiovascular and pulmonary comorbidity because of their drinking and smoking habits and have a high incidence of death from causes unrelated to their head and neck cancer.
Supplementary Material
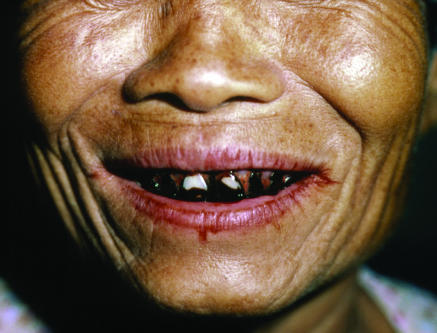
Figure 3.
SUE FORD/SPL
Oral cancers are common in Asia. One cause is chewing betel nuts, with the site of the cancer related to the site at which the nut is chewed
Box 4.
Ongoing research
- Optimisation of fractionation and chemotherapy or sensitisers
- Intensity modulated radiotherapy
- Novel therapies—for example, oncolytic viruses29
- Expanding role of laser
- Sensate flaps in reconstruction
Acknowledgments
We thank D Collie, consultant neuroradiologist, Western General Hospital, Edinburgh.
Footnotes
Competing interests: None declared.
References
- 1.Quinn M. Cancer trends in England and Wales 1950-1999. London: Stationery Office; 2001. . (Studies on medical and population subjects No 66.) [Google Scholar]
- 2. Scottish Cancer Intelligence Unit. Trends in cancer survival in Scotland 1971-1995. Edinburgh: Information and statistics division, SCIU: 2000.
- 3.van der Riet P, Nawroz H, Hruban RH, Corio R, Tokino K, Koch W, et al. Frequent loss of chromosome 9p21-22 early in head and neck cancer progression. Cancer Res. 1994;54:1156–1158. [PubMed] [Google Scholar]
- 4.Merino OR, Lindberg RD, Fletcher GH. An analysis of distant metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1977;40:145–151. doi: 10.1002/1097-0142(197707)40:1<145::aid-cncr2820400124>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Panosetti E, Luboinski B, Marmelle G, Richard JM. Multiple synchronous and metachronous cancers of the upper autodigestive tract: a nine-year study. Laryngoscope. 1989;99:1267–1273. doi: 10.1288/00005537-198912000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Department of Health. Referral guidelines for suspected cancer. London: DoH; 2000. p. 29.www.doh.gov.uk/pub/docs/doh/guidelines.pdf (accessed 20 Aug 2002). [Google Scholar]
- 7.McGuirt WF, McCabe BF. Significance of node biopsy before definitive treatment of cervical metastatic carcinoma. Laryngoscope. 1978;88:594–597. doi: 10.1002/lary.1978.88.4.594. [DOI] [PubMed] [Google Scholar]
- 8. Lodi G, Sardella A, Bez C, Demarosi F, Carrassi A. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev 2002;(1):CD001829. [DOI] [PubMed]
- 9.International Union against Cancer. New York: Wiley-Liss; 1997. Classification of malignant tumours. 5th ed. . (Sobin LH, Wittekind C, eds.) [Google Scholar]
- 10.British Association of Otorhinolaryngologists Head and Neck Surgeons. Effective head and neck cancer management. London: BAOHNS; 2000. www.baoms.org.uk/download/cancer/baorl-hns/hnc.pdf (accessed 20 Aug 2002). [Google Scholar]
- 11.Northrop MF, Fletcher GH, Jesse RH, Lindberg RP. Evolution of neck disease in patients with primary squamous cell carcinoma of the oral tongue, floor of mouth and palatine arch and clinically positive neck nodes neither fixed nor bilateral. Cancer. 1972;29:23–30. doi: 10.1002/1097-0142(197201)29:1<23::aid-cncr2820290104>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Steiner W. Results of curative laser microsurgery of laryngeal carcinoma. Am J Otolaryngol. 1993;14:116–121. doi: 10.1016/0196-0709(93)90050-h. [DOI] [PubMed] [Google Scholar]
- 13.Steiner W, Ambrosch P, Hess CF, Kron M. Organ preservation by trans-oral laser microsurgery in piriform fossa carcinoma. Otolaryngol Head Neck Surg. 2001;124:58–67. doi: 10.1067/mhn.2001.111597. [DOI] [PubMed] [Google Scholar]
- 14.Department of Veteran Affairs Laryngeal Cancer Study Group. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 15.Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, Sahmoud T. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. 1996;88:890–899. doi: 10.1093/jnci/88.13.890. [DOI] [PubMed] [Google Scholar]
- 16.Newkirk KA, Cullen KJ, Harter KW, Picken CA, Sessions RB, Davidson BJ. Planned neck dissection for advanced primary head and neck malignancy treated with organ preservation therapy: disease control and survival outcomes. Head Neck. 2001;23:73–79. doi: 10.1002/1097-0347(200102)23:2<73::aid-hed1001>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Pignon JP, Bourhis J, Domenge C, Designe L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355:949–955. [PubMed] [Google Scholar]
- 18.Tobias JS, Monson KM, Gladholm J, et al. UKHAN 1: a prospective multi-centre randomised trial investigating chemotherapy as part of initial management in advanced head and neck cancer. Radiother Oncol. 2001;58(suppl 1):S16. [Google Scholar]
- 19.Browman GP, Hodson DI, Mackenzie RJ, Bestic N, Zuraw L Cancer Care Ontario Practice Guideline Initiative Head and Neck Cancer Disease Site Group. Choosing a concomitant chemotherapy and radiotherapy regimen for squamous cell head and neck cancer: a systematic review of the published literature with sub group analysis. Head Neck. 2001;23:579–589. doi: 10.1002/hed.1081. [DOI] [PubMed] [Google Scholar]
- 20.Henk JM. Concomitant chemoradiation for head and neck cancer: saving lives or grays. Clin Oncol (R Coll Radiol) 2001;13:333–335. doi: 10.1053/clon.2001.9284. [DOI] [PubMed] [Google Scholar]
- 21.Capizzi Rl, Oster W. Chemoprotective and radioprotective effects of Amiphostine: an update of clinical trials. Int J Hematol. 2000;72:425–435. [PubMed] [Google Scholar]
- 22.Jeremic B, Shibamoto Y, Stanisavljevic B, Milojevic I, Milicic B, Niklic N. Radiation therapy alone or with concurrent low dose daily either cisplatin or carboplatin in locally advanced unresectable squamous cell carcinoma of the head and neck: a prospective randomised trial. Radiother Oncol. 1997;43:29–37. doi: 10.1016/s0167-8140(97)00048-0. [DOI] [PubMed] [Google Scholar]
- 23.Overgaard J, Hansen HS, Overgaard M, Bastholt L, Bertelsen A, Specht L, et al. A randomised double-blind phase III study of nimorazole as a hypoxic radiosensitiser of primary radiotherapy in supraglottic larynx and pharynx carcinoma: results of the Danish head and neck cancer study (DAHANCA) protocol 5-85. Radiother Oncol. 1998;48:344–346. doi: 10.1016/s0167-8140(97)00220-x. [DOI] [PubMed] [Google Scholar]
- 24.Thames HD, Jr, Withers HR, Peters LJ, Fletcher GH. Changes in early and late radiation responses with altered dose fractionation: implications for dose-survival relationships. Int J Radiat Oncol Biol Phys. 1982;8:219–226. doi: 10.1016/0360-3016(82)90517-x. [DOI] [PubMed] [Google Scholar]
- 25.Nutting C, Dearnaley DP, Webb S. Intensity modulated radiotherapy: a clinical review. Br J Radiol. 2000;73:459–469. doi: 10.1259/bjr.73.869.10884741. [DOI] [PubMed] [Google Scholar]
- 26.Hammerlid E, Bjordal K, Ahlner-Elmqvist M, Boysen M, Evenson JF, Biorklund A, et al. A prospective study of quality of life in head and neck cancer patients. I: At diagnosis. Laryngoscope. 2001;111:669–680. doi: 10.1097/00005537-200104000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Bjordal K, Ahlner-Elmqvist M, Hammerlid E, Boysen M, Evenson JF, Biorklund A, et al. A prospective study of quality of life in head and neck cancer patients. II. Longitudinal data. Laryngoscope. 2001;111:1440–1452. doi: 10.1097/00005537-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Caponigro F, Massa E, ManZione L, Rosati G, Biglietto M, De Lucia L, et al. Docetaxel and cisplatin in locally advanced or metastatic squamous-cell carcinoma of the head and neck: a phase II study of the southern Italy cooperative oncology group (SICOG) Ann Oncol. 2001;12:199–202. doi: 10.1023/a:1008322415335. [DOI] [PubMed] [Google Scholar]
- 29.Khuri FR, Nemunaitis J, Ganly I, Arseneay J, Tannock IF, Romel L, et al. A controlled trial of intratumoral ONYX-015, a selectively replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]