Abstract
The purpose of this study was to determine which regions of the VP6 protein of the murine rotavirus strain EDIM are able to elicit protection against rotavirus shedding in the adult mouse model following intranasal (i.n.) immunization with fragments of VP6 and a subsequent oral EDIM challenge. In the initial experiment, the first (fragment AB), middle (BC), or last (CD) part of VP6 that was genetically fused to maltose-binding protein (MBP) and expressed in Escherichia coli was examined. Mice (BALB/c) immunized with two 9-μg doses of each of the chimeras and 10 μg of the mucosal adjuvant LT(R192G) were found to be protected against EDIM shedding (80, 92, and nearly 100% reduction, respectively; P ≤ 0.01) following challenge. Because CD produced almost complete protection, we prepared four E. coli-expressed, MBP-fused chimeras containing overlapping fragments of the CD region (i.e., CD1, CD2, CD3, and CD4) whose lengths ranged from 61 to 67 amino acid residues. Following i.n. immunization, CD1, CD2, and CD4 induced significant (P ≤ 0.004) protection (88, 84, and 92% reduction, respectively). In addition, 11 peptides (18 to 30 residues) of the CD region with between 0 and 13 overlapping amino acids were synthesized. Two 50-μg doses of each peptide with LT(R192G) were administered i.n. to BALB/c mice. Five peptides were found to elicit significant (P ≤ 0.02) protection. Moreover, a 14-amino-acid region within peptide 6 containing a putative CD4+ T-cell epitope was found to confer nearly complete protection, suggesting a protective role for CD4+ T cells. Mice that were protected by fragments BC and CD1 and four of the five protective synthetic peptides did not develop measurable rotavirus antibodies in serum or stool, implying that protection induced by these domains was not dependent on antibody. Together, these observations suggest that multiple regions of VP6 can stimulate protection, a region of VP6 as small as 14 amino acids containing a CD4+ T-cell epitope can stimulate nearly complete protection, and protection mediated by a subset of epitopes in the VP6 protein does not require antibodies in BALB/c mice.
Rotavirus infections are the primary cause of severe gastroenteritis in young children and are the cause of nearly one million deaths worldwide each year (3, 20, 32). Several rotavirus vaccine candidates have been evaluated in clinical trials with various degrees of success. All have been live, attenuated rotavirus strains that are delivered orally to mimic the excellent protection normally associated with natural rotavirus infection (4, 5, 13, 17, 18, 24–26, 30). The most studied of these vaccine candidates (Rotashield) consistently provided approximately 50% protection against all rotavirus diarrhea and 75% protection against severe rotavirus disease (5, 17, 18, 26). In 1998, this vaccine was recommended for routine childhood immunization in the United States but was withdrawn in less than 1 year after being associated with intussusception, a rare form of bowel blockage found most frequently in young children (9). Although it is unknown why Rotashield increased the risk of intussusception, all future rotavirus vaccine candidates are expected to be even more carefully scrutinized prior to their general usage.
To minimize possible association with intussusception, nonreplicating rotavirus vaccine candidates represent an obvious alternative. In 1990, we developed an adult mouse model with which to evaluate possible novel rotavirus vaccines (33). Using this model, we recently immunized BALB/c and B-cell-deficient μMt mice intranasally (i.n.) with an Escherichia coli-expressed chimeric protein containing the rotavirus group antigen VP6 of murine rotavirus strain EDIM (12). When challenged with EDIM, both strains of immunized mice shed approximately 97% less rotavirus than did unimmunized controls during the subsequent week. In μMt mice, this excellent protection was not dependent upon anti-VP6 antibody because these mice made no detectable rotavirus antibody following VP6 immunization. Protection was highly dependent on coadministration of attenuated E. coli heat-labile toxin LT(R192G) as an adjuvant. The possible usefulness of chimeric VP6 as a vaccine candidate was enhanced by the observation that protection remained constant for at least 3 months after immunization and equal protection was induced by 1, 2, or 3 i.n. doses of this protein (12).
Because of the potential for VP6 to be developed into a rotavirus vaccine, it was of immediate interest to determine the locations of the protective epitopes within the VP6 protein. Once these are identified, it may be possible to study the immune effector mechanisms they induce. At the same time, identification of protective epitopes within VP6 may advance the development of the subunit VP6 vaccine into peptide vaccines. The purpose of this study was to use deletion mutant forms of VP6 and synthetic peptides to compare the abilities of different regions of the VP6 protein to elicit protection in the adult mouse model.
MATERIALS AND METHODS
Virus.
The murine EDIM strain of rotavirus that was used throughout this study was originally isolated from a fecal specimen of an infected mouse (obtained from M. Collins, Microbiological Associates, Bethesda, Md.). It was adapted to grow in cell culture by serial passage in MA-104 cells, a monkey kidney-derived cell line. After the ninth passage, the virus was triply plaque purified. This preparation was used for the construction of recombinant plasmids containing the entire VP6 gene or deletion mutant forms of VP6. Cell culture-adapted EDIM (passage 9) was used to challenge mice after immunization. This virus preparation had a titer of 107 focus-forming units (FFU)/ml.
Construction of recombinant pMAL-c2X plasmids.
The bacterial expression plasmid pMAL-c2X (New England Biolabs, Beverly, Mass.) was used to construct recombinant plasmids pMAL-c2X/EDIM6, pMAL-c2X/EDIM6AB, pMAL-c2X/EDIM6BC, pMAL-c2X/EDIM6CD, pMAL-c2X/EDIM6CD1, pMAL-c2X/EDIM6CD2, pMAL-c2X/EDIM6CD3, and pMAL-c2X/EDIM6CD4 containing VP6 or deletion mutant forms of VP6 (i.e., AB, BC, CD, CD1, CD2, CD3, and CD4, respectively) using standard cloning procedures (1). Briefly, cDNAs were synthesized by PCR using pcDNA1/EDIM6 (11) as the template and gene-specific primers (Table 1) and were inserted into the XmnI restriction site of pMAL-c2X. The inserted sequences were placed downstream from the E. coli malE gene, which encodes the maltose-binding protein (MBP), and the factor Xa proteolytic cleavage site (Ile-Glu-Gly-Arg). Recombinant pMAL-c2X plasmids were transformed into BL21(DE3), a protease-deficient strain of E. coli. Bacterial colonies containing deletion mutant forms of VP6 were identified by blue-to-white selection.
TABLE 1.
Oligonucleotide primers used in PCRs to construct chimeric VP6 proteins
| Protein or fragment | Amino acid position in VP6 | Primer sequence
|
|
|---|---|---|---|
| Forward | Reverse | ||
| VP6 | 1–397 | ATG GAT GTG CTG TAC TCT ATC | TCA CTT TAC CAG CAT GCT TCT AAT |
| AB | 1–196 | ATG GAT GTG CTG TAC TCT ATC | TCA CGA GTA GTC GAA TCC TGC AAC |
| BC | 97–299 | ATG GAT GAA ATG ATG CGA GAG TCA | TCA GAA TGG CGG TCT CAT CAA TTG |
| CD | 197–397 | TGC GCA ATT GCT CCA GCT | TCA CTT TAC CAG CAT GCT TCT AAT |
| CD1 | 197–263 | TGC GCA ATT GCT CCA GCT | GAA CTC AAC TTC TAC ATT ATT TGG |
| CD2 | 244–310 | GCA ACT ACA TGG TAC TTC AAC CCA | ATT TGG GAA AAG TGC AGT CAC TGC |
| CD3 | 291–351 | TCA TTT CAA TTG ATG AGA CCG CCA | TTG TCT GAC TGA CGT CAC ATT GGC |
| CD4 | 332–397 | GAA TCA GTT CTC GCG GAT GCA AGT | TCA CTT TAC CAG CAT GCT TCT AAT |
Induction of recombinant proteins.
Induction of expression of MBP-based chimeric proteins was performed by the method of Jarrett and Foster (16) as previously described (12). A single colony of recombinant bacteria expressing each chimeric protein was grown as an overnight culture (37°C) in 50 ml of rich broth (10 g of tryptone per liter, 5 g of yeast extract per liter, 5 g of NaCl per liter, 2 g of glucose per liter, 100 mg of ampicillin per liter). On the following day, 10 ml of the overnight cell culture was inoculated into 1 liter of rich broth. When the A600 reached approximately 0.6, IPTG (isopropyl-β-d-thiogalactopyranoside) was added to give a final concentration of 0.3 mM to induce expression of chimeric proteins. At 3 h postinduction, the cell suspension was centrifuged (4,000 × g, 20 min, 4°C) to harvest the cells, which were washed in phosphate-buffered saline and again centrifuged. The supernatant was discarded, and the cell pellet was frozen at −20°C.
Preparation of soluble chimeric proteins by affinity chromatography.
The procedure used to prepare chimeric VP6 proteins has been described elsewhere (12). In short, the frozen bacterial pellets were thawed and resuspended in 50 ml of buffer L (5 mM NaH2PO4, 10 mM Na2HPO4, 30 mM NaCl, 10 mM β-mercaptoethanol, 0.2% Tween 20, 200 mg of lysozyme per liter). After digestion (15 min, room temperature), the suspensions were sonicated (Bronwill BioSonic IV, 50% power setting, three 30-s bursts; VWR Scientific, Piscataway, N.J.) in an ice-and-water bath. NaCl and RNase A (final concentrations of 26.5 mg/ml and 5 μg/ml, respectively) were then added. The lysates were centrifuged (54,000 × g, 30 min) to separate insoluble cell debris (which included inclusion bodies containing insoluble chimeric VP6 protein) from supernatants (soluble fraction) that contained soluble chimeric rotavirus proteins. Amylose resin was prepared by placing 25 ml of the packed resin (New England Biolabs) in a 250-ml centrifuge tube and washing it twice with 8 volumes of buffer C (buffer L containing 0.5 M NaCl). The mixture was rocked for 30 min at 4°C, and the resin was recovered by centrifugation (2,100 × g, 5 min). The soluble fractions were mixed with amylose resin for 2 h in a 500-ml flask on a magnetic stirrer. After centrifugation (2,100 × g, 5 min), the resin was recovered and then resuspended in 50 ml of buffer C, rocked for 30 min, and centrifuged to recover the resin. The resin was washed in this manner three times and finally washed overnight with 500 ml of buffer C. On the following day, the resin was recovered by centrifugation (2,100 × g, 5 min), resuspended in 50 ml of buffer D (50 mM Tris-HCl [pH 7.5], 50 mM NaCl, 1 mM EDTA, 10 mM β-mercaptoethanol), and rocked for 30 min. The resin was pelleted by centrifugation (2,100 × g, 5 min), and the bound fusion protein was eluted from the resin with 250 ml of 15 mM maltose in buffer D for 2 h. The resin was removed by centrifugation (2,100 × g, 5 min), and the buffer in the supernatant containing the fusion proteins was replaced with phosphate-buffered saline and simultaneously concentrated by ultrafiltration using a stirred-cell concentrator (model 8400; Amicon Inc., Beverly, Mass.). The concentration of preparations of chimeric VP6 protein or chimeric VP6 fragments was maintained at 180 μg/ml (i.e., 9 μg/50-μl dose), since further concentration led to gradual precipitation of the purified proteins. Concentrations of purified proteins were measured by the method described by Bradford (6).
Western blot analyses of chimeric rotavirus proteins.
Preparations of affinity chromatography purified chimeric VP6, AB, BC, and CD proteins were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). Samples were suspended in gel loading buffer (50 mM Tris [pH 6.8], 10% glycerol, 5% SDS, 5% β-mercaptoethanol, 0.005% bromophenol blue), heated (95°C, 5 min), and subjected to SDS–PAGE. Following SDS-PAGE, separated proteins were blotted to nitrocellulose sheets which were then blocked with 5% skim milk in TBS (25 mM Tris-HCl [pH 7.5], 0.9% NaCl). The sheets were then incubated with a rabbit anti-MBP serum (1:10,000 dilution; New England Biolabs). After washing with TTBS (0.1% Tween 20 in Tris-buffered saline [TBS]), the sheet was incubated with goat anti-rabbit immunoglobulin G (IgG) conjugated to alkaline phosphatase (1:3,000; Life Technologies, Gaithersburg, Md.). The sheet was washed with TTBS and then incubated with 5-bromo-4-chloro-3-indolylphosphate (BCIP; 0.25 mg/ml) and nitroblue tetrazolium (0.25 mg/ml; Life Technologies) to visualize bound antibodies.
Western blot analyses of immune sera.
To determine whether the immune sera obtained from mice vaccinated with chimeric deletion mutant form AB, BC, or CD of VP6 generated antibodies against the specific rotavirus proteins, triple-layered rotavirus particles were subjected to SDS-PAGE as described above. Following SDS-PAGE, separated rotavirus proteins were electrophoretically transferred to nitrocellulose sheets, which were cut into strips. The strips were blocked with 5% skim milk in TBS. The strips were then incubated with antisera obtained from mice immunized with chimeric VP6, AB, BC, or CD, which was used at a 1:100 dilution. After washing with TTBS, the strips were incubated with goat anti-mouse IgG conjugated to alkaline phosphatase. The strips were washed with TTBS and then incubated with nitroblue tetrazolium and BCIP to visualize bound antibodies as described above.
Synthetic peptides.
Peptide synthesis was performed by Quality Control Biochemicals, Inc. (Hopkinton, Mass.), or by Sigma-Genosys (The Woodlands, Tex.). The peptides designated 1 through 11 were derived from the amino acid sequence of the last half of VP6 (amino acids 197 to 397; Table 2). The lengths of the peptides ranged from 18 to 30 residues, and the overlapping sequences ranged from 0 to 13 residues in length. Peptide 6-14, a 14-amino-acid peptide contained within 25-amino-acid peptide 6, was also synthesized. The traditional solid-phase method of peptide synthesis was employed utilizing orthogonally protected amino acids. Cleavage and deprotection were performed with aqueous trifluoroacetic acid in the presence of scavengers. The purity of the peptides was greater than 95% according to mass spectral and reverse-phase high-pressure liquid chromatography analyses.
TABLE 2.
Amino acid sequences of synthetic peptides used to map protective epitopes in the CD region of VP6
| Peptide no. | No. of residues | Location in VP6 | Amino acid sequencea |
|---|---|---|---|
| 1 | 25 | 197–221 | CAINAPANIQQFEHIVQLRRVLTTA |
| 2 | 18 | 227–244 | PDAERFSFPRVINSADGA |
| 3 | 30 | 232–261 | FSFPRVINSADGATTWYFNPVILRPNNVEV |
| 4 | 29 | 249–277 | FNPVILRPNNVEVEFLLNGQVINTYQARF |
| 5 | 30 | 266–295 | NGQVINTYQARFGTIVARNFDTIRLSFQLM |
| 6 | 25 | 283–307 | RNFDTIRLSFQLMRPPNMTPAVTAL |
| 7 | 30 | 300–329 | MTPAVTALFPNAQPFEHHATVGLTLRIDSA |
| 8 | 29 | 317–346 | HATVGbLTLRIDSAICESVLADASETMLANV |
| 9 | 22 | 335–355 | VLADASETMLANVTSVRQEYAI |
| 10 | 30 | 351–380 | QEYAIPVGPVFPPGMNWTDLITNYSPSRED |
| 11 | 30 | 368–397 | TDLITNYSPSREDNLQRVFTVASIRSMLVK |
| 6-14 | 14 | 289–302 | RLSFQLMRPPNMTP |
Overlapping residues between successive peptides are in boldface.
The glycine residue was excluded from the peptide due to technical difficulties in synthesis.
Mouse strains.
Rotavirus antibody-free BALB/c mice were used in these studies. They were purchased from Harlan-Sprague-Dawley (Indianapolis, Ind.) when 6 weeks of age. All mice were housed in groups of three or four in sterile microisolation cages. All procedures were carried out in accordance with protocols reviewed and approved by the Children's Hospital Research Foundation Institutional Animal Care and Use Committee.
Immunization of mice with chimeric rotavirus proteins or synthetic peptides.
Immunization of mice (i.n.) was carried out under light sedation, after placing them in a closed vessel with Metafane (methoxyflurane; Pitman-Moore, Inc., Mundelein, Ill.) until they could no longer stand, by administration of two 60-μl doses (30 μl per nostril) of immunogen separated by 2 weeks. In an earlier study (12), we found that one dose of 9 μg of chimeric MBP-VP6 in a 50-μl volume (i.e., 2 μM) was sufficient to induce 99.5% protection. Since priming followed by one or two boosts did not further stimulate the protection obtained with a single dose, it is not likely that increasing the antigen dose would improve protection higher than 99.5%. Nevertheless, to ensure maximum protection, a two-dose rather a one-dose regimen was implemented for all immunizations in the present study. In every case, each dose of immunogen consisted of 9 μg of one of the purified chimeric proteins or 50 μg of one of the synthetic peptides along with 10 μg (10 μl) of the attenuated E. coli heat-labile toxin LT(R192G) adjuvant in a 60-μl volume. The adjuvant LT(R192G) carries a mutation in the proteolytic site of its A subunit at amino acid 192 with the replacement of the arginine with a glycine residue. The mutation abrogates cleavage of LT(R192G) and attenuates the toxicity of the protein (14). To compare the protective efficacies of the chimeric AB, BC, and CD fragments, 9 μg (50 μl of 2.8 μM) of the chimeric AB, BC, or CD region of VP6 was used to inoculate mice. To compare the efficacies of the chimeric CD1, CD2, CD3, and CD4 fragments, 9 μg (50 μl of approximately 3.4 μM) of each chimeric fragment was used for immunization. To determine the peptides that could elicit protection, mice were inoculated with 50 μg (50 μl) with concentrations of each peptide ranging from 288 to 592 μM (see Table 6). In a previous study, we showed that immunization of mice with 10 μg of LT(R192G) alone did not produce rotavirus antibodies or stimulate protection (12).
TABLE 6.
Protection of mice from shedding of rotavirus antigen after i.n. immunization with overlapping synthetic peptides and LT(R192G)a
| Peptide | Concn (μM) | % Reduction in shedding relative to control group |
|---|---|---|
| None | ||
| VP6 | 2 | 98 (97–99)bc |
| CD | 2.8 | 98 (96–99)bc |
| 1 | 356 | 6 |
| 2 | 513 | 70c |
| 3 | 292 | 57c |
| 4 | 294 | 77c |
| 5 | 288 | 0 |
| 6 | 346 | 85 (74–93)bc |
| 7 | 312 | 18 |
| 8 | 328 | 51 |
| 9 | 420 | 0 |
| 10 | 294 | 0 |
| 11 | 294 | 64c |
| 6-14 | 592 | 96 (93–98)bc |
Groups of eight BALB/c mice were immunized i.n. with two 50-μg doses (50 μl) of one of the synthetic peptides at the indicated concentration and 10 μg of LT(192G). Immunized mice were challenged with EDIM 4 weeks after the last immunization. Shedding of rotavirus antigen (nanograms per mouse per day) was determined by ELISA during the subsequent 7 days.
Average reduction in shedding (range) of three experiments is shown.
Shedding was significantly (P ≤ 0.02) less than in the mock-immunized control group.
Challenge of mice with EDIM rotavirus.
Four weeks after the last immunization, mice were orally (gavage) challenged with 4 × 104 FFU (focus-forming units), which was equivalent to 100 50% shedding doses of passage 9 EDIM.
Detection of rotavirus antigen in stools.
Two fecal pellets were collected from each mouse for 7 or more days following EDIM challenge and kept in 1 ml of Earle's balanced salt solution. Samples were stored frozen until analyzed, at which time they were homogenized and centrifuged (1,500 × g, 5 min, 4°C) to remove debris. Quantities of rotavirus antigen in the fecal samples were determined by enzyme-linked immunosorbent assay (ELISA) as nanograms per milliliter per specimen using methods already described (19).
Determination of rotavirus antibody titers.
Blood samples were collected by retro-orbital capillary plexus puncture before the first immunization, before challenge, and 3 weeks after challenge. Stool specimens were collected at the same periods. Titers of rotavirus IgG and IgA in serum, as well as fecal rotavirus IgA, were determined by ELISA using EDIM lysate as the antigen as previously described (12, 19).
Statistical methods.
Statistical analyses of differences in the amounts of shed rotavirus antigen and titers of rotavirus-specific antibodies between groups of mice immunized with different chimeric proteins or synthetic peptides and mock-vaccinated groups were performed by Students t test (analysis of variance). Differences between groups were considered significant when the probability (P) level was ≤0.05.
RESULTS
Protective epitopes are present in the first, middle, and last parts of VP6.
Three plasmids that expressed large overlapping recombinant proteins encompassing the entire VP6 molecule were constructed to determine the locations of protective epitopes in VP6. These recombinant plasmids, which were constructed by deleting portions of the VP6 gene, expressed approximately the first (amino acids 1 to 196; fragment AB), middle (amino acids 97 to 299; fragment BC), and last (amino acids 197 to 397; fragment CD) parts of VP6. The gene sequences encoding these VP6 fragments were cloned into plasmid pMAL/c2X. They were then expressed in E. coli as chimeric proteins with the carboxyl terminus of MBP, encoded by the plasmid, genetically fused to the amino terminus of the protein fragments. The chimeric proteins were purified by affinity chromatography using amylose resin and analyzed by Western blotting as described for chimeric VP6 (12). Polypeptides migrating with the expected mobility of chimeric fragments AB (65.2 kDa), BC (66.1 kDa), and CD (65.14 kDa) were detected. As in the case of chimeric VP6 (12), truncated MBP-containing polypeptides were also obtained even though the proteins were expressed in a protease-deficient strain of E. coli. Furthermore, the amount of truncated products was equivalent for each of the expressed chimeric protein, as determined by Western blot analysis (results not shown).
To determine whether any of these VP6 fragments, i.e., VP6 deletion mutant proteins, contained protective epitopes, groups of BALB/c mice were immunized i.n. with two 9-μg inoculations of chimeric VP6 fragments (50 μl of 2.8 μM) or unmodified VP6 protein (50 μl of 2 μM). In every case, 10 μg of the mucosal adjuvant LT(R192G) was included in the inoculum. The mice were then orally challenged with live murine rotavirus strain EDIM 4 weeks after the second immunization. Stool specimens were collected from the immunized and mock-immunized groups between 1 and 7 days after challenge. The quantities of rotavirus antigen in the stools were measured by ELISA. The protective efficacy of each VP6 fragment was calculated as the reduction in rotavirus shedding in each immunized group relative to the mock-immunized group. Mice immunized with each of the VP6 fragments (i.e., AB, BC, or CD) were protected against EDIM shedding (P ≤ 0.01, Table 3). However, reductions in shedding in the groups immunized with fragments AB and BC (79.4 and 92.5%, respectively) were significantly (P ≤ 0.005) less than that in the CD-immunized group (99.8%). Therefore, protective epitopes were present in all three VP6 fragments but the CD fragment elicited the best protection.
TABLE 3.
Protection of mice from shedding of rotavirus antigen after i.n. immunization with overlapping fragments of VP6 and LT(R192G)a
| Inoculum | Mean quantity (ng) ± SEM of rotavirus antigen shed/mouse/day | % Reduction in shedding relative to control group |
|---|---|---|
| None | 42.7 ± 12.99 | |
| VP6 | 1.0 ± 0.49b | 97.7 |
| AB | 8.8 ± 2.05bc | 79.4 |
| BC | 3.2 ± 0.84bc | 92.5 |
| CD | 0.1 ± 0.13b | 99.8 |
Groups of seven or eight BALB/c mice were immunized i.n. with two 9-μg doses of either chimeric VP6, AB, BC, or CD protein and with 10 μg of LT(192G). Four weeks after the last immunization, mice were challenged by oral gavage with 4 × 104 FFU of passage 9 EDIM. Stool samples were collected for the subsequent 7 days. Quantities of rotavirus antigen shed were determined by ELISA. The total quantity shed (nanograms per mouse per day) by each group was compared with the total quantity shed by the mock-immunized control group and expressed as percent reduction in shedding relative to the level of shedding of the control group.
Significantly (P ≤ 0.01) less than that of the mock-immunized group.
Significantly (P ≤ 0.005) greater than that of the CD-immunized group.
Fragment BC does not induce detectable rotavirus antibody.
The largest antibody responses following rotavirus infection have been consistently against the VP6 protein (12). Furthermore, i.n. immunization with chimeric VP6 was found to induce high ELISA titers of serum rotavirus antibodies (12). Although it was subsequently observed that protection following VP6 immunization appeared not to depend on antibody (12), it was still of interest to determine whether rotavirus antibody responses were stimulated by each of the three VP6 fragments and whether these responses correlated in any way with the protection elicited by the fragments.
To make this determination, blood and stool specimens collected 4 weeks after the second i.n. immunization (the day prior to challenge) were analyzed for rotavirus antibodies by ELISA. As previously reported, chimeric VP6 administered with LT(R192G) elicited high titers of rotavirus IgG in serum, moderate titers of rotavirus IgA in serum, and very low titers of rotavirus IgA in stool (12; Table 4). Fragment CD induced significantly higher titers of rotavirus IgG and IgA in serum, and rotavirus IgA in stool than did fragment AB (P ≤ 0.01). Unexpectedly, none of the mice administered fragment BC developed rotavirus antibody detectable by ELISA in serum or stool. To verify that rotavirus antibody generated in BC-immunized mice was not detectable merely due to its inability to bind to VP6 contained in the EDIM lysate used in the ELISA, Western blot analysis was performed using antisera collected from the VP6-, AB-, BC-, and CD-immunized groups. In this assay, the antibody must recognize denatured rather than native VP6. Again, rotavirus antibody was readily detected in mice immunized with VP6 or its AB and CD fragments but not in the BC-immunized animals (data not shown). Since BC stimulated nearly complete protection, this result demonstrates that antibody was not absolutely required for protection of mice immunized with a VP6 fragment. Clearly, therefore, ELISA antibody titers can only be used as markers of protection following i.n. immunization with VP6 or the AB and CD fragments.
TABLE 4.
Geometric mean titers of rotavirus antibodies induced by i.n. immunization with deletion mutant forms of VP6 and LT(R192G)a
| Inoculum | Geometric mean titer (U/ml) ± SEM of:
|
||
|---|---|---|---|
| IgG in serum | IgA in serum | IgA in stool | |
| None | <100 | <100 | <5 |
| VP6 | 122,839 ± 14,067 | 256 ± 60 | 24 ± 12 |
| AB | 43,652 ± 7,816b | 222 ± 40b | 20 ± 7 |
| BC | <100b | <100b | <5b |
| CD | 129,920 ± 11,971 | 716 ± 64 | 70 ± 15 |
Groups of eight BALB/c mice were immunized i.n. with two 9-μg doses of chimeric VP6, AB, BC, or CD protein and 10 μg of LT(192G). Serum and stool specimens were collected 4 weeks after the last immunization. Rotavirus IgG and IgA in serum and IgA in stool were determined by ELISA as units per milliliter.
Significantly lower than that of the CD-immunized group (P ≤ 0.01).
Mapping of protective domains within the CD fragment of VP6.
Since the CD region was found to elicit the best protection of the VP6 fragments, this portion of VP6 was further analyzed for protective epitopes. To delineate the distribution of protective epitopes within this region, we first constructed four deletion mutant proteins containing overlapping fragments within the CD region. These deletion mutant proteins, which were designated CD1 (amino acids 197 to 263), CD2 (amino acids 244 to 310), CD3 (amino acids 291 to 351), and CD4 (amino acids 332 to 397) were 67, 67, 61, and 65 residues in length. Again, these VP6 fragments were expressed in E. coli as chimeric proteins with the VP6 regions fused to the carboxyl terminus of MBP. Following two 9-μg i.n. immunizations [50 μl of 3.4 μM with 10 μg of LT(R192G)] separated by a 2-week interval, the mice were orally challenged with EDIM. Fragments CD1, CD2, CD3, and CD4 induced 88, 84, 19, and 92% reductions in shedding, respectively (Table 5). The reductions induced by CD1, CD2, and CD4 were all found to be significant (P ≤ 0.004), but protection induced by fragment CD4 was not significantly better than that induced by CD1 (P = 0.053) and CD2 (P = 0.057). ELISA titers of rotavirus antibodies were again measured to determine whether there was any association between rotavirus antibodies and protection. Fragments CD1 and CD3 were found not to induce antibodies, while CD2 and CD4 induced high titers of rotavirus IgG in serum (data not shown). Therefore, no association between rotavirus antibodies and protection was obtained with fragment CD1.
TABLE 5.
Protection of mice from shedding of rotavirus antigen after i.n. immunization with overlapping fragments of the CD region of VP6 and LT(R192G)a
| Immunogen | Avg quantity (ng) ± SEM of rotavirus antigen shed/mouse/day | % Reduction in shedding relative to control group |
|---|---|---|
| None | 207 ± 57 | |
| CD | 9 ± 4b | 96 |
| CD1 | 24 ± 7bc | 88 |
| CD2 | 32 ± 7bd | 84 |
| CD3 | 167 ± 51 | 19 |
| CD4 | 17 ± 5b | 92 |
Groups of eight BALB/c mice were immunized i.n. with two 9-μg doses of chimeric CD, CD1, CD2, CD3, or CD4 protein and 10 μg of LT(R192G). Immunized mice were challenged with EDIM 4 weeks after the last immunization. Shedding of rotavirus antigen (nanograms per mouse per day) was determined by ELISA during the subsequent 7 days.
Significantly (P ≤ 0.004) less than that of the mock-immunized group.
Not significantly (P = 0.053) more than that of the CD4-immunized group.
Not significantly (P = 0.057) more than that of the CD4-immunized group.
To better delineate the distribution of protective epitopes within the CD portion of VP6, 11 peptides derived from this region were synthesized (Table 2). The lengths of these peptides ranged from 18 to 30 amino acids, with overlapping regions of 0 to 13 residues between adjacent peptides. Unlike the recombinant VP6 fragments expressed in E. coli, these synthetic peptides were not conjugated to MBP. Therefore, any potential immunogenic effects exerted by the carrier, MBP, were avoided. Groups of mice were immunized i.n. with two 50-μg doses (50 μl, concentrations ranging from 288 to 592 μM, 2 weeks apart) of each peptide with 10 μg of LT(R192G) and then challenged with EDIM 4 weeks after the second immunization. Five (peptides 2, 3, 4, 6, and 11) of the 11 peptides induced significant (P ≤ 0.02) reductions in EDIM shedding during the 7 days following challenge (Table 6). These peptides reduced shedding between 57 and 93%, clearly indicating that regions of VP6 with as few as 18 amino acids could stimulate good protection in this model. In a subsequent experiment, the effective antigen doses for two of these peptides were examined. It was found that reduction of antigen doses to 10 or 2 μg for peptide 3 or 6 induced the same level of protection obtained with 50 μg (results not shown). Therefore, the difference in protection observed with each peptide was not likely due to administration of suboptimal antigen doses.
Only one of the five protective peptides (peptide 3) and one of the other six peptides (peptide 1) induced detectable rotavirus IgG in serum as determined by ELISA using the synthetic peptides to capture the antibody (results not shown). These results, therefore, demonstrate a lack of association between protection and ELISA-detectable rotavirus antibodies for peptides 2, 4, 6, and 11.
Protection against rotavirus shedding is induced by i.n. immunization with a VP6 peptide containing a putative CD4+ T-cell epitope.
Porcine rotavirus strain YM has been reported (2) to contain a CD4+ T-cell epitope for BALB/c mice within a 14-amino-acid region of VP6 (i.e., amino acids 289 to 302). The same region of EDIM VP6 with nearly the same sequence is contained within 25-amino-acid synthetic peptide 6 (i.e., RLSFQLMRPPNMTP), where the first methionine replaces a valine residue in the YM strain. Furthermore, peptide 6 stimulated good protection against EDIM shedding following i.n. immunization of BALB/c mice (Table 6). Because CD4+ T cells may be effectors of protection in this model, the 14-amino-acid peptide containing the putative CD4+ T-cell epitope was synthesized and used for i.n. immunization of BALB/c mice. Two 50-μg doses (50 μl of 592 μM) of this 14-mer peptide (peptide 6-14) stimulated between 93 and 98% reductions in EDIM shedding in three separate experiments (Table 6). This result established that a region of VP6 consisting of only 14 amino acids is sufficient to stimulate protection and suggests that CD4+ T cells are at least one of the effectors of protection stimulated by i.n. immunization with VP6.
DISCUSSION
We recently reported that i.n. immunization of mice with an E. coli-expressed chimera composed of MBP and the VP6 protein of murine rotavirus strain EDIM (i.e., MBP-VP6) stimulated nearly complete protection against shedding following subsequent oral challenge with live EDIM. Protection was dependent on inclusion of a mucosal adjuvant during immunization, and the adjuvant utilized for most studies was the attenuated E. coli heat-labile toxin LT(R192G). Based on the possibility that results obtained with the VP6 protein in this mouse model may be useful in the development of a human rotavirus vaccine, it was of interest to determine what regions of the VP6 protein stimulate protection. This information should not only be useful in uncovering the mechanism of protection but also help define regions of VP6 that might be included as a possible peptide vaccine.
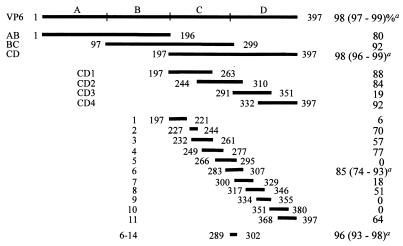
The study plan was to compare the abilities of different regions of the VP6 protein to stimulate protection in BALB/c mice as the sizes of these regions were gradually reduced. The results of this study are summarized in Fig. 1. Although the first, middle, and last parts of VP6 each stimulated excellent protection in this model, the last 50% (i.e., the CD region) was significantly (P < 0.001) more protective than the first 50% (i.e., the AB region) of this protein. When the protective epitopes within the CD region were further delineated using two sets of either four overlapping protein fragments or 11 overlapping synthetic peptides, both protective and nonprotective regions were identified. Fragments CD1, CD2, and CD4 were all protective, while CD3 was not. Immunization with the 11 peptides provided even more definitive data. Five of those peptides contained epitopes that stimulated significant protection, while six did not. Because peptides 2 and 3 contained 11 overlapping residues which were of sufficient length to harbor a major histocompatibility complex (MHC)-binding epitope (7, 10, 22, 23, 27), it is not certain whether the two peptides contain distinct protective epitopes or share a common epitope. Similarly, it is uncertain whether peptides 3 and 4 share a common epitope. Since peptides 2 and 4 do not share common sequences, at least two epitopes are located in the region spanned by these three peptides.
FIG. 1.
Schematic summary of the AB, BC, and CD fragments of VP6 and synthetic peptides (1 through 11 and 6-14) used to map protective domains and epitopes in VP6 and in its CD region. The numbers in front of and following the bars indicate the locations of the regions in the primary sequence of VP6. The protective efficacies of VP6 and the two groups of VP6 fragments (i.e., AB, BC, and CD and CD1, CD2, CD3, and CD4) and synthetic peptides are indicated as percent reduction in rotavirus shedding relative to mock-immunized mice. a Average percent reduction in shedding (range) of three experiments.
Peptide 6 was also found to be protective (74 to 93%) and contained a 14-amino-acid sequence (amino acids 289 to 302) that was almost identical to the one found in VP6 of the porcine YM strain of rotavirus (2). The porcine sequence was reported to contain an epitope that could recognize CD4+ T-cell hybridomas in the context of an MHC class II IEd molecule. Interestingly, the EDIM VP6-derived 14-mer synthetic peptide was found to induce excellent protection (93 to 98%). Therefore, we have located a very small region of VP6 that can stimulate almost complete protection. The binding groove of the MHC class II molecule is open at both ends, allowing naturally processed peptides of various lengths (10 to 30 residues) to bind within the groove (10, 22). The peptides that bind class II molecules have a core binding region of 9 amino acids with certain key pockets in the groove accommodating peptide side chains in a fashion similar to that of the class I molecules (7, 15, 22, 27). Because the core peptides appear to be as small as nine residues in length, it will be of interest to determine whether smaller MHC class II-binding epitopes can be further identified in this 14-amino-acid region.
Fragments CD2 and CD4 induced high titers of rotavirus antibodies, whereas detectable titers of antibodies were induced by only two synthetic peptides (peptides 1 and 3). The poor humoral responses obtained with the synthetic peptides were not unexpected, because most small peptides by themselves do not stimulate B-cell responses. To increase immunogenicity, peptides containing B-cell epitopes are often coupled to carrier proteins containing T-cell epitopes (21, 28). A number of studies have found that synthesis of T-cell epitopes contiguous with B-cell epitopes also increases the immunogenicity of the B-cell epitopes (8, 31).
The extraordinary magnitude of protection afforded by the 14-amino-acid stretch of VP6 might be mediated by the reported CD4+ T-cell epitope alone and not require epitopes such as those recognized by specific immunoglobulin receptors on B cells. This contention is supported by the observation (12) that B-cell-deficient μMt mice were fully protected following i.n. immunization with MBP-VP6. Thus, antibody was not required for protection in these mice. It should be noted that μMt mice belong to the H-2b haplotype, while BALB/c mice, which were used in this study with the 14-mer peptide, belong to the H-2d haplotype. Therefore, the results obtained in studies using μMt mice may not be directly applicable to mice having different haplotypes. Nevertheless, fragments BC and CD1 of VP6, as well as several peptides of VP6, stimulated good protection in BALB/c mice but induced no detectable rotavirus antibody in these mice. These results reinforce the hypothesis that antibody is not required for protection following i.n. immunization with VP6 or its peptides in BALB/c mice and, possibly, any mouse strain. It also establishes that antibody titers cannot be used as reliable markers of immunity, at least following i.n. immunization with some of the VP6 deletion mutant polypeptides or synthetic peptides.
It should be noted that 100% congruity was found between regions of CD that elicited significant (P ≤ 0.05) protection using the four CD fragments and the 11 synthetic peptides. That is, the only fragment that was not significantly protective was CD3 and this fragment contained only nonprotective peptides 7 and 8 in their entirety. Furthermore, the three protective fragments (CD1, CD2 and CD4) all contained at least one complete protective synthetic peptide. Two other interesting features of CD3 should also be noted. The first is that peptide 8, which CD3 contained in its entirety, stimulated 51% protection. Although that was not significant (P = 0.07) by our criterion, it was marginally so. Peptide 8 was difficult to synthesize and was therefore modified by deletion of the glycine residue at position 321. Had this glycine residue been present, peptide 8 might have been either more or less protective. Had the former occurred and the authentic peptide 8 actually stimulated significant protection, there would have been a lack of congruity between the protection stimulated by CD3 and peptide 8, possibly due to improper processing of CD3 for antigen presentation. The second feature of interest is that CD3 contained 17 of the 25 amino acid residues within protective peptide 6, including 12 of the 14 residues in protective peptide 6-14. Since CD3 lacks only the first two amino acids of the 14-mer peptide, it appeared likely that these two residues are critical for this peptide to stimulate protection. This possibility will be further examined through truncation studies with the 14-mer peptide.
From the results presented, it is clear that multiple regions of VP6 can stimulate protection against rotavirus shedding in adult BALB/c mice following i.n. immunization. Since so many regions of VP6 can elicit protection in H-2d mice, it is likely that all haplotypes of adult mice will be protected by responses against at least some regions of this protein. As already noted (12), both H-2b (μMt) and H-2d (BALB/c) mice are equally protected following i.n. immunization with VP6. Taking this a step further, if VP6 is eventually found to stimulate immunity against disease in humans following immunization by this route, it follows that persons of many, if not all, HLA types may be protected. Finally, because VP6 is a very conserved protein among group A rotaviruses, with generally >90% homology at the amino acid level (29), immunization with VP6 from any group A rotavirus strain may protect against all group A rotaviruses. These possibilities need to be verified, but if they are shown to be true, VP6 may constitute a highly effective vaccine against rotavirus.
ACKNOWLEDGMENT
This work was funded in part by NIH-NIAID contract NO1 AI 45252, which was awarded to the Children's Hospital Medical Center, Cincinnati, Ohio.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smit J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates, Inc., and John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 2.Banos D M, Lopez S, Arias C F, Esquivel F R. Identification of a T-helper cell epitope on the rotavirus VP6 protein. J Virol. 1997;71:419–426. doi: 10.1128/jvi.71.1.419-426.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bern C, Martines J, de Zoysa I, Glass R I. The magnitude of the global problem of diarrhoeal disease: a ten-year update. Bull W H O. 1992;70:705–714. [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein D I, Smith V E, Sander D S, Pax K A, Schiff G M, Ward R L. Evaluation of WC3 rotavirus vaccine and correlates of protection in healthy infants. J Infect Dis. 1990;162:1055–1062. doi: 10.1093/infdis/162.5.1055. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein D I, Glass R I, Rodgers G, Davidson B L, Sack D A. Evaluation of rhesus rotavirus monovalent and tetravalent reassortant vaccines in US children. US Rotavirus Vaccine Efficacy Group. JAMA. 1995;273:1191–1196. [PubMed] [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Brown J H, Jardetzky T S, Gorga J C, Stern L J, Urban R G, Strominger J L, Wiley D C. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993;364:33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- 8.Brumeanu T D, Casares S, Bot A, Bot S, Bona C A. Immunogenicity of a contiguous T-B synthetic epitope of the A/PR/8/34 influenza virus. J Virol. 1997;71:5473–5480. doi: 10.1128/jvi.71.7.5473-5480.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Withdrawal of rotavirus vaccine recommendation. Morbid Mortal Weekly Rep. 1999;48:1007. [PubMed] [Google Scholar]
- 10.Chicz R M, Urban R G, Gorga J C, Vignali D A, Lane W S, Strominger J L. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi A H, Knowlton D R, McNeal M M, Ward R L. Particle bombardment-mediated DNA vaccination with rotavirus VP6 induces high levels of serum rotavirus IgG but fails to protect mice against challenge. Virology. 1997;232:129–138. doi: 10.1006/viro.1997.8552. [DOI] [PubMed] [Google Scholar]
- 12.Choi A H, Basu M, McNeal M M, Clements J D, Ward R L. Antibody-independent protection against rotavirus infection of mice stimulated by intranasal immunization with chimeric VP4 or VP6 protein. J Virol. 1999;73:7574–7581. doi: 10.1128/jvi.73.9.7574-7581.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark H F, Offit P A, Ellis R W, Eiden J J, Krah D, Shaw A R, Pichichero M, Treanor J J, Borian F E, Bell L M, Plotkin S A. The development of multivalent bovine rotavirus (strain WC3) reassortant vaccine for infants. J Infect Dis. 1996;174(Suppl. 1):S73–S80. doi: 10.1093/infdis/174.supplement_1.s73. [DOI] [PubMed] [Google Scholar]
- 14.Dickinson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammer J, Sturniolo T, Sinigaglia F. HLA class II peptide binding specificity and autoimmunity. Adv Immunol. 1997;66:67–100. doi: 10.1016/s0065-2776(08)60596-9. [DOI] [PubMed] [Google Scholar]
- 16.Jarrett H W, Foster J L. Alternate binding of actin and calmodulin to multiple sites on dystrophin. J Biol Chem. 1995;270:5578–5586. doi: 10.1074/jbc.270.10.5578. [DOI] [PubMed] [Google Scholar]
- 17.Lanata C F, Midthun K, Black R E, Butron B, Huapaya A, Penny M E, Ventura G, Gil A, Jett-Goheen M, Davidson B L. Safety, immunogenicity, and protective efficacy of one and three doses of the tetravalent rhesus rotavirus vaccine in infants in Lima, Peru. J Infect Dis. 1996;174:268–275. doi: 10.1093/infdis/174.2.268. [DOI] [PubMed] [Google Scholar]
- 18.Linhares A C, Gabbay Y B, Mascarenhas J D, de Freitas R B, Oliveira C S, Bellesi N, Monteiro T A, Lins-Lainson Z, Ramos F L, Valente S A. Immunogenicity, safety and efficacy of tetravalent rhesus-human, reassortant rotavirus vaccine in Belem, Brazil. Bull W H O. 1996;74:491–500. [PMC free article] [PubMed] [Google Scholar]
- 19.McNeal M M, Rae M N, Bean J A, Ward R L. Antibody-dependent and -independent protection following intranasal immunization of mice with rotavirus particles. J Virol. 1999;73:7565–7573. doi: 10.1128/jvi.73.9.7565-7573.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray C J, Lopez A D. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 21.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou W M, Fiers W A. Universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999;5:1157–1163. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 22.Rammensee H-G. Chemistry of peptides associated with MHC class I and class II molecules. Curr Opin Immunol. 1995;7:85–96. doi: 10.1016/0952-7915(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 23.Rammensee H G, Friedem T, Stevanoviic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 24.Rennels M B, Glass R I, Dennehy P H, Bernstein D I, Pichichero M E, Zito E T, Mack M E, Davidson B L, Kapikian A Z. Safety and efficacy of high-dose rhesus-human reassortant rotavirus vaccines—report of the National Multicenter Trial. United States Rotavirus Vaccine Efficacy Group. Pediatrics. 1996;97:7–13. [PubMed] [Google Scholar]
- 25.Santosham M, Letson G W, Wolff M, Reid R, Gahagan S, Adams R, Callahan C, Sack R B, Kapikian A Z. A field study of the safety and efficacy of two candidate rotavirus vaccines in a Native American population. J Infect Dis. 1991;163:483–487. doi: 10.1093/infdis/163.3.483. [DOI] [PubMed] [Google Scholar]
- 26.Santosham M, Moulton L H, Reid R, Croll J, Weatherholt R, Ward R, Forro J, Zito E, Mack M, Brenneman G, Davidson B L. Efficacy and safety of high-dose rhesus-human reassortant rotavirus vaccine in Native American populations. J Pediatr. 1997;131:632–638. doi: 10.1016/s0022-3476(97)70076-3. [DOI] [PubMed] [Google Scholar]
- 27.Stern L J, Brown J H, Jardetzky T S, Gorga J C, Urban R G, Strominger J L, Wiley D C. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature. 1994;368:215–221. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- 28.Sun J B, Mielcarek N, Lakew M, Grzych J M, Capron A, Holmgren J, Czerkinsky C. Intranasal administration of a Schistosoma mansoni glutathione S-transferase-cholera toxoid conjugate vaccine evokes antiparasitic and antipathological immunity in mice. J Immunol. 1999;163:1045–1052. [PubMed] [Google Scholar]
- 29.Tang B, Gilbert J M, Matsui S M, Greenberg H B. Comparison of the rotavirus gene 6 from different species by sequence analysis and localization of subgroup-specific epitopes using site-directed mutagenesis. Virology. 1997;13:89–96. doi: 10.1006/viro.1997.8762. [DOI] [PubMed] [Google Scholar]
- 30.Vesikari T. Clinical trials of live oral rotavirus vaccines: the Finnish experience. Vaccine. 1993;11:255–261. doi: 10.1016/0264-410x(93)90026-t. [DOI] [PubMed] [Google Scholar]
- 31.Volpina O M, Surovoy A Y, Zhmak M N, Kuprianova M A, Koroev D O, Chepurkin A V, Toloknov A S, Ivanov V T. A peptide construct containing B-cell and T-cell epitopes from the foot-and-mouth disease viral VP1 protein induces efficient antiviral protection. Vaccine. 1999;12:577–584. doi: 10.1016/s0264-410x(98)00236-9. [DOI] [PubMed] [Google Scholar]
- 32.Walsh J A, Warren K S. Selective primary health care: an interim strategy for disease control in developing countries. N Engl J Med. 1979;301:967–974. doi: 10.1056/NEJM197911013011804. [DOI] [PubMed] [Google Scholar]
- 33.Ward R L, McNeal M M, Sheridan J F. Development of an adult mouse model for studies on protection against rotavirus. J Virol. 1990;64:5070–5075. doi: 10.1128/jvi.64.10.5070-5075.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]