Abstract
Context
The presence of metabolic dysfunction–associated steatotic liver disease (MASLD) in patients with diabetes mellitus (DM) is associated with a high risk of cardiovascular disease, but is often underdiagnosed.
Objective
To develop machine learning (ML) models for risk assessment of MASLD occurrence in patients with DM.
Methods
Feature selection determined the discriminative parameters, utilized to classify DM patients as those with and without MASLD. The performance of the multiple logistic regression model was quantified by sensitivity, specificity, and percentage of correctly classified patients, and receiver operating characteristic (ROC) curve analysis. Decision curve analysis (DCA) assessed the model's net benefit for alternative treatments.
Results
We studied 2000 patients with DM (mean age 58.85 ± 17.37 years; 48% women). Eight parameters: age, body mass index, type of DM, alanine aminotransferase, aspartate aminotransferase, platelet count, hyperuricaemia, and treatment with metformin were identified as discriminative. The experiments for 1735 patients show that 744/991 (75.08%) and 586/744 (78.76%) patients with/without MASLD were correctly identified (sensitivity/specificity: 0.75/0.79). The area under ROC (AUC) was 0.84 (95% CI, 0.82-0.86), while DCA showed a higher clinical utility of the model, ranging from 30% to 84% threshold probability. Results for 265 test patients confirm the model's generalizability (sensitivity/specificity: 0.80/0.74; AUC: 0.81 [95% CI, 0.76-0.87]), whereas unsupervised clustering identified high-risk patients.
Conclusion
A ML approach demonstrated high performance in identifying MASLD in patients with DM. This approach may facilitate better risk stratification and cardiovascular risk prevention strategies for high-risk patients with DM at risk of MASLD.
Keywords: diabetes, metabolic dysfunction–associated steatotic liver disease, machine learning, risk prediction
Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases; its prevalence has grown over recent decades and it now affects approximately 30% of adults globally (1). Because NAFLD does not take into account all underlying pathophysiologic conditions, Eslam et al proposed in 2020 to replace this terminology with metabolic dysfunction–associated fatty liver disease (MAFLD), as this highlighted the metabolic abnormalities in the condition (2). However, MAFLD still seemed to stigmatize patients with the term fatty and raised concerns related to mixing etiologies. Therefore, the Delphi consensus recently changed the nomenclature again and proposed metabolic dysfunction–associated steatotic liver disease (MASLD) (3).
One of the most important metabolic diseases among comorbidities associated with liver steatosis is type 2 diabetes mellitus (T2DM) (4). T2DM is also one of the criteria for MASLD classification. Despite the high prevalence of NAFLD (the prevalence of MASLD is not known yet since the term was introduced in June 2023) in patients with T2DM, the diagnosis is often unrecognized in clinical practice, and current risk prediction is suboptimal. The European Associations for the Study of the Liver, Diabetes and Obesity (EASL, EASD, EASO) recommended screening patients with T2DM for the presence of NAFLD because of high risk of disease progression (5) and its associations with an elevated risk of cardiovascular (CV) mortality in the long-term (6). Since CV disease (CVD) is a more common cause of death than liver disease in patients with NAFLD (7), early diagnosis is important. For the detection of liver steatosis, ultrasound is the recommended first-line diagnostic method. However, it may be difficult to encourage every patient suspected of MASLD to undergo it. The diagnostic process could be streamlined if patients are informed that new technology or refined risk stratification suggests that they are at elevated risk for liver steatosis and thus increased CV risk.
Such new technology is machine learning (ML), which may quickly analyze the most discriminative predictors differentiating patients with and without diseases, refining risk stratification (8-10). Recently, we demonstrated that a ML approach based on easy-to-obtain parameters can accurately identify patients with DM at a high risk of new CV events (11) and MAFLD patients with prevalent CVD (12). Such an approach in clinical practice can help identify patients who are at risk of MASLD, who could then be targeted for detailed liver examinations for confirmation. To the best of our knowledge, there has been no study performed to develop ML models for assessing the risk of MASLD occurrence in a well-profiled cohort of patients with DM.
Materials and Methods
Study Design
This is a single-center, observational study, examining patients with DM, hospitalized in the diabetology ward in Zabrze, Poland, in 2015 to 2020. This study is a part of the Silesia Diabetes-Heart Project (registered on ClinicalTrials.gov: NCT05626413), previously detailed in (11). We enrolled patients with type 1 DM (T1DM) or T2DM into the study, and exclusion criteria were terminal stages of cancer or in-hospital mortality. For this analysis, only the baseline hospitalization was considered.
Ethical Approval
Upon admission to the hospital, all patients provided informed consent. Given that the data analyzed were sourced from an anonymized registry, further consent was not required and ethical approval was granted by the Medical University of Silesia Ethics Committee (PCN/0022/KB/126/20). The study adhered to the principles outlined in the Declaration of Helsinki. Any tests or interventions conducted during the hospitalization were part of routine medical care.
MASLD Diagnosis
Diagnosis of MASLD was established through hepatic ultrasonography demonstrating steatosis, in conjunction with at least one of the following criteria: T2DM, a body mass index (BMI) of 25 kg/m2 or greater, a blood pressure reading of 130/85 mmHg or higher, pharmacological treatment for hypertension, plasma triglycerides equal to or greater than 1.7 mmol/L, or plasma high-density lipoprotein cholesterol levels lower than 1.0 mmol/L in men and 1.3 mmol/L in women or current lipid-lowering therapy (3). The ultrasonographic assessment of liver steatosis was performed using the ARIETTA 750 ultrasound system (Hitachi) with a C253 transducer. The full description of the methods is presented in Supplementary material (13).
Predicting MASLD Using ML in Patients With DM
The process of predicting the occurrence of MASLD in patients with DM included the analysis of 80 patient parameters, including demographic characteristics (2 parameters), clinical data (DM-related [3 parameters], vascular disease-related [10], diabetic complications [3], general [3], and concomitant disease [5]), together with laboratory parameters (30) and pharmacological parameters (24) (Table 1). Prior to developing the ML methods, missing data was imputed using factorial analysis (14).
Table 1.
Clinical patient parameters of dataset A
| Parameter | Patients without MASLD (n = 744) | Patients with MASLD (n = 991) | P value |
|---|---|---|---|
| Demographic parameters | |||
| Age [years] | 54.53 ± 20.61 (57.50) | 62.48 ± 13.47 (63.00) | <.001 |
| Men, [n (%)] | 307 (41.26%) | 509 (51.36%) | <.001 |
| Clinical parameters | |||
| Diabetes-related | |||
| BMI [kg/m2] | 27.30 ± 6.58 (26.01) | 32.66 ± 6.69 (32.35) | <.001 |
| Duration of diabetes [years] | 12.00 ± 10.13 (10.00) | 10.59 ± 8.24 (10.00) | .053 |
| Type of diabetes [% of type 1] | 299 (40.19%) | 71 (7.16%) | <.001 |
| Cardiovascular-related | |||
| Atrial fibrillation | 64 (8.86%) | 95 (9.87%) | .482 |
| Carotid arteries stenosis | 14 (1.88%) | 15 (1.51%) | .554 |
| Coronary artery disease | 225 (30.24%) | 388 (39.15%) | <.001 |
| Heart failure | 123 (16.53%) | 194 (19.58%) | .104 |
| Hypertension | 443 (59.62%) | 823 (83.05%) | <.001 |
| Mean diastolic blood pressure [mmHg] | 75.92 ± 7.58 (76.00) | 76.59 ± 7.43 (78.00) | .018 |
| Mean heart rate [bpm] | 80.23 ± 15.42 (80.00) | 80.36 ± 15.00 (80.00) | .936 |
| Mean systolic blood pressure [mmHg] | 125.87 ± 14.92 (124.00) | 129.83 ± 14.74 (130.00) | <.001 |
| Peripheral artery disease | 31 (4.17%) | 50 (5.05%) | .391 |
| Stroke | 64 (8.60%) | 80 (8.07%) | .692 |
| Diabetic complications | |||
| Diabetic foot disease | 19 (2.56%) | 31 (3.13%) | .479 |
| Diabetic peripheral neuropathy | 68 (9.15%) | 84 (8.48%) | .629 |
| Retinopathy | 296 (39.78%) | 332 (33.50%) | .007 |
| General | |||
| Current smoker [% of yes] | 119 (15.99%) | 199 (20.08%) | .029 |
| Emergency admission [% of yes] | 221 (29.70%) | 244 (24.65%) | .018 |
| Number of days of hospital stay | 7.05 ± 2.89 (7.00) | 7.44 ± 2.73 (7.00) | .007 |
| Concomitant diseases | |||
| Chronic kidney disease | 125 (16.87%) | 197 (19.92%) | .103 |
| Degenerative disease of the spine | 199 (26.78%) | 456 (46.01%) | <.001 |
| Hypercholesterolemia | 419 (56.32%) | 694 (70.03%) | <.001 |
| Hypertriglyceridemia | 180 (24.19%) | 471 (47.53%) | <.001 |
| Hyperuricemia | 154 (20.70%) | 350 (35.32%) | <.001 |
| Laboratory parameters | |||
| Alanine aminotransaminase [U/L] | 23.48 ± 24.34 (18.30) | 41.18 ± 84.07 (26.25) | <.001 |
| Aspartate aminotransaminase [U/L] | 21.28 ± 12.30 (18.50) | 41.25 ± 110.22 (25.55) | <.001 |
| Basophil count [109/L] | 0.04 ± 0.05 (0.02) | 0.04 ± 0.06 (0.03) | .003 |
| Creatinine [mmol/L] | 90.60 ± 42.38 (80.00) | 94.05 ± 40.38 (82.00) | .013 |
| CRP [mg/L] | 19.01 ± 52.50 (2.51) | 19.89 ± 54.09 (4.03) | <.001 |
| eGFR [mL/min/1.73m2] | 82.35 ± 33.10 (81.29) | 77.07 ± 31.09 (76.54) | .001 |
| Eosinophil count [109/L] | 0.19 ± 0.22 (0.15) | 0.20 ± 0.53 (0.14) | .137 |
| HbA1c [%] | 9.10 ± 2.45 (8.66) | 9.13 ± 2.33 (8.90) | .323 |
| HCT [%] | 39.45 ± 5.68 (39.90) | 40.27 ± 6.06 (40.90) | <.001 |
| Hgb [g/dL] | 13.40 ± 2.09 (13.50) | 13.72 ± 2.19 (14.00) | <.001 |
| Ketones—urine sample | 195 (26.97%) | 160 (16.31%) | <.001 |
| Lymphocyte count [109/L] | 2.11 ± 1.89 (1.94) | 2.38 ± 3.77 (2.10) | .004 |
| MCH [pg] | 30.42 ± 2.84 (30.40) | 30.88 ± 2.75 (30.80) | <.001 |
| MCHC [g/dL] | 33.86 ± 1.27 (33.90) | 33.98 ± 1.33 (34.00) | .036 |
| MCV [fL] | 89.65 ± 6.31 (89.60) | 90.85 ± 6.96 (90.50) | <.001 |
| Mean fast. glycemia [mg/dL] first day | 193.02 ± 79.69 (179.00) | 197.92 ± 85.45 (179.50) | .324 |
| Mean fast. glycemia [mg/dL] last day | 133.68 ± 38.12 (129.50) | 137.58 ± 34.62 (134.00) | .003 |
| Mean post. glycemia [mg/dL] first day | 173.37 ± 73.00 (163.00) | 178.84 ± 60.32 (166.00) | .027 |
| Mean post. glycemia [mg/dL] last day | 138.86 ± 29.71 (136.00) | 139.80 ± 30.35 (136.00) | .631 |
| Monocyte count [109/L] | 0.64 ± 0.39 (0.55) | 0.66 ± 0.60 (0.56) | .578 |
| Neutrophil count [109/L] | 6.15 ± 3.91 (5.14) | 5.79 ± 4.03 (4.91) | .028 |
| Platelet count [109/L] | 278.95 ± 92.69 (265.00) | 226.94 ± 85.19 (219.00) | <.001 |
| Potassium [mmol/L] | 4.61 ± 0.57 (4.56) | 4.59 ± 0.58 (4.57) | .597 |
| Protein—urine sample | 297 (40.80%) | 411 (41.73%) | .515 |
| Red blood cell count [1012/L] | 4.44 ± 0.79 (4.49) | 4.47 ± 0.71 (4.54) | .051 |
| Sodium [mmol/L] | 138.56 ± 10.31 (139.00) | 140.12 ± 41.32 (139.00) | <.001 |
| Total cholesterol [mmol/L] | 4.59 ± 1.22 (4.49) | 4.64 ± 1.68 (4.43) | .388 |
| Triglyceride [mmol/L] | 1.47 ± 0.98 (1.22) | 2.18 ± 2.02 (1.69) | <.001 |
| Uric acid [mmol/L] | 306.55 ± 115.03 (284.50) | 343.09 ± 110.25 (329.00) | <.001 |
| White blood cell count [109/L] | 9.10 ± 4.61 (8.10) | 8.86 ± 5.20 (8.00) | .243 |
| Pharmacotherapy | |||
| ACEi/ARB | 299 (40.19%) | 612 (61.76%) | <.001 |
| Allopurinol | 123 (16.53%) | 263 (26.54%) | <.001 |
| Alpha blocker | 63 (8.47%) | 121 (12.21%) | .012 |
| Amiodarone | 3 (0.40%) | 7 (0.71%) | .409 |
| ASA | 289 (38.84%) | 562 (56.71%) | <.001 |
| Beta blocker | 315 (42.34%) | 596 (60.14%) | <.001 |
| Calcium blocker | 156 (20.97%) | 317 (31.99%) | <.001 |
| Clopidogrel | 35 (4.70%) | 46 (4.64%) | .951 |
| Digoxin | 12 (1.61%) | 16 (1.61%) | .998 |
| DPP-4 inhibitors | 111 (14.92%) | 162 (16.35%) | .419 |
| Fibrate | 4 (0.54%) | 28 (2.83%) | <.001 |
| GLP-1 agonist | 13 (1.75%) | 22 (2.22%) | .488 |
| Heparin | 37 (4.97%) | 57 (5.75%) | .478 |
| Insulin | 605 (81.32%) | 761 (76.79%) | .023 |
| PPI | 203 (27.28%) | 312 (31.48%) | .058 |
| Loop diuretic | 190 (25.54%) | 325 (32.80%) | .001 |
| Metformin | 241 (32.39%) | 599 (60.44%) | <.001 |
| NOAC | 40 (5.38%) | 65 (6.56%) | .307 |
| Non-loop diuretics | 71 (9.54%) | 202 (20.38%) | <.001 |
| Potassium-sparing diuretics | 55 (7.39%) | 118 (11.91%) | .002 |
| SGLT2 inhibitor | 50 (6.72%) | 153 (15.44%) | <.001 |
| Statin | 303 (40.73%) | 607 (61.25%) | <.001 |
| Sulfonylureas | 195 (26.21%) | 278 (28.05%) | .394 |
| VKA | 23 (3.09%) | 30 (3.03%) | .939 |
For each parameter (if applicable), mean ± SD is reported together with the median (in parentheses). For each binary parameter, we calculated the total number of ones and the percentage of ones. The P values were obtained using either Mann-Whitney U-test or χ2 test as appropriate. The most discriminative predictors are bolded.
Abbreviations: ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; ASA, acetylsalicylic acid; BMI, body mass index; CRP, C-reactive protein; DPP-4, dipeptidyl peptidase 4; eGFR, estimated glomerular filtration rate; GLP-1, glucagon-like peptide-1; HbA1c, hemoglobin A1C; HCT, hematocrit; Hgb, hemoglobin; NOAC, novel oral anticoagulants; MASLD, metabolic dysfunction–associated steatotic liver disease; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; PPI, proton-pump inhibitor; SGLT-2, sodium-glucose cotransporter 2; VKA, vitamin K antagonists.
To determine the most discriminative predictors differentiating patients with and without MASLD, denoted as MASLD(+) and MASLD(-), we utilized feature selection using a χ2 test. The stability of the selected features was ensured by repeating the Monte-Carlo simulation 1000 times: in each experiment, we randomly selected 80% of all patients from the MASLD(+) and MASLD(-) groups, for whom the most discriminative features were selected. For those predictors, the P value obtained by a χ2 test was less than 0.05, and the final set of features included the predictors which were selected as discriminative in all 1000 independent runs.
The most discriminative parameters were used to predict the occurrence of MASLD by a multiple logistic regression (MLR) model, and the cutoff value was extracted from the receiver operating characteristic curve (ROC) using the Index of Union technique (15). Unsupervised hierarchical clustering was performed for all patients using the most discriminative predictors. The optimal number of groups was determined using the Calinski-Harabasz criterion (16).
To evaluate the performance of the algorithms, we use sensitivity, specificity, and the percentage of correctly classified (CC) patients with and without MASLD. For the MLR model, the ROC curve was determined and the area under this curve (AUC) was calculated, together with its 95% CI. The clinical usefulness of the proposed model was assessed using decision curve analysis (DCA). The MLR models were also validated using an independent test set including patients who were never used at any stage of developing the ML model. Clusters were investigated by comparing the parameter values in the obtained groups, as well as the percentage of MASLD(+) patients in each cluster. Statistical analysis, feature selection and visualizations were carried out in MATLAB R2023a.
Results
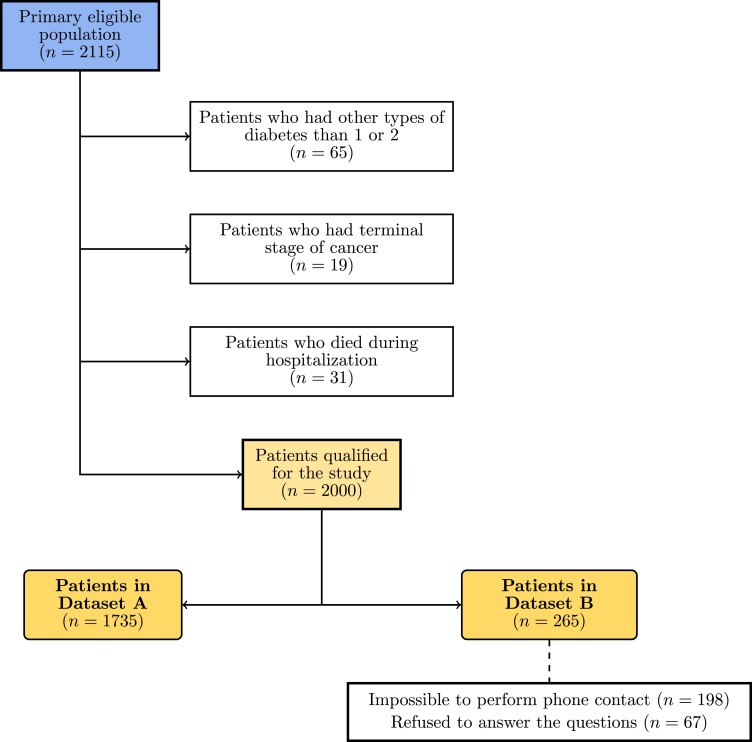
Out of 2115 eligible patients with DM, we included 2000 patients in this study (mean age, 58.85 ± SD 17.37 years; 48% women) (Fig. 1). There were 1735 patients who served for feature selection and for training the models using the most discriminating features (Dataset A) (Table 1). A subset of 265 patients (Dataset B) (Supplementary Table S2) was used as a test set to verify the generalizability of the models (13). Among Dataset A patients (n = 1735), there were 370 (21%) patients with T1DM and 1383 with T2DM; 991 (57%) patients were diagnosed with MASLD. Dataset B (n = 265) consisted of 58 (22%) patients with T1DM and 207 (78%) with T2DM. Here, MASLD was diagnosed in 137 (52%) patients.
Figure 1.
Patient flowchart.
Feature Selection
Feature selection resulted in 8 most discriminative predictors—their values significantly differ across the MASLD(+) and MASLD(-) groups (Table 1 and Supplementary Fig. S1): age, BMI, type of DM, alanine aminotransaminase (ALT), aspartate aminotransaminase (AST), platelet count, treatment with metformin, and hyperuricemia (13). The performance of the MLR model for identifying MASLD(+) and MASLD(-) patients is presented in Table 2.
Table 2.
The results of predicting MASLD in patients with diabetes based on 8 most discriminative and all (80) features using the MLR model
| Subset | Method | Sensitivity | Specificity | CC with MASLD [%] | CC without MASLD [%] | CC all [%] |
|---|---|---|---|---|---|---|
|
Dataset A
(n = 1735) |
MLR (8 features) | 0.75 | 0.79 | 75.08% | 78.76% | 76.66% |
| MLR (80 features) | 0.82 | 0.75 | 82.24% | 75.00% | 79.14% | |
|
Dataset B
(n = 265) |
MLR (8 features) | 0.80 | 0.74 | 79.56% | 74.56% | 74.22% |
| MLR (80 features) | 0.77 | 0.74 | 77.37% | 74.23% | 74.21% |
Abbreviations: CC, correctly classified; MASLD, metabolic dysfunction–associated steatotic liver disease; MLR, multiple logistic regression.
Evaluating the Multiple Logistic Regression ML Model
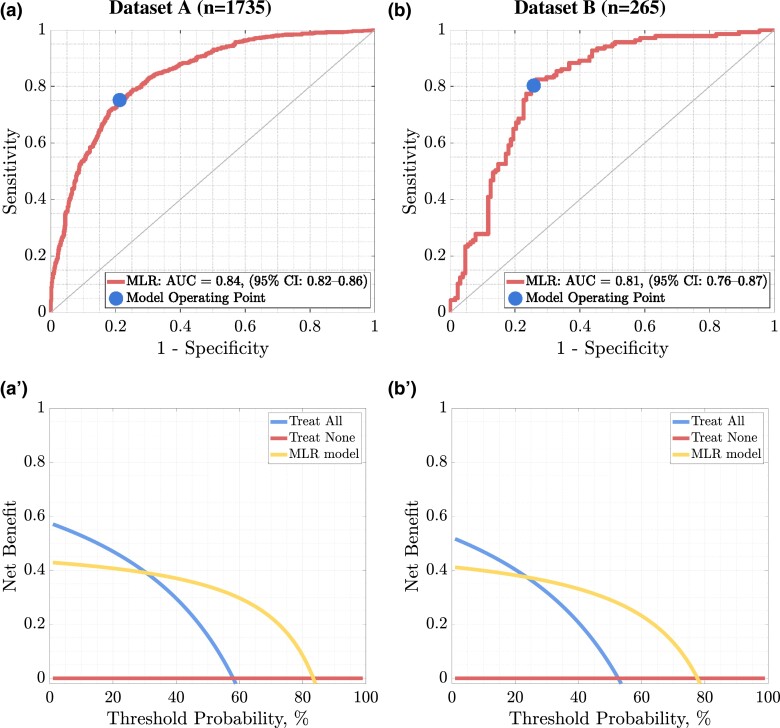
Of the cohort, 744/991 (75.08%) MASLD(+) and 586/744 (78.76%) MASLD(-) patients were correctly identified, which amounts to 1330/1735 (76.66%) of the correct predictions among all Dataset A patients (hence, for 1330/1735 patients, their MASLD status was correctly identified using a ML model). The high performance of the MLR model is also reflected in its sensitivity (0.75) and specificity (0.79). The classifier was evaluated using ROC (Fig. 2a and 2a′) and DCA (Fig. 2b and 2b′) curves. AUC was 0.84 (95% CI, 0.82-0.86), while the clinical utility based on DCA shows a higher utility of the model in terms of net benefit for alternative strategies (treating none and all patients), ranging from 30% to 84% threshold probability.
Figure 2.
The ROC curve (a) together with the DCA (a′) determined for the results of the MLR model fitted to the 8 selected features for dataset a, while the ROC (b) and the DCA (b′) curves were obtained for dataset b. The 45° curve through the origin in the case of the ROC curve shows the discriminant ability of the classifier no better than random selection.
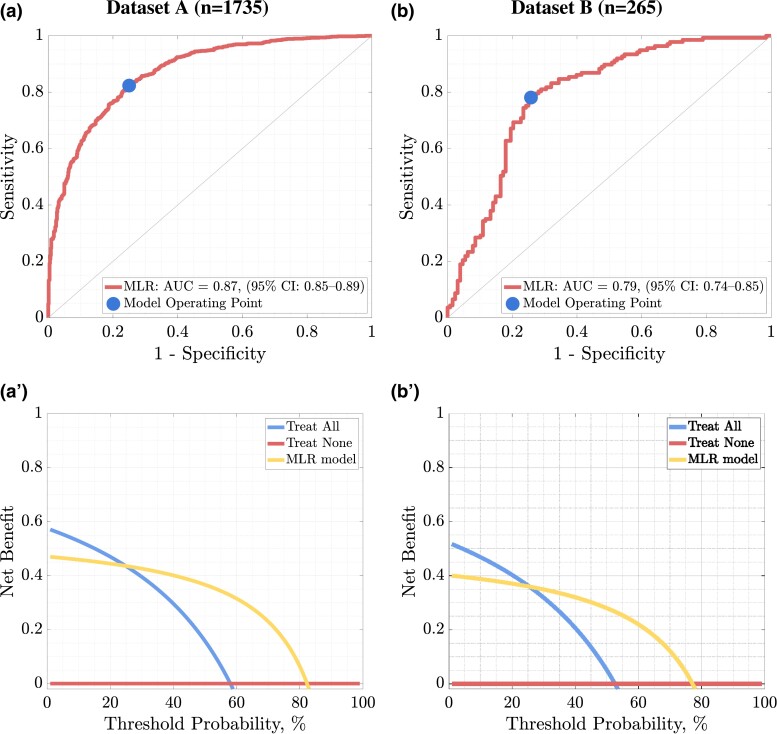
Utilizing all 80 predictors led to AUC of 0.87 (95% CI, 0.86-0.89), with 815/991 (82.24%) and 586/744 (75.00%) correctly identified MASLD(+) and MASLD(-) patients; thus, 1373/1735, 79.14% of all patients were appropriately classified as those with or without MASLD (Table 2). In Dataset B, we obtained an AUC of 0.81 (95% CI, 0.76-0.87) for MLR operating on the 8 most discriminative features, and AUC of 0.79 (95% CI, 0.74-0.85) for the model exploiting all available predictors; this shows the importance of feature selection (Fig. 3). DCA shows a higher utility of the ML model in terms of net benefit for alternative treatment strategies, ranging from 24% to 78% threshold probability for Dataset B.
Figure 3.
The ROC curve (a) together with the DCA (a′) determined for the results of the MLR model fitted to all (80) features for dataset a, while the ROC (b) and the DCA (b′) curves were obtained for dataset b. The 45° curve through the origin in the case of the ROC curve shows the discriminant ability of the classifier no better than random selection.
Unsupervised Hierarchical Clustering
The patients of both datasets were subjected to hierarchical clustering based on the 8 most discriminative features, with the optimal number of 10 clusters. The distribution of those parameters for patients included in all clusters are in Table 3 and Supplementary Fig. S3 for Dataset A, and in Supplementary Table S2 and Supplementary Fig. S4 for Dataset B (13). This showed that there are clusters corresponding to higher-risk MASLD patients which are consistently elaborated for both sets. Such profiling of patients, based on 8 easy-to-obtain parameters, may help better design the treatment pathway to those patients who are at a higher MASLD risk.
Table 3.
The results of hierarchical patient (dataset A) clustering based on 8 most discriminative predictors (the clusters are indicated as C1-C10).
| Parameter | C1 (n = 303) | C2 (n = 400) | C3 (n = 1) | C4 (n = 44) | C5 (n = 153) | C6 (n = 618) | C7 (n = 13) | C8 (n = 201) | C9 (n = 1) | C10 (n = 1) | P value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age [years] | 52.99 ± 18.34 (54.00) | 57.36 ± 18.43 (61.00) | 67.00 ± 0.00 (67.00) | 56.09 ± 18.72 (55.00) | 64.13 ± 15.32 (67.00) | 62.52 ± 15.33 (64.00) | 70.62 ± 10.87 (72.00) | 57.03 ± 17.41 (59.00) | 44.00 ± 0.00 (44.00) | 81.00 ± 0.00 (81.00) | <.001 |
| Alanine aminotransaminase [U/L] | 20.83 ± 9.24 (19.30) | 29.11 ± 20.27 (22.80) | 909.00 ± 0.00 (909.00) | 180.48 ± 85.08 (164.00) | 30.56 ± 18.73 (25.40) | 29.44 ± 18.39 (24.05) | 27.72 ± 18.95 (23.90) | 29.12 ± 22.69 (21.70) | 546.80 ± 0.00 (546.80) | 2170.00 ± 0.00 (2170.00) | <.001 |
| Aspartate aminotransaminase [U/L] | 20.97 ± 8.32 (19.40) | 26.83 ± 16.62 (22.05) | 241.90 ± 0.00 (241.90) | 154.29 ± 82.17 (134.05) | 37.81 ± 26.48 (28.40) | 27.19 ± 14.35 (22.65) | 26.21 ± 20.36 (18.10) | 30.03 ± 23.44 (22.80) | 1340.00 ± 0.00 (1340.00) | 3023.00 ± 0.00 (3023.00) | <.001 |
| BMI [kg/m2] | 28.87 ± 6.66 (27.90) | 30.33 ± 7.04 (29.82) | 25.28 ± 0.00 (25.28) | 29.57 ± 5.87 (29.48) | 30.42 ± 6.39 (30.43) | 31.32 ± 6.75 (31.01) | 27.25 ± 4.98 (27.7) | 28.98 ± 7.22 (28.53) | 25.60 ± 0.00 (25.60) | 23.96 ± 0.00 (23.96) | <.001 |
| Hyperuricemia | 59 (19.47%) | 126 (31.50%) | 0 (0.00%) | 10 (22.73%) | 56 (36.60%) | 194 (31.39%) | 3 (23.08%) | 55 (27.36%) | 0 (0.00%) | 1 (100.00%) | .003 |
| Metformin | 129 (42.57%) | 193 (48.25%) | 0 (0.00%) | 5 (11.36%) | 64 (41.83%) | 362 (58.58%) | 6 (46.15%) | 81 (40.30%) | 0 (0.00%) | 0 (0.00%) | <.001 |
| Platelet count [109/L] | 249.61 ± 16.99 (252.00) | 298.84 ± 22.40 (299.00) | 156.00 ± 0.00 (156.00) | 189.30 ± 81.59 (186.50) | 115.01 ± 35.81 (128.00) | 197.76 ± 24.49 (197.00) | 716.54 ± 142.54 (683.00) | 395.65 ± 53.68 (385.00) | 66.00 ± 0.00 (66.00) | 113.00 ± 0.00 (113.00) | <.001 |
| Type of diabetes [% of type 1] | 119 (39.27%) | 100 (25.00%) | 0 (0.00%) | 3 (6.82%) | 13 (8.50%) | 85 (13.75%) | 1 (7.69%) | 48 (23.88%) | 1 (100.00%) | 0 (0.00%) | <.001 |
| MASLD | 117 (38.61%) | 188 (47.00%) | 1 (100.00%) | 40 (90.91%) | 140 (91.50%) | 416 (67.31%) | 3 (23.08%) | 84 (41.79%) | 1 (100.00%) | 1 (100.00%) | <.001 |
For each parameter (if applicable), mean ± SD is reported together with the median (in parentheses). For each binary parameter, we calculated the total number of ones and the percentage of ones. The P values were obtained using the Kruskal-Wallis test with post hoc Dunn. The row with the target (MASLD) is boldfaced.
Discussion
The key findings of our investigation are as follows: (i) we determine 8 (out of 80) most discriminative patient parameters (age, BMI, type of DM, ALT, AST, hyperuricemia, platelet count, and metformin treatment) which enabled to identify patients who are most likely to present with MASLD using a MLR model; and (ii) on the basis of 8 parameters, we clustered individuals with similar phenotypes in order to stratify risk of MASLD presence.
The potential clinical utility of our approach lies in the independent role of MASLD as predictor of CVD, which is distinct and additive to the risks associated with T2DM (17). The successful identification of MASLD in DM patients using our ML model indicates an elevated CVD risk, surpassing the inherent risks of DM. Hence, accurate identification of MASLD enables more nuanced CVD risk stratification, essential for tailoring preventive and therapeutic strategies in DM patients. This becomes particularly actionable for patients with DM with the availability of SGLT2 inhibitors and GLP-1 receptor agonists, proven to reduce CVD risk (18).
Since MASLD is only recently established nomenclature, we will refer to studies examining NAFLD/MAFLD given that those studying MASLD are not available yet. All of the parameters that were determined in our study are easy to obtain and interpret in clinical practice and mainly related to the metabolic aspect of MASLD. Age was one of the parameters determined in feature selection implicating that the older age is related to a higher MASLD risk. Data from the studies performed recently are not uniform in this term, where the incidence rate of NAFLD assessed annually was higher in people at least 50 years of age (5.5%) compared with those younger than 50 years (3.5%) (19), and varied across age groups in Chinese (20) and Israeli population (21). It can therefore be assumed that the NAFLD incidence rate may be race-related and would differ by age; however, the data related to MASLD are not available yet.
Excessive body weight is a factor included in the definition of MASLD. In the present analysis, BMI was identified as an important parameter, where higher BMI indicated the elevated risk of MASLD. This confirms what is already known for NAFLD, as an increase in BMI was a risk factor for new onset of NAFLD (19), and that the risk increases with an increasing BMI (22). Moreover, our model pinpoints the type of diabetes as a relevant parameter, specifically highlighting associations between T2DM and MASLD risk. Our analysis also brings attention to T1DM, a condition where NAFLD is much less prevalent (23) but is especially important in relation to recent data revealing that the 10-year estimated CVD risk is much higher in T1DM patients with hepatic steatosis and significant fibrosis when compared to those with hepatic steatosis alone or without steatosis (24). Moreover, prior cross-sectional studies indicate an association between NAFLD and an increased risk of CVD in T1DM, even after adjusting for traditional risk factors (25, 26). Furthermore, in patients with T1DM, NAFLD has been linked to chronic kidney disease (27), and a connection between MASLD and retinopathy has been established (28). This suggests that the ability to diagnose MASLD in T1DM could facilitate more comprehensive or frequent screening for these complications in this high-risk population.
Other parameters determined as those identifying patients with MASLD were higher values of ALT and AST. This is important, given that incorporating liver enzymes activity into assessment of patients with NAFLD can help categorize them as low or high risk for advanced fibrosis stage and metabolic-associated comorbidities (29). Also, a lower platelet count was another factor related to the presence of MASLD. This has a pathophysiological basis as platelet synthesis is regulated by thrombopoietin, a glycoprotein hormone synthesized in the liver and an inverse correlation has been reported between the degree of hepatitis and the platelet count (30). Indeed, thrombocytopenia has been related to NAFLD (31) and platelet counts could even serve as biomarker of the severity of fibrosis in NAFLD patients (32).
The most important factor associated with prognosis in patients with NAFLD is liver fibrosis (33). The gold standard of fibrosis diagnosis remains liver biopsy (34), but it is an invasive procedure so difficult to implement in all patients with NAFLD. Thus, there has been a clinical scoring tool developed, that is, the fibrosis-4 index, and recommended for noninvasive detection of advanced liver fibrosis (29, 35). In the light of presented outcomes, our model identified patients based on all these parameters and more, that constituted the screening tool for advanced fibrosis. While this is a notable observation, it does not imply that the model possesses the capability to assess fibrosis risk per se, which needs elastography/liver biopsy. Another important metabolic abnormality is hyperuricemia which we identified as a parameter determining MASLD. It confirms prior observations that elevated uric acid was a risk factor for NAFLD (36) and an independent predictor for NAFLD (37).
Our feature selection discriminated also a parameter related to DM pharmacotherapy where high-risk patients for MASLD were those who were treated with metformin. This association seems surprising because metformin has been shown to have a positive impact on liver function in NAFLD (38). On the other hand, other studies analyzing long-term metformin therapy did not show any protective effects on the liver histology (39) or a moderate one (40). Our results also confirm a real-world study, where metformin treatment caused a higher risk of developing NAFLD (41).
Finally, our predictive model based on 8 parameters correctly classified over 76% of patients, indicating its substantial capability in identifying MASLD. Beyond this metric, the model's discriminative power is further evidenced by an AUC of 0.84. The significance of this model extends into its clinical utility, as demonstrated by DCA, which reveals the model's applicability across a wide range of threshold probabilities (30%-84%). This indicates the model's relevance in varying clinical contexts, enabling healthcare professionals to apply it effectively across different patient risk profiles. For instance, in a clinical context where early detection is prioritized and diagnostic tests available, a lower threshold probability might be chosen, making the model sensitive to even moderate risks of MASLD.
When all 80 parameters were utilized by the model, the accuracy of identifying patients with MASLD were higher, but such large number of parameters is difficult to obtain in clinical settings, and the model is more likely to overfit (due to the ratio of the number of features to the number of observations) and unable to generalize. The MLR model was verified using an independent test set of 265 patients, for which the obtained metrics confirm the generalization capabilities of the model operating on 8 patients’ parameters. The clinical utility determined using DCA further highlights the clinical usefulness of the ML model.
Apart from determining the features indicating the risk for MASLD patients, our cluster analysis performed for 1735 patients from Dataset A divided them into 10 clusters. Of these clusters, C3, C9, and C10 containing a single patient appeared to be of the highest risk for MASLD and differed significantly in the analyzed parameters compared to the rest of the cohort. Such significantly different clusters were similarly visible in the test set containing patients from Dataset B, where the number of clusters was kept consistent with the optimal number of clusters determined for Dataset A (ie, 10). Albeit the differences in patient parameters are not necessarily statistically significant, qualitative analysis reveals that 3 clusters (C3, C9, and C10) contained patients with visibly different clinical characteristics than the other clusters. These differences in parameters in those 3 clusters are related to elevated activity of liver enzymes and low platelet count which are the parameters inseparable of the liver metabolic function and are used in fibrosis-4 index for assessing the fibrosis risk (42).
Our unsupervised ML distinguished the cluster of patients at a high risk of MASLD based on these parameters. This analysis highlights the importance of unsupervised hierarchical clustering in profiling patients with DM, and the possibility of extracting patient group clusters with discriminative clinical parameters. Moreover, other clusters indicate patients who are not at high risk of MASLD (clusters C1, C2, C7, and C8), where the risk of having MASLD was lower than 50%. For the 265 patients from Dataset B, clustering resulted in similarly consistent groups, indicating the patients who are at a higher risk of MASLD.
Limitations
Although the results obtained over the set of 265 test patients confirm the generalizability of the models, this was a single-center study, hence validating the models over a larger and more general cohort could robustify our findings. Moreover, neither elastography nor liver biopsy was performed; therefore, there is no information about the stage of MASLD. Ultrasonography offers the qualitative rather than quantitative assessment of liver fat content and cannot assess fibrosis, limiting the understanding of the disease's severity. We acknowledge that the lack of waist circumference and high-density lipoprotein cholesterol measurements might lead to a slight underestimation of MASLD. However, the majority of enrolled patients are at least overweight or have T2DM which classifies them for MASLD diagnosis if other criteria are fulfilled.
Conclusion
A ML approach demonstrated high performance in identifying MASLD in patients with DM. This approach may facilitate better risk stratification and cardiovascular risk prevention strategies for high-risk patients with DM at risk of MASLD.
Abbreviations
- ALT
alanine aminotransferase
- AUC
area under the curve
- AST
aspartate aminotransferase
- BMI
body mass index
- CV
cardiovascular
- CVD
cardiovascular disease
- DCA
decision curve analysis
- DM
diabetes mellitus
- MAFLD
metabolic-associated fatty liver disease
- MASLD
metabolic dysfunction–associated steatotic liver disease
- ML
machine learning
- MLR
multiple logistic regression
- NAFLD
nonalcoholic fatty liver disease
- ROC
receiver operating characteristic
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
Contributor Information
Katarzyna Nabrdalik, Department of Internal Medicine, Diabetology and Nephrology, Faculty of Medical Sciences in Zabrze, Medical University of Silesia, 40-055 Katowice, Poland; Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart & Chest Hospital, Liverpool L69 3BX, UK.
Hanna Kwiendacz, Department of Internal Medicine, Diabetology and Nephrology, Faculty of Medical Sciences in Zabrze, Medical University of Silesia, 40-055 Katowice, Poland.
Krzysztof Irlik, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart & Chest Hospital, Liverpool L69 3BX, UK; Students’ Scientific Association by the Department of Internal Medicine, Diabetology and Nephrology, Faculty of Medical Sciences in Zabrze, Medical University of Silesia, 40-055 Katowice, Poland.
Mirela Hendel, Students’ Scientific Association by the Department of Internal Medicine, Diabetology and Nephrology, Faculty of Medical Sciences in Zabrze, Medical University of Silesia, 40-055 Katowice, Poland.
Karolina Drożdż, Department of Internal Medicine, Diabetology and Nephrology, Faculty of Medical Sciences in Zabrze, Medical University of Silesia, 40-055 Katowice, Poland.
Agata M Wijata, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart & Chest Hospital, Liverpool L69 3BX, UK; Faculty of Biomedical Engineering, Silesian University of Technology, 41-800 Zabrze, Poland.
Jakub Nalepa, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart & Chest Hospital, Liverpool L69 3BX, UK; Department of Algorithmics and Software, Silesian University of Technology, 44-100 Gliwice, Poland.
Oliwia Janota, Department of Internal Medicine, Diabetology and Nephrology, Faculty of Medical Sciences in Zabrze, Medical University of Silesia, 40-055 Katowice, Poland.
Wiktoria Wójcik, Students’ Scientific Association by the Department of Internal Medicine, Diabetology and Nephrology, Faculty of Medical Sciences in Zabrze, Medical University of Silesia, 40-055 Katowice, Poland.
Janusz Gumprecht, Department of Internal Medicine, Diabetology and Nephrology, Faculty of Medical Sciences in Zabrze, Medical University of Silesia, 40-055 Katowice, Poland.
Gregory Y H Lip, Liverpool Centre for Cardiovascular Science at University of Liverpool, Liverpool John Moores University and Liverpool Heart & Chest Hospital, Liverpool L69 3BX, UK; Danish Center for Health Services Research, Department of Clinical Medicine, Aalborg University, 9220 Aalborg, Denmark.
Funding
A.M.W. and J.N. were supported by the Silesian University of Technology funds through the Excellence Initiative—Research University program (grant 02/080/SDU/10-21-01). A.M.W. was partially supported by the Silesian University of Technology funds through the grant for maintaining and developing research potential (grant 07/010/BKM23/1028). The publication fee was covered by Medical University of Silesia.
Author Contributions
K.N., H.K., J.G., and G.Y.H.L.: substantial contribution to the conception and design of the work; K.N. and H.K.: project administration; K.I., M.H., K.D., O.J., and W.W.: collected the data; H.K. and A.M.W.: prepared the dataset for statistical analysis; A.M.W.: conceptualization of data analysis; A.M.W. and J.N.: designed the machine learning algorithms; A.M.W.: implemented and verified the machine learning algorithms; A.M.W.: visualization of the experimental results; A.M.W. and J.N.: performed the computational experiments, data analysis and prepared the tables and figures; K.N.: drafted the manuscript; A.M.W. and J.N.: drafted the manuscript (data analysis & statistical methods and results); J.G.: substantively revised the work; K.N., J.N., A.M.W., K.I., J.G., and G.Y.H.L.: review & editing of the original draft. K.N. and J.N. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All of the authors have read and approved the submitted version of the manuscript.
Disclosure
The authors have nothing to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77(4):1335‐1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eslam M, Sanyal AJ, George J, International Consensus Panel . MAFLD: a consensus-driven proposed Nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999‐2014.e1. [DOI] [PubMed] [Google Scholar]
- 3. Rinella ME, Lazarus JV, Ratziu V, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78(6):1966‐1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2(3):262‐265. [DOI] [PubMed] [Google Scholar]
- 5. EASL–EASD–EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388‐1402. [DOI] [PubMed] [Google Scholar]
- 6. Calori G, Lattuada G, Ragogna F, et al. Fatty liver index and mortality: the cremona study in the 15th year of follow-up. Hepatology. 2011;54(1):145‐152. [DOI] [PubMed] [Google Scholar]
- 7. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363(14):1341‐1350. [DOI] [PubMed] [Google Scholar]
- 8. Ross EG, Jung K, Dudley JT, Li L, Leeper NJ, Shah NH. Predicting future cardiovascular events in patients with peripheral artery disease using electronic health record data. Circ Cardiovasc Qual Outcomes. 2019;12(3):e004741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cho SY, Kim SH, Kang SH, et al. Pre-existing and machine learning-based models for cardiovascular risk prediction. Sci Rep. 2021;11(1):8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meng J, Xing R. Inside the “black box”: embedding clinical knowledge in data-driven machine learning for heart disease diagnosis. Cardiovasc Digit Health J. 2022;3(6):276‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nabrdalik K, Kwiendacz H, Drożdż K, et al. Machine learning predicts cardiovascular events in patients with diabetes: the silesia diabetes-heart project. Curr Probl Cardiol. 2023;48(7):101694. [DOI] [PubMed] [Google Scholar]
- 12. Drożdż K, Nabrdalik K, Kwiendacz H, et al. Risk factors for cardiovascular disease in patients with metabolic-associated fatty liver disease: a machine learning approach. Cardiovasc Diabetol. 2022;21(1):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nabrdalik K, Kwiendacz H, Irlik K, et al. Machine learning identifies metabolic dysfunction associated steatotic liver disease in patients with diabetes mellitus. Cardiovasc Diabetol. 2023;22(1):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Audigier V, Husson F, Josse J. A principal component method to impute missing values for mixed data. Adv Data Anal Classif. 2016;10(1):5‐26. [Google Scholar]
- 15. Unal I. Defining an optimal cut-point value in ROC analysis: an alternative approach. Comput Math Methods Med. 2017;2017:3762651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rousseeuw PJ. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math. 1987;20:53‐65. [Google Scholar]
- 17. Moon JH, Jeong S, Jang H, Koo BK, Kim W. Metabolic dysfunction-associated steatotic liver disease increases the risk of incident cardiovascular disease: a nationwide cohort study. eClinicalMedicine. 2023;65:102292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of Major adverse cardiovascular and renal outcomes in type 2 diabetes Mellitus. Circulation. 2019;139(17):2022‐2031. [DOI] [PubMed] [Google Scholar]
- 19. Lin Y, Feng X, Cao X, et al. Age patterns of nonalcoholic fatty liver disease incidence: heterogeneous associations with metabolic changes. Diabetol Metab Syndr. 2022;14(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu W, Feng A, Ma W, et al. Worldwide long-term trends in the incidence of nonalcoholic fatty liver disease during 1990-2019: a joinpoint and age-period-cohort analysis. Front Cardiovasc Med. 2022;9:891963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aneni EC, Saeed GJ, Bittencourt MS, et al. Cardiometabolic disorders, inflammation and the incidence of non-alcoholic fatty liver disease: a longitudinal study comparing lean and non-lean individuals. PLoS One. 2022;17(4):e0266505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loomis AK, Kabadi S, Preiss D, et al. Body mass Index and risk of nonalcoholic fatty liver disease: two electronic health record prospective studies. J Clin Endocrinol Metab. 2016;101(3):945‐952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cusi K, Sanyal AJ, Zhang S, et al. Non-alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes Metabol. 2017;19(11):1630‐1634. [DOI] [PubMed] [Google Scholar]
- 24. Mantovani A, Morieri ML, Palmisano L, et al. Hepatic steatosis with significant fibrosis is associated with an increased 10-year estimated risk of cardiovascular disease in adults with type 1 diabetes mellitus. Cardiovasc Diabetol. 2023;22(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Targher G, Bertolini L, Padovani R, et al. Prevalence of non-alcoholic fatty liver disease and its association with cardiovascular disease in patients with type 1 diabetes. J Hepatol. 2010;53(4):713‐718. [DOI] [PubMed] [Google Scholar]
- 26. Mertens J, Van Gaal LF, Francque SM, De Block C. NAFLD in type 1 diabetes: overrated or underappreciated? Ther Adv Endocrinol. 2021;12:20420188211055557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Targher G, Mantovani A, Pichiri I, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of chronic kidney disease in patients with type 1 diabetes. Diabetes Care. 2014;37(6):1729‐1736. [DOI] [PubMed] [Google Scholar]
- 28. Mantovani A, Morieri ML, Aldigeri R, et al. MASLD, hepatic steatosis and fibrosis are associated with the prevalence of chronic kidney disease and retinopathy in adults with type 1 diabetes mellitus. Diabetes Metab. 2023;50(1):101497. [DOI] [PubMed] [Google Scholar]
- 29. Shirin D, Peleg N, Sneh-Arbib O, et al. The pattern of elevated liver function tests in nonalcoholic fatty liver disease predicts fibrosis stage and metabolic-associated comorbidities. Digest Dis. 2018;37(1):69‐76. [DOI] [PubMed] [Google Scholar]
- 30. Murali AR, Attar BM, Katz A, Kotwal V, Clarke PM. Utility of platelet count for predicting cirrhosis in alcoholic liver disease: model for identifying cirrhosis in a US population. J Gen Intern Med. 2015;30(8):1112‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rivera-Álvarez M, Córdova-Ramírez AC, Elías-De-La-Cruz GD, et al. Non-alcoholic fatty liver disease and thrombocytopenia IV: its association with granulocytopenia. Hematol Transfus Cell Ther. 2022;44(4):491‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoneda M, Fujii H, Sumida Y, et al. Platelet count for predicting fibrosis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46(11):1300‐1306. [DOI] [PubMed] [Google Scholar]
- 33. Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but No other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389‐397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67(1):328. [DOI] [PubMed] [Google Scholar]
- 35. Park H, Yoon EL, Ito T, et al. Diagnostic performance of the fibrosis-4 Index and nonalcoholic fatty liver disease fibrosis score in lean adults with nonalcoholic fatty liver disease. JAMA Network Open. 2023;6(8):e2329568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang C, Yang S, Xu W, Zhang J, Fu W, Feng C. Association between the hyperuricemia and nonalcoholic fatty liver disease risk in a Chinese population: a retrospective cohort study. PLoS One. 2017;12(5):e0177249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wei F, Li J, Chen C, et al. Higher Serum uric acid level predicts non-alcoholic fatty liver disease: a 4-year prospective cohort study. Front Endocrinol (Lausanne). 2020;11:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Rep. 2013;1(1):57‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shields WW, Thompson KE, Grice GA, Harrison SA, Coyle WJ. The effect of metformin and standard therapy versus standard therapy alone in nondiabetic patients with insulin resistance and nonalcoholic steatohepatitis (NASH): a pilot trial. Therap Adv Gastroenterol. 2009;2(3):157‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Loomba R, Lutchman G, Kleiner DE, et al. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29(2):172‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang KH, Lee CH, Cheng YD, et al. Correlation between long-term use of metformin and incidence of NAFLD among patients with type 2 diabetes mellitus: a real-world cohort study. Front Endocrinol (Lausanne). 2022;13:1027484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Han S, Choi M, Lee B, et al. Accuracy of noninvasive scoring systems in assessing liver fibrosis in patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gut Liver. 2022;16(6):952‐963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.