Abstract
Fluorescent protein (FP) tags are extensively used to visualize and characterize the properties of biomolecular condensates despite a lack of investigation into the effects of these tags on phase separation. Here, we characterized the dynamic properties of µNS, a viral protein hypothesized to undergo phase separation and the main component of mammalian orthoreovirus viral factories. Our interest in the sequence determinants and nucleation process of µNS phase separation led us to compare the size and density of condensates formed by FP::µNS to the untagged protein. We found an FP-dependent increase in droplet size and density, which suggests that FP tags can promote µNS condensation. To further assess the effect of FP tags on µNS droplet formation, we fused FP tags to µNS mutants to show that the tags could variably induce phase separation of otherwise noncondensing proteins. By comparing fluorescent constructs with untagged µNS, we identified mNeonGreen as the least artifactual FP tag that minimally perturbed µNS condensation. These results show that FP tags can promote phase separation and that some tags are more suitable for visualizing and characterizing biomolecular condensates with minimal experimental artifacts.
The findings deepen our understanding of mammalian orthoreovirus viral factory formation by defining the nonstructural reoviral protein µNS as a phase-separating biomolecule that nucleates dynamic liquid-like condensates.
The authors used analytical microscopy techniques and a sensitive fluorescent tag screening strategy to decouple the intrinsic properties of µNS from an array of artifacts that can complicate dynamic protein measurements.
The advances indicate that fluorescent protein tags can promote the condensation of biomolecular condensates and can recover phase separation of noncondensing mutants.
INTRODUCTION
Fluorescent proteins (FP) are a critical tool to study phase separation. Seminal studies using green fluorescent protein (GFP) as a marker for protein localization enabled researchers to visualize proteins in living systems (Chalfie et al., 1994; Wang and Hazelrigg, 1994). The concept of phase separation as a mechanism of biomolecular compartmentalization was initially proposed from observations of the behavior of GFP-labeled P granule components in living Caenorhabditis elegans (Brangwynne et al., 2009). Fluorescent P granules demonstrated liquid-like characteristics such as coalescence and viscous relaxation, deformation under mechanical stress, and dynamic internal molecular rearrangement, properties that have since been observed in highly diverse biological systems. Phase separation is now understood to be a common mechanism underlying the formation of membraneless structures independent of molecular composition, function, localization, and host species, prompting the unifying name biomolecular condensate to collectively refer to phase-separated compartments (Banani et al., 2017). A key function of phase separation in biology is the concentration of enzymes and their substrates into distinct foci to enhance reaction rates through mass action and physical organization (Peeples and Rosen, 2021). Many publications now implicate phase separation in the biogenesis of cellular organelles, signaling complexes, neuropathogenesis, cancer, and infectious diseases (Wang et al., 2021).
Despite the pervasive use of genetically encoded FPs to study condensates, we know surprisingly little about the effects of FPs on phase separation. There is evidence that FPs alter the susceptibility of condensates to 1,6-hexanediol (1,6-HD) (Uebel and Phillips, 2019) and can change the appearance of fixation artifacts (Irgen-Gioro et al., 2022). Perhaps the most striking is a report that GFP lowers the nucleation barrier of FIB1 in vitro and can alter its droplet fusion kinetics (Feric et al., 2016). Although effects of FP tags on phase separation have been described, this phenomenon has not been investigated until recently (Zhou and Narlikar, 2023; Pandey et al., 2024). Pandey et al. found that phase separation of Httex1(Q25) was dependent on the RFP tag and that untagged Httex1(Q25) did not phase separate under the same conditions (Pandey et al., 2024). This is a consequential finding because it shows that FP tags can drive phase separation, leading to the mischaracterization of biomolecular condensates.
Viruses concentrate their proteins and nucleic acids within replication compartments during infection and phase separation is an underlying mechanism driving the formation of replication compartments for pathogens like ebolavirus (Bodmer et al., 2023), SARS-CoV-2 (Savastano et al., 2020), rabies virus (Nikolic et al., 2017), measles virus (Guseva et al., 2020), influenza virus (Alenquer et al., 2019), and rotavirus (Geiger et al., 2021). Like rotaviruses, mammalian orthoreoviruses (ReoV) have a segmented double-stranded RNA genome and are members of the Reoviridae family. ReoV infects the gastrointestinal tract and has been associated with loss of oral tolerance to dietary antigens and the onset of celiac disease (Bouziat et al., 2017). Reovirus mRNAs and proteins localize to cytoplasmic membraneless organelles called viral factories, where genome replication and virion assembly are thought to occur. Recently, viral factories and viroplasms from related viruses in the Reoviridae family were characterized as biomolecular condensates (Geiger et al., 2021; Durinova et al., 2023), motivating our investigation into the biophysical properties of ReoV viral factories.
The reoviral nonstructural protein μNS forms the “matrix” of ReoV viral factories and is required for viral replication (Broering et al., 2004; Takeshi et al., 2006). μNS is encoded by the M3 genome segment and its mRNA is highly transcribed early in infection (Nanoyama et al., 1974). Functional studies identified the carboxyl-terminal region spanning residues 471-721 as the minimal region of µNS required to form viral factory-like structures (VFLS) that resemble viral factories (Broering et al., 2005). Many viral proteins concentrate in the factory via specific nonoverlapping interactions with regions of the amino-terminal 221 residues of µNS (Miller et al., 2010). Point mutations at residues H570 and C572 or a small truncation of the C-terminal five to eight amino acids prevent VFLS formation by µNS and recombinant viruses bearing these mutations cannot be recovered (Broering et al., 2005; Arnold et al., 2008; Takeshi et al., 2009), suggesting that the formation of viral factories is required for productive ReoV infection.
This study shows that μNS, the viral nonstructural protein that forms ReoV viral factories, self-assembles into phase-separated compartments with dynamic liquid-like properties. By comparing the full-length μNS protein with its minimal region variant, we identified an important role for the N-terminal 471 residues of μNS in the nucleation process of phase separation as well as the diffusion of μNS molecules within inclusions. This led us to investigate other factors involved in μNS nucleation, including the role of FP tags, which revealed an FP-dependent increase in droplet formation. We then developed a panel of constructs encoding established deleterious mutations to demonstrate the recovery of μNS phase separation in an FP-dependent manner. We show that FP tags variably promoted µNS condensation and that mNeonGreen caused minimal perturbation. It is unlikely that these artifacts are exclusive to μNS, as similar conclusions were recently reported with other proteins (Zhou and Narlikar, 2023; Pandey et al., 2024); therefore, a strategy is presented herein that could help condensate researchers circumvent the adverse influences of fluorescent labels. Here we characterize the phase-separating properties of μNS, identify novel sequences involved in μNS nucleation, and show that FP tags affect the condensation properties of μNS.
RESULTS
The spherical morphology of μNS inclusions is indicative of interfacial tension
Multivalency is the fundamental driving force of biological phase separation (Li et al., 2012). In canonical liquid-liquid phase separation (LLPS), constituent biomolecules form a multitude of weak homotypic and heterotypic interactions involving proteins enriched with intrinsically disordered regions (IDRs) (Nott et al., 2015; Uversky et al., 2015; Harmon et al., 2017). While IDRs are ubiquitous in many systems that exhibit LLPS, the requisite multivalency for phase separation can also be achieved through structured protein domains. Although the structure of µNS has not been determined, computational sequence analysis tools indicate a divergence from IDR-driven multivalency. The ColabFold (Jumper et al., 2021; Mirdita et al., 2022) predicted structure of µNS from ReoV strain type 1 Lang (T1L) suggests the protein is mostly structured. This was corroborated with disorder annotations generated by IUPred3 (Erdős et al., 2021) and PONDR-FIT (Xue et al., 2010) predictors (Figure 1A). These models predict that only the extreme N- and C-terminal sequences of µNS are likely to be disordered regions. Within the minimal region for VFLS formation (471-721 µNS), there are two alpha helices spanning residues 521-559 and 590-690 that are predicted in the ColabFold structure. These align with predicted coiled-coil domains at residues 523-560 and 622-662, as annotated by MARCOIL (Delorenzi and Speed, 2002; Zimmermann et al., 2018; Gabler et al., 2020). These interacting domains may be sufficient for full-scale phase separation (Rios et al., 2024), and have been proposed as principal interacting elements for µNS self-assembly (McCutcheon et al., 1999; Broering et al., 2005).
FIGURE 1:
moxGFP::µNS VFLS are morphologically spherical. (A) ColabFold predicted structure of ReoV µNS (T1L, top). The model was sequentially colored from blue (N-terminus) to red (C-terminus). Residues 1, 472, and 721 were annotated. Structural disorder prediction scores (bottom) from PONDR-FIT and IUPred3 indicate that µNS is likely to be highly ordered, mostly lacking IDRs. Coiled coil domains predicted by MARCOIL were indicated in gold bands. (B) Representative immunofluorescence images of ReoV (T1L) infection with or without microtubule depolymerization, and transfected cells expressing untagged or moxGFP-tagged µNS after 24 h. Infected cells were treated with 10 µM nocodazole to disrupt microtubules, or an equal volume of DMSO as a vehicle control at 1 h postinfection. Bar, 10 µm. (C) The circularity and roundness of µNS VFLS and filamentous or globular MRV (T1L) viral factories. Measurements were constrained to particles with >1 µm area. Confocal microscopy images as shown in B were analyzed and the global mean for all replicates (N = 38–60 per sample) was plotted from three independent experiments. NCZ = nocodazole. (D) Maximum-intensity projection of a representative CV-1 cell expressing moxGFP::µNS(1-721) at 24 h post-transfection, acquired by LLSM. (E) Corresponding x-z (top) and x-y (bottom) cross-sectional slices from D show the spherical nature of inclusion bodies in isotropic resolution. Bar, 2 µm. (F) 3D measurements of inclusion body volume and sphericity from the image represented in D indicate highly spherical VFLS, even among large inclusion bodies.
To investigate the biophysical mechanism of µNS self-assembly in living cells, we encoded a moxGFP fluorescent tag to the N-terminus of full-length µNS (1-721 µNS) and its minimal region for VFLS formation (471-721 µNS). moxGFP is a monomeric GFP superfolder that lacks cysteine residues, thereby reducing the potential for FP dimerization, protein misfolding, and disulfide bond formation (Costantini et al., 2015). moxGFP was fused to the N-terminus of µNS because C-terminal GFP fusions were reported to interfere with µNS VFLS formation (Broering et al., 2002). We generated a full-length µNS construct and a minimal(er) region construct missing a single N-terminal residue (472-721 µNS). When expressed in cells, the moxGFP::µNS chimeras formed puncta that are partially and reversibly susceptible to 4% (w/v) 1,6-HD (Supplemental Figure S1, A and B). 1,6-HD sensitivity is an indicator of phase separation (Kroschwald et al., 2017) but is considered inconclusive for describing LLPS as there are numerous caveats to interpretation (Alberti et al., 2019).
Biomolecular condensates have a characteristic spherical morphology from surface tension forces that minimize interfacial area (Brangwynne et al., 2011), a feature universal to phase-separated polymer systems (Xia et al., 1992). ReoV (T1L) viral factories nonetheless have a distinctly filamentous structure because µNS interacts with the microtubule-associated viral protein µ2 (Broering et al., 2002; Parker et al., 2002). Thus, filamentous viral factories arise from association with the host cytoskeleton. In contrast, µ2 from ReoV strain type 3 Dearing does not bind to microtubules and forms globular viral factories that are phenocopied after microtubule depolymerization in ReoV (T1L) infection (Broering et al., 2002; Parker et al., 2002). Without µ2, VFLS in µNS-transfected cells are morphologically identical to globular viral factories seen during infection with the type 3 Dearing strain, but those formed by µNS(471-721) are described as having an elongated shape (Broering et al., 2005). We compared the morphology of moxGFP-tagged and untagged µNS inclusions with globular and filamentous viral factories by measuring the degree of circularity and roundness based on the perimeter, area, and aspect ratio (Cox, 1927; Takashimizu and Iiyoshi, 2016) of inclusions in two-dimensional immunofluorescence images (Figure, 1B and C). These shape descriptors correlate and tend to increase with liquidity (Etibor et al., 2023). As we expected, depolymerizing microtubules by nocodozole treatment led to globular viral factories in cells infected with the ReoV (T1L) strain. These globular factories were highly round and circular, similar to the VFLS formed in cells expressing µNS alone. Unlike previous descriptions of the elongated morphology of µNS(471-721) VFLS (Broering et al., 2005), we found that inclusions formed by μNS(471-721) were globular. Furthermore, we found that fusing moxGFP to the N-terminus of µNS did not markedly decrease the circularity and roundness of µNS VFLS, therefore, the morphology of fluorescent moxGFP::µNS inclusions were comparable to those formed by untagged µNS and globular viral factories seen during infection.
The three-dimensional (3D) morphology of µNS inclusions in live cells is ideally examined using fast, volumetric imaging with isotropic spatial resolution. We used lattice light sheet microscopy (LLSM) to examine the 3D morphology of moxGFP::µNS(1-721) inclusions in CV-1 cells (Figure 1D). Orthogonal slices of representative VFLS demonstrated a smooth interface of µNS inclusions (Figure 1E) and 3D measurements of sphericity and volume indicated highly spherical structures (Figure 1F). Dynamic observation of moxGFP::µNS inclusions from time course LLSM imaging provided further evidence for interfacial tension, as we observed coalescence of colliding VFLS (Supplemental Video S1). This liquid-like fusion behavior of ReoV viral factories has also been described during infection (Bussiere et al., 2017). The movement of moxGFP::µNS VFLS was partially dependent on an intact microtubule network (Supplemental Figure S1C), presumably due to vesicular trafficking as described for ReoV viral factories (Eichwald et al., 2018). In time-lapse confocal imaging, we observed fragmented linear motion of hollow spots traversing larger VFLS, assumed to be occupied by vesicles undergoing motor-driven transport (Supplemental Videos S2 and S3). These events caused viscoelastic deformation to the VFLS structure, as well as fission with a characteristic liquid bridge (Supplemental Figure S1D) (Brangwynne et al., 2009, 2011). Both the moxGFP::µNS(1-721) and moxGFP::µNS(472-721) constructs formed inclusions that showed evidence of liquid-like behaviors including fusion (Supplemental Figure S1E) and fission, although the constructs differed in the frequency of these events (Supplemental Figure S1F). We followed these observations with a quantitative assessment of interfacial tension and internal molecular rearrangement to study the dynamic properties of µNS inclusions.
Movie S1.
Lattice light sheet microscope imaging of a CV-1 cell transfected with moxGFP::μNS(1-721).
Movie S2.
Dynamics of moxGFP::µNS(1-721) inclusions
Movie S3.
Dynamics of moxGFP::µNS(472-721) inclusions
moxGFP::µNS inclusions coalesce with liquid-like characteristics
Quantifying the change in aspect ratio of coalescing droplets can give valuable insight into the biophysical properties of condensates (Brangwynne et al., 2009). moxGFP::µNS fusion events occurred within seconds, so we employed spinning disk confocal microscopy to resolve moxGFP::µNS fusion kinetics. Both moxGFP::µNS(1-721) and moxGFP::µNS(472-721) inclusions underwent fusion and relaxation to a spherical shape (Figure 2A) with conservation of volume (Supplemental Figure S2). The characteristic relaxation time τ for two droplets to fuse and assume a spherical shape is roughly set by droplet size ℓ and the inverse capillary velocity η/γ, where η is droplet viscosity and γ is surface tension (Brangwynne et al., 2009, 2011). The shape aspect ratio of coalescing moxGFP::µNS inclusions fit an exponential decay function which is expected for liquid condensates (Figure 2B) (Brangwynne et al., 2009, 2011). The characteristic relaxation time τ was solved from the exponential decay function of 105 fusion events and was plotted against the average droplet radius ℓ prior to fusion (Figure 2C). Given τ≈η/γ ∙ ℓ, the slope of the linear regression is the inverse capillary velocity η/γ which did not statistically differ between moxGFP::µNS(1-721) and moxGFP::µNS(472-721) (analysis of covariance [ANCOVA], p = 0.6157), indicating that they fused on similar timescales and their bulk and interfacial properties did not vary substantially. The inverse capillary velocity η/γ was 6 s/µm, 95% confidence interval (CI) [3, 9] for moxGFP::µNS(1-721) and with this, theoretical arguments were used to approximate surface tension γ and solve for an estimated viscosity η of 255 Pa·s (see Materials and Methods) (Brangwynne et al., 2011). During the fission of moxGFP::µNS(1-721) and moxGFP::µNS(472-721) inclusions, a thin liquid bridge was observed that ruptured and retracted in roughly 1 s, further reflecting the liquid properties of condensates (Brangwynne et al., 2011).
FIGURE 2:
moxGFP::µNS inclusions undergo fusion and fission with liquid-like characteristics. (A) Representative frames of moxGFP::µNS VFLS coalescence events from spinning disk confocal microscopy time-lapse videos of live transfected cells at 24 h post-transfection. 100X objective fields were captured at a 100 ms acquisition rate. Bar, 1 µm. (B) Example plot of the measured shape aspect ratio of two fusing moxGFP::µNS inclusions at similar length scales. Two VFLS make contact and the aspect ratio is ∼2, then after a short delay, it exponentially decayed to an A.R. of ∼1. (C) Inverse capillary velocity (ICV) plot of ReoV µNS. moxGFP::µNS(1-721) (N = 75) (in blue) and moxGFP::µNS(472-721) (N = 29) (in yellow) fusion events were observed with one outlier excluded from the analysis (see statistics and reproducibility). The ICV was determined from the slope of a simple linear regression fit to the data, which is 5.814 s/µm, 95% CI [3.044, 8.583] for moxGFP::µNS(1-721) and 4.6 s/µm, 95% CI [−0.2344, 9.435] for moxGFP::µNS(472-721). 95% CI bands are shown. moxGFP::µNS(1-721) is significantly nonzero (p < 0.0001) while moxGFP::µNS(472-721) is insignificant (p = 0.0613). The slopes of the linear regressions do not significantly differ (p = 0.6157). (D) Example of a rupturing liquid bridge observed during droplet fission. The ROI shows bridge formation, rupture, and retraction in roughly 1 s. Bar, 1 µm.
The diffusion of µNS(472-721) was faster than wild-type µNS within inclusions
Coalescence is a hallmark of LLPS; however, nonliquid internal structure, glass-like phases, or gel-like phases may not be revealed in these observations. We generated a multicolor VFLS model, employing the UV-photoconvertible Dendra2 fluorophore, which enabled visualization of µNS flux to resolve any constraints in protein diffusion within inclusion bodies. Utilizing a scanning laser confocal fluorescence microscope, we selectively irradiated individual Dendra2::µNS(1-721) and Dendra2::µNS(472-721) inclusion bodies with gentle 405 nm light, then imaged fusion events between unconverted and converted VFLS in red and green fluorescence channels simultaneously (Figure 3A). We observed complete homogenization of photoconverted Dendra2::µNS across the fused VFLS, and a spatially uniform gradient in the dye front, so no internal obstructions or structure were observable. Furthermore, no photoconverted Dendra2::µNS was observed leaving the dense phase for the dilute phase or unirradiated VFLS. These multicolor fusion events are strong support for the LLPS model of µNS self-assembly, as they emulate Fickian diffusion behavior and exhibit behaviors of interfacial tension and unrestricted internal rearrangement that gel, glass, solid-aggregate (Hyman et al., 2014), or scaffold-binding-hub (Mir et al., 2018) alternative models of µNS condensation cannot explain.
FIGURE 3:
µNS inclusion bodies are liquid compartments exhibiting slow diffusion rates. (A) Laser-scanning confocal microscope imaging of photoconverted Dendra2::µNS droplets fusing after selective 405 nm photoconversion (“PC”) demonstrated isotropic diffusion, redistributing the construct homogeneously. Example images of live cells were captured using a Zeiss LSM880 confocal microscope at 22–26 h post-transfection. Timestamps display minutes and seconds. (B) Schematic of MP-FRAP acquisition. A focused 920 nm beam parked in a condensate is rapidly modulated by Pockels Cell actuation, enabling diffusive fluorescence recovery monitoring in a two-photon excitation spot at micron and microsecond resolution. SR-430 photon counter traces are displayed and fit to a model of diffusive recovery in custom-written MP-FRAP software. The example trace (from acquisition highlighted in Supplemental Figure S3) normalized fluorescence recovery and fitting results are displayed. The unitless quantity F/F0 is the sum of photon counts per time bin, normalized to the prebleach mean. β is the unitless bleach depth parameter, D is the diffusion coefficient in μm²/s, and the immobile fraction is unitless. (C) Summary of Levenberg-Marquardt fitting results of moxGFP::μNS(1-721) (N = 67) and moxGFP::μNS(472-721) (N = 8) traces acquired 20–26 h after transfection. Statistics performed by Conover-Iman rank test with Holm-Bonferroni correction after rejection of null hypothesis in Kruskal–Wallis H-test.
While homogenization is observed in both minimal region and full-length constructs of Dendra2::µNS, we noted a significant change in the rates of mixing between these constructs. In representative time-course images of ∼2 µm diameter photoconverted droplets, the speed of interfacial area minimization appeared similar (as observed in Figure 2B), but the internal rearrangement of Dendra2::µNS(1-721) took several minutes, while the mixture of Dendra2::µNS(472-721) homogenized within about 30 s of fusion (Figure 3A; Supplemental Videos S4 and S5). This difference was unexpected given the 2-fold difference in the molecular weight between Dendra2::µNS(1-721) and Dendra2::µNS(472-721), so we sought to measure the diffusion rates of both constructs in the dense phase in live cells.
Movie S4.
Unconverted and photoconverted Dendra2::μNS(1-721) VFLS fusion events show slow liquid-like behavior
Movie S5.
Unconverted and photoconverted Dendra2::μNS(472-721) VFLS fusion events show faster liquid-like behavior
Some of the pitfalls of employing fluorescence recovery after photobleaching (FRAP) to study transport in biomolecular condensates have been carefully examined (Taylor et al., 2019). Notably, these include a significant discrepancy in measurement results when different boundary conditions are assumed, and a loss of measurement fidelity when the length scale of a bleached volume approaches the length scale of the condensate under study. To this, we add a concern for addressing the stochastic impacts that intracellular motion imparts to droplets. To avoid these pitfalls, we carried out multiphoton-FRAP (MP-FRAP) measurements at the center of large VFLS (Figure 3B). In MP-FRAP, fluorescent molecules within a subfemtoliter two-photon 3D excitation volume were photobleached using short bleaching pulses, and the recovery of fluorescence within the focal volume was recorded at a low, nonbleaching monitoring power (Brown et al., 1999). To mitigate VFLS motion during MP-FRAP acquisitions, we stabilized VFLS motion by treating the cells with nocodazole. Using this method, we measured diffusion constants for moxGFP::µNS(1-721) traces to have 4 ± 5 µm2/s (N = 67) and moxGFP::µNS(472-721) traces to have 30 ± 8 µm2/s (N = 8), each between 20 and 26 h post-transfection. In agreement with observations from photoconverted Dendra2::µNS imaging, the measured diffusion coefficient of the minimal region mutant construct was significantly greater than the full-length construct.
The N-terminal 471 residues of µNS enhance droplet nucleation
Another key difference that we observed between the moxGFP::µNS(1-721) and moxGFP::µNS(472-721) constructs was the size and number of inclusions. moxGFP::µNS(1-721) demonstrated constant and efficient droplet formation over 24 h, whereas moxGFP::µNS(472-721) formed a lower density of larger inclusions (Figure 4A). This indicated that the nucleation of moxGFP::µNS droplets differed between constructs.
FIGURE 4:
Droplet nucleation and growth kinetics show the first 471 residues of µNS are important for nucleation. (A) The radius and density of moxGFP::µNS droplets were quantified at 6, 12, and 24 h post-transfection in live cells. The mean number of droplets per µm3 (middle) and their mean radius (left) in each independent experiment were plotted with a connecting line through the grand means. moxGFP::µNS(1-721) nucleated a higher density of droplets at 6 h post-transfection (right). An unpaired two-tailed t test yielded a statistically significant difference (p < 0.0001) between the two samples. N = 55 cells were analyzed for moxGFP::µNS(1-721) (in blue) and N = 50 cells for moxGFP::µNS(472-721) (in yellow). (B) Representative frames of the emergence of nascent moxGFP::µNS puncta were recorded using multi-area time-lapse imaging. Bar, 20 µm. (C) Droplet density and growth were measured over the first hour of phase separation in N = 48 moxGFP::µNS(1-721) - (in blue) and N = 66 moxGFP::µNS(472-721) - (in yellow) transfected cells over three independent experiments. All measurements were fit with a simple linear regression with 95% CI bands. Droplet growth (left) and nucleation (right) rates statistically differed (ANCOVA, p < 0.0001). L.O.D. = limit of detection. (D) The relative fluorescence mean gray value (MGV) (a proxy for Csat) was measured one frame before moxGFP::µNS diffraction-limited puncta spontaneously emerged. MGV measurements from background-subtracted images were standardized by the median MGV of moxGFP::µNS(1-721) for N = 18 cells per sample. An unpaired two-tailed t test reached statistical significance (p < 0.0001).
Nucleation is the initial thermodynamic process of forming a new phase, the rate of which is commonly modeled by classical nucleation theory (Karthika et al., 2016). Classical nucleation theory has been used to explain the formation of biomolecular condensates (Narayanan et al., 2019; Shimobayashi et al., 2021). It predicts the spontaneous self-assembly of nanoscale clusters that are unstable at thermodynamic equilibrium, yet stochastic protein dynamics can overcome a characteristic Gibbs free energy barrier if the condensate grows to a critical radius Rc, beyond which the droplet becomes stable. We reasoned that the first 471 residues of µNS may play a role in nucleation since moxGFP::µNS(1-721) had a higher droplet density at 6 h after transfection (Figure 4A). To record the earliest moments of moxGFP::µNS VFLS formation, we initiated multi-area time-lapse imaging at 3 to 4 h post-transfection (Figure 4B; Supplemental Videos S6 and S7) to quantify the density and radius of puncta formed over the first hour of phase separation (Figures 4C).
Movie S6.
moxGFP::µNS(1-721) droplet formation
Movie S7.
moxGFP::µNS(472-721) droplet formation
The nucleation rate J is expressed as a change in droplet density over time, specifically, J = dρ(t)/dt|t=t0, where ρ is droplet density expressed as the number of droplets per cubic micron, t is time in seconds, and t0 is the first frame droplets are observed (Shimobayashi et al., 2021). We found that the nucleation rate of moxGFP::µNS(1-721), J = 1.255 × 10−5 µm−3 s−1, was 5-fold higher than moxGFP::µNS(472-721) at J = 2.537 × 10−6 µm−3 s−1 (Figure 4C). These values fall within the range of 10−6 to 10−4 µm−3 s−1 which is typical of biomolecular condensates (Shimobayashi et al., 2021). We similarly calculated the droplet growth rates by measuring the change in radius over time. We found that moxGFP::µNS(472-721), with an increase in radius over time of 0.87 µm/h, had a greater droplet growth rate than moxGFP::µNS(1-721) (0.33 µm/h). These findings suggest that the first 471 residues of µNS are important for droplet nucleation and that the increased rate of nucleation comes at the expense of droplet growth.
The presence of a defined saturation concentration Csat is strong evidence of phase separation because Csat is a distinct property of phase separation that is not seen in other forms of biomolecular self-association (Alberti et al., 2019). Csat is the equilibrium concentration in a homogeneous system required to form a dense phase. As a proxy for Csat, we measured the mean gray value in cells one frame before t0, representing the concentration of moxGFP::µNS less than 5 min before the spontaneous emergence of nascent diffraction-limited puncta (Figure 4D). The relative Csat for moxGFP::1-721 µNS was 3-fold lower than moxGFP::472-721 µNS, which is consistent with their nucleation propensities. Furthermore, this elevated fluorescence intensity in moxGFP::µNS(472-721)-transfected cells appeared to be maintained in the dilute phase during supersaturated conditions (Figure 4B). Importantly, these results suggest that there is a defined Csat for moxGFP::µNS phase separation.
FP tags affect the formation and distribution of µNS condensates
To identify factors involved in µNS nucleation, we considered the intrinsic properties of moxGFP::µNS including the role of the fluorescent tag. To test whether the moxGFP tag affected µNS nucleation, we compared the droplet density of moxGFP::µNS with that of untagged µNS (Supplemental Figure S5A). We found that untagged full-length µNS nucleated a higher density of droplets than the minimal region; however, both constructs formed fewer droplets than their moxGFP-tagged counterparts, suggesting the moxGFP tag was promoting condensate formation.
Because some biomolecular condensates react to paraformaldehyde (PFA) fixation and display artifacts that would confound droplet density measurements (Irgen-Gioro et al., 2022), we first examined the effects of fixation on moxGFP::µNS droplet density. We quantified the droplet density of moxGFP::µNS droplets in maximum intensity Z-stack projections of transfected cells before and after the addition of 4% PFA (Figure 5A). We found that fixation with 4% PFA reduced moxGFP fluorescence (Supplemental Figure S5B), leading to the disappearance of some droplets; however, this could be corrected by increasing the laser intensity of the microscope. Immunofluorescence staining of the same cells confirmed that moxGFP::µNS inclusion density was not affected by PFA fixation, therefore the increase in moxGFP::µNS droplet density compared with untagged µNS (Supplemental Figure S5A) could not be explained by fixation artifacts.
FIGURE 5:
N-terminal FP tags enhance the droplet density of µNS inclusions. (A) moxGFP::µNS-expressing cells were fixed with 4% PFA at 24 h post-transfection. Images were collected of live cells before fixation (live), 10 min after fixation (fixed), and with the laser intensity increased by a factor of 5 (fixed +) in the EGFP channel. The same cells were relocated after immunofluorescence (IF) staining and imaged in the Alexa Fluor 647 channel (stained). Droplet densities were quantified in cells transiently expressing moxGFP::µNS(1-721) (N = 19) or moxGFP::µNS(472-721) (N = 19) over three independent experiments. For each sample, values were made relative to the droplet density of each cell before fixation, then the mean for each experiment was plotted. An ordinary two-way ANOVA indicated a statistical difference between conditions (p = 0.0004) but not constructs (p = 0.5851), nor is there an interaction (p = 0.5718). We applied Dunnett’s test to compare the droplet density of each condition to that of live cells. Bar, 10 µm. (B) Fixation does not affect FusionRed::µNS(1-721) and Dendra2::µNS(1-721) droplet density in transfected CV-1 cells at 24 h post-transfection. The mean droplet density was quantified in live cells before and after the addition of 4% PFA for 10 min over three independent experiments and reached statistical insignificance by ordinary two-way ANOVA (p > 0.05). The laser power was increased after fixation for Dendra2::µNS(1-721)-transfected samples, but not FusionRed::µNS(1-721)-transfected samples because fluorescence reduction was not observed. (C) The moxGFP tag, but not Dendra2 or FusionRed tags, enhanced µNS(1-721) droplet density. IF images of samples expressing untagged µNS or FP::µNS constructs were fixed at 24 h post-transfection and the mean droplet density in three independent experiments was quantified. An ordinary two-way ordinary ANOVA was applied with Dunnett’s test comparing the droplet density of each FP::µNS fusion to that of untagged µNS. Representative IF images are shown on the right. Bar, 10 µm. (D) Quantification of FP::µNS(1-721) droplet density in live cells with three different FP tags. Transfected cells (N = 20–29) expressing moxGFP::µNS(1-721), Dendra2::µNS(1-721) or FusionRed::µNS(1-721) were randomly sampled at 24 h post-transfection to measure droplet densities. Not all samples were normally distributed, so the median for each experiment is reported with a line at the mean, and an ordinary one-way ANOVA (p = 0.0002) with Tukey’s correction for multiple comparisons was applied.
Given the effect of the moxGFP tag on μNS droplet formation, we included two other FP-tagged µNS constructs, specifically Dendra2::µNS(1-721) and FusionRed::µNS(1-721), to test whether this result was specific to the moxGFP tag. Fixation artifacts in phase-separated systems can be FP tag-dependent (Irgen-Gioro et al., 2022), so we first confirmed the absence of fixation artifacts on the droplet density of Dendra2::µNS(1-721) and FusionRed::µNS(1-721)-transfected cells (Figure 5B). While FusionRed::µNS was unaffected by fixation, Dendra2::µNS showed a small but insignificant decrease in droplet density from fluorescence reduction (Supplemental Figure S5C). When assessed by immunofluorescence imaging, we concluded that the Dendra2::µNS(1-721) droplet density was not affected by 4% PFA fixation (Supplemental Figure S5D). As the droplet density of all three FP::µNS chimeras was not influenced by fixation, we proceeded to compare the droplet density of FP::µNS(1-721) with untagged µNS(1-721) in fixed cells (Figure 5C). We found that the moxGFP tag enhanced µNS(1-721) nucleation by an order of magnitude, while the Dendra2 and FusionRed tags had little effect and these values were consistent in live cells (Figure 5D). This finding adds to our understanding of the effects of FP tags on phase separation (Uebel and Phillips, 2019; Irgen-Gioro et al., 2022; Zhou and Narlikar, 2023; Pandey et al., 2024) by demonstrating an FP-dependent influence on the observed density of condensates.
We used chicken antiserum to detect untagged µNS by immunofluorescence imaging (Supplemental Figure S5E). However, we recognize that the availability of antibodies can be a barrier to detecting other untagged proteins. We found that the minimal epitope tag hemagglutinin (HA) could be used to detect and quantify µNS puncta in immunofluorescence images as an alternative to µNS antisera (Supplemental Figure S5, F and G).
FP tags can rescue phase separation of mutant µNS constructs
Several mammalian and avian orthoreovirus µNS mutants cannot form condensates. However, fusion of GFP to these constructs rescues VFLS formation (Broering et al., 2005; Alberto et al., 2010). With our current understanding of mammalian and avian µNS as phase-separating biomolecules (Durinova et al., 2023), this outcome could now be interpreted as FP-driven phase separation. To test this hypothesis, we used an established histidine to glutamine substitution at residue 570 (H570Q) that is known to abrogate VFLS formation (Broering et al., 2005) to test whether FP tags could rescue phase separation of soluble µNS mutants. We chose Dendra2 as a fluorescent tag because of its brightness and marginal effect on wild-type full-length µNS droplet density (Figure 5D). Untagged µNS(1-721)H570Q was diffusely distributed in cells (Supplemental Figure S6A), but remarkably, we found that Dendra2::µNS(1-721)H570Q efficiently formed phase-separated droplets (Supplemental Video S8). Similarly, µNS(1-721)H570Q condensation was also rescued by a moxGFP tag (Supplemental Video S9). These FP-tagged µNS(1-721)H570Q inclusions displayed viscoelastic properties, indicating a phenotype of FP-induced phase separation.
Movie S8.
Phase separation of Dendra2::µNS(1-721)H570Q
Movie S9.
Phase separation of moxGFP::µNS(1-721)H570Q
Next, we hypothesized that truncation of the first 471 residues of μNS from the Dendra2::µNS(1-721)H570Q construct would render the protein soluble due to the apparent role of this region in µNS droplet nucleation (Figure 4). Indeed, Dendra2::µNS(472-721)H570Q was diffuse in most transfected cells; however, phase separation was observed in roughly 5% of the transfected cell population (Supplemental Video S10; Supplemental Figure S6B). We also noted that the Dendra2::µNS(1-721)H570Q and Dendra2::µNS(472-721)H570Q constructs had an elevated fluorescence intensity in the dilute phase compared with their wild-type counterparts (Supplemental Figure S6C).
Movie S10.
Phase separation of Dendra2::µNS(472-721)
To expand on these initial observations, we generated a panel of N-terminal moxGFP- and Dendra2-tagged µNS mutants carrying three deleterious mutations both individually and in combination. The three mutations are the H570Q point mutation, an N-terminal truncation of the first 471 residues, and a C-terminal deletion of the last eight amino acids (1-713 µNS) which is also known to perturb VFLS formation (Broering et al., 2005). As expected, without an FP tag, µNS(1-721) and µNS(471-721) formed VFLS, while µNS mutants harboring the H570Q point mutation and/or C-terminal truncation were diffuse (Figure 6A). However, efficient phase separation was rescued in several of these constructs when they were fused with N-terminal Dendra2 or moxGFP tags (Figure 6B). Removal of the first 471 residues of µNS entirely depleted all signs of phase separation in the FP-tagged mutants, except for Dendra2::µNS(472-721)H570Q, which strengthens our conclusion that this region is important for µNS condensation. Phase separation was apparent in these FP::µNS mutants as evidenced by coalescence (Figure 6C) and viscoelastic behavior like that of the wild-type µNS constructs.
FIGURE 6:
FP tags rescue condensation of µNS mutant constructs that do not phase separate. (A) Untagged µNS mutants harboring the H570Q substitution or C-terminal deletion of residues 714-721 were diffusely distributed in cells. Representative widefield microscopy IF images of CV-1 cells at 24 h post-transfection that were fixed and stained with µNS antiserum were imaged in the Alexa Fluor 594 channel. Bar, 10 µm. (B) Dendra2 and moxGFP tags rescued condensation of µNS mutants. Representative confocal microscopy images of live cells transiently expressing the annotated construct were captured at 22–26 h post-transfection. Bar, 10 µm. (C) FP::µNS mutant constructs formed puncta that exhibited characteristics of phase separation. Time, seconds. Bar, 2 µm. (D) Phase separation–positive cells were scored in the transfected cell population based on the unambiguous presence of one or two phases. (E) The percentage of fluorescent signal in the dense phase (punctate percentage) was quantified in N = ∼60 phase-separated cells and the grand mean (X-axis) was plotted with respect to the mean estimation of the population proportion of phase sepration–positive cells (Y-axis) from D. (F) The radius (X-axis) and density (Y-axis) of droplets were also quantified and the grand mean of three independent experiments was plotted.
To quantify the degree of phase separation in this panel of FP::µNS mutants, fluorescent cells were scored based on the presence of one or two phases to estimate the population proportion of transfected cells containing phase separation (Figure 6D). Among phase separation–positive cells, punctate percentage (the percentage of fluorescent signal in the concentrated phase) was measured and plotted on a separate axis (Figure 6E). Punctate percentage is an indicator of phase separation propensity (Irgen-Gioro et al., 2022) and we found that it roughly correlated with the population proportion of phase separation–positive cells. Interestingly, these measures did not correlate with the size and density of droplets (Figure 6F). Mutant Dendra2::µNS condensates were more prevalent in the transfected population, had a greater punctate percentage, and were generally larger compared with their moxGFP::µNS counterparts. These differences were unexpected, given that moxGFP had a stronger influence on wild-type full-length µNS (Figure 5, C and D). Altogether, the simplest interpretation is that FP tags can create unpredictable outcomes on phase behavior, including nucleation, droplet size distribution, and punctate percentage. Our finding that FP tags can induce phase separation of otherwise soluble proteins shows that the phenomenon of phase separation can be an artifact of experimental design.
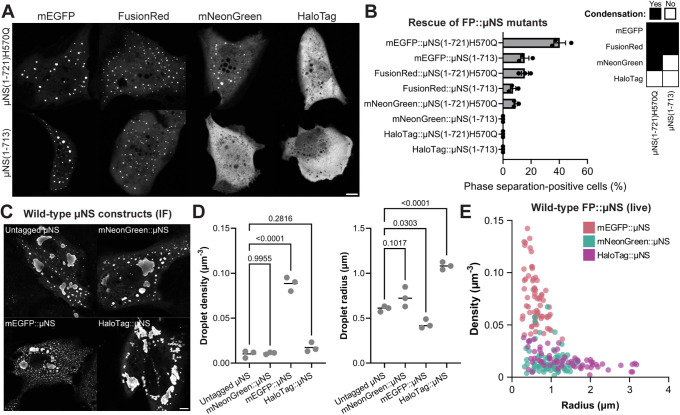
mNeonGreen is a suitable fluorescent tag for ReoV µNS
In this panel of soluble µNS mutants, the µNS(1-721)H570Q and µNS(1-713) constructs were the most sensitive to FP-mediated phase separation and µNS(1-713) was more dependent on FP tag choice (Figure 6, D and E). We chose to employ these mutants as a platform to screen diverse FP tags, with the goal of identifying FP tags that minimally recover µNS phase separation. We reasoned that these two sensitive mutants were particularly advantageous over the wild-type full-length µNS construct because the mutant model is an on-off phenotype of phase separation controlled by the FP tag. mEGFP was selected as a prototypical FP that is considered a highly monomeric GFP variant (Zacharias et al., 2002; Cranfill et al., 2016). mNeonGreen has low sequence similarity to GFP but similar structural and functional properties (Shaner et al., 2013). FusionRed is an mKate2 derivative that is purely monomeric under high performance liquid chromatography analysis at concentrations up to 10 mg/mL (Shemiakina et al., 2012). HaloTag, which is a modified bacterial hydrolase (Los et al., 2008), was included to emphasize structural and functional diversity, as well as three small FPs; smURFP (Rodriguez et al., 2016), miRFP670nano (Oliinyk et al., 2019), and miniGFP2 (Liang et al., 2022). These tags were N-terminally fused to µNS(1-713) and µNS(1-721)H570Q constructs with a GS linker and were transiently expressed in CV-1 cells (Figure 7A; Supplementary Figure S7A).
FIGURE 7:
mNeonGreen::µNS and untagged µNS inclusions have a similar size and density. (A) Representative images are shown from three independent experiments of live cells expressing GFP-like proteins or HaloTag fused to µNS(1-721)H570Q or µNS(1-713) at 24 h post-transfection. Scale bar, 10 µm. (B) Estimation of the population proportion of phase separation–positive cells from three independent experiments, with a line at the mean and SEM error bars. (C) Representative immunofluorescence images show transfected cells expressing wild-type FP::µNS or untagged µNS that were stained with chicken µNS antiserum (1:1000 dilution) and Alexa Fluor 594 secondary antibodies (1:800 dilution) at 24 h post-transfection. Scale bar, 10 µm. (D) The average droplet density (left) and radius (right) of inclusions per cell per experiment were measured and plotted with a line at the mean. Over three independent experiments, images of cells transfected with wild-type untagged µNS (N = 85), mNeonGreen::µNS (N = 94), mEGFP::µNS (N = 81), or HaloTag::µNS (N = 81), as shown in C, were processed in Fiji. The mean droplet density and radius were calculated per cell and the median from each experiment was plotted. A one-way ANOVA with Dunnett’s test was applied with p values shown. (E) Live cells transiently expressing wild-type mEGFP::µNS (N = 69), mNeonGreen::µNS (N = 76), or HaloTag::µNS (N = 66) were imaged over three independent experiments at 24 h post-transfection and the mean droplet density and radius for each cell is shown.
We scored the population proportion of phase separation–positive cells to assess the impact of different FP tags on µNS(1-721)H570Q and µNS(1-713) condensation (Figure 7B). Of the GFP-like proteins, mEGFP, FusionRed, and mNeonGreen induced puncta that were viscoelastic and capable of fusion (Supplemental Figure S7B), indicating a liquid-like state no different from what was observed with moxGFP::µNS and Dendra2::µNS. mNeonGreen was the least consequential GFP-like protein tag, as it did not produce phase separation in the µNS(1-713) construct and had the least effect on µNS(1-721)H570Q condensation compared with mEGFP and FusionRed. We found that HaloTag produced no signs of phase separation when fused to either µNS construct. This shows that not all FP tags could rescue the µNS mutants, which suggests that some may be more suitable to visualize biomolecular condensates with minimal FP-driven phase separation.
We reserve our conclusions on the effect of small FPs on phase separation because miRFP670nano, smURFP, and miniGFP2 tags formed puncta on both µNS mutant constructs (Supplemental Figure S7A); however, the biophysical properties of these inclusions appeared different from those formed by the GFP-like protein tags. The miRFP670nano::µNS and smURFP::µNS inclusions displayed evidence of fusion (Supplemental Figure S7C), although some inclusions appeared refractory to coalescence and deformation. miniGFP2::µNS mutants formed puncta in half of the transfected cell population and showed little evidence of fusion or viscoelasticity (Supplemental Figure 7C), which was also observed in the wild-type miniGFP2::µNS construct (Supplemental Figure 7, D and E). The miniGFP2::µNS compartments were not misfolded protein aggregates (Supplemental Figure 7F), suggesting the miniGFP2 tag modulated the material properties of the µNS condensates.
Altogether, of the nine FP tags that were screened for their propensity to rescue µNS phase separation, we found that mNeonGreen and HaloTag caused minimal recovery of µNS mutant constructs. Next, we generated wild-type µNS constructs fused to mEGFP, mNeonGreen, or HaloTag and compared the size and density of inclusions with untagged µNS (Figure 7C). Consistent with their propensity to rescue the µNS mutant constructs, mNeonGreen and HaloTag did not affect the wild-type µNS droplet density, but mEGFP strongly promoted inclusion formation and this difference could not be explained by fixation artifacts (Figure 7, D and E; Supplemental Figure S7G). Intriguingly, HaloTag led to much larger µNS condensates (Figure 7, D and E). Concerning both the size and density of the condensates, mNeonGreen::µNS closely reflected untagged µNS (Figure 7D; Supplemental Figure S7H).
Summary of FP-induced artifacts
To summarize the effects of GFP-like tags and HaloTag on µNS phase separation, we consolidated our measurements of condensate size and density across experiments (Supplemental Figure S8A), as well as the population proportion of rescued µNS mutant phase separation (Supplemental Figure S8B). The effect size between FP::µNS and untagged µNS was expressed as a fold change value from the pooled data (Supplemental Figure S8C). mEGFP and moxGFP were the most consequential tags for µNS phase separation, as they formed ∼6.6-fold more inclusions and caused the greatest rescue of µNS mutant constructs. FusionRed and Dendra2 moderately rescued the µNS mutants and increased wild-type µNS droplet density but did not affect their size. HaloTag did not rescue the µNS mutants and did not increase wild-type µNS droplet density but caused aberrant inclusion growth. These results suggest that the influence of FP tags on nucleation and growth may occur through different mechanisms.
Toward mechanistic insight on the effects of FP tags on µNS phase separation, we calculated the predicted charge and molecular weight for each FP tag and found no correlation between these properties and the effect of the tags on µNS condensation (Supplemental Figure S8C). This suggests that neither overall charge (Yeong et al., 2022), nor the additional molecular weight of the tag (Zhou and Narlikar, 2023) are dominant factors, although the surface distribution of charged patches could play a role in promoting complex coacervation (Kapelner and Obermeyer, 2019; Zervoudis and Obermeyer, 2021). Another concern is the tendency for FPs to dimerize. Biomolecular condensates can concentrate proteins well into the millimolar range which is an ideal condition for FP dimerization (Levin et al., 2021). The standard method to assess FP dimerization in cells is the organized smooth endoplasmic reticulum (OSER) assay (Costantini et al., 2012). We performed the OSER assay in a single-blind study and found that the greatest source of variation was the participant and not the FP tag; therefore, we could not interpret meaningful data about FP–FP interactions from this method (Supplemental Figure S8D), though FP–FP interactions remain a possible contributing mechanism. We coexpressed FPs alone with untagged µNS and found modest colocalization in fixed cells (Supplemental Figure S8E), although this was not as apparent in live cells (Supplemental Figure S8F). This could indicate the presence of FP–µNS interactions or at least a lack of exclusion of the FPs from untagged µNS condensates. Careful experimental design is required to disentangle the contributions of each of these factors, as well as consideration for other mechanisms, so any mechanistic interpretation of the effect of FP tags on µNS phase separation is at the moment speculative.
DISCUSSION
ReoV viral factories have shown properties consistent with biomolecular condensates (Bussiere et al., 2017) and it has been speculated that phase separation underpins their formation (Guo and Parker, 2021; Lee et al., 2021). Here we confirm that the major protein constituting ReoV viral factories, µNS, forms liquid-like phase-separated droplets. We quantified the intrinsic properties of µNS, including nucleation kinetics, inverse capillary velocity, and molecular diffusion. This work adds ReoV to the growing list of viruses that form phase-separated compartments (Etibor et al., 2021). The field of viral phase separation is now advancing toward understanding the mechanistic roles of phase separation during viral infection. It has recently been shown that liquid compartments promote viral self-assembly processes (Guseva et al., 2020; Charman et al., 2023). However, the importance of a liquid microenvironment for viral assembly is currently not well understood, but theoretical models have proposed that mechanisms of capsid assembly could reflect a crystallization process coupled to liquid-liquid phase-separated compartments (Hagan and Mohajerani, 2023).
The expression of µNS in transfected cells and ReoV infection are both high and thus our model of plasmid overexpression mimics the concentration of µNS during infection and does not create artificially supersaturated conditions. However, ReoV viral factories in infected cells have a complex composition that includes viral proteins and mRNAs, viral core particles, virions, fragmented endoplasmic reticulum (ER) tubules (Raquel et al., 2018), and host proteins (Susanne et al., 2012; Stanifer et al., 2017), some of which are absent in µNS-transfected cells.
We identified an important and previously unrecognized role for the first 471 residues of µNS in the nucleation process of ReoV viral factory formation. This region lowers the apparent saturation concentration Csat (Figure 4D) but is not required for phase separation. The mechanism of µNS nucleation is not understood but could involve extrinsic host factors. According to classical nucleation theory, the critical radius Rc and the degree of supersaturation can be reduced by nucleating on a foreign structure, referred to as heterogeneous nucleation (Karthika et al., 2016). If a region in the N-terminal 471 residues of µNS interacts with a host factor, this could influence the nucleation and growth kinetics of µNS condensates and explain the disparity in droplet nucleation observed between µNS(1-721) and µNS(472-721). A candidate nucleation surface is the endoplasmic reticulum (Lee et al., 2020; Snead et al., 2022) because it is known to interact with µNS (Raquel et al., 2018). Alternatively, host condensates like stress granules (Wheeler et al., 2016; Yang et al., 2020) that share components with ReoV viral factories through interactions with residues 78 and 79 of µNS (Carroll et al., 2014) could also enhance viral factory formation (Jacobs, 2021).
Biomolecular condensates can be modeled by the sticker and spacer framework borrowed from the theory of associative polymers (Holehouse and Pappu, 2018; Wang et al., 2018). Stickers are associative motifs that lower the saturation concentration Csat, while spacers impart flexibility but are not major determinants of phase separation. It is important to recognize that FP tags may create an artificial sticker through transient FP–FP or FP–µNS interactions. Combinations of FP tags and deleterious µNS mutations lead to unequivocal yet unpredictable outcomes on phase behavior and the distribution of µNS droplets. While further work is needed to identify and characterize the proposed stickers within µNS, we unambiguously demonstrated that FP tags can generate artifacts and even invert the phenotype of phase-separated proteins.
In this study, FP-mediated condensation was a phenomenon that we characterized exclusively in ReoV µNS, therefore our conclusions are limited to this individual phase-separating system. There is an example of GFP promoting FIB1 droplet nucleation (Feric et al., 2016) as well as a recent finding that RFP drives Httex1(Q25) phase separation (Pandey et al., 2024). This suggests that the FP-mediated condensation artifacts described here and elsewhere (Pandey et al., 2024) are generalizable, and other proteins could be mischaracterized as biomolecular condensates in the absence of untagged controls.
Another consideration is the position of the FP tag on the construct. C-terminal µNS(1-721)::GFP fusion was reported to partially interfere with VFLS formation by reducing the population of transfected cells that contained inclusions to 80% (Broering et al., 2002). In comparison, we found that N-terminal moxGFP fusion condensed µNS in nearly 100% of transfected cells (Figure 6D). Considering this population-scale metric appears to be useful for comparing relative phase separation efficiency (Figure 6E), one interpretation is that C-terminal FP tags were inhibitory to µNS condensation. However, as N-terminal FP tags were shown to promote µNS condensation (Figures 5–7), it could be that C-terminal FP tags are less consequential to µNS phase separation and that C-terminal µNS fusions may be more representative of untagged µNS. Whether C-terminal GFP tags can rescue condensation of µNS variants that do not phase separate is another question. In functional mutagenesis studies of avian µNS, both an N- and C-terminal GFP tag recovered inclusion formation in a mutant analogous to µNS(1-713) used in this study (Alberto et al., 2010), suggesting that FP tags can affect µNS phase separation regardless of their position in the construct.
Our findings demonstrate the functional role of FP tags in promoting the condensation of a typical biomolecular condensate. When developing a model to study phase separation that involves FPs, the choice of FP tag should not be a slapdash decision, otherwise, there is a risk that the fusion protein will not accurately recapitulate its native counterpart. We encourage researchers to exercise greater caution when utilizing FP tags to study biomolecular condensates. The methods and pitfalls in condensate research are evolving. Experimental systems must be paired with analytical measurements to properly model the physiology or pathology of interest (Alberti et al., 2019; McSwiggen et al., 2019; Taylor et al., 2019; Irgen-Gioro et al., 2022). Based on our µNS rescue model, we identified mNeonGreen as a suitable FP tag to visualize and characterize ReoV µNS. Whether mNeonGreen is an ideal tag for other phase-separating proteins remains to be seen. We focused on the FP-dependent modulation of µNS condensation; however, it is possible that FP tags can affect other aspects of phase separation. The effects that an FP tag imparts on phase separation likely depend on the protein that it is fused to because GFP tags inhibit HP1α phase separation (Larson et al., 2017; Zhou and Narlikar, 2023) but promote µNS condensation. Without a complete understanding of the mechanistic roles of FP tags in phase-separated systems, there should be greater caution in the generation and interpretation of FP-labeled condensates. With more insight, FP tags could be used as a tool to intentionally modify the properties of biomolecular condensates to ask biologically meaningful questions.
Based on the work herein and recent publications (Irgen-Gioro et al., 2022; Zhou and Narlikar, 2023; Pandey et al., 2024), we advocate that it is critical to validate fluorescent constructs with untagged controls. Our approach to this end involved quantitative immunofluorescence imaging of transfected cells, which required antigen-specific antibodies to detect puncta formed by untagged µNS. For proteins that lack available antibodies, minimal epitope tags such as HA could be used instead (Supplemental Figure S5G); however, we caution that the effects of epitope tags on phase-separated systems are understudied. Immunofluorescence imaging of transfected cells requires fixation, which was recently shown to modulate the appearance of some condensates in an FP-dependent manner (Irgen-Gioro et al., 2022). Therefore, it is important to also assess the effects of fixation on fluorescent constructs to support the analysis and interpretation of immunofluorescence experiments. Alternatively, fluorescent constructs can be compared with the untagged protein in vitro (Feric et al., 2016; Pandey et al., 2024), which would bypass the need for fixative and antigen-specific antibodies. Given the variable and unpredictable artifacts caused by FP tags reported here and elsewhere (Feric et al., 2016; Uebel and Phillips, 2019; Irgen-Gioro et al., 2022; Zhou and Narlikar, 2023; Pandey et al., 2024) and the current lack of mechanistic understanding, we recommend testing several FP tags and reiterate the importance of comparing these constructs to the untagged protein.
MATERIALS AND METHODS
Request a protocol through Bio-protocol.
Computational sequence analysis and structural predictions of ReoV M3 (T1L)
The open reading frame of ReoV M3 (T1L) (accession number AF174382.1) (McCutcheon et al., 1999) was input into the ColabFold v1.5.3 (Mirdita et al., 2022) Jupyter Notebook to generate a predicted structure of µNS that was visualized with ChimeraX v1.5rc202210312311 (Goddard et al., 2018; Pettersen et al., 2021). A sequential colorblind-friendly palette (https://github.com/smsaladi/chimerax_viridis) was imported into ChimeraX to improve accessibility. The degree of predicted disorder in short regions of µNS was assessed using IUPred3 (short disorder) (Erdős et al., 2021) and PONDR-FIT (Xue et al., 2010). The sequence was analyzed for coiled coil motifs with MARCOIL through the Max Planck Institute bioinformatics toolkit (Delorenzi and Speed, 2002; Zimmermann et al., 2018; Gabler et al., 2020).
Cell culture, constructs, expression, and mutagenesis
African green monkey kidney fibroblasts (CV-1) cells were used in all microscopy experiments except for the OSER assay. CV-1 cells were cultured in Eagle’s minimum essential medium with Earle’s salts (EMEM) (50-011-PC, Corning, Manassas, VA) supplemented with 1% sodium pyruvate (25-000-CI, Corning, Manassas, VA), MEM nonessential amino acids (25-025-CI, Corning, Manassas, VA), L-glutamine (25-005-CI, Corning, Manassas, VA) and 10% FBS (FS-0050-AD, Atlas Biologicals, Fort Collins, CO). U-2 OS osteosarcoma cells were used in the OSER assay. The U-2 OS cells were cultured in McCoy’s 5A (Iwakata & Grace modification) medium supplemented with 10% FBS. All cell cultures were maintained at 37°C with 5% CO2.
µNS was expressed from a pCI-neo mammalian expression vector driven by a cytomegalovirus (CMV) promoter. Mutagenesis of the µNS sequence was performed using Gibson assembly (Gibson et al., 2009) (E2611L) or KLD reaction (M0554S) with reagents from New England BioLabs, Ipswich, MA. Sequences were verified by Sanger sequencing and/or whole plasmid nanopore sequencing (Plasmidsaurus, Oregon). µNS constructs that were N-terminally fused to mEGFP, mNeonGreen, HaloTag, miRFP670nano, smuRFP, and miniGFP2 were ordered as a synthetic construct cloned into the pTWIST vector under the CMV promoter with a GS linker (Twist Bioscience, California).
Plasmids were transfected with Lipofectamine 3000 transfection reagent (L3000008, Invitrogen, Carlsbad, California) using the manufacturer’s recommended protocol. Briefly, 125 µl of Opti-MEM I reduced serum medium (31985062, Gibco, Thermo Fisher Scientific) was mixed with 5 µl P3000 reagent and 2.5 µg of plasmid DNA, and another 125 µl of Opti-MEM was mixed with 3.75 µl lipofectamine 3000 in a separate tube. The tubes were combined, mixed, and incubated for 20 min at room temperature before adding the transfection complexes to the cells dropwise. Transfected cells were maintained at 37°C with 5% CO2 until imaging or sample collection.
Immunofluorescence microscopy
CV-1 cells were seeded in 35 mm glass-bottom dishes (P35G-1.5-20-C, MatTek Corporation, Ashland, MA). 24 h after transfection or infection, the media was removed and replaced with room temperature 4% PFA in 1X PBS, pH 7.4 (AM9625, Ambion) for 10 min with agitation. The fixative was removed, followed by 3X PBS washes before storing the samples at 4°C or proceeding with immunofluorescence staining. PBS containing 1% BSA (A3803-50G, Sigma-Aldrich, St. Louis, MO) and 0.1% triton X-100 (X100-500ML, Sigma-Aldrich, St. Louis, MO) was used as permeabilization and antibody incubation buffer. Permeabilization for 20 min at room temperature with agitation was followed by 1-h incubations with primary and secondary antibodies with 3X PBS washes in between. Chicken µNS antiserum was used to probe for µNS at a 1:1000 dilution. Anti-chicken secondary antibodies conjugated to Alexa Fluor 680 (703-625-155), Alexa Fluor 647 (703-605-155), or Alexa Fluor 594 (703-585-155) from Jackson ImmunoResearch Laboratories, West Grove, PA were also used at 1:1000 dilutions. Rabbit µNS antiserum was used to probe for µNS at a 1:4000 dilution in Supplemental Figures S5F and S8F. HaloTag polyclonal antibodies (G2928A, Promega) were used to detect HaloTag in Supplemental Figure 8F at a 1:500 dilution. Donkey anti-rabbit secondary antibodies conjugated to Alexa Fluor 488 (711-545-152) or Alexa Fluor 647 (711-605-152) from Jackson ImmunoResearch Laboratories were used at a 1:800 dilution to detect the rabbit µNS antiserum and HaloTag antibodies. Rat anti-HA high affinity (clone 3F10) antibodies were used at a 1:2000 dilution along with donkey anti-rat secondary antibodies conjugated to Alexa Fluor 488 (712-545-153, Jackson ImmunoResearch Laboratories, 1:800 dilution). Mouse monoubiquitin and polyubiquitin monoclonal antibodies (clone FK2) (PW8810, Biomol International LP) were used at a 1:1000 dilution along with Donkey anti-mouse secondary antibodies conjugated to Alexa Fluor 488 (715-545-150, Jackson ImmunoResearch Laboratories, 1:800 dilution).
Quantification of droplet distribution, morphology, and punctate percentage
1024 × 1024 resolution images collected on an Olympus Fluoview FV3000 laser scanning confocal microscope with an open pinhole were processed using custom macros (https://github.com/RussellBarkley) in Fiji (version 1.54b) (Schindelin et al., 2012). For measurements of droplet distribution, the border of the cell was first outlined using a sensitive threshold to measure its area, then with a suitable automatic threshold, the number and area of particles were quantified. Droplet density is obtained by dividing the total number of particles by cell area. Radius is calculated from particle area assuming circularity using the formula = SQRT(Area/PI) in Microsoft Excel. Circularity and roundness shape descriptors (Cox, 1927; Takashimizu and Iiyoshi, 2016) are based on the area, perimeter, and aspect ratio of particles. Punctate percentage, as previously shown (Irgen-Gioro et al., 2022), was calculated by dividing the integrated fluorescence density of the entire cell (IntDentotal) by that of the dense phase (IntDendense),
LLSM
CV-1 cells were transfected with moxGFP::µNS(1-721) in wells of Nunc Lab-Tek 4-chambered #1 cover glass slide (#155383, Thermo Fisher Scientific Company L.L.C., Pittsburgh, PA) 18 h before imaging. Samples were left to incubate in EMEM after transfection. The incubation conditions of 37°C and 5% CO2 were set on the stage of an LLS7 (Carl Zeiss GmbH, Oberkochen, Germany) lattice light sheet microscope before imaging. Using 488 nm excitation in a dithered 15 μm long x 650 nm thick lattice light sheet with ∼16 μm focal length, we imaged live cells at various volumes and acquisition speeds. After deskewing and deconvolving the raw data, the resolution was 290 nm x 290 nm x 650 nm and the image voxel size was 145 nm x 145 nm x 145 nm (each X-res x Y-res x Z-res), so images are closer to isotropic resolution than in other modes of live-cell 3D imaging.
Quantification of droplet morphology from LLSM imaging
3D imaging datasets were first processed with Fiji, where the biovoxxel3Dbox plugin’s Voronoi Threshold Labeler was used to generate uniquely indexed 3D connected component volumes. This result was transferred to the Dragonfly software package (Object Research Systems Inc, Montreal, Canada), in which a multi-ROI (region of interest) was generated for all VFLS. Measurements including sphericity and volume were computed for each VFLS and exported.
Treatment of moxGFP::µNS-transfected cells with 1,6-HD
CV-1 cells were transfected in 35 mm glass-bottom dishes (P35G-1.5-20-C, MatTek Corporation, Ashland, MA) and images of moxGFP::µNS transfected cells were acquired at 24 h post-transfection on an Olympus Fluoview FV3000 laser scanning confocal microscope equipped with a heated stage at 1024 × 1024 pixel resolution. A water bath set to 37°C was brought to the microscope to warm reagents like 1,6-HD–supplemented EMEM (88571-100ML-F, Sigma-Aldrich, St. Louis, MO) and untreated EMEM. Media was aspirated from the dish and placed on the heated stage, then the cells were wet with 100 µl of untreated EMEM. The locations of transfected cells were mapped with coordinates for multi-area time-lapse imaging with Z-drift compensation. Images were collected before and immediately after the addition of 400 µl of prewarmed 4% (w/v) 1,6-HD–containing media. With little delay, the 1,6-HD–conditioned media was diluted with 3 mL prewarmed untreated media, and images were collected at 0, 5, and 10 min after washout. Punctate percentage was quantified manually using Fiji.
Particle tracking
moxGFP::µNS-transfected CV-1 cells in 35 mm glass-bottom dishes (P35G-1.5-20-C, MatTek Corporation, Ashland, MA) had their media replaced at 5 h post-transfection with prewarmed media containing 10 µM nocodazole or an equal volume of DMSO as a vehicle control. Time-lapse recordings at 6 h post-transfection with a 5-s framerate were analyzed using the Fiji TrackMate plugin (Tinevez et al., 2017) and the average velocity and displacement of all tracks in each cell are plotted.
Spinning disk confocal microscopy to determine the inverse capillary velocity
Transfected CV-1 cells were imaged on an Andor Revolution spinning disk microscope (Oxford Instruments Plc, Abingdon, United Kingdom) equipped with a temperature and CO2-controlled chamber (iNU and GM-8000, Tokai Hit USA Inc, Bala Cynwyd, PA). At settings for 37°C media temperature and 5% CO2, cells were imaged by 488 nm excitation through a 100x/1.4NA objective lens and 1.6x optivar. Fields of view containing a high density of fluorescent inclusions were imaged for 3 min with 100 ms exposure, and the use of the stream-to-RAM function enabled the 10-Hz acquisition required to observe droplet fusion and fission events. We followed a useful guide (Ceballos et al., 2018) to quantify the inverse capillary velocity from droplet fusion events. Using Fiji, a ROI was drawn around two fusing droplets, and their shape aspect ratio was measured starting from the time of contact through to complete fusion and relaxation. The average droplet radius ℓ was calculated from their area immediately before contact. Radius was calculated from area assuming circularity with the equation = SQRT(Area/PI) in Microsoft Excel. For each fusion event, a nonlinear regression exponential decay function was fit to the shape aspect ratio over time to isolate the time constant τ using GraphPad Prism 9 (GraphPad Software). τ was then plotted with respect to the mean droplet radius, and the inverse capillary velocity η/γ was determined from the slope of a simple linear regression fit to the data. An ANCOVA was used to statistically test the difference between slopes of the linear regressions.
Viscosity estimate of moxGFP::µNS(1-721) condensates
Complementary experimental techniques are effective at quantifying condensate viscosity (Elbaum-Garfinkle et al., 2015; Taylor et al., 2019; H. Wang et al., 2021), but theoretical arguments can be used to calculate a ballpark estimate of viscosity from the inverse capillary velocity η/γ (Brangwynne et al., 2011). If we assume each moxGFP::µNS molecule is a perfect sphere with a diameter d, then the surface tension γ ≈ κBT/d2 (Aarts et al. 2004) where κB is the Boltzmann constant and T is the temperature in kelvin. The radius of moxGFP::µNS(1-721) can be estimated from its molecular weight ∼108 kDa using the equation Rmin = (4V/3π)1/3 = 0.066M1/3 where M is daltons and Rmin is in nanometers (Erickson, 2009). Assuming moxGFP::µNS(1-721) is a sphere with a radius of 3.13 nm, the surface tension γ would be ≈ 0.44 µN/m. Given η/γ = 5.814 s/µm, viscosity η is 255 Pa·s.
Photoconverted condensate fusion imaging
Dendra2::µNS(1-721)- and Dendra2::µNS(472-721)-transfected CV-1 cell samples in 35 mm glass-bottom dishes (P35G-1.5-20-C, MatTek Corporation, Ashland, MA) were imaged with a scanning laser confocal microscope (LSM 880, Carl Zeiss GmbH, Oberkochen, Germany) with 488 nm and 561 nm excitation through a 63x/1.4 NA Plan-Apochromat objective lens. After identifying closely positioned droplets with the potential to fuse, the Zen software (Zeiss) photobleaching module was used to irradiate a user-defined ROI with gentle 405 nm light in 10–40 passes, which efficiently photoconverted the originally green FP to a stable red fluorescence state. Subsequent time course imaging of the fusion event was captured for the 488 nm and 561 nm confocal fluorescence channels, in which transport behavior of the red and green Dendra2::µNS populations could be observed.
MP-FRAP instrumentation
Multiphoton fluorescence photobleaching recovery measurements allow for the determination of the 3D diffusion coefficient of a fluorescently-labeled protein, due to the use of a closed bleaching region that can be accurately modeled as a 3D Gaussian volume. When using high numerical aperture objective lenses, the bleached volume can be on the order of a μm3 or less. Like confocal FRAP, a high-intensity bleaching pulse is used to bleach fluorophores within a time less than the characteristic diffusion time, after which the laser intensity is set to a low level for monitoring the recovery of fluorescence. Due to the small volume bleached, MP-FRAP recovery traces are typically acquired using photon counting methods and trace averaging. A Pockels Cell electro-optic modulator (Model-350-80-LABK EOM and Model 302 Driver, Conoptics, Danbury, CT) placed in the multiphoton laser (InSight, Spectra-Physics, Milpitas, CA) beam path is used to deliver high-intensity pulses (as short as ∼1 μs) and then return to a low monitoring level. For the measurements shown in this work, we used a Zeiss multiphoton/confocal microscope (LSM 880, Carl Zeiss GmbH, Oberkochen, Germany) with the EOM added to the beam path, a multichannel scaler (SR430, Stanford Research Systems, Sunnyvale, CA) for MP-FRAP trace acquisition, and lab-built control hardware and analysis software. The MP-FRAP traces were acquired using a lab-built MP-FRAP PMT assembly designed to slide into the housing of the Zeiss non-descanned detectors (NDDs). The assembly has an adjustable diverting mirror to first route the fluorescence to a GaAsP PMT in the NDD unit for laser-scanned imaging to locate the ROI (e.g., a VFLS). Once an x,y position within the image frame is chosen the LSM 880 is used in “spot” mode to park the multiphoton beam at the x,y position, and the fluorescence signal is diverted into the MP-FRAP PMT (HC-125 PMT, Hamamatsu Corporation, Bridgewater, NJ) which is connected to the SR-430 for photon count trace acquisition. All timing control of the EOM and SR-430 is carried out using lab-built electronics and data transfer from the SR-430 and trace analysis is done using a software package written in C/C++ under Visual Studio 2022. Typical MP-FRAP acquisition parameters were a 50 μs bleaching pulse followed by 4–5 ms of photon count acquisition with a 5.12 μs channel bin width (800–1000 data points of recovery). The laser wavelength was 920 nm and the bleach pulse intensity ranged from 50–70 mW during the 50 μs pulse, and 10–20 mW during the recovery monitoring phase. The bleach pulse/monitor sequences were delivered at 10 Hz, and 60–300 traces were averaged per measurement. The bleaching volume was chosen to be at the center of the moxGFP::µNS VFLS, to avoid interface-proximity artifacts described by Sullivan and Brown (Sullivan and Brown, 2011).
MP-FRAP experiments
moxGFP::µNS-transfected CV-1 cells in 35 mm glass-bottom dishes (P35G-1.5-20-C, MatTek Corporation, Ashland, MA) had their media replaced 1 h before imaging with prewarmed media containing 10 µM nocodazole. The microscope stage incubator and housing units were set for 37°C media temperature and 5% CO2. Widefield fluorescence was used to locate transfected cells with sufficiently large VFLS, after which the instrument was switched to laser-scanning mode to carry out the MP-FRAP measurements. The external detection path mirror was positioned for two-photon imaging to find the midplane of the targeted VFLS. Once the x-y coordinate was selected and the stationary spot set (“Spot Select” on Zen), the external detection path was rerouted to the FRAP-PMT for photon counting with the SR-430. Using the control software (Supplemental Figure S3) the bleaching pulse sequence (50 μs pulses at 10 Hz) was started. MP-FRAP acquisition was stopped after enough photon counts were collected to provide a fit-able recovery curve, and the VFLS was reimaged to ensure it had not moved. All MP-FRAP measurements were carried out using excitation at 920 nm (80 MHz repetition rate and ∼100 fs pulse width) delivered through a Plan-Apochromat 63x/1.40 Oil immersion objective lens (Carl Zeiss GmbH, Oberkochen, Germany).
MP-FRAP analysis
SR-430 acquired time-binned photon counting data were downloaded to the MP-FRAP control/fitting software and the data fit to the model of 3D fluorescence recovery traces detailed in Brown et al. (1999). The Marquardt-Levenberg least-squares algorithm was used to fit the fluorescence recovery with the series solution carried out for six terms. A model assuming one 3D diffusion coefficient with an immobile fraction was used:
 |
1 |
Where F(t) is the measured fluorescence at time t, F0 is the prebleach fluorescence level, D is the diffusion coefficient, β is the bleach depth parameter, and Cimmobile fraction is the fraction of fluorescence not recovered on the timescale of the experiment. Although we are using the standard “immobile fraction” terminology, the failure of the recovery to reach 1.0 is most likely due to depletion of the fluorophore pool within the closed volume, rather than an immobile fraction. m and b are excitation-related parameters, where b = m = 2 for two-photon bleaching (b) and monitoring (m). ωr and ωz are the radial and axial 1/e2 radii of the two-photon point spread function which are calculated based on the numerical aperture and wavelength using equations from Zipfel et al. (2003).
The fitting algorithm returns an estimate for diffusion coefficient D, bleach depth parameter β, and immobile fraction. The resulting estimates of D were compiled for statistical treatment, utilizing the SciPy library and scikit-posthocs package for python, as follows. The data was compared to the normal distribution CDF by the Kolmogorov–Smirnov test, which rejected the null, that the datasets were normally distributed, at the 95% confidence level. The nonparametric Kruskal–Wallis test of overall differences then revealed that there were overall differences, and this was confirmed directly by the nonparametric Conover-Iman rank test for pair-wise differences. This test’s P-value is displayed in Figure 3C.
Droplet nucleation time-lapse experiments
To observe the initial moments of moxGFP::µNS VFLS formation, CV-1 cells cultured in 35 mm glass bottom dishes (P35G-1.5-20-C, MatTek Corporation, Ashland, MA) had their plasma membrane labeled with Vybrant DiD cell-labeling solution (V22887, Molecular Probes, Eugene, OR) for 20 min at 37°C. Meanwhile, lipofectamine 3000 (L3000008, Invitrogen, Carlsbad, CA) transfection complexes containing 2.5 µg moxGFP::µNS plasmid DNA were prepared. The cells were washed thrice with PBS before adding 2 ml of prewarmed media and the transfection complexes dropwise. After 4 to 5 h of incubation at 37°C, the media was supplemented with 25 mM HEPES buffer (15630080, Thermo Fisher Scientific, Waltham, MA) and the sample was imaged on a temperature-controlled stage using the Olympus Fluoview FV3000 laser scanning confocal microscope. Multi-area time-lapse images of a seven-by-seven grid of fields with a 60X objective were imaged at a 5-min framerate in the brightfield, FITC, and Alexa Fluor 647 channels for 5 h. To maintain focus on the cells before the emergence of fluorescent VFLS, the Z-drift compensator was used to focus on the fluorescently-labeled plasma membrane. Quantification of droplet density and radius were manually analyzed in Fiji using the analyze particles feature starting from the first frame t0 of observable diffraction limited puncta and for 1 h thereafter. The mean gray value, used as a proxy for Csat, was measured in the cell one background-subtracted frame before t0 and all measurements were made relative to the median of moxGFP::µNS(1-721).
Assessment of PFA fixation artifacts in µNS-transfected cells
moxGFP::µNS-transfected CV-1 cells were cultured in 35 mm gridded glass-bottom dishes (P35G-1.5-14-C-GRD.S, Mattek Corporation, Ashland, MA) for 24 h. 4% PFA diluted in 10X PBS (AM9625, Ambion) to a final 1X concentration was kept warm in a 37°C water bath along with untreated media. Media was removed from the cells and the dish was placed on a heated stage on the Olympus Fluorview FV3000 laser scanning confocal microscope. The cells were then wet with 100 µl of prewarmed media and coordinates for five to 10 100X objective fields were mapped and their position in the grid was noted. Z-stacks with an open pinhole were collected before and after the addition of 1 ml prewarmed fixative for 10 min without adjusting laser intensity. We designated these samples as “live” and “fixed.” Additional images of the same coordinates were collected after increasing the laser intensity by a factor of 3 to 5, which we called “fixed +.” The samples were subsequently processed for immunofluorescence staining with µNS antiserum and the same cells were relocated and imaged in the Alexa Fluor 647 channel, denoted as “stained.” Quantification of droplet density in Fiji was performed on maximum intensity Z-stack projections using a custom macro (https://github.com/RussellBarkley). The same protocol and analysis were performed on Dendra::µNS-transfected cells in separate experiments. Fluorescence quenching was assessed by quantifying the mean gray value in background-subtracted cells before and after 10 min of 4% PFA fixation in a single plane with an open pinhole and Z-drift compensation.
OSER assay
CyTERM-mGFP was a gift from Erik Snapp (Addgene plasmid # 62237; http://n2t.net/addgene:62237; RRID: Addgene_62237). This mammalian expression vector encodes an ER-targeted transmembrane domain fused to monomeric EGFP oriented toward the cytoplasm. mEGFP in this plasmid carries the L221K substitution. L221K and A206K mutations are frequently used to disrupt the dimer interface (Zacharias et al., 2002) and both mutants are considered some of the most monomeric variants of EGFP (Cranfill et al., 2016). mEGFP in this construct was replaced with other FPs through Gibson assembly (Gibson et al., 2009) (E2611L, New England BioLabs, Ipswich, MA). The OSER assay was performed according to the primary literature (Costantini et al., 2012) in U-2 OS cells cultured in 35 mm glass-bottom dishes (P35G-1.5-20-C, MatTek Corporation, Ashland, MA) at 24 h post-transfection. FPs were expressed and retained on the cytoplasmic face of the endoplasmic reticulum, which concentrates and orients the FPs parallel to each other. Transient FP–FP interactions can bend the ER into a distinct spiral structure called a whorl and the proportion of transfected cells containing whorls is used to infer the degree of FP dimerization. A percent monomeric (%) score was calculated by dividing the whorl-negative cells by the total sampled population. The experiment was performed twice. In the first experiment, Participant 1 was not blinded and scored the transfected population at 30 h post-transfection. In the second experiment, Participants 2 and 3 were single-blinded and scored the same samples in reverse order at 24 h post-transfection.
Estimation of the population proportion of phase separation–positive transfected cells
CV-1 cells cultured in 35 mm glass-bottom dishes (P35G-1.5-20-C, MatTek Corporation, Ashland, MA) were transfected with FP::µNS mutants for 24 h and the transfected cell population was evaluated through the eyepiece of an Olympus Fluoview FV3000 laser scanning confocal microscope in the FITC or TRITC channels. For far-red small FPs, transfected cells were viewed using the appropriate filter through the live image preview. Using a dual tally counter, transfected cells were scored based on the unequivocal presence or absence of condensates (diffuse or phase-separated). Some cells contained small misfolded protein aggregates and in these cases, the cell was not scored to avoid ambiguity. The sampling of the population was N = 150–250 cells per sample.
Statistics and reproducibility
Unless otherwise stated, all statistical analyses were performed on GraphPad Prism 9 or 10 (GraphPad Software). One outlier was excluded from the inverse capillary analysis. This moxGFP::µNS(1-721) fusion event had an average droplet radius of 0.73 µm and a characteristic relaxation time τ of 8.26, which strongly influenced the slope of the linear regression (7.746 s/µm) compared with 5.814 s/µm with the outlier excluded. In either case, the conclusions from the statistical analysis do not change; p = 0.1874 with the outlier and p = 0.6157 without it. For most experiments measuring droplet size and density, morphology, or punctate percentage, a random sampling of 15–30 cells were imaged in each of three independent experiments and the analysis was conducted after data collection for all biological replicates. The mean from each independent experiment was calculated and often log10 transformed and tested for normality before plotting all biological replicates with a line at the mean and a suitable parametric statistical test with SEM error bars. For experiments that fail one or more tests of normality, we used a nonparametric statistical test and plotted a line at the median. Several supplemental figures were performed in one or two independent experiments and frequently failed tests of normality, therefore box and whisker plots with lines at the min, max, quartiles, and median were plotted to describe the distribution of technical replicates. Representative images were always selected from just one of the biological replicates. In some multivariable plots involving an abundance of samples and measurements, the grand mean was plotted to simplify and reduce information. Dunnett’s test for multiple comparisons was applied in Figures 5, A and C, and 7D to explicitly compare the droplet density in fixed cells with that of live cells, and the droplet density of FP::µNS fusions with untagged µNS.
Supplementary Material
Acknowledgments
We thank Meleana Hinchman for preparing samples for imaging experiments. The funding resources for this work included a Genomics Innovation Seed Grant and the Cornell Physical Sciences Oncology Center on the Physics of Cancer Metabolism (NCI U54CA210184). We thank R. Williams and the Cornell Biotechnology Resource Center (BRC) Imaging Facility (NYSTEM C029155, NIH S10OD018516, NIH S10OD010605) for microscopy experiments. We thank the Cornell BRC Genomics Facility (RRID: SCR_021727) at the Cornell Institute for Biotechnology for plasmid sequencing. We would also like to thank the Baker Institute for Animal Health at Cornell University for their generous financial support with this project.
Abbreviations used:
- FP
Fluorescent protein
- ReoV
Mammalian orthoreovirus
- T1L
Mammalian orthoreovirus strain type 1 Lang
- VFLS
Viral factory-like structure
- LLPS
Liquid-liquid phase separation
- 1,6-HD
1,6-hexanediol
- MP-FRAP
Multiphoton fluorescence recovery after photobleaching
- PFA
Paraformaldehyde
- IF
Immunofluorescence
- OSER
Organized smooth endoplasmic reticulum
- C sat
Saturation concentration
- MGV
Mean gray value.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E24-01-0013) on May 29, 2024.
REFERENCES
- Aarts DGAL, Schmidt M, Lekkerkerker HNW (2004). Direct visual observation of thermal capillary waves. Science 304, 847–850. [DOI] [PubMed] [Google Scholar]
- Alberti S, Gladfelter A, Mittag T (2019). Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 176, 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberto B-N, Menaya-Vargas R, Benavente J, Martinez-Costas J (2010). Avian reovirus μNS protein forms homo-oligomeric inclusions in a microtubule-independent fashion, which involves specific regions of its C-terminal domain. J Virol 84, 4289–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenquer M, Vale-Costa S, Etibor TA, Ferreira F, Sousa AL, Amorim MJ (2019). Influenza A virus ribonucleoproteins form liquid organelles at endoplasmic reticulum exit sites. Nat Commun 10, 1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold MM, Murray KE, Nibert ML (2008). Formation of the factory matrix is an important, though not a sufficient function of nonstructural protein μNS during reovirus infection. Virology 375, 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banani SF, Lee HO, Hyman AA, Rosen MK (2017). Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 18, 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer BS, Vallbracht M, Ushakov DS, Wendt L, Chlanda P, Hoenen T (2023). Ebola virus inclusion bodies are liquid organelles whose formation is facilitated by nucleoprotein oligomerization. Emerg Microbes Infect 12, 2223727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouziat R, Hinterleitner R, Brown JJ, Stencel-Baerenwald JE, Ikizler M, Mayassi T, Meisel M, Kim SM, Discepolo V, Pruijssers AJ, et al. (2017). Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 356, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Jülicher F, Hyman AA (2009). Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, Mitchison TJ, Hyman AA (2011). Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc Natl Acad Sci USA 108, 4334–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broering TJ, Parker JSL, Joyce PL, Kim J, Nibert ML (2002). Mammalian reovirus nonstructural protein μNS forms large inclusions and colocalizes with reovirus microtubule-associated protein μ2 in transfected cells. J Virol 76, 8285–8297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broering TJ, Kim J, Miller CL, Piggott CDS, Dinoso JB, Nibert ML, Parker JSL (2004). Reovirus nonstructural protein μNS recruits viral core surface proteins and entering core particles to factory-like inclusions. J Virol 78, 1882–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broering TJ, Arnold MM, Miller CL, Hurt JA, Joyce PL, Nibert ML (2005). Carboxyl-proximal regions of reovirus nonstructural protein μNS necessary and sufficient for forming factory-like inclusions. J Virol 79, 6194–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EB, Wu ES, Zipfel W, Webb WW (1999). Measurement of molecular diffusion in solution by multiphoton fluorescence photobleaching recovery. Biophys J 77, 2837–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussiere LD, Choudhury P, Bellaire B, Miller CL (2017). Characterization of a replicating mammalian orthoreovirus with tetracysteine-tagged μNS for live-cell visualization of viral factories. J Virol 91, e01371-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll K, Hastings C, Miller CL (2014). Amino acids 78 and 79 of Mammalian Orthoreovirus protein µNS are necessary for stress granule localization, core protein λ2 interaction, and de novo virus replication. Virology 448, 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos AV, McDonald CJ, Elbaum-Garfinkle S (2018). Chapter Two - Methods and strategies to quantify phase separation of disordered proteins. In: Methods in Enzymology, ed. Rhoades E., Cambridge, MA: Academic Press; 31–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC (1994). Green fluorescent protein as a marker for gene expression. Science 263, 802–805. [DOI] [PubMed] [Google Scholar]
- Charman M, Grams N, Kumar N, Halko E, Dybas JM, Abbott A, Lum KK, Blumenthal D, Tsopurashvili E, Weitzman MD (2023). A viral biomolecular condensate coordinates assembly of progeny particles. Nature 616, 332–338. [DOI] [PubMed] [Google Scholar]
- Costantini LM, Fossati M, Francolini M, Snapp EL (2012). Assessing the tendency of fluorescent proteins to oligomerize under physiologic conditions. Traffic 13, 643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini LM, Baloban M, Markwardt ML, Rizzo MA, Guo F, Verkhusha VV, Snapp EL (2015). A palette of fluorescent proteins optimized for diverse cellular environments. Nat Commun 6, 7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox EP (1927). A method of assigning numerical and percentage values to the degree of roundness of sand grains. J Paleontol 1, 179–183. [Google Scholar]
- Cranfill PJ, Sell BR, Baird MA, Allen JR, Lavagnino Z, de Gruiter HM, Kremers G-J, Davidson MW, Ustione A, Piston DW (2016). Quantitative assessment of fluorescent proteins. Nat Methods 13, 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorenzi M, Speed T (2002). An HMM model for coiled-coil domains and a comparison with PSSM-based predictions. Bioinformatics 18, 617–625. [DOI] [PubMed] [Google Scholar]
- Durinova E, Mojzes P, Bily T, Franta Z, Fessl T, Borodavka A, Tuma R (2023). Chapter Five - Shedding light on reovirus assembly—Multimodal imaging of viral factories. In: Advances in Virus Research, ed. Finke S., Ushakov D., Cambridge, MA: Academic Press, 173–213. [DOI] [PubMed] [Google Scholar]
- Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, Kriwacki RW, Pappu RV, Brangwynne CP (2016). Coexisting liquid phases underlie nucleolar subcompartments. Cell 165, 1686–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichwald C, Ackermann M, Nibert ML (2018). The dynamics of both filamentous and globular mammalian reovirus viral factories rely on the microtubule network. Virology 518, 77–86. [DOI] [PubMed] [Google Scholar]
- Elbaum-Garfinkle S, Kim Y, Szczepaniak K, Chen CC-H, Eckmann CR, Myong S, Brangwynne CP (2015). The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc Natl Acade Sci USA 112, 7189–7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdős G, Pajkos M, Dosztányi Z (2021). IUPred3: prediction of protein disorder enhanced with unambiguous experimental annotation and visualization of evolutionary conservation. Nucleic Acids Res 49, W297–W303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson HP (2009). Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol Proced Online 11, 32–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etibor TA, Vale-Costa S, Sridharan S, Bras D, Becher I, Hugo VH, Ferreira F, Alenquer M, Savitski MM, Amorim M-J (2023). Defining basic rules for hardening influenza A virus liquid condensates. eLife 12, e85182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etibor TA, Yamauchi Y, Amorim MJ (2021). Liquid biomolecular condensates and viral lifecycles: review and perspectives. Viruses 13, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabler F, Nam S-Z, Till S, Mirdita M, Steinegger M, Söding J, Lupas AN, Alva V (2020). Protein sequence analysis using the MPI bioinformatics toolkit. Curr Protoc Bioinformatics 72, e108. [DOI] [PubMed] [Google Scholar]
- Geiger F, Acker J, Papa G, Wang X, Arter WE, Saar KL, Erkamp NA, Qi R, Bravo JP, Strauss S, et al. (2021). Liquid–liquid phase separation underpins the formation of replication factories in rotaviruses. EMBO J 40, e107711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang R-Y, Venter JC, Hutchison CA, Smith HO (2009). Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6, 343–345. [DOI] [PubMed] [Google Scholar]
- Goddard TD, Huang CC, Meng EC, Pettersen EF, Couch GS, Morris JH, Ferrin TE (2018). UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci 27, 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Parker JSL (2021). The paradoxes of viral mRNA translation during mammalian orthoreovirus infection. Viruses 13, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseva S, Milles S, Jensen MR, Salvi N, Kleman J-P, Maurin D, Ruigrok RWH, Blackledge M (2020). Measles virus nucleo- and phosphoproteins form liquid-like phase-separated compartments that promote nucleocapsid assembly. Sci Adv 6, eaaz7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan MF, Mohajerani F (2023). Self-assembly coupled to liquid-liquid phase separation. PLoS Comput Biol 19, e1010652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon TS, Holehouse AS, Rosen MK, Pappu RV (2017). Intrinsically disordered linkers determine the interplay between phase separation and gelation in multivalent proteins. eLife 6, e30294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holehouse AS, Pappu RV (2018). Functional implications of intracellular phase transitions. Biochemistry 57, 2415–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman AA, Weber CA, Jülicher F (2014). Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol 30, 39–58. [DOI] [PubMed] [Google Scholar]
- Irgen-Gioro S, Yoshida S, Walling V, Chong S (2022). Fixation can change the appearance of phase separation in living cells. eLife 11, e79903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs WM (2021). Self-assembly of biomolecular condensates with shared components. Phys Rev Lett 126, 258101. [DOI] [PubMed] [Google Scholar]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, et al. (2021). Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapelner RA, Obermeyer AC (2019). Ionic polypeptide tags for protein phase separation. Chem Sci 10, 2700–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthika S, Radhakrishnan TK, Kalaichelvi P (2016). A review of classical and nonclassical nucleation theories. Cryst Growth Des 16, 6663–6681. [Google Scholar]
- Kobayashi T, Chappell JD, Danthi P, Dermody TS (2006). Gene-specific inhibition of reovirus replication by RNA interference. J Virol 80, 9053–9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Ooms LS, Chappell JD, Dermody TS (2009). Identification of functional domains in reovirus replication proteins μNS and μ2. J Virol 83, 2892–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschwald S, Maharana S, Simon A (2017). Hexanediol: a chemical probe to investigate the material properties of membrane-less compartments. Matters [Preprint], 10.19185/matters.201702000010. [DOI] [Google Scholar]
- Larson AG, Elnatan D, Keenen MM, Trnka MJ, Johnston JB, Burlingame AL, Agard DA, Redding S, Narlikar GJ (2017). Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature 547, 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Raghunathan K, Taylor GM, French AJ, Tenorio R, Fernández de Castro I, Risco C, Parker JSL, Dermody TS (2021). Reovirus nonstructural protein σNS recruits viral RNA to replication organelles. mBio 12, e0140821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Cathey PI, Wu H, Parker R, Voeltz GK (2020). Endoplasmic reticulum contact sites regulate the dynamics of membraneless organelles. Science 367, eaay7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin B, Mittasch M, Gomes BF, Manteiga J, Patel A, Zamudio A, Beutel O, Mitrea DM (2021). Chapter 1 - Harnessing the power of fluorescence to characterize biomolecular condensates. In: Methods in Microbiology, ed. Gurtler V., Academic Press, 1–47. [Google Scholar]
- Li P, Banjade S, Cheng H-C, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, King DS, Banani SF, et al. (2012). Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G-T, Lai C, Yue Z, Zhang H, Danyang L, Chen Z, Lu X, Tao L, Subach FV, Piatkevich KD (2022). Enhanced small green fluorescent proteins as a multisensing platform for biosensor development. Front Bioeng Biotechnol 10, 1039317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohana RF, Urh M, et al. (2008). HaloTag: A novel protein labeling technology for cell imaging and protein analysis. ACS Chem Biol 3, 373–382. [DOI] [PubMed] [Google Scholar]
- McCutcheon AM, Broering TJ, Nibert ML (1999). Mammalian reovirus M3 gene sequences and conservation of coiled-coil motifs near the carboxyl terminus of the μNS protein. Virology 264, 16–24. [DOI] [PubMed] [Google Scholar]
- McSwiggen DT, Mir M, Darzacq X, Tjian R (2019). Evaluating phase separation in live cells: diagnosis, caveats, and functional consequences. Genes Dev 33, 1619–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL, Arnold MM, Broering TJ, Hastings CE, Nibert ML (2010). Localization of mammalian orthoreovirus proteins to cytoplasmic factory-like structures via nonoverlapping regions of μNS. J Virol 84, 867–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir M, Stadler MR, Ortiz SA, Hannon CE, Harrison MM, Darzacq X, Eisen MB (2018). Dynamic multifactor hubs interact transiently with sites of active transcription in Drosophila embryos. eLife 7, e40497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirdita M, Schütze K, Moriwaki Y, Heo L, Ovchinnikov S, Steinegger M (2022). ColabFold: making protein folding accessible to all. Nat Methods 19, 679–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanoyama M, Millward S, Graham AF (1974). Control of transcription of the reovirus genome. Nucleic Acids Res 1, 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan A, Meriin A, Andrews JO, Spille J-H, Sherman MY, Cisse II (2019). A first order phase transition mechanism underlies protein aggregation in mammalian cells. eLife 8, e39695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic J, Le Bars R, Lama Z, Scrima N, Lagaudrière-Gesbert C, Gaudin Y, Blondel D (2017). Negri bodies are viral factories with properties of liquid organelles. Nat Commun 8, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott TJ, Petsalaki E, Farber P, Jervis D, Fussner E, Plochowietz A, Craggs TD, Bazett-Jones DP, Pawson T, Forman-Kay JD, Baldwin AJ (2015). Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol Cell 57, 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliinyk OS, Shemetov AA, Pletnev S, Shcherbakova DM, Verkhusha VV (2019). Smallest near-infrared fluorescent protein evolved from cyanobacteriochrome as versatile tag for spectral multiplexing. Nat Commun 10, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey NK, Varkey J, Ajayan A, George G, Chen J, Langen R (2024). Fluorescent protein tagging promotes phase separation and alters the aggregation pathway of huntingtin exon-1. J Biol Chem 300, 105585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JS, Broering TJ, Kim J, Higgins DE, Nibert ML (2002). Reovirus core protein μ2 determines the filamentous morphology of viral inclusion bodies by interacting with and stabilizing microtubules. J Virol 76, 4483–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeples W, Rosen MK (2021). Mechanistic dissection of increased enzymatic rate in a phase-separated compartment. Nat Chem Biol 17, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, Croll TI, Morris JH, Ferrin TE (2021). UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci 30, 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios MU, Bagnucka MA, Ryder BD, Gomes BF, Familiari NE, Yaguchi K, Amato M, Stachera WE, Joachimiak ŁA, Woodruff JB (2024). Multivalent coiled-coil interactions enable full-scale centrosome assembly and strength. J Cell Biol 223, e202306142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez EA, Tran GN, Gross LA, Crisp JL, Shu X, Lin JY, Tsien RY (2016). A far-red fluorescent protein evolved from a cyanobacterial phycobiliprotein. Nat Methods 13, 763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savastano A, Ibáñez de Opakua A, Rankovic M, Zweckstetter M (2020). Nucleocapsid protein of SARS-CoV-2 phase separates into RNA-rich polymerase-containing condensates. Nat Commun 11, 6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner NC, Lambert GG, Chammas A, Ni Y, Cranfill PJ, Baird MA, Sell BR, Allen JR, Day RN, Israelsson M, et al. (2013). A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum. Nat Methods 10, 407–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemiakina II, Ermakova GV, Cranfill PJ, Baird MA, Evans RA, Souslova EA, Staroverov DB, Gorokhovatsky AY, Putintseva EV, Gorodnicheva TV, et al. (2012). A monomeric red fluorescent protein with low cytotoxicity. Nat Commun 3, 1204. [DOI] [PubMed] [Google Scholar]
- Shimobayashi SF, Ronceray P, Sanders DW, Haataja MP, Brangwynne CP (2021). Nucleation landscape of biomolecular condensates. Nature 599, 503–506. [DOI] [PubMed] [Google Scholar]
- Snead WT, Jalihal AP, Gerbich TM, Seim I, Hu Z, Gladfelter AS (2022). Membrane surfaces regulate assembly of ribonucleoprotein condensates. Nat Cell Biol 24, 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanifer ML, Kischnick C, Rippert A, Albrecht D, Boulant S (2017). Reovirus inhibits interferon production by sequestering IRF3 into viral factories. Sci Rep 7, 10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KD, Brown EB (2011). Multiphoton fluorescence recovery after photobleaching in bounded systems. Phys Rev E Stat Nonlin Soft Matter Phys 83, 051916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susanne K, Coffey CM, Parker JS (2012). The cellular chaperone Hsc70 is specifically recruited to reovirus viral factories independently of its chaperone function. J Virol 86, 1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashimizu Y, Iiyoshi M (2016) New parameter of roundness R: circularity corrected by aspect ratio. Prog in Earth and Planet Sci 3, 2. [Google Scholar]
- Taylor NO, Wei M-T, Stone HA, Brangwynne CP (2019). Quantifying dynamics in phase-separated condensates using fluorescence recovery after photobleaching. Biophys J 117, 1285–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenorio R, Fernández de Castro I, Knowlton JJ, Zamora PF, Lee CH, Mainou BA, Dermody TS, Risco C (2018). Reovirus σNS and μNS proteins remodel the endoplasmic reticulum to build replication neo-organelles. mBio 9, e01253–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinevez J-Y, Perry N, Schindelin J, Hoopes GM, Reynolds GD, Laplantine E, Bednarek SY, Shorte SL, Eliceiri KW (2017). TrackMate: An open and extensible platform for single-particle tracking. Methods 115, 80–90. [DOI] [PubMed] [Google Scholar]
- Uebel C, Phillips CM (2019). Phase-separated protein dynamics are affected by fluorescent tag choice. MicroPubl Biol 219, 143. [PMC free article] [PubMed] [Google Scholar]
- Uversky VN, Kuznetsova IM, Turoverov KK, Zaslavsky B (2015). Intrinsically disordered proteins as crucial constituents of cellular aqueous two phase systems and coacervates. FEBS Lett 589, 15–22. [DOI] [PubMed] [Google Scholar]
- Wang B, Zhang L, Dai T, Qin Z, Lu H, Zhang L, Zhou F (2021). Liquid–liquid phase separation in human health and diseases. Signal Transduct Target Ther 6, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Kelley FM, Milovanovic D, Schuster BS, Shi Z (2021). Surface tension and viscosity of protein condensates quantified by micropipette aspiration. Biophys Rep 1, 100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Choi J-M, Holehouse AS, Lee HO, Zhang X, Jahnel M, Maharana S, Lemaitre R, Pozniakovsky A, Drechsel D, et al. (2018). A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell 174, 688–699.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Hazelrigg T (1994). Implications for bcd mRNA localization from spatial distribution of exu protein in Drosophila oogenesis. Nature 369, 400–403. [DOI] [PubMed] [Google Scholar]
- Wheeler JR, Matheny T, Jain S, Abrisch R, Parker R (2016). Distinct stages in stress granule assembly and disassembly. eLife 5, e18413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia K-Q, Franck C, Widom B (1992). Interfacial tensions of phase-separated polymer solutions. J Chem Phys 97, 1446–1454. [Google Scholar]
- Xue B, Dunbrack RL, Williams RW, Dunker AK, Uversky VN (2010). PONDR-FIT: A meta-predictor of intrinsically disordered amino acids. Biochim Biophys Acta 1804, 996–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Mathieu C, Kolaitis R-M, Zhang P, Messing J, Yurtsever U, Yang Z, Wu J, Li Y, Pan Q, et al. (2020). G3BP1 is a tunable switch that triggers phase separation to assemble stress granules. Cell 181, 325–345. e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeong V, Wang J-W, Horn JM, Obermeyer AC (2022). Intracellular phase separation of globular proteins facilitated by short cationic peptides. Nat Commun 13, 7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias DA, Violin JD, Newton AC, Tsien RY (2002). Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296, 913–916. [DOI] [PubMed] [Google Scholar]
- Zervoudis NA, Obermeyer AC (2021). The effects of protein charge patterning on complex coacervation. Soft Matter 17, 6637–6645. [DOI] [PubMed] [Google Scholar]
- Zhou ZK, Narlikar GJ (2023). Understanding how genetically encoded tags affect phase separation by Heterochromatin Protein HP1α. bioRxiv 2023.12.04.569983. Available at: 10.1101/2023.12.04.569983. [DOI] [Google Scholar]
- Zimmermann L, Stephens A, Nam S-Z, Rau D, Kübler J, Lozajic M, Gabler F, Söding J, Lupas AN, Alva V (2018). A completely reimplemented MPI bioinformatics toolkit with a new HHpred server at its core. J Mol Biol 430, 2237–2243. [DOI] [PubMed] [Google Scholar]
- Zipfel WR, Williams RM, Webb WW (2003). Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol 21, 1369–1377. [DOI] [PubMed] [Google Scholar]