Abstract
Background
Cholera outbreaks in Ethiopia necessitate frequent mass oral cholera vaccine (OCV) campaigns. Despite this, there is a notable absence of a comprehensive summary of these campaigns. Understanding national OCV vaccination history is essential to design appropriate and effective cholera control strategies. Here, we aimed to retrospectively review all OCV vaccination campaigns conducted across Ethiopia between 2019 and 2023.
Methods
The OCV request records from 2019 to October 2023 and vaccination campaign reports for the period from 2019 to December 2023 were retrospectively accessed from the Ethiopia Public Health Institute (EPHI) database. Descriptive analysis was conducted using the retrospective data collected.
Results
From 2019 to October 2023, Ethiopian government requested 32 044 576 OCV doses (31 899 576 doses to global stockpile; 145 000 doses to outside of stockpile). Around 66.3% of requested doses were approved; of which 90.4% were received. Fifteen OCV campaigns (12 reactive and 3 pre-emptive) were conducted, including five two-dose campaigns with varying dose intervals and single-dose campaigns partially in 2019 and entirely in 2021, 2022 and 2023. Overall vaccine administrative coverage was high; except for Tigray region (41.8% in the 1st round; 2nd round didn't occur). The vaccine administrative coverage records were documented, but no OCV coverage survey data was available.
Conclusions
This study represents the first comprehensive review of OCV campaigns in Ethiopia spanning the last five years. Its findings offer valuable insights into informing future cholera control strategies, underscoring the importance of monitoring and evaluation despite resource constraints. Addressing the limitations in coverage survey data availability is crucial for enhancing the efficacy of future campaigns.
Keywords: Ethiopia, Cholera, OCV, global OCV stockpile, OCV dosing schedules
INTRODUCTION
Vaccination is a feasible rapid intervention to prevent the emergence of cholera and contain further spread during outbreaks [1]. Among three World Health Organization (WHO)-prequalified oral cholera vaccines (OCVs) (Dukoral®, Shanchol™, and Euvichol-Plus®), Shanchol™ and Euvichol-Plus® have been supplied to the global OCV stockpile and used for mass vaccination campaigns [2]. A Gavi supported global stockpile of OCV was established in 2013 to support the OCV use in outbreak responses (through the International Coordinating Group [ICG] approval process) [1]. In 2017, the “Ending Cholera: A Global Roadmap to 2030” was launched and the use of OCV was expanded to include preventive campaigns with approval of requests initially made by the WHO Global Task Force on Cholera Control (GTFCC) [3]. Currently, access to OCV for preventive campaigns requires a multi-year plan and application through Gavi [4]. Since the 2019 cholera outbreaks, the Ethiopian government has submitted several official requests to the ICG for reactive and preventive use of OCV doses from the global stockpile [5] and an additional request was made to the government of the Republic of Korea bilaterally in response to the mass cholera outbreaks [6]. In parallel, the “Multi-sectoral Cholera Elimination Plan, Ethiopia 2022–2028' was developed by the Ethiopian government, which has been endorsed nationally and announced at the 75th World Health Assembly in 2022; encompassing the potential future use of OCVs based on the mapping of cholera “hotspots” [7].
In 2019, GTFCC OCV Working Group published a review paper on the global OCV use for 5 years, since the establishment of stockpile in 2013, to draw lessons from global OCV deployments and related campaigns [8]. The review paper demonstrated that many countries were using OCVs for both outbreak response and endemic cholera prevention with high coverage in general [8]. However, some campaigns showed relatively lower coverage in adult males and some decreased coverage was observed during the second round of vaccination [8]. In addition, there was a delay between onset of outbreak and implementation of vaccination and/or between the first round and second round [8]. According to this report, Ethiopia is one of the countries that received more than 1 million doses of OCV from the global stockpile between 2013 and 2018 [8]. Since 2019, however, the number of received doses has significantly increased to approximately 18 million doses [5]. To trace and understand this increased OCV deployment in Ethiopia, documenting and reviewing OCV campaigns conducted in Ethiopia would be important. The GTFCC cholera research agenda suggests evidence-generation on OCV use, including the community level duration of protection per dosing schedule, the impact of OCV vaccination timing on outbreak prevention and control, and potential delivery strategies to optimize OCV coverage [9]. A comprehensive review of past use of OCVs in the country could provide the groundwork for future research to address these gaps and policies. Moreover, analyzing such vaccination history data including coverage will also provide an opportunity to identify barriers to uptake and characterize acceptance towards cholera vaccines [10, 11], and lessons learned regarding feasibility and efficiency of strategies, and ultimately these impacts could improve future campaigns.
Here we aimed to conduct a comprehensive retrospective review of all OCV vaccination campaigns conducted in Ethiopia from 2019 to 2023. The GTFCC monitoring and reporting guidelines on the use of OCV recommends the tracking of: OCV administrative coverage per vaccination campaigns; proportion of hotspots targeted by the vaccination plans; and the proportion of doses used in campaigns to respond to an outbreak compared to doses administered during preventive campaigns [12]. Our review focused on the OCV doses requested to the ICG by vaccination type, approved, and delivered; OCV dose numbers and dose intervals applied in each campaign and rolled-out per region, zone, and woreda; OCV vaccination periods per campaign; OCV coverage rates per region, zone, and woreda in Ethiopia; and sex and age-stratified information on the OCV vaccinated populations.
METHODS
Dataset Description
The OCV request records from 2019 to October 2023 and vaccination campaign reports for the period from 2019 to December 2023 were retrospectively accessed from the Ethiopia Public Health Institute (EPHI) database. The data prior to 2019 were scattered and challenging to obtain and therefore not included in this analysis. Ethiopia officially has 4 levels of administrative division: Region, Zone, Woreda, and Kebele in descending size order. Ethiopia uses an excel-based and paper-based data collection method to document all OCV vaccination-related data at kebele (ward)- and woreda (district)-level, and subsequently compiled into a national database.
Regional level data are collected using the Open Data Kit (ODK) system. This data set included: date and number of OCV doses requested, approved, and received; OCV vaccination campaigns conducted per region, zone, woreda with planned and actual number of doses administered; OCV vaccination administrative coverage based on actual doses administered and number of population targeted per vaccination target areas (based on the latest administrative census); and sex- and age-stratified data on populations administered with cholera vaccines. Because not all kebeles in targeted woredas were always selected for vaccination, total population number per woreda and population of vaccination targeted kebeles were separately reported in the paper.
Data Analysis
The request numbers refer to each OCV request made by the Ethiopian government to the ICG, according to the EPHI records. For non-stockpile requests, a discernible request number was given in our analysis. Total population of woredas represent population in the year when OCV vaccination campaigns were conducted. In most past OCV campaigns, the target population was the population in kebeles planned for vaccine administration, excluding infants under 1 year old. The OCV administrative coverage was calculated based on the actual number of people vaccinated (numerator) out of the OCV target populations (denominator) in each round of vaccination per region, zone, and woreda levels in Ethiopia.
RESULTS
OCV Doses Requested, Approved, Received, and Used in Ethiopia
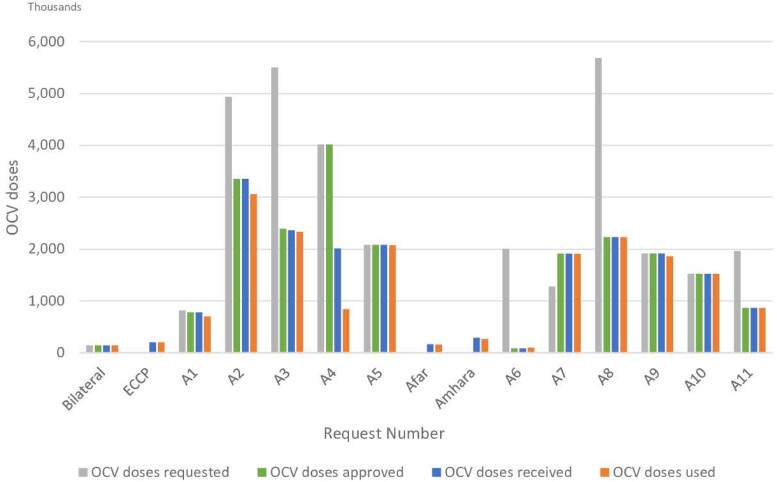
From 2019 until October 2023, a total of 32 044 576 OCV doses were requested by the Ethiopian government with 31 899 576 doses (11 requests: A1–A11) to the ICG and 1 request for 145 000 doses through the government of the Republic of Korea. The OCV doses (202 491) used in a preemptive vaccination in May 2022 [13] as part of the earlier planned Ethiopia Cholera Control and Prevention (ECCP) project is not counted in this total number of OCV doses requested to the ICG because these doses were procured as part of a joint research collaboration between the International Vaccine Institute (IVI) and Armauer Hansen Research Institute (AHRI) under the Ministry of Health in Ethiopia. The ICG approved 66.3% (21 148 800/31 899 576) of the requested OCVs and 90.4% (19 113 386/21 148 800) of the approved doses were delivered to Ethiopia; therefore, 59.9% (19 113 386/31 899 576) of the total requested OCV doses were received in Ethiopia (Table 1, Figure 1). The request A6, which was almost immediately after the ICG changed to a single dose recommendation for reactive campaigns in October 2022, shows only 4.3% (86 910/2 000 466) of the requested OCV doses were approved and received in Ethiopia for this outbreak response due to extreme shortage of vaccine in the global stockpile.
Table 1.
OCV Doses Requested and Made Available in Ethiopia From 2019 to October 2023
| OCV Doses Requested and Made Available | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Request Numbera | Request Type b | OCV Requested | OCV Request Approved | Approved OCV Doses Received in Ethiopia | OCV Doses Used & Leftover | |||||
| OCV Doses Requested (n) |
Date of OCV Doses Requested (YYYY/MM/DD) |
OCV Doses Approved (n) |
Date of OCV Doses Approved (YYYY/MM/DD) |
OCV Doses Received at Entry port in Ethiopiac (n) |
Date of OCV Doses Received at Entry Port in Ethiopia (YYYY/MM/DD) |
OCV Doses Used (n) |
OCV Doses Unused Due to VVM Change (n) |
OCV Doses Returned Unused and in Good Conditiond (n) |
||
| Request outside of Global OCV Stockpile | ||||||||||
| Bilaterale | Reactive | 145 000 | n.a | 145 000 | n.a | 145 000 | 2019/06/14 | 145 000 | n.a | n.a |
| ECCP | Preemptive/Preventive | n.a | n.a | n.a | n.a | 202 360 | 2021/11/18 | 202 491f | n.a | n.a |
| Total | 145 000 | n.a | 145 000 | n.a | 347 360 | n.a | 347 491 | n.a | n.a | |
| Request to Global OCV Stockpile | ||||||||||
| A1g | Preemptive/Preventive | 817 705 | 2019/05/07 | 778 766 | 2019/05/10 | 778 766 | 2019/05/17 (1st round)h 2019/12/03 (2nd round) |
698 970 | 496 | 32 311 |
| A2 | Reactive | 4 938 802 | 2020/11/16 | 3 354 278 | 2020/11/19 | 3 354 400 | 2020/12/06 (1st round) 2021/02/21 (2nd round) |
3 059 678 | 22 | n.a |
| A3 | Reactive | 5 501 446 | 2021/03/03 | 2 390 454 | 2021/03/05 | 2 363 164 | 2021/03/18 (1st round) 2021/07/09 (2nd round) |
2 332 578 | 751 | 26 545 |
| A4 | Preemptive/Preventive | 4 017 218 | 2021/05/11 | 4 017 218 | 2021/05/13 | 2 008 650 | 2021/05/22 | 840 774 | n.a | n.a |
| A5 | Reactive | 2 077 959 | 2021/11/04 | 2 077 959 | 2021/11/06 | 2 077 958 | 2021/11/18 | 2 073 728 | 296 | 3583 |
| A6 | Reactive | 2 000 466 | 2022/11/30 | 86 910 | 2022/12/02 | 86 910 | 2022/12/15 | 100 713 | n.a | 5687 |
| A7 | Reactive | 1 275 898 | 2023/03/01 | 1 910 416 | 2023/03/29 | 1 910 416 | 2023/04/05 | 1 909 405 | 53 | 957 |
| A8 | Reactive | 5 867 505 | 2023/06/13 | 2 230 038 | 2023/07/09 | 2 230 038 | 2023/07/20 | 2 229 941 | 68 | 169 |
| A9 | Reactive | 1 917 914 | 2023/08/01 | 1 917 914 | 2023/08/03 | 1 917 914 | 2023/09/12 | 1 858 472 | 85 | 5389 |
| A10 | Reactive | 1 522 495 | 2023/09/29 | 1 522 495 | 2023/10/04 | 1 522 495 | 2023/10/20 | 1 522 407 | 246 | 1283 |
| A11 | Reactive | 1 962 168 | 2023/10/15 | 862 352 | 2023/10/17 | 862 675 | 2023/10/27 | 862 326 | 16 | 333 |
| Total | 31 899 576 | n.a | 21 148 800 | n.a | 19 113 386 | n.a | 17 488 992 | 2033 | 76 257 | |
| Grand total | 32 044 576 | n.a | 21 293 800 | n.a | 19 460 746 | n.a | 17 836 483 | 2033 | 76 257 | |
Abbreviations: ECCP, Ethiopia Cholera Control and Prevention; n.a, not applicable; OCV, oral cholera vaccine; VVM, Vaccine Vial Monitor.
The bold values refer to the total or subtotal amount.
aRequest number refers to each OCV request made by the Ethiopian government.
bRequest Type refers to a preemptive/preventive or reactive campaign per the Ethiopian government's OCV request dossiers.
cAll OCV doses approved were delivered to Addis Ababa, the entry port in Ethiopia.
dRemaining OCV doses are returned to Ethiopian Pharmaceuticals Supply Agency (EPSA) for storage and stored doses are used for other vaccination campaigns.
eBilateral: Ethiopian government's bilateral request to the government of the Republic of Korea for OCV doses for cholera outbreak control in 2019.
gOCV doses were requested for preemptive/preventive vaccination, but doses were partially used for reactive vaccination and partially for preemptive vaccination at the moment of campaign.
fTotal 202 491 doses were used: 202 360 doses procured and delivered under ECCP and additional 131 available doses from health centers.
hApproved OCV doses were shipped in a separate batch per round according to the campaign schedule in the case of 2 rounds of vaccination.
Figure 1.
OCV doses made available in Ethiopia from 2019 to October 2023. The figure shows OCV doses requested, approved, and received in Ethiopia from 2019 to October 2023, and doses used in last 5 years. “Request number” refers to each OCV request made by the Ethiopian government to the ICG (A1-A11) and non-stockpile requests (Bilateral, ECCP). “Bilateral” represents the Ethiopian government's bilateral request to the government of the Republic of Korea for OCV doses for cholera outbreak control in 2019. “ECCP” represents “Ethiopia Cholera Control and Prevention” project. “Afar” and “Amhara” represent preventive vaccination campaigns conducted in Afar and Amhara region, respectively using remaining OCV doses from other vaccination campaigns. Abbreviation: OCV, oral cholera vaccine.
Generally, all approved doses were delivered to Ethiopia, but the request A4 for reactive vaccination campaign in Tigray region was exceptional (4 017 218 requested and approved; 2 008 650 delivered). When a campaign is designed for 2 rounds, approved OCV doses are usually shipped in a separate batch per round according to the campaign schedule, and OCV doses for second round are delivered after a technical report from first round is submitted to the ICG. In the case of Tigray campaign, the first round's technical report concluded that the second round could not be implemented due to insecurity in the region and the second-round doses were not sent although the doses for 2 rounds had been approved. Of the total 31 899 576 doses (requests A1–A11) requested, 21 148 800 doses were approved, of which 19 113 386 doses were delivered to Ethiopia and 17 488 992 doses were administered. Based on the available records, 2033 doses were not used due to vaccine vial monitor (VVM) change signifying lack of integrity of the cold chain which occurred in the hard-to-reach areas under conflict. 76 257 unused doses were returned to the Ethiopian Pharmaceuticals Supply Agency (EPSA) for cold chain storage, to be used for future reactive vaccination, such as campaigns conducted in Afar and Amhara region in 2022 (Table 2). Among the gap of 1 546 104 doses between delivered and administered, 1 167 876 doses could not be used in Tigray campaign as planned and have not been retrieved to EPSA yet. The remaining gap of 378 228 doses is attributed to remaining doses from prior campaigns but marked as “not applicable” in the database or some damaged vials which were not described in Table 1.
Table 2.
OCV Vaccination Campaigns in Ethiopia From 2019 to 2023
| Request Numbera | Region | OCV Target Populationb | Dose Regimen SD/2D |
1st Round | Dose Interval (If 2 Doses Administered) |
2nd Round | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Campaign Start Date (YYYY/MM/DD) |
Campaign End Date (YYYY/MM/DD) |
Number of People Vaccinated with OCV (n) | Administrative Coveragec (%) | Campaign Start Date (YYYY/MM/DD) |
Campaign End Date (YYYY/MM/DD) |
Number of People Vaccinated with OCV (n) | Administrative Coverage (%) | |||||
| A1 | Oromia | 298 343 | 2D | 2019/07/01 | 2019/07/05 | 290 740 | 97.5 | 24 wks | 2019/12/14 | 2019/12/24 | 286 203 | 95.9 |
| Addis Ababa | 17 324 | SD | 2019/07/01 | 2019/07/05 | 17 324 | 100.0 | n.a | n.a | n.a | n.a | n.a | |
| Afar | 92 070 | SD | 2019/07/01 | 2019/07/05 | 81 381 | 88.4 | n.a | n.a | n.a | n.a | n.a | |
| Sidama | 23 322 | SD | 2019/07/01 | 2019/07/05 | 23 322 | 100.0 | n.a | n.a | n.a | n.a | n.a | |
| A1-total | 431 059 | SD/2D (Oromia) | 2019/07/01 | 2019/07/05 | 412 767 | 95.8 | 24 wks | 2019/12/14 | 2019/12/24 | 286 203 | 95.9 | |
| A2 | Gambella | 27 128 | 2D | 2020/12/21 | 2020/12/29 | 24 226 | 89.3 | 9 wks | 2021/02/28 | 2021/03/06 | 24 226 | 89.3 |
| Oromia | 503 823 | 2D | 2020/12/21 | 2020/12/29 | 483 581 | 96.0 | 9 wks | 2021/02/28 | 2021/03/06 | 482 341 | 95.7 | |
| Sidama | 359 176 | 2D | 2020/12/21 | 2020/12/29 | 352 189 | 98.1 | 9 wks | 2021/02/28 | 2021/03/06 | 351 845 | 98.0 | |
| SNNPR | 598 201 | 2D | 2020/12/21 | 2020/12/29 | 559 605 | 93.5 | 9 wks | 2021/02/28 | 2021/03/06 | 534 199 | 89.3 | |
| Somali | 113 991 | 2D | 2020/12/21 | 2020/12/29 | 124 863 | 109.5 | 9 wks | 2021/02/28 | 2021/03/06 | 122 603 | 107.6 | |
| A2-total | 1 602 319 | 2D | 2020/12/21 | 2020/12/29 | 1 544 464 | 96.4 | 9 wks | 2021/02/28 | 2021/03/06 | 1 515 214 | 94.6 | |
| A3 | Oromia | 151 227 | 2D | 2021/05/20 | 2021/05/26 | 148 201 | 98.0 | 12 wks | 2021/08/18 | 2021/08/25 | 148 201 | 100.0 |
| SNNPR | 1 044 000 | 2D | 2021/05/20 | 2021/05/26 | 1 019 738 | 97.7 | 12 wks | 2021/08/18 | 2021/08/25 | 1 016 438 | 99.7 | |
| A3-total | 1 195 227 | 2D | 2021/05/20 | 2021/05/26 | 1 167 939 | 97.7 | 12 wks | 2021/08/18 | 2021/08/25 | 1 164 639 | 99.7 | |
| A4 | Tigray | 2 010 680 | SD | 2021/06/10 | 2021/06/17 | 840 774 | 41.8 | n.a | n.a | n.a | n.a | n.a |
| A5 | Oromia | 518 379 | 2D | 2021/12/23 | 2022/01/01 | 516 834 | 99.7 | 13 wks | 2022/03/25 | 2022/03/31 | 516 834 | 99.7 |
| Somali | 520 600 | 2D | 2021/12/23 | 2022/01/01 | 520 035 | 99.9 | 13 wks | 2022/03/25 | 2022/03/31 | 520 025 | 99.9 | |
| A5-total | 1 038 979 | 2D | 2021/12/23 | 2022/01/01 | 1 036 869 | 99.8 | 13 wks | 2022/03/25 | 2022/03/31 | 1 036 859 | 99.8 | |
| N/Ad | Afar | 163 544 | SD | 2022/04/23 | 2022/04/30 | 160 340 | 98.0 | n.a | n.a | n.a | n.a | n.a |
| Amhara | 291 594 | SD | 2022/05/23 | 2022/05/31 | 265 188 | 90.9 | n.a | n.a | n.a | n.a | n.a | |
| N/A-total | 455 138 | SD | n.a | n.a | 425 528 | 93.5 | n.a | n.a | n.a | n.a | n.a | |
| A6 | Oromia | 76 400 | SD | 2023/01/13 | 2023/01/20 | 76 226 | 99.8 | n.a | n.a | n.a | n.a | n.a |
| Somali | 24 487 | SD | 2023/01/13 | 2023/01/20 | 24 487 | 100 | n.a | n.a | n.a | n.a | n.a | |
| A6-total | 100 887 | SD | 2023/01/13 | 2023/01/20 | 100 713 | 99.8 | n.a | n.a | n.a | n.a | n.a | |
| A7 | Oromia | 1 467 612 | SD | 2023/05/15 | 2023/05/24 | 1 466 698 | 99.9 | n.a | n.a | n.a | n.a | n.a |
| Somali | 442 806 | SD | 2023/05/15 | 2023/05/24 | 442 707 | 100.0 | n.a | n.a | n.a | n.a | n.a | |
| A7-total | 1 910 418 | SD | 2023/05/15 | 2023/05/24 | 1 909 405 | 99.9 | n.a | n.a | n.a | n.a | n.a | |
| A8 | Oromia | 571 259 | SD | 2023/08/10 | 2023/08/20 | 571 102 | 100.0 | n.a | n.a | n.a | n.a | n.a |
| Sidama | 343 158 | SD | 2023/08/10 | 2023/08/20 | 343 156 | 100.0 | n.a | n.a | n.a | n.a | n.a | |
| SNNPR | 1 315 761 | SD | 2023/08/10 | 2023/08/20 | 1 315 683 | 100.0 | n.a | n.a | n.a | n.a | n.a | |
| A8-total | 2 230 178 | SD | 2023/08/10 | 2023/08/20 | 2 229 941 | 100.0 | n.a | n.a | n.a | n.a | n.a | |
| A9 | Amhara | 1 867 912 | SD | 2023/09/16 | 2023/09/23 | 1 858 472 | 99.5 | n.a | n.a | n.a | n.a | n.a |
| A10 | Afar | 524 923 | SD | 2023/11/11 | 2023/11/21 | 524 173 | 99.9 | n.a | n.a | n.a | n.a | n.a |
| Sidama | 365 488 | SD | 2023/11/11 | 2023/11/21 | 365 270 | 99.9 | n.a | n.a | n.a | n.a | n.a | |
| SNNPR | 633 652 | SD | 2023/11/11 | 2023/11/21 | 632 964 | 99.9 | n.a | n.a | n.a | n.a | n.a | |
| A10-total | 1 524 063 | SD | 2023/11/11 | 2023/11/21 | 1 522 407 | 99.9 | n.a | n.a | n.a | n.a | n.a | |
| A11 | Amhara | 131 134 | SD | 2023/11/29 | 2023/12/05 | 130 803 | 99.7 | n.a | n.a | n.a | n.a | n.a |
| Oromia | 731 541 | SD | 2023/11/29 | 2023/12/05 | 731 523 | 100.0 | n.a | n.a | n.a | n.a | n.a | |
| A11-total | 862 675 | SD | 2023/11/29 | 2023/12/05 | 862 326 | 100.0 | n.a | n.a | n.a | n.a | n.a | |
| Grand total | 15 229 535 | n.a | n.a | n.a | 13 911 605 | 91.3 | n.a | n.a | n.a | 4 002 915 | 97.1 | |
Abbreviations: n.a, not applicable; OCV, oral cholera vaccine; SD, single dose; SNNPR: Southern Nations, Nationalities, and Peoples’ Region; 2D, 2 doses; wks, weeks.
The bold values refer to the total or subtotal amount.
aRequest number refers to each OCV request made by the Ethiopian government.
bIn first round. OCV target population is the population of OCV vaccination targeted kebeles in each woreda excluding infants (ie, < 1 y).
cAdministrative coverage (%) was calculated based on the actual number of people vaccinated in each round (numerator) out of the OCV target population (denominator) in each round.
dIt used remaining OCV doses from previous OCV vaccination campaigns.
OCV Vaccination Dose Intervals and Coverage per Region, Zone, and Woreda in Ethiopia
Based on the OCV doses received from requests A1-A11, 17 488 992 people were vaccinated with either a single dose (SD) or 2-doses (2D) of OCV with different dosing schedules (Table 2, Supplementary Table 1, Figure 2). The OCV doses from request A1 were administered as 2D with 24-week dose interval in Oromia region with 97.5% administrative coverage in first round and 95.9% in second round, but SD strategy was used in other cholera vulnerable regions due to the shortage of the vaccine. OCV doses were requested for pre-emptive vaccination in Tigray region, a site of ongoing conflict. Tigray campaign was conducted in the setting of insecurity or armed conflict, resulting in the lowest OCV administrative coverage across all vaccination campaigns conducted in Ethiopia from 2019 to December 2023 (Table 2). Reactive vaccination campaigns implemented between 2020 and 2021 (request A2, A3, A5) used recommended 2D regimen but dose intervals were more than 2 weeks. In April and May 2022, OCV vaccinations were carried out in Afar and Amhara regions to respond to the outbreak in each region. Leftover doses from other OCV vaccination campaigns conducted prior to April 2022, which have been stored at EPSA, were used for SD strategy to cover more people (Table 2). Six reactive vaccinations in 2023 administered SD reflecting the ICG recommendation in global OCV shortage setting.
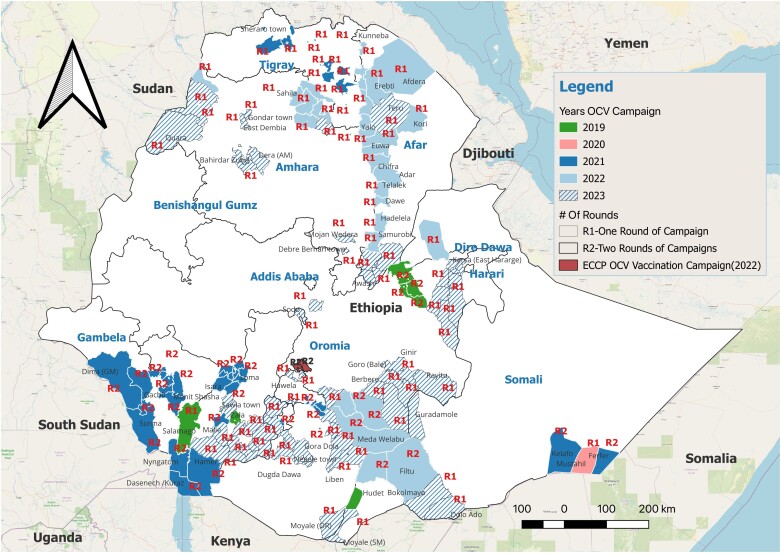
Figure 2.
Map of OCV vaccination campaign areas. The map comprehensively shows woredas where OCV vaccination campaigns have been conducted from 2019 to 2023. Number of vaccination rounds conducted in each woreda is represented as either R1 or R2. Abbreviations: ECCP, Ethiopia Cholera Control and Prevention; OCV, oral cholera vaccine.
Sex- and Age-Stratified OCV Vaccination in Ethiopia
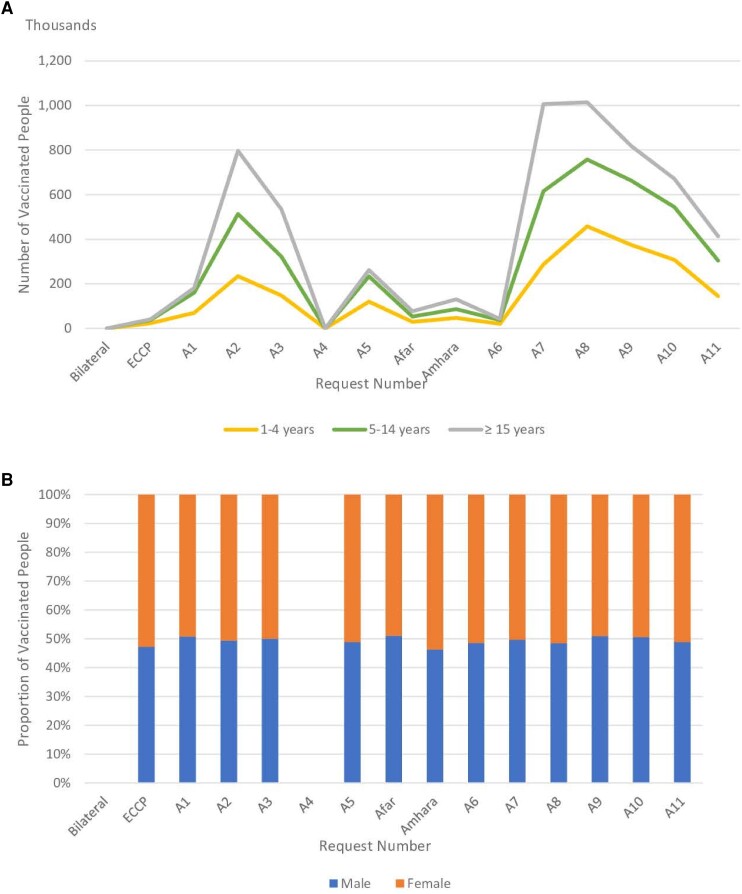
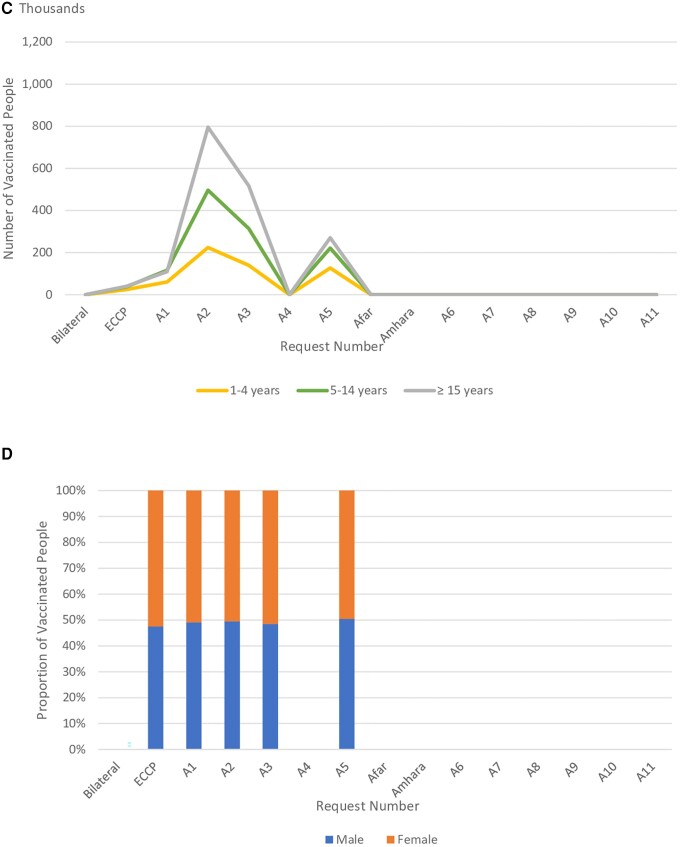
The sex- and age-stratified data on the OCV vaccinated populations for each vaccination campaign from 2019 to 2023 were described (Table 3, Supplementary Table 2, and Figure 3). There was no sex and age data for the campaign conducted in Southern Nations, Nationalities, and Peoples’ Region (SNNPR) and Somali regions in 2019 through bilateral request and Tigray campaign (request A4). Overall, local populations aged 15 years and older received the largest number of doses of OCV regardless of SD or 2D administration (Table 3), both first (Figure 3A) and second round (Figure 3C); followed by children aged between 5–14 years and 1–4 years. An exception was in the A1 request related OCV use during the second round of vaccination campaign, where children aged 5–14 years (116 610) were vaccinated slightly more than those aged 15 years and older (109 626) (Table 3 and Supplementary Table 2). The proportion of male and female populations vaccinated with OCV was similar regardless of dosing schedules or campaign types (Figure 3B and Figure 3D).
Table 3.
Age-Group and Sex-Stratified Data on OCV Vaccination Campaigns in Ethiopia From 2019 to 2023
| Request Numbera |
Region | Number of People Vaccinated With OCVb (n) | 1st Round | 2nd Round | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–4 yc | 5–14 yd | ≥15 y | 1–4 y | 5–14 y | ≥15 y | |||||||||
| M | F | M | F | M | F | M | F | M | F | M | F | |||
| A1 | Oromia | 290 740 | 28 959 | 28 698 | 57 610 | 57 790 | 58 907 | 58 776 | 29 955 | 30 012 | 56 963 | 59 647 | 53 650 | 55 976 |
| Addis Ababa | 17 324 | 405 | 320 | 4208 | 3770 | 4500 | 4121 | n.a | n.a | n.a | n.a | n.a | n.a | |
| Afar | 81 381 | 5998 | 5998 | 17 861 | 17 861 | 20 284 | 13 379 | n.a | n.a | n.a | n.a | n.a | n.a | |
| Sidama | 23 322 | 0 | 0 | 810 | 800 | 10 212 | 11 500 | n.a | n.a | n.a | n.a | n.a | n.a | |
| A1-total | 412 767 | 35 362 | 35 016 | 80 489 | 80 221 | 93 903 | 87 776 | 29 955 | 30 012 | 56 963 | 59 647 | 53 650 | 55 976 | |
| A1-total per age group | 70 378 | 160 710 | 181 679 | 59 967 | 116 610 | 109 626 | ||||||||
| A2 | Gambella | 24 226 | 1487 | 1546 | 3111 | 3107 | 8868 | 6107 | 1487 | 1546 | 3111 | 3107 | 8868 | 6107 |
| Oromia | 483 581 | 42 899 | 43 033 | 78 596 | 79 435 | 116 846 | 122 772 | 43 122 | 43 033 | 79 954 | 75 636 | 117 824 | 122 772 | |
| Sidama | 352 189 | 18 868 | 19 933 | 63 243 | 63 568 | 93 039 | 93 538 | 19 090 | 16 931 | 64 412 | 65 274 | 92 017 | 94 121 | |
| SNNPR | 559 605 | 44 550 | 43 414 | 83 773 | 86 314 | 147 948 | 153 606 | 38 388 | 39 775 | 78 105 | 81 158 | 145 990 | 150 783 | |
| Somali | 124 863 | 8789 | 10 259 | 23 942 | 28 166 | 26 576 | 27 131 | 10 190 | 10 242 | 20 657 | 24 635 | 26 512 | 30 367 | |
| A2-total | 1 544 464 | 116 593 | 118 185 | 252 665 | 260 590 | 393 277 | 403 154 | 112 277 | 111 527 | 246 239 | 249 810 | 391 211 | 404 150 | |
| A2-total per age group | 234 778 | 513 255 | 796 431 | 223 804 | 496 049 | 795 361 | ||||||||
| A3 | Oromia | 148 201 | 8300 | 8639 | 24 364 | 25 358 | 40 354 | 41 186 | 8300 | 8639 | 24 364 | 25 358 | 40 354 | 41 186 |
| SNNPR | 1 019 738 | 57 862 | 60 192 | 168 388 | 175 229 | 276 990 | 281 077 | 56 928 | 59 251 | 167 099 | 173 917 | 276 769 | 282 474 | |
| A3-total | 1 167 939 | 66 162 | 68 831 | 192 752 | 200 587 | 317 344 | 322 263 | 65 228 | 67 890 | 191 463 | 199 275 | 317 123 | 323 660 | |
| A3-total per age group | 134 993 | 393 339 | 639 607 | 133 118 | 390 738 | 640 783 | ||||||||
| A4 | Tigray | 840 774 | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
| A5 | Oromia | 516 834 | 48 959 | 52 988 | 93 152 | 93 264 | 116 209 | 112 262 | 48 975 | 52 990 | 93 152 | 93 255 | 116 205 | 112 257 |
| Somali | 520 035 | 47 940 | 42 695 | 98 068 | 110 178 | 101 326 | 119 828 | 47 944 | 42 695 | 98 068 | 110 186 | 101 328 | 119 804 | |
| A5-total | 1 036 869 | 96 899 | 95 683 | 191 220 | 203 442 | 217 535 | 232 090 | 96 919 | 95 685 | 191 220 | 203 441 | 217 533 | 232 061 | |
| A5-total per age group | 192 582 | 394 662 | 449 625 | 192 604 | 394 661 | 449 594 | ||||||||
| N/Ae | Afar | 160 340 | 15 552 | 14 026 | 27 462 | 25 904 | 38 888 | 38 508 | n.a | n.a | n.a | n.a | n.a | n.a |
| Amhara | 265 188 | 22 908 | 24 614 | 40 552 | 45 939 | 59 313 | 71 862 | n.a | n.a | n.a | n.a | n.a | n.a | |
| N/A-total | 425 528 | 38 460 | 38 640 | 68 014 | 71 843 | 98 201 | 110 370 | n.a | n.a | n.a | n.a | n.a | n.a | |
| N/A-total per age group | 77 100 | 139 857 | 208 571 | n.a | n.a | n.a | ||||||||
| A6 | Oromia | 76 226 | 7344 | 7809 | 13 442 | 14 526 | 16 056 | 17 049 | n.a | n.a | n.a | n.a | n.a | n.a |
| Somali | 24 487 | 2956 | 2910 | 4436 | 4701 | 4664 | 4820 | n.a | n.a | n.a | n.a | n.a | n.a | |
| A6-total | 100 713 | 10 300 | 10 719 | 17 878 | 19 227 | 20 720 | 21 869 | n.a | n.a | n.a | n.a | n.a | n.a | |
| A6-total per age group | 21 019 | 37 105 | 42 589 | n.a | n.a | n.a | ||||||||
| A7 | Oromia | 1 466 698 | 108 619 | 112 184 | 233 817 | 238 020 | 385 638 | 388 420 | n.a | n.a | n.a | n.a | n.a | n.a |
| Somali | 442 707 | 33 352 | 33 211 | 71 526 | 72 158 | 116 395 | 116 065 | n.a | n.a | n.a | n.a | n.a | n.a | |
| A7-total | 1 909 405 | 141 971 | 145 395 | 305 343 | 310 178 | 502 033 | 504 485 | n.a | n.a | n.a | n.a | n.a | n.a | |
| A7-total per age group | … | 287 366 | 615 521 | 1 006 518 | n.a | n.a | n.a | |||||||
| A8 | Oromia | 571 102 | 57 544 | 62 358 | 97 712 | 95 258 | 126 752 | 131 478 | n.a | n.a | n.a | n.a | n.a | n.a |
| Sidama | 343 156 | 43 255 | 44 984 | 57 601 | 60 320 | 64 868 | 72 128 | n.a | n.a | n.a | n.a | n.a | n.a | |
| SNNPR | 1 315 683 | 122 069 | 127 788 | 217 282 | 229 514 | 294 082 | 324 948 | n.a | n.a | n.a | n.a | n.a | n.a | |
| A8-total | 2 229 941 | 222 868 | 235 130 | 372 595 | 385 092 | 485 702 | 528 554 | n.a | n.a | n.a | n.a | n.a | n.a | |
| A8-total per age group | 457 998 | 757 687 | 1 014 256 | n.a | n.a | n.a | ||||||||
| A9 | Afar | 1 858 472 | 187 787 | 187 774 | 322 197 | 341 420 | 437 115 | 382 179 | n.a | n.a | n.a | n.a | n.a | n.a |
| A9-total per age group | 375 561 | 663 617 | 819 294 | n.a | n.a | n.a | ||||||||
| A10 | Afar | 524 173 | 52 489 | 53 289 | 91 216 | 96 769 | 121 130 | 109 280 | n.a | n.a | n.a | n.a | n.a | n.a |
| Sidama | 365 270 | 36 547 | 37 447 | 61 932 | 67 787 | 85 661 | 75 896 | n.a | n.a | n.a | n.a | n.a | n.a | |
| SNNPR | 632 964 | 63 863 | 63 663 | 110 118 | 116 455 | 147 337 | 131 528 | n.a | n.a | n.a | n.a | n.a | n.a | |
| A10-total | 1 522 407 | 152 899 | 154 399 | 263 266 | 281 011 | 354 128 | 316 704 | n.a | n.a | n.a | n.a | n.a | n.a | |
| A10-total per age group | 307 298 | 544 277 | 670 832 | n.a | n.a | n.a | ||||||||
| A11 | Amhara | 130 803 | 5156 | 5563 | 20 037 | 20 803 | 39 435 | 39 809 | n.a | n.a | n.a | n.a | n.a | n.a |
| Oromia | 731 523 | 64 779 | 69 605 | 131 506 | 132 057 | 160 635 | 172 941 | n.a | n.a | n.a | n.a | n.a | n.a | |
| A11-total | 862 326 | 69 935 | 75 168 | 151 543 | 152 860 | 200 070 | 212 750 | n.a | n.a | n.a | n.a | n.a | n.a | |
| A11-total per age group | 145 103 | 304 403 | 412 820 | n.a | n.a | n.a | ||||||||
| Grand total per age group | 2 304 176 | 4 524 433 | 6 242 222 | 609 493 | 1 398 058 | 1 995 364 | ||||||||
Abbreviations: n.a, not applicable; OCV, oral cholera vaccine; SNNPR: Southern Nations, Nationalities, and Peoples’ Region; y: years.
The bold values refer to the total or subtotal amount.
aRequest number refers to each OCV request made by the Ethiopian government.
bIn first round.
c12–59 months.
d60–179 months.
eIt used remaining OCV doses from previous OCV vaccination campaigns.
Figure 3.
Age group and sex-stratified OCV vaccinated populations from 2019 to 2023. Panels A–D show age groups and sex of OCV vaccinated populations in each vaccination campaigns. “Request number” refers to each OCV request made by the Ethiopian government to the ICG (A1–A11) and non-stockpile requests (Bilateral, ECCP). “Bilateral” represents the Ethiopian government's bilateral request to the government of the Republic of Korea for OCV doses for cholera outbreak control in 2019. “ECCP” represents “Ethiopia Cholera Control and Prevention” project. “Afar” and “Amhara” represent preventive vaccination campaigns conducted in Afar and Amhara region respectively using remaining OCV doses from other vaccination campaigns. Age and sex stratified data of OCV vaccinated populations from Bilateral and A4 were not available. Afar, Amhara, and A6–A11 administered single dose (SD) strategy; there was no second round information. Panels A and B exhibit age group and sex stratifications of populations vaccinated respectively in the 1st round; Panels C and D exhibit age group and sex stratifications of populations vaccinated respectively in the 2nd round. Abbreviation: OCV, oral cholera vaccine.
DISCUSSION
Overall, 15 OCV mass vaccination campaigns were conducted in Ethiopia from 2019 to 2023. 11 campaigns (9 reactive and 2 preemptive campaigns) used OCV doses received through requests made to the global stockpile of OCVs and 2 reactive campaigns in Afar and Amhara region in 2022 used some remaining doses from prior campaigns. OCV doses obtained from outside of global stockpile (a bilateral channel and a research project) were used for 1 reactive campaign and 1 preemptive vaccination, respectively. This preemptive vaccination campaign result under the research project is presented in this CID Supplement [13]. Most of the OCV requests were deployed in response to cholera outbreaks, and dose intervals were more than standard 2 weeks in the case of 2-rounds campaigns. Regardless of dose regimen, all areas targeted showed high administrative coverage except for Tigray region, but formal post-campaign coverage surveys have not been conducted in Ethiopia. Generally, population aged 15 years and older were vaccinated more compared to children aged below 15 years, and there was not much difference between the proportion of sex in vaccinated population. No adverse events following immunization (AEFI) were reported throughout all vaccination campaigns.
During the vaccination campaign implemented in 2019, the Ethiopian government used a SD strategy to cover broader outbreak area due to shortage of the vaccine, except parts of Oromia region where 2D vaccination was rolled-out considering the severity of outbreak in the region [14]. The ICG SD recommendation was announced in October 2022 in response to large scale global cholera epidemics and the shortage in global OCV stockpile [15]. Debates on the effectiveness and impact of SD are ongoing with limited research available thus far. However, some modeling studies demonstrate a speedy vaccination with SD of OCV under a limited vaccine supply may avert more cholera cases and deaths in outbreak setting compared to a standard 2D regimen [16, 17]. Nonetheless, the effectiveness, duration of protection and impact of SD OCV intervention may differ in populations living in different environments with varying degrees of cholera endemicity [18]; as well as between age-groups as children under 5 years may receive limited benefit from an SD [19, 20]. All mass OCV vaccination campaigns conducted in Ethiopia from 2022 used SD strategy (except for the campaign under the ECCP project in May 2022), but no follow-up studies have been performed to investigate SD effectiveness or to explore how long protection would last or optimal timing of catch-up second dose vaccination.
In addition to dose regimen, optimal and feasible dosing schedule is another point to be considered for effective vaccination. The standard dose interval of OCV is 2 weeks [21], but it is not feasible to strictly follow in most emergency settings. Among 5 OCV vaccination campaigns with 2D regimen conducted in Ethiopia from 2019, only 1 vaccination campaign implemented in Shashemene area in May 2022 (under the ECCP research project) adopted the standard 14-day dose interval [13]. The remaining 2D vaccination campaigns showed varying dose intervals from 9 weeks to 24 weeks. Similar to the SD, there are limited studies on OCV dosing schedules. Several studies have explored immunogenicity of alternative dose intervals and found generally comparable immune responses at intervals of 1 or 6 months [22, 23]. A SD strategy may be able to reduce the urgent risk of cholera infection in an ongoing outbreak for the short term; but an extended interval dose is recommended to make protection robust even with delays in the second dose [20, 23, 24]. Nevertheless, this may not be an easy task in the real-world setting. A study conducted in Lusaka, Zambia, suggested the long delay between doses can make people miss an opportunity to get full 2-doses OCV because of population movement [25]. However, further operational research on a longer dosing interval is warranted to answer questions on the effectiveness and impact in real-world settings.
One of the priorities in the GTFCC cholera research agenda is finding vaccination delivery strategies for “hard-to-reach populations” such as internally displaced people (IDP) [9]. This facet is also important to consider because areas of insecurity may be at risk for cholera outbreaks and difficult to provide immunization [26–28]. In Ethiopia, the government conducted campaigns to prevent cholera outbreaks amid the conflict region in Tigray regional state in 2021 [29], targeting populations living in the conflict affected communities and IDP. However, significantly lower vaccination administrative coverage (41.8% in the first round; and second round did not occur) was reported from all woredas in Tigray region during this period as a result of the challenging operational situation on the ground. For instance, barriers to sufficient community engagement and sensitization, limited access to health centers and posts, and management of overall mass vaccination campaigns with proper record keeping and documentations. EPHI uses a mixed vaccination campaign strategy (door-to-door; fixed posts), and campaigns are integrated with other response pillars including surveillance and water, sanitation, and hygiene interventions. Yet it underscores how difficult it can be to implement vaccination effectively in a conflict setting and may infer necessity of identifying and building logistical and operational strategy to equitably administer OCVs to hard-to-reach and vulnerable populations [30].
No post-campaign coverage surveys have been conducted in Ethiopia due to the constraints of manpower and time in the face of the upsurge in outbreaks. Coverage surveys are important to understand the actual coverage estimates of SD or 2D which can differ from administrative coverage [31]. Indeed, one coverage survey data was available from 2D pre-emptive vaccination in 2022, implemented under ECCP project. The 2D coverage estimate was approximately 80% (78% in Shashemene Town; 83% in Shashemene Woreda) whereas administrative coverages of first and second round reached nearly 100% [13]. Coverage surveys including age and sex demographics provide important information regarding vaccine acceptance as well as reasons for non-vaccination which are important for improving future campaigns [32–36]. In addition, OCV is known to provide indirect or herd protection enhancing the overall impact, but which can vary with coverage rate [37, 38]. With the data available currently in Ethiopia, it is difficult to know actual coverage per dose or have data to inform improvement in future campaigns. Fortunately, as of November 2023, EPHI is conducting coverage studies in Afar, Oromia, South and Central Ethiopia regions to collect comprehensive coverage data using a pre-developed protocol. The coverage survey will be implemented within 2 weeks of campaign end date for every future OCV campaign.
CONCLUSION
To our knowledge, this is the first comprehensive review paper documenting all OCV requests made by the Ethiopian government from 2019 to October 2023 and mass vaccination campaigns implemented across the country in the last 5 years. Five full approvals and six partial approvals were made by the ICG, and 66.3% of OCV requests were approved. Of the approved OCV doses, 90.4% were delivered to Ethiopia. In spite of many challenges, the Ethiopian government was able to conduct numerous campaigns both reactive and preemptive in many settings, achieving high administrative coverage. Because of the inherent inaccuracies in administrative coverage data, coverage surveys should be carried out in future campaigns. A comprehensive review of past OCV vaccinations implemented in Ethiopia not only supports better planning of effective national OCV vaccination in the future but also generates the framework for future research areas.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Moti Edosa, Public Health Emergency Management, Ethiopia Public Health Institute, Addis Ababa, Ethiopia.
Yeonji Jeon, Clinical, Assessment, Regulatory, Evaluation (CARE) Unit, International Vaccine Institute, Seoul, Republic of Korea.
Abel Gedefaw, Clinical, Assessment, Regulatory, Evaluation (CARE) Unit, International Vaccine Institute, Seoul, Republic of Korea; College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia.
Dejene Hailu, Clinical, Assessment, Regulatory, Evaluation (CARE) Unit, International Vaccine Institute, Seoul, Republic of Korea; School of Public Health, Hawassa University, Hawassa, Ethiopia.
Edlawit Mesfin Getachew, Clinical Trials Directorate, Armauer Hansen Research Institute, Addis Ababa, Ethiopia.
Ondari D Mogeni, Clinical, Assessment, Regulatory, Evaluation (CARE) Unit, International Vaccine Institute, Seoul, Republic of Korea.
Geun Hyeog Jang, Biostatistics and Data Management (BDM) Department, International Vaccine Institute, Seoul, Republic of Korea.
David Mukasa, Biostatistics and Data Management (BDM) Department, International Vaccine Institute, Seoul, Republic of Korea.
Biruk Yeshitela, Bacterial and Viral Disease Research Directorate, Armauer Hansen Research Institute, Addis Ababa, Ethiopia.
Tomas Getahun, Clinical Trials Directorate, Armauer Hansen Research Institute, Addis Ababa, Ethiopia.
Julia Lynch, Cholera Program Director, International Vaccine Institute, Seoul, Republic of Korea.
Malika Bouhenia, Global Task Force on Cholera Control (GTFCC), World Health Organization (WHO), Geneva, Switzerland.
Yeshambel Worku Demlie, Public Health Emergency Management, Ethiopia Public Health Institute, Addis Ababa, Ethiopia.
Mukemil Hussen, Public Health Emergency Management, Ethiopia Public Health Institute, Addis Ababa, Ethiopia.
Mesfin Wossen, Public Health Emergency Management, Ethiopia Public Health Institute, Addis Ababa, Ethiopia.
Mekonnen Teferi, Clinical Trials Directorate, Armauer Hansen Research Institute, Addis Ababa, Ethiopia.
Se Eun Park, Clinical, Assessment, Regulatory, Evaluation (CARE) Unit, International Vaccine Institute, Seoul, Republic of Korea; Department of Global Health and Disease Control, Yonsei University Graduate School of Public Health, Seoul, Republic of Korea.
Notes
Author contributions. Y. J., M. E., M. T., and S. E. P. conceptualized the overall manuscript. Database review, data analysis, and manuscript drafting were led by Y. J. with M. E. in discussion with S. E. P. and M. T. All authors read and provided feedback and approved the final draft.
Acknowledgments . The authors acknowledge the EPHI (M. E., Y. W., M. H., M. W.) for the access to the national health data system. We thank health professionals and local and regional government officials in each woreda and regional health bureau for their effort to supervise oral cholera vaccine (OCV) vaccination and collect the data.
Data sharing. All data relevant to the study are included in this manuscript.
Disclaimer. The findings and conclusions are the authors' own and do not necessarily reflect the positions of donors.
Financial support . This study was supported by the Korea Support Committee for International Vaccine Institute (CHMTD05083-010), LG Electronics (CHMTD05083-020), and Community Chest of Korea (CHMTD05083-030). The International Vaccine Institute (IVI) acknowledges its donors, including the government of the Republic of Korea and the Swedish International Development Cooperation Agency.
Supplement sponsorship. This article appears as part of the supplement “Ethiopia Cholera Control and Prevention (ECCP): Evidence-Generation Towards Global Roadmap to Ending Cholera,” sponsored by the IVI.
References
- 1. Wierzba TF. Oral cholera vaccines and their impact on the global burden of disease. Hum Vacci Immunother 2019; 15:1294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Cholera vaccine. Available at: https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases/cholera. Accessed 15 December 2023.
- 3. Global Task Force on Cholera Control . Ending cholera a global roadmap to 2030. Available at: https://www.gtfcc.org/wp-content/uploads/2019/10/gtfcc-ending-cholera-a-global-roadmap-to-2030.pdf. Accessed 11 December 2023.
- 4. GTFCC OCV Working Group Annual Meeting . Gavi support for OCV programme. Available at: https://www.gtfcc.org/wp-content/uploads/2022/08/9th-meeting-of-gtfcc-wg-ocv-2022-gavi-support-allyson-russel-olivia-bullock.pdf. Accessed 11 December 2023.
- 5. Global Task Force on Cholera Control . GTFCC OCV dashboard. Available at: https://apps.epicentre-msf.org/public/app/gtfcc. Accessed 10 December 2023.
- 6. Wosen M. Use of cholera vaccine; Ethiopia 2021. EPHI. Available at: https://www.gtfcc.org/wp-content/uploads/2022/01/8th-meeting-of-the-gtfcc-working-group-on-ocv-2021-day-1-mesfin-wossen.pdf. Accessed 26 June 2023.
- 7. ReliefWeb . Ethiopia—multi-sectorial cholera elimination plan 2022–2028. Available at: https://reliefweb.int/report/ethiopia/ethiopia-multi-sectorial-cholera-elimination-plan-2022-2028. Accessed 7 February 2023.
- 8. Pezzoli L; on behalf of the Oral Cholera Vaccine Working Group of the Global Task Force on Cholera Control. Global oral cholera vaccine use, 2013–2018. Vaccine 2020; 38:A132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Global Task Force on Cholera Control . Cholera roadmap research agenda. Available at: https://www.gtfcc.org/wp-content/uploads/2021/01/gtfcc-global-roadmap-research-agenda-full-report-with-methodology.pdf. Accessed January 2021.
- 10. Kayser V, Ramzan I.. Vaccines and vaccination: history and emerging issues. Hum Vaccin Immunother 2021; 17:5255–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trolle H, Forsberg B, King C, et al. . A scoping review of facilitators and barriers influencing the implementation of surveillance and oral cholera vaccine interventions for cholera control in lower- and middle-income countries. BMC Public Health 2023; 23:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Global Task Force on Cholera Control . Monitoring and reporting | National Cholera Plan. Available at: https://ncp.gtfcc.org/book-page/monitoring-and-reporting. Accessed 19 June 2023.
- 13.Park SE, Gedefaw A, Hailu D, et al. Coverage of two–dose preemptive cholera mass vaccination campaign in high–priority hotspots in Shashemene, Oromia Region, Ethiopia. Clin Infect Dis 2024; 79(S1):S33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EPHI Public Health Emergency Management. Summary report on oral cholera vaccine (OCV) in Ethiopia. Addis Ababa, Ethiopia: EPHI, 2020.
- 15. World Health Organization . Shortage of cholera vaccines leads to temporary suspension of two-dose strategy, as cases rise worldwide. Available at: https://www.who.int/news/item/19-10-2022-shortage-of-cholera-vaccines-leads-to-temporary-suspension-of-two-dose-strategy–as-cases-rise-worldwide. Accessed 19 October 2022.
- 16. Azman AS, Luquero FJ, Ciglenecki I, Grais RF, Sack DA, Lessler J. The impact of a one-dose versus two-dose oral cholera vaccine regimen in outbreak settings: a modeling study. PLoS Med 2015; 12:e1001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leung T, Eaton J, Matrajt L. Optimizing one-dose and two-dose cholera vaccine allocation in outbreak settings: a modeling study. PLoS Negl Trop Dis 2022; 16:e0010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Azman AS, Luquero FJ, Rodrigues A, et al. . Urban cholera transmission hotspots and their implications for reactive vaccination: evidence from Bissau City, Guinea Bissau. PLoS Negl Trop Dis 2012; 6:e1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qadri F, Ali M, Lynch J, et al. . Efficacy of a single-dose regimen of inactivated whole-cell oral cholera vaccine: results from 2 years of follow-up of a randomised trial. Lancet Infect Dis 2018; 18:666–74. [DOI] [PubMed] [Google Scholar]
- 20. Lopez AL, Deen J, Azman AS, et al. . Immunogenicity and protection from a single dose of internationally available killed oral cholera vaccine: a systematic review and metaanalysis. Clin Infect Dis 2018; 66:1960–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization . Cholera vaccines: WHO position paper—August 2017. Available at: https://www.who.int/publications-detail-redirect/who-wer9234-477-500. Accessed 25 August 2017. [DOI] [PubMed]
- 22. Kanungo S, Desai SN, Nandy RK, et al. . Flexibility of oral cholera vaccine dosing—a randomized controlled trial measuring immune responses following alternative vaccination schedules in a cholera hyper-endemic zone. PLoS Negl Trop Dis 2015; 9:e0003574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mwaba J, Chisenga CC, Xiao S, et al. . Serum vibriocidal responses when second doses of oral cholera vaccine are delayed 6 months in Zambia. Vaccine 2021; 39:4516–23. [DOI] [PubMed] [Google Scholar]
- 24. Chowdhury F, Bhuiyan TR, Akter A, et al. . Augmented immune responses to a booster dose of oral cholera vaccine in Bangladeshi children less than 5 years of age: revaccination after an interval of over three years of primary vaccination with a single dose of vaccine. Vaccine 2020; 38:1753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferreras E, Matapo B, Chizema-Kawesha E, et al. . Delayed second dose of oral cholera vaccine administered before high-risk period for cholera transmission: cholera control strategy in Lusaka, 2016. PLoS One 2019; 14:e0219040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. UNICEF . Amidst insecurity in Haiti, new cholera upsurge puts 1.2 million children at risk. Available at: https://www.unicef.org/press-releases/amidst-insecurity-haiti-new-cholera-upsurge-puts-12-million-children-risk. Accessed 4 October 2022.
- 27. Charnley GE, Jean K, Kelman I, Gaythorpe KA, Murray KA. Association between conflict and cholera in Nigeria and the Democratic Republic of the Congo. Emerg Infect Dis 2022; 28:2472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dureab FA, Shibib K, Al-Yousufi R, Jahn A. Yemen: cholera outbreak and the ongoing armed conflict. J Infect Dev Countries 2018; 12:397–403. [DOI] [PubMed] [Google Scholar]
- 29.WHO | Regional Office for Africa. Ethiopia to vaccinate 2 million against cholera in Tigray region. Available at: https://www.afro.who.int/news/ethiopia-vaccinate-2-million-against-cholera-tigray-region. Accessed 12 June 2021.
- 30. Heyerdahl LW, Ngwira B, Demolis R, et al. . Innovative vaccine delivery strategies in response to a cholera outbreak in the challenging context of Lake Chilwa: a rapid qualitative assessment. Vaccine 2018; 36:6491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cutts FT, Claquin P, Danovaro-Holliday MC, Rhoda DA. Monitoring vaccination coverage: defining the role of surveys. Vaccine 2016; 34:4103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elias Chitio JJ, Baltazar CS, Langa JP, et al. . Pre-emptive oral cholera vaccine (OCV) mass vaccination campaign in Cuamba District, Niassa Province, Mozambique: feasibility, vaccination coverage and delivery costs using CholTool. BMJ Open 2022; 12:e053585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qayum M, Billah MM, Sarker MFR, et al. . Oral cholera vaccine coverage evaluation survey: forcibly displaced Myanmar nationals and host community in Cox's bazar, Bangladesh. Front Public Health 2023; 11:1147563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gelormini M, Gripenberg M, Marke D, et al. . Coverage survey and lessons learned from a pre-emptive cholera vaccination campaign in urban and rural communities affected by landslides and floods in Freetown Sierra Leone. Vaccine 2023; 41:2397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khan AI, Levin A, Chao DL, et al. . The impact and cost-effectiveness of controlling cholera through the use of oral cholera vaccines in urban Bangladesh: a disease modeling and economic analysis. PLoS Negl Trop Dis 2018; 12:e0006652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ali M, Qadri F, Kim DR, et al. . Effectiveness of a killed whole-cell oral cholera vaccine in Bangladesh: further follow-up of a cluster-randomised trial. Lancet Infect Dis 2021; 21:1407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Song KR, Lim JK, Park SE, et al. . Oral cholera vaccine efficacy and effectiveness. Vaccines 2021; 9:1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khatib AM, Ali M, von Seidlein L, et al. . Effectiveness of an oral cholera vaccine in Zanzibar: findings from a mass vaccination campaign and observational cohort study. Lancet Infect Dis 2012; 12:837–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.