Abstract
The etiological heterogeneity of depression poses a challenge for prevention and intervention efforts. One solution is to map unique etiological pathways for subgroups defined by a singular risk factor. A relevant population for this approach is women who carry the premutation of the fragile X messenger ribonucleoprotein 1 (FMR1) gene, who are at high risk for adult-onset depression. This study explores a candidate neurophysiological marker of depression risk: reduced reward sensitivity, indexed by the reward positivity (RewP). The RewP has been linked to depression risk in the general population, but is unexplored within FMR1 premutation carriers. 16 women with the FMR1 premutation and a matched control group completed a simple guessing task while the electroencephalogram was recorded. Among premutation carriers, RewP difference score (win versus loss) was reduced. These preliminary finding suggest that the FMR1 premutation may confer increased risk for depression in part through abnormal neural sensitivity to rewards.
Keywords: Event-related potentials, Reward processing, FMR1 premutation, Depression, Fragile X
1. Introduction
For decades, the field searched for a universal cause of major depressive disorder (MDD). It is now clear that MDD follows the process of equifinality, whereby it is the common outcome of a wide range of genetic, physiological, and environmental factors [1]. Yet for any one individual, it is not clear what unique combination of factors causes MDD to develop. Mapping specific pathways to MDD is necessary for tailored prevention efforts and intervention targets that will optimize public health outcomes. One promising approach for isolating such pathways is to focus on genetically homogenous subtypes that are likely characterized by unique pathophysiological processes [2]. A recent genome-wide association study yielded 19 functionally distinct genetic pathways that confer MDD risk, each of which is a unique starting point for mapping MDD etiology [3].
Critically, one such genetic pathway is regulated by the fragile X messenger ribonucleoprotein 1 gene (FMR1). The fragile X premutation is a 55–199 CGG repeat expansion in the FMR1 promotor region, and it occurs in approximately 1:200 women [4]. Women who are carriers of the Fragile X premutation (PMC) are at high risk for adult-onset MDD, with 54% developing depression by age 50, independent of parental status [5]. The pathophysiological processes through which this premutation confers increased risk for depression, however, are unknown.
To begin to explore candidate processes of high depression risk among PMC, we tested neural sensitivity to reward delivery, as measured by the reward positivity (RewP). The RewP is an event-related potential that occurs within 300 ms following reward delivery [6], captures the initial evaluation of outcome valence [7], and correlates with reward-related BOLD signal within the medial prefrontal cortex and ventral striatum [8,9]. In the general population, reduced RewP amplitude has been linked to current MDD diagnosis [10,11], symptom severity [12,13,14,15], and prospectively predicts MDD onset in at-risk cohorts [16,17,18]. Thus, reduced RewP can be understood as a neurophysiological indicator of depression vulnerability. The RewP is thus far unexplored in PMC. Given findings linking the RewP to MDD vulnerability in the general population, as well as the established finding of high MDD prevalence in PMC, we hypothesized that the RewP might also be blunted in PMC. Reduced RewP amplitude in PMC would suggest a candidate pathophysiological process that is consistent with the known increased risk of MDD in this population, and that is shared with MDD vulnerability in the general population.
2. Material and methods
2.1. Participants
Data were collected at Purdue University from 16 female-sexed PMC, all of whom self-identified as women, and a control group of 16 women with no personal or family history of fragile X syndrome, autism, or intellectual disability. Two PMC participants were sisters, and three others were from the same family (sisters, daughter). PMC participants were recruited regionally through online advertisements, support groups, and the National Fragile X Foundation. Groups were matched on age and education level. Exclusion criteria for both groups were history of serious head injury, psychosis, neurological illness, or past-year substance use disorder. Participants were not specifically recruited based upon MDD status, although lifetime MDD history was assessed during study procedures.
Study procedures were approved by the Institutional Review Board at Purdue University and were in accordance with the Declaration of Helsinki. One participant was excluded from analyses because of poor quality ERP data. Thus, data were available from 15 controls and 16 PMC.
2.2. Genetic protocol
Whole blood samples were collected from PMC and sent to Rush University Medical Center (EBK), where FMR1 genotyping for CGG repeat length and Fragile X messenger ribonucleoprotein (FMRP) measurement were done. Genotyping was performed with the AmplideX® FMR1 and FMR1 mPCR reagents (Asuragen, Inc.) and FMRP was assayed using the Luminex-based method [19].
2.3. Cognitive and clinical assessment
Cognitive functioning was assessed using the Wechsler Abbreviated Scale of Intelligence-II (WASI-II) [20]. Lifetime history of MDD was determined using the Mini International Neuropsychiatric Interview for DSM-5 (MINI) [21], administered by Master’s-level interviewers and supervised by DF.
2.4. Neurophysiological assessment
The continuous electroencephalogram (EEG) was recorded from 32 Ag/AgCl active scalp electrodes with an actiCHAmp amplifier (Brain Products) while participants completed a simple guessing task using Presentation software (Neurobehavioral Systems, Inc., Berkeley, CA) to elicit the RewP [10]. On each trial, participants were shown an image of two doors and were asked to choose a door. Participants then received feedback indicating whether they won ($.40, ‘↑’) or lost money ($.20, ‘↓’) on that trial. Unbeknownst to participants, feedback was pseudo-random such that they won on 50% of trials (25 out of 50 trials). Stimulus timing was as follows: doors were presented until a behavioral response was made; a fixation mark (‘+’) for 1000 ms; a feedback stimulus for 2000 ms; a fixation mark for 1500 ms; “Click for the next round” instructions until a behavioral response was made.
Offline EEG analysis was performed in BrainVision Analyzer software (Brain Products). EEG data were referenced to the average mastoid (TP9/TP10), filtered from .1–30 Hz, and segmented relative to feedback onset (−200 to 800 ms). Data were corrected for blinks and eye movements [22]. Artifacts were identified as a step of 50 μV between samples, > 200 μV difference within 200 ms intervals, or < 0.5 μV change within 100 ms intervals; additional artifacts were identified by visual inspection. Segmented data were then averaged separately for wins and losses, and baseline corrected relative to the pre-stimulus interval (−200 to 0 ms). The RewP was scored separately for wins (RewP-win) and losses (RewP-loss) as the average amplitude from 275–325 ms3 at each of two frontocentral electrodes (Fz and Cz). The RewP-diff reflects the difference between conditions (win minus loss).
2.5. Statistical analysis
Group comparisons in sample characteristics were performed using t-tests and Fisher’s exact test. RewP amplitudes were compared across groups using a repeated-measures ANOVA with factors of Valence (win versus loss), Electrode (Fz versus Cz) and Group (PMC versus Control); analogous ANCOVA models adjusted for effects of cognitive ability and lifetime MDD. Significant higher-order interactions were followed up with independent-samples t-tests.
2.6. Data Availability
De-identified data that support the findings of this study are available from the corresponding author upon reasonable request.
3. Results
3.1. Sample characteristics
Sample characteristics are reported in Table 1. Premutation status was confirmed for all carriers (CGG repeat length: M=97.38, SD=16.88, Range: 79–150). The groups had similar demographic characteristics. Lifetime MDD was somewhat more common in the control group, but current MDD and past month psychiatric medication status were similar between groups. Of those currently taking psychiatric medications, 75% were taking a single selective serotonin reuptake inhibitor. One PMC and one control reported polytherapy of psychiatric medication, and both participants reported as-needed use of a benzodiazepine medication. PMC had lower Verbal Comprehension but similar Matrix Reasoning and Full-Scale IQ.
Table 1.
Sample characteristics and group comparisons between PMC and controls.
| PMC | Controls | Comparison | |||
|---|---|---|---|---|---|
| (n = 16) | (n = 15) | ||||
| N | % | N | % | p-value | |
| Ethnicity | 1.000 | ||||
| Hispanic/Latino | 1 | 6.3 | 1 | 6.3 | |
| Not Hispanic/Latino | 14 | 87.5 | 14 | 93.3 | |
| Missing | 1 | 6.3 | 0 | 0.0 | |
| Race | .315 | ||||
| Euro American | 15 | 93.8 | 11 | 81.3 | |
| Other | 1 | 6.3 | 3 | 18.8 | |
| Missing | 0 | 0.0 | 1 | 3.7 | |
| Household Income | .264 | ||||
| < $75,000 | 7 | 43.8 | 10 | 66.7 | |
| $75,000 or more | 8 | 50.0 | 4 | 26.7 | |
| Preferred not to answer | 1 | 6.3 | 1 | 6.7 | |
| Education | .220 | ||||
| At least some college | 10 | 62.5 | 13 | 86.7 | |
| Post-graduate education | 6 | 37.5 | 2 | 13.3 | |
| Lifetime MDD | .073 | ||||
| Present | 6 | 37.5 | 11 | 73.3 | |
| Absent | 10 | 62.5 | 4 | 26.7 | |
| Current MDD | .157 | ||||
| Present | 2 | 12.5 | 0 | 0.0 | |
| Absent | 14 | 87.5 | 15 | 100.0 | |
| Past-month psychiatric medication | .193 | ||||
| Yes | 3 | 18.8 | 6 | 40.0 | |
| No | 13 | 81.3 | 9 | 60.0 | |
| M | SD | M | SD | p-value | |
| Age (years) | 39.56 | 9.54 | 43.47 | 11.42 | .309 |
| Cognitive Functioning | |||||
| Verbal Comprehension | 52.56 | 5.39 | 59.13 | 9.80 | .027 |
| Matrix Reasoning | 56.00 | 5.48 | 54.73 | 6.71 | .568 |
| Full Scale IQ | 107.31 | 7.37 | 112.00 | 11.75 | .191 |
| Reward processing (Fz/Cz average in μV) | |||||
| RewP-wins | 15.44 | 5.66 | 14.18 | 6.85 | .290 |
| RewP-losses | 14.58 | 5.11 | 11.11 | 5.90 | .045 |
| RewP-diff | 0.86 | 2.99 | 3.07 | 2.27 | .014 |
3.2. Reward sensitivity
RewP amplitude and scalp topography for the PMC and control groups are shown in Fig. 1.
Fig. 1.
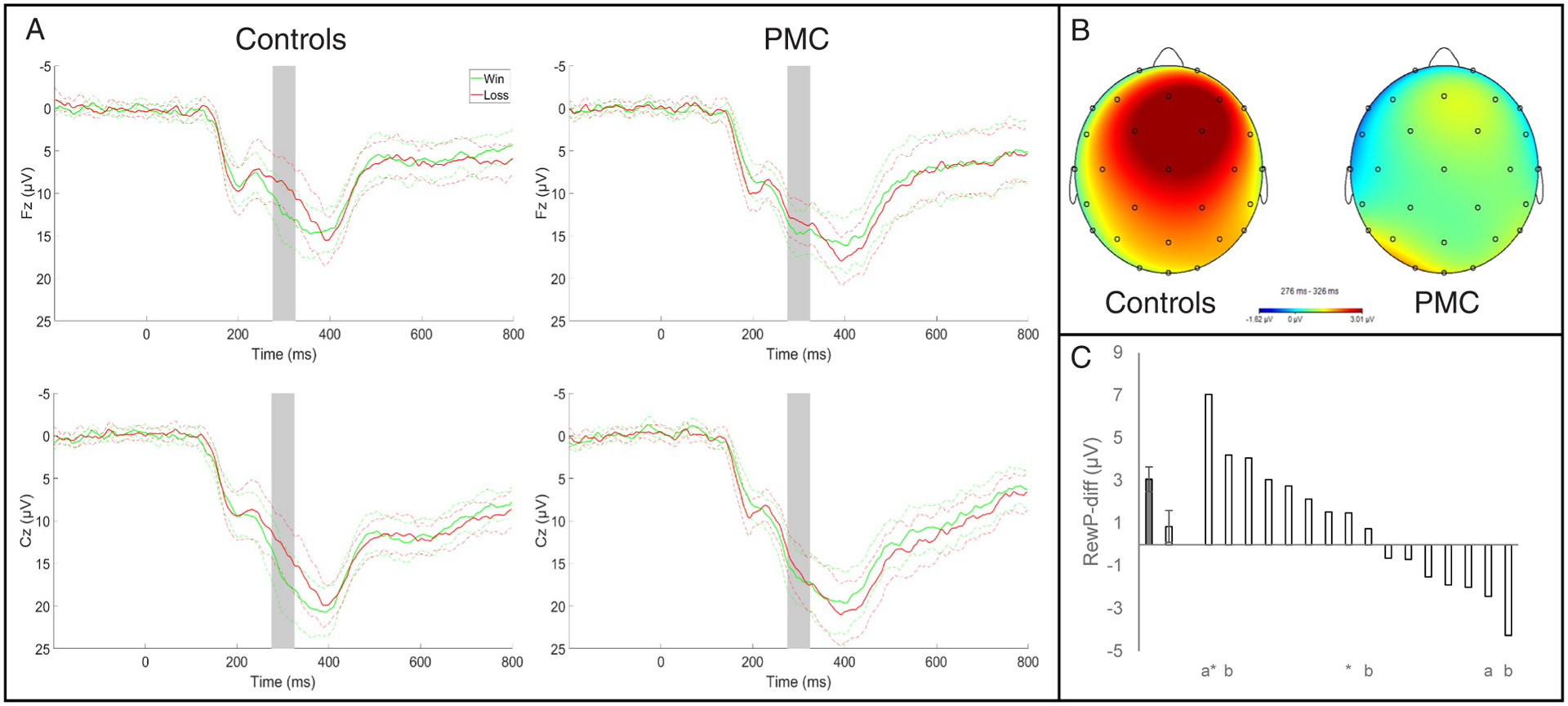
A) Waveforms are presented separately for monetary wins and losses; solid lines represent mean activity and dotted lines represent the 95% confidence interval. Shaded gray bars indicate the time window where the RewP was scored (275–325 ms). B) Headmaps depict the contrast of win versus loss conditions (i.e. RewP-diff). C) Average RewP-diff amplitude for controls (gray bar, left) and PMC (first white bar). Error bars show the standard error of the mean for each group. RewP-diff amplitude for individual PMC are depicted by subsequent white bars. Nested data for family members among the premutation group are indicated by a (family 1) and b (family 2); * ‘s indicate participants who were currently in a major depressive episode at the time of data collection.
Main effects of Valence (F1,29= 16.85, p < .001, η2=.368) and Electrode (F1,29= 51.44, p < .001, η2=.639) significantly predicted RewP amplitude; the main effect of premutation status was not significant (F1,29= 1.32, p = .261, η2=.043). Critically, the interaction between premutation status and outcome valence was significant (F1,29= 5.34, p = .028, η2=.156), and this interaction remained significant after controlling for Verbal Comprehension and lifetime MDD (F1,27 =5.36, p = .028, η2=.166). Effects did not vary by electrode site (Valence x Electrode: F1,29= 0.34, p = .565, η2=.012; Electrode x Group: F1,29= 3.71, p = .064, η2=.113; Valence x Electrode x Group: F1,29= 0.67, p = .420, η2=.023).
Follow-up t-tests in the control group yielded significant differences in RewP amplitude between wins and losses (Fz: t14 =5.30, p < .001; Cz: t14 =4.72, p < .001). Among PMC, the RewP was not significantly different between win and loss conditions (Fz: t15 =1.41, p = .179; Cz: t15 =0.74, p = .474). Altogether these results show reduced differentiation of wins versus losses among PMC. Panel C of Fig. 1 displays the mean RewP-diff for controls and PMC next to the individual RewP-diff scores for each of the PMC; 11 of 16 PMC (68.75%) exhibited RewP-diff scores that were less than the standard error of the control group.
To explore whether reduced RewP amplitude was explained by premutation status versus the CGG repeat length, we analyzed variability among the PMC group. The bivariate correlation between RewP-diff and the number of CGG repeats was not significant (Spearman’s ρ.14, p = .613). Other studies have observed nonlinear associations between CGG repeats and phenotypic characteristics of PMC [23,24]; following this, we calculated the correlation between CGG repeats and RewP-diff amplitude within mid-range (79–100) and high-range (>100) expansions. Associations were similarly small and non-significant (mid-range: n = 10, ρ−.25, p = .487; high-range: n = 6; ρ−.06, p = .913). Finally, we tested the mean difference in the RewP-diff amplitude between the mid-range and high-range groups. The mid-range group had a numerically smaller RewP-diff amplitude (M=0.25, SD=2.72) compared to the high-range group (M=1.87, SD=3.38) with a medium effect size (d=−0.54), but this group difference was not significant (t14=−1.05, p = .310).
4. Discussion
This study shows for the first time that neural sensitivity to reward delivery, as measured by RewP amplitude, may be affected by PMC status in adult women. These preliminary findings are notable considering genome-wide association studies in the general population have linked depression risk to a genetic pathway regulated by FMRP [3], the protein product of FMR1, and the established finding that PMC are at high risk of adult-onset MDD [5]. In general population, blunted RewP amplitudes indicate MDD vulnerability [12,16,17,25]. Together with the current findings, this raises the possibility that reduced differentiation in the RewP is a downstream consequence of genetic variability in FMR1, which warrants further investigation.
Thus, the RewP may represent a candidate process of MDD vulnerability relevant to PMC and shared with the general population. Abnormal RewP may help to explain how PMC status confers increased risk for adult-onset MDD. Additionally, the RewP was elicited using a simple task that is brief (<10 min) and has been used effectively in a wide range of populations [26], making it reasonable to administer to a large cohort with a range of cognitive functioning. If replicated in larger samples, the RewP may be leveraged alongside other MDD risk factors in order to construct a tailored etiological model of MDD specific to PMC.
MDD occurs in PMC alongside comorbid anxiety disorders, obsessive compulsive disorder, and substance use disorders, as well as a wide range of physical health problems [27]. This complexity makes it important to identify intermediate phenotypes that can explain how the FMR1 premutation confers risk to targeted clinical outcomes. In the general population, reduced RewP differentiation specifically predicts MDD onset and not comorbid anxiety disorders [18]. Future work should test whether reduced RewP differentiation prospectively predicts depression onset in PMC, the clinical specificity of this relationship, and links with genetic variability among PMC (e.g., possible non-linear associations with CGG repeat length). This would help clarify if reduced RewP differentiation is a feature of PMC that accounts for increased risk for MDD or is instead a phenotypic characteristic of PMC unrelated to incidence of MDD. Future work in larger sample should also clarify whether abnormal RewP in PMC is driven by abnormal reactivity to wins, losses, or both.
The current study is the first to identify a candidate pathophysiological process of high depression risk among PMC: altered reward processing, indicated by indicated by reduced RewP amplitude. While limited by the small sample size and the absence of genetic information from controls, this preliminary finding lays a foundation for future research to improve the prediction of MDD risk among PMC.
Acknowledgements
This project was supported by the Indiana Clinical and Translational Sciences Institute, funded in part by grant #UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award (DF). Additional support received from NIH (K23MH111955 to BK) and Purdue University (Center for Research on Brain, Behavior, and Neuro-Rehabilitation to DF; Executive Vice President for Research and Partnerships to DF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
EBK has received funding from Seaside Therapeutics, Novartis, Roche, Alcobra, Neuren, Cydan, Fulcrum, GW, Neurotrope, Marinus, Zynerba, BioMarin, Ovid, Yamo, Acadia, Ionis, Ultragenyx, GeneTx, Neurogene, and Lumos Pharmaceuticals to consult on trial design or development strategies and/or conduct clinical studies in FXS or other NNDs or neurodegenerative disorders; from Vtesse/Sucampo/Mallinckrodt Pharmaceuticals to conduct clinical trials in NP-C; and from Asuragen Inc. to develop testing standards for FMR1 testing. All funding to EBK is directed to Rush University Medical Center (RUMC) in support of rare disease programs. EBK receives no personal funds. RH, BK, KN, WSN, TS, TL, TG, and DF report no disclosures.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dan Foti reports financial support was provided by National Institutes of Health. Bridgette Kelleher reports financial support was provided by National Institutes of Health. Dan Foti reports financial support was provided by Purdue University. Elizabeth Berry-Kravis reports a relationship with Seaside Therapeutics that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with Novartis that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with Roche that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with Alcobra that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with Neuren that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with Cydan Development Inc that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with Fulcrum that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with GW that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with Neurotrope that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with Marinus Pharmaceuticals Inc that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with Zynerba Pharmaceuticals, Inc that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with BioMarin Pharmaceutical Inc that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with Ovid Therapeutics Inc that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with Yamo that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with ACADIA Pharmaceuticals Inc that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with Ionis Pharmaceuticals Inc that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with Ultragenyx Pharmaceutical Inc that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with GeneTx Biotherapeutics that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with Neurogene that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with Lumos Pharma that includes: consulting or advisory and funding grants. Elizabeth Berry-Kravis reports a relationship with Vtesse that includes: funding grants. Elizabeth Berry-Kravis reports a relationship with Sucampo that includes: funding grants. Elizabeth Berry-Kravis reports a relationship with Mallinckrodt that includes: funding grants. Elizabeth Berry-Kravis reports a relationship with Asuragen Inc that includes: consulting or advisory. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
We chose this time window by inspecting the grand average of the difference waveform while blind to group status. Because a 100 ms time window is often used in the literature, we also analyzed the data using a 100 ms time window from 250–350 ms, and, separately, a 100 ms time window around the peak of the difference wave for each participant using a semi-automated peak detection algorithm. Results were not substantively changed by use of the wider time window either at 250–350 ms or using peak detection. Thus, we report results from the more conservative 50 ms time window.
References
- [1].Belmaker RH, Agam G. Major depressive disorder. N Engl J Med Jan. 2008;vol. 358 (1). 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- [2].Flint J, Kendler KS. The genetics of major depression. Neuron 2014;vol. 81(3). 10.1016/j.neuron.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wray NR, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet May 2018;vol. 50(5). 10.1038/s41588-018-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tassone F, et al. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med 2012;vol. 4(12). 10.1186/gm401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Roberts JE, Tonnsen BL, McCary LM, Ford AL, Golden RN, Bailey DB. Trajectory and predictors of depression and anxiety disorders in mothers with the FMR1 Premutation. Biol Psychiatry 2016;vol. 79(10). 10.1016/j.biopsych.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Proudfit GH. The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology 2015;vol. 52(4). 10.1111/psyp.12370. [DOI] [PubMed] [Google Scholar]
- [7].Foti D, Weinberg A, Dien J, Hajcak G. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity. Hum Brain Mapp Dec. 2011;vol. 32(12). 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Becker MPI, Nitsch AM, Miltner WHR, Straube T. A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. J Neurosci 2014;vol. 34(8). 10.1523/JNEUROSCI.3684-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. Neuroimage 2011;vol. 57 (4). 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- [10].Foti D, Carlson JM, Sauder CL, Proudfit GH. Reward dysfunction in major depression: multimodal neuroimaging evidence for refining the melancholic phenotype. Neuroimage 2014;vol. 101. 10.1016/j.neuroimage.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Klawohn J, Burani K, Bruchnak A, Santopetro N, Hajcak G. Reduced neural response to reward and pleasant pictures independently relate to depression. Psychol Med 2021;vol. 51(5). 10.1017/S0033291719003659. [DOI] [PubMed] [Google Scholar]
- [12].Ait Oumeziane B, Foti D. Reward-related neural dysfunction across depression and impulsivity: a dimensional approach. Psychophysiology 2016;vol. 53(8). 10.1111/psyp.12672. [DOI] [PubMed] [Google Scholar]
- [13].Bress JN, Smith E, Foti D, Klein DN, Hajcak G. Neural response to reward and depressive symptoms in late childhood to early adolescence. Biol Psychol Jan. 2012;vol. 89(1). 10.1016/j.biopsycho.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Novak BK, Novak KD, Lynam DR, Foti D. Individual differences in the time course of reward processing: Stage-specific links with depression and impulsivity. Biol Psychol 2016;vol. 119. 10.1016/j.biopsycho.2016.07.008. [DOI] [PubMed] [Google Scholar]
- [15].Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology 2009;vol. 46(3). 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- [16].Foti D, Kotov R, Klein DN, Hajcak G. Abnormal neural sensitivity to monetary gains versus losses among adolescents at risk for depression. J Abnorm Child Psychol 2011;vol. 39(7). 10.1007/s10802-011-9503-9. [DOI] [PubMed] [Google Scholar]
- [17].Bress JN, Foti D, Kotov R, Klein DN, Hajcak G. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology 2013;vol. 50(1). 10.1111/j.1469-8986.2012.01485.x. [DOI] [PubMed] [Google Scholar]
- [18].Nelson BD, Perlman G, Klein DN, Kotov R, Hajcak G. Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. Am J Psychiatry 2016;vol. 173(12). 10.1176/appi.ajp.2016.15121524. [DOI] [PubMed] [Google Scholar]
- [19].Lafauci G, et al. Fragile X screening by quantification of FMRP in dried blood spots by a luminex immunoassay. J Mol Diagn 2013;vol. 15(4). 10.1016/j.jmoldx.2013.02.006. [DOI] [PubMed] [Google Scholar]
- [20].McCrimmon AW, Smith AD. Review of the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-II). J Psychoeduc Assess 2013;vol. 31(3). 10.1177/0734282912467756. [DOI] [Google Scholar]
- [21].Sheehan DV, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998. [PubMed] [Google Scholar]
- [22].Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artifact. Electro Clin Neurophysiol 1983. 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- [23].Sullivan AK, et al. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod 2005;vol. 20(2). 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- [24].Ennis S, Ward D, Murray A. Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. Eur J Hum Genet 2006;vol. 14(2). 10.1038/sj.ejhg.5201510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Weinberg A, Liu H, Shankman SA. Blunted neural response to errors as a trait marker of melancholic depression. Biol Psychol 2016;vol. 113. 10.1016/j.biopsycho.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Belden AC, et al. Neural correlates of reward processing in depressed and healthy preschool-age children. J Am Acad Child Adolesc Psychiatry 2016;vol. 55(12). 10.1016/j.jaac.2016.09.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hagerman RJ, Protic D, Rajaratnam A, Salcedo-Arellano MJ, Aydin EY, Schneider A. Fragile X-Associated Neuropsychiatric Disorders (FXAND). Front Psychiatry 2018;vol. 9. 10.3389/fpsyt.2018.00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
De-identified data that support the findings of this study are available from the corresponding author upon reasonable request.


