Venous thromboembolism is a common complication among hospital inpatients and contributes to longer hospital stays, morbidity, and mortality. Some venous thromboembolisms may be subclinical, whereas others present as sudden pulmonary embolus or symptomatic deep vein thrombosis. Ultrasonic Doppler and venographic techniques have shown deep vein thrombosis of the lower limb to occur in half of all major lower limb orthopaedic operations performed without antithrombotic prophylaxis. Deep vein thrombosis of the lower limb is also seen in a quarter of patients with acute myocardial infarction, and more than half of patients with acute ischaemic stroke.
Deep vein thrombosis of the lower limb normally starts in the calf veins. About 10-20% of thromboses extend proximally, and a further 1-5% go on to develop fatal pulmonary embolism. Appropriate antithrombotic measures can reduce this complication. Until recently, some clinicians were reluctant to provide such prophylaxis routinely. As unfounded fears of major bleeding complications from anticoagulant regimens wane, preventive treatments are used more often with medical and surgical patients. However, the risk of bleeding can be serious and this has particular bearing in postoperative patients.
Venous thromboembolism can also arise spontaneously in ambulant individuals particularly if they have associated risk factors such as thrombophilia, previous thrombosis, or cancer. However, in over half of these patients, no specific predisposing factors can be identified at presentation.
Venous thromboembolism often manifests clinically as deep vein thrombosis or pulmonary embolism, and is possibly one of the preventable complications that occur in hospitalised patients
Pathophysiology
Thrombus formation and propagation depend on the presence of abnormalities of blood flow, blood vessel wall, and blood clotting components, known collectively as Virchow's triad. Abnormalities of blood flow or venous stasis normally occur after prolonged immobility or confinement to bed. Venous obstruction can arise from external compression by enlarged lymph nodes, bulky tumours, or intravascular compression by previous thromboses. Increased oestrogens at pharmacological levels, as seen with oral contraceptive use and with hormone replacement therapy in postmenopausal women, have been associated with a threefold increased risk in the small initial risk of venous thromboembolism. Cancers, particularly adenocarcinomas and metastatic cancers, are also associated with increased venous thromboembolism. Indeed, on presentation, some idiopathic venous thromboembolisms have revealed occult cancers at follow up. Both oestrogens at pharmacological levels and cancer can also activate the clotting system.
Risk factors and conditions predisposing to venous thromboembolism
History of venous thromboembolism
Prolonged immobility
Prolonged confinement to bed or lower limb paralysis
Surgery, particularly lower limb orthopaedic operations, and major pelvic or abdominal operations
Trauma—For example, hip fractures and acute spinal injury
Obesity
Major medical illnesses such as acute myocardial infarction, ischaemic stroke, congestive cardiac failure, acute respiratory failure
Oestrogen use in pharmacological doses—For example, oral contraception pills, hormone replacement therapy
Cancer, especially metastatic adenocarcinomas
Age >40 years
Aquired hypercoagulable states—Lupus anticoagulant and antiphospholipid antibodies, hyperhomocysteinaemia, dysfibrinogenaemia, myeloproliferative disorders such as polycythaemia rubra vera
Inherited hypercoaguable states—Activated protein C resistance (factor V Leiden mutation), protein C deficiency, protein S deficiency, antithrombin deficiency, prothrombin gene mutation
Clinical presentation and diagnosis
Deep vein thrombosis
Deep vein thrombosis commonly presents with pain, erythema, tenderness, and swelling of the affected limb. Thus, in lower limb deep vein thrombosis, the affected leg is usually swollen with the circumference of the calf larger than the unaffected side. Other causes of leg swelling, erythema, and tenderness include a ruptured Baker's cyst and infective cellulitis. The diagnosis of deep vein thrombosis is therefore more likely when risk factors are present and less so if there are features suggesting alternative diagnoses. For example, ruptured Baker's cysts commonly appear in the context of osteoarthritis and rheumatoid arthritis. Infective cellulitis is unlikely to be bilateral, with clearly demarcated areas of erythema extending proximally. Breaks in the skin, particularly between the toes, and coexistent fungal infection are additional clues to cellulitis.
Modified pretest probability for deep vein thrombosis
| Clinical feature | Score |
|---|---|
| Tenderness along entire deep vein system | 1.0 |
| Swelling of the entire leg | 1.0 |
| Greater than 3 cm difference in calf circumference | 1.0 |
| Pitting oedema | 1.0 |
| Collateral superficial veins | 1.0 |
| Risk factors present: | |
| Active cancer | 1.0 |
| Prolonged immobility or paralysis | 1.0 |
| Recent surgery or major medical illness | 1.0 |
| Alternative diagnosis likely (ruptured Baker's cyst in rheumatoid arthritis, superficial thrombophlebitis, or infective cellulitis) | −2.0 |
Score >3=high probablility; 1-2=moderate probability <0=low probability
Objective diagnosis of venous thromboembolism is important for optimal management. Although the clinical diagnosis of venous thromboembolism is imprecise, various probability models based on clinical features have proved to be practical and reliable (interobserver reliability, κ=0.85) in predicting the likelihood of venous thromboembolism. These models should be used in conjunction with objective diagnostic tests.
Compression ultrasonography remains the non-invasive investigation of choice for the diagnosis of clinically suspected deep vein thrombosis. It is highly sensitive in detecting proximal deep vein thrombosis although less accurate for isolated calf deep vein thrombosis. In patients with suspected thrombosis and a negative compression ultrasound result, the test should be repeated in seven days because studies have shown that patients with two or more negative tests over a week who are untreated have a less than 2% risk of proximal extension or subsequent deep vein thrombosis.
Impedance plethysmography is slightly less specific and sensitive than ultrasonography but may still have a role in pregnant women and suspected recurrent deep vein thrombosis. The gold standard is invasive contrast venography, which is still used when a definitive answer is needed. Newer imaging techniques are being developed, and tools such as magnetic resonance venography or computed tomography could possibly detect pelvic vein thromboses, but further testing is needed to establish their role in the diagnosis of deep vein thrombosis.
Blood tests such as fibrin D-dimer add to the diagnostic accuracy of the non-invasive tests. In one study, the sensitivity and specificity of a D-dimer concentration of >500 μg/l for the presence of pulmonary embolism were 98% and 39%, respectively, which give positive and negative predictive values of 44% and 98%. The sensitivity of the test even remained high at three and seven days after presentation (96% and 93%).
Investigations for suspected venous thromboembolism by pretest clinical probability
| Pretest probability | Fibrin D-dimer | Other investigations | Comment |
|---|---|---|---|
| Deep vein thrombosis: | |||
| Low | Negative | No further investigations needed | |
| Low or moderate | Positive | Negative ultrasound compression | Consider venography or repeat ultrasound after a week |
| Moderate or high | Positive | Positive ultrasound compression | Treat with anticoagulants |
| High | Positive | Negative ultrasound compression | Consider venography to rule out deep vein thrombosis, especially in high risk patients and those with recurrent pulmonary emboli |
| Pulmonary embolism: | |||
| Low | Negative | No | No further investigation needed |
| Low or moderate | Positive | Proceed to ventilation-perfusion scan | If non-diagnostic ventilation-perfusion scan consider serial compression ultrasound over two weeks to rule out venous thromboembolism |
| High | Positive | Proceed to ventilation-perfusion scan (or ultrasound) | If non-diagnostic ventilation-perfusion scans, proceed to venography or pulmonary angiography as needed |
Pulmonary embolism
Patients presenting with acute pulmonary embolism often complain of sudden onset of breathlessness with haemoptysis or pleuritic chest pain, or collapse with shock in the absence of other causes. Deep vein thrombosis may not be suspected clinically, but its presence, along with thrombotic risk factors, will make the diagnosis of pulmonary embolism more likely. A similar clinical probability model to that for deep vein thrombosis has been developed for pulmonary embolism.
Clinical probability for pulmonary embolism
| Clinical feature | Score |
|---|---|
| Deep vein thrombosis suspected: | |
| Clinical features of deep vein thrombosis | 3.0 |
| Recent prolonged immobility or surgery | 1.5 |
| Active cancer | 1.0 |
| History of deep vein thrombosis or pulmonary embolism | 1.5 |
| Haemoptysis | 1.0 |
| Resting heart rate >100 beats/min | 1.5 |
| No alternative explanation for acute breathlessness or pleuritic chest pain | 3.0 |
>6=high probability (60%); 2-6=moderate probability (20%); <1.5=low probability (3-4%)
Pulmonary angiography is the gold standard investigation for pulmonary embolism, but it is invasive and associated with 0.5% mortality. A ventilation-perfusion scan using technetium DTPA (ditriaminopentaric acid) is more widely used. However this investigation is non-specific, and is diagnostic in only 30% of cases. Spiral computed tomography scans are more reliable but diagnosis is limited to emboli in larger vessels only. Measurement of fibrin D-dimer levels, used for deep vein thrombosis, is helpful, as is compression ultrasound in the detection of occult deep vein thrombosis.
Prevention strategies
An appropriate strategy for the prevention of venous thromboembolism include pharmacological or physical methods. To optimise treatment, patients should be stratified into risk categories to allow the most appropriate prophylactic measure to be used.
Thromboembolic risk stratification for surgery patients
• Low risk—Uncomplicated surgery in patients aged <40 years with minimal immobility postoperatively and no risk factors
• Moderate risk—Any surgery in patients aged 40-60 years, major surgery in patients <40 years and no other risk factors, minor surgery in patients with one or more risk factors
• High risk—Major surgery in patients aged >60 years, major surgery in patients aged 40-60 years with one or more risk factors
• Very high risk—Major surgery in patients aged >40 years with previous venous thromboembolism, cancer or known hypercoagulable state, major orthopaedic surgery, elective neurosurgery, multiple trauma, or acute spinal cord injury
Prophylactic drugs include unfractionated heparin, low molecular weight heparin, oral anticoagulants (such as coumarins), thrombin inhibitors (such as hirudin), and specific factor Xa inhibitors (such as fondaparinux). The recently approved fondaparinux reduces the risk of venous thromboembolism after orthopaedic surgery by more than half compared with low molecular weight heparin and seems likely to become the treatment of choice after universal availability.
Prophylactic physical methods include the use of compression elastic stockings, intermittent pneumatic compression (which provides rythmic external compression at 35-40 mm Hg for about 10 seconds every minute), and early mobilisation to improve venous blood flow in conditions predisposing to venous stasis.
General surgery
Patients at low risk undergoing general surgery do not need specific prophylaxis other than early mobilisation. In moderate risk patients, fixed low doses of unfractionated heparin (5000 IU every 12 hours) or low molecular weight heparin (3400 anti-Xa units or equivalent) once daily is sufficient. Higher doses of low molecular weight heparin (more than 3400 IU anti-Xa daily) should be reserved for high risk general surgery and orthopaedic operations. Compression elastic stockings and intermittent pneumatic compression may protect high risk patients when used with anticoagulants. They are also effective when used alone in moderate risk patients where anticoagulants are contraindicated.
Orthopaedic surgery
In very high risk patients, such as those undergoing major orthopaedic operations, high dose low molecular weight heparin or warfarin is appropriate. The current recommended length of anticoagulant prophylaxis is 7-10 days with low molecular weight heparin or warfarin. Extended use may provide additional benefit. Routine screening with duplex ultrasonography is not helpful. Hirudin seems to be superior to low molecular weight heparin and low dose unfractionated heparin as prophylaxis in patients undergoing elective hip replacements but is still not universally available.
Key points
• Understanding Virchow's triad aids treatment of venous thromboembolism
• Numerous situations and risk factors can contribute to venous thromboembolism
• Diagnosis of venous thromboembolism depends upon combination of history, risk factors, and investigations
• Antithrombotic prophylaxis is safe and effective
Neurosurgery, multiple traumas, and spinal cord injuries
Intermittent pneumatic compression is the prophylaxis of choice for elective neurosurgery. Among the low molecular weight heparins, only enoxaparin 30 mg twice daily has been shown to reduce venous thromboembolism without excess bleeding after elective neurosurgery, multiple traumas, or spinal cord injuries and so may be used in these situations. Other low molecular weight heparins have either not been tested or not conclusively shown to be of benefit in this setting.
Evidence based use of antithrombotic prophylaxis
General surgery
• Low risk— Early mobilisation
• Moderate risk—UH 5000 IU 12 hourly starting two hours before surgery, or low molecular weight heparin <3400 anti-Xa IU daily*, or compression elastic stockings, or intermittent pneumatic compression
• High risk—Low molecular weight heparin >3400 anti-Xa IU daily† plus compression elastic stockings, or unfractionated heparin 5000 IU eight hourly starting two hours before surgery plus compression elastic stockings, or intermittent pneumatic compression if anticoagulation contraindicated
• Very high risk—Perioperative warfarin (INR 2-3), low molecular weight heparin >3400 anti-Xa IU daily† plus compression elastic stockings, or prolonged low molecular weight heparin therapy plus compression elastic stockings
Major orthopaedic surgery
• Elective hip replacement—Recombinant hirudin 15 mg twice daily, unfractionated heparin 3500 IU eight hourly with postoperative adjustments (APTT 1.2-1.5), or low molecular weight heparin >3400 anti-Xa IU daily†, or perioperative warfarin (INR 2-3), or fondaparinux 2.5 mg daily
• Elective knee replacement—Low molecular weight heparin >3400 anti-Xa IU daily†, or perioperative warfarin (INR 2-3), or fondaparinux 2.5 mg daily, intermittent pneumatic compression
• Surgery for hip fracture—Low molecular weight heparin >3400 anti-Xa IU daily†, or perioperative warfarin (INR 2-3), or fondaparinux 2.5 mg daily
Elective neurosurgery
• Intermittent pneumatic compression, enoxaparin 30 mg twice daily
Acute spinal cord injury
• Enoxaparin 30 mg twice daily
Trauma
• Enoxaparin 30 mg twice daily
Acute myocardial infarction
• Low dose unfractionated heparin 5000 IU twice daily, full dose unfractionated heparin 40 000 IU infusion over 24 hours, elastic stockings, and early mobilisation
Ischaemic stroke
• Low dose unfractionated heparin 5000 IU twice daily
Other medical conditions including congestive heart failure
• Enoxaparin 40 mg once daily or 30 U twice daily, dalteparin 2500 IU daily, low dose unfractionated heparin 5000 IU twice daily
Cancer patients receiving chemotherapy
• Low dose warfarin (INR <2), dalteparin 2500 IU daily
*Dalteparin 2500 IU once daily starting two hours before surgeryEnoxaparin 20 mg once daily starting two hours before surgeryNadroparin 3100 IU once daily starting two hours before surgeryTinzaparin 3500 IU once daily starting two hours before surgery†Dalteparin 5000 IU once daily starting 10-12 hours before surgeryDanaparoid 750 IU twice daily starting one to two hours before surgeryEnoxaparin 40 mg once daily starting 10-12 hours before surgery or 30 mg twice daily starting after surgeryTinzaparin 50 IU/kg once daily starting two hours before surgery
Medical conditions
In general medical patients including heart failure and respiratory failure patients, both unfractionated heparin and low molecular weight heparin have been shown to be effective in reducing the risk of venous thromboembolism. Low molecular weight heparin has been shown to be more effective than heparin in stroke. Low dose heparin has been shown to be effective in acute myocardial infarction but this is now largely historic because myocardial infarction patients receive therapeutic dose anticoagulants.
Other considerations
Combined approaches using drugs and physical methods may be better at preventing thromboembolism than physical methods alone. However, compression elastic stockings and intermittent pneumatic compression may be used for moderate or high risk patients when anticoagulation is contraindicated or best avoided. Inferior vena cava filter placement should be reserved for patients at very high risk of venous thromboembolism where anticoagulation as well as physical methods are contraindicated. Inferior vena cava filter placement tends to cause a long term increase of recurrent deep vein thrombosis, even though the immediate risk of postoperative pulmonary embolism is reduced.
Further reading
• Haemostasis and Thrombosis Task Force of the British Society for Haematology. Guidelines on anticoagulation: third edition. Br J Haematol 1998;101:374-87
• Hyers TM, Agnelli G, Hull RD, Morris TA, Samama M, Tapson V, et al. Antithrombotic therapy for venous thromboembolic disease. Chest 2001;119:176-93S
• Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery.
• Simonneau G, Sors H, Charbonnier B, Page Y, Labaan JP, Azarian R et al. A comparison of low molecular weight heparin with unfractionated heparin for acute pulmonary embolism. N Engl J Med 1997:337;663-9
• Turpie AG, Bauer KA, Eriksson BI, Lassen MR. Fondaparinus vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of 4 randomised double blind studies. Arch Intern Med 2002;162:1833-40
• Walker ID, Greaves M, Preston FE. Guideline: Investigation and management of heritable thrombophilia. Br J Haematol 2001:114;512-28
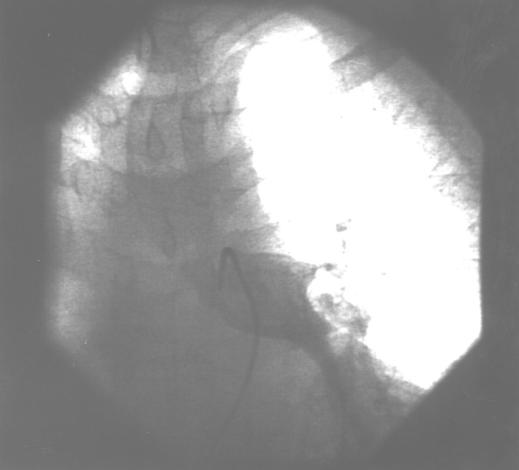
Figure.
Pulmonary angiography showing large pulmonary embolus in left pulmonary artery
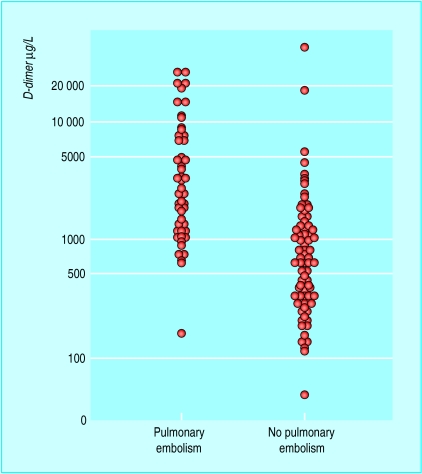
Figure.
Plasma D-dimer concentrations on day of presentation according to final diagnosis
Figure.
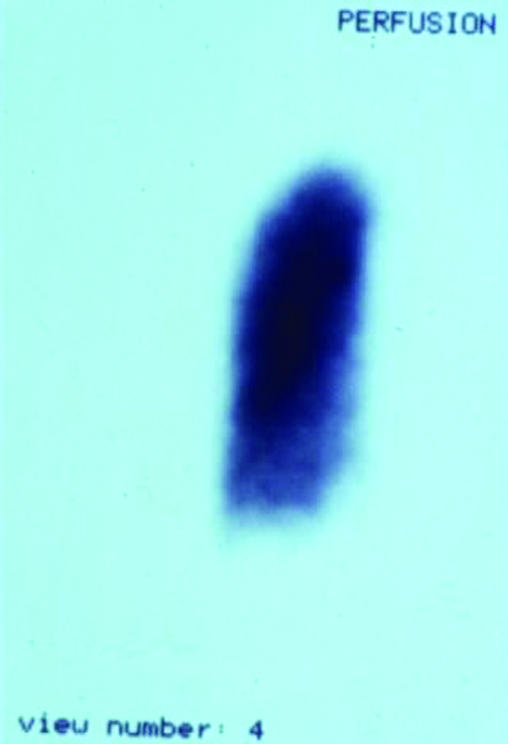
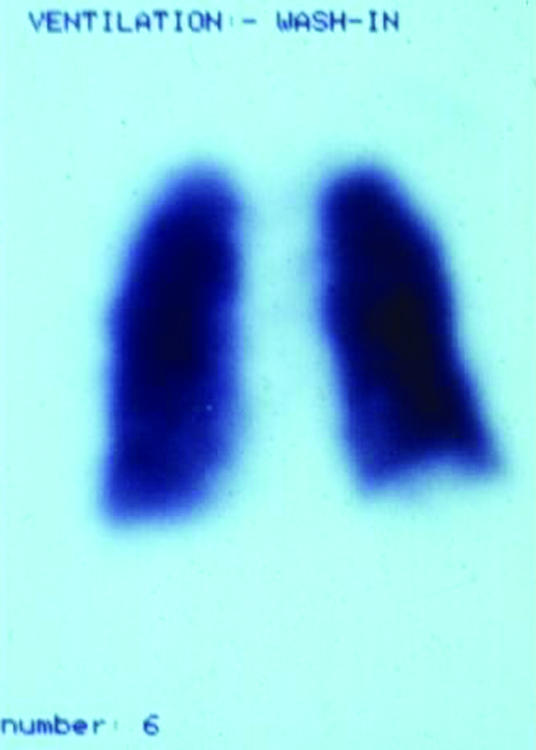
Ventilation-perfusion scan showing massive pulmonary thromboembolism, showing a mismatch between (left) perfusion and (right) ventilation scans
Acknowledgments
The box showing evidence based use of antithrombotic prophylaxis is adapted from the 6th ACCP guidelines Geerts WH, et al. Chest 2001;119:132-75S. The figure showing Plasma D-dimer concentrations on day of presentation according to final diagnosis is adapted from Bounameaux H, et al. Lancet 1991;337:196-200.
Footnotes
Alexander G G Turpie is professor of medicine, McMaster University, Hamilton, Canada. Bernard S P Chin is research fellow and Gregory Y H Lip is professor of cardiovascular medicine at the haemostasis thrombosis and vascular biology unit, university department of medicine, City Hospital, Birmingham.
The ABC of antithrombotic therapy is edited by Gregory Y H Lip and Andrew D Blann, senior lecturer in medicine at the haemostasis thrombosis and vascular biology unit, university department of medicine, City Hospital, Birmingham. The series will be published as a book in spring 2003.